Lactoferrin Induces the Synthesis of Vitamin B6 and Protects HUVEC Functions by Activating PDXP and the PI3K/AKT/ERK1/2 Pathway
Abstract
:1. Introduction
2. Results
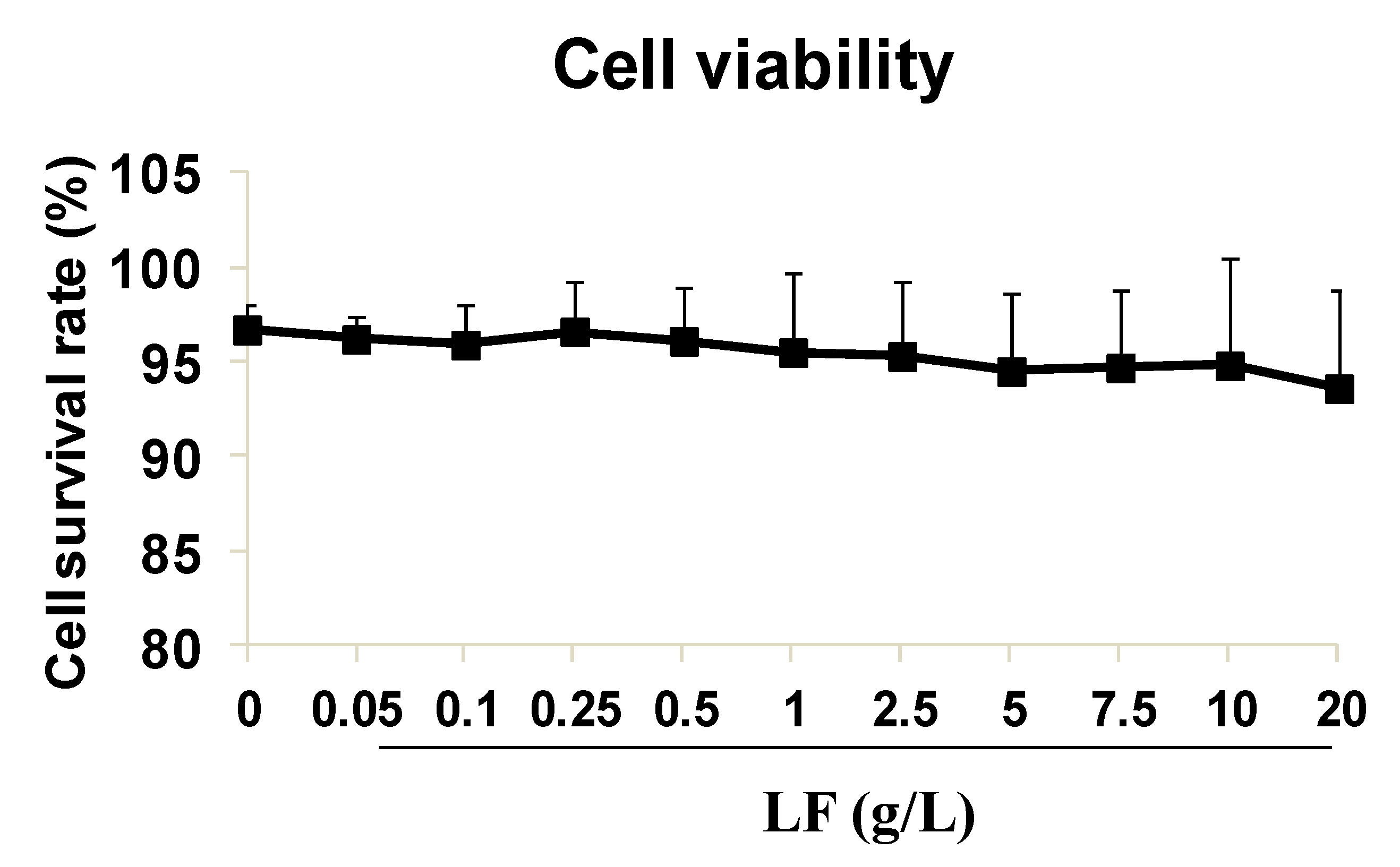
2.1. High-Dose Lactoferrin Shows No Obvious Inhibition on HUVEC Viability
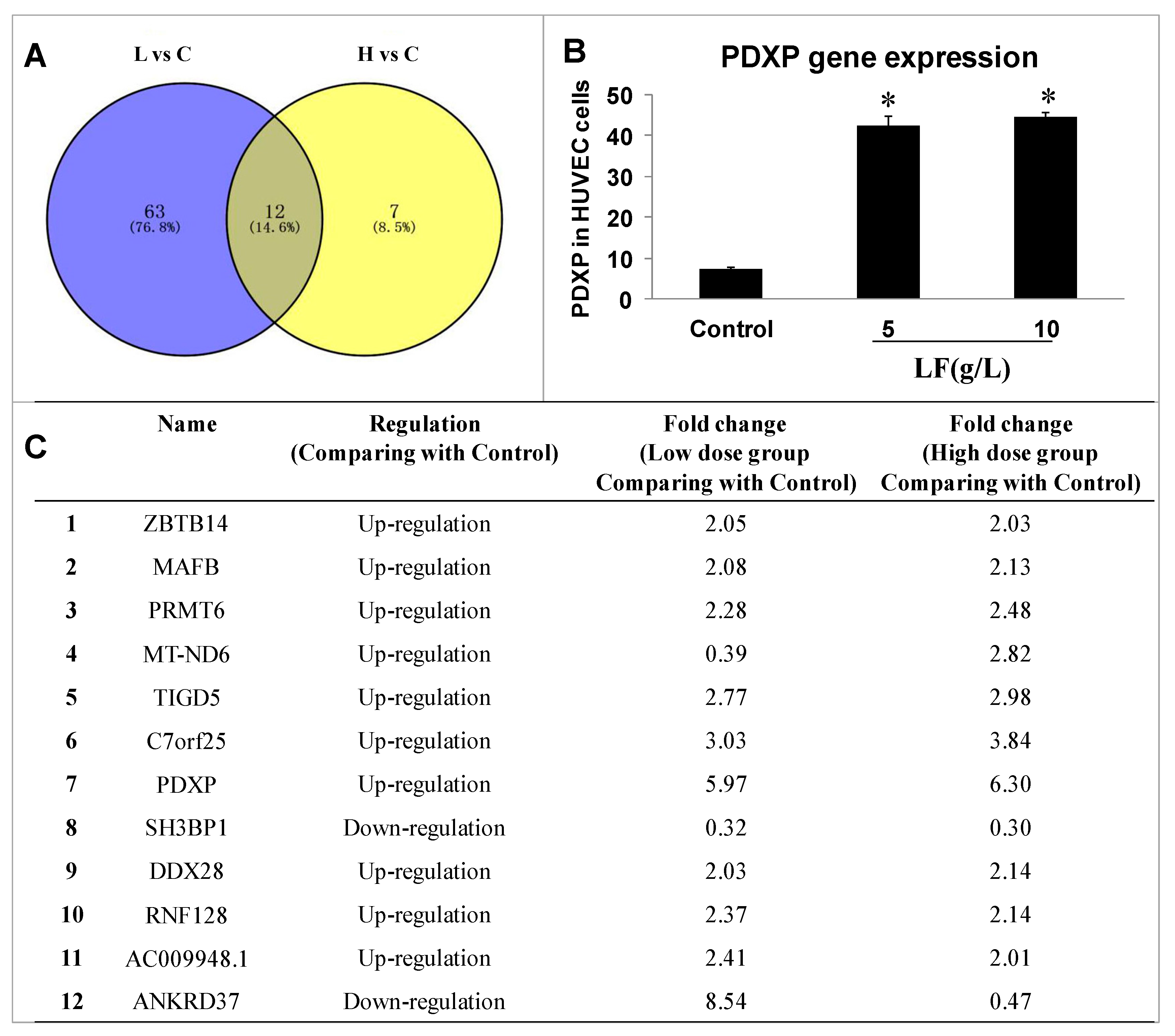
2.2. Transcriptomic Analysis of HUVECs and Data Analysis
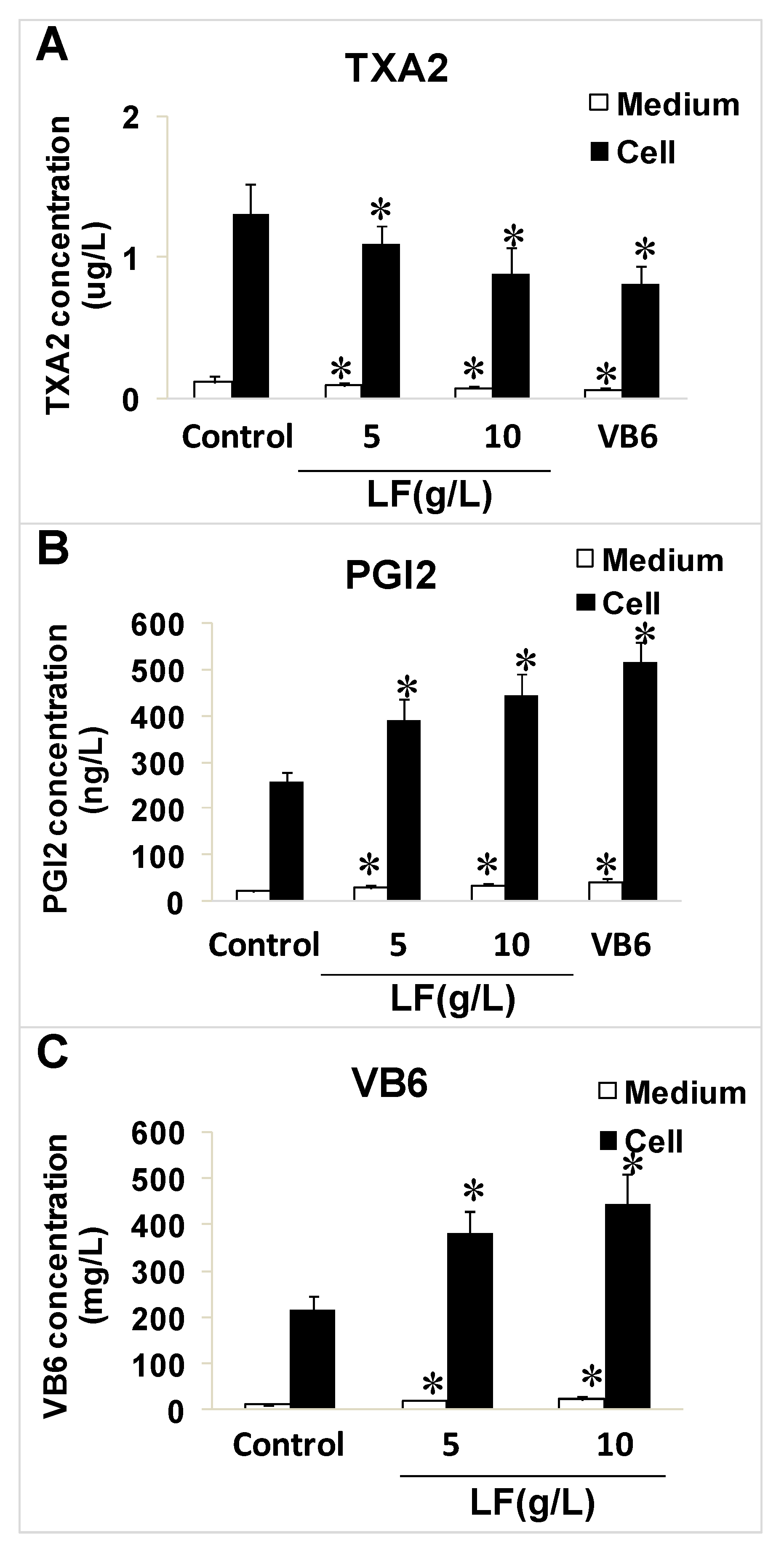
2.3. Effects of Lactoferrin and VB6 on the Expression of Thromboxane A2 (TXA2) and Prostacyclin (PGI2) in HUVECs with Enzyme-Linked Immunosorbent Assay (ELISA) Kits
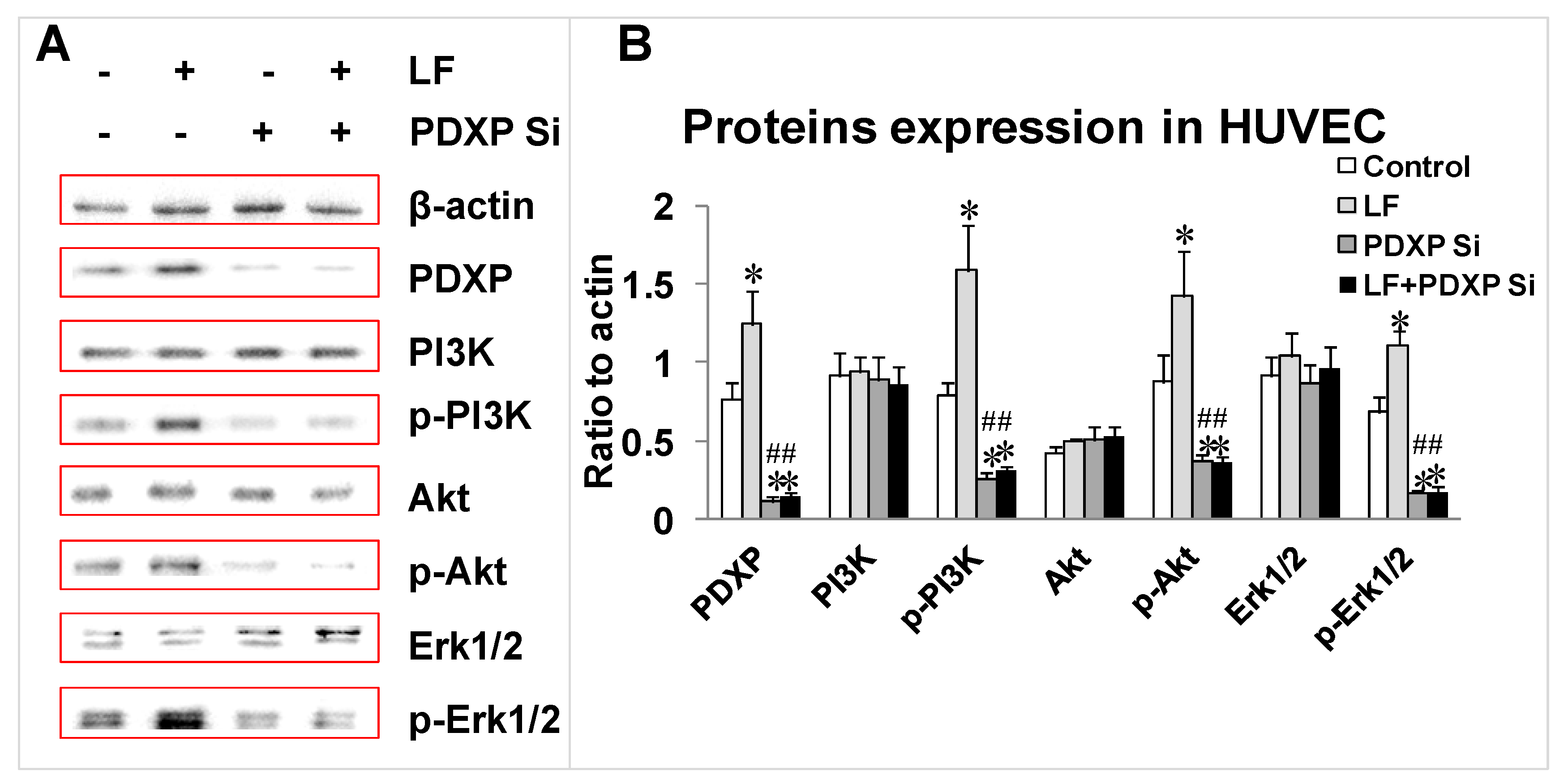
2.4. Effects of Lactoferrin and PDXP siRNA on the Expression of PI3K, AKT, and ERK1 and Their Phosphorylated Forms in HUVECs
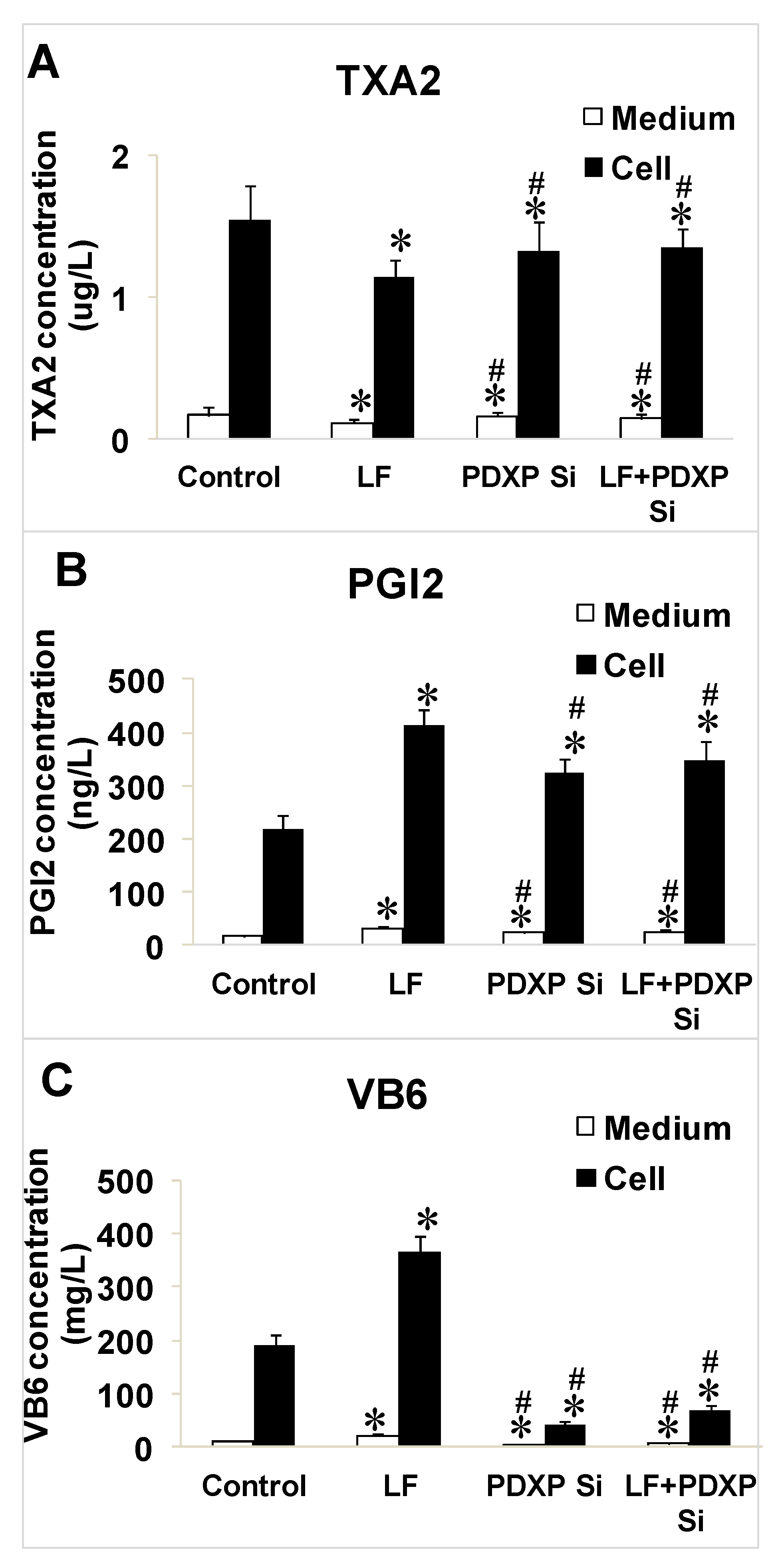
2.5. Effects of Lactoferrin and PDXP siRNA on the Expressions of VB6, TXA2, and PGI2 in HUVECs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture and Viability Testing
4.3. Transcriptomic and Data Analyses
4.4. siRNA Treatment of Cells
4.5. ELISA Detection of TXA2, PGI2, and VB6
4.6. Western Blotting Detection of Proteins
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LF | lactoferrin |
| HUVEC | human umbilical vein endothelial cells |
| PDXP | pyridoxal phosphatase |
