Extraction and Nutritional Value of Soybean Meal Protein Isolate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
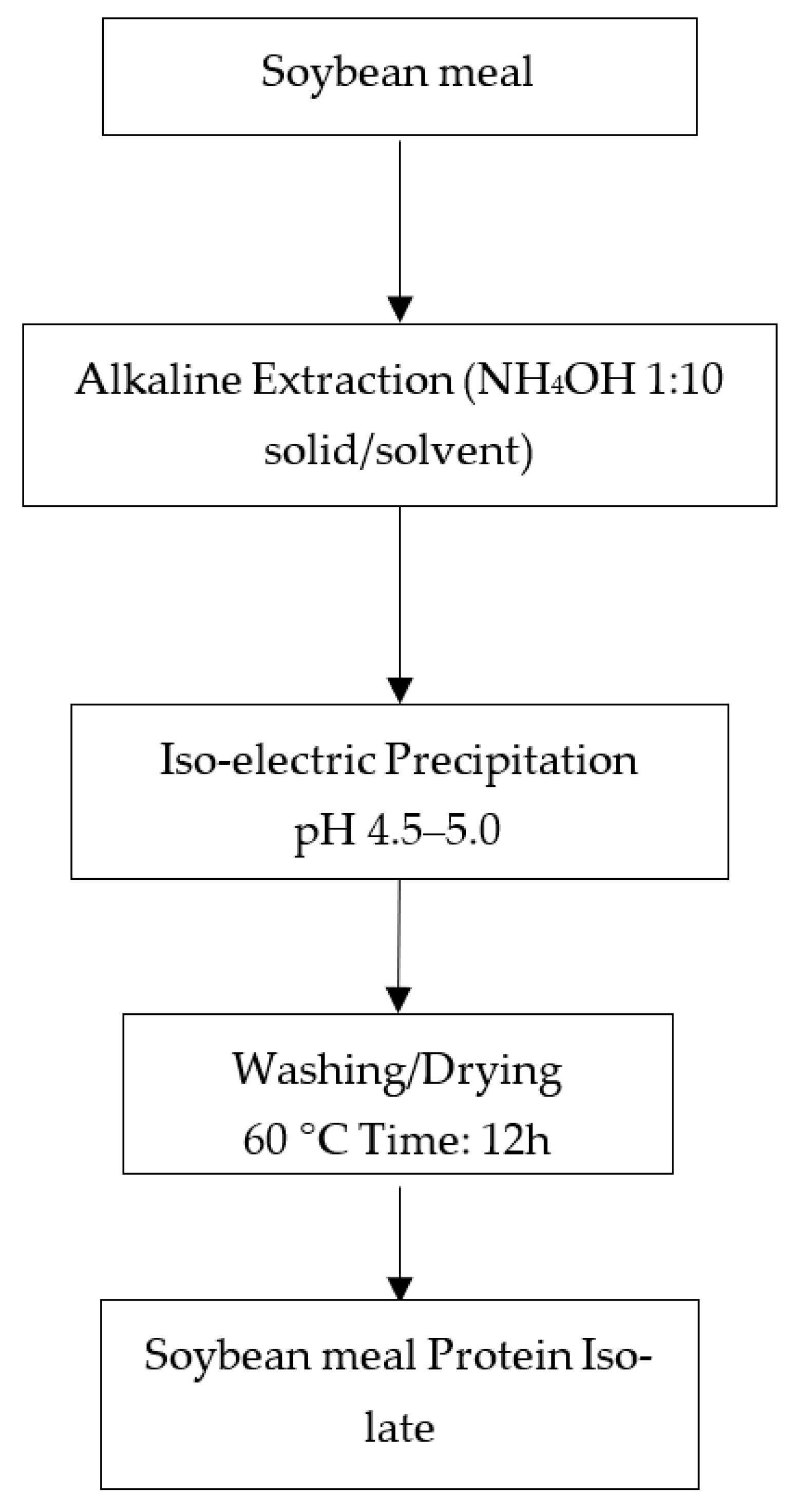
2.2. Extraction of Protein Isolates
2.3. Proximate Analysis
2.3.1. Moisture Content
2.3.2. Ash Content
2.3.3. Fat Content
2.3.4. Total Protein
2.3.5. Total Carbohydrate
2.4. Enzymatic Hydrolysis of Soybean Meal Protein Isolates
