Conservation of Soil Organic Carbon and Nitrogen Fractions in a Tallgrass Prairie in Oklahoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Soil Sampling and Analyses
2.3. Statistical Analyses
3. Results and Discussion
3.1. Total Organic C and N
3.2. Particulate Organic C and N
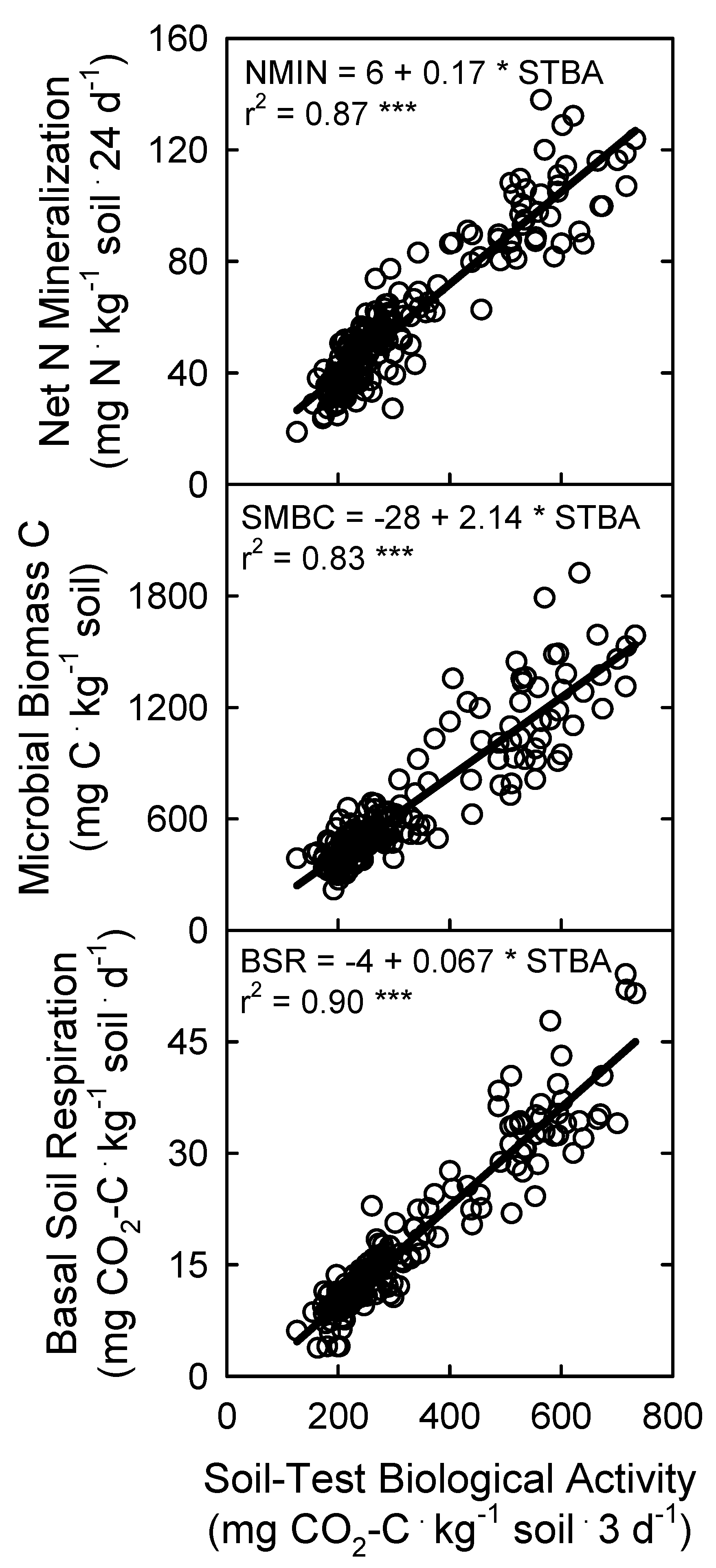
3.3. Soil Microbial Biomass and Activity
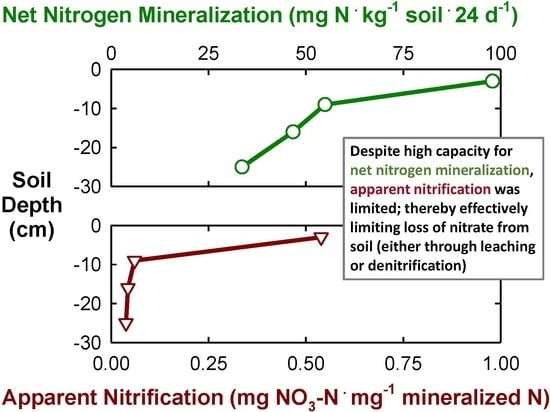
3.4. Residual Inorganic N and Net N Mineralization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stefferud, A. (Ed.) Grass: The 1948 Yearbook of Agriculture; U.S. Govt. Print. Office: Washington, DC, USA, 1948; 892p.
