Assessment of Binding Interaction between Bovine Lactoferrin and Tetracycline Hydrochloride: Multi-Spectroscopic Analyses and Molecular Modeling
Abstract
1. Introduction
2. Results
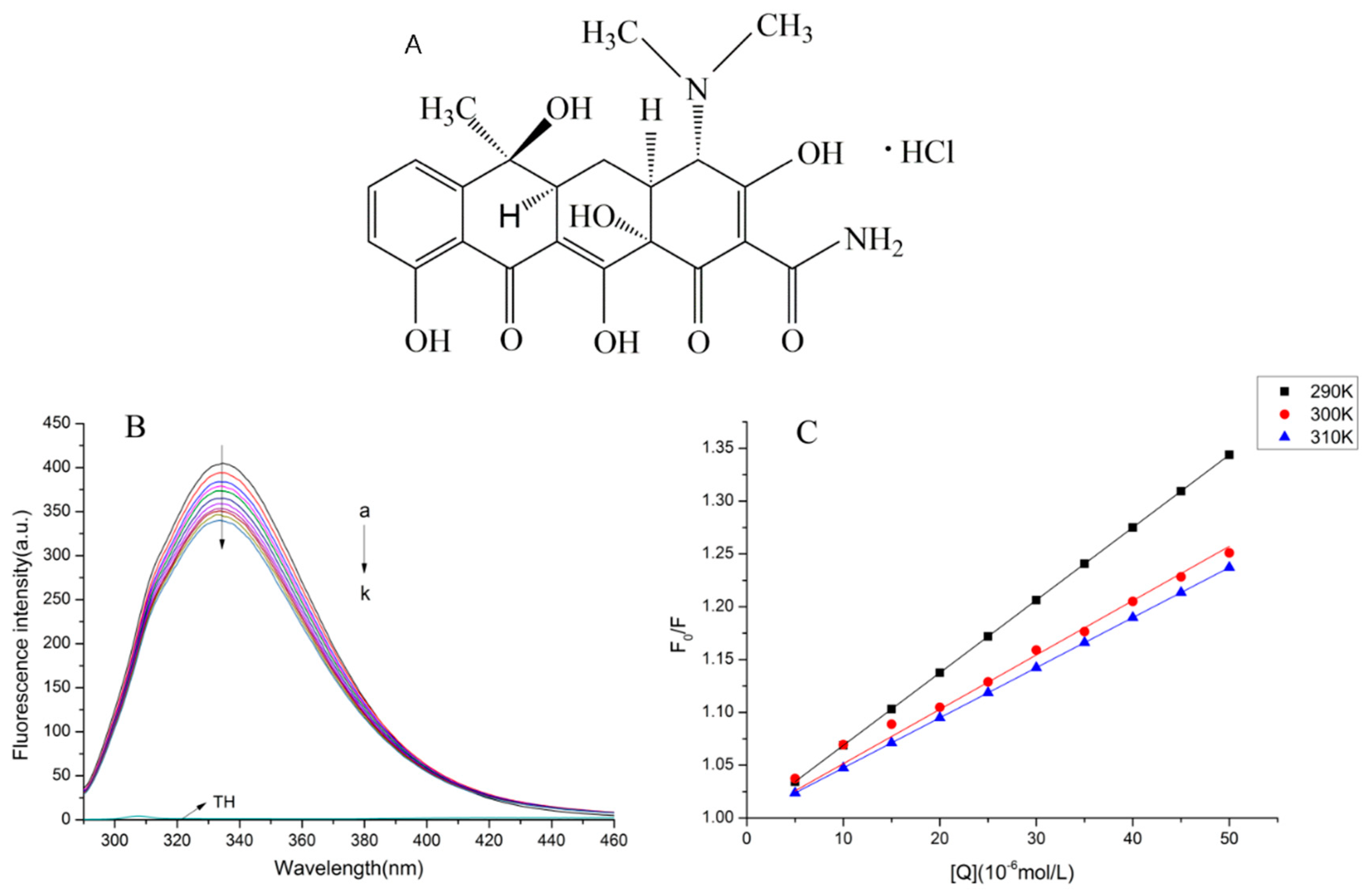
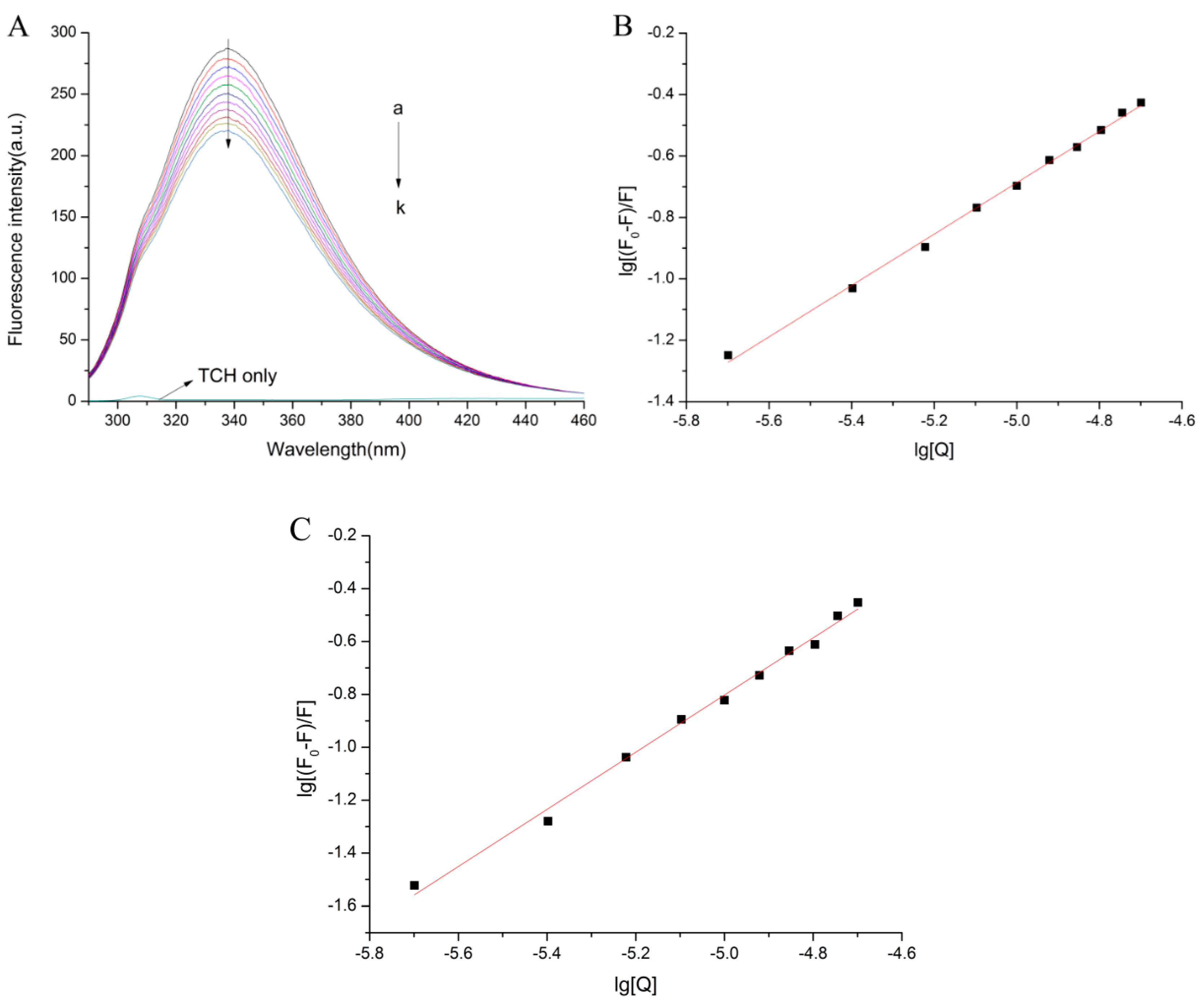
2.1. Normal Fluorescence Spectra
2.2. The Fluorescence Quenching Mechanism
2.3. Binding Parameters and Thermodynamic Parameters
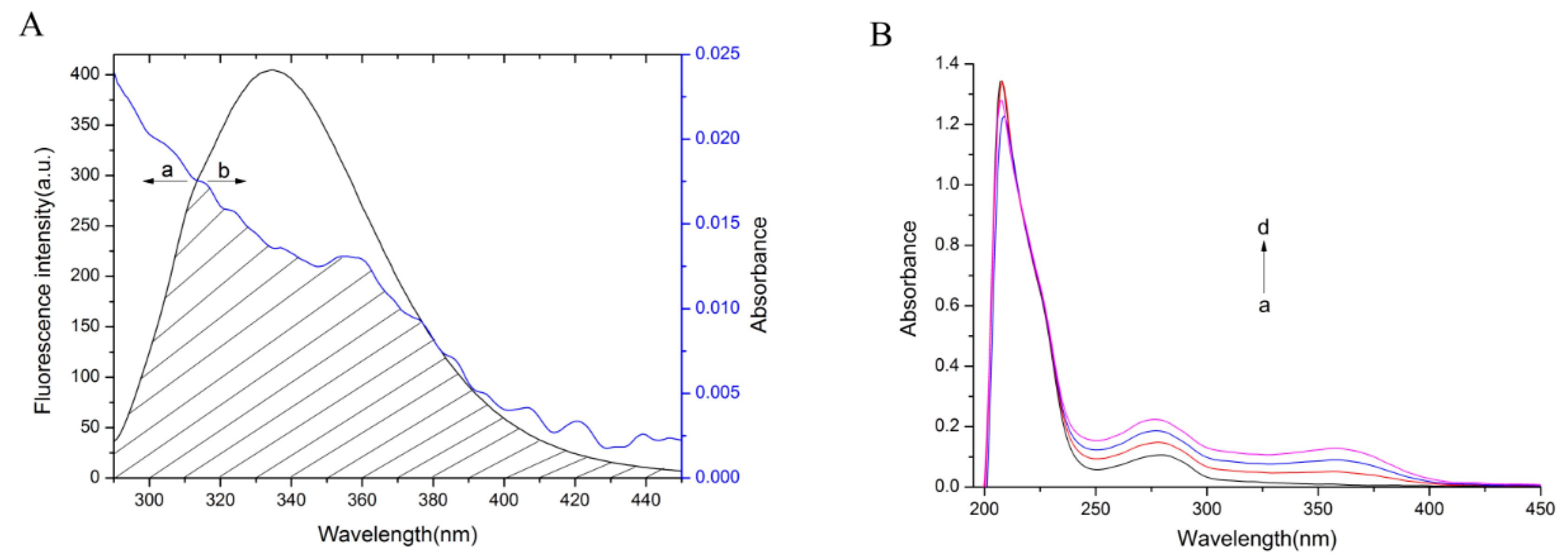
2.4. Energy Transfer between bLf and TCH
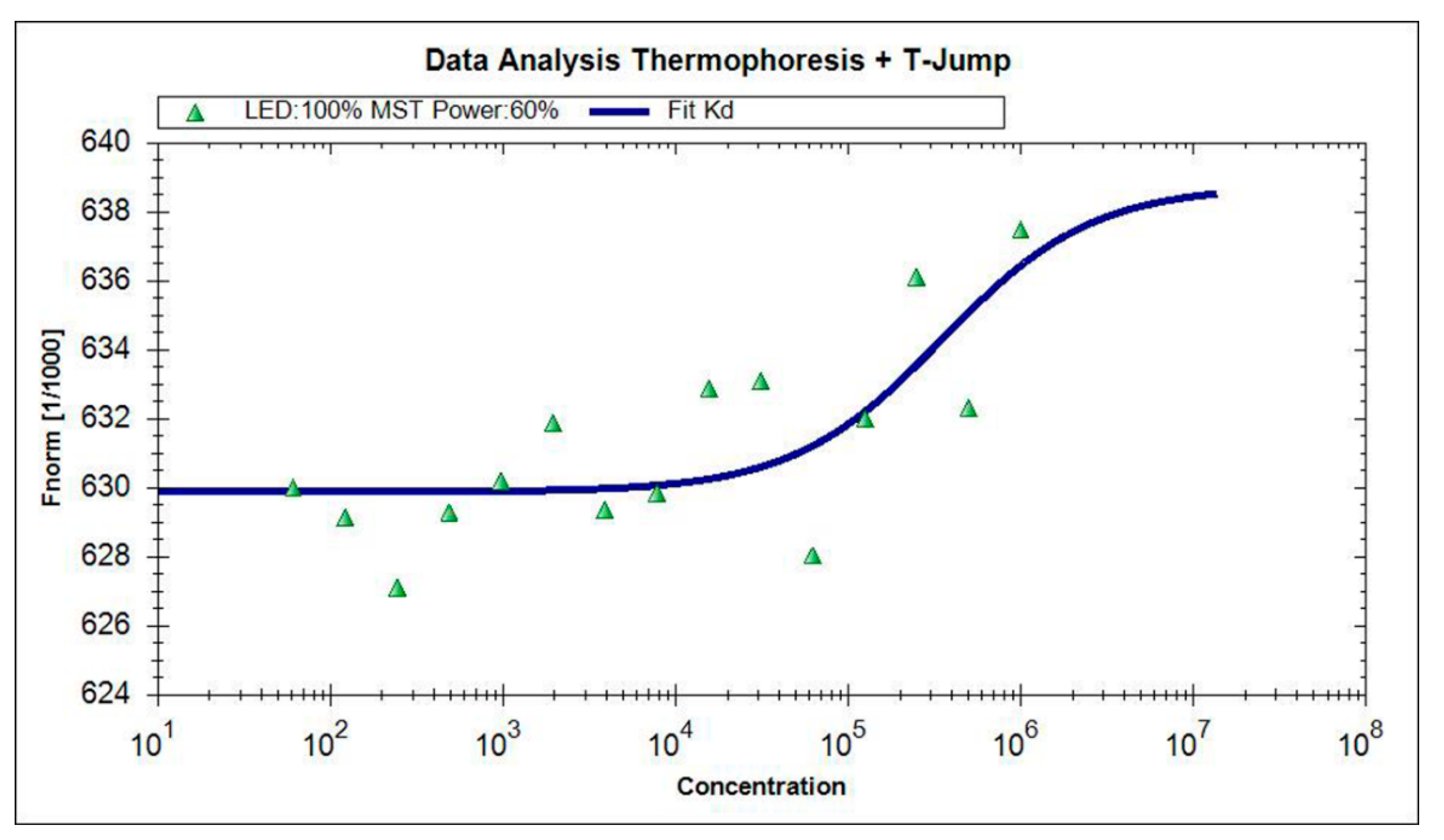
2.5. BLf-TCH Interaction Studies by Microscale Thermophoresis
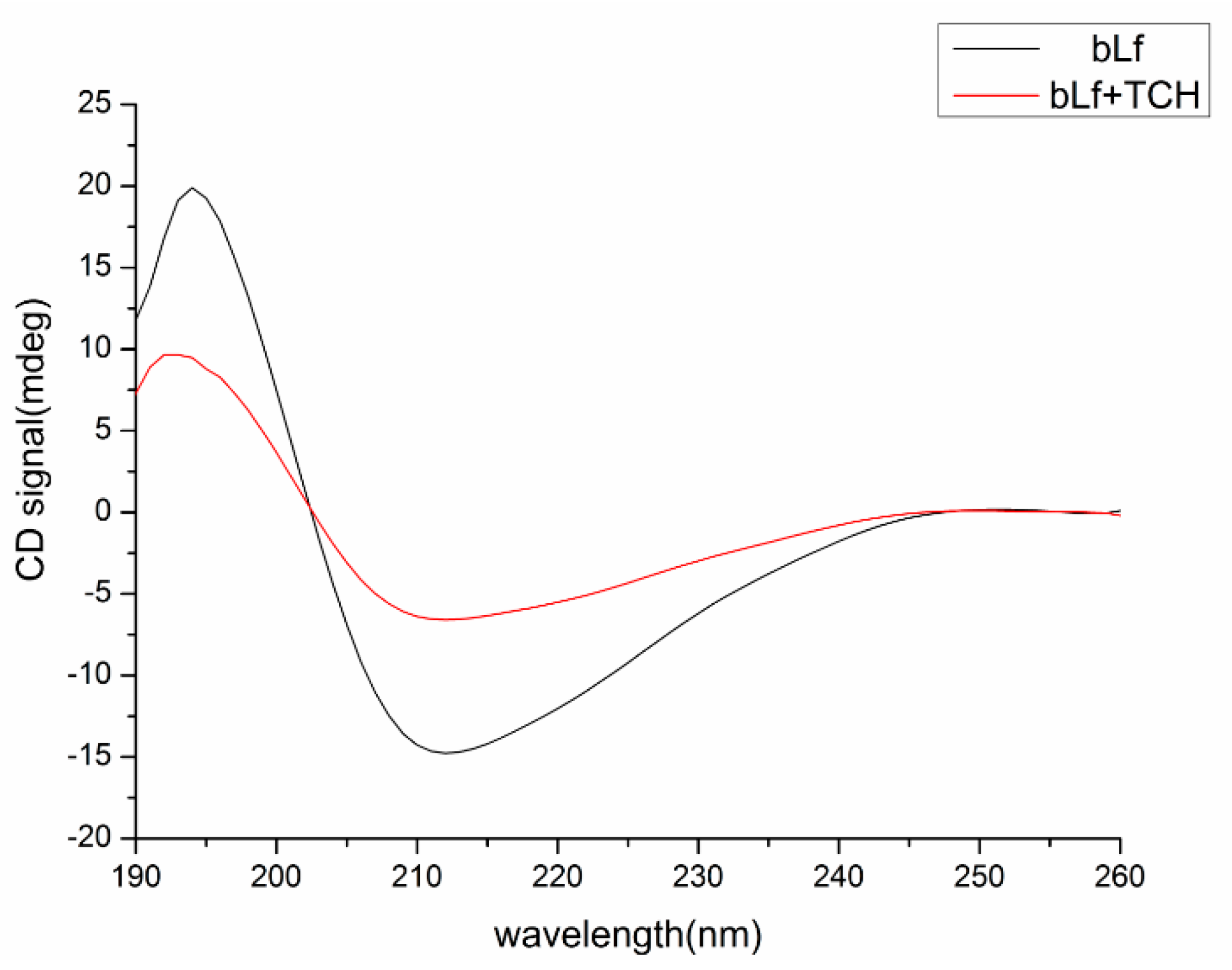
2.6. Effect of the TCH on the bLf Conformation
2.6.1. Conformational Investigation by UV-vis Absorption Spectra
2.6.2. Conformational Investigation by Synchronous Fluorescence
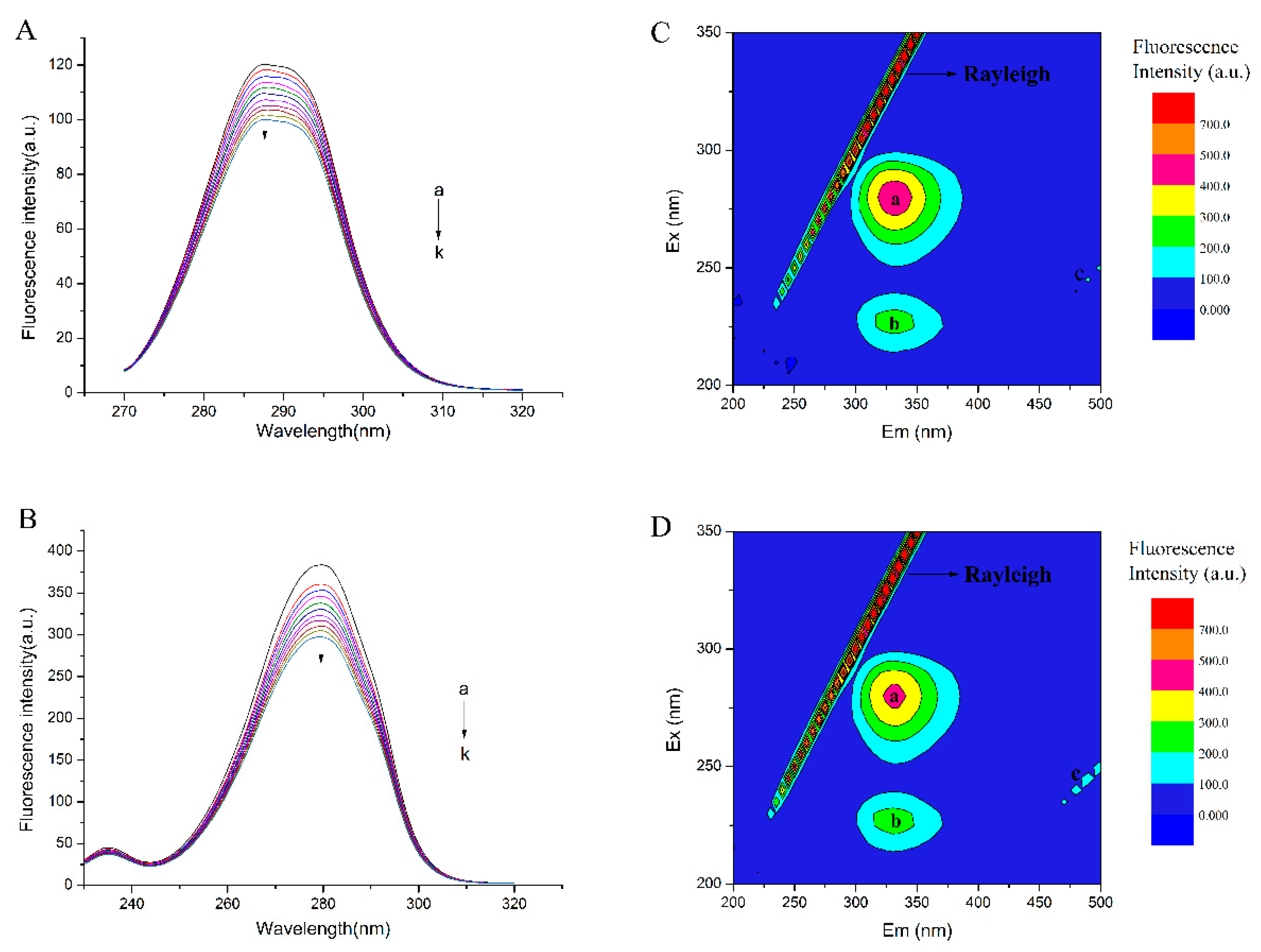
2.6.3. Conformational Investigation by Fluorescence EEM Spectra
2.6.4. Conformational Investigation by CD Spectra
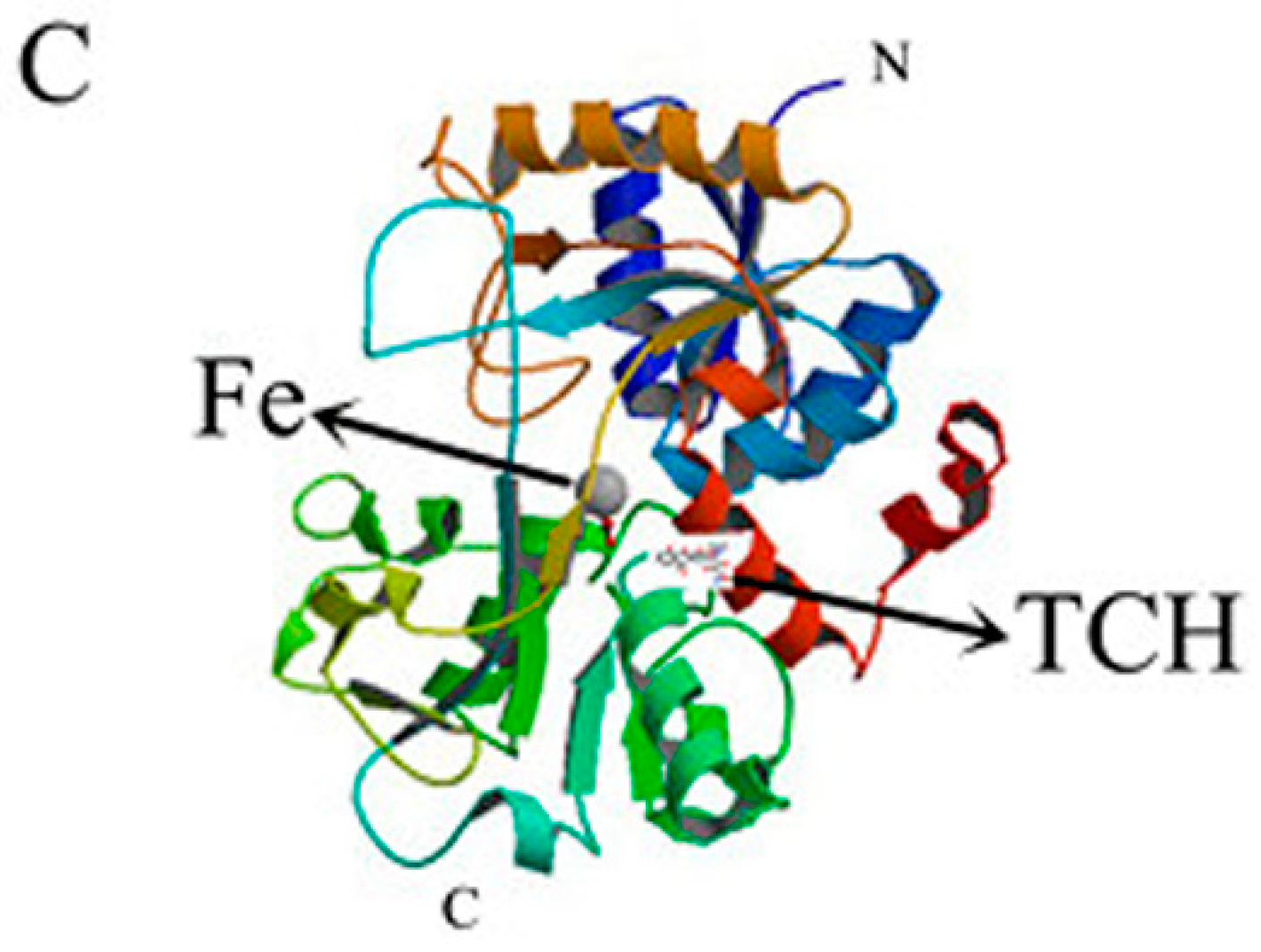
2.6.5. Conformational Investigation by Molecular Modeling
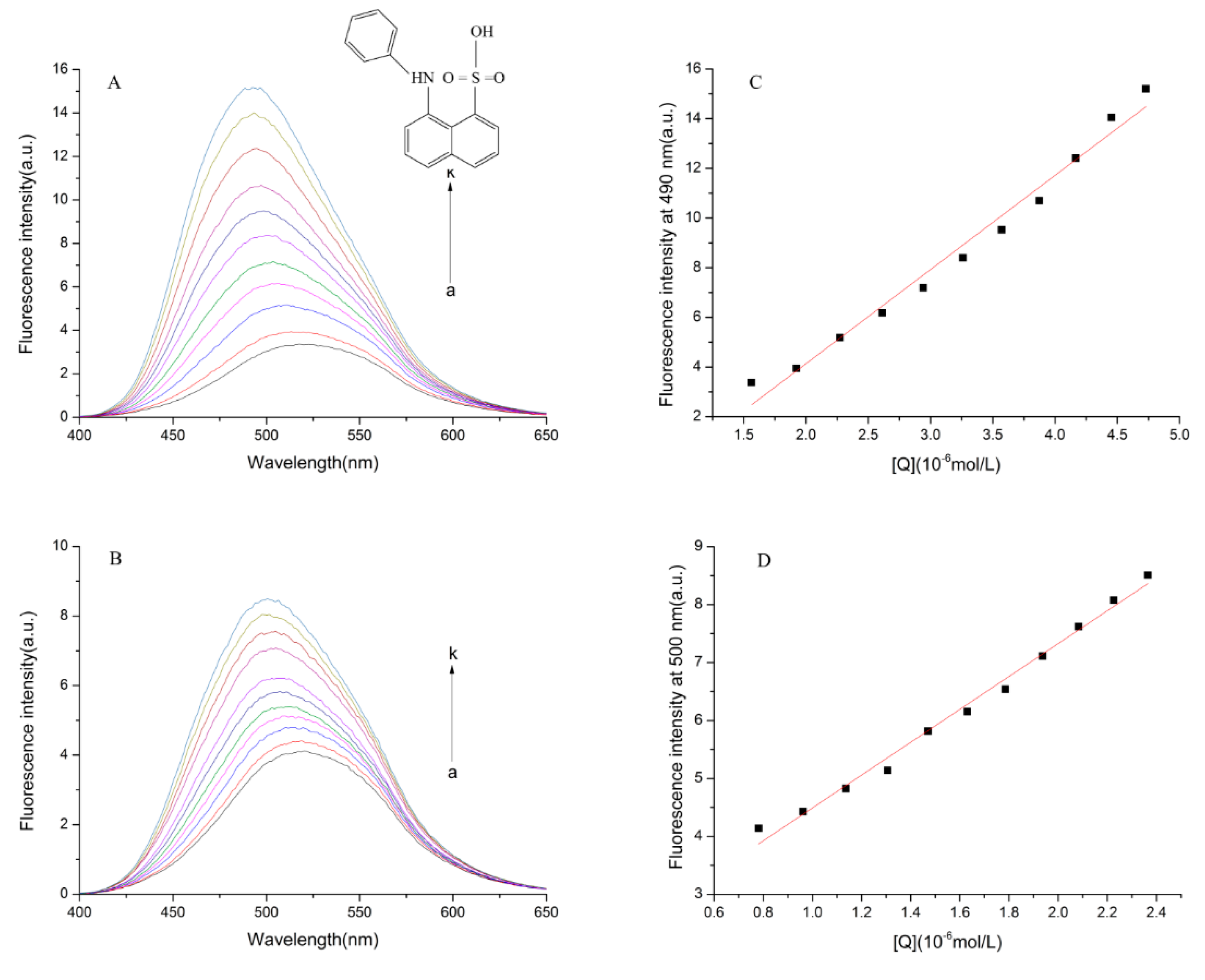
2.6.6. ANS Surface Hydrophobicity Determination
2.7. Effect of TCH on the Interaction between bLf and Fe3+ Ion
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Apparatus and Procedures
4.2.1. Fluorescence Spectroscopy
4.2.2. Microscale Thermophoresis (MST) Study
4.2.3. UV-vis Absorption Spectroscopy
4.2.4. CD Spectroscopy
4.2.5. Molecular Docking
4.2.6. ANS Surface Hydrophobicity Determination
4.2.7. Impact of Fe3+ on the Interactions between TCH and bLf
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Tetracycline hydrochloride | TCH |
| Bovine lactoferrin | bLf |