Amine Quantification
2.5. FTIR
2.6. SDS-PAGE Electrophoresis
2.7. Amino Acid Profiling of SMPI
2.8. Differential Scanning Measurement
2.9. Statistical Analysis
3. Results and Discussion
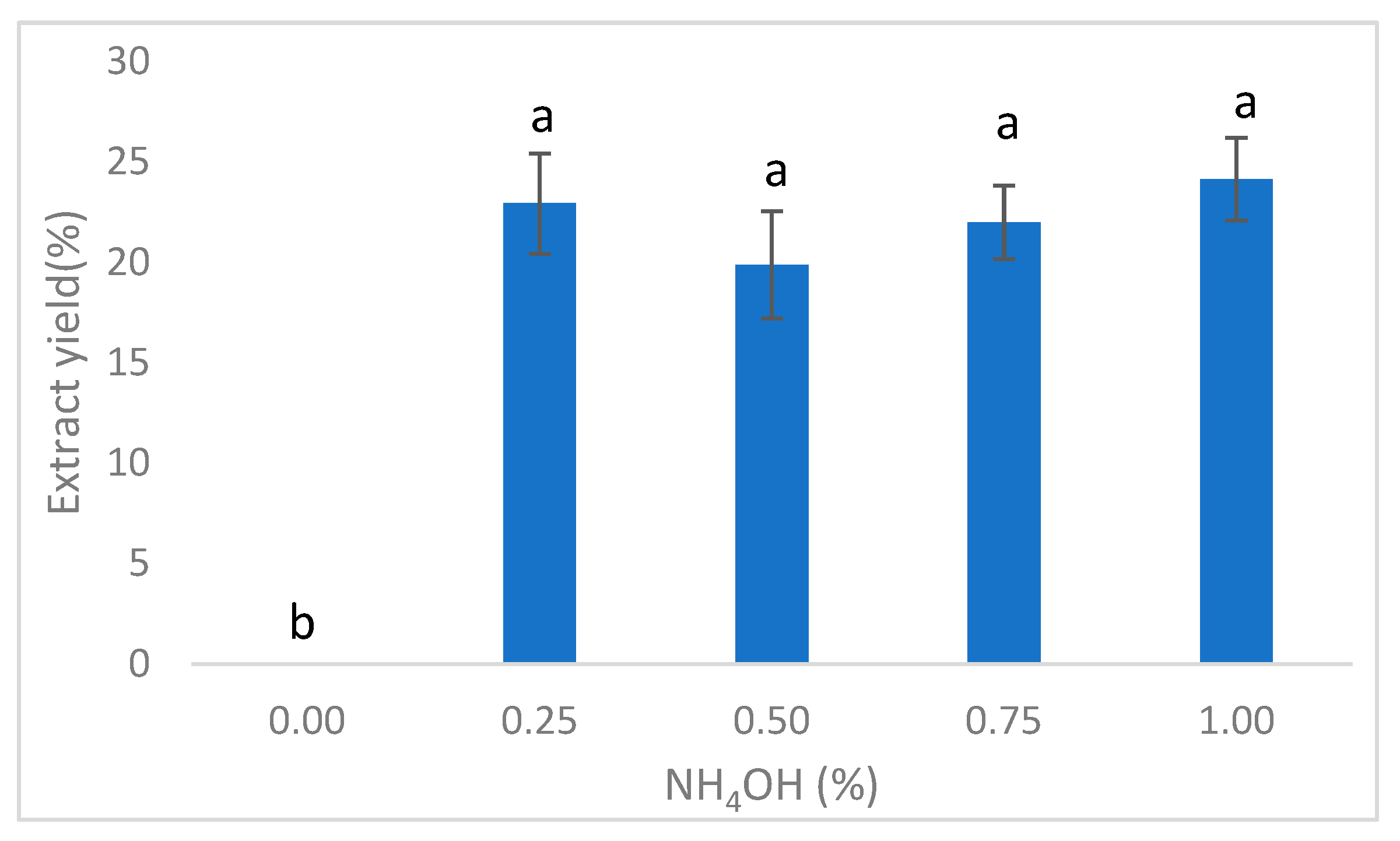
3.1. Extractability of Soybean Meal Protein
3.2. Proximate Composition
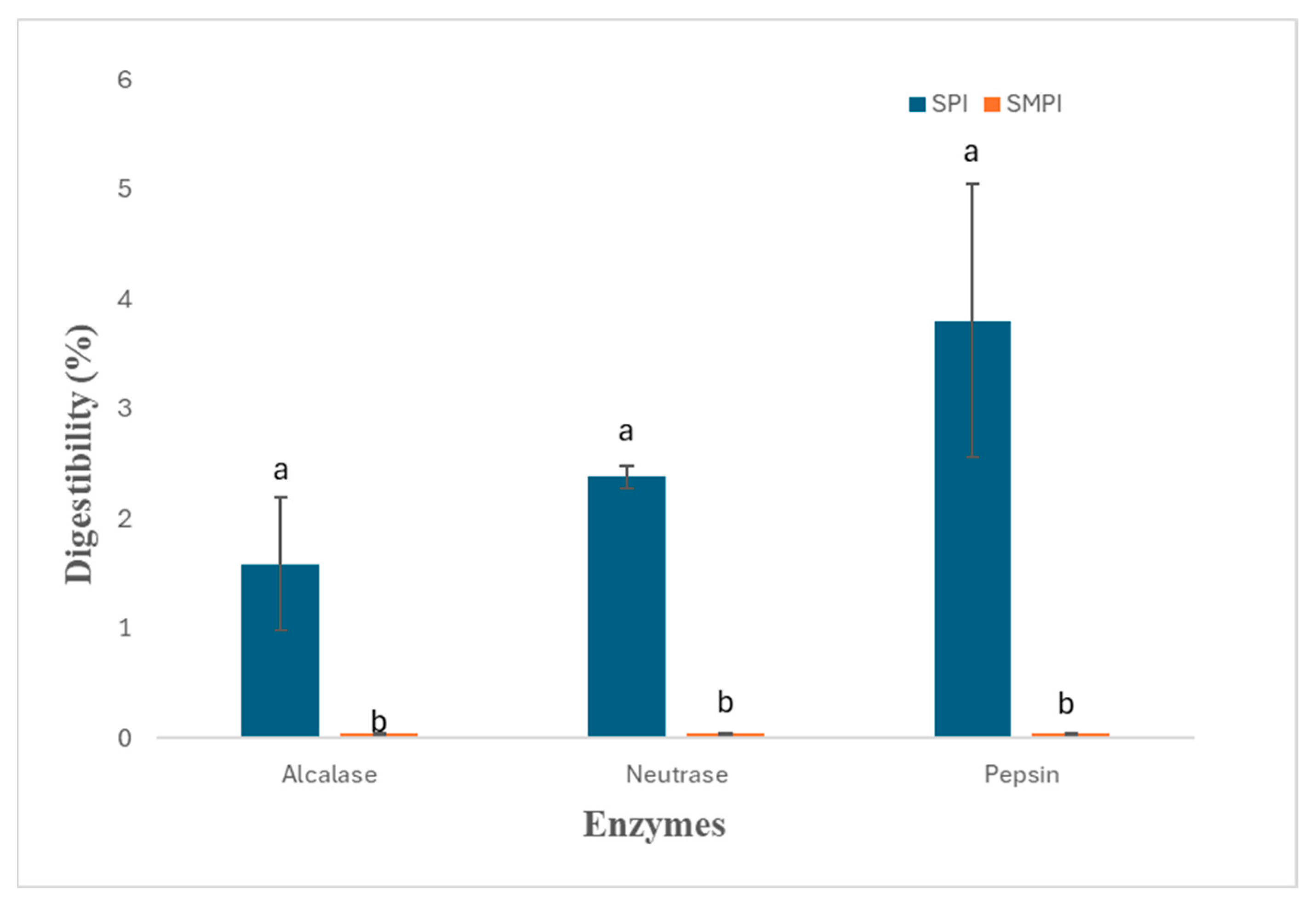
