Protein Hydrolysates from Fenugreek (Trigonella foenum graecum) as Nutraceutical Molecules in Colon Cancer Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fenugreek Protein Hydrolysates Preparation
2.2. Preparation of FP Hydrolysates (FPHs)
2.3. Hydrolysates Proximate Composition
2.4. Amino Acid Analysis
2.5. Cell Culture
2.6. Cell Treatment and Antiproliferative Property Analysis
2.7. Apoptosis Measurement
2.8. Propidium Iodide Staining of DNA Content and Cell Cycle Analysis
2.9. Mitochondrial Membrane Potential Assay by Flow Cytometry
2.10. Determination of Caspase 3 and Cytochrome C
2.11. Intracellular Levels of Reactive Oxygen Species (ROS)
2.12. Thioredoxin Reductase 1 (TrxR1) Activity Assay
2.13. Statistical Analysis
3. Results
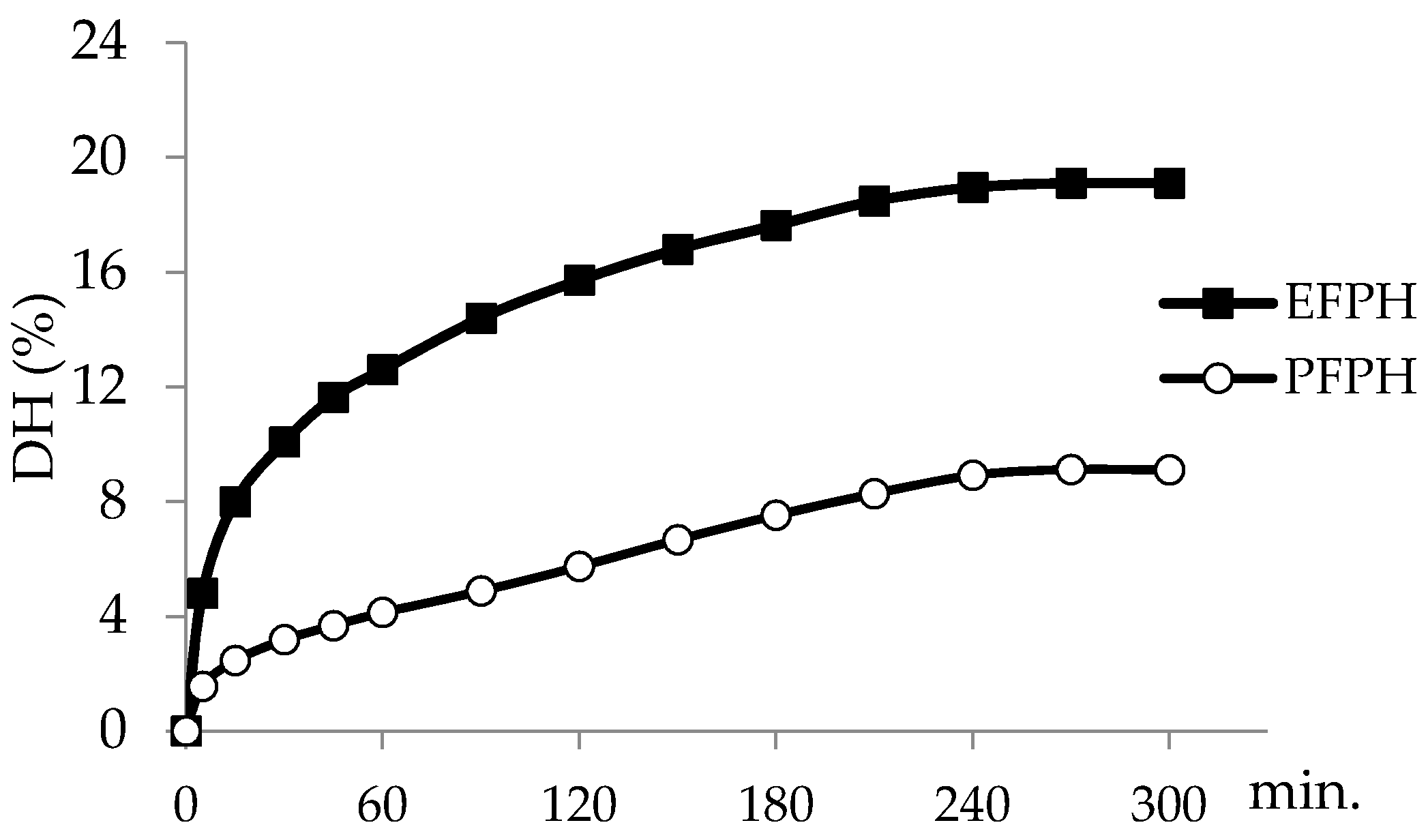
3.1. Kinetic and Degree of Hydrolysis
3.2. Chemical and Amino Acids Composition of FP and FPHs
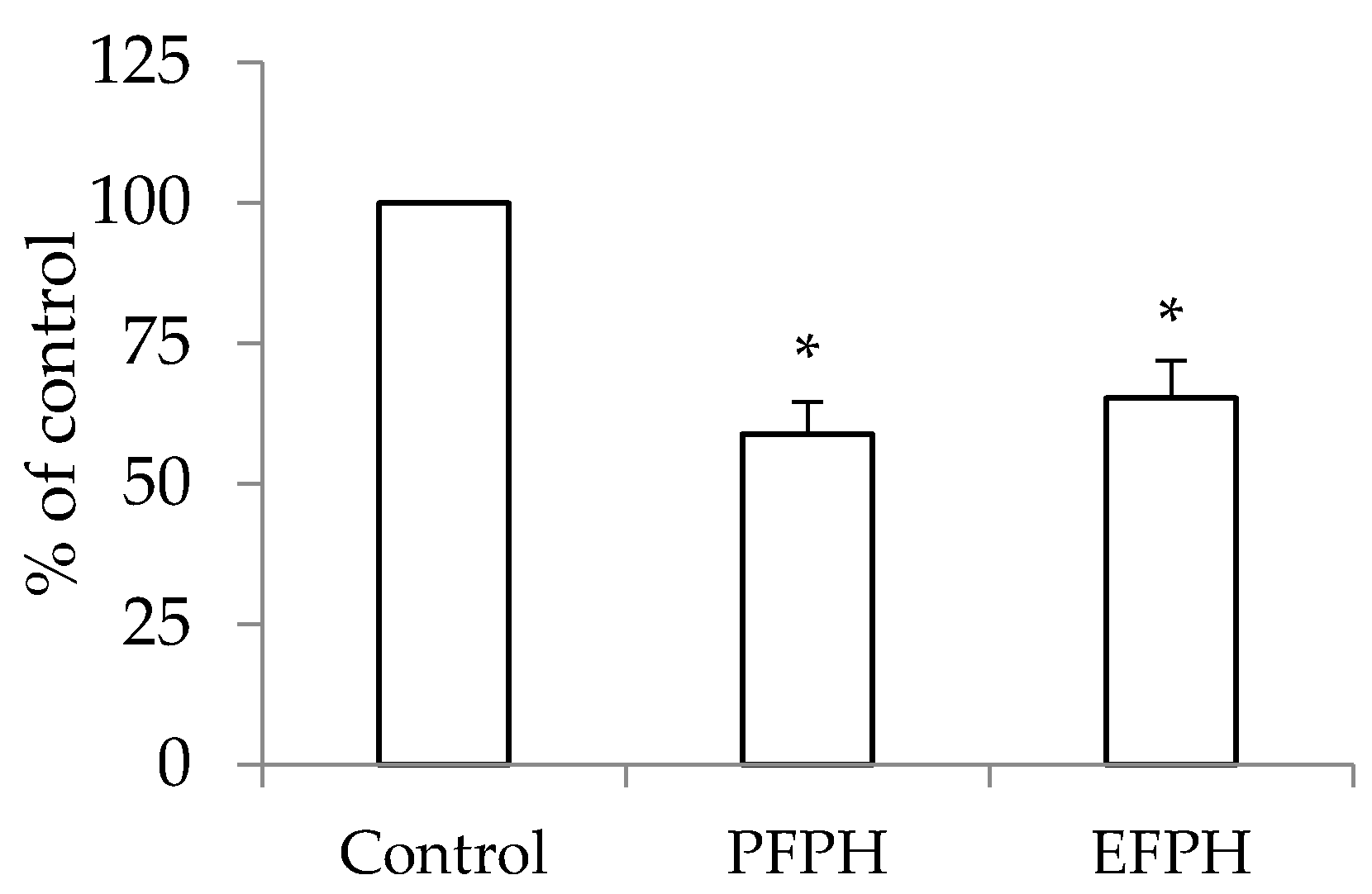
3.3. Antiproliferative Activity
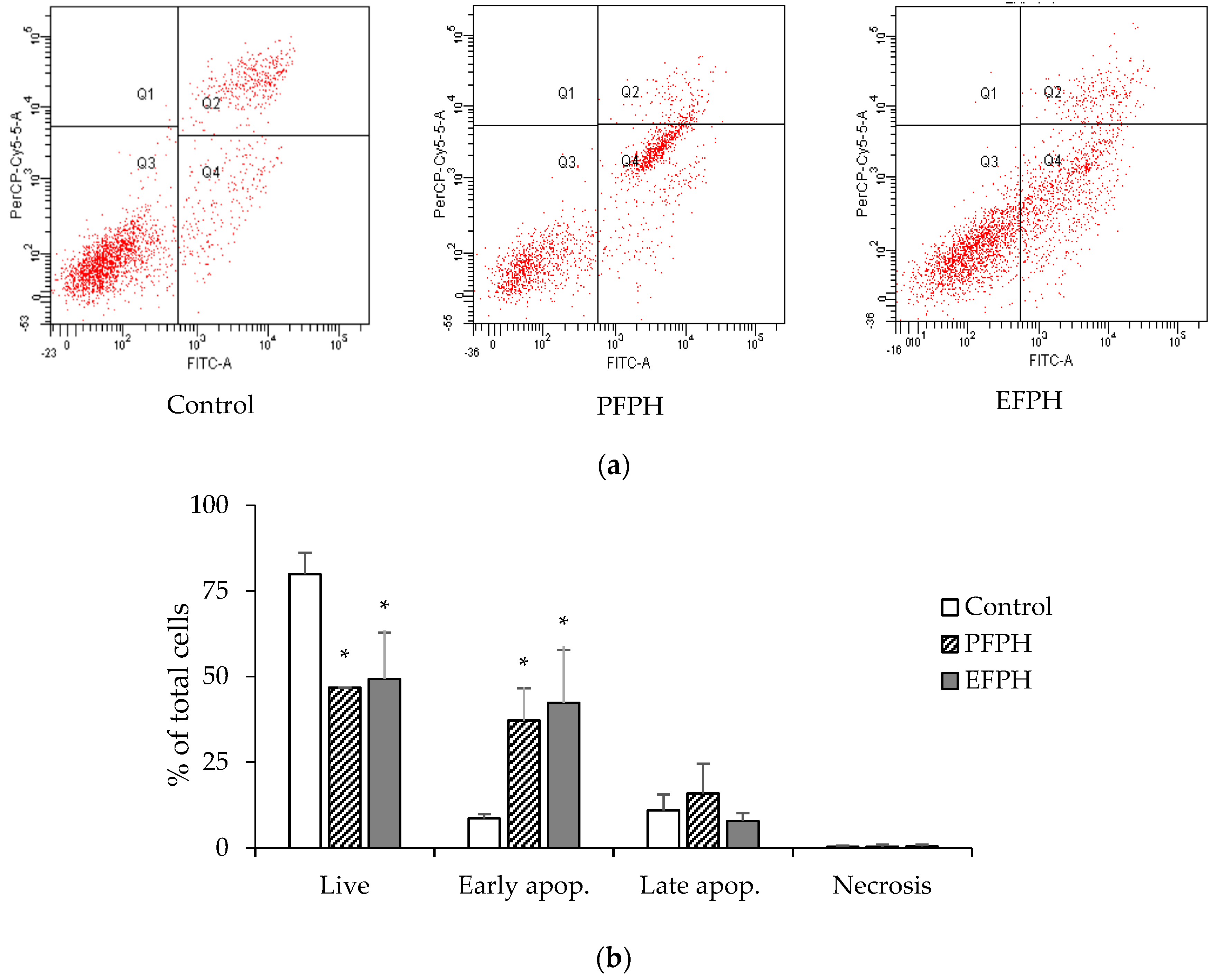
3.4. Apoptosis Analysis
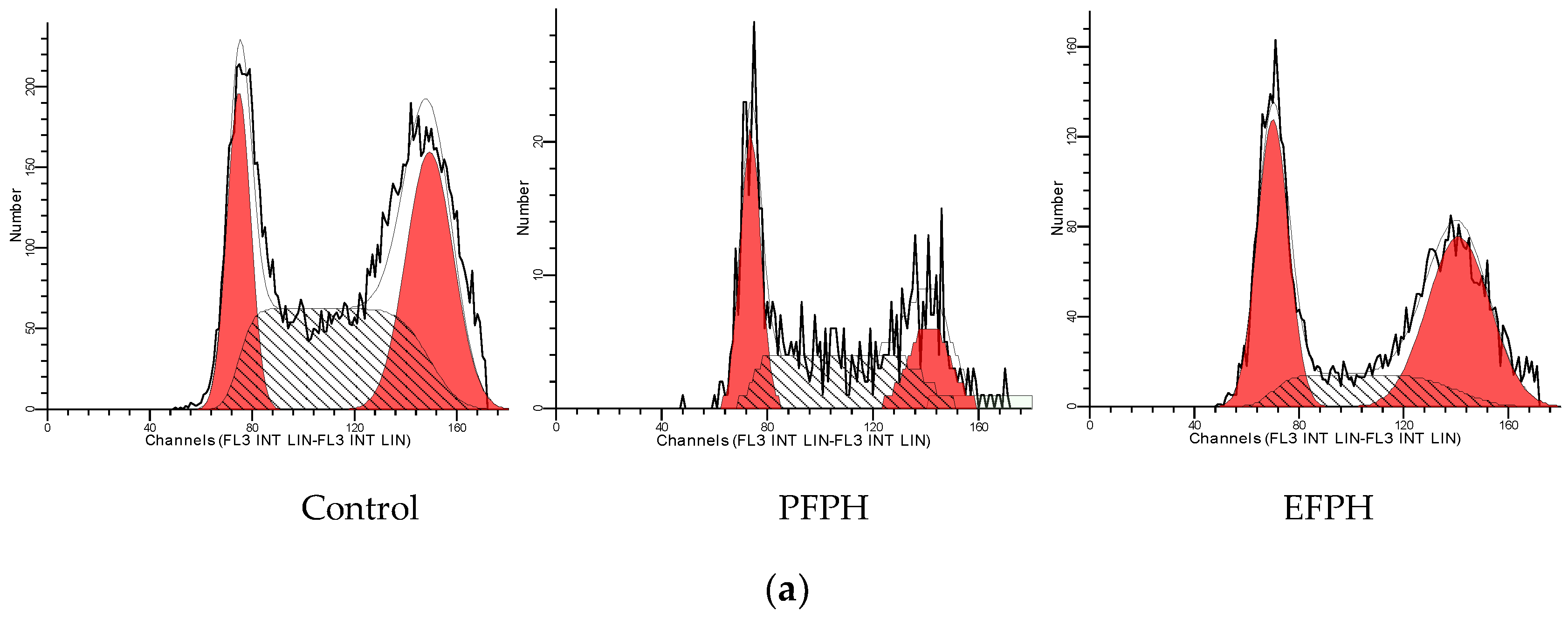
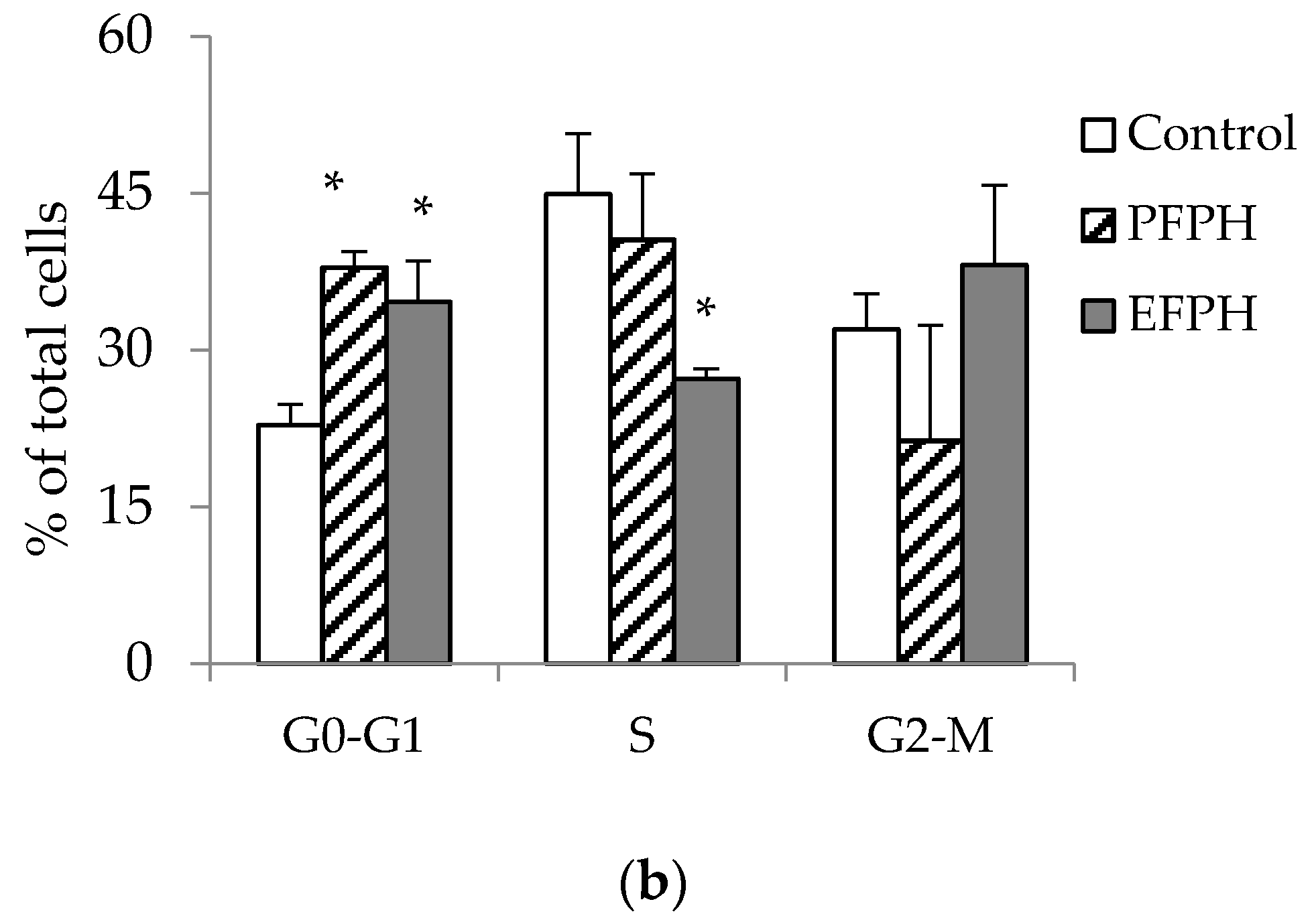
3.5. Cell Cycle Analysis
3.6. Analysis of Mitochondrial Membrane Potential Change, Cytochrome C Release, and Caspase-3 Activation
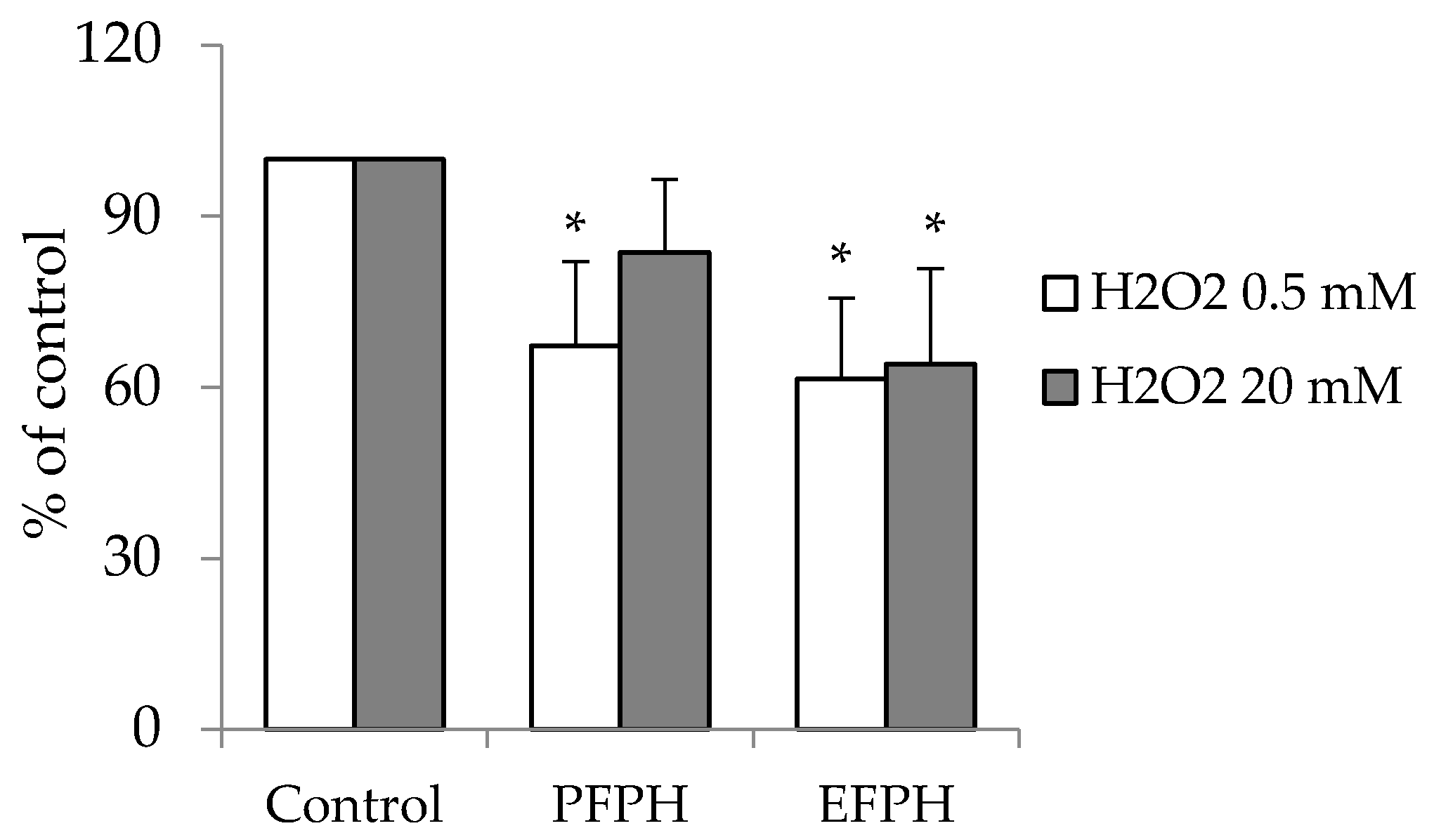
3.7. Antioxidant Activity of FPH in Caco2 Cells
3.8. Thioredoxin Reductase 1 Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meghwal, M.; Goswami, T.K. A Review on the functional properties, nutritional content, medicinal utilization and potential application of fenugreek. J. Food Process. Technol. 2012, 3, 181–190. [Google Scholar] [CrossRef]
