Abstract
High-density lipoprotein (HDL) has been proposed to provide cardio-protective properties through the functionality of its anti-inflammatory and antioxidant enzymatic machinery. Within this article, the beneficial effects of several functional foods on HDL levels and functionality for cardio-protection are thoroughly reviewed. Emphasis is given to functional foods and their antioxidant and anti-inflammatory health-promoting effects for the cardiovascular system through their benefits on HDL, which act either solely or synergistically as an adjuvant approach with well-established anti-atherogenic therapies. Promising outcomes from both in vitro and in vivo studies in animal models and clinical trials, which outline the beneficial effects of such functional foods on HDL levels and functionality, are thoroughly discussed. The mechanisms of the obtained antioxidant, anti-inflammatory, antithrombotic, and cardio-protective effects on HDL activities of functional foods containing natural bioactives are also outlined. Limitations and future perspectives on the overall benefits that these natural bioactive compounds exert as important ingredients in functional foods to induce HDL-related benefits and to strengthen cardiovascular health are also discussed.
Keywords:
cholesterol; LDL; HDL; inflammation; thrombosis; cardiovascular; cardio-protection; anti-inflammatory; antithrombotic; antioxidant 1. Introduction
Cardiovascular diseases (CVDs) are the main cause of morbidity globally and a significant contributor to disability, especially stroke and coronary heart disease (CHD) [1]. Data retrieved from the World Health Organization have shown that in the last two decades, the number of deaths caused by CVDs among adults surpassed 350,000,000 globally [2]. Classic cardiovascular risk evaluation involves the evaluation of the plasma levels of triglycerides, cholesterol, and plasma lipoproteins that function as carriers of such hydrophobic lipid molecules (cholesterol, cholesterol esters, and triglycerides), including chylomicrons, very-low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), and especially low-density lipoprotein (LDL) and high-density lipoprotein (HDL) [3]. These lipoproteins contain apo-proteins, some of which are structural elements, others possess enzymatic activities, and some possess both functions, a surface of polar lipids (mainly phospholipids, but also glycolipids), and a core rich in the hydrophobic lipid molecules they carry. The classic structures of such lipoproteins are presented in Figure 1.
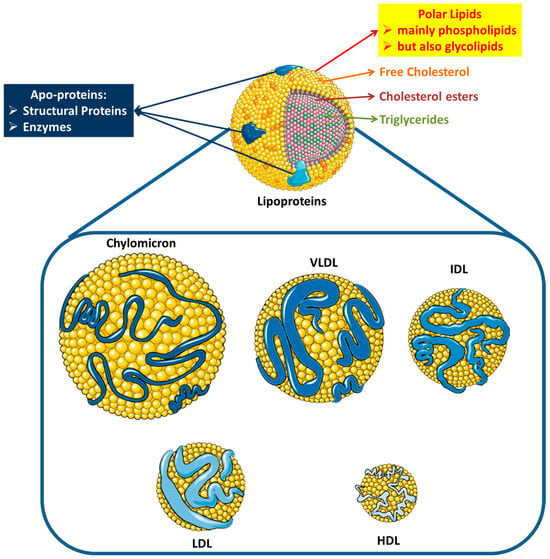
Figure 1.
Structures of plasma lipoproteins. Abbreviations: VLDL = very-low-density lipoprotein; IDL = intermediate-density lipoprotein; LDL = low-density lipoprotein; HDL = high-density lipoprotein (images for each individual lipoprotein were retrieved from: https://smart.servier.com; date of access 28 August 2024).
Apart from the levels of total cholesterol and low-density lipoprotein cholesterol (LDL-C), a clear relationship exists between HDL levels and the probability of developing cardiovascular diseases (CVDs): having healthy levels of HDL-C (>60 mg/dL) is strongly associated with a potentially lower risk of CVDs, while low levels of high-density lipoprotein cholesterol (HDL-C; <40 mg/dL for men and <50 mg/dL for women) are associated with an increased danger, among both healthy and not healthy individuals, in both sexes, with no exception [4,5].
According to the National Health and Nutrition Examination Survey, a 3-year investigation from 2015 to 2018 showed that the prevalence of high total cholesterol in U.S. adults was 11.4%, of which the group with the highest rate was men (10.5%) and women (12.2%) over 40 years old. Apart from that, the reported prevalence of low HDL-C in men was equal to 26.6%, and for women 8.5%, indicating the higher risk of development of CVDs in the male population [6]. Nevertheless, preserving healthy HDL-C levels does not seem to be an adequate way to reduce the risk for CVDs, most notably if the functionality of this particle is not sufficient.
The most used explanation of how HDL-C protects the human body from CVDs is that it operates by mediating the uptake of peripheral cholesterol (cholesterol efflux capacity, CEC), returning it to the liver for its re-absorption by the liver and its excretion into the bile and the intestines (reverse cholesterol transport, RCT). CEC has gained attention as a biomarker proposed to reflect the function of HDL and improve CVD risk prediction, as it measures the ability of HDL to receive cholesterol from macrophages, which is the first key step of RCT, a primary function of HDL in which peripherally deposited cholesterol is taken up and carried by HDL to the liver for excretion (Figure 2 and Figure 3). Apart from CEC and RCT, it seems that other potentially beneficial functions of HDL also contribute to its cardio-protection, including its effects on macrophage foam cell formation, endothelial activation of endothelial nitric oxide synthase and endothelial nitric oxide stimulation through its vasodilator ability, inhibition of reactive oxygen species (ROS), reducing the oxidation of LDL-C and blocking the expression of adhesion molecules in the endothelial cells, monocyte adhesion, and platelet aggregation. All the above functions of HDL play a crucial role in the prevention of atherosclerosis and other CVDs, while it is now estimated that a 1% increase in HDL-C results in a 2% reduction in the incidence of major cardiovascular problems [4,7,8]. Interestingly, specific types of dietary functional foods rich in bioactive compounds beneficially affect both RCT and CEC (Figure 2 and Figure 3), as well as several of all the other functions of HDL, thus providing cardio-protective benefits.
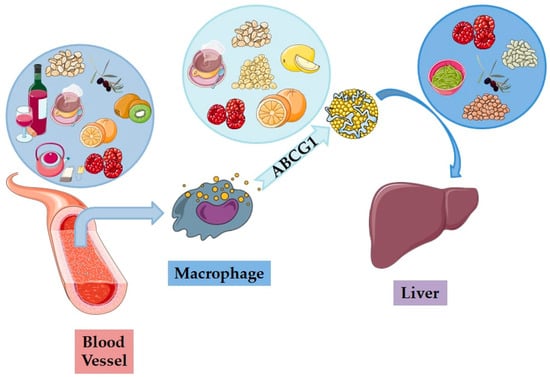
Figure 2.
The beneficial impacts of functional foods on the reverse cholesterol transport (RST) process: differing foods positively influence different stages of the process, enhancing the removal of cholesterol from blood vessels via macrophages and the ABCG1 (ATP-binding cassette subfamily G member 1) protein, ultimately destined for metabolism in the liver (images for each individual food, biomolecule, and organ were retrieved from: https://smart.servier.com; date of access 19 September 2024).
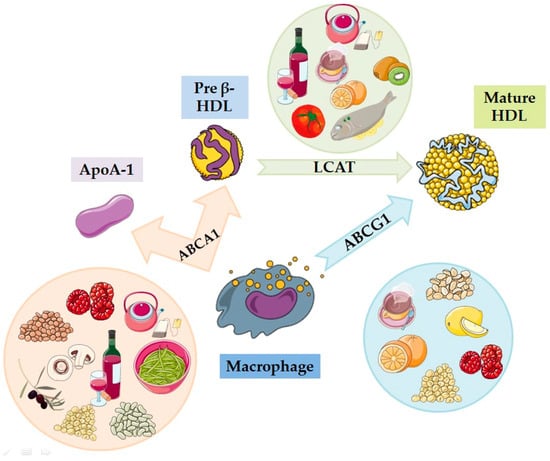
Figure 3.
The beneficial actions of functional foods in improving cholesterol efflux capacity (CEC): different foods enhance the cholesterol removal process from macrophages through the transporters ABCA1 and ABCG1, contributing to the formation of mature HDL by the action of the enzyme LCAT. HDL = high-density lipoprotein; LCAT = lecithin–cholesterol acyltransferase; ABCA1 = ATP-binding cassette transporter A1; ABCG1 = ATP-binding cassette subfamily G member 1; Apo-AI = apolipoprotein AI (images for each individual food and biomolecule were retrieved from: https://smart.servier.com; date of access 19 September 2024).
Even though there is no precise definition concerning functional foods, this term is used to describe foods that provide health benefits over and above nutrition because of the presence of specific bioactive compounds, unlike regular foods, due to the variety of approaches [9,10,11]. The perspective offered by Dr. Mingruo Guo is the most widely accepted, as it encompasses a wide range of views. Particularly, he points out that, “A food can serve three primary functions: (1) providing energy through carbohydrates, proteins, and/or lipids, along with essential nutrients, (2) providing sensory pleasure through aroma, color, and taste, and (3) health benefits. A functional food, while looking like or being a conventional food, consumed as part of a regular diet, provides physiological benefits and/or reduces the risk of chronic disease, in addition to providing basic nutrition” [10,12]. Functional foods are associated specifically with health beneficial effects due to the presence of specific components, including antioxidant and anti-inflammatory compounds, reducing the risk of developing CVDs and enhancing the immunological response, all in all promoting overall health [10,13].
Experts in the fields of genetics, nutrition, and metabolism evidently support the significant relationship between nutrition and HDL-C levels, pointing out, in particular, individuals with cholesterol-related diseases. Over the last 10 years, there has been increasing evidence of the beneficial effects of functional foods and bioactive compounds on improving the functionality and regulating the levels of HDL-C and LDL-C, leading to lower CVD development risks [14]. Clinical evidence supports the use of various foods containing advantageous substances in patients with different diseases, such as dyslipidemia and diabetes. To this day, there is no specific target for HDL-C defined in clinical trials, although raised HDL-C is associated with atherosclerosis, while low HDL-C levels are linked to incidence and morbidity in CHD, even at low LDL-C concentrations [15]. Despite this fact, only a small number of long-term studies have been completed, leading to a lack of evidence that could help in the development of a high-precision dietary guideline for the treatment of HDL-C concentration and function, as well as clinical end points, such as morbidity and cardiac incidents [16].
In this review, data and results of clinical studies and clinical trials on the effect of functional foods on HDL-C have been collected, highlighting their importance for human health and protection. In addition, our primary goal was to describe the available evidence to date, to further support the understanding of dietary compounds on HDL-C functionality.
2. Methodology and Materials
The methodological approach for this narrative review was performed using Scopus, PubMed, and ScienceDirect databases, by utilizing research publications and clinical trials updated to August 2024. The search terms that were included in the title and abstract were “dietary compounds”, “functional foods”, “HDL-C functionality”, “CVDs”, and “cardiovascular health”, by using the terms “AND” and/or “OR” for all these keywords. For particular functional foods and dietary compounds, which were defined in the first selection, the criteria below were also applied after the name of each food or compound. The total number of articles was over 8000 results in all databases used. Thus, this narrative review is a summary of the available data of the last 5 years (2019–2024), after excluding duplicates, reviews, books, book chapters (apart from some few important ones) and non-relevant articles, which reduced this number to approximately 200 articles, while some few important articles prior to 2019 were included as they contained relevant information that have not previously been thoroughly reviewed.
Manual search was undertaken in the reference list of each publication to guarantee that no important research articles were skipped. We emphasized the included clinical trials that were examined for the most recent publications through the last 5 years, to present the most recent evidence. Our review also included a number of important earlier studies that have not been reported in other relevant reviews, as well as clinical research, the outcomes of which have not been thoroughly developed in previous review studies. We selected all studies in English in full text, in vivo and in vitro trials, and clinical studies with major outcomes for the efficiency of functional foods in HDL-C functionality and improvement. The methods and editorial validity of all articles included in this review were thoroughly detailed by the authors.
3. HDL-C Physiology and Pathophysiology
Present evidence indicates that the maintenance of healthy HDL-C levels is not adequate when the circulating HDL-C is non-functional [17]. New research emphasizes the importance of HDL-C functionality, especially in CEC, being a much more accurate and precise indicator of cardiovascular protection, highlighting it much more than its sole concentration [18,19]. The main cardio-protective effects of HDL-C are CEC, RCT, and the inhibition of the expression of adhesion molecules, such as E-selectin [20]. HDL-C and apolipoprotein A-1 (apoA-1) protect against atherosclerosis through multiple mechanisms, the most researched and widely acknowledged being their key role in RCT, a highly complex physiological process in which HDL-C molecules deliver cholesterol from the exogenous tissues to the liver, for processing and removal from the body [21]. This procedure consists of a number of different phases. At first, a lipid-poor apoA-1 is coupled to the ATP-binding cassette ABCA-1 in macrophages, and it adsorbs cholesterol from the lipid-laden membrane of arterial wall cells, leading to the formation of pre-β HDL bodies. These immature particles interface with various enzymes and gain additional lipids (extra cholesterol and phospholipids) from the peripheral tissues, resulting in the formation of large HDL particles. The next step is the esterification of the surface of HDL-C particles by lecithin–cholesterol acyltransferase (LCAT), generating mature HDL particles (α-HDL). Lastly, the mature esterified cholesterol is delivered to the liver by the scavenger class B type 1 receptor (SR-B1), from where it will be excreted in the bile to be removed from the human body [21,22,23].
There are also other potentially beneficial properties of HDL-C. Its anti-inflammatory, antioxidant, and antithrombotic effects are proven facts [24,25,26]. It is evidently proven that HDL-C has the ability to inhibit ROS and LDL-C oxidation, and induce the synthesis of vasodilators, such as nitric oxides (NO), helping to reduce the dysfunction of the endothelium. Besides its lipid modulating functions, HDL has been identified as a regulating molecule in the function of blood platelets [27]. Research studies point to immediate interaction mechanisms between HDL and platelets, through which HDL blocks platelet aggregation in responding to a variety of triggers. These findings highlight the versatility of the impact of HDL-C on platelet activity, pointing to its potency as a novel therapeutic agent for the prevention of thrombotic incidents in CVDs [28,29,30].
The cardio-protective effects of HDL-C are widely known, not only on account of RCT, but also because of its inhibiting role in the expression of endothelial cell genes associated with high inflammation risk [31]. Epidemiological research has indicated an inverse relationship between HDL-C and the increased risk of CVDs, specifically atherosclerosis [32]. HDL-C is deemed to be atheroprotective, as it is capable of binding to serum paraoxonases (PONs), aiming at the protection of HDL from oxidation [33]. HDL-C also modulates the chronic inflammatory response by inhibiting IL-1β and TNFα (pro-inflammatory cytokines) formation by blocking the interaction of monocytes with activated T-cells, a procedure responsible for atheromatic plaque formation and tissue damage [34,35].
Finally, HDL-C and its enzymatic activities, including the PAF-AH, seem to protect against these manifestations. Efforts have mainly been focused on increasing HDL levels as one of the main goals of dietary interventions and drug administration for cardio-protection. The past classical pharmacological approaches and treatments to increase the functionality and levels of HDL-C, as well as to reduce LDL-C and ox-LDL-C levels, include the intake of cholesterol absorption inhibitors [36]. According to the 2019 ESC/EAS guidelines, individuals with a high risk of developing CVDs are suggested to undertake high-intensity statin therapy (atorvastatin 40-80 mg or rosuvastatin 20–40 mg daily), while moderate-risk ones could use moderate-intensity statins, such as simvastatin 20-40 mg per day. Daily dosage is suggested because of the long half-life and effectiveness of the drugs [37]. Statins act by inhibiting the enzymatic action of HMG-CoA reductase, a molecule responsible for cholesterol production in the liver, resulting in decreasing LDL-C and ox-LDL-C levels, increasing those of HDL-C and improving its functionality in CEC [37,38]. In addition to pharmacological guidelines, a heart-healthy diet is suggested. A nutritional diet rich in fruit and vegetables, whole grains, and poor in trans and saturated fats, as well as refined sugars, helps reduce LDL-C levels and improve HDL-C levels and functionality [37]. However, pharmacological treatments sometimes cause unfavorable side effects. Statins, for example, lead to liver dysfunction and an elevated risk of developing type 2 diabetes and chronic pain syndromes [39,40,41]. Apart from that, not all users display the same effective impact of statins, as adjustment of the dosage is very essential for each individual [42].