3.3. Evaluation of SMPI Digestibility Using Various Industrial Proteases
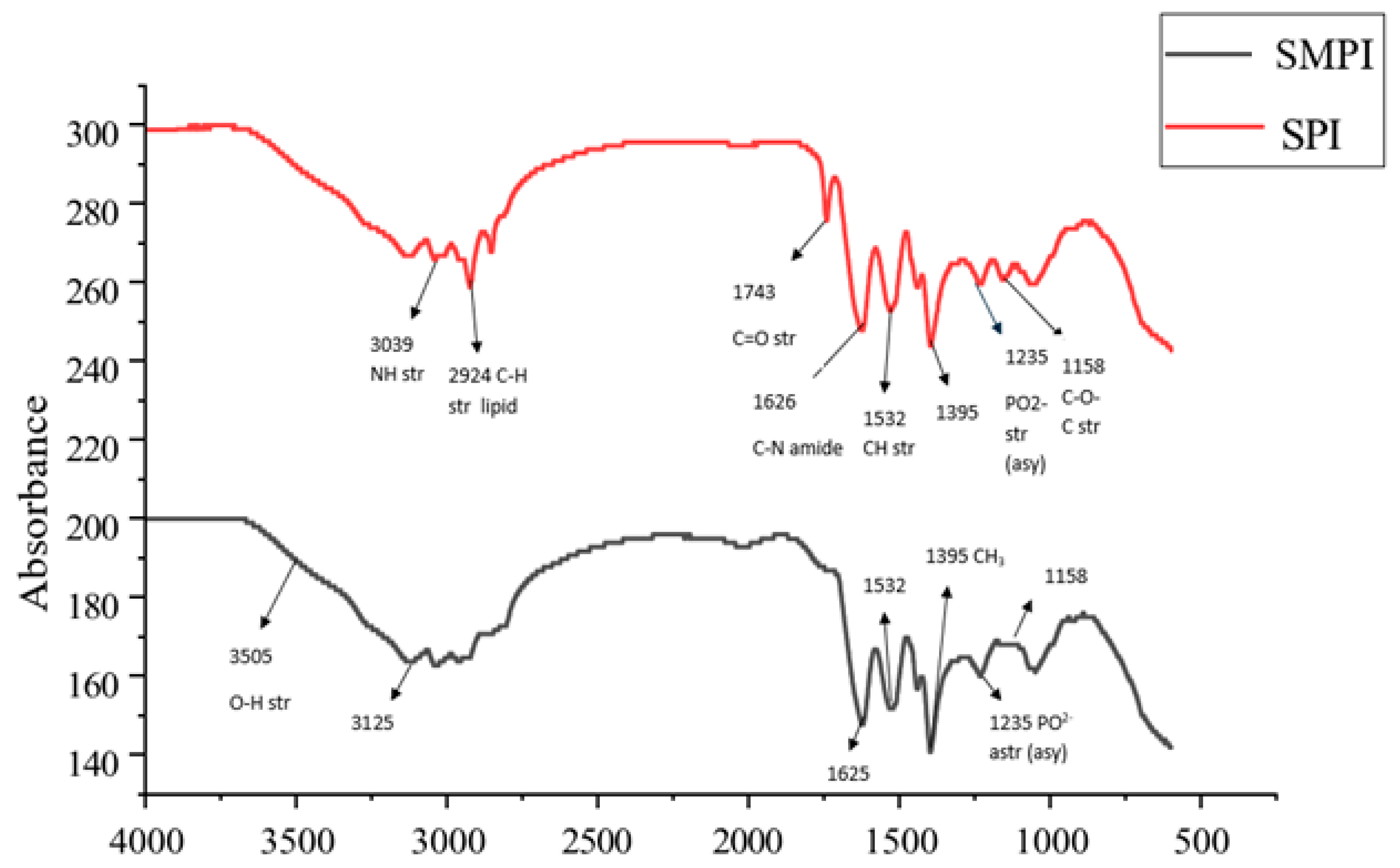
3.4. FTIR Spectroscopy
3.5. SDS-PAGE Banding Pattern of Soybean and Soybean Meal Protein Isolates
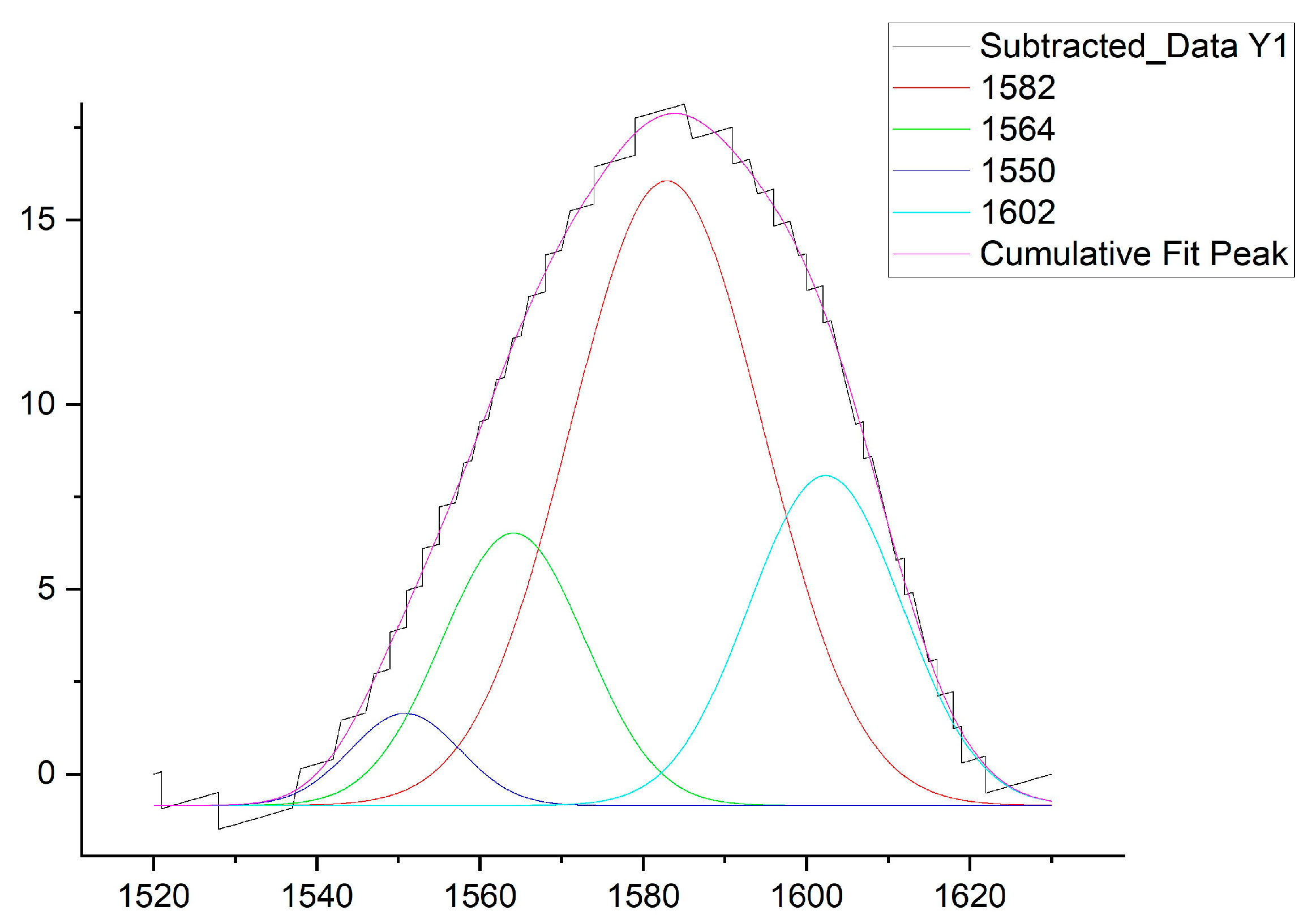
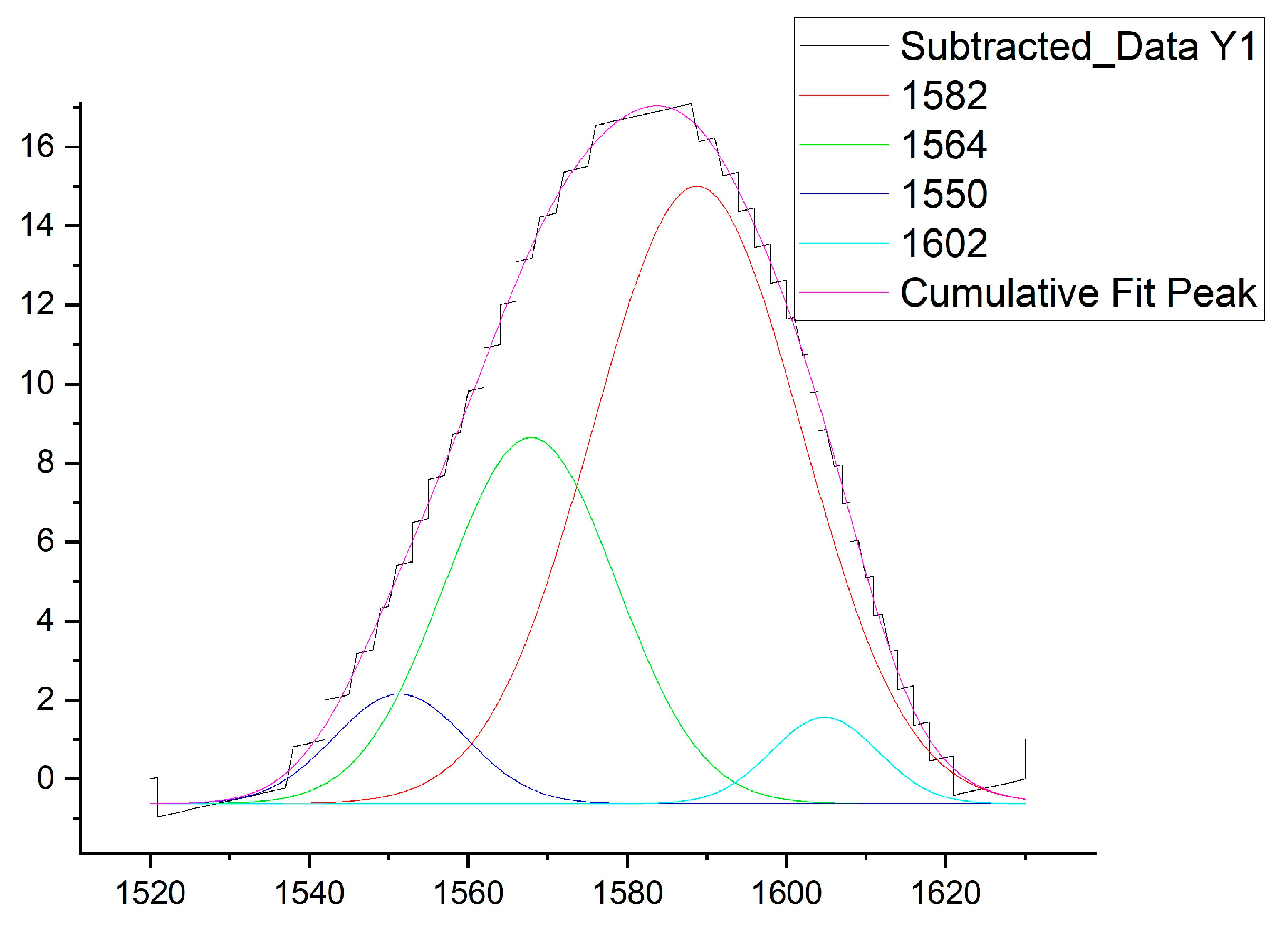
3.6. Deconvolution of Amide II Regions of FTIR spectra
3.7. Differential Scanning Calorimetry (DSC)
3.8. Amino Acid Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruiz, N.; Parsons, C.M.; Stein, H.H.; Coon, C.N.; Eys, J.; Miles, R.D. A Review: 100 Years of Soybean Meal; ADM: Chicago, IL, USA, 2020. [Google Scholar]
- USDA ERS—Oil Crops Sector at a Glance. Available online: https://www.ers.usda.gov/topics/crops/soybeans-and-oil-crops/oil-crops-sector-at-a-glance/ (accessed on 22 February 2024).