| Phosphate buffer solution | PBS |
| 8-Anilino-1-naphthalenesulfonic acid | ANS |
| MicroScale Thermophoresis | MST |
| Ultraviolet visible absorption spectra | UV-vis absorption spectra |
| Fluorescence Excitation-Emission Matrix spectra | fluorescence EEM spectra |
| Circular dichroism spectra | CD spectra |
References
- Spisso, B.F.; de Oliveira e Jesus, A.L.; de Araújo Júnior, M.A.G.; Monteiro, M.A. Validation of a high-performance liquid chromatographic method with fluorescence detection for the simultaneous determination of tetracyclines residues in bovine milk. Anal. Chim. Acta 2007, 581, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, H.; He, H.B.; Wang, C.; Gao, Y.; Zhong, Q.; Wang, X.; Zeng, Y. Study on the hemolysin phenotype and the genetype distribution of Staphyloccocus aureus caused bovine mastitis in Shandong dairy farms. Int. J. Appl. Res. 2009, 9, 416–421. [Google Scholar]
- Chi, Z.; Liu, R.; You, H.; Wang, D. Binding of the veterinary drug tetracycline to bovine hemoglobin and toxicological implications. J. Environ. Sci. Health Part B 2014, 49, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; He, Y.; Feng, S.; Sun, D.-W. Study on infrared spectroscopy technique for fast measurement of protein content in milk powder based on LS-SVM. J. Food Eng. 2008, 84, 124–131. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Kelly, A.L.; O’Mahony, J.A.; Fenelon, M.A. Sensitivity of emulsions stabilised by bovine β-casein and lactoferrin to heat and CaCl2. Food Hydrocoll. 2014, 35, 420–428. [Google Scholar] [CrossRef]
- Agostoni, C.V.; Bresson, J.-L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on bovine lactoferrin. EFSA J. 2012, 10, 2701–2726. [Google Scholar]