- Khorshidian, N.; Asli, M.Y.; Arab, M.; Mirzaie, A.A.; Mortazavian, A.M. Fenugreek: Potential applications as a functional food and nutraceutical. Nutr. Food Sci. Res. 2016, 3, 5–16. [Google Scholar] [CrossRef]
- Belguith-Hadriche, O.; Bouaziz, M.; Jamoussia, K.; Simmonds, M.; El Feki, A.; Makni-Ayedi, F. Comparative study on hypocholesterolemic and antioxidant activities of various extracts of fenugreek seeds. Food Chem. 2013, 138, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Subhashini, N.; Thangathirupathi, A.; Lavanya, N. Antioxidant activity of Trigonella foenum graecum using various in vitro and ex vivo models. J. Pharm. Pharm. Sci. 2011, 3, 96–102. [Google Scholar]
- Madhava Naidu, M.; Shyamala, B.N.; Pura Naik, J.; Sulochanamma, G.; Srinivas, P. Chemical composition and antioxidant activity of the husk and endosperm of fenugreek seeds. Food Sci. Technol. 2011, 44, 451–456. [Google Scholar] [CrossRef]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Girón-Calle, J.; Vioque, J.; Pedroche, J.; Alaiz, M.; Yust, M.M.; Megías, C.; Millán, F. Chickpea protein hydrolysate as a substitute for serum in cell culture. Cytotechnology 2008, 57, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Zambrowicz, A.; Timmer, M.; Polanowski, A.; Lubec, G.; Trziszka, T. Manufacturing of peptides exhibiting biological activity. Amino Acids 2013, 44, 315–320. [Google Scholar] [CrossRef] [PubMed]