- Challenges Ahead For Soybean-Based Renewable Fuels Demand | Biodiesel Magazine. Available online: https://biodieselmagazine.com//articles/challenges-ahead-for-soybean-based-renewable-fuels-demand-2518696 (accessed on 22 February 2024).
- Mizutani, Y.; Shibata, M.; Yamada, S.; Nambu, Y.; Hirotsuka, M.; Matsumura, Y. Effects of Heat Treatment under Low Moisture Conditions on the Protein and Oil in Soybean Seeds. Food Chem. 2019, 275, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Dufaure, C.; Leyris, J.; Rigal, L.; Mouloungui, Z. A Twin-Screw Extruder for Oil Extraction: I. Direct Expression of Oleic Sunflower Seeds. J. Am. Oil Chem. Soc. 1999, 76, 1073–1079. [Google Scholar] [CrossRef]
- Herkelman, K.L.; Cromwell, G.L.; Stahly, T.S. Effects of Heating Time and Sodium Metabisulfite on the Nutritional Value of Full-Fat Soybeans for Chicks. J. Anim. Sci. 1991, 69, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Sá, A.G.A.; Laurindo, J.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Influence of Emerging Technologies on the Utilization of Plant Proteins. Front. Nutr. 2022, 9, 809058. Available online: https://www.frontiersin.org/articles/10.3389/fnut.2022.809058 (accessed on 22 February 2024). [CrossRef]
- Grieshop, C.M.; Fahey, G.C., Jr. Comparison of Quality Characteristics of Soybeans from Brazil, China, and the United States. J. Agric. Food Chem. 2001, 49, 2669–2673. [Google Scholar] [CrossRef]
- Salim, R.; Nehvi, I.B.; Mir, R.A.; Tyagi, A.; Ali, S.; Bhat, O.M. A Review on Anti-Nutritional Factors: Unraveling the Natural Gateways to Human Health. Front. Nutr. 2023, 10, 1215873. [Google Scholar] [CrossRef]
- Gerliani, N.; Hammami, R.; Aïder, M. Extraction of Protein and Carbohydrates from Soybean Meal Using Acidic and Alkaline Solutions Produced by Electro-Activation. Food Sci. Nutr. 2020, 8, 1125–1138. [Google Scholar] [CrossRef]
- Pope, M.; Borg, B.; Boyd, R.; Holzgraefe, D.; Rush, C.; Sifri, M. Quantifying the Value of Soybean Meal in Poultry and Swine Diets. J. Appl. Poult. Res. 2023, 32, 100337. [Google Scholar] [CrossRef]
- Grant, G. Anti-nutritional effects of soyabean: A review. Prog. Food Nutr. Sci. 1989, 13, 317–348. [Google Scholar]
- Sun, Z.W.; Qin, G.X.; Lou, Y.J. Soybean antigens and its influence on piglets and calves. Acta Zoonutrim. Sin. 2005, 17, 20–24. [Google Scholar]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Kleekayai, T.; Khalesi, M.; Amigo-Benavent, M.; Cermeño, M.; Harnedy-Rothwell, P.; FitzGerald, R.J. Enzyme-Assisted Extraction of Plant Proteins. In Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development; Hernández-Álvarez, A.J., Mondor, M., Nosworthy, M.G., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 131–178. [Google Scholar] [CrossRef]
- Bello, I.; Adeniyi, A.; Mukaila, T.; Hammed, A. Optimization of Soybean Protein Extraction with Ammonium Hydroxide (NH4OH) Using Response Surface Methodology. Foods 2023, 12, 1515. [Google Scholar] [CrossRef] [PubMed]
- Nielson, P.M. Functionality of protein hydrolysates. In Food Proteins and Their Applications, 1st ed.; Damodaran, S., Paraf, A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 443–472. [Google Scholar]
- Dourado, L.R.B.; Pascoal, L.A.F.; Sakomura, N.K.; Costa, F.G.P.; Biagiotti, D. Soybeans (Glycine max) and Soybean Products in Poultry and Swine Nutrition. In Recent Trends for Enhancing the Diversity and Quality of Soybean Products; Krezhova, D., Ed.; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Lilleeng, E.; Frøystad, M.K.; Vekterud, K.; Valen, E.C.; Krogdahl, Å. Comparison of Intestinal Gene Expression in Atlantic Cod (Gadus morhua) Fed Standard Fish Meal or Soybean Meal by Means of Suppression Subtractive Hybridization and Real-Time PCR. Aquaculture 2007, 267, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, T. Nutritional Value of Soybean Meal. In Soybean and Nutrition; El-Shemy, H., Ed.; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.A.; Kumar, S.; Bhat, H.F. Thermal Processing Implications on the Digestibility of Meat, Fish and Seafood Proteins. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4511–4548. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, S.; Kalashnikova, O.; Ivanova, S.; Prosekov, A.; Krol, O.; Kriger, O.; Fedovskikh, N.; Babich, O. Evaluating the Influence of Microbial Fermentation on the Nutritional Value of Soybean Meal. Fermentation 2022, 8, 458. [Google Scholar] [CrossRef]
- Feng, J.; Liu, X.; Xu, Z.R.; Wang, Y.Z.; Liu, J.X. Effects of Fermented Soybean Meal on Digestive Enzyme Activities and Intestinal Morphology in Broilers. Poult. Sci. 2007, 86, 1149–1154. [Google Scholar] [CrossRef]
- Bello, I.; Adeniyi, A.; Mukaila, T.; Hammed, A. Developing and Modelling of Sustainable Protein Extraction Using Ammonium Hydroxide—A Recoverable and Reusable Solvent. Food Bioprod. Process. 2023, 140, 16–28. [Google Scholar] [CrossRef]