| TXA2 | thromboxane A2 |
| PGI2 | prostacyclin |
References
- Neville, M.C. Lactoferrin Secretion into milk: Comparison between bovine, murine and human milk. J. Anim. Sci. 2000, 78, 26–35. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, M.; Luo, C.C.; Wang, J.Q.; Zheng, N. Lactoferrin exerts antitumor effects by inhibiting angiogenesis in a HT29 human colon tumor model. J. Agric. Food Chem. 2017, 65, 10464–10472. [Google Scholar] [CrossRef]
- Chung, S.H.; Kang, H.B.; Kim, J.W.; Yoon, S.S.; Nam, M.S. The biological effects of bovine lactoferrin on inflammatory cytokine expression in the PMA stimulated cells. Korean J. Food Sci. Anim. 2012, 32, 364–368. [Google Scholar] [CrossRef]
- Koshu, O.; Mako, K.; Yasuteru, U.; Hiroshi, N.; Jan, M.; Herter, J.M.; Tanya, M.; Daigoro, H.; Kazuo, S.; Hiroshi, K.; et al. Lactoferrin supresses neutrophil extracellular traps release in inflammation. Ebiomedicine 2016, 10, 204–215. [Google Scholar]
- Redwan, E.M.; El-Baky, N.A.; Al-Hejin, A.M.; Baeshen, M.N.; Almehdar, H.A.; Elsaway, A.; Gomaa, A.B.; Al-Masaudi, S.B.; Al-Fassi, F.A.; AbuZeid, I.E.; et al. Significant antibacterial activity and synergistic effects of camel lactoferrin with antibiotics against methicillin-resistant staphylococcus aureus (MRSA). Res. Microbiol. 2016, 167, 480–491. [Google Scholar] [CrossRef]