Recent research, however, has also highlighted the importance of affecting the bio-functionality of HDL-C and not only its levels, and especially its enzymatic activities toward reducing thrombo-inflammation [43]. For example, dietary intake of phenolic antioxidants and bioactive anti-inflammatory polar lipids (PLs), and especially those PLs that bear unsaturated fatty acids (UFA) in their structures, such as the monounsaturated fatty acid (MUFA), oleic acid (OA), and/or the omega-3 (ω-3) polyunsaturated fatty acids (PUFA), alpha linolenic acid (ALA; 18:3ω3), eicosapentaeinoic acid (EPA; 20:5ω3), and docosahexaenoic acid (DHA; 22:6ω3), induce the increase in the plasma levels of HDL-C. At the same time, the incorporation of such anti-inflammatory dietary PLs and antioxidant dietary phenolics to HDL-C also facilitates the increase in HDL-C enzymatic activities, such as increasing the enzymatic activity of platelet-activating factor (PAF) acetylhydrolase (PAF-AH), also termed lipoprotein-associated phospholipase A2 (LpPLA2), which degrades the thrombo-inflammatory mediator PAF and the oxidized phospholipids (Ox-PLs) of both oxidized and LDL-C (ox-LDL-C) in plasma and, thus, deactivates and reduces oxidative and inflammatory signaling and their associated manifestations.
Overall, such dietary bioactives provide an additional protective mechanism by increasing plasma PAF-AH activity and protecting HDL-C biomolecules and enzymes, including the phospholipids, PAF-AH, and antioxidant enzymes, from oxidative-stress-induced deactivations and unfavorable molecular modifications. This is in agreement with the beneficial in vitro and in vivo effects of several dietary PLs, especially on PAF metabolism and HDL levels and bio-functionality (increased levels and anti-inflammatory and antioxidant enzymatic activities of HDL-C) toward reducing PAF levels and cardio-protection [43].
3.1. Impacting Factors for HDL-C Function and Morphology
The composition and functionality of HDL-C particles can be affected by a number of parameters, including environmental and genetic factors, dietary habits, and lifestyle [17]. Daily unhealthy habits, including smoking, extreme alcohol intake, and sedentary lifestyle, have been clinically documented to negatively affect the functionality of HDL-C and increase the possibilities of CVD development [44,45]. Although numerous studies indicate that alcohol consumption has a negative impact on health in general, alcohol intake in moderation has shown an increase in the levels of HDL-C, in relation to abusive consumption [45,46].
Concerning ethnic diversity in lipid levels, most data indicate differences in HDL-C regulation, with the higher indexes appearing in African populations and lower being observed in Asians, with these variations being present in childhood, seeming to be unrelated to obesity and underlining genetic racial markers [47].
Genetic profile is an important factor when it comes to diseases associated with changes in HDL-C function inheritance, such as familial hypercholesterolemia, CVDs, or even type II diabetes [48,49]. Polymorphisms of the genes of important molecules known to be involved in HDL-C modification and operability, for example, apoA-1, LCAT, and ABCA1, are crucial for the determination of the HDL-C concentration in blood serum, indicating the critical impact of heredity and the genetic profile on the potential for the development of an HDL-related disease, by negatively affecting the functionality and the morphology of the molecule [48,50,51].
3.2. Inflammation Impact on HDL-C
Inflammation is a highly significant factor with a damaging influence on HDL-C’s functionality, thus increasing the possibilities of emerging CVDs [52,53,54]. During inflammation, the body’s defense mechanisms are being activated, releasing antibodies and proteins to attack the foreign substance, causing the excretion of macrophage cells and proteins at the inflammation zone [54]. Extensive systemic inflammation has negative health effects by modifying the cardiovascular system, as well as the HDL-C particles’ number and structure. Upon the emergence of acute inflammation, HDL-C composition is altered, and it loses its ability to carry out the CEC, as it normally should. Additionally, HDL-C takes a pro-inflammatory form, promoting monocyte adhesion during the immune response, resulting in losing its anti-inflammatory and antioxidant properties as a consequence of a lack of antioxidant factors as well as the increased concentration of oxidized phospholipids on its surface [54,55].
The dysfunctionality of HDL-C during an acute inflammation response can be explained by the increased levels of serum amyloidal A (SAA), which can replace apoA-1 in the HDL-C molecule. This can lead to the formation of dysfunctional HDL-C particles with much less CEC than the normal rate [56]. Apart from this molecule, other acute-phase proteins, such as α-1-acid-glycoprotein 1, α-2-HS-glycoprotein, and fibrinogen, have also been detected in modified HDL-C particles, making it dysfunctional [56]. In addition, the lack or the deficiency of LCAT evidently enhances the attenuation of lipopolysaccharide (LPS)-induced inflammation, resulting in HDL-C modifications, both in HDL-C function and regulation [57].
Overall, the inflammation changes in RCT enzymes result in lower HDL-C levels, characterized by more rapid degradation rates than usual and faster removal from the blood circulation, having an undesirable impact in the main atheroprotective function of HDL-C in RCT [58,59]. This HDL-C level drop causes a counterbalancing reaction, characterized by higher aggregation of VLDL, resulting in other health complications, for example, hypertriglyceridemia. The previously mentioned deviations from normal baselines can explain clinical incidents, where patients display low apoA-1 and HDL-C levels upon an inflammatory response [60].
4. Dietary Compounds and HDL-C
Nutrition patterns and dietary compounds are strongly associated with chronic diseases linked to diversification from the physiological morphology and levels of HDL-C, including CVDs in general, type 2 diabetes, and obesity [61,62]. Recent and contemporary research has shown the capabilities of nutritional intercessions for the prevention and control of these kinds of diseases [62]. In July 2023, the World Health Organization (WHO) published updated guidelines on the healthy diet designation, with a specific focus on carbohydrates and total and certain types of fat, underlining that a wholesome eating pattern must include a complex mix of food groups [63]. According to the WHO, carbohydrate intake should constitute 40–70% of the total calorie intake and should mainly derive from minimally processed whole grains, fruit, and vegetables (400 g of fruit and vegetables and 25 g of fibers on a daily basis), dietary compounds associated with a low risk of morbidity. Regarding total fat intake, it should account for a maximum of 30% of the total calorie ingestion to prevent adverse effects, whereas saturated fatty acids should be limited to a maximum of 10%, if not to be replaced with polyunsaturated fatty acids, and trans fats should be only 1% or less of the total calorie intake [63].
Dieticians recommend a well-balanced, nutritious, and versatile diet based on frequent consumption of foods rich in unsaturated fats, fibers, and anti-inflammatory and antioxidant substances, to guarantee the support of the cardiovascular system and the prevention and protection of CVDs [64]. The best known diets are the Mediterranean diet and DASH-type diets (Dietary Approaches to Stop Hypertension) [65,66]. These two dietary models prioritize nutrient-rich foods and emphasize the importance of consuming healthy fats (monounsaturated (MUFAs) and polyunsaturated fatty acids (PUFAs)), whole grains, and minimally processed foods to support cardiovascular health [67,68,69].
The consumption of beneficial dietary compounds has a positive impact on the functionality and regulation of HDL-C and, therefore, the promotion of human health in numerous ways. Their beneficial effects include the promotion of RCT and endothelial function, increase of antioxidant capacity of HDL-C, and reduction of oxidative damage and inflammatory acute responses. In this review, we summarize the most recent data on HDL-C functionality and its association with specific functional foods.
4.1. Cholesterol Efflux Capacity—HDL-C Improvement
Reverse cholesterol transfer (RCT) is a vital procedure for sustaining cardiovascular health, as it entails the removal of surplus cholesterol from peripheral cells for it to be sent to the liver [22]. The most important phase of this procedure is the CEC, the capability of HDL-C to excrete cholesterol from the peripheral membranes, macrophage, and foam cells, with the contribution of different transmitters, including ABCA-1 [22,70]. Therefore, the CEC can be used as an index for determining the functionality of HDL-C, as well as an indicator of cardiovascular and other diseases’ occurrence [71,72].
The literature provides evidence about the association between functional foods and bioactive ingredients regarding CEC regulation (Figure 3), including fruit, nuts, legumes, fish, extra virgin olive oil, fungi, alcohol, cocoa, coffee, and tea, as depicted in Figure 4 and presented in Table 1.
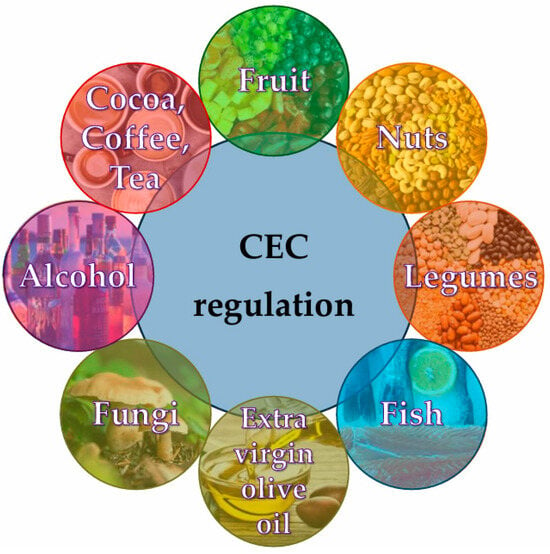
Figure 4.
Representative dietary factors affecting CEC regulation.

Table 1.
Studies on functional foods affecting the CEC regulation.
4.1.1. Phenol-Rich Fruit
The beneficial effects of fruit consumption are well known, as fruit contain loads of useful substances that promote cardiovascular health, by advancing the CEC of HDL-C. Several fruits are rich in flavonoids, polyphenols, and antioxidants, contributing to the protection and boosting the functionality of HDL-C particles [88,89].
Berries are fruit rich in polyphenols, the consumption of which is believed to be beneficial for cardiovascular health. A double-blind controlled trial, including 102 prohypertensive patients, took place in a 12-week concurrent randomized study to examine the cardiometabolic effects of the consumption of aronia berries, as well as the gut microbiota. Participants were randomly allocated to daily consumption of either an encapsulated aronia berry extract rich in polyphenols, or a placebo of maltodextrin. The research findings implied that the arterial function and the flow-mediated dilation (FMD) were improved due to the consumption of aronia berry extract by the individuals of the aronia group, in comparison to the placebo group. Other major differences in secondary outcomes, such as cardiac pace and blood lipid levels, we not observed. Alterations from the baseline value were considerable for HDL-C and cortisol levels only for the aronia group (HDL-C = +0.07; 95% confidence interval: 0.004–0.141 mmol/L). Apart from these results, there were no notable influences on the CEC [73].
Another study of the same type took place to examine the influence of aronia berries on the cardiovascular system, as well as the intestinal microbiota, in 66 healthy men, who were randomly divided into 3 groups to consume an Aronia melanocarpa extract rich in polyphenols for a period of 12 weeks (n = 23; 75 g of berries, 116 milligrams), a powdered whole fruit (n = 23; 10 g of berries, 12 milligrams), or a maltodextrin placebo (n = 20). The aronia berry consumption increased the FMD in relation to the control group (0.9% ± 0.4%; 95% CI: 0.13%, 1.72%, and 1.2% ± 0.4%; 95% CI: 0.36%, 1.97%) and improved the endothelial function in healthy men. HDL means and standard deviation (SD) were increased by 1.5 ± 0.3 mmol/L for the whole fruit group, whereas for the aronia extract and the placebo groups, the means and SDs were equal, 1.3 ± 0.2 mmol/L. The overall HDL mean and SD increase was 0.4 ± 0.3 [90].
Grapes are rich in fibers, polyphenols, and flavonoids and are a potent controller of obesity and inflammation, especially grape seed extract (GSE). In a randomized, double-blind clinical trial, researchers randomly allocated 40 obese individuals to consume either 300 milligrams of GSE per day or placebos, along with a calorie-restricted diet for 12 weeks. HDL-C levels as well as the ratio of HDL-C to LDL-C were markedly increased in the GSE group, in relation to the placebo group, with p-values equal to 0.03 and 0.008, respectively. In addition, a clear reduction in LDL-C, total cholesterol, and triglycerides was observed in the GSE group [74].
Another study was designed to review the influence of a daily intake of 46 g of whole grape powder on the cholesterol/bile acid metabolism in healthy individuals. The trial consisted of two phases: a four-week formulation of a low-polyphenol diet and a following four-week formulation of 46 g of grape powder consumption, maintaining the low-polyphenol diet. The consumption of grape powder markedly lowered the total cholesterol by 6.1%, HDL-C by 7.6%, and LDL-C by 5.9%. The reduction in the total bile acid was equal to 40.9%, indicating the modification of cholesterol/bile acid metabolism [91].
4.1.2. Nuts
Nuts’ consumption is significantly beneficial for health maintenance, as they are a food rich in MUFAs and PUFAs, whether or not they are processed. In particular, hazelnut kefir is found to contain the highest total of MUFAs and walnut kefir contains the highest total of polyunsaturated fatty acids [92]. Their high amount of unsaturated fats has a positive impact on maintaining cardiovascular health [92].
A 1-year study on 296 individuals with a high cardiovascular risk took place to determine the correlation between cardio-protective foods, including nuts, and HDL-C functionality. The researchers noticed that elevations in nut consumption were linked to an increase in PON1 (paraoxonase-1), improving CEC and cardiovascular health onwards [93]. Another group of researchers tested the effects of mixed nuts’ consumption on inflammation markers on high cardiovascular risk individuals. During a 16-week randomized control trial, 29 obese or overweight volunteers consumed 42.5 g of mixed nuts on a daily basis or 69 g of isocaloric pretzels per day. The ingestion of mixed nuts reduced the percentage of body fat, even though inflammation markers, such as 8-oxo-deoxyguanose, had no significant decreasing tendencies. No major differences in HDL-C were noted among the two groups [75].
4.1.3. Legumes
Legumes are an exquisite provider of vitamins, fibers, and other bioactive compounds, such as phenol and saponin compounds. The most commonly reported phenol compounds in legumes are flavonoids, in particular in colored seed coats. These bioactive compounds modulate glucose metabolism and are characterized by anti-inflammatory and antioxidant properties, thus providing a positive effect in inhibiting the progression of several chronic diseases, including cardiovascular diseases [94].
An observational trial took place to examine the dietary effects of legumes on the body weight of 400 obese Korean volunteers, divided into a group of legume-enriched diet (168 individuals) and a usual diet group (232 individuals). After the end of the study, researchers observed that HDL-C levels were increased in both groups, especially in the legume-enriched group [95]. Another clinical study aimed to examine the impact of nuts (peanuts, almonds, walnuts, pistachios, and hazelnuts) and legumes’ (lentils, beans, and peas) consumption in association with cardiovascular risk factors and the Framingham Risk Score (FRS), which was used to estimate the 10-year probability of CVD occurrence in elderly men. The study included 267 men aged 60–70 using a verified food intake questionnaire. Both nuts and legumes’ consumption resulted in an increase in HDL-C levels. In particular, legumes’ consumption was correlated with lower LDL-C levels (odds ratio (OR): 0.28, 95% CI: 0.15, 0.49, p-value < 0.001) and higher HDL-C levels (OR: 2.14, 95% CI: 1.20, 3.72, p = 0.005). The researchers did not detect any correlation between nuts and legumes regarding FRS or CVD risk factors [76].
4.1.4. Fish
Fish, and especially fatty fish, such as salmon and sardines, are frequently recommended as beneficial for cardiovascular health, due to their naturally high content of ω-3 PUFA, EPA, and DHA, in particular. Since the 1990s, fish and n-3 PUFA intake have been linked to a reduced risk of CHD [96,97,98]. Soon after, n-3 PUFA intake was found to lower cholesterol levels, LDL-C levels, and triglycerides, while also slightly increasing HDL-C levels [92]. Omega-3 PUFAs found in fish, in particular EPA and DHA, are of crucial importance for cardiovascular health, as not only do they lower LDL-cholesterol and triglycerides, but they also enhance the functionality of HDL-C particles, which promotes RCT. Thereby, this mechanism contributes to the removal of cholesterol from the arteries, transporting it to the liver for elimination, reducing the risk of atherosclerosis and cardiovascular disease (CVD). Moreover, fish also contain other bioactive lipids, such as marine phospholipids (PL), which can further support cardiovascular health by reducing inflammation and improving HDL functionality. All these benefits extend beyond heart health, as fish lipids also reduce inflammation markers, reduce oxidative stress, and improve vascular function. Fish and their bioactive lipid consumption are associated with reduced risks of chronic inflammatory conditions, such as metabolic syndrome, diabetes, and obesity. Nevertheless, cooking procedures can affect the effectiveness of these lipids, as heat can oxidize them and reduce their effectiveness. Consequently, mild cooking techniques and sustainable fish sourcing are essential to preserve their nutritional value and maintain the long-term viability of fish oils in promoting heart health. Overall, including omega-3 PUFAs and marine phospholipids in nutrition offers comprehensive cardiovascular protection and anti-inflammatory effects [96]. There is evidence proving their impact on increasing HDL-C levels and promoting HDL-C particles’ functionality by assisting in RCT and boosting its cardio-protective role [97,98,99].