- Adeniyi, A.; Bello, I.; Mukaila, T.; Monono, E.; Hammed, A. Enzyme-Driven Bioprocessing for Enhanced Bio-Ammonia Production from Soybean Meal Protein Isolate. Biomass Convers. Biorefinery 2023, 1–10. [Google Scholar] [CrossRef]
- Verfaillie, D.; Janssen, F.; Van Royen, G.; Wouters, A.G. A Systematic Study of the Impact of the Isoelectric Precipitation Process on the Physical Properties and Protein Composition of Soy Protein Isolates. Food Res. Int. 2023, 163, 112177. [Google Scholar] [CrossRef]
- Gerzhova, A.; Mondor, M.; Benali, M.; Aider, M. Study of Total Dry Matter and Protein Extraction from Canola Meal as Affected by the pH, Salt Addition and Use of Zeta-Potential/Turbidimetry Analysis to Optimize the Extraction Conditions. Food Chem. 2016, 201, 243–252. [Google Scholar] [CrossRef]
- Oxford University Press. Official Methods of Analysis of AOAC INTERNATIONAL; Oxford University Press: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- He, Z.; Zhang, H.; Olk, D.C.; Shankle, M.; Way, T.R.; Tewolde, H. Protein and fiber profiles of cottonseed from upland cotton with different fertilizations. Mod. Appl. Sci. 2014, 8, 97. [Google Scholar] [CrossRef]
- Hunsakul, K.; Laokuldilok, T.; Sakdatorn, V.; Klangpetch, W.; Brennan, C.S.; Utama-Ang, N. Optimization of enzymatic hydrolysis by alcalase and flavourzyme to enhance the antioxidant properties of jasmine rice bran protein hydrolysate. Sci. Rep. 2022, 12, 12582. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Zhao, M.; Yuan, B.; Zhang, Y.; Ren, J. Effect of pH and pepsin limited hydrolysis on the structure and functional properties of soybean protein hydrolysates. J. Food Sci. 2013, 78, C1871–C1877. [Google Scholar] [CrossRef] [PubMed]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Panesar, P.S.; Saini, C.S. pH Shifting Treatment of Ultrasonically Extracted Soybean Meal Protein Isolate: Effect on Functional, Structural, Morphological and Thermal Properties. Process. Biochem. 2022, 120, 227–238. [Google Scholar] [CrossRef]
- Tömösközi, S.; Lásztity, R.; Haraszi, R.; Baticz, O. Isolation and study of the functional properties of pea proteins. Food/Nahrung 2001, 45, 399–401. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Enhanced alkaline extraction techniques for isolating and modifying plant-based proteins. Food Hydrocoll. 2023, 145, 109132. [Google Scholar] [CrossRef]
- Barbosa, L.R.; Ortore, M.G.; Spinozzi, F.; Mariani, P.; Bernstorff, S.; Itri, R. The Importance of Protein-Protein Interactions on the pH-Induced Conformational Changes of Bovine Serum Albumin: A Small-Angle X-Ray Scattering Study. Biophys. J. 2010, 98, 147–157. [Google Scholar] [CrossRef]
- Mauri, A.N.; Añón, M.C. Effect of Solution pH on Solubility and Some Structural Properties of Soybean Protein Isolate Films. J. Sci. Food Agric. 2006, 86, 1064–1072. [Google Scholar] [CrossRef]
- Dissanayake, M.; Ramchandran, L.; Donkor, O.; Vasiljevic, T. Denaturation of Whey Proteins as a Function of Heat, pH and Protein Concentration. Int. Dairy J. 2013, 31, 93–99. [Google Scholar] [CrossRef]
- Sari, Y.W.; Mulder, W.J.; Sanders, J.P.M.; Bruins, M.E. Towards plant protein refinery: Review on protein extraction using alkali and potential enzymatic assistance. Biotechnol. J. 2015, 10, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Deleu, L.J.; Lambrecht, M.A.; Van de Vondel, J.; Delcour, J.A. The Impact of Alkaline Conditions on Storage Proteins of Cereals and Pseudo-Cereals. Curr. Opin. Food Sci. 2019, 25, 98–103. [Google Scholar] [CrossRef]
- Hou, F.; Ding, W.; Qu, W.; Oladejo, A.O.; Xiong, F.; Zhang, W.; He, R.; Ma, H. Alkali Solution Extraction of Rice Residue Protein Isolates: Influence of Alkali Concentration on Protein Functional, Structural Properties and Lysinoalanine Formation. Food Chem. 2017, 218, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dossou, S.; Wang, W.; Zhang, X.; Shadrack, R.S.; Mzengereza, K.; Zhu, K.; et al. Optimization of Soybean Meal Fermentation for Aqua-Feed with Bacillus subtilis natto Using the Response Surface Methodology. Fermentation 2021, 7, 306. [Google Scholar] [CrossRef]
- Gallo, J.-A.Q.; Debeaufort, F.; Callegarin, F.; Voilley, A. Lipid Hydrophobicity, Physical State and Distribution Effects on the Properties of Emulsion-Based Edible Films. J. Membr. Sci. 2000, 180, 37–46. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Zuidam, N.J. Whole Soybean Protein Extraction Processes: A Review. Innov. Food Sci. Emerg. Technol. 2017, 43, 163–172. [Google Scholar] [CrossRef]
- Serrato, A.G. Extraction of Oil from Soybeans. J. Am. Oil Chem. Soc. 1981, 58 Pt 1, 157–159. [Google Scholar] [CrossRef]