- Dominique, L. Overview of lactoferrin as a natural immune modulator. J. Pediatr. 2016, 173, 10–15. [Google Scholar]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2008, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Zimecki, M.; Paprocka, M.; Kruzel, M.L. Orally administered lactoferrin restores humoral immune response in immunocompromised mice. Immunol. Lett. 2003, 89, 9–15. [Google Scholar] [CrossRef]
- Sun, J.; Ren, F.Z.; Xiong, L.; Zhao, L.; Guo, H.Y. Bovine lactoferrin suppresses high-fat diet induced obesity and modulates gut microbiota in C57BL/6J mice. J. Funct. Foods 2006, 22, 189–200. [Google Scholar] [CrossRef]
- Sharon, M.D. The role of lactoferrin in gastrointestinal and immune development and function: A Preclinical Perspective. J. Pediat. 2016, 173, 16–28. [Google Scholar]
- Liu, F.; Zhang, S.; Li, J.; McClements, D.J.; Liu, X. Recent development of lactoferrin-based vehicles for the delivery of bioactive compounds: Complexes, emulsions, and nanoparticles. Trends Food Sci. Technol. 2018, 79, 67–77. [Google Scholar] [CrossRef]
- Korpela, R. Milk peptides and cardiovascular health: Effects on blood pressure and beyond. J. Dairy Technol. 2009, 64, 26–28. [Google Scholar]