The Health Planning Center of Nihon University Hospital conducted a multisite study over a period of 11 months, from April 2019 to March 2020, including 6841 adults, in order to examine the relations between fish consumption, lifestyle, and the monocytes–HDL-C ratio (MHR), a promising biomarker of CVD risk. Results showed that a high MHR was associated with high serum LDL-C levels, leading to further atherosclerosis progression. The increased MHR was explained by the fact that the monocytes levels were high, meanwhile HDL-C levels were low. The researchers concluded that higher fish consumption leads to lower CVD occurrence, in particular, atherosclerotic cardiovascular disease (ASCVD) [100].
Another cross-sectional study was designed to investigate the variation of frequent fish consumption between men and women and their non-HDL-C levels. In a study that took place at the Health Center of Nihon University Hospital from April 2018 to March 2019, 2479 men and 1570 women participated. The average weekly frequency of fish consumption was 2.58 ± 1.39 days for men and 2.42 ± 1.36 days for women. The results showed a significant decrease in the serum non-HDL-C regarding men with increasing weekly fish intake frequency (days: 0–1, 2–3, 4–5, and 6–7; p < 0.0001), while such associations were not observed in women. Additionally, in men, the serum LDL-C levels as well as the LDL-C-to-HDL-C ratio were markedly reduced, whereas HDL-C levels increased as the frequency of weekly fish consumption increased. No corresponding results were indicated for women [77].
A 28-day research study aimed to investigate the effects of fish meat and oil ingestion of two different fish, Hamri (Barbus luteus) and Balaout (Chondrostoma regium), on the lipid profile of male experimental rats. The rats were divided into three groups: the T1 control group (normal feeding), the T2 group (6% Hamri), and the T3 group (6% Balaout). After 28 days, the results showed a decrease in total cholesterol, triglycerides, and blood sugar in the T2 and T3 groups, in comparison to the T1 control group. The diet containing 6% Hamri and Balaout oil consumption resulted in a significant increase in HDL-C levels, 25.09 mg/Dl and 26.06 mg/dL, respectively, suggesting that these fish oils could be utilized in the human diet for corresponding health benefits [101].
4.1.5. Extra Virgin Olive Oil
Extra virgin olive oil (EVOO) has been reported to have positive effects on HDL-C due to its contained phenol compounds, tocopherols, beta-carotene, and MUFAs, particularly oleic acid [102].
A 2021 study investigated the effect of EVOO intake on the CEC and the clarification of the underlying mechanisms of EVOO consumption in improving the antithrombotic properties of HDL-C. In this study, 84 healthy men and women participated, allocated by age into 2 groups. The first group consisted of 28 young (23–45 years old) and the second group included 56 older participants (65–85 years old). The participants of both groups were given 25 mL of EVOO on a daily basis for 12 weeks. The CEC level of HDL-C from the elderly participants was 11.12% (p < 0.0001) lower than the CEC level from the younger group, while the older people’s CEC level returned to normal at the end of the clinical trial. The analysis of HDL-C subtype allocation indicated that HDL-C of the older group contained lower levels of large HDL-C (L-HDL, p < 0.03) and higher levels of small HDL-C (S-HDL, p < 0.002), when compared to the HDL-C consistency of the younger group.
In addition, it was indicated that there was a strong positive association between L-HDL levels and the CEC (r = 0.35, p < 0.001) and a negative one between S-HDL levels and the CEC (r = −0.27, p < 0.01), an independent result of the involvement of different factors. This age-associated variation in the CEC was partially attributed to an alteration in the allocation of HDL-C subtypes. Overall, the obtained results demonstrated that a short-term 12-week supplementation with EVOO-rich nutrition enhanced the HDL-C’s CEC to a similar level to the young group and optimized the subclass of HDL-C [103].
Another research team aimed to examine the efficacy of extra virgin high-polyphenol olive oil (HPOO) compared to low-polyphenol olive oil (LPOO) on the capacity of CEC promotion of HDL-C in Australian healthy adults. The researchers conducted a double-blind, randomized controlled trial, including 50 volunteers: 33 women and 27 men. A daily dose of HPOO (60 mL, 320 mg/kg polyphenols) or LPOO (60 mL, 86 mg/kg polyphenols) was given to all of the participants for three weeks. All the participants experienced a switch to the alternate option at the end of a two-week period. There were no major group differences observed regarding CEC and circulating lipids within the two treatments. LPOO and HPOO mean shifts were 0–54% (95% CI: 0–29, 1–37) and 0–10% (95% CI: 0–74, 0–94), respectively. Serum HDL-C was significantly raised after LPOO and HPOO, by 0–13 mmol/L (95% CI: 0–04, 0–22) and 0–10 mmol/L (95% CI: 0–02, 0–19), correspondingly [78].
4.1.6. Fungi
Edible fungi consumption, including mushrooms, is suggested to be beneficial for cardiovascular health because of some of their bioactive compounds. They include β-glucans, potentially affecting HDL-C levels and functionality [104,105]. The potential effectiveness of fungi and their contained substances has not been extensively studied, compared to those of other nutrition compounds. However, they are expected to be examined in more depth in the future.
A 2023 study was designed to examine the impact of Natokinase Monascus Supplements (NMS) over cardiovascular biomarkers in dyslipidemic patients. A total of 113 suitable participants were randomly allocated to intake NMSs (55 participants) or placebos (58 participants) for 120 days. After the trial was over, there were statistically significant mean absolute alterations in total cholesterol (−0.52 mmol/L, 95% CI: −0.51 to −0.54), LDL-C (−0.43 mmol/L, 95% CI: −0.45 to −0.41), non-HDL-C (−0.52 mmol/L, 95% CI: −0.52 to −0.52), and the LDL-C-to-HDL-C ratio (−0.29 mmol/L, 95% CI: −0.3 to −0.28). Major changes regarding triglycerides and HDL-C were not observed, resulting in the improvement of the overall lipid profile by consumption of fungal-originated substances [79].
4.1.7. Alcohol
Light to moderate alcohol consumption has been shown to positively affect the HDL-C’s functionality and levels. More specifically, certain types of alcohol, such as red wine, are associated with an increase in HDL-C levels due to their effect on lipid metabolism and apoA-1 production [106]. We should emphasize that extreme alcohol consumption endangers negative effects, not only for cardiovascular health but also health in general [46,107]. Excessive alcohol consumption is strongly associated with an elevated risk of arrhythmias, cardiomyopathy, and several other CVDs, dramatically increasing the likelihood of unfavorable cardiovascular outcomes, such as CHD and stroke [108,109,110]. Additionally, heavy drinking has a negative effect on liver function, leading to cirrhosis and hepatitis, and is also associated with several cancers, for example, liver, mouth, and breast cancer, as well as neurological disorders, including peripheral neuropathy [111,112,113]. On a molecular level, continuous long-term alcohol consumption can reduce HDL-C functionality by promoting oxidative stress and inflammation, thus reducing its protective ability against CVDs, worsening atherosclerosis and systemic inflammation [114,115,116].
Another research group conducted a study to determine the effect of moderate alcohol consumption in patients with peripheral arterial disease (PAD), including 153 male PAD patients, aged 47 to 93. The participants were divided into three groups according to the frequency of alcohol intake: the non-drinkers group, the occasional drinkers (four days or more), and the regular drinkers (five or more days). The alcohol types were not taken into consideration for the sorting of the groups. The results showed that HDL-C levels were higher in the regular drinker’s group than those of the non-drinkers, while LDL-C, total cholesterol, and triglycerides levels showed no significant differences. The probability ratios of low HDL-C for regular drinkers and non-drinkers were markedly below the reference level [80].
Another study aimed to further investigate how alcohol intake is linked to ASCVD and various HDL-C biomarkers, as well as the modification of these biomarkers via alcohol. In total, 2919 participants aged between 30 and 65 years old were examined, 43% of which were men and 57% women, 30% were white, 49% black, and 18% Hispanic. Alcohol consumption was quantified continuously as grams per week and sorted on a category scale as “never (0 g for both genders)/past (0 alcohol in the past 12 months for both genders)/light (for women: 0–49 g per week, for men: 0–98 g per week)/moderate (for women: 49–98 g per week, for men: 98–196 g per week)/heavy (for women: 98–392 g per week, for men: 196–490 g per week)/excessive (for women: over 392 g per week, for men: over 490 g per week)”. The results showed that the increased HDL-C levels and size, apoA-1, and CEC were associated with increasing alcohol intake, while elevated HDL-C levels were associated with moderate alcohol drinkers. Light drinkers’ HDL-C levels did not increase as much.
When divided by sex, the association factors for all HDL-C biomarkers were arithmetically higher for men than women (about two times higher). Black participants were found to have higher apoA-1 levels in contrast to white participants, while CEC differentiations were not significant among the two groups. The researchers were unable to determine the correlation between HDL-C markers and ASCVD [45].
4.1.8. Cocoa, Coffee, and Tea
Research studies indicate that cocoa components, quercetin, polyphenols, and flavonoids, in particular, have a potential beneficial impact on overall health. These compounds contribute to improving endothelial function, having antioxidant and anti-inflammatory effects, leading to a boosted HDL-C function [117,118]. Long-term coffee consumption is linked to slightly improving HDL-C levels, while it is strongly associated with a lower obesity risk in healthy individuals, due to its high amounts of caffeine, trigonelline, and other compounds [119]. Tea, especially green and black tea, is known for its cardiovascular protective effects, due to its richness in polyphenol flavonoids, especially catechins [120]. All these dietary compounds are highly believed to be CVD risk reductive [121].
A research group conducted a randomized, double-blind, 2-phase clinical trial including 60 participants (aged from 55 to 70 years old) to investigate the effect of a flavonoid-rich cocoa supplement in the improvement of plasma oxidative stress and inflammation biomarkers. The participants were randomized into three groups: the flavonoid-free highly alkaline group (NF), the flavonoid-rich natural cocoa group (F), and the placebo group. All individuals were assigned to ingest the beverages once a day for up to 12 weeks. The F group demonstrated improvement in the lipid profile. The HDL-C levels significantly increased (3.2 ± 4.3 mg/dL), the triglycerides levels decreased by 23.6 ± 38 mg/dL, and the triglycerides-to-HDL-C ratio showed a major decrease as well. LDL-C levels were decreased for all groups, with a greater drop regarding group F (11.5 ± 18.6 mg/dL). The overall results of this study indicate that frequent flavonoids consumption had a positive influence on the lipid profile, oxidative stress, and inflammation [122].
A double-blind, randomized clinical trial aimed to examine the effects of standard caffeinated and decaffeinated coffee on endothelium function and arterial stiffness in obese and overweight people. Volunteers aged between 25 and 59 years old with a low or mediate cardiovascular risk participated in this study. The participants were not allowed to consume certain types of foods and drinks, including caffeine, high-fat concentrations, and vasoactive compounds, to avoid collateral influence on the investigation. The researchers measured the blood pressure and endothelial function of the volunteers before and after the ingestion of a cup of coffee (220 mL) by blood sample testing in the laboratory. The individuals consuming caffeinated coffee underwent a one-week clearance period and then proceeded to take the other coffee type, and vice versa. The results showed that decaffeinated coffee had no influence on the lipid profiles, besides reducing the triglycerides levels. Caffeinated coffee had no influence on the lipid profile, as well. Caffeinated coffee caused an increase in blood pressure and the pulse waves rate, while it also improved the hyperemia-induced endothelial function. No such findings were noted for the decaffeinated coffee [81].
Another study aimed to evaluate the effect of consuming different amounts of tea or coffee during breakfast on the cardiometabolic risk factors in healthy individuals. The study included 18 volunteers. They had to stay unfed for 8 h overnight, after which they were assigned to consume water and different types of coffee (iced, frozen, and spray) or tea (black, green, and oolong), plus one egg and 180 g of fried dough. The researchers collected blood samples at certain times (h = 0, 0.5, 1, 2, and 3). Results showed a remarkable decrease in triglyceride levels due to the green tea, compared to the black and oolong tea. Green and oolong tea had a major decreasing impact on total cholesterol levels (p < 0.05 and p < 0.01, respectively). Furthermore, oolong tea consumption had a negative impact on the levels of HDL-C, as HDL-C levels were significantly low, in comparison to black tea digestion (p < 0.01 and p < 0.05, respectively). The same was observed for the LDL-C levels [123].
4.2. Antioxidant Impact
One of HDL-C’s abilities is the prevention of LDL-C oxidation and the neutralization of various other oxidizing agents, with apoA-1 being the primary antioxidant constituent of HDL-C molecules [124]. ApoA-1 antioxidant capacity mainly owes to the promoting mechanisms of the antioxidant efficacy of PON1. In diseases associated with lipid profile abnormalities, such as certain CVDs, diabetes, and dyslipidemia, as well as in inflammatory conditions, PON1’s activity is significantly reduced, as it loses its ability to associate with the HDL-C particles [125,126].
Diet plays a crucial role in modulating the levels and functionality of PON1 and onwards in a better HDL-C function. Some dietary compounds, included in fruit, nuts, fish, and legumes, are considered to contribute to increasing the enzyme secretion and function, as depicted in Figure 5 and presented in Table 2 [127,128].
Figure 5.
Substances with an antioxidant impact.

Table 2.
Studies on functional foods with antioxidant properties.
4.2.1. Fruit, Fermented Beverages, and By-Products
Fruits are rich in antioxidant plant-based bioactive compounds, comprising polyphenols, vitamins, and minerals. These substances are able to positively affect HDL-C antioxidant capacity, by either reducing oxidative stress, eliminating free radicals, or by preserving the infrastructure of lipoproteins [138,139].
Fruit by-products, commonly perceived as waste in the food-processing industry, have attracted widespread interest for their high potential as valuable sources of bioactive compounds and nutrients. Fruit pomace, which is the solid remains after the extraction of juice or oil from fruits, such as apples, kiwis, and citrus fruit, is mostly rich in dietary fiber, polyphenols, and other bioactive compounds [140,141]. These byproducts, as opposed to being rejected, are a sustainable option for the improvement and the protection of health. Utilizing fruit pomace and remains in the development of functional foods not only reduces food waste, but also unlocks the nutritional and therapeutic benefits inherent in these ingredients [142]. Apple pomace, a by-product of apple treatment, is a rich source of bioactive phenolics, dietary fiber, and probiotics, which confer potent anti-inflammatory, antioxidant, and antithrombotic activity [143]. Similarly, avocado by-products, including the peels and seeds, are high in bioactive phenolics, unsaturated fatty acids, and polar lipids, thus providing remarkable anti-inflammatory and antioxidant properties [144]. The kiwifruit skin, leaves, and pomace are plentiful in polyphenols and flavonoids, providing strong antioxidant and anti-inflammatory properties, and reinforcing its potential for functional foods and nutraceuticals [145]. Watermelon by-products are also a valuable source of vital nutrients and bioactive compounds, providing support for the development of functional health-promoting products [146].
In a similar way, both the fermented alcoholic beverages, such as wine and beer, and their by-products, which include grape skins, grape stems, grape seeds, spent yeasts, seeds, and grape hops, have been identified as a valuable source of bio-functional compounds with significant health benefits [147,148,149]. Wine holds a very important role in cardiovascular health thanks to its rich content of bioactive compounds, such as phenols, unsaturated fatty acids, and polar lipids. These compounds have strong antioxidant, anti-inflammatory, and antithrombotic properties that contribute to their therapeutic potential [150,151]. Equally, beer and its by-products have also gained attention recently for their rich content of bioactive compounds. Utilized yeasts, grains, and hops are naturally rich in antioxidants, anti-inflammatory agents, and antithrombotic ingredients, which reinforce their potential value as functional compounds in new foods, supplements, as well as in cosmetics [152]. Taken all together, fermented beverages and their by-products have both health and environmental benefits through bioactive compounds and provide viability and sustainability potential by using agricultural and industrial waste. The incorporation of these by-products into functional food and nutritional products advocates waste reduction and maximizes nutritional value. Further research should further improve the extraction and use of these bioactive substances to enhance their health benefits and overcome current limitations.