- Sethi, S.; Yadav, D.; Snigdha, S.; Gupta, A. Optimization of Process Parameters for Extraction of Protein Isolates from Khesari dhal (Lathyrus sativus L). LWT 2021, 137, 110368. [Google Scholar] [CrossRef]
- L’Hocine, L.; Boye, J.I.; Arcand, Y. Composition and Functional Properties of Soy Protein Isolates Prepared Using Alternative Defatting and Extraction Procedures. J. Food Sci. 2006, 71, C137–C145. [Google Scholar] [CrossRef]
- Kang, H.-C.; Kim, J.-B.; Roh, K.H.; Yoon, S.-H. A New Method of Extracting Whole Cell Proteins from Soil Microorganisms Using Pre-treatment of Ammonium Hydroxide. J. Appl. Biol. Chem. 2013, 56, 171–177. [Google Scholar] [CrossRef]
- Etiosa, O.R.; Chika, N.B.; Benedicta, A. Mineral and Proximate Composition of Soya Bean. Asian J. Phys. Chem. Sci. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Ogata, S.-I.; Lloyd, K.O. Mild Alkaline Borohydride Treatment of Glycoproteins—A Method for Liberating Both N- and O-Linked Carbohydrate Chains. Anal. Biochem. 1982, 119, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein Quality Evaluation Twenty Years after the Introduction of the Protein Digestibility Corrected Amino Acid Score Method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Ogawa, Y. In Vitro Protein Digestibility and Biochemical Characteristics of Soaked, Boiled and Fermented Soybeans. Sci. Rep. 2021, 11, 14257. [Google Scholar] [CrossRef] [PubMed]
- Liener, I.E. Factors Affecting the Nutritional Quality of Soya Products. J. Am. Oil Chem. Soc. 1981, 58, 406–415. [Google Scholar] [CrossRef]
- Delgado-Andrade, C. Maillard Reaction Products: Some Considerations on Their Health Effects. Clin. Chem. Lab. Med. 2014, 52, 53–60. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Rahma, E.H.; El-Bedawy, A.A.; Sobihah, T.Y. Effect of Soaking Process on Nutritional Quality and Protein Solubility of Some Legume Seeds. Food/Nahrung 2000, 44, 339–343. [Google Scholar] [CrossRef]
- Kathuria, D.; Dhiman, A.K.; Attri, S.; Kumar, M. Effect of Processing Method on Quality Characteristics of Harit Soybean (Glycine max): In Vitro Protein Digestibility, Hplc, Ftir Analysis. Nutr. Food Sci. 2021, 52, 684–697. [Google Scholar] [CrossRef]
- Shi, L.; Mu, K.; Arntfield, S.D.; Nickerson, M.T. Changes in Levels of Enzyme Inhibitors during Soaking and Cooking for Pulses Available in Canada. J. Food Sci. Technol. 2017, 54, 1014–1022. [Google Scholar] [CrossRef]
- Kayembe, N.; van Rensburg, C.J. Germination as a Processing Technique for Soybeans in Small-Scale Farming. S. Afr. J. Anim. Sci. 2013, 43, 167–173. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Zhang, C.; Kong, X.; Hua, Y. The Heat-Induced Protein Aggregate Correlated with Trypsin Inhibitor Inactivation in Soymilk Processing. J. Agric. Food Chem. 2012, 60, 8012–8019. [Google Scholar] [CrossRef] [PubMed]
- Gilani, G.S.; Cockell, K.A.; Sepehr, E. Effects of Antinutritional Factors on Protein Digestibility and Amino Acid Availability in Foods. J. AOAC Int. 2005, 88, 967–987. [Google Scholar] [CrossRef] [PubMed]
- Zahir, M.; Fogliano, V.; Capuano, E. Effect of Soybean Processing on Cell Wall Porosity and Protein Digestibility. Food Funct. 2020, 11, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Byler, D.M.; Susi, H. Examination of the Secondary Structure of Proteins by Deconvolved FTIR Spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Tatulian, S.A. FTIR Analysis of Proteins and Protein–Membrane Interactions. In Lipid-Protein Interactions: Methods and Protocols; Kleinschmidt, J.H., Ed.; Springer: New York, NY, USA, 2019; pp. 281–325. [Google Scholar] [CrossRef]
- Hoffmann, D.H. Assessment of Soy Cake Protein Quality for Broiler Feeding through Infrared Spectroscopy Calibration Validated by In-Vitro Feed Analyses and In-Vivo Feeding Studies. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2019. Available online: https://mediatum.ub.tum.de/1468716 (accessed on 22 February 2024).
- Priyadarshini, K.M.; Chandramohan, A.; Babu, G.A.; Ramasamy, P. Synthesis, crystal growth, spectral, optical, thermal and dielectric studies of dichloro (4-hydroxy-l-proline) cadmium (II) single crystals. Optik 2014, 125, 1390–1395. Available online: https://www.sciencedirect.com/science/article/pii/S0030402613011637?casa_token=elMauyLKqScAAAAA (accessed on 22 February 2024). [CrossRef]
- Brimas, E.; Raudonis, R.; Kareiva, A. Analytical methods used for the characterisation of specific features of biological tissues related with obesity: A review. Chemija 2022, 33. Available online: https://www.lmaleidykla.lt/ojs/index.php/chemija/article/view/4809 (accessed on 22 February 2024). [CrossRef]
- Chen, N.; Zhao, M.; Chassenieux, C.; Nicolai, T. Data on the Characterization of Native Soy Globulin by SDS-Page, Light Scattering and Titration. Data Brief 2016, 9, 749–752. [Google Scholar] [CrossRef]