- Alejandro, A.; Robert, B.; Chad, C.; Nathan, D.; Phillip, D.; Paula, D.; Juan, F.; Eugene, F.; Guntupalli, K.K.; Huang, D.T.; et al. A phase 2 randomized, double-blind, placebo-controlled study of the safety and efficacy of talactoferrin in patients with severe sepsis. Crit. Care Med. 2013, 41, 706–716. [Google Scholar]
- Visioli, F.; Strata, A. Milk, dairy products, and their functional effects in humans: A narrative review of recent evidence. Adv. Nutr. 2014, 5, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Mao, J.; Rasmussen, G.T.; Serody, J.S.; Britigan, B.E. Interaction of Lactoferrin and Lipopolysaccharide (LPS): Effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J. Infect. Dis. 1992, 166, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.M.; Connellan, P.A.; Oliver, C.J.; Morris, C.A.; Stevenson, L.M. Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males. Nutr. Res. 2008, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Letchoumy, P.V.; Mohan, K.V.P.C.; Stegeman, J.J.; Gelboin, H.V.; Hara, Y.; Nagini, S. In vitro antioxidative potential of lactoferrin and black tea polyphenols and protective effects in vivo on carcinogen activation, DNA damage, proliferation, invasion, and angiogenesis during experimental oral carcinogenesis. Oncol. Res. 2008, 17, 193. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, J. Bovine lactoferrin-derived ACE inhibitory tripeptide LRP also shows antioxidative and anti-inflammatory activities in endothelial cells. J. Funct. Foods 2016, 25, 375–384. [Google Scholar] [CrossRef]
- Xiang, W.; Lian, H.U.; Jian, D.U. Effect of captopril on platelet cytosolic [Ca2+] i and plasma TXA 2/PGI 2 in renovascular hypertensive rats. Acta Pharmacol. Sinica 1998, 19, 89–91. [Google Scholar]
- Wang, Z.J.; Yu, Y.H.; Chen, J.; Zhao, Y.T.; Zhang, Y. Correlation between oligohydramnios and abnormal expressions of TXA2, PGI2 and TXA2R in the umbilical arterial blood and placenta. J. South. Med. Univ. 2009, 29, 1917. [Google Scholar]
- Machlin, L.J. Handbook of Vitamins (Vol. 14); Marcel Dekker Inc.: New York, NY, USA, 1991. [Google Scholar]
- Choi, S.Y.; Churchich, J.E.; Zaiden, E.; Kwok, F. Brain pyridoxine5-phosphate oxidase. Modulation of its by reaction with pyridoxal 5-phosphate and analogs. J. Biol. Chem. 1987, 262, 12013–12017. [Google Scholar] [PubMed]
- Kim, D.W.; Hong, J.W.; Eum, W.S.; Choi, H.S.; Choi, S.H.; Kim, S.Y.; Lee, B.R.; An, J.J.; Lee, S.H.; Lee, S.R.; et al. Inactivation of brain myo-inositol monophosphate phosphatase by pyridoxal-5’-phosphate. J. Biochem. Mol. Biol. 