- Voswinkel, L.; Vogel, T.; Kulozik, U. Impact of the iron saturation of bovine lactoferrin on adsorption to a strong cation exchanger membrane. Int. Dairy J. 2016, 56, 134–140. [Google Scholar] [CrossRef]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef] [PubMed]
- Ripolles, D.; Harouna, S.; Parrón, J.A.; Calvo, M.; Pérez, M.D.; Carramiñana, J.J.; Sánchez, L. Antibacterial activity of bovine milk lactoferrin and its hydrolysates prepared with pepsin, chymosin and microbial rennet against foodborne pathogen Listeria monocytogenes. Int. Dairy J. 2015, 45, 15–22. [Google Scholar] [CrossRef]
- Kanyshkova, T.G.; Buneva, V.N.; Nevinsky, G.A. Lactoferrin and Its biological functions. Biochemistry 2001, 66, 1–7. [Google Scholar] [PubMed]
- Chen, X.; Fan, J.-C.; Wang, Y.; Fan, C.-P.; Shang, Z.-C. Fluorometric Study on the Interaction between Lomefloxacin and Bovine Lactoferrin. Anal. Sci. 2006, 22, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, F.; Xu, C.; Yuan, F.; Gao, Y. Molecular interaction between (−)-epigallocatechin-3-gallate and bovine lactoferrin using multi-spectroscopic method and isothermal titration calorimetry. Food Res. Int. 2014, 64, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Huang, J.M.; Feng, M.Y.; Ju, Z.H.; Wang, C.F.; Yang, G.W.; Yuan, J.D.; Zhong, J.F. Regulatory mutations in the A2M gene are involved in the mastitis susceptibility in dairy cows. Anim. Genet. 2013, 45, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhou, J. Aberrant regulation of autophagy in mammalian diseases. Biol. Lett. 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-L.; Tian, F.-F.; Ge, Y.