Abstract
Angiogenesis, the process of forming new blood vessels from existing ones, is crucial in both physiological and pathological conditions, such as tumor growth, metastasis, and inflammatory disorders. Targeting angiogenesis has emerged as a promising therapeutic strategy. Recent research has increasingly focused on the role of bioactive components found in food in regulating angiogenesis, although there are certain limitations. This review provides a comprehensive examination of the origins, composition, pharmacological activities, and mechanisms of action of these components in medicinal foods, reflecting the growing intersection of medicine and nutrition. The goal is to aid in preventing angiogenesis-related complications and fostering healthier habits. The insights offered in this review aim to advance the development of effective, low-toxicity antiangiogenic drugs. Additionally, there has been a notable rise in interest in plant-derived compounds with antiangiogenic properties. This review investigates the potential of phytoconstituents from plants as drug candidates targeting angiogenesis, exploring their mechanisms of action, the research conducted thus far, and the challenges associated with transitioning these compounds into clinical applications.
1. Introduction
1.1. Angiogenesis Process and Its Role in Disease Progression
Angiogenesis is the natural process of forming new blood vessels from existing ones. It plays vital roles in normal bodily functions like embryonic development, wound healing, and the menstrual cycle. This complex process is tightly regulated by a delicate balance of pro- and antiangiogenic factors, which ensures proper tissue growth and repair under normal circumstances. When this balance is disrupted, pathological angiogenesis can occur, which contributes to the progression of various diseases [1]. However, when angiogenesis goes away, it can worsen various diseases such as cancer, cardiovascular issues, and inflammatory conditions [2].
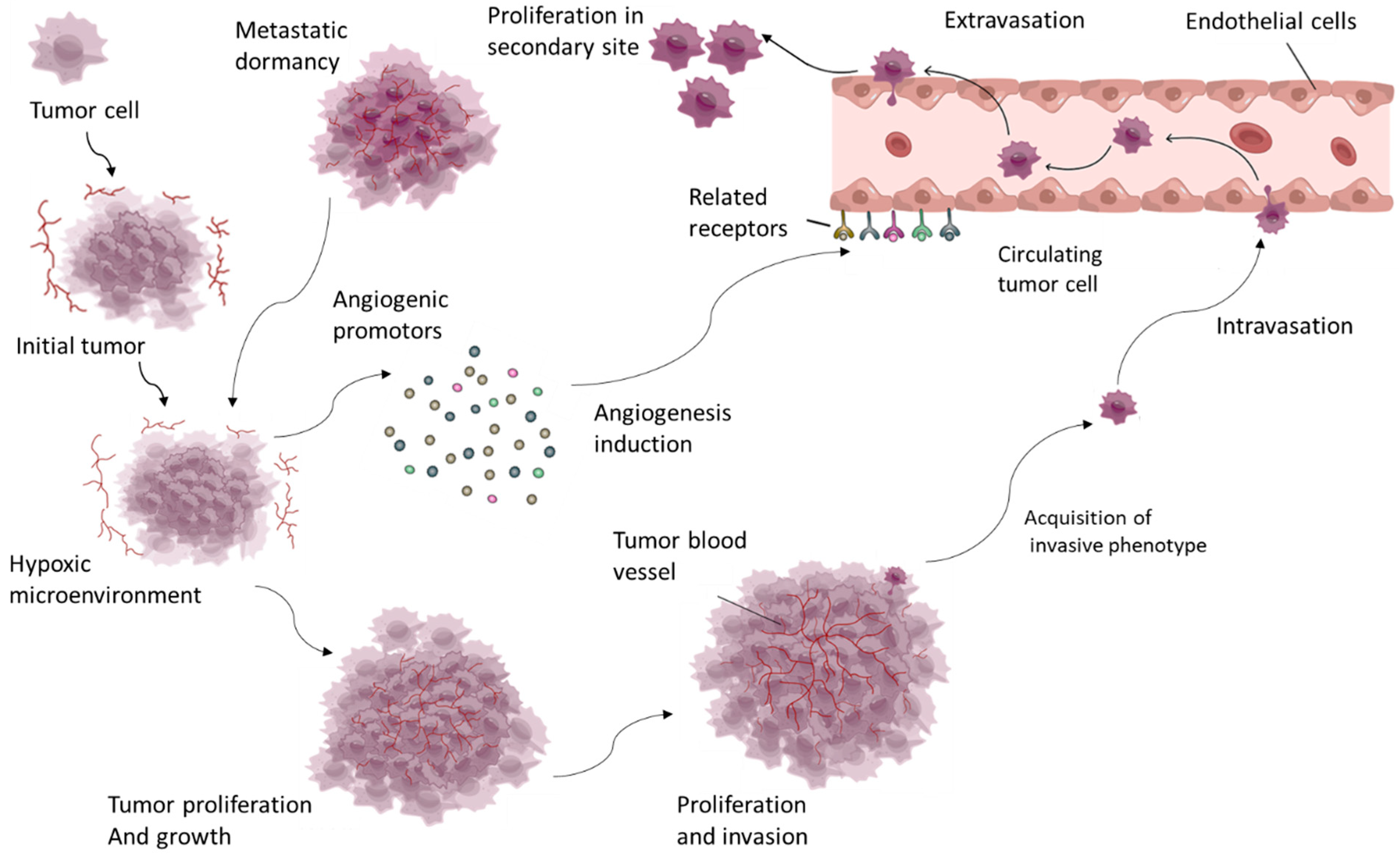
Angiogenesis is especially important in cancer because it provides growing tumors with the oxygen and nutrients they require, allowing tumors to spread and metastasize. Tumor cells can hijack the angiogenic process by secreting pro-angiogenic factors such as vascular endothelial growth factor (VEGF), which stimulates endothelial cell proliferation and migration, leading to the formation of new blood vessels [3] (Figure 1).
Figure 1.
Tumor growth induces a hypoxic microenvironment, which triggers excessive angiogenesis through factors such as VEGF and PDGF. This angiogenesis supports tumor survival and proliferation, enabling continued growth, invasion, and metastasis.