2005, 38, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Hong, M.L.; Kim, D.W.; Park, J.; Kang, T.C.; Won, M.H.; Baek, N.I.; Moon, B.J.; Choi, S.Y.; Kwon, O.S. Genomic organization, tissue distribution and deletion mutation of human pyridoxine5’-phosphate oxidase. Eur. J. Biochem. 2004, 271, 2452–2461. [Google Scholar] [CrossRef] [PubMed]
- Gudmundson, P.; Alpman, J.; Chang, S.J. Vitamin B6 protects vascular endothelial injury by activated platelets. Nutr. Res. 1999, 19, 1613–1624. [Google Scholar]
- Mahfouz, M.M.; Zhou, S.Q.; Kummerow, F.A. Vitamin B-6 compounds are capable of reducing the superoxide radical and lipid peroxide levels induced by H2O2 in vascular endothelial cells in culture. Int. J. Vitam. Nutr. Res. 2009, 79, 218. [Google Scholar] [CrossRef] [PubMed]
- Francesco, D.; Fiallo, P.; Ghio, M.; Flora, S.D. Chemoprevention of doxorubicin-induced alopecia in mice by dietary administration ofl-cystine and vitamin B6. Arch. Dermatol. Res. 2013, 305, 25–34. [Google Scholar]
- Chen, C.; Yang, T.L. TXA2/PGI2 and cardiovascular disease. Prog. Mod. Biomed. 2008, 8, 2166–2168. [Google Scholar]