A gender-related study took place to evaluate the different impact of fruit and vegetable consumption on plasma lipids and their biomarkers, on either sex. The volunteers were both men (35 participants) and women (48 participants) aged between 30 and 50. The participants were assigned to fill in a 15-day dietary questionnaire, including the fruit (150 g daily) and vegetable (50 g per day referring to salads, and 250 g per day referring to vegetables) consumption, to evaluate the nutritional intake. By the end of the trial, the researchers verified the gender-related variations in dietary habits. Women’s vitamin C and β-carotene levels were higher than those of the male participants. Overall, fruit and vegetable consumption was significantly associated with lower oxidized LDL-C and oxidative stress levels, due to the higher intake of lutein and carotenoids. However, there was no significant difference in the activity and levels of PON1 among the two groups (82 ± 31 for men and 72 ± 35 for women). PON1 activity was not linked to fruit and vegetable consumption, while the plasma ox-LDL-C levels were not associated with PON1 competence or gender variation [153].
A randomized crossover trial was undertaken to examine the effect of Acai and Jucara fruit juices on the lipid profile and oxidative stress biomarkers. In this study, 30 healthy individuals participated, instructed to consume 200 mL of one of the two juices assigned per day for a four-week period, followed by a four-week washout time. By the end of the trial, both juices significantly increased HDL-C levels, up to 7.7% and 11.4% for Acai and Jucara juice, respectively (p < 0.05). Moreover, Acai juice consumption enhanced substantial elevations in the total antioxidant capacity by 66.7%, and the antioxidant enzyme, catalase (CAT), activity by 275.1%, while it reduced the oxidative stress index by 55.7%. On the other hand, Jucara juice raised the CAT activity by approximately 15% in comparison to the baseline (p < 0.05) [129].
Quercetin is one of over 4000 known phenolic compounds present in plants. It belongs to the major flavonoid family, and it is one of the most common dietary antioxidant substances [154]. Foods rich in quercetin include green tea, whole grains, and berries [88]. Quercetin and its derivatives promote cardiovascular health by inhibiting blood thickening and clotting [155].
A placebo-controlled, randomized trial was performed to define the effects of consumption of bread enriched with a 0.05% quercetin–epicatechin mixture (1:1; bread with flavonoid mixture (BF)) on both anthropometric and biochemical variables of Mexican people. In this study, 156 Mexican adults (30–50 years old) suffering from metabolic syndrome participated. The participants were assigned to either intake 30 g of BF bread or control (placebo) bread (CN) on a daily basis for a three-month period, followed by a washout week. Blood samples were tested at the end of every month to measure plasma lipoproteins and glucose. By the end of the study, the BF resulted in reducing the total cholesterol and triglycerides count, as well as lower LDL-C and plasma glucose levels. There were also reduced nuclear abnormalities in the epithelial tissue of the mouth (15.8 ± 3.2 to 8.3 ± 1.0), suggesting a genoprotective effect of the flavonoids. The flavonoids’ antioxidant capacity was significantly higher in the enriched bread than the control one (5.83 ± 0.07 μmol/g versus 3.01 ± 0.05 μmol/g, respectively), while the percentage of retention of flavonoids in the BF was 88%, beyond the baking process. Overall, the added flavonoids modified the cellular redox environment in vivo and improved the biochemical variables and cellular deformities in buccoid cells [133].
4.2.2. Nuts
Nuts are rich in a variety of bioactive compounds, including MUFAs and PUFAs, minerals, dietary fibers, vitamins, and polyphenols, improving health and lowering the CVD occurrence risk [156].
Cashews are characterized by their high amount of unsaturated fats and, therefore, are primarily linked to reducing CVD risk. A research group conducted a study on the beneficial effects of cashews on the lipid profile. In this study, 50 type II diabetes patients participated, aged between 30 and 75 years, and they were randomly allocated to the interventional or the control group. The intervention group had to receive 10% of the total calorie intake from cashew nuts on a daily basis, for a period of 8 weeks. After the end of the trial, the LDL-C-to-HDL-C ratio significantly reduced in the intervention group, in comparison to the control group (p = 0.01 and p = 0.04, respectively). There was also a great increase on PON1 activity in the cashew-consuming group, even though PON1 levels between groups were not majorly different [130].
4.2.3. Legumes
Legumes contain a variety of phytochemicals, the most known being polyphenols, dietary fibers, vitamins, proteins, minerals, and carbohydrates. Most of legumes’ bioactive compounds are characterized by antioxidant activities. Some of these are flavonoids, tannins, and certain phenolic acids [157,158].
An eight-week clinical trial was conducted to examine the antioxidant and anti-inflammatory impact of Baru almonds, a legume seed, on obese and overweight women. In total, 46 women, aged 30 to 50 years old, were randomly allocated into two different groups. The first group (24 participants) was assigned to 20 g of Baru almonds, in addition to a determined diet for 8 weeks, while the second group (22 participants) had to comply with equienergetic placebo nutritional habits. At baseline, both groups showed comparable levels of oxidative and inflammatory terms. By the end of the trial, the Baru almond group was found to have an increased plasma copper concentration (p = 0.037), as well as increased glutathione peroxidase (+0.08 U/mg, 95% CI: +0.05 to +0.12, versus −0.07, 95% CI: −0.12 to −0.03, p < 0.01), compared to the placebo group. The increased GPx activity and high copper levels in the Baru almond group indicate a positive improvement in the antioxidant mechanisms of the participants, as well as a potential contribution to the oxidative stress reduction [131].
4.2.4. Fish
There are not as many studies indicating fish’s antioxidant capacity as for fruit, or other dietary compounds. However, fish contain a few essential antioxidants with beneficial impacts on overall health. For example, fish are a high source of carotenoids, ω-3 fatty acids, and vitamin E, key compounds for the reduction of oxidative stress and free radicals’ neutralization [159].
A double-blind, randomized controlled clinical trial was designed to evaluate the effect of ω-3 fatty acids on depressive disorder by validating the relationship between signs of this psychiatric disorder, lipids, and the lipid profile. The participants included 58 children and teenagers of both sexes, aged from 11 to 17 years old, with 31 suffering from depression disorder (DD) and mixed anxiety and depressive disorder (MADD). The participants were randomly allocated to be treated daily with either 20 mL of a fish oil emulsion rich in ω-3 fatty acids (2400 mg of ω-3 fatty acids) or a similar emulsion from sunflowers, rich in ω-6 fatty acids (2467 mg of ω-3 linoleic acids), for a period of 12 weeks, to be followed by a 4-week washout period. The ω-3 fatty acids group demonstrated a significant improvement in DD and MADD symptoms, in contrast to the ω-6 fatty acids group. There was no major variation in PON1 arylesterase (PON-A) or lactonase (PON-L) activities between the patients, while neither supplementation affected the main PON1 enzyme activities.
This research group was the first to ever present that ω-3 fatty acids increase the anti-atherogenic L-HDL-C subtypes by also decreasing the pro-atherogenic S-LDL-C subfractions in depressed underage individuals after a 12-week complementary supplementation. Overall, this study indicates the positive effect of ω-3 fatty acids on the antioxidant capacity of HDL-C, particularly by increasing the levels of anti-atherogenic subtypes and decreasing the pro-atherogenic ones [132].
Besides n-3 PUFA esters, various bioactive nutrients in fish lipids have an important role in cardiovascular protection. Among these are lipid vitamins (A, D, and E), marine carotenoids, and amphiphilic phenols, and especially polar lipids, such as phospholipids and glycolipids, which are rich in n-3 PUFA. These compounds all contribute in a collective way to the anti-inflammatory, antithrombotic, and antioxidant properties of fish lipids, which help to reduce the risk of cardiovascular disease (CVD) [96].
Furthermore, the natural antioxidants present in fish and fish oils, such as free radical and active oxygen scavengers, metal chelators, enzymes, antioxidant-stable polar lipids, lipid vitamins, and carotenoids, are critically important in preventing oxidation of both monounsaturated and polyunsaturated fatty acids. In addition to the antioxidant effects, lipid vitamins and carotenoids extracted from fish also present additional health benefits, underlining the importance of their cumulative presence in extracts containing bioactive fish lipids. In particular, vitamins A and E are known for their antioxidant effects, protecting the body from the accumulation of harmful free radicals that can lead to oxidative stress. However, their benefits extend beyond antioxidant activity, as they possess bio-functional and cardio-protective properties found in fish and shark oils [96].
4.2.5. Vitamin E
Fat-soluble vitamin E is most commonly found in various fish oils, including those derived from shark liver. It is mainly known for its powerful antioxidant properties. Considerable research has investigated the role of vitamin E in fish, indicating its importance for the nervous development and overall growth of marine species [160].
Addition of external tocopherols at low concentrations in extracted fish oils contributes to maintaining the quality of both muscle and oils during storage [161]. Nevertheless, tocopherols incorporated in the fish diet (endogenous tocopherols) are more efficient in preventing lipid breakdown, as they are integrated into the lipids of the muscle membrane, extending the seafood lifespan. Alpha-tocopherol, an isomer of vitamin E, also provides protection against oxidation in fish phospholipids and contributes to the formation of stable liposomes, making these ingredients valuable for food and feed applications [161]. In addition, vitamin E is recycled by other natural antioxidants, preventing the accumulation of oxidized molecules [162]. Tocopherols are important not only from a nutritional but also a physiological point of view, as they participate in various metabolic processes in the human body. Epidemiological studies show that vitamin E helps prevent LDL oxidation and reduces the risk of cardiovascular disease. In addition to its antioxidant function, vitamin E enhances the immune system, helps prevent blood clots, and has demonstrated antithrombotic and anti-inflammatory properties [163,164].
Past studies have extensively reviewed the effects of fish oil and vitamin E co-ingestion on cardiovascular outcomes and mortality in dialysis patients, highlighting the need for more robust trials to confirm these health benefits [165]. Moreover, the combination of fish oils high in n-3 PUFA with vitamin E has shown potential benefits for several conditions, such as dysmenorrhea. For example, in female students, daily co-administration of n-3 PUFA-rich fish oil and vitamin E significantly relieved menstrual pain. This suggests that the anti-inflammatory properties of these bioactive compounds could reduce dysmenorrhea pain and could serve as safer alternatives to non-steroidal anti-inflammatory drugs, which often carry risks [166].
4.3. Anti-Inflammatory Impact
Bioactive compounds contained in functional foods have shown significant anti-inflammatory effects, promoting cardiovascular health and positively affecting HDL-C functionality and levels, thereby reducing oxidative stress and inflammation. Polyphenols, antioxidants, omega-3 fatty acids, and certain minerals, such as magnesium and potassium, have been found to reduce inflammation indexes and improve endothelial function. Additionally, dietary fibers and probiotics in functional foods contribute to gut health, which is closely linked to systemic inflammation and cardiovascular risk reduction. These bioactive components not only promote general health but also enhance the cardio-protective activity of HDL-C by improving its antioxidant capacity, as depicted in Figure 6 and presented in Table 3 [10,167,168,169]. Furthermore, specific polyphenols, such as flavonoids, found in berries and dark chocolate, have been shown to inhibit lipid peroxidation, adding another layer of protection against cardiovascular diseases [10].
Figure 6.
Substances with an anti-inflammatory impact.

Table 3.
Studies on functional foods with anti-inflammatory properties.
4.3.1. Fruit, Fermented Beverages, By-Products, and Flavonoids
Fruit peels are a unique and valuable source of phytoactive substances, particularly polyphenols, proven to enhance metabolic disorders. These by-products, in combination with various other fruit leftovers, are an important contributor to the development of functional foods, providing significant anti-inflammatory and protective properties. Utilizing them does not only reduce food waste but also helps release their full nutritional value, boosting the overall wellness via novel applications in functional and nutritional products [177,178,179,180]. For example, apple [143], watermelon [146], and kiwi [145] pomace, and other by-products rich in vital bioactive compounds and nutrients, demonstrate significant anti-inflammatory and antithrombotic properties, reducing the inflammatory response and enhancing cardiovascular and general health. In addition, avocado skin and seed remains are a great source of anti-inflammatory bioactive compounds, such as phenols and unsaturated fatty acids, strengthening their possibility of being considered a very important functional, nutritional source [144]. Citrus fruit by-products, for example, orange peels, are rich in anti-inflammatory compounds, such as vitamin C and phenolic compounds, lending further endorsement for their use in the development of functional products that contribute positively to cardiovascular health [181]. Overall, fruit by-products are not only providing an environmentally friendly solution to reduce food waste, but also unveil a rich source of valuable bioactive compounds.
The various bioactive compounds found in fermented alcoholic beverages, such as wine and beer, as well as their relevant by-products, such as grape stems and seeds, spent yeasts, grains, and hops, have attracted more attention for their potential in functional food and health applications. Wine, a historically important part of nutrition in various cultures, has been linked to health benefits from antiquity, and present-day research is revealing the synergistic effects of its bioactive compounds in the regulation of inflammation and chronic disorders [150,151]. Likewise, beer fermentation products and brewery by-products are identified for their anti-inflammatory, antithrombotic, and antioxidant properties, which are associated with the content of bioactive compounds derived from the fermentation process. Both wine and beer by-products present great promise for the development of functional foods, supplements, and nutritional products that promote health, while contributing to waste reduction [149,152].
A 2020 in vivo clinical study aimed to examine the cardiovascular protective effects of Ananas comosus peel extract (PEAC) on male Wistar rats fed either a high-fat or normal diet for a total of nine weeks. The rats were orally treated with the PEAC (200 mg/kg), beginning from the sixth week of the trial. PEAC was found to have a positive impact on the lipid profile, reducing the inflammation biomarker IL-6 and oxidative stress. Its activity improved the blood and cerebral antioxidant ranking by decreasing malondialdehyde and increasing glutathione levels and CAT activity. Overall, the results indicate the atheroprotective, antioxidant, and anti-inflammatory effects of PEAC [179].
Root and skin extracts from Broussonetia papyrifera are proven to have anti-inflammatory and antioxidant effects on the lipid profile. An experimental project was planned to investigate the impact of total flavonoids of Broussonetia papyrifera (TFBP) on inflammation and oxidative stress. The researchers conducted a series of in vitro experiments, during which they used free fatty acids to induce HepG2 cells’ stimulation. Additionally, they created an in vivo non-alcoholic fatty liver disease (NAFLD) model in mice, randomly allocated into six groups. The mice were fed either a high-fat diet (HFD) or an intraperitoneally tyloxapol-injected diet (TY), while also being administered with 0.2 mL of TFBP per day. TFBP intake significantly reduced oleic acid lipid aggregation, total cholesterol, triglycerides, and LDL-C levels, while it promoted the HDL-C levels in blood serum and regulated Nrf2 (nuclear factor erythroid-2-related factor 2)/AMPK (AMP-activated protein kinase). Overall, the results indicated the anti-inflammatory effects of TFBP for the first time [182].
Also, many clinical studies have tried to analyze the impact of flavonoid consumption on cancer. In a randomized study, 1063 women over 75 years of age were selected in order to assess the all-cause, cancer, and cardiovascular mortalities. An estimation of flavonoid consumption was carried out through two comprehensive food composition databases. During the five-year period, twelve percent of deaths were documented. Finally, the results indicated that women with a high flavonoid intake presented with a lower risk of all-cause mortality than women with a low intake of flavonoids. Similar results were found for cancer and CVD mortality, respectively [173].
Another double-blinded 6-week trial conducted by Stull et al. comprised chronic dosing of 45 g of freeze-dried blueberry powder per day in 32 obese men and women who had insulin resistance. The blueberry powder (daily amount was equivalent to two cups of fresh fruit) was blended into a yogurt plus milk beverage. At the end of the trial, no differences in changes in circulating hs-CRP, TNF-α, or MCP-1 were observed in the blueberry group, in comparison to the control group [170].
4.3.2. Nuts
Selenium is an antioxidant microelement present in nuts, such as Brazil nuts, almonds, and walnuts, characterized by atheroprotective activity [183]. It plays a fundamental role in promoting human health by reducing inflammation and oxidative stress, as well as intermediating the lipid profile, through selenoprotein activity. Sufficient selenium intake is associated with lower occurrence risks for CVD and some types of cancer [184,185].