-S.; Jiang, F.-L.; Lai, L.; Li, D.-W.; Yu, Q.-L.; Wang, J.; Lin, C.; Liu, Y. Spectroscopic, structural and thermodynamic properties of chlorpyrifos bound to serum albumin: A comparative study between BSA and HSA. J. Photochem. Photobiol. B 2012, 109, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mehranfar, F.; Bordbar, A.-K.; Parastar, H. A combined spectroscopic, molecular docking and molecular dynamic simulation study on the interaction of quercetin with β-casein nanoparticles. J. Photochem. Photobiol. B 2013, 127, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-J.; Yang, R.; Liang, H.; Qu, L.-B. Molecular interactions of flavonoids to pepsin: Insights from spectroscopic and molecular docking studies. Spectrochim. Acta Part A 2015, 151, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-M.; Zhang, J.; Bai, C.-L.; Wang, X.; Qiu, X.-Z.; Wang, X.-L.; Ji, H.; Liu, B. Spectroscopic study on flavonoid–drug interactions: Competitive binding for human serum albumin between three flavonoid compounds and ticagrelor, a new antiplatelet drug. J. Lumin. 2015, 168, 69–76. [Google Scholar] [CrossRef]
- Tang, L.; Li, S.; Bi, H.; Gao, X. Interaction of cyanidin-3-O-glucoside with three proteins. Food Chem. 2016, 196, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, L.; Bi, H. Study on the interaction between pelargonidin-3-O-glucoside and bovine serum albumin using spectroscopic, transmission electron microscopy and molecular modeling techniques. Luminescence 2015, 31, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Liu, R.; Teng, Y.; Fang, X.; Gao, C. Binding of Oxytetracycline to Bovine Serum Albumin: Spectroscopic and Molecular Modeling Investigations. J. Agric. Food Chem. 2010, 58, 10262–10269. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Liu, R. Phenotypic Characterization of the Binding of Tetracycline to Human Serum Albumin. Biomacromolecules 2011, 12, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, W.D.; Peter Snyder, A. Measurement of distance between fluorescent amino acid residues and metal ion binding sites. Quantitation of energy transfer between tryptophan and terbium(III) or europium(III) in thermolysin. Biochem. Bioph. Res. Commun. 1981, 100, 111–117. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Instrumentation for fluorescence spectroscopy. In Principles of Fluorescence Spectroscopy; Springer: Berlin, Germany, 1999; pp. 25–61. [Google Scholar]
- Kang, J.; Liu, Y.; Xie, M.-X.; Li, S.; Jiang, M.; Wang, Y.-D. Interactions of human serum albumin with chlorogenic acid and ferulic acid. Biochim. Biophys. Acta 2004, 1674, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Zhang, H.-M.; Zhang, G.-C.; Liu, S.-X.; Zhou, Q.-H.; Fei, Z.-H.; Liu, Z.-T. Studies of the interaction between paraquat and bovine hemoglobin. Int. J. Biol. Macromol. 2007, 41, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kronman, M.J.; Sinha, S.; Brew, K. Characteristics of the binding of Ca2+ and other divalent metal ions to bovine alpha-lactalbumin. J. Biol. Chem. 1981, 256, 8582–8587. [Google Scholar] [PubMed]
- Lu, J.-Q.; Jin, F.; Sun, T.-Q.; Zhou, X.-W. Multi-spectroscopic study on interaction of bovine serum albumin with lomefloxacin–copper(II) complex. Int. J. Biol. Macromol. 