- Zhou, H.C.; Fan, G.L.; Mu, X.; Hu, G.; Suo, Z.W. Effects of active various concentrations of hyperoside on PGI2, TXA2 and PAF production induced by SLT-II e in rat intestinal microvascular endothelial cells. J. Northwest A&F Univ. (Nat. Sci. Ed.) 2007, 35, 6–10. [Google Scholar]
- Zhou, H.C.; Fan, G.L.; Mu, X. Effects of astragaloside IV on the secretions of NO, ET-1, PGI2, TXA2, P-selectin and sICAM-1 in rat intestinal microvascular endothelial cells induced by SLT-II e. Vet. Sci. China 2009, 10, 922–927. [Google Scholar]
- Taylor, R.G.; Jerome, W.G.; Lewis, J.C. Ultrastructural events associated with endothelial cell changes during the initiation and early progression of atherosclerosis. Adv. Exp. Med. Biol. 1990, 273, 89. [Google Scholar]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef] [PubMed]
- Hennig, B.; Lei, W.; Arzuaga, X.; Ghosh, D.D.; Saraswathi, V.; Toborek, M. Linoleic acid induces proinflammatory events in vascular endothelial cells via activation of PI3K/Akt and ERK1/2 signaling. J. Nutr. Biochem. 2006, 17, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Xing, L.; Zhao, N.; Wang, J.Q.; Zheng, N. Furosine induced apoptosis by the regulation of STAT1/STAT2 and UBA7/UBE2L6 genes in HepG2 cells. Int. J. Mol. Sci. 2018, 19, 1629. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, K.; Chang, J.; Sun, J. Osteogenesis and angiogenesis induced by porous β-CaSiO (3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials 2013, 34, 64–77. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, Y.; Yang, H.; Liu, L.; Wang, J.; Zheng, N. Lactoferrin Induces the Synthesis of Vitamin B6 and Protects HUVEC Functions by Activating PDXP and the PI3K/AKT/ERK1/2 Pathway. Int. J. Mol. Sci. 2019, 20, 587. https://doi.org/10.3390/ijms20030587
Li H, Wang Y, Yang H, Liu L, Wang J, Zheng N. Lactoferrin Induces the Synthesis of Vitamin B6 and Protects HUVEC Functions by Activating PDXP and the PI3K/AKT/ERK1/2 Pathway. International Journal of Molecular Sciences. 2019; 20(3):587. https://doi.org/10.3390/ijms20030587
Chicago/Turabian StyleLi, Huiying, Yizhen Wang, Huaigu Yang, Li Liu, Jiaqi Wang, and Nan Zheng. 2019. "Lactoferrin Induces the Synthesis of Vitamin B6 and Protects HUVEC Functions by Activating PDXP and the PI3K/AKT/ERK1/2 Pathway" International Journal of Molecular Sciences 20, no. 3: 587. https://doi.org/10.3390/ijms20030587
APA StyleLi, H., Wang, Y., Yang, H., Liu, L., Wang, J., & Zheng, N. (2019). Lactoferrin Induces the Synthesis of Vitamin B6 and Protects HUVEC Functions by Activating PDXP and the PI3K/AKT/ERK1/2 Pathway. International Journal of Molecular Sciences, 20(3), 587. https://doi.org/10.3390/ijms20030587