Selenium-enriched Brazil nuts and their anti-inflammatory impact in obese individuals were examined in a randomized, controlled clinical trial. In this study, 55 obese women participated (18–55 years of age). The participants were equally divided into two groups and were randomized to either consume one Brazil nut (BN) per day, equal to 1.261 mg of selenium intake, or completely refrain from any kind of food containing Brazil nuts (CO) over the same time period of two months. By the end of the trial, both groups showed no selenium deficiency, yet the BN group demonstrated a significant increase in selenium biomarkers, as well as a positive association between selenium and the gene expression of almost all pro-inflammation biomarkers, including IL-6 (r = −0.679, p < 0.001) and inflammation regulation cytokine IFN-γ (r = −0.621, p = 0.001). This result poses concerns about the potential long-term effects of high selenium intake from Brazil nuts in obesity and other similar health conditions, highlighting the need for further research on this topic [171].
In addition, nut-derived dairy alternative products and fermented nut-derived yogurt alternatives have also shown strong in vitro anti-inflammatory and antithrombotic potency against mediators of inflammation and thrombosis in human platelets, such as PAF, thrombin, and ADP, and their associated thrombo-inflammatory signaling [186].
4.3.3. Spices
Hyperglycemia caused by consuming a high-saturated fats and carbohydrates meal (HFCM) is a major risk factor of CVD occurrence, as it can cause after-ingestion inflammation. Spices are known to reduce the inflammatory response. The effect of a specific spice mixture in inflammatory responses of cytokines was examined by a clinical trial on 12 obese or overweight adult men (aged 40 to 65 years). The participants were assigned to consume three different diet programs: a HFCM meal containing 2 g of the spice mixture, a HFCM meal containing 6 g of the mixture, and a no-mixture HFCM meal, in no specific order, with at least a three-day washout period between each part of the trial. By the end of the trial, the 6 g spice blend had the best effect on after-meal inflammation, as it reduced postprandial cytokines IL1β, IL-6, and IL-8 excretion, indicating the anti-inflammatory activity of spices [172].
4.3.4. Fish
Fish consumption positively contributes to the reduction in inflammation, primarily due to their high content of essential nutrients and bioactive compounds. Omega-3 polyunsaturated fatty acids (PUFAs), such as EPA and DHA, found in fish, have documented anti-inflammatory effects through modulation of the eicosanoid pathways. These bioactive lipids, as well as monounsaturated fatty acids (MUFAs), carotenoids, such as astaxanthin, and fat-soluble vitamins, for example, vitamin E, all contribute to the reduction in oxidative stress and inflammation. Additionally, fish-derived polar lipids, with their amphiphilic properties, provide additional benefits, such as antithrombotic and cardio-protective effects, further reinforcing their role in the prevention of inflammation-related diseases, such as cardiovascular disease, diabetes, and others [96].
The cardiovascular health benefits of vitamin E and fish oil have been extensively examined, most notably under their combined effects. Vitamin E is well known for its antioxidant properties, contributing to protection against oxidative stress, while fish oil, rich in omega-3 PUFAs, has been shown to provide significant cardiovascular benefits. It is suggested that the co-administration of vitamin E and fish oil can enhance each other’s positive effect on heart health. While there is evidence suggesting that omega-3 PUFAs and fish oil may increase LDL-cholesterol levels and possibly enhance LDL oxidizability, vitamin E supplementation has been found to neutralize these adverse effects. This combination can not only help to modulate adverse lipid modifications but also contributes to anti-atherogenic changes, such as increasing HDL-cholesterol levels, reducing triacylglycerol-rich lipoproteins, and reducing postprandial lipemia and residue concentrations [187,188]. For example, in animal studies involving rabbits fed with a diet rich in high cholesterol, the vitamin E and fish oil combination effectively reduced atherosclerosis, proving that these supplements can work synergistically to improve cardiovascular outcomes [187].
Marine phospholipids (PL), particularly from fish species, such as sea bass and sea bream, have remarkable anti-inflammatory properties. They reduce platelet aggregation triggered by platelet-activating factor (PAF) and work by inhibiting enzymes involved in PAF biosynthesis [189,190]. Moreover, these PL also aid in reduced atherosclerotic plaque formation and improved HDL-cholesterol levels, both of which are vital for cardiovascular health. Additionally, they can reduce PAF levels and attenuate associated inflammatory and thrombotic processes, thus reducing the risk of chronic diseases, such as atherosclerosis, cardiovascular disease (CVD), and cancer [43,189,191].
Phosphatidylcholines from fish eggs also have a positive impact on cardiovascular health, as well as health in general. A 1999 study aimed to evaluate the potential benefits of n-3 polyunsaturated fatty acids (PUFA) in the form of phosphatidylcholine (PC) for the prevention of liver fibrosis in patients with chronic liver disease. More specifically, the research centered on salmon phospholipids, mainly composed of PC, containing 30% docosahexaenoic acid (22:6 n-3) and 10% eicosapentaenoic acid (20:5 n-3). During a period of six months, six chronic liver disease patients—four with hepatitis B (three with cirrhosis and one with chronic hepatitis), one with hepatitis C cirrhosis, and one with alcoholic cirrhosis—were administered a daily dose of 1600 mg of these phospholipids. While the study did not detect any significant changes in the overall blood chemistry of liver function, there was a notable decrease in serum globulin levels and significant increases in HDL-cholesterol, apolipoprotein A-I, and apolipoprotein E. Overall, these findings suggested that n-3 PUFA PC supplementation can have beneficial effects in the treatment of chronic liver disease, despite the study’s small sample size [192].
4.3.5. Algae
Algae are a diverse group of photosynthetic organisms that have received major interest for their nutritional and therapeutic potential, most notably for the lipid extracts they contain. These lipid-rich compounds are loaded with bioactive molecules, including omega-3 and omega-6 polyunsaturated fatty acids (PUFAs), which provide promising health benefits. Lipids derived from marine algae are known for their potent anti-inflammatory properties, playing a key role in regulating the body’s inflammatory response [193,194]. They also contribute to cardiovascular health by improving the functionality of HDL-cholesterol. As a sustainable and nutrient-dense source, algal lipids have promising potential for the prevention and management of chronic inflammation, which is a major underlying factor in several non-communicable diseases, and for enhancing the cardio-protective effects of HDL-cholesterol [194].
In a double-blind, placebo-controlled, parallel-group randomized trial study, researchers aimed to investigate the effects of a polyphenol-rich seaweed (Fucus vesiculosus) extract on cardiovascular disease (CVD) risk markers. Thirty-four obese and overweight adults with increased LDL-cholesterol levels were randomized to either a treatment group receiving 2000 mg/day of the seaweed extract or a placebo group for twelve weeks. The study results indicated that the polyphenol-rich seaweed extract led to a significant increase in HDL-cholesterol compared to the placebo group. Specifically, the algae group saw a 9.5% increase in HDL-cholesterol from baseline to week 12, whereas the placebo group saw only a 1.7% increase (p = 0.045). Although, the actual change in HDL-cholesterol levels between the two groups did not reach statistical significance (p = 0.073). As for other cholesterol markers, there were no significant differences between the algae and placebo groups for changes in LDL-cholesterol, total cholesterol, or the total cholesterol ratio. In particular, in the algae group, there was a significant reduction in the total cholesterol ratio between baseline and week 6 (p = 0.007), although this effect was not maintained at week 12. Overall, seaweed extract showed potential to increase HDL-cholesterol, but did not induce significant changes in other lipid markers [195].
4.3.6. Dairy Products
Chronic inflammation’s involvement in the pathogenesis of incommunicable diseases, such as CVDs, diabetes, and obesity, has raised interest in the future development of foods with anti-inflammatory properties. Dairy products, being traditionally acknowledged for their nutritional value, have been highlighted as a potentially promising platform for the delivery of bioactive compounds that can modulate inflammatory processes. New studies have highlighted the potential of integrating functional components—such as polyphenols, omega-3 fatty acids, and dietary fiber—from agricultural- and industrial-derived by-streams into dairy formulations. These bioactive ingredients have displayed anti-inflammatory effects through mechanisms that include the reduction of pro-inflammatory cytokines and oxidative stress. By enhancing dairy products with these compounds, it is possible to develop functional foods that not only provide essential nutrients but also help in preventing and managing inflammation-related diseases. This strategy is aligned with sustainability objectives, promoting the circular use of resources, while providing novel solutions to improve public health [196].
A seven-year observational cohort study was undertaken to investigate the association between dairy intake and the metabolic dysfunction associated with steatotic liver disease (MASLD) and its relationship with inflammation. In this study, 316 participants were recruited, with MASLD assessed using ultrasound or the controlled attenuation parameter (CAP) and liver fibrosis assessed using FibroTest or Fibroscan. The analysis focused on the effect of low-fat and low-sugar dairy products versus high-fat and low-sugar dairy products on MASLD incidence and fibrosis markers. In a complementary manner, a 12-week animal study in male C57BL/6j mice compared high-fat diets of lard, soybean oil, and milk fat to evaluate their effects on metabolic dysfunction, inflammation, serum advanced glycation end products (AGEs), and liver injury. The results demonstrated that high consumption of low-to-medium fat and low-sugar dairy products was associated with a significantly reduced risk of metabolic impairment, associated with steatotic liver disease (MASLD) and its persistence over time (OR = 0.42, p = 0.037 and OR = 0.58, p = 0.039, respectively). On the contrary, high consumption of high-fat and low-sugar dairy products was associated with an increased risk of new onset and MASLD progression and persistence. While low- and high-fat dairy intake did not influence liver fibrosis markers either, milk fat was found to elevate serum cholesterol levels and advanced glycation end products (AGEs) more than lard or soybean oil. Across the animal study, all high-fat diets resulted in similar weight gain and liver steatosis, without affecting liver enzyme levels [197].
5. Conclusions
In this review, scientific data and literature studies were provided, regarding the mechanisms through which functional foods help promote the functionality of HDL-C and the elevation of its levels. These mechanisms include their impact in reverse cholesterol transfer and the enhancement of antioxidant and anti-inflammatory effects of HDL-C. In the comparative examination of the so far published trials, it was indicated that the uptake of functional foods and bioactive compounds contributes to improvements in HDL-C activities in individuals of high cardiovascular risk and other relevant diseases. It must be noted that in the majority of the discussed clinical studies, the main objectives did not include the examination of HDL-C functionality or levels. The reviewed findings were generated from additional analyses.
However, the current shortage of evidence on the precise mechanisms of functional foods’ impact on HDL-C highlights the necessity for further research on this topic. Human studies are crucial for understanding the specific effects of functional foods and their bioactive compounds on HDL-C metabolism. By conducting more studies in humans, valuable findings can be obtained on the relationship between dietary compounds and HDL-C function, as well as determining the optimum therapeutic doses for the prevention of cardiovascular disease. These studies may provide the basis for the development of nutritionally valid recommendations that could help inform dietary guidelines and interventions aimed at reducing the global burden of cardiovascular disease. In summary, furthering the understanding of the role of dietary compounds in the modulation of HDL-C functionality can lead to more individualized and optimally effective strategies for the prevention and treatment of CVDs, thus improving health outcomes for public health worldwide.
Author Contributions
Conceptualization, A.T.; methodology, A.T.; software, all authors; validation, A.T.; investigation, all authors; writing—original draft preparation, A.V., E.P. and A.T.; writing—review and editing, V.P. and A.T.; visualization, A.T.; supervision, A.T.; project administration, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the School of Chemistry of the Faculty of Science, of the Democritus University of Thrace, for the continuous support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases (accessed on 10 April 2024).
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Cardiovascular Risk: Assumptions, Limitations, and Research. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–266. ISBN 978-0-12-813792-5. [Google Scholar]
- Wu, Z.; Huang, Z.; Lichtenstein, A.H.; Jin, C.; Chen, S.; Wu, S.; Gao, X. Different Associations between HDL Cholesterol and Cardiovascular Diseases in People with Diabetes Mellitus and People without Diabetes Mellitus: A Prospective Community-Based Study. Am. J. Clin. Nutr. 2021, 114, 907–913. [Google Scholar] [CrossRef]
- HDL Cholesterol: How to Boost Your “Good” Cholesterol. Available online: https://www.mayoclinic.org/diseases-conditions/high-blood-cholesterol/in-depth/hdl-cholesterol/art-20046388 (accessed on 11 April 2024).
- Carroll, M.D.; Fryar, C.D. Total and High-Density Lipoprotein Cholesterol in Adults: United States, 2015–2018; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020.
- Marcason, W. What Role Does HDL Cholesterol Have in CVD and What Is the Most Effective Way to Increase It? J. Am. Diet. Assoc. 2011, 111, 1266. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High Density Lipoprotein as a Protective Factor against Coronary Heart Disease. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Balthazar, C.F.; Silva, R.; Rocha, R.S.; Graça, J.S.; Esmerino, E.A.; Silva, M.C.; Sant’Ana, A.S.; Duarte, M.C.K.H.; Freitas, M.Q.; et al. Impact of Probiotics and Prebiotics on Food Texture. Curr. Opin. Food Sci. 2020, 33, 38–44. [Google Scholar] [CrossRef]
- Zabetakis, I.; Lordan, R.; Tsoupras, A.; Ramji, D. Functional Foods and Their Implications for Health Promotion; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-12-823811-0. [Google Scholar]
- Entry Details | FAO Terminology Portal | Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faoterm/viewentry/en/?entryId=170967 (accessed on 16 September 2024).
- Guo, M. Introduction. In Functional Foods; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–8. ISBN 978-1-84569-592-7. [Google Scholar]
- Mashau, M.E.; Ramashia, S.E.; Mashau, M.E.; Ramashia, S.E. Role of Functional Food in Treating and Preventing Cardiovascular Diseases. In Functional Foods—Phytochemicals and Health Promoting Potential; IntechOpen: London, UK, 2021; ISBN 978-1-83968-933-8. [Google Scholar]
- Baumgartner, S.; Bruckert, E.; Gallo, A.; Plat, J. The Position of Functional Foods and Supplements with a Serum LDL-C Lowering Effect in the Spectrum Ranging from Universal to Care-Related CVD Risk Management. Atherosclerosis 2020, 311, 116–123. [Google Scholar] [CrossRef]
- Raygor, V.; Khera, A. New Recommendations and Revised Concepts in Recent Guidelines on the Management of Dyslipidemias to Prevent Cardiovascular Disease: The 2018 ACC/AHA and 2019 ESC/EAS Guidelines. Curr. Cardiol. Rep. 2020, 22, 87. [Google Scholar] [CrossRef]
- Hunter, P.M.; Hegele, R.A. Functional Foods and Dietary Supplements for the Management of Dyslipidaemia. Nat. Rev. Endocrinol. 2017, 13, 278–288. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-Density Lipoprotein (HDL) Functionality and Its Relevance to Atherosclerotic Cardiovascular Disease. Drugs Context 2018, 7, 212525. [Google Scholar] [CrossRef]
- Soria-Florido, M.T.; Schröder, H.; Grau, M.; Fitó, M.; Lassale, C. High Density Lipoprotein Functionality and Cardiovascular Events and Mortality: A Systematic Review and Meta-Analysis. Atherosclerosis 2020, 302, 36–42. [Google Scholar] [CrossRef]
- Damasceno, N.R.T.; De Freitas, M.C.P. High Density-Lipoprotein Functionality Score Predicts Cardiovascular Risk Estimates and Subclinical Atherosclerosis in Brazilian Individuals. Atherosclerosis 2022, 355, 69–70. [Google Scholar] [CrossRef]
- Ben-Aicha, S.; Badimon, L.; Vilahur, G. Advances in HDL: Much More than Lipid Transporters. Int. J. Mol. Sci. 2020, 21, 732. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, R.; Mu, H.; Wang, X.; Yao, Q.; Chen, C. Reverse Cholesterol Transport and Cholesterol Efflux in Atherosclerosis. QJM 2005, 98, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Favari, E.; Chroni, A.; Tietge, U.J.F.; Zanotti, I.; Escolà-Gil, J.C.; Bernini, F. Cholesterol Efflux and Reverse Cholesterol Transport. In High Density Lipoproteins: From Biological Understanding to Clinical Exploitation; Handbook of Experimental Pharmacology Series; Springer: Berlin/Heidelberg, Germany, 2015; Volume 224, pp. 181–206. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Zhu, L.; Wang, Q. Reverse Cholesterol Transport Pathway and Cholesterol Efflux in Diabetic Retinopathy. J. Diabetes Res. 2021, 2021, 8746114. [Google Scholar] [CrossRef] [PubMed]
- Translation of High-Density Lipoprotein Function into Clinical Practice. Available online: https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.113.000962 (accessed on 12 April 2024).