These newly formed vessels are frequently abnormal, with irregular structure and function, which complicates disease progression by allowing cancer cells to enter the bloodstream and spread to distant sites [4]. Angiogenesis also plays an important role in other pathological conditions, such as chronic inflammatory diseases like rheumatoid arthritis, in which excessive blood vessel formation causes inflammation and tissue damage. Similarly, in ocular diseases like age-related macular degeneration, abnormal angiogenesis causes vision loss by forming fragile, leaky blood vessels in the retina [5]. Given its critical role in disease progression, angiogenesis has emerged as a key target in the development of therapeutic strategies. Inhibiting angiogenesis, particularly in cancer, has emerged as a promising strategy, with several antiangiogenic drugs already in clinical trials (Figure 1). These treatments seek to normalize or block the blood supply to tumors, depriving them of the nutrients required for growth and survival [4]. Understanding the molecular mechanisms that drive angiogenesis and its role in disease progression is critical for developing more effective treatments. This ongoing research reveals new targets and strategies, which could lead to innovative therapies for managing and treating angiogenesis-driven diseases [1].
Like normal tissue, malignant tumors require an unremitting supply of nutrients and the removal of waste to sustain their growth. This vital exchange is predominantly accomplished through the phenomenon of tumor neovascularization, a process known as angiogenesis. This intricate process entails the creation of new blood vessels from the pre-existing vascular network. However, malignancies separate from the typically inactive and well-organized course of angiogenesis observed in healthy tissue. Instead, they exploit this process to facilitate unhindered malignant proliferation [6]. In normal circumstances, angiogenesis supports a. embryonic development: creating blood vessels for growing tissues and organs; b. wound healing: providing essential nutrients and immune cells to repair injured areas; and c. menstrual cycle: regulating the development and shedding of the uterine lining [2].
The orchestration of cancer-related angiogenesis is profoundly influenced by various mediators within the tumor microenvironment and is expedited by localized tissue degradation. Among the array of angiogenic mediators, the principal signaling protein implicated in this angiogenic cascade is vascular endothelial growth factor (VEGF). The total amount of VEGFR2 proteins remains similar during angiogenesis, even though they are quickly degraded by VEGF stimulation after signal transduction. The important factor is the localization of VEGFR2 by the trafficking system. This binding event results in the upregulation of genes accountable for facilitating the proliferation and migration of endothelial cells. At the same time, it promotes the survival and permeability of the emerging vasculature [7].
However, targeting angiogenesis can be challenging due to its complexity and essential roles in normal physiology. Achieving a balance between therapeutic benefits and potential side effects is a critical consideration. Overall, understanding angiogenesis’s molecular mechanisms leading to the development of targeted therapies for diseases where abnormal blood vessel formation plays a pivotal role in disease progression.
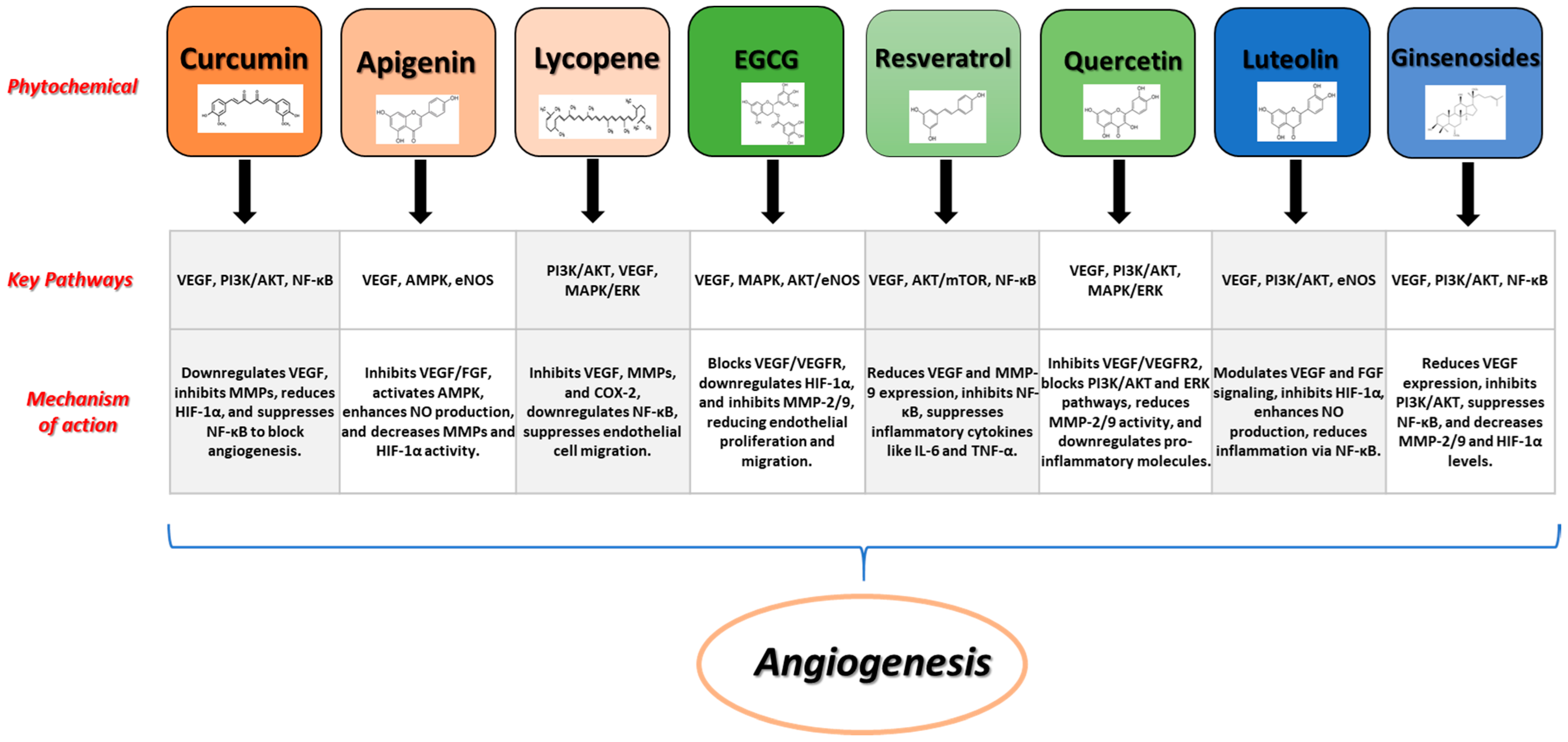
Figure 2 shows some examples of prominent phytochemicals together with their signaling pathways, and processes involved in the angiogenic process.