- Ma, C.Y.; Harwalkar, V.R. Thermal Analysis of Food Proteins. In Advances in Food and Nutrition Research; Kinsella, J.E., Ed.; Academic Press: Cambridge, MA, USA, 1991; Volume 35, pp. 317–366. [Google Scholar] [CrossRef]
- Damodaran, S. Refolding of thermally unfolded soy proteins during the cooling regime of the gelation process: Effect on gelation. J. Agric. Food Chem. 1988, 36, 262–269. [Google Scholar] [CrossRef]
- Tang, C.-H.; Chen, Z.; Li, L.; Yang, X.-Q. Effects of Transglutaminase Treatment on the Thermal Properties of Soy Protein Isolates. Food Res. Int. 2006, 39, 704–711. [Google Scholar] [CrossRef]
- Souza, A.C.P.; Gurak, P.D.; Marczak, L.D.F. Maltodextrin, Pectin and Soy Protein Isolate as Carrier Agents in the Encapsulation of Anthocyanins-Rich Extract from Jaboticaba Pomace. Food Bioprod. Process. 2017, 102, 186–194. [Google Scholar] [CrossRef]
- Zhao, Q.; Selomulya, C.; Xiong, H.; Chen, X.D.; Li, X.; Wang, S.; Bai, C.; Peng, H.; Zhou, Q.; Sun, W. Rice Dreg Protein as an Alternative to Soy Protein Isolate: Comparison of Nutritional Properties. Int. J. Food Prop. 2014, 17, 1791–1804. [Google Scholar] [CrossRef]
- Singh, J.; Karmakar, S.; Banerjee, R. Extraction and Nutritional Properties of Protein Derived from Rice (Oryza sativa) Based Distillery Byproducts:A Potential Substrate for Food Formulation. Biocatal. Agric. Biotechnol. 2019, 22, 101364. [Google Scholar] [CrossRef]
- FAO; WHO; UNU. Energy and Protein Requirements; WHO Technical Report Series 724; WHO: Geneva, Switzerland, 1985; p. 209. [Google Scholar]
- Gorissen, S.H.; Crombag, J.J.; Senden, J.M.; Waterval, W.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]



| Parameters | SMPI (%) | SPI (%) |
|---|---|---|
| Moisture content a | 14 ± 0.58 | 11 ± 1.00 |
| Ash b | 2.12 ± 2.47 | 1.66 ± 0.28 |
| Fat a | 0.02 ± 0.00 | 8.67± 0.01 |
| Protein a | 68 ± 4.50 | 56.8 ± 0.80 |
| Carbohydrate a | 15.86 ± 3.01 | 21.87 ± 0.34 |
| Amino Acid | SMPI | Soy a | Pea a | Wheat a | FAO/WHO (1985) | ||
|---|---|---|---|---|---|---|---|
| Child | Adult | ||||||
| Essential | |||||||
| Phenylalanine + Tyrosine | 7.94 | 5.4 | 6.3 | 6.1 | 6.3 | 1.9 | |
| Histidine | 2.12 | 1.5 | 1.6 | 1.4 | 1.9 | 1.6 | |
| Isoleucine | 4.3 | 1.9 | 2.3 | 2.0 | 2.8 | 1.3 | |
| Leucine | 6.61 | 5 | 5.7 | 5.0 | 6.6 | 1.9 | |
| Lysine | 4.8 | 3.4 | 4.7 | 1.1 | 5.8 | 1.6 | |
| Methionine + cysteine | 1.65 | 0.5 | 0.5 | 1.4 | 2.5 | 1.7 | |
| Threonine | 2.81 | 2.3 | 2.5 | 1.8 | 3.4 | 0.9 | |
| Valine | 4.07 | 2.2 | 2.7 | 2.3 | 3.5 | 1.3 | |
| Tryptophan | 1.25 | 1.6 | 0.8 | 1.2 | 1.1 | 0.5 | |
| ∑EAA | 35.55 | 23.8 | 27.1 | 22.3 | 33.9 | 12.7 | |
| Non-essential | |||||||
| Alanine | 3.13 | 2.8 | 3.2 | 1.8 | |||
| Arginine | 6.47 | 4.8 | 5.9 | 2.4 | |||
| Glycine | 3.44 | 2.7 | 2.8 | 2.4 | |||
| Proline | 4.53 | 3.3 | 3.1 | 8.8 | |||
| Serine | 3.78 | 3.4 | 3.6 | 3.5 | |||
| Glutamic acid | 17.79 | 12.4 | 12.9 | 26.9 | |||
| Aspartic acid | 10.35 | ||||||
| Hydroxyproline | 0.06 | ||||||
| Hydroxylysine | 0.03 | ||||||
| Taurine | 0.04 | ||||||
| Lanthionine | 0.19 | ||||||
| Ornithine | 0.27 | ||||||
| ∑NEAA | 50.08 | ||||||
| Hydrophobic AAs | 32.96 | 23 | 24.6 | 27.9 | |||
| Uncharged polar | 10.55 | 8.1 | 8.9 | 8.4 | |||
| Basic | 13.39 | 9.7 | 12.2 | 4.9 | |||
| Acid | 17.78 | ||||||
| Composition | EAA (mg/g) | Reference Pattern (mg/g) [72] | AAS (%) |
|---|---|---|---|
| Histidine | 21.2 | 19 | 111.6 |
| Lysine | 48 | 58 | 82.8 |
| Tryptophan | 12.5 | 11 | 113.6 |
| Phenylalanine + Tyrosine | 79.4 | 63 | 126 |
| Methionine + Cystine | 16.5 | 25 | 66 |
| Threonine | 28.1 | 34 | 82.2 |
| Leucine | 66.1 | 66 | 100.2 |
| Isoleucine | 43 | 28 | 153.6 |
| Valine | 40.7 | 35 | 116.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarape, K.; Adeniyi, A.; Ayodele, T.; Bello, I.A.; Sarker, N.C.; Clementson, C.; Hammed, A. Extraction and Nutritional Value of Soybean Meal Protein Isolate. Nutraceuticals 2024, 4, 503-521. https://doi.org/10.3390/nutraceuticals4040029
Alarape K, Adeniyi A, Ayodele T, Bello IA, Sarker NC, Clementson C, Hammed A. Extraction and Nutritional Value of Soybean Meal Protein Isolate. Nutraceuticals. 2024; 4(4):503-521. https://doi.org/10.3390/nutraceuticals4040029
Chicago/Turabian StyleAlarape, Kudirat, Adewale Adeniyi, Tawakalt Ayodele, Ibrahim Adebayo Bello, Niloy Chandra Sarker, Clairmont Clementson, and Ademola Hammed. 2024. "Extraction and Nutritional Value of Soybean Meal Protein Isolate" Nutraceuticals 4, no. 4: 503-521. https://doi.org/10.3390/nutraceuticals4040029
APA StyleAlarape, K., Adeniyi, A., Ayodele, T., Bello, I. A., Sarker, N. C., Clementson, C., & Hammed, A. (2024). Extraction and Nutritional Value of Soybean Meal Protein Isolate. Nutraceuticals, 4(4), 503-521. https://doi.org/10.3390/nutraceuticals4040029