- Daniil, G.; Phedonos, A.A.P.; Holleboom, A.G.; Motazacker, M.M.; Argyri, L.; Kuivenhoven, J.A.; Chroni, A. Characterization of Antioxidant/Anti-Inflammatory Properties and apoA-I-Containing Subpopulations of HDL from Family Subjects with Monogenic Low HDL Disorders. Clin. Chim. Acta 2011, 412, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Cardioprotective Functions of HDLs-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0022227520350252 (accessed on 12 April 2024).
- Egea, J.C.L. HDL Lipoproteins: A Fluid and Changing Relationship between Quantity and Functionality. Clin. Investig. Arter. 2022, 34, 120–121. [Google Scholar] [CrossRef]
- Lordan, R.; Walsh, A.M.; Crispie, F.; Finnegan, L.; Cotter, P.D.; Zabetakis, I. The Effect of Ovine Milk Fermentation on the Antithrombotic Properties of Polar Lipids. J. Funct. Foods 2019, 54, 289–300. [Google Scholar] [CrossRef]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Zabetakis, I.; Murray, P.; Kumar Saha, S. Anti-Inflammatory and Antithrombotic Properties of Polar Lipid Extracts, Rich in Unsaturated Fatty Acids, from the Irish Marine Cyanobacterium Spirulina Subsalsa. J. Funct. Foods 2022, 94, 105124. [Google Scholar] [CrossRef]
- Tsouka, A.N.; Tellis, C.; Tselepis, A. The Effect of LDL or HDL-Associated PCSK9 on Platelet Aggregation and Inflammatory Stimulation of Endothelial Cells, in Vitro. Atherosclerosis 2023, 379, S3–S4. [Google Scholar] [CrossRef]
- Marsche, G.; Heine, G.H.; Stadler, J.T.; Holzer, M. Current Understanding of the Relationship of HDL Composition, Structure and Function to Their Cardioprotective Properties in Chronic Kidney Disease. Biomolecules 2020, 10, 1348. [Google Scholar] [CrossRef]
- Pavanello, C.; Zheng, K.H.; Schnitzler, J.G.; Kroon, J.; Versloot, M.; Levels, J.H.; Calabresi, L.; Stroes, E.S.; Bekkering, S. Low Hdl-Cholesterol Levels Are Associated With A Decreased Monocyte Activity And Inflammation In Carriers Of Lcat Mutation. Atherosclerosis 2019, 287, e23–e24. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Ortiz-Rodriguez, M.; Medina-Briseño, L.; Carreón-Torres, E.; Izquierdo-Vega, J.; Sharma, A.; Cancino-Díaz, J.; Pérez-Méndez, O.; Belefant-Miller, H.; Betanzos-Cabrera, G. Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease. Molecules 2018, 23, 2730. [Google Scholar] [CrossRef] [PubMed]
- Chyu, K.-Y.; Shah, P.K. HDL/ApoA-1 Infusion and ApoA-1 Gene Therapy in Atherosclerosis. Front. Pharmacol. 2015, 6, 187. [Google Scholar] [CrossRef]
- High-Density Lipoprotein and Inflammation and Its Significance to Atherosclerosis—The American Journal of the Medical Sciences. Available online: https://www.amjmedsci.com/article/S0002-9629(16)30371-8/abstract (accessed on 12 April 2024).
- Kastelein, J.J.P.; Van Der Steeg, W.A.; Holme, I.; Gaffney, M.; Cater, N.B.; Barter, P.; Deedwania, P.; Olsson, A.G.; Boekholdt, S.M.; Demicco, D.A.; et al. Lipids, Apolipoproteins, and Their Ratios in Relation to Cardiovascular Events with Statin Treatment. Circulation 2008, 117, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, B.J.; Boekholdt, S.M. Clinical and Biological Relevance of Statin-Mediated Changes in HDL Metabolism. Curr. Atheroscler. Rep. 2014, 16, 379. [Google Scholar] [CrossRef]
- Sampson, U.K.; Linton, M.F.; Fazio, S. Are Statins Diabetogenic? Curr. Opin. Cardiol. 2011, 26, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Sikarwar, P.; Khurana, S.; Sharma, J. Assessing the Incidence of New-Onset Diabetes Mellitus with Statin Use: A Systematic Review of the Systematic Reviews and Meta-Analyses. touchREVIEWS Endocrinol. 2022, 18, 96. [Google Scholar] [CrossRef]
- Cohen, H.; Sheinin, R.; Amital, H.; Bitzur, R. The Link between Statin Associated Muscle Pain and Chronic Pain Syndromes: A Cross-Section Study. Atherosclerosis 2019, 287, e273. [Google Scholar] [CrossRef]
- Paraskevas, K.I.; Mikhailidis, D.P.; Veith, F.J. Optimal Statin Type and Dosage for Vascular Patients. J. Vasc. Surg. 2011, 53, 837–844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Sourlas, A.; Guzman, E.; Kostara, C.E. Environmental Factors Modifying HDL Functionality. Curr. Med. Chem. 2022, 29, 1687–1701. [Google Scholar] [PubMed]
- Badia, R.R.; Pradhan, R.V.; Ayers, C.R.; Chandra, A.; Rohatgi, A. The Relationship of Alcohol Consumption and HDL Metabolism in the Multiethnic Dallas Heart Study. J. Clin. Lipidol. 2023, 17, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Naharci, M.I. Is Very High HdL-Cholesterol Level Related to Cardiovascular Mortality Affected by Chronic Kidney Disease and Alcohol Use? Comment on Palatini et Al. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 913–914. [Google Scholar] [CrossRef] [PubMed]
- Gazzola, K.; Reeskamp, L.; van den Born, B.-J. Ethnicity, Lipids and Cardiovascular Disease. Curr. Opin. Lipidol. 2017, 28, 225. [Google Scholar] [CrossRef] [PubMed]
- Sviridov, D.; Nestel, P.J. Genetic Factors Affecting HDL Levels, Structure, Metabolism and Function. Curr. Opin. Lipidol. 2007, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Furtado, J.D.; Jensen, M.K. Protein-Based HDL Subspecies: Rationale and Association with Cardiovascular Disease, Diabetes, Stroke, and Dementia. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2022, 1867, 159182. [Google Scholar] [CrossRef] [PubMed]
- Afshoon, Z.; Bahiraee, A.; Esmaeili, F.; Mansouri, E.; Emami, M.A.; Montaseri, M.; Davoodian, N.; Ebrahimi, R.; Eftekhar, E. Study of the Relationship between Endothelial Lipase Gene Polymorphism and Serum Levels of HDL-C, Apo A-I and Severity of Stenosis in Non-Diabetic Coronary Artery Disease Patients. Gene Rep. 2020, 21, 100831. [Google Scholar] [CrossRef]
- Baik, I.; Lee, S.; Kim, S.H.; Shin, C. A Lipoprotein Lipase Gene Polymorphism Interacts with Consumption of Alcohol and Unsaturated Fat to Modulate Serum HDL-Cholesterol Concentrations1, 2, 3. J. Nutr. 2013, 143, 1618–1625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feingold, K.R.; Grunfeld, C. Effect of Inflammation on HDL Structure and Function. Curr. Opin. Lipidol. 2016, 27, 521–530. [Google Scholar] [CrossRef]
- Spieker, L.E.; Ruschitzka, F.; Lüscher, T.F.; Noll, G. HDL and Inflammation in Atherosclerosis. Curr. Drug Targets Immune Endocr. Metab. Disord. 2004, 4, 51–57. [Google Scholar] [CrossRef]
- Inflammation, Not Cholesterol, Is a Cause of Chronic Disease-PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5986484/ (accessed on 12 April 2024).
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, Inflammation and Innate Immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Marsche, G.; Saemann, M.D.; Heinemann, A.; Holzer, M. Inflammation Alters HDL Composition and Function: Implications for HDL-Raising Therapies. Pharmacol. Ther. 2013, 137, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Petropoulou, P.-I.; Berbée, J.F.P.; Theodoropoulos, V.; Hatziri, A.; Stamou, P.; Karavia, E.A.; Spyridonidis, A.; Karagiannides, I.; Kypreos, K.E. Lack of LCAT Reduces the LPS-Neutralizing Capacity of HDL and Enhances LPS-Induced Inflammation in Mice. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2015, 1852, 2106–2115. [Google Scholar] [CrossRef]
- O’Neill, F.; Riwanto, M.; Charakida, M.; Colin, S.; Manz, J.; McLoughlin, E.; Khan, T.; Klein, N.; Kay, C.W.M.; Patel, K.; et al. Structural and Functional Changes in HDL with Low Grade and Chronic Inflammation. Int. J. Cardiol. 2015, 188, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Grunfeld, C. The Acute Phase Response Inhibits Reverse Cholesterol Transport1. J. Lipid Res. 2010, 51, 682–684. [Google Scholar] [CrossRef]
- Esteve, E.; Ricart, W.; Fernández-Real, J.M. Dyslipidemia and Inflammation: An Evolutionary Conserved Mechanism. Clin. Nutr. 2005, 24, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Jing, W.; Qian, W.; Fan, L.; Cheng, J. Association between Dietary Patterns and Cardiovascular Diseases: A Review. Curr. Probl. Cardiol. 2024, 49, 102412. [Google Scholar] [CrossRef]
- WHO Updates Guidelines on Fats and Carbohydrates. Available online: https://www.who.int/news/item/17-07-2023-who-updates-guidelines-on-fats-and-carbohydrates (accessed on 16 April 2024).
- Sikand, G.; Severson, T. Top 10 Dietary Strategies for Atherosclerotic Cardiovascular Risk Reduction. Am. J. Prev. Cardiol. 2020, 4, 100106. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, Its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Dastmalchi, L.N.; Gulati, M.; Michos, E.D. A Heart-Healthy Diet for Cardiovascular Disease Prevention: Where Are We Now? Vasc. Health Risk Manag. 2023, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Wickman, B.E.; Enkhmaa, B.; Ridberg, R.; Romero, E.; Cadeiras, M.; Meyers, F.; Steinberg, F. Dietary Management of Heart Failure: DASH Diet and Precision Nutrition Perspectives. Nutrients 2021, 13, 4424. [Google Scholar] [CrossRef]
- Karpouzas, G.A.; Papotti, B.; Ormseth, S.R.; Palumbo, M.; Hernandez, E.; Adorni, M.P.; Zimetti, F.; Budoff, M.J.; Ronda, N. Statins Influence the Relationship between ATP-Binding Cassette A1 Membrane Transporter-Mediated Cholesterol Efflux Capacity and Coronary Atherosclerosis in Rheumatoid Arthritis. J. Transl. Autoimmun. 2023, 7, 100206. [Google Scholar] [CrossRef]
- Ferraz-Amaro, I.; Hernández-Hernández, M.V.; Armas-González, E.; Sánchez-Pérez, H.; Machado, J.D.; Díaz-González, F. HDL Cholesterol Efflux Capacity Is Related to Disease Activity in Psoriatic Arthritis Patients. Clin. Rheumatol. 2020, 39, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Narita, I.; Kotani, K. The Macrophage and Its Related Cholesterol Efflux as a HDL Function Index in Atherosclerosis. Clin. Chim. Acta 2016, 457, 117–122. [Google Scholar] [CrossRef]
- Le Sayec, M.; Xu, Y.; Laiola, M.; Gallego, F.A.; Katsikioti, D.; Durbidge, C.; Kivisild, U.; Armes, S.; Lecomte, M.; Fança-Berthon, P.; et al. The Effects of Aronia Berry (Poly)Phenol Supplementation on Arterial Function and the Gut Microbiome in Middle Aged Men and Women: Results from a Randomized Controlled Trial. Clin. Nutr. 2022, 41, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, R.; Parandoosh, M.; Khorsandi, H.; Hosseinzadeh, N.; Madani Tonekaboni, M.; Saidpour, A.; Babaei, H.; Ghorbani, A. Grape Seed Extract Supplementation along with a Restricted-calorie Diet Improves Cardiovascular Risk Factors in Obese or Overweight Adult Individuals: A Randomized, Placebo-controlled Trial. Phytother. Res. 2021, 35, 987–995. [Google Scholar] [CrossRef]
- Nora, C.L.; Zhang, L.; Castro, R.J.; Marx, A.; Carman, H.B.; Lum, T.; Tsimikas, S.; Hong, M.Y. Effects of Mixed Nut Consumption on LDL Cholesterol, Lipoprotein(a), and Other Cardiometabolic Risk Factors in Overweight and Obese Adults. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Daneshzad, E.; Jafari, A.; Bellissimo, N.; Azadbakht, L. Association of Nut and Legume Consumption with Framingham 10 Year Risk of General Cardiovascular Disease in Older Adult Men: A Cross-Sectional Study. Clin. Nutr. ESPEN 2021, 42, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Matsuo, R.; Imatake, K.; Suzuki, Y.; Yagi, T.; Takahashi, A.; Matsumoto, N.; Okumura, Y. Gender Differences in the Associations among Fish Intake, Lifestyle, and Non-HDL-C Level in Japanese Subjects over the Age of 50 Years: Anti-Atherosclerotic Effect of Fish Consumption. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Sarapis, K.; George, E.S.; Marx, W.; Mayr, H.L.; Willcox, J.; Powell, K.L.; Folasire, O.S.; Lohning, A.E.; Prendergast, L.A.; Itsiopoulos, C.; et al. Extra Virgin Olive Oil Improves HDL Lipid Fraction but Not HDL-Mediated Cholesterol Efflux Capacity: A Double-Blind, Randomised, Controlled, Cross-over Study (OLIVAUS). Br. J. Nutr. 2023, 130, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zeng, X.; Mahe, J.; Guo, K.; He, P.; Yang, Q.; Zhang, Z.; Li, Z.; Wang, D.; Zhang, Z.; et al. The Effect of Nattokinase-Monascus Supplements on Dyslipidemia: A Four-Month Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 4239. [Google Scholar] [CrossRef] [PubMed]
- Sotoda, Y.; Hirooka, S.; Orita, H.; Wakabayashi, I. Associations of Habitual Alcohol Drinking with HDL Cholesterol and D-Dimer in Patients with Peripheral Arterial Disease. Clin. Chim. Acta 2023, 547, 117422. [Google Scholar] [CrossRef] [PubMed]
- Pavão, T.P.; Chemello, D.; Ferigollo, A.; Saffi, M.A.L.; Moresco, R.N.; dos Santos Stein, C.; Emanuelli, T.; Somacal, S.; Moriguchi, E.H.; Badimon, L.; et al. Acute Effect of Coffee on Arterial Stiffness and Endothelial Function in Overweight and Obese Individuals: A Randomized Clinical Trial. Clin. Nutr. ESPEN 2022, 50, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Amadi, P.U.; Agomuo, E.N.; Amadi, J.A.; Bob-Chile Agada, A.I.; Njoku, U.C.; Ogunwa, C.S.; Odika, P.C.; Osuoha, J.O.; Ogbolosingha, A.J.; Adumekwe, C.W.; et al. Efficacy of Using Walnuts as Statin Adjuvants in Hypertension Management. Clin. Exp. Hypertens. 2022, 44, 419–426. [Google Scholar] [CrossRef]
- Dusanov, S.; Svendsen, M.; Ruzzin, J.; Kiviranta, H.; Gulseth, H.L.; Klemsdal, T.O.; Tonstad, S. Effect of Fatty Fish or Nut Consumption on Concentrations of Persistent Organic Pollutants in Overweight or Obese Men and Women: A Randomized Controlled Clinical Trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Dikariyanto, V.; Smith, L.; Francis, L.; Robertson, M.; Kusaslan, E.; O’Callaghan-Latham, M.; Palanche, C.; D’Annibale, M.; Christodoulou, D.; Basty, N.; et al. Snacking on Whole Almonds for 6 Weeks Improves Endothelial Function and Lowers LDL Cholesterol but Does Not Affect Liver Fat and Other Cardiometabolic Risk Factors in Healthy Adults: The ATTIS Study, a Randomized Controlled Trial. Am. J. Clin. Nutr. 2020, 111, 1178–1189. [Google Scholar] [CrossRef]