2007, 40, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Pasdar, N.; Li-Chan, E.C.Y. Comparison of Protein Surface Hydrophobicity Measured at Various pH Values Using Three Different Fluorescent Probes. J. Agric. Food Chem. 2000, 48, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Haskard, C.A.; Li-Chan, E.C.Y. Hydrophobicity of Bovine Serum Albumin and Ovalbumin Determined Using Uncharged (PRODAN) and Anionic (ANS-) Fluorescent Probes. J. Agric. Food Chem. 1998, 46, 2671–2677. [Google Scholar] [CrossRef]
- Gaucheron, F. Iron fortification in dairy industry. Trends Food Sci. Technol. 2000, 11, 403–409. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Förster, T. Delocalized Excitation and Excitation Transfer; Florida State University: Tallahassee, FL, USA, 1965. [Google Scholar]
- Mao, Y.; Yu, L.; Yang, R.; Qu, L.-B.; Harrington, P.d.B. A novel method for the study of molecular interaction by using microscale thermophoresis. Talanta 2015, 132, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Jerabek-Willemsen, M.; Wienken, C.J.; Braun, D.; Baaske, P.; Duhr, S. Molecular Interaction Studies Using Microscale Thermophoresis. ASSAY Drug Dev. Technol. 2011, 9, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhu, J.; Jin, J.; Yao, X. Studies on the binding of nevadensin to human serum albumin by molecular spectroscopy and modeling. J. Mol. Struct. 2007, 846, 34–41. [Google Scholar] [CrossRef]
- Farrell, H.M.; Wickham, E.D.; Unruh, J.J.; Qi, P.X.; Hoagland, P.D. Secondary structural studies of bovine caseins: temperature dependence of β-casein structure as analyzed by circular dichroism and FTIR spectroscopy and correlation with micellization. Food Hydrocoll. 2001, 15, 341–354. [Google Scholar] [CrossRef]