- Savory, A.; Butterfield, J. Holistic Management, 2nd ed.; Island Press: Washington, DC, USA, 1999; 616p. [Google Scholar]
- Wedin, W.F.; Fales, S.L. (Eds.) Grassland: Quietness and Strength for a New American Agriculture; American Society for Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2009; 256p. [Google Scholar]
- Franzluebbers, A.J. Ecosystem services from forages. In Cool Forages: Advanced Management of Temperate Forages; Bittman, S., Hunt, D., Eds.; Pacific Field Corn Association: Agassiz, BC, Canada, 2013; pp. 2–6. [Google Scholar]
- Sollenberger, L.E.; Kohmann, M.M.; Dubeux, J.C.B.; Silveira, M.L. Grassland management affects delivery of regulating and supporting ecosystem services. Crop Sci. 2019, 59, 441–459. [Google Scholar] [CrossRef]
- Briske, D.D.; Derner, J.D.; Brown, J.R.; Fuhlendorf, S.D.; Teague, W.R.; Havstad, K.M.; Gillen, R.L.; Ash, A.J.; Willms, W.D. Rotational grazing on rangelands: Reconciliation of perception and experimental evidence. Rangel. Ecol. Manag. 2008, 61, 3–17. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Paine, L.K.; Winsten, J.R.; Krome, M.; Sanderson, M.A.; Ogles, K.; Thompson, D. Well-managed grazing systems: A forgotten hero of conservation. J. Soil Water Conserv. 2012, 67, 100A–104A. [Google Scholar] [CrossRef]
- Nelson, C.J. (Ed.) Conservation Outcomes from Pastureland and Hayland Practices: Assessment, Recommendations, and Knowledge Gaps; Allen Press, Inc.: Lawrence, KS, USA, 2012; 362p. [Google Scholar]
- Franzluebbers, A.J. Grass roots of soil carbon sequestration. Carbon Manag. 2012, 3, 9–11. [Google Scholar] [CrossRef]
- Parton, W.J.; Stewart, J.W.B.; Cole, C.V. Dynamics of C, N, P and S in grassland soils: A model. Biogeochemistry 1988, 5, 109–131. [Google Scholar] [CrossRef]
- Burke, I.C.; Yonker, C.M.; Parton, W.J.; Cole, C.V.; Flach, K.; Schimel, D.S. Texture, climate and cultivation effects on soil organic matter content in U.S. grassland soils. Soil Sci. Soc. Am. J. 1989, 53, 800–805. [Google Scholar] [CrossRef]
- Steinfeld, H.; Wassenaar, T. The role of livestock production in carbon and nitrogen cycles. Ann. Rev. Environ. Resour. 2007, 32, 271–294. [Google Scholar] [CrossRef]
- Taboada, M.A.; Rubio, G.; Chaneton, E.J. Grazing impacts on soil physical, chemical and ecological properties in forage production systems. In Soil Management: Building a Stable Base for Agriculture; Hatfield, J.L., Sauer, T.J., Eds.; American Society for Agronomy, Soil Science Society of America: Madison, WI, USA, 2011; pp. 301–320. [Google Scholar]
- Soussana, J.-F.; Lemaire, G. Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agric. Ecosyst. Environ. 2014, 190, 9–17. [Google Scholar] [CrossRef]
- Abdalla, K.; Mutema, M.; Chivenge, P.; Everson, C.; Chaplot, V. Grassland degradation significantly enhances soil CO2 emission. Catena 2018, 167, 284–292. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Hons, F.M.; Zuberer, D.A. In situ and potential CO2 evolution from a Fluventic Ustochrept in southcentral Texas as affected by tillage and cropping intensity. Soil Tillage Res. 1998, 47, 303–308. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Achieving soil organic carbon sequestration with conservation agricultural systems in the southeastern United States. Soil Sci. Soc. Am. J. 2010, 74, 347–357. [Google Scholar] [CrossRef]