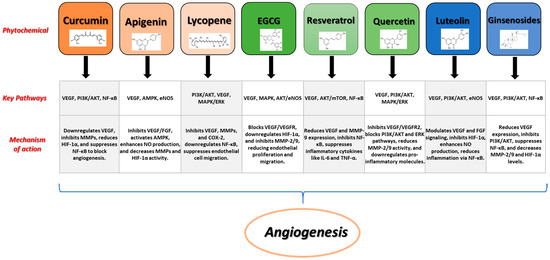
Figure 2.
Examples of prominent phytochemicals, signaling pathways, and mechanisms involved in angiogenesis.
1.2. Current Angiogenesis in Cancer Treatment
In the early 1970s, Dr. Folkman proposed the concept that targeting angiogenesis could have therapeutic potential. The first pharmaceutical agents with these properties were not approved until the early 2000s. Bevacizumab, a monoclonal antibody that binds to circulating VEGF, was the pioneering angiogenesis inhibitor to receive FDA approval [8]. When used alongside standard chemotherapy, it demonstrated improved survival rates for patients with metastatic colorectal cancer. Similarly, patients with advanced non-small cell lung cancer (NSCLC) experienced enhanced survival when bevacizumab was incorporated into their standard chemotherapy regimen. In general, pharmaceutical VEGF inhibitors have shown modest benefits when combined with standard care and have proven to be a valuable addition under specific circumstances [9].
Drawing from data from randomized controlled clinical trials involving 17,396 NSCLC participants, the introduction of approved angiogenesis inhibitors led to significant improvements in progression-free survival (PFS), overall survival (OS), objective response rate (ORR), and disease control rate (DCR) when compared to non-angiogenesis inhibitor treatments. Additionally, approved angiogenesis inhibitors have shown enhanced survival outcomes in patients with ovarian and gastric cancers. However, a meta-analysis of seven randomized controlled trials with 1322 participants exploring the use of angiogenesis inhibitors in small-cell lung cancer (SCLC) indicated that angiogenesis inhibitors did not significantly impact prognosis, highlighting the potential for the clinical impact to vary with different cancer types [10].
While pharmaceutical angiogenesis inhibitors offer clinical benefits, they come with notable side effects. Potential serious adverse events include hemorrhage, hypertension, neutropenia, thromboembolic events, and impaired wound healing. Balancing the positive effects on prognosis with the impact on patients’ quality of life and the burden of side effects is an essential consideration within the evidence-based medicine model and should inform clinical decision-making [11].
2. Exploring Natural Compounds as a Source for Lead Phytoconstituents
Phytochemicals are natural bioactive compounds found in plants, and they are responsible for various physiological effects in both plants and humans. These compounds are not essential nutrients like vitamins and minerals, but they have gained significant attention for their potential health benefits and their role in disease prevention [12]. Phytochemicals come in a diverse range of classes, each with unique properties and potential health-promoting effects.
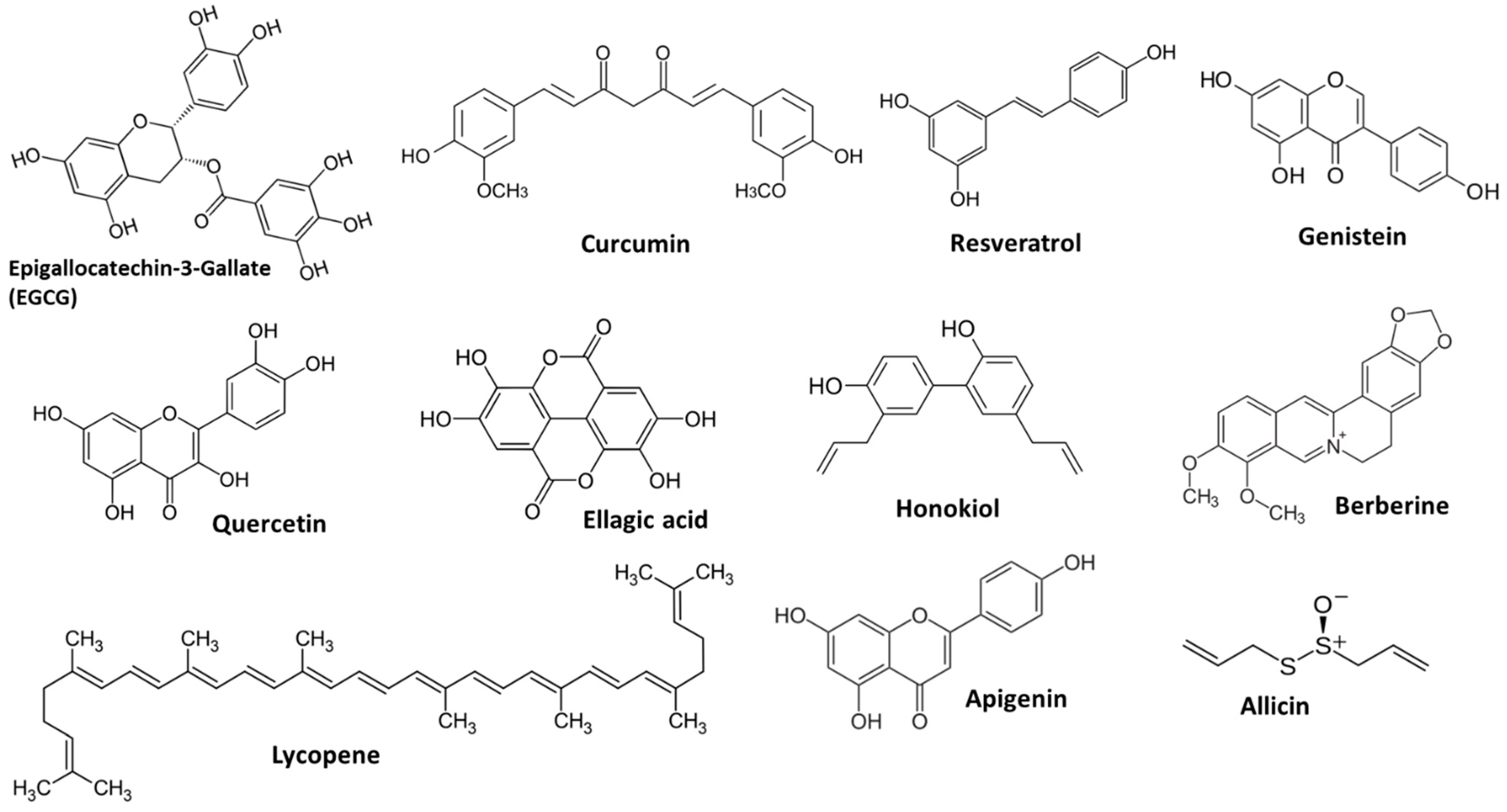
Some naturally occurring compounds exhibit antiangiogenic properties when consumed. Exploring and developing agents derived from phytochemical sources to target a key aspect of cancer progression—angiogenesis—may provide additional options alongside traditional cancer treatments [13]. Careful examination of the existing evidence to identify promising phytochemicals for further study would promote efficient and collaborative efforts, ultimately aiding in the discovery of new therapeutic choices for cancer patients [14]. Figure 3 displays the structure of the main phytochemicals with antiangiogenic properties. In this review, we highlight specific phytochemicals that currently possess robust evidence of clinically significant angiogenesis inhibition.