- León-Espinosa, E.B.; Sánchez-Chino, X.; Garduño-Siciliano, L.; Álvarez-González, R.I.; Dávila-Ortiz, G.; Madrigal-Bujaidar, E.; Téllez-Medina, D.I.; Jiménez-Martínez, C. Hypocholesterolemic and anticarcinogenic effect of Vicia faba protein hydrolyzates. Nutr. Cancer 2016, 68, 856–864. [Google Scholar] [CrossRef]
- Luna Vital, D.A.; González de Mejía, E.; Dia, V.P.; Loarca-Piña, G. Peptides in common bean fractions inhibit human colorectal cancer cells. Food Chem. 2014, 157, 347–355. [Google Scholar] [CrossRef]
- Kannan, A.; Hettiarachchy, N.S.; Lay, J.O.; Liyanage, R. Human cancer cell proliferation inhibition by a pentapeptide isolated and characterized from rice bran. Peptides 2010, 31, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Martinez, M.; de Mejia, E.G.; García-Lara, S.; Aguilar, O.; Lopez-Castillo, L.M.; Otero-Pappatheodorou, J.T. Antiproliferative effect of peptide fractions isolated from a quality protein maize, a white hybrid maize, and their derived peptides on hepatocarcinoma human HepG2 cells. J. Funct. Foods 2017, 34, 36–48. [Google Scholar] [CrossRef]
- Xue, Z.; Wen, H.; Zhai, L.; Yu, Y.; Li, Y.; Yu, W.; Cheng, A.; Wang, C.; Kou, X. Antioxidant activity and anti-proliferative effect of a bioactive peptide from chickpea (Cicer arietinum L.). Food Res. Int. 2015, 77, 75–81. [Google Scholar] [CrossRef]
- Gao, C.; Sun, R.; Xie, Y.R.; Jiang, A.L.; Lin, M.; Li, M.; Chen, Z.W.; Zhang, P.; Jin, H.; Feng, J.P. The soy-derived peptide Vglycin inhibits the growth of colon cancer cells in vitro and in vivo. Exp. Boil. Med. 2017, 242, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Allaoui, A.; Barranquero, C.; Yahia, S.; Herrera-Marcos, L.V.; Benomar, S.; Jridi, M.; Navarro-Ferrando, M.