- Hewins, D.B.; Lyseng, M.P.; Schoderbek, D.F.; Alexander, M.; Willms, W.D.; Carlyle, C.N.; Chang, S.X.; Bork, E.W. Grazing and climate effects on soil organic carbon concentration and particle-size association in northern grasslands. Sci. Rep. 2018, 8, 1336. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Casanovas, N.; DeLucia, N.J.; Bernacchi, C.J.; Boughton, E.H.; Sparks, J.P.; Chamberlain, S.D.; DeLucia, E.H. Grazing alters net ecosystem C fluxes and the global warming potential of a subtropical pasture. Ecol. Appl. 2018, 28, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.L.; Hons, F.M.; Rouquette, F.M. Long-term management impacts on soil carbon and nitrogen dynamics of grazed bermudagrass pastures. Soil Biol. Biochem. 2004, 36, 1809–1816. [Google Scholar] [CrossRef]
- Teague, W.R.; Dowhower, S.L.; Baker, S.A.; Haile, N.; DeLaune, P.B.; Conover, D.M. Grazing management impacts on vegetation, soil biota and soil chemical, physical and hydrological properties in tall grass prairie. Agric. Ecosyst. Environ. 2011, 141, 310–322. [Google Scholar] [CrossRef]
- Sollenberger, L.E.; Agouridis, C.T.; Vanzant, E.S.; Franzluebbers, A.J.; Owens, L.B. Prescribed grazing on pasturelands. In Conservation Outcomes from Pastureland and Hayland Practices: Assessment, Recommendations, and Knowledge Gaps; Nelson, C.J., Ed.; Allen Press, Inc.: Lawrence, KS, USA, 2012; pp. 111–204. [Google Scholar]
- Briske, D.D.; Sayer, N.F.; Huntsinger, L.; Fernandez-Gimenez, M.; Budd, B.; Derner, J.D. Origin, persistence, and resolution of the rotations grazing debate: Integrating human dimensions into rangeland research. Rangel. Ecol. Manag. 2011, 64, 325–334. [Google Scholar] [CrossRef]
- Teague, R.; Provenza, F.; Kreuter, U.; Steffens, T.; Barnes, M. Multi-paddock grazing on rangelands: Why the perceptual dichotomy between research results and rancher experience? J. Environ. Manag. 2013, 128, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Chaplot, V.; Dlamini, P.; Chivenge, P. Potential of grassland rehabilitation through high density-short duration grazing to sequester atmospheric carbon. Geoderma 2016, 271, 10–17. [Google Scholar] [CrossRef]
- Sanderman, J.; Reseigh, J.; Wurst, M.; Young, M.-A.; Austin, J. Impacts of rotational grazing on soil carbon in native grass-based pastures in southern Australia. PLoS ONE 2015, 10, e0136157. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.L.; Starks, P.J.; Neel, J.P.S.; Northup, B.; Turner, K.E.; Gowda, P.; Coleman, S.; Brown, M. Managing Tallgrass Prairies for Productivity and Ecological Function: A Long Term Grazing Experiment in the Southern Great Plains, USA; USDA-Agricultural Research Service: Raleigh, NC, USA, 2019.