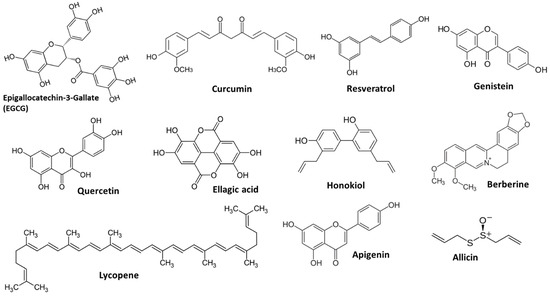
Figure 3.
Examples of main phytochemicals with angiogenic potency.
Investigating natural compounds as primary sources of leading phytoconstituents for angiogenesis control has become a prominent focus in medical and pharmaceutical research. Derived from various natural sources, these bioactive compounds encompass flavonoids, polyphenols, and terpenoids found in herbs, fruits, vegetables, and medicinal plants, offering potential in modulating abnormal blood vessel formation [15]. For instance, resveratrol and curcumin have demonstrated antiangiogenic effects, with the advantage of fewer side effects compared to synthetic drugs. Their multifaceted targeting of angiogenesis pathways and synergy with conventional treatments make them attractive candidates for drug development. These compounds can be integrated into nutraceuticals and functional foods, promoting long-term health benefits. While challenges such as bioavailability and standardization exist, ongoing research in this field holds promise for the development of safer and more effective therapies for angiogenesis-related diseases, including cancer and inflammatory disorders [16].
Prominent phytochemicals supported by evidence for their antiangiogenic effects through various pathways include resveratrol, catechins, curcumin, and ginsenosides, among others. These compounds target different aspects of angiogenesis and can be categorized into five main groups: polyphenols, flavonoids, tannins, glucosinolates, and brassinosteroids. Among these, select compounds have been subjected to more extensive research, positioning them for immediate clinical investigation and thoughtful clinical consideration on a case-by-case basis. For each of the following compounds, the collated preclinical evidence, bioavailability, and clinical trial data offer a comprehensive assessment, providing valuable insights for potential future research [17].
Green tea, derived from the Camellia sinensis plant, has been extensively researched due to its potential health benefits, primarily attributed to its catechin content, notably (-)-epigallocatechin-3-gallate (EGCG). Epidemiological studies have shown a link between green tea consumption and a decreased risk of various cancers and cardiovascular diseases [18,19]. At the cellular level, EGCG has demonstrated the ability to inhibit VEGF expression and activity in several cancer cell lines. Preclinical research has delved into the antiangiogenic properties of green tea catechins, revealing their ability to inhibit cancer cell invasion, neovascularization, and VEGF expression [19]. However, translating these findings to clinical practice faces challenges, primarily related to the limited bioavailability of EGCG in humans, resulting in lower plasma concentrations compared to preclinical models. Approaches to enhance EGCG absorption include fasting before intake, nanostructure carriers, molecular modification, and coadministration with bioactive components. Clinical studies have shown mixed results, with some indicating a reduction in VEGF levels in conditions like prostate cancer and dermatological issues [18]. However, further research is needed to establish the clinical efficacy of green tea catechins as antiangiogenic agents.
2.1. Polyphenolics
Polyphenols are one of the largest and most studied classes of phytochemicals. They are found in a wide range of plant-based foods, i.e., fruits, vegetables, and beverages like tea [20]. Polyphenolic compounds, which encompass flavonoids, phenolic acids, tannins, curcumin, resveratrol, and gallocatechins, are recognized for their anticancer properties. These compounds can interfere with various signaling pathways involved in angiogenesis, including the suppression of vascular endothelial growth factor (VEGF) expression [21]. Resveratrol, present in foods like red grapes and derivatives, peanuts, and certain berries, and gallocatechins found in green tea, are examples of such compounds. Dietary incorporation of polyphenols is believed to enhance health and reduce cancer risk due to their natural antioxidant properties. These compounds have demonstrated cytotoxicity against various cancer cells and exhibit antioxidant characteristics. Their role in apoptosis induction, potentially mediated by regulating the mobilization of copper ions bound to chromatin, leading to DNA fragmentation, is noteworthy. Additionally, polyphenols interfere with proteins within cancer cells, affecting their growth. For example, curcumin has been observed to suppress tumor necrosis factor (TNF) expression in various cancer cell lines by interacting with diverse stimuli [22].
2.2. Flavonoids
Flavonoids, a subgroup of polyphenolic compounds, represent a vast family of plant secondary metabolites with around 10,000 known structures. These compounds have attracted scientific interest for their physiological activity in plants and potential health benefits. Investigations into flavonoid-rich plants and their effects on cancer cells have included various species and traditional Chinese medicinal plants like the litchi leaf. Plants often contain a rich diversity of flavonoid compounds, including anthocyanins, chalcones, flavones, and flavonols, among others. Research has shown that flavonoids possess cytotoxicity against cancer cells and exhibit high free radical scavenging activity [23]. Pure isolated flavonoids have also demonstrated anticancer activities against various human cancers, including hepatoma, cervical carcinoma, and breast cancer. These compounds can induce apoptosis through intrinsic and extrinsic signaling pathways and impact mitochondrial membrane potential, leading to cancer cell death [24].
2.3. Glucosinolates
Glucosinolates are sulfur-containing compounds found in cruciferous vegetables like broccoli, cauliflower, and kale. They have been associated with cancer prevention due to their role in detoxification and antioxidative processes. Ginsenosides, triterpene saponins predominantly found in the roots of red ginseng (Panax ginseng), exhibit antiangiogenic effects. Classified using an “Rx” system, over 30 ginsenosides have been identified [25]. Research in this area emphasizes standardized extraction processes for improved consistency, with particular focus on Rg3 as the most intensively studied form.