- Wang, X.; Ju, Z.; Huang, J.; Hou, M.; Zhou, L.; Qi, C.; Zhang, Y.; Gao, Q.; Pan, Q.; Li, G.; et al. The relationship between the variants of the bovine MBL2 gene and milk production traits, mastitis, serum MBL-C levels and complement activity. Vet. Immunol. Immunopathol. 2012, 148, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Elcoroaristizabal, S.; Callejón, R.M.; Amigo, J.M.; Ocaña-González, J.A.; Morales, M.L.; Ubeda, C. Fluorescence excitation-emission matrix spectroscopy as a tool for determining quality of sparkling wines. Food Chem. 2016, 206, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Kasoju, N.; Bora, U. Fluorescence study of the curcumin− casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules 2008, 9, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Zhang, G.; Ding, Y.; Ran, L.; Luo, L.; Wu, J.; Hu, D.; Song, B. Binding interactions between enantiomeric α-aminophosphonate derivatives and tobacco mosaic virus coat protein. Int. J. Biol. Macromol. 2017, 94, 603–610. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Compound | T (K) | Ksv (104 L/mol) | Kq (1012 L/mol/s) | R2 |
|---|---|---|---|---|
| 290 | 6.88 | 6.88 | 0.9999 | |
| bLf + TCH | 300 | 5.14 | 5.14 | 0.9997 |
| 310 | 4.74 | 4.74 | 0.9999 |
| Compound | T (K) | Ka (104 L/mol) | n | R2 | △H (KJ/mol) | △G (KJ/mol) | △S (J/mol/K) |
|---|---|---|---|---|---|---|---|
| 290 | 5.154 | 0.978 | 0.9970 | −107.99 | |||
| bLf + TCH | 300 | 2.551 | 1.316 | 0.9765 | −87.56 | −108.70 | 70.45 |
| 310 | 0.495 | 0.814 | 0.9932 | −109.40 |
| Compound | T (K) | Ka (104 L/mol) | E | n | R (nm) | R0 (nm) |
|---|---|---|---|---|---|---|
| bLf + TCH | 300 | 2.551 | 0.053 | 1.32 | 2.81 | 1.74 |