- Guarneiri, L.L.; Paton, C.M.; Cooper, J.A. Pecan-Enriched Diets Alter Cholesterol Profiles and Triglycerides in Adults at Risk for Cardiovascular Disease in a Randomized, Controlled Trial. J. Nutr. 2021, 151, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- García-Cordero, J.; Mateos, R.; González-Rámila, S.; Seguido, M.A.; Sierra-Cinos, J.L.; Sarriá, B.; Bravo, L. Dietary Supplements Containing Oat Beta-Glucan and/or Green Coffee (Poly)Phenols Showed Limited Effect in Modulating Cardiometabolic Risk Biomarkers in Overweight/Obese Patients without a Lifestyle Intervention. Nutrients 2023, 15, 2223. [Google Scholar] [CrossRef]
- Nova, E.; San Mauro-Martín, I.; Díaz-Prieto, L.E.; Marcos, A. Wine and Beer within a Moderate Alcohol Intake Is Associated with Higher Levels of HDL-c and Adiponectin. Nutr. Res. 2019, 63, 42–50. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL Function. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of Aronia Berry (Poly)Phenols on Vascular Function and Gut Microbiota: A Double-Blind Randomized Controlled Trial in Adult Men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- Yang, J.; Kurnia, P.; Henning, S.M.; Lee, R.; Huang, J.; Garcia, M.C.; Surampudi, V.; Heber, D.; Li, Z. Effect of Standardized Grape Powder Consumption on the Gut Microbiome of Healthy Subjects: A Pilot Study. Nutrients 2021, 13, 3965. [Google Scholar] [CrossRef] [PubMed]
- Comak Gocer, E.M.; Koptagel, E. Production of Milks and Kefir Beverages from Nuts and Certain Physicochemical Analysis. Food Chem. 2023, 402, 134252. [Google Scholar] [CrossRef]
- Hernáez, Á.; Sanllorente, A.; Castañer, O.; Martínez-González, M.Á.; Ros, E.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Alonso-Gómez, Á.M.; et al. Increased Consumption of Virgin Olive Oil, Nuts, Legumes, Whole Grains, and Fish Promotes HDL Functions in Humans. Mol. Nutr. Food Res. 2019, 63, 1800847. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.V.; Guzzetti, L.; Panzeri, D.; De Giuseppe, R.; Coccetti, P.; Labra, M.; Cena, H. Bioactive Compounds in Legumes: Implications for Sustainable Nutrition and Health in the Elderly Population. Trends Food Sci. Technol. 2021, 117, 139–147. [Google Scholar] [CrossRef]
- Han, Y.; Kim, A.R.; Lee, J.H.; Kim, M. Adiponectin and 8-Epi-PGF2α as Intermediate Influencing Factors in Weight Reduction after Legume Consumption: A 12-Week Randomised Controlled Trial. Br. J. Nutr. 2022, 127, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar. Drugs 2022, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, N.R.T.; Cartolano, F.D.C.; Dias, G.D.; Ventura, A.O.C.; Mello, A.P.D.Q. Can A Nutritional Factor Modulate The Hdl Functionality: Role Of Omega-3 Fatty Acid. Atherosclerosis 2019, 287, e226. [Google Scholar] [CrossRef]
- Tutor, A.; O’Keefe, E.L.; Lavie, C.J.; Elagizi, A.; Milani, R.; O’Keefe, J. Omega-3 Fatty Acids in Primary and Secondary Prevention of Cardiovascular Diseases. Prog. Cardiovasc. Dis. 2024, 84, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.B.; Reisdorph, R.M.; Rasolofomanana-Rajery, S.; Michel, C.; Khajeh-Sharafabadi, M.; Doenges, K.A.; Weaver, N.; Quinn, K.; Sutliff, A.K.; Tang, M.; et al. Salmon Food-Specific Compounds and Their Metabolites Increase in Human Plasma and Are Associated with Cardiometabolic Health Indicators Following a Mediterranean-Style Diet Intervention. J. Nutr. 2024, 154, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Atsumi, W.; Imatake, K.; Suzuki, Y.; Yagi, T.; Takahashi, A.; Matsumoto, N.; Okumura, Y. Associations of Higher Fish Consumption and Lifestyle with Lower Monocyte/HDL-C Ratio in a Japanese Population: Implication for the Anti-Atherosclerotic Effect of Fish Consumption. J. Cardiol. 2022, 80, 402–409. [Google Scholar] [CrossRef]
- Albashr, T.K.; Khidhir, Z.K.; Namiq, K.; Hamadamin, A.; Alhabib, F.; Khalaf, W. Study on Chemical Composition and Physical Properties of the Hamri (Barbus luteus) and Balaout (Chondrostoma regium) Fish Meat, Oil and Impact of Its Oils on Cholesterol, Triglyceride, HDL and Blood Sugar of Laboratory Rats. Saudi J. Biol. Sci. 2022, 29, 261–265. [Google Scholar] [CrossRef]
- Sgroi, F.; Sciortino, C.; Giamporcaro, G.; Modica, F. Exploring the Impact of Beliefs and Experiential Factors on Extra Virgin Olive Oil Consumption. J. Agric. Food Res. 2024, 15, 101056. [Google Scholar] [CrossRef]
- Otrante, A.; Trigui, A.; Walha, R.; Berrougui, H.; Fulop, T.; Khalil, A. Extra Virgin Olive Oil Prevents the Age-Related Shifts of the Distribution of HDL Subclasses and Improves Their Functionality. Nutrients 2021, 13, 2235. [Google Scholar] [CrossRef]
- Pilafidis, S.; Tsouko, E.; Sougleri, G.; Diamantopoulou, P.; Gkatzionis, K.; Ioannou, Z.; Sarris, D. Submerged Cultivation of Selected Macro-Fungi to Produce Mycelia Rich in β-Glucans and Other Bioactive Compounds, Valorizing Side Streams of the Food Industry. Carbon Resour. Convers. 2024, 7, 100198. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, Z.; Cai, G.; Chen, B.; Yang, H. Improving the Hypoglycemic Activity of Phenolic Extracts from Dendrobium officinale Leaves Using the Solid-State Fermentation of Edible Fungi. Food Biosci. 2024, 58, 103828. [Google Scholar] [CrossRef]
- Wilkens, T.L.; Sørensen, H.; Jensen, M.K.; Furtado, J.D.; Dragsted, L.O.; Mukamal, K.J. Associations between Alcohol Consumption and HDL Subspecies Defined by ApoC3, ApoE and ApoJ: The Cardiovascular Health Study. Curr. Probl. Cardiol. 2023, 48, 101395. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Isath, A.; Rosenson, R.S.; Khawaja, M.; Wang, Z.; Fogg, S.E.; Virani, S.S.; Qi, L.; Cao, Y.; Long, M.T.; et al. Alcohol Consumption and Cardiovascular Health. Am. J. Med. 2022, 135, 1213–1230.e3. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Huang, C.; Li, J.; Liu, F.; Huang, K.; Liu, Z.; Yang, X.; Liu, X.; Cao, J.; Chen, S.; et al. Causal Associations of Alcohol Consumption with Cardiovascular Diseases and All-Cause Mortality among Chinese Males. Am. J. Clin. Nutr. 2022, 116, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.; Paré, G.; Leong, D.P. Alcohol and Cardiovascular Disease: How Much Is Too Much? Curr. Atheroscler. Rep. 2017, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Medling, T.; Gobeil, K.; Sawalha, K.; Abozenah, M.; Tavares, P.; Friedmann, P.; Naimi, T.; Pack, Q. Relation of Patient’s Opinion of Alcohol’s Health Effects and Drinking Habits Among Hospitalized Patients with Cardiovascular Disease. Am. J. Cardiol. 2022, 179, 31–38. [Google Scholar] [CrossRef]
- Mandrekar, P.; Mandal, A. Pathogenesis of Alcohol-Associated Liver Disease. Clin. Liver Dis. 2024, in press. [Google Scholar] [CrossRef]
- Reile, R.; Innos, K. Alcohol-Related Cancer Risk Awareness and Support for Cancer Warning Labelling among Adults in Estonia. Public Health 2024, 236, 247–249. [Google Scholar] [CrossRef]
- Paul, P.; Campbell, G.; Zekeridou, A.; Mauermann, M.; Naddaf, E. Diagnosing Peripheral Neuropathy in Patients with Alcohol Use Disorder. Mayo Clin. Proc. 2024, 99, 1299–1305. [Google Scholar] [CrossRef]
- Sushma; Mishra, S.; Kanchan, S.; Divakar, A.; Jha, G.; Sharma, D.; Kapoor, R.; Rath, S.K. Alcohol Induces ER Stress and Apoptosis by Inducing Oxidative Stress and Disruption of Calcium Homeostasis in Glial Cells. Food Chem. Toxicol. 2023, 182, 114192. [Google Scholar] [CrossRef]
- Yeligar, S.M.; Harris, F.L.; Hart, C.M.; Brown, L.A.S. Ethanol Induces Oxidative Stress in Alveolar Macrophages via Upregulation of NADPH Oxidases. J. Immunol. 2012, 188, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhai, Q.; Shi, X. Alcohol-Induced Oxidative Stress and Cell Responses. J. Gastroenterol. Hepatol. 2006, 21, S26–S29. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- Yun-Soo, S.; Ok-Hwa, K.; Sung-Bae, K.; Su-Hyun, M.; Da-Hye, K.; Da-Wun, Y.; Jang-Gi, C.; Young-Mi, L.; Dae-Kil, K.; Ho-Seog, L.; et al. Quercetin Prevents Adipogenesis by Regulation of Transcriptional Factors and Lipases in OP9 Cells. Int. J. Mol. Med. 2015, 35, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H. The Sources and Mechanisms of Bioactive Ingredients in Coffee. Food Funct. 2019, 10, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New Insights into the Mechanisms of Polyphenols beyond Antioxidant Properties; Lessons from the Green Tea Polyphenol, Epigallocatechin 3-Gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hu, G.-L.; Qiu, M.-H.; Cao, J.; Xiong, W.-Y. Coffee, Tea, and Cocoa in Obesity Prevention: Mechanisms of Action and Future Prospects. Curr. Res. Food Sci. 2024, 8, 100741. [Google Scholar] [CrossRef]
- Munguia, L.; Rubio-Gayosso, I.; Ramirez-Sanchez, I.; Ortiz, A.; Hidalgo, I.; Gonzalez, C.; Meaney, E.; Villarreal, F.; Najera, N.; Ceballos, G. High Flavonoid Cocoa Supplement Ameliorates Plasma Oxidative Stress and Inflammation Levels While Improving Mobility and Quality of Life in Older Subjects: A Double-Blind Randomized Clinical Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1620–1627. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Li, D.; Hu, X. Effects of Coffee and Tea on Postprandial Cardiometabolic Risk Factors in Healthy Individuals: A Randomized Crossover Trial. Asia Pac. J. Clin. Nutr. 2024, 33, 102–110. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Z.; Xu, Y. HDL and Oxidation. In HDL Metabolism and Diseases; Zheng, L., Ed.; Springer Nature: Singapore, 2022; pp. 63–77. ISBN 978-981-19159-2-5. [Google Scholar]
- Durrington, P.N.; Bashir, B.; Soran, H. Paraoxonase 1 and Atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1065967. [Google Scholar] [CrossRef]
- Ponce-Ruiz, N.; Murillo-González, F.E.; Rojas-García, A.E.; Bernal Hernández, Y.Y.; Mackness, M.; Ponce-Gallegos, J.; Barrón-Vivanco, B.S.; Hernández-Ochoa, I.; González-Arias, C.A.; Ortega Cervantes, L.; et al. Phenotypes and Concentration of PON1 in Cardiovascular Disease: The Role of Nutrient Intake. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lou-Bonafonte, J.M.; Gabás-Rivera, C.; Navarro, M.A.; Osada, J. PON1 and Mediterranean Diet. Nutrients 2015, 7, 4068–4092. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Volkova, N.; Aviram, M. Postprandial Serum Triacylglycerols and Oxidative Stress in Mice after Consumption of Fish Oil, Soy Oil or Olive Oil: Possible Role for Paraoxonase-1 Triacylglycerol Lipase-like Activity. Nutrition 2006, 22, 922–930. [Google Scholar] [CrossRef] [PubMed]
- De Liz, S.; Cardoso, A.L.; Copetti, C.L.K.; Hinnig, P.D.F.; Vieira, F.G.K.; Da Silva, E.L.; Schulz, M.; Fett, R.; Micke, G.A.; Di Pietro, P.F. Açaí (Euterpe oleracea Mart.) and Juçara (Euterpe edulis Mart.) Juices Improved HDL-c Levels and Antioxidant Defense of Healthy Adults in a 4-Week Randomized Cross-over Study. Clin. Nutr. 2020, 39, 3629–3636. [Google Scholar] [CrossRef] [PubMed]
- Damavandi, R.D.; Mousavi, S.N.; Shidfar, F.; Mohammadi, V.; Rajab, A.; Hosseini, S.; Heshmati, J. Effects of Daily Consumption of Cashews on Oxidative Stress and Atherogenic Indices in Patients with Type 2 Diabetes: A Randomized, Controlled-Feeding Trial. Int. J. Endocrinol. Metab. 2019, 17, e70744. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.G.M.; Gomes, A.C.; Navarro, A.M.; da Cunha, L.C.; Silva, M.A.C.; Junior, F.B.; Mota, J.F. Baru Almonds Increase the Activity of Glutathione Peroxidase in Overweight and Obese Women: A Randomized, Placebo-Controlled Trial. Nutrients 2019, 11, 1750. [Google Scholar] [CrossRef]
- Katrenčíková, B.; Vaváková, M.; Waczulíková, I.; Oravec, S.; Garaiova, I.; Nagyová, Z.; Hlaváčová, N.; Ďuračková, Z.; Trebatická, J. Lipid Profile, Lipoprotein Subfractions, and Fluidity of Membranes in Children and Adolescents with Depressive Disorder: Effect of Omega-3 Fatty Acids in a Double-Blind Randomized Controlled Study. Biomolecules 2020, 10, 1427. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Soto, A.; Alejandra Chavez-Santoscoy, R.; Porras, O.; Hidalgo-Ledesma, M.; Serrano-Medina, A.; Alejandra Ramírez-Rodríguez, A.; Alejandra Castillo-Martinez, N. Epicatechin and Quercetin Exhibit in Vitro Antioxidant Effect, Improve Biochemical Parameters Related to Metabolic Syndrome, and Decrease Cellular Genotoxicity in Humans. Food Res. Int. 2021, 142, 110101. [Google Scholar] [CrossRef] [PubMed]
- Schincaglia, R.M.; Cuppari, L.; Neri, H.F.S.; Cintra, D.E.; Sant’Ana, M.R.; Mota, J.F. Effects of Baru Almond Oil (Dipteryx alata Vog.) Supplementation on Body Composition, Inflammation, Oxidative Stress, Lipid Profile, and Plasma Fatty Acids of Hemodialysis Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Complement. Ther. Med. 2020, 52, 102479. [Google Scholar] [CrossRef]
- Boshagh, K.; Khorvash, F.; Sahebkar, A.; Majeed, M.; Bahreini, N.; Askari, G.; Bagherniya, M. The Effects of Curcumin-Piperine Supplementation on Inflammatory, Oxidative Stress and Metabolic Indices in Patients with Ischemic Stroke in the Rehabilitation Phase: A Randomized Controlled Trial. Nutr. J. 2023, 22, 69. [Google Scholar] [CrossRef]
- Desai, T.; Roberts, M.; Bottoms, L. Effects of Short-Term Continuous Montmorency Tart Cherry Juice Supplementation in Participants with Metabolic Syndrome. Eur. J. Nutr. 2021, 60, 1587–1603. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Tajmiri, S. The Effects of Powdered Black Cumin Seeds on Markers of Oxidative Stress, Intracellular Adhesion Molecule (ICAM)-1 and Vascular Cell Adhesion Molecule (VCAM)-1 in Patients with Hashimoto’s Thyroiditis. Clin. Nutr. ESPEN 2020, 37, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, N.; Ktenioudaki, A.; Smyth, T.P.; McLoughlin, P.; Doran, L.; Auty, M.A.E.; Arendt, E.; Gallagher, E. Physicochemical Assessment of Two Fruit By-Products as Functional Ingredients: Apple and Orange Pomace. J. Food Eng. 2015, 153, 89–95. [Google Scholar] [CrossRef]
- Kumar, H.; Guleria, S.; Kimta, N.; Nepovimova, E.; Dhalaria, R.; Dhanjal, D.S.; Sethi, N.; Alomar, S.Y.; Kuca, K. Selected Fruit Pomaces: Nutritional Profile, Health Benefits, and Applications in Functional Foods and Feeds. Curr. Res. Food Sci. 2024, 9, 100791. [Google Scholar] [CrossRef] [PubMed]
- Unveiling Novel Applications of Fruit Pomace for Sustainable Production of Value-Added Products and Health Benefits: A Review-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S2212429224009635 (accessed on 31 August 2024).