Preclinical evidence demonstrates the antiangiogenic potential of Rg3. In various cancer models, including thyroid, gastric, and esophageal cancers, Rg3 inhibits cell migration, invasion, and VEGF expression under hypoxic conditions. Moreover, Rg3 has shown efficacy in a rat endometriosis model by reducing ectopic endometrial growth through downregulation of VEGF, p-Akt, and p-mTOR pathways [26].
Understanding the bioavailability of ginsenosides is complex. While the mechanisms of absorption and metabolism remain subjects of study, research suggests that specific bacterial families are involved in converting ginsenosides into active secondary metabolites. Bioavailability varies between different ginsenoside forms and necessitates further investigation [27].
Clinical research reveals the potential antiangiogenic effects of ginsenosides. Studies on leukemia, gastric cancer, non-small cell lung cancer (NSCLC), and esophageal cancer patients illustrate the benefits of Rg3 supplementation in conjunction with chemotherapy. Notably, Rg3 has been associated with reduced serum VEGF levels in leukemia patients and improved survival outcomes in gastric and esophageal cancer patients. However, the results vary between different cancer types, emphasizing the need for tailored antiangiogenic therapies [28].
2.4. Brassinosteroids
Brassinosteroids are naturally occurring plant compounds with essential roles in regulating cell growth, differentiation, and responses to disease and stress. Two specific BRs, 28-homocastasterone (28-homoCS) and 24-epibrassinolide (24-epiBL), have been studied for their anticancer effects on various cell lines, including T-lymphoblastic leukemia, multiple myeloma, cervical carcinoma, lung carcinoma, osteosarcoma, breast cancer, and prostate cancer [29]. These BRs have demonstrated the ability to induce growth inhibition and apoptosis in cancer cells by interacting with cell cycle processes. They have also shown the capability to bind to key proteins like estrogen receptor (ER), epidermal growth factor receptor (EGFR), human EGFR-2 (HER-2), and androgen receptor (AR), contributing to the inhibition of hormone-sensitive and hormone-insensitive cancer cell growth. BRs can alter the balance of apoptotic and anti-apoptotic proteins, leading to programmed cell death in cancer cells. Importantly, BRs exhibit different responses in normal and cancer cells, with minimal cytotoxicity to normal cells, making them potential candidates for cancer-specific therapeutic applications [30,31].
2.5. Carotenoids
Carotenoids are responsible for the red, orange, and yellow colors in many fruits and vegetables. Carotenoids, which include lycopene, beta-carotene, astaxanthin, and zeaxanthin, are natural pigments with antioxidant qualities that may influence angiogenesis, or the creation of new blood vessels. Angiogenesis is important in many disorders, including cancer and cardiovascular disease. Carotenoids might influence this process by altering critical signaling pathways and lowering oxidative stress, thereby providing therapeutic benefits in disorders linked with aberrant blood vessel formation [32,33]. Lycopene, present in tomatoes and red fruits, has been demonstrated to suppress angiogenesis by downregulating vascular endothelial growth factor (VEGF) and its receptors, reducing endothelial cell proliferation and vessel formation. Lycopene’s antiangiogenic properties have been connected to its capacity to suppress VEGF expression [34].
Similarly, beta-carotene, a precursor to vitamin A, inhibits angiogenesis via antioxidant characteristics that reduce oxidative stress and inflammation. Beta-carotene has been shown to diminish angiogenic activity in a variety of cancer models, indicating its possible function in suppressing aberrant blood vessel creation [35].
Astaxanthin and zeaxanthin also have strong antiangiogenic properties. Astaxanthin, present in certain algae and shellfish, inhibits angiogenesis by targeting oxidative stress and inflammatory pathways [36]. Zeaxanthin, found in green leafy foods, reduces angiogenesis via regulating cell signaling and lowering oxidative damage [37]. These findings imply that carotenoids have substantial potential as natural angiogenesis inhibitors, which could be useful in the prevention and treatment of illnesses characterized by aberrant blood vessel growth [38].
2.6. Anthraquinones as Angiogenesis Inhibitors
Angiogenesis inhibitory potential has been demonstrated by a class of naturally occurring compounds called anthraquinones, which are primarily derived from plants. Angiogenesis, the process by which new blood vessels develop from pre-existing ones, is an essential mechanism in a number of physiological functions, such as the development of embryos and the healing of wounds. Aberrant angiogenesis, however, also serves as a marker for diseases like cancer, where it promotes the growth and metastasis of tumors [39]. Therefore, one therapeutic approach to treating cancer is to inhibit angiogenesis, and anthraquinones have shown promise in this regard. Anthropogenic processes are suppressed by anthraquinones via various means. They have the ability to disrupt the signaling pathways that control the growth, migration, and tube formation of endothelial cells—all essential processes in the angiogenesis process. As an example, certain anthraquinones block the vascular endothelial growth factor (VEGF) pathway, which is an essential factor in tumor angiogenesis [17]. These substances can effectively stop the growth of tumors by preventing the formation of new blood vessels that supply nutrients to them by blocking VEGF or its receptors. Moreover, anthraquinones have been demonstrated to cause endothelial cells to undergo programmed cell death, which adds to their antiangiogenic properties. Because of these characteristics, anthraquinones are desirable candidates for the creation of cutting-edge anticancer treatments that target angiogenesis.
Angiogenesis inhibitors, a class of naturally occurring compounds present in many plants, have shown promise, especially in the treatment of cancer. Inhibiting angiogenesis, the process of creating new blood vessels, is a critical oncology therapeutic approach because it plays a critical role in tumor growth and metastasis. A number of anthraquinones have been found to have the ability to inhibit angiogenesis by a variety of methods, including emodin, aloe-emodin, rhein, and chrysophanol [40]. Plants such as rhubarb and aloe vera contain emodin, which inhibits the VEGF signaling pathway, a necessary pathway for the formation of new blood vessels [41]. Emodin is useful in lung, breast, and liver cancer models because it inhibits the growth and migration of endothelial cells and triggers apoptosis by lowering the expression of VEGF and its receptor VEGFR2. Aloe-emodin, which is also present in aloe vera, targets the VEGF pathway in a similar manner and also affects matrix metalloproteinases (MMPs), which are enzymes that break down the extracellular matrix, which is a process that is essential for angiogenesis. Research on gliomas and melanoma has demonstrated the promise of this compound, indicating that it may be useful in the treatment of highly vascularized tumors. Another rhubarb anthraquinone called rhein inhibits the VEGF pathway, which prevents angiogenesis. It also has anti-inflammatory qualities that further minimize pro-angiogenic factors. Rhein’s effects have been investigated in glioblastoma and colorectal cancer [42].