- Bundy, L.G.; Meisinger, J.J. Nitrogen availability indices. In Methods of Soil Analysis, Part 2; Weaver, R.W., Angle, J.S., Bottomley, P.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 951–984. [Google Scholar]
- Franzluebbers, A.J.; Stuedemann, J.A. Early response of soil organic fractions to tillage and integrated crop-livestock production. Soil Sci. Soc. Am. J. 2008, 72, 613–625. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Stuedemann, J.A. Particulate and non-particulate fractions of soil organic carbon under pastures in the Southern Piedmont USA. Environ. Pollut. 2002, 116, S53–S62. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Stuedemann, J.A. Temporal dynamics of total and particulate organic carbon and nitrogen in cover crop grazed cropping systems. Soil Sci. Soc. Am. J. 2014, 78, 1404–1413. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Stuedemann, J.A. Bermudagrass management in the Southern Piedmont USA. III. Particulate and biologically active soil carbon. Soil Sci. Soc. Am. J. 2003, 67, 132–138. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Pershing, M.R.; Crozier, C.; Osmond, D.; Schroeder-Moreno, M. Soil-test biological activity with the flush of CO2: I. C and N characteristics of soils in corn production. Soil Sci. Soc. Am. J. 2018, 82, 685–695. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Pershing, M.R. Soil-test biological activity with the flush of CO2: II. Greenhouse growth bioassay from soils in corn production. Soil Sci. Soc. Am. J. 2018, 82, 696–707. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Pehim-Limbu, S.; Poore, M.H. Soil-test biological activity with the flush of CO2: IV. Fall-stockpiled tall fescue yield response to applied nitrogen. Agron. J. 2018, 110, 2033–2049. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Yoshihashi, T.; Worthington, M.; Nakahara, K.; Ando, Y.; Sahrawat, K.L.; Rao, I.M.; Lata, J.-C.; Kishii, M.; Braun, H.-J. Suppression of soil nitrification by plants. Plant Sci. 2015, 233, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, R.C.; Nunez, J.; Arenas, L.; Rao, I.; Trujillo, C.; Alvarez, C.; Arango, J.; Rasche, F.; Chirinda, N. Biological nitrification inhibition by Brachiaria grasses mitigates soil nitrous oxide emissions from bovine urine patches. Soil Biol. Biochem. 2017, 107, 156–163. [Google Scholar] [CrossRef]

| Soil Depth (cm) | 2009 | 2012 | 2017 * | |||
|---|---|---|---|---|---|---|
| Continuous | Rotational | Continuous | Rotational | Continuous | Rotational | |
| 0−6 | 3.05 | 2.84 | 2.57 | 2.49 | 1.98 | 1.82 |
| 6−12 | 1.55 | 1.52 | 1.37 | 1.38 | ||
| 12−20 | 1.23 | 1.22 | 1.15 | 1.16 | 0.99 | 1.02 |
| 20−30 | 1.01 | 0.97 | 1.00 | 1.00 | ||
| LSD (p < 0.05) | 0.40 | 0.08 | 0.39 | |||
| Soil Depth (cm) | 2009 | 2012 | 2017 * | |||
|---|---|---|---|---|---|---|
| Continuous | Rotational | Continuous | Rotational | Continuous | Rotational | |
| 0–6 | 18.9 | 16.4 | 10.3 | 9.9 | 4.5 | 4.3 |
| 6–12 | 2.6 | 2.9 | 2.0 | 2.0 | ||
| 12–20 | 1.6 | 1.7 | 1.4 | 1.5 | 0.9 | 1.0 |
| 20–30 | 1.2 | 1.1 | 1.1 | 1.1 | ||