- Tsoupras, A.; Gkika, D.A.; Markopoulos, T.; Curran, R.; Scallon, C.; Karali, M.; Kyzas, G.Z. Apple Products (Apple Juice and Cider) and By-Products (Apple Pomace): Bioactive Compounds and Biological Properties. In Natural Products in Beverages: Botany, Phytochemistry, Pharmacology and Processing; Mérillon, J.-M., Riviere, C., Lefèvre, G., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–42. ISBN 978-3-031-04195-2. [Google Scholar]
- Marra, A.; Manousakis, V.; Zervas, G.P.; Koutis, N.; Finos, M.A.; Adamantidi, T.; Panoutsopoulou, E.; Ofrydopoulou, A.; Tsoupras, A. Avocado and Its By-Products as Natural Sources of Valuable Anti-Inflammatory and Antioxidant Bioactives for Functional Foods and Cosmetics with Health-Promoting Properties. Appl. Sci. 2024, 14, 5978. [Google Scholar] [CrossRef]
- Moysidou, A.M.; Cheimpeloglou, K.; Koutra, S.I.; Finos, M.A.; Ofrydopoulou, A.; Tsoupras, A. A Comprehensive Review on the Antioxidant and Anti-Inflammatory Bioactives of Kiwi and Its By-Products for Functional Foods and Cosmetics with Health-Promoting Properties. Appl. Sci. 2024, 14, 5990. [Google Scholar] [CrossRef]
- Meghwar, P.; Ghufran Saeed, S.M.; Ullah, A.; Nikolakakis, E.; Panagopoulou, E.; Tsoupras, A.; Smaoui, S.; Mousavi Khaneghah, A. Nutritional Benefits of Bioactive Compounds from Watermelon: A Comprehensive Review. Food Biosci. 2024, 61, 104609. [Google Scholar] [CrossRef]
- Sodhi, G.K.; Kaur, G.; George, N.; Walia, H.K.; Sillu, D.; Rath, S.K.; Saxena, S.; Rios-Solis, L.; Dwibedi, V. Waste to Wealth: Microbial-Based Valorization of Grape Pomace for Nutraceutical, Cosmetic, and Therapeutic Applications to Promote Circular Economy. Process Saf. Environ. Prot. 2024, 188, 1464–1478. [Google Scholar] [CrossRef]
- Phenolic and Nutritional Profiles, and Antioxidant Activity of Grape Pomaces and Seeds from Lacrima Di Morro d’Alba and Verdicchio Varieties-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S2212429223004595 (accessed on 31 August 2024).
- Dziedziński, M.; Stachowiak, B.; Kobus-Cisowska, J.; Kozłowski, R.; Stuper-Szablewska, K.; Szambelan, K.; Górna, B. Supplementation of Beer with Pinus sylvestris L. Shoots Extracts and Its Effect on Fermentation, Phenolic Content, Antioxidant Activity and Sensory Profiles. Electron. J. Biotechnol. 2023, 63, 10–17. [Google Scholar] [CrossRef]
- Tsoupras, A.; Ni, V.L.J.; O’Mahony, É.; Karali, M. Winemaking: “With One Stone, Two Birds”? A Holistic Review of the Bio-Functional Compounds, Applications and Health Benefits of Wine and Wineries’ By-Products. Fermentation 2023, 9, 838. [Google Scholar] [CrossRef]
- Muñoz-Bernal, Ó.A.; Coria-Oliveros, A.J.; de la Rosa, L.A.; Rodrigo-García, J.; del Rocío Martínez-Ruiz, N.; Sayago-Ayerdi, S.G.; Alvarez-Parrilla, E. Cardioprotective Effect of Red Wine and Grape Pomace. Food Res. Int. 2021, 140, 110069. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Panagopoulou, E.A.; Kyzas, G.Z. Anti-Inflammatory, Antithrombotic and Anti-Oxidant Bioactives of Beer and Brewery by-Products, as Ingredients of Bio-Functional Foods, Nutraceuticals, Cosmetics, Cosmeceuticals and Pharmaceuticals with Health Promoting Properties. AIMS Agric. Food 2024, 9, 568–606. [Google Scholar] [CrossRef]
- Bacchetti, T.; Turco, I.; Urbano, A.; Morresi, C.; Ferretti, G. Relationship of Fruit and Vegetable Intake to Dietary Antioxidant Capacity and Markers of Oxidative Stress: A Sex-Related Study. Nutrition 2019, 61, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic Potential of Quercetin as a Cardiovascular Agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. Factors Modulating Bioavailability of Quercetin-Related Flavonoids and the Consequences of Their Vascular Function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Pegg, R.B. Tree Nuts and Peanuts as a Source of Natural Antioxidants in Our Daily Diet. Curr. Pharm. Des. 2020, 26, 1898–1916. [Google Scholar] [CrossRef] [PubMed]
- Alcázar-Valle, M.; Lugo-Cervantes, E.; Mojica, L.; Morales-Hernández, N.; Reyes-Ramírez, H.; Enríquez-Vara, J.N.; García-Morales, S. Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species. Molecules 2020, 25, 3528. [Google Scholar] [CrossRef]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive Proteins and Phytochemicals from Legumes: Mechanisms of Action Preventing Obesity and Type-2 Diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef]
- Power, R.; Nolan, J.M.; Prado-Cabrero, A.; Roche, W.; Coen, R.; Power, T.; Mulcahy, R. Omega-3 Fatty Acid, Carotenoid and Vitamin E Supplementation Improves Working Memory in Older Adults: A Randomised Clinical Trial. Clin. Nutr. 2022, 41, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Head, B.; La Du, J.; Tanguay, R.L.; Kioussi, C.; Traber, M.G. Vitamin E Is Necessary for Zebrafish Nervous System Development. Sci. Rep. 2020, 10, 15028. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Jiménez, G.M.; López-Saiz, C.M.; Ramírez-Guerra, H.E.; Ezquerra-Brauer, J.M.; Ruiz-Cruz, S.; Torres-Arreola, W. Role of Endogenous and Exogenous Tocopherols in the Lipid Stability of Marine Oil Systems: A Review. Int. J. Mol. Sci. 2016, 17, 1968. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.S.; Nielsen, N.S.; Baron, C.P.; Jacobsen, C. Oxidative Stability of Marine Phospholipids. In Proceedings of the 10th ILPS Phospholipids Congress, Rotterdam, The Netherlands, 16–18 September 2011. [Google Scholar]
- Oxidation of LDL by Myeloperoxidase and Reactive Nitrogen Species | Arteriosclerosis, Thrombosis, and Vascular Biology. Available online: https://www.ahajournals.org/doi/full/10.1161/01.ATV.20.7.1716 (accessed on 30 August 2024).
- Nutrients | Free Full-Text | Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Available online: https://www.mdpi.com/2072-6643/13/2/462 (accessed on 30 August 2024).
- Associations of Fish Oil and Vitamin B and E Supplementation with Cardiovascular Outcomes and Mortality in People Receiving Haemodialysis: A Review-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26283325/ (accessed on 30 August 2024).
- Sadeghi, N.; Paknezhad, F.; Rashidi Nooshabadi, M.; Kavianpour, M.; Jafari Rad, S.; Khadem Haghighian, H. Vitamin E and Fish Oil, Separately or in Combination, on Treatment of Primary Dysmenorrhea: A Double-Blind, Randomized Clinical Trial. Gynecol. Endocrinol. 2018, 34, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Prado, Y.; Aravena, D.; Gatica, S.; Llancalahuen, F.M.; Aravena, C.; Gutiérrez-Vera, C.; Carreño, L.J.; Cabello-Verrugio, C.; Simon, F. From Genes to Systems: The Role of Food Supplementation in the Regulation of Sepsis-Induced Inflammation. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2024, 1870, 166909. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-Inflammatory Effects in Humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Duarte, G.B.S.; Reis, B.Z.; Rogero, M.M.; Vargas-Mendez, E.; Júnior, F.B.; Cercato, C.; Cozzolino, S.M.F. Consumption of Brazil Nuts with High Selenium Levels Increased Inflammation Biomarkers in Obese Women: A Randomized Controlled Trial. Nutrition 2019, 63–64, 162–168. [Google Scholar] [CrossRef]
- Oh, E.S.; Petersen, K.S.; Kris-Etherton, P.M.; Rogers, C.J. Spices in a High-Saturated-Fat, High-Carbohydrate Meal Reduce Postprandial Proinflammatory Cytokine Secretion in Men with Overweight or Obesity: A 3-Period, Crossover, Randomized Controlled Trial. J. Nutr. 2020, 150, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Emamat, H.; Zahedmehr, A.; Asadian, S.; Nasrollahzadeh, J. The Effect of Barberry (Berberis integerrima) on Lipid Profile and Systemic Inflammation in Subjects with Cardiovascular Risk Factors: A Randomized Controlled Trial. BMC Complement. Med. Ther. 2022, 22, 59. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. The Impact of an 8-Week Supplementation with Fermented and Non-Fermented Aronia Berry Pulp on Cardiovascular Risk Factors in Individuals with Type 2 Diabetes. Nutrients 2023, 15, 5094. [Google Scholar] [CrossRef]
- Clark, J.S.; Dyer, K.A.; Davis, C.R.; Shivappa, N.; Hébert, J.R.; Woodman, R.; Hodgson, J.M.; Murphy, K.J. Adherence to a Mediterranean Diet for 6 Months Improves the Dietary Inflammatory Index in a Western Population: Results from the MedLey Study. Nutrients 2023, 15, 366. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Huang, L.L.; Luo, H.L.; Huang, Y.C.; Huang, X.Y.; Chen, G.; Gui, J.; Liu, Z.L.; Yang, L.; Liu, X.Z. Passion Fruit Peel and Its Zymolyte Enhance Gut Function in Sanhuang Broilers by Improving Antioxidation and Short-Chain Fatty Acids and Decreasing Inflammatory Cytokines. Poult. Sci. 2023, 102, 102672. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.M.; John, K.A.; Emmanuel, I.B.; Chidebe, E.O.; Adedapo, A.D.A. High-Fat Diet-Induced Memory Impairment and Anxiety-like Behavior in Rats Attenuated by Peel Extract of Ananas comosus Fruit via Atheroprotective, Antioxidant and Anti-Inflammatory Actions. Metab. Open 2021, 9, 100077. [Google Scholar] [CrossRef]
- Mokhtari, I.; Moumou, M.; Harnafi, M.; Milenkovic, D.; Amrani, S.; Harnafi, H. Loquat Fruit Peel Extract Regulates Lipid Metabolism and Liver Oxidative Stress in Mice: In Vivo and in Silico Approaches. J. Ethnopharmacol. 2023, 310, 116376. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A. The Anti-Inflammatory and Antithrombotic Properties of Bioactives from Orange, Sanguine and Clementine Juices and from Their Remaining By-Products. Beverages 2022, 8, 39. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Y.; Wang, Y.; Yu, Z.; Qin, H.; Zhao, L.; Cheng, J.; Shen, B.; Jin, M.; Feng, H. Total Flavonoids of Broussonetia papyrifera Alleviate Non-Alcohol Fatty Liver Disease via Regulating Nrf2/AMPK/mTOR Signaling Pathways. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2024, 1869, 159497. [Google Scholar] [CrossRef]
- Alcântara, D.B.; Dionísio, A.P.; Artur, A.G.; Silveira, B.K.S.; Lopes, A.F.; Guedes, J.A.C.; Luz, L.R.; Nascimento, R.F.; Lopes, G.S.; Hermsdorff, H.H.M.; et al. Selenium in Brazil Nuts: An Overview of Agronomical Aspects, Recent Trends in Analytical Chemistry, and Health Outcomes. Food Chem. 2022, 372, 131207. [Google Scholar] [CrossRef]
- Adam-Dima, E.I.; Balas, M.; Anastasescu, M.; Purdel, C.; Margină, D. Synthesis of Homogeneous Spherical Selenium Nanoparticles through a Chemical Method for Cancer Therapy Applications. Toxicol. In Vitro 2024, 95, 105765. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Chiodi, L.; Boccoli, G.; Costarelli, L.; Piacenza, F.; Provinciali, M.; Malavolta, M. Reduced Levels of Plasma Selenium Are Associated with Increased Inflammation and Cardiovascular Disease in an Italian Elderly Population. Exp. Gerontol. 2021, 145, 111219. [Google Scholar] [CrossRef]
- Glenn-Davi, K.; Hurley, A.; Brennan, E.; Coughlan, J.; Shiels, K.; Moran, D.; Saha, S.K.; Zabetakis, I.; Tsoupras, A. Fermentation Enhances the Anti-Inflammatory and Anti-Platelet Properties of Both Bovine Dairy and Plant-Derived Dairy Alternatives. Fermentation 2022, 8, 292. [Google Scholar] [CrossRef]
- Chen, M.-F.; Hsu, H.-C.; Liau, C.-S.; Lee, Y.-T. The Role of Vitamin E on the Anti-Atherosclerotic Effect of Fish Oil in Diet-Induced Hypercholesterolemic Rabbits. Prostaglandins Other Lipid Mediat. 1999, 57, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J. Fish Oil and Cardiovascular Disease: Lipids and Arterial Function12. Am. J. Clin. Nutr. 2000, 71, 228S–231S. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Fragopoulou, E.; Nomikos, T.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Characterization of the de Novo Biosynthetic Enzyme of Platelet Activating Factor, DDT-Insensitive Cholinephosphotransferase, of Human Mesangial Cells. Mediat. Inflamm. 2007, 2007, 27683. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Nomikos, T.; Demopoulos, C.A.; Zabetakis, I. Comparison of Antiatherogenic Properties of Lipids Obtained from Wild and Cultured Sea Bass (Dicentrarchus labrax) and Gilthead Sea Bream (Sparus aurata). Food Chem. 2007, 100, 560–567. [Google Scholar] [CrossRef]
- Tsoupras, A.; Iatrou, C.; Frangia, C.; Demopoulos, C. The Implication of Platelet Activating Factor in Cancer Growth and Metastasis: Potent Beneficial Role of PAF-Inhibitors and Antioxidants. Infect. Disord. Drug Targets 2009, 9, 390–399. [Google Scholar] [CrossRef]
- Hayashi, H.; Tanaka, Y.; Hibino, H.; Umeda, Y.; Kawamitsu, H.; Fujimoto, H.; Amakawa, T. Beneficial Effect of Salmon Roe Phosphatidylcholine in Chronic Liver Disease. Curr. Med. Res. Opin. 1999, 15, 177–184. [Google Scholar] [CrossRef]
- Conde, T.A.; Zabetakis, I.; Tsoupras, A.; Medina, I.; Costa, M.; Silva, J.; Neves, B.; Domingues, P.; Domingues, M.R. Microalgal Lipid Extracts Have Potential to Modulate the Inflammatory Response: A Critical Review. Int. J. Mol. Sci. 2021, 22, 9825. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R.; et al. Algae as an Emerging Source of Bioactive Pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Dordevic, A.L.; Cox, K.; Scholey, A.; Ryan, L.; Bonham, M.P. Twelve Weeks’ Treatment with a Polyphenol-Rich Seaweed Extract Increased HDL Cholesterol with No Change in Other Biomarkers of Chronic Disease Risk in Overweight Adults: A Placebo-Controlled Randomized Trial. J. Nutr. Biochem. 2021, 96, 108777. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Carocho, M.; Barros, L.; Zabetakis, I.; Mocan, A.; Tsoupras, A.; Cruz, A.G.; Pimentel, T.C. Implementation of Sustainable Development Goals in the Dairy Sector: Perspectives on the Use of Agro-Industrial Side-Streams to Design Functional Foods. Trends Food Sci. Technol. 2022, 124, 128–139. [Google Scholar] [CrossRef]
- Tirosh, O.; Verman, M.; Ivancovsky-Wajcman, D.; Grinshpan, L.S.; Fliss-Isakov, N.; Webb, M.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. Differential Effects of Low or High-Fat Dairy and Fat Derived from Dairy Products on MASLD. JHEP Rep. 2024, 101194, in press. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).