3. Preclinical Studies and Mechanism of Action
3.1. In Vitro Studies: Cell-Based Assays and Molecular Mechanisms
Various tumor-established cell lines have been used to evaluate the effect of phytochemicals and elucidate their action mechanism (see Table 1).
Several in vitro studies focused on the antiangiogenic effect of curcumin. In [43], the antiproliferative effects of curcumin were examined in human cervical cancer cell models (HeLa). The cell viability was evaluated by MTT assay and cell cycle was evaluated by flow cytometry. The expression levels of Wnt/β-catenin and NF-kB pathways were evaluated to determine the related mechanism of antiproliferative activities of curcumin. It was shown that curcumin efficiently inhibits the proliferation and invasion of human cervical cancer cells through impairing Wnt/β-catenin and NF-kB pathways [44].
Vascular endothelial growth factor (VEGF) is the most important angiogenic factor with proven significance in breast cancer [45]. With the aim of investigating the effect of curcumin on VEGF expression in triple-negative breast cancer using the 4T1 cell line, cytotoxic evaluation was conducted by using the MTT assay, and the expression of VEGF was studied by RT-PCR. It was shown that the VEGF mRNA expression was significantly lowered upon curcumin treatment [46]. In a further study, the curcumin compound was examined for the effects on transforming growth factor β1 (TGF-β1)-induced myofibroblast differentiation and on the pro-angiogenic activities of orbital fibroblasts [47]. For that, the researchers assessed the effects of curcumin on TGF-β1-induced orbital fibroblasts through cell viability assay and tried to detect the expression of myofibroblast differentiation markers (connective tissue growth factor (CTGF) and α-smooth muscle actin (α-SMA)). The trans well migration assay and tube formation assay were carried out to evaluate the pro-angiogenic potential of curcumin-treated orbital fibroblasts. The results showed that curcumin (1–5 ng/mL) was able to suppress TGF-β1-induced myofibroblast differentiation and to attenuate the pro-angiogenic activities of the primary culture of orbital fibroblasts [48].
Tian and co-workers (2021) [48] used human liver cancer cell lines (HepG2, Huh-7 and MHCC-97H) to test the effect of curcumin on the growth of liver cancer. Cell Counting Kit 8 assay was carried out and the results revealed that treatment of HepG2, Huh-7 and MHCC-97H cells with curcumin inhibited their viability.
Resveratrol effect was reported by Qin et al. (2020) [49], in which pancreatic cancer cells (Panc-1, MiaPaCa-2, BxPC-3, CF PAC-1, and SW1990) were assayed through Western blot analysis, immunofluorescence staining, stable lentiviral transfection, spheroid formation, cell invasion, and scratch assays. Nutrient-deprivation autophagy factor 1 (NAF-1), an important genetic locus for regulating oxidative stress and autophagy, was found to be expressed in pancreatic cancer tissue and correlated with the progression of pancreatic cancer. Furthermore, NAF-1 inhibition significantly hindered the stem cell characteristics and the invasion and migration abilities of pancreatic cancer cells.
Epigallocatechin was assayed for its bioactivity against tumor angiogenesis using human umbilical vein endothelial cells [50]. Western blotting analysis, sulforhodamine B assay, cell migration and invasion assays, and tube formation analysis and molecular docking studies were carried out and the results showed that epigallocatechin-3-gallate inhibits tumor angiogenesis through the involvement of endoglin/Smad1 signaling in human umbilical vein endothelium cells. In further research, epigallocatechin gallate (EGCG) was tested on human umbilical vein endothelial cells (HUVECs) to assess its activity on angiogenesis, cell viability, and markers of endothelial dysfunction [51]. It was found that EGCG mediated effects on the production of antiangiogenic factors, cell viability, and endothelial dysfunction through downregulating high-mobility group box 1 (HMGB1). Moreover, to overcome the shortages of EGCG for antiangiogenic antitumor usage, a study developed a novel EGCG derivate Y6 (5,3′,4′,3″,4″,5″-6-0-ethyl-EGCG). Epigallocatechin gallate and its derivate displayed in vitro antiangiogenetic and antitumor effects against hepatocellular carcinoma [52]. The methods adopted in that study were Western blot, immunohistochemistry, and quantitative real-time PCR.
It has been validated that genistein can effectively inhibit the angiogenesis of several tumors. An in vitro study was carried out to investigate the effect of this compound in vascular endothelial growth factor expression and angiogenesis induced by the inflammatory environment of rheumatoid arthritis. In that study, MH7A cells were used to verify the inhibiting effect of genistein on the expression of VEGF in MH7A cells under inflammatory conditions and to demonstrate the mechanism of action. EA.hy926 cells were used to verify the inhibiting effect of genistein on the migration and tube formation of vascular endothelial cells in an inflammatory environment. The research showed that genistein inhibits angiogenesis developed during rheumatoid arthritis through the IL-6/JAK2/STAT3/VEGF signaling pathway [53]. In another study, the human breast cancer cell lines MDA-MB231 and T-47D were used to investigate whether genistein could reduce HIF-1α in breast cancer cells [54]. The findings revealed that that genistein downregulates HIF-1α in breast cancer cells, and molecular docking analysis also revealed that genistein binds to the FIH-1 binding site of HIF-1α protein [55].
Quercetin is a generic flavonoid and has been investigated for its ability to inhibit angiogenesis in different types of cancers. Given that MALAT1 and MIAT lncRNAs are associated with the angiogenesis process and that MALAT1 induces hypoxia-driven angiogenesis via the overexpression of angiogenic genes, [56] studied the antiangiogenic activity of quercetin on human umbilical vein endothelial cells via the expression of MALAT1 and MIAT genes. The results of the study showed that quercetin treatment had an antiangiogenic effect on HUVEC cells, at least partially via the downregulation of MALAT1 and MIAT LncRNA gene expression. In more recent studies, direct angiogenic effects of quercetin were studied in vitro in HUVECs. It was found that quercetin may directly induce angiogenesis and decrease myocardial oxidative stress [57]. In addition, another study examined the action of quercetin on the secretion of angiogenic factors from murine mast cells in vitro and revealed that quercetin significantly inhibited the production of angiogenetic factors induced by IgE-dependent mechanisms at 5.0 µM or more [58], and this result may be implicated in the therapeutic action of quercetin on allergic diseases, especially allergic rhinitis. Moreover, quercetin was investigated for invasion and angiogenesis of esophageal cancer cells. It was found that quercetin suppressed the invasion and angiogenesis of esophageal cancer cells, and the effects were associated with the decreased expression of VEGF-A, MMP2, and MMP9 [59].