| LSD (p < 0.05) | 4.9 | 1.2 | 1.4 | |||
| Soil Depth (cm) | 2009 | 2012 | 2017 * | |||
|---|---|---|---|---|---|---|
| Continuous | Rotational | Continuous | Rotational | Continuous | Rotational | |
| 0–6 | 1306 | 915 | 1171 | 1274 | 719 | 800 |
| 6–12 | 647 | 670 | 560 | 588 | ||
| 12–20 | 333 | 410 | 472 | 510 | 260 | 297 |
| 20–30 | 383 | 448 | 401 | 389 | ||
| LSD (p < 0.05) | 370 | 129 | 193 | |||
| Soil Depth (cm) | 2009 | 2012 | 2017 * | |||
|---|---|---|---|---|---|---|
| Continuous | Rotational | Continuous | Rotational | Continuous | Rotational | |
| 0–6 | 575 | 565 | 535 | 581 | 497 | 475 |
| 6–12 | 280 | 297 | 278 | 293 | ||
| 12–20 | 207 | 225 | 235 | 241 | 203 | 210 |
| 20–30 | 189 | 174 | 207 | 209 | ||
| LSD (p < 0.05) | 72 | 60 | 161 | |||
| Soil Depth (cm) | 2009 | 2012 | 2017 * | |||
|---|---|---|---|---|---|---|
| Continuous | Rotational | Continuous | Rotational | Continuous | Rotational | |
| Residual soil ammonium (mg NH4-N kg−1 soil) | ||||||
| 0–6 | 7 | 14 | 24 | 23 | 15 | 9 |
| 6–12 | 8 | 9 | 14 | 14 | ||
| 12–20 | 14 | 7 | 10 | 10 | 9 | 6 |
| 20–30 | 11 | 6 | 8 | 8 | ||
| LSD (p < 0.05) | 8 | 2 | 3 | |||
| Residual soil nitrate (mg NO3-N kg−1 soil) | ||||||
| 0–6 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6–12 | 1 | 1 | 2 | 2 | ||
| 12–20 | 1 | 1 | 1 | 1 | <1 | <1 |
| 20–30 | 1 | 1 | 1 | 1 | ||
| LSD (p < 0.05) | 1 | <1 | 1 | |||
| Soil Depth (cm) | 2009 | 2012 | 2017 * | |||
|---|---|---|---|---|---|---|
| Continuous | Rotational | Continuous | Rotational | Continuous | Rotational | |
| 0–6 | 0.44 | 0.58 | 0.57 | 0.51 | 0.12 | 0.15 |
| 6–12 | 0.25 | 0.19 | 0.08 | 0.05 | ||
| 12–20 | 0.12 | 0.12 | 0.04 | 0.04 | 0.03 | 0.03 |
| 20–30 | 0.11 | 0.09 | 0.04 | 0.04 | ||
| LSD (p < 0.05) | 0.34 | 0.14 | 0.11 | |||
| Soil Depth (cm) | 2009 | 2012 | 2017 * | |||
|---|---|---|---|---|---|---|
| Continuous | Rotational | Continuous | Rotational | Continuous | Rotational | |
| 0–6 | 110 | 120 | 97 | 99 | 89 | 82 |
| 6–12 | 76 | 65 | 55 | 55 | ||
| 12–20 | 46 | 54 | 46 | 47 | 33 | 38 |
| 20–30 | 32 | 37 | 33 | 34 | ||
| LSD (p < 0.05) | 35 | 7 | 3 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzluebbers, A.J.; Starks, P.J.; Steiner, J.L. Conservation of Soil Organic Carbon and Nitrogen Fractions in a Tallgrass Prairie in Oklahoma. Agronomy 2019, 9, 204. https://doi.org/10.3390/agronomy9040204
Franzluebbers AJ, Starks PJ, Steiner JL. Conservation of Soil Organic Carbon and Nitrogen Fractions in a Tallgrass Prairie in Oklahoma. Agronomy. 2019; 9(4):204. https://doi.org/10.3390/agronomy9040204
Chicago/Turabian StyleFranzluebbers, Alan J., Patrick J. Starks, and Jean L. Steiner. 2019. "Conservation of Soil Organic Carbon and Nitrogen Fractions in a Tallgrass Prairie in Oklahoma" Agronomy 9, no. 4: 204. https://doi.org/10.3390/agronomy9040204
APA StyleFranzluebbers, A. J., Starks, P. J., & Steiner, J. L. (2019). Conservation of Soil Organic Carbon and Nitrogen Fractions in a Tallgrass Prairie in Oklahoma. Agronomy, 9(4), 204. https://doi.org/10.3390/agronomy9040204