Á.; Rodriguez-Yoldi, M.J.; Nasri, M.; Osada, J.; et al. Fenugreek proteins and their hydrolysates prevent hypercholesterolemia and enhance the HDL antioxidant properties in rats. Nutr. Food Sci. 2018, 48, 973–989. [Google Scholar] [CrossRef]
- Adler-Nissen, J. A review of food hydrolysis specific areas. In Enzymic Hydrolysis of Food Proteins; Adler-Nissen, J., Ed.; Elsevier Applied Science Publishers: Copenhagen, Dermark, 1986; pp. 57–109. [Google Scholar]
- Horwitz, W. Food composition; additives; natural contaminants. In Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., AOAC International, Eds.; AOAC International: Gaithersburg, MD, USA, 2000; Volume 2. [Google Scholar]
- Yust, M.M.; Pedroche, J.; Girón-Calle, J.; Vioque, J.; Millán, F.; Alaiz, M. Determination of tryptophan by high-performance liquid chromatography of alkaline hydrolysates with spectrophotometric detection. Food Chem. 2004, 85, 317–320. [Google Scholar] [CrossRef]
- Sánchez-de-Diego, C.; Mármol, I.; Pérez, R.; Gascón, S.; Rodriguez-Yoldi, M.J.; Cerrada, E. The anticancer effect related to disturbances in redox balance on Caco-2 cells caused by an alkynyl gold(I) complex. J. Inorg. Biochem. 2017, 166, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.E.; Jansen, E.S.; Sanchez, W.; Waterhouse, N.J. Flow cytometry based assays for the measurement of apoptosis-associated mitochondrial membrane depolarisation and cytochrome c release. Methods 2013, 61, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Leal, M.; George, S. An in vitro procedure for evaluation of early stage oxidative stress in an established fish cell line applied to investigation of PHAH and pesticide toxicity. Mar. Environ. Res. 2004, 58, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Ortiz-Martinez, M.; Winkler, R.; García-Lara, S. Preventive and therapeutic potential of peptides from cereals against cancer. J. Proteom. 2014, 111, 165–183. [Google Scholar] [CrossRef]
- Sbroggio, M.F.; Montilha, M.S.; de Figueiredo, V.R.G.; Georgetti, S.R.; Kurozawa, L.E. Influence of the degree of hydrolysis and type of enzyme on antioxidant activity of okara protein hydrolysates. Food Sci. Technol. 2016, 36, 375–381. [Google Scholar] [CrossRef]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef]
- Mundi, S.; Aluko, R.E. Inhibitory properties of kidney bean protein hydrolysate and its membrane fractions against renin, angiotensin converting enzyme, and free radicals. Austin J. Nutr. Food Sci. 2014, 2, 1008. [Google Scholar]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Yust, M.d.M.; Millán-Linares, M.d.C.; Alcaide-Hidalgo, J.M.; Millán, F.; Pedroche, J. Hypocholesterolaemic and antioxidant activities of chickpea (Cicer arietinum L.) protein hydrolysates. J. Sci. Food Agric. 2012, 92, 1994–2001. [Google Scholar] [CrossRef]
- Li, J.T.; Zhang, J.L.; He, H.; Ma, Z.L.; Nie, Z.K.; Wang, Z.Z.; Xu, X.G. Apoptosis in human hepatoma HepG2 cells induced by corn peptides and its anti-tumor efficacy in H22 tumor bearing mice. Food Chem. Toxicol. 2013, 51, 297–305. [Google Scholar] [CrossRef]
- Kumar, S.; Kapoor, V.; Gill, K.; Singh, K.; Xess, I.; Das, S.N.; Dey, S. Antifungal and antiproliferative protein from Cicer arietinum: A bioactive compound against emerging pathogens. BioMed Res. Int. 2014, 2014, 387203. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. Apoptosis of human breast cancer cells induced by hemagglutinin from Phaseolus vulgaris cv. Legumi secchi. Food Chem. 2011, 126, 595–602. [Google Scholar] [CrossRef]
- Blaszczyk, U.; Duda-Chodak, A. Magnesium: Its role in nutrition and carcinogenesis. Roczniki Państwowego Zakładu Hig. 2013, 64, 165–171. [Google Scholar]
- Whitfield, J. Calcium, calcium-sensing receptor and colon cancer. Cancer Lett. 2009, 275, 9–16. [Google Scholar] [CrossRef]
- Kasprzak, K.S. Effects of calcium, magnesium, zinc, and iron on nickel carcinogenesis: Inhibition versus enhancement. In Cytotoxic, Mutagenic and Carcinogenic Potential of Heavy Metals Related to Human Environment; Hadjiliadis, N.D., Ed.; Springer: Dordrecht, The Netherlands, 1997; Volume 26, pp. 93–106. [Google Scholar]
- Zhang, F.L.; Wang, P.; Liu, Y.H.; Liu, L.B.; Liu, X.B.; Li, Z.; Xue, Y.X. Topoisomerase I inhibitors, shikonin and topotecan, inhibit growth and induce apoptosis of glioma cells and glioma stem cells. PLoS ONE 2013, 8, e81815. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Boil. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Boil. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef]
- Galluzzi, L.; López-Soto, A.; Kumar, S.; Kroemer, G. Caspases connect cell-death signaling to organismal homeostasis. Immunity 2016, 44, 221–231. [Google Scholar] [CrossRef]
- Bhatia, M.; McGrath, K.L.; Di Trapani, G.; Charoentong, P.; Shah, F.; King, M.M.; Clarke, F.M.; Tonissen, K.F. The thioredoxin system in breast cancer cell invasion and migration. Redox Biol. 2016, 8, 68–78. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; del María, C.M.; Isidra, R.; Manuel, A.; Javier, V. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tong, X.; Sui, X.; Wang, Z.; Qi, B.; Li, Y.; Jiang, L. Antioxidant activity and protective effects of Alcalase-hydrolyzed soybean hydrolysate in human intestinal epithelial Caco-2 cells. Food Res. Int. 2018, 111, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, Z.R.; Luo, H.Y. Influence of amino acid compositions and peptide profiles on antioxidant capacities of two protein hydrolysates from skipjack tuna (Katsuwonus pelamis) dark muscle. Mar. Drugs 2015, 13, 2580–2601. [Google Scholar] [CrossRef] [PubMed]

| PFPH | EFPH | |
|---|---|---|
| (%) | (%) | |
| Proteins | 89.9 ± 0.2 | 92.3 ± 0.5 * |
| HAA | 49.4 | 49.0 |
| AAA | 16.8 | 17.2 |
| PCAA | 12.2 | 12.5 |
| Lipids | 2.8 ± 0.2 | 3.3 ± 0.4 * |
| Total fiber | 3.0 ± 0.1 | 2.0 ± 0.1 * |
| Carbohydrates # | 1.5 | ˂1 |
| Moisture | 1.0 ± 0.1 | 1.1 ± 0.1 |
| Ash | 1.8 ± 0.2 | 1.3 ± 0.3 |
| Mineral composition | ||
| Potassium (mg/100 g) | 986 ± 5 | 1001 ± 1 * |
| Phosphorus (µg/g) | 1933 ± 7 | 1927 ± 11 |
| Sulphide (µg/g) | 1128 ± 5 | 1268 ± 48 * |
| Magnesium (µg/g) | 1013 ± 4 | 985 ± 9 * |
| Calcium (µg/g) | 636 ± 2 | 502 ± 14 * |
| Sodium (µg/g) | 122 ± 4 | 110 ± 3 * |
| Selenium (µg/kg) | 53 ± 1 | 52 ± 1 |
| Number of Cells (/100 Cells) | Control | PFPH | EFPH |
|---|---|---|---|
| Cells with positive MMP | 28.7 ± 10.9 | 69.2 ± 11.4 * | 70.4 ± 4.1 * |
| Cells with mitochondrial cytochrome C | 90.7 ± 1.6 | 71.3 ± 1.2 * | 61.0 ± 9.8 * |
| Cells with active caspase-3 | 1.4 ± 0.0 | 33.3 ± 4.3 * | 18.5 ± 3.8 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allaoui, A.; Gascón, S.; Benomar, S.; Quero, J.; Osada, J.; Nasri, M.; Rodríguez-Yoldi, M.J.; Boualga, A. Protein Hydrolysates from Fenugreek (Trigonella foenum graecum) as Nutraceutical Molecules in Colon Cancer Treatment. Nutrients 2019, 11, 724. https://doi.org/10.3390/nu11040724
Allaoui A, Gascón S, Benomar S, Quero J, Osada J, Nasri M, Rodríguez-Yoldi MJ, Boualga A. Protein Hydrolysates from Fenugreek (Trigonella foenum graecum) as Nutraceutical Molecules in Colon Cancer Treatment. Nutrients. 2019; 11(4):724. https://doi.org/10.3390/nu11040724
Chicago/Turabian StyleAllaoui, Amine, Sonia Gascón, Souhila Benomar, Javier Quero, Jesús Osada, Moncef Nasri, María Jesús Rodríguez-Yoldi, and Ahmed Boualga. 2019. "Protein Hydrolysates from Fenugreek (Trigonella foenum graecum) as Nutraceutical Molecules in Colon Cancer Treatment" Nutrients 11, no. 4: 724. https://doi.org/10.3390/nu11040724
APA StyleAllaoui, A., Gascón, S., Benomar, S., Quero, J., Osada, J., Nasri, M., Rodríguez-Yoldi, M. J., & Boualga, A. (2019). Protein Hydrolysates from Fenugreek (Trigonella foenum graecum) as Nutraceutical Molecules in Colon Cancer Treatment. Nutrients, 11(4), 724. https://doi.org/10.3390/nu11040724