Table 1.
Phytoconstituents with antiangiogenesis and anticancer in vivo and in vitro activities together with mechanism of action.
Table 1.
Phytoconstituents with antiangiogenesis and anticancer in vivo and in vitro activities together with mechanism of action.
| Phytochemical | Study | Cell/Animal Model and Tumor Xenograph | Assays | Findings/Mechanisms | Reference |
|---|---|---|---|---|---|
| Curcumin | In vitro | Triple-negative breast cancer cells | - Cell viability assay (MTT) - RT-PCR | Curcumin inhibits VEGF expression in murine triple-negative breast cancer. | [45] |
| Curcumin | In vitro | Human orbital fibroblasts | - Cell viability assay - Western blot - Transwell migration assay - Tube formation assay | Curcumin reduces TGF-β1-induced myofibroblast differentiation and pro-angiogenic activity in orbital fibroblasts. | [46] |
| Curcumin | In vitro | Human liver cancer cell lines | - Cell Counting Kit 8 assay | Curcumin inhibited the viability of Huh-7, MHCC-97H and HepG2 cells. | [47] |
| Curcumin | In vivo | HepG2 xenograft nude mouse model | - Flow cytometry - Western blot - immunohistochemistry and immunofluorescence - ELISA assay | Curcumin reduced the number of MDSCs in mouse xenograft tumors. Curcumin inhibited the TLR4/NF-κB signaling pathway and the expression of inflammatory factors, including IL-6, IL-1β, prostaglandin E2 and cyclooxygenase 2, in mouse xenograft tumors. Downregulation of the expression levels of vascular endothelial growth factor, CD31 and α-smooth muscle actin. | [48] |
| Resveratrol | In vitro | Human Panc-1, MiaPaCa-2, BxPC-3, CF PAC-1, and SW1990 pancreatic cancer cells | - Western blot analysis - Immunofluorescence staining - Spheroid formation assay - Cell invasion assay - Stable lentiviral transfection - Scratch assay | NAF-1 is expressed in pancreatic cancer tissue and correlated with the progression of pancreatic cancer. NAF-1 inhibition significantly inhibits the stem cell characteristics and the invasion and migration abilities of pancreatic cancer cells. | [49] |
| Resveratrol | In vivo | Nude mice | - Tumor volume - H&E staining | Resveratrol inhibited the expression of NAF-1, thereby inhibiting tumor growth. | [49] |
| Epigallocatechin gallate | In vitro | Human umbilical vein endothelial cells (JEG-3 cell line and HUVECs) | - Cell proliferation assay - ELISA - Western blot assay - Real-time quantitative PCR | EGCG-mediated effects on the production of antiangiogenic factors, cell viability, and endothelial dysfunction through downregulating HMGB1. | [51] |
| Epigallocatechin gallate and its derivate (5,3′,4′,3″,4″,5″-6-0-ethyl-EGCG) | In vitro | Hepatocellular carcinoma (HCC) cells | - Western blot - Immunohistochemistry - Quantitative real-time PCR | EGCG and EGCG derivate Y6 (5,3′,4′,3″,4″,5″-6-0-ethyl-EGCG) displayed antiangiogenetic and antitumor effects against HCC cells. | [52] |
| Epigallocatechin gallate and its derivate (5,3′,4′,3″,4″,5″-6-0-ethyl-EGCG) | In vivo | HepG2 xenograft model | HepG2 xenograft model and chorioallantoic membrane (CAM) | EGCG and EGCG derivate Y6 (5,3′,4′,3″,4″,5″-6-0-ethyl-EGCG) displayed antiangiogenetic and antitumor effects against HCC cells. | [60] |
| Epigallocatechin-3-gallate | In vitro | Human umbilical vein endothelial cells (HUVECs; H-UV001) | - Western blot analysis - Sulforhodamine B assay - Cell migration and invasion assays - Tube formation analysis - Molecular docking studies | Epigallocatechin-3-gallate inhibits tumor angiogenesis: involvement of endoglin/Smad1 signaling in human umbilical vein endothelium cells. | [50] |
| Epigallocatechin-3-gallate | In vivo | BALB/C-nu/nu nude mice KBV200 xenograft model | - Chick embryo chorioallantoic membrane assay - Immunohistochemistry, - ELISA - RT-PCR analysis | Epigallocatechin-3-gallate sensitizes multidrug-resistant oral carcinoma xenografts to vincristine sulfate, and this may occur through the inhibition of angiogenesis via VEGF downregulation. | [51] |
| Genistein | In vitro | - MH7A cells - EA.hy926 cells | - Cytotoxicity tests - RNA extraction, reverse transcription, and real time quantitative polymerase chain reaction - Enzyme-linked immunosorbent assay - Western blot - Transwell assay - Wound healing assay - Confocal laser scanning fluorescence microscopy | Genistein inhibits angiogenesis developed during rheumatoid arthritis through the IL-6/JAK2/STAT3/VEGF signaling pathway. | [60] |
| Genistein | In vitro | Human breast cancer cell lines MDA-MB231 and T-47D | - Docking simulation - Western blot | - Genistein downregulates HIF-1α in breast cancer. - Genistein binds to the FIH-1 binding site of HIF-1α protein. | [54] |
| Quercetin | In vitro | Mast cells (1 × 105 cells/mL) | ELISA | Quercetin significantly inhibited the production of angiogenetic factors induced by IgE-dependent mechanisms at 5.0 µM or more. | [56] |
| Quercetin | In vivo | BALB/c male mice | - Assay for nasal symptoms - ELISA | Oral administration of 25.0 mg/kg quercetin into the mice suppressed the appearance of angiogenetic factors in nasal lavage fluids, along with the attenuation of nasal symptoms. | [56] |
| Quercetin | In vitro | Human esophageal cancer cell line Eca109 | - Colony formation assay - wound healing assay - Transwell assays - Tube formation assay - Western blot | Quercetin suppressed the invasion and angiogenesis of esophageal cancer cells, and the effects were associated with the decreased expression of VEGF-A, MMP2, and MMP9. | [57] |
| Quercetin | In vitro | HUVECs | - MTT assay - real-time PCR | Quercetin treatment had an antiangiogenic effect on HUVEC cells, at least partially via the down regulation of MALAT1 and MIAT LncRNA gene expression. | [55] |
| Quercetin | In vitro | HUVECs | Formation of tube-like structures | - Quercetin may directly induce angiogenesis and decrease myocardial oxidative stress | [56] |