| Compound | Peak Position λex/λem (nm/nm) | Intensity F0 | Peak Position λex/λem (nm/nm) | Intensity F0 |
|---|---|---|---|---|
| bLf | 280/332 | 468.0 | 225/331 | 239.5 |
| bLf + TCH | 280/332 | 431.1 | 225/331 | 247.5 |
| Molar Ratio [bLf]/[TCH] | α-helix | β-sheet | Antiparallel | Parallel | Random Coil |
|---|---|---|---|---|---|
| 1:0 | 26.6 | 18.3 | 16.1 | 10.0 | 36.3 |
| 1:1 | 20.9 | 19.6 | 28.1 | 11.7 | 40.7 |
| Compound | S0 | nm | R2 |
|---|---|---|---|
| BLF + ANS | 3.791 | 490 | 0.9908 |
| BLF + ANS + TCH | 2.836 | 500 | 0.9953 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Gao, X.; Bi, H.; Xie, Y.; Tang, L. Assessment of Binding Interaction between Bovine Lactoferrin and Tetracycline Hydrochloride: Multi-Spectroscopic Analyses and Molecular Modeling. Molecules 2018, 23, 1900. https://doi.org/10.3390/molecules23081900
Sun Q, Gao X, Bi H, Xie Y, Tang L. Assessment of Binding Interaction between Bovine Lactoferrin and Tetracycline Hydrochloride: Multi-Spectroscopic Analyses and Molecular Modeling. Molecules. 2018; 23(8):1900. https://doi.org/10.3390/molecules23081900
Chicago/Turabian StyleSun, Qifan, Xin Gao, Hongna Bi, Yingbo Xie, and Lin Tang. 2018. "Assessment of Binding Interaction between Bovine Lactoferrin and Tetracycline Hydrochloride: Multi-Spectroscopic Analyses and Molecular Modeling" Molecules 23, no. 8: 1900. https://doi.org/10.3390/molecules23081900
APA StyleSun, Q., Gao, X., Bi, H., Xie, Y., & Tang, L. (2018). Assessment of Binding Interaction between Bovine Lactoferrin and Tetracycline Hydrochloride: Multi-Spectroscopic Analyses and Molecular Modeling. Molecules, 23(8), 1900. https://doi.org/10.3390/molecules23081900