| Quercetin | In vivo | Male C57BL/6J mice | Cardiac function | - Quercetin normalized heart weight and triglycerides. - Quercetin prevented cardiac fat accumulation and reduced HFD-induced cardiac fibrosis, cardiomyocyte hypertrophy, oxidative stress, and vascular rarefaction. | [56] |
| Honokiol | In vivo | Male Balb/c nude mice | Immunohistochemical staining | - Honokiol decreased the vascular endothelial growth factor. expression, tumor angiogenesis, and tumor development. | [58] |
| Honokiol | In vitro | Human lung cancer cell lines A549 and H460 | - Cell viability assay - Real-time PCR - Western blot analysis - Enzyme-linked immunosorbent assay - Immunofluorescence staining - Wound healing assay - Capillary tube formation analyses - Cell invasion assay | - Honokiol decreased the A549 and H460 cell viability. - Honokiol reduced the production of vascular endothelial growth factor. - Honokiol blocked the NF-κB signaling pathway. | [58] |
| Honokiol | In vitro | Human breast cancer cell lines MCF7 (hormone-dependent), MDA-MB-231, and SKBR3 (hormone-independent) | - MTT test - Immunoblotting analysis | - Honokiol demonstrated significant antiproliferative activity against both hormone-dependent breast cancer cells and lines with primary and acquired hormone resistance. - The accumulation of cleaved PARP and a decrease in the expression of Bcl-2 and ERα in MCF7/HT were induced following the combination of honokiol with metformin. | [59] |
| Berberine | In vitro | Human umbilical vein endothelial cells (HUVEC) | - Cell Counting Kit 8 (CCK-8) assays - Western blot | Berberine decreased HUVEC proliferation in a dose-dependent manner and inhibited the expression ofphospho-ERK1/2 in HUVECs. | [61] |
| Berberine | In vivo | C57BL/6J mice | - Biochemical analysis - Immunohistochemistry analysis - Histological analysis - Reverse transcription and quantitative real-time PCR - Western blot | - Berberine reduced the incidence of tumors. - Berberine reduced the levels of alanine aminotransferase (ALT), aspartate amino- transferase (AST), glucose (GLU), high-density lipoprotein (HDL), low-density lipoprotein (LDL) and total cholesterol (TC). - Berberine suppressed the expressions of genes related to lipogenesis, inflammation, fibrosis and angiogenesis. - Berberine suppressed phosphorylation of p38MAPK and ERK as well as COX2 expression significantly. | [62] |
Honokiol has received attention regarding its ability to inhibit tumor reproduction, induce tumor cell apoptosis, and control tumor migration and invasion [58]. It exerts an inhibitory effect on tumor angiogenesis [59]. In recent research, hypothesizing that HNK could inhibit angiogenesis induced by inflammatory factors such as IL-1 in the lung cancer microenvironment, a study evaluated the antiangiogenic effect of honokiol using the human lung cancer cell lines A549 and H460 and determined the mechanism by which honokiol inhibited tumor angiogenesis induced by cytokines secreted from the microenvironment [49]. In other study, honokiol inhibited the growth of hormone-resistant human breast cancer cell lines, where its IC50 ranged from 12 to 20 μM. The accumulation of cleaved PARP and a decrease in the expression of Bcl-2 and ERα in MCF7/HT were induced following the combination of honokiol with metformin [59].
The antitumor activity of berberine on various cancers was reviewed recently. A study by Wen and collaborators (2023) [61] was carried out on human umbilical vein endothelial cells. Using methylthiazolyldiphenyl-tetrazolium bromide and CCK-8 assays to assess HUVEC proliferation, the results showed that berberine decreased HUVEC proliferation in a dose-dependent manner. Moreover, Western blot analysis revealed that berberine inhibited the expression of phospho-ERK1/2 in HUVECs. PD98059, a specific inhibitor of ERK1/2 signaling, and attenuated the BBR-induced decrease in the proliferation of HUVECs [63].
3.2. In Vivo Studies: Animal Models and Tumor Xenografts
Various in vivo studies have been reported to evaluate the effect of phytochemicals and elucidate their action mechanism (see Table 1).
The evaluation of curcumin’s effect on xenograft experiments on BALB/c-nu nude mice revealed that curcumin reduced the number of MDSCs in mouse xenograft tumors as determined by flow cytometry analysis [64]. In that study, the compound inhibited the TLR4/NF-κB signaling pathway and the expression of inflammatory factors, including IL-6, IL-1β, prostaglandin E2 and cyclooxygenase 2, in mouse xenograft tumors. Furthermore, curcumin suppressed the secretion of granulocyte–macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF), which are essential factors for MDSC modulation in tumor tissues. Western blotting, immunohistochemistry and immunofluorescence experiments demonstrated that curcumin induced downregulation of the expression levels of vascular endothelial growth factor, CD31, and α-smooth muscle actin.
An in vivo study on nude mice was carried out to study the effect of resveratrol on pancreatic cancer. The results revealed that resveratrol inhibited the expression of NAF-1, thereby inhibiting tumor growth [49].
EGCG and the EGCG derivate Y6 (5,3′,4′,3″,4″,5″-6-0-ethyl-EGCG) displayed antiangiogenetic and antitumor effects against HCC cells through a HepG2 xenograft model and the chorioallantoic membrane [52]. Another study investigated the effect of EGCG combined with vincristine sulfate on the growth, angiogenic activity and VEGF expression using a BALB/C-nu/nu nude mice KBV200 xenograft model [65]. EGCG and vincristine sulfate strongly inhibited tumor growth and angiogenesis and lowered the protein levels in the KBV200 xenograft group treated with the combined EGCG and vincristine sulfate compared to those in the group treated with vincristine sulfate alone.
BALB/c male mice were used to evaluate the effect of quercetin on angiogenic factor secretion in a murine allergic rhinitis model. After ELISA and assaying for nasal symptoms, it was found that oral administration of 25.0 mg/kg quercetin to the mice suppressed the appearance of angiogenetic factors in nasal lavage fluids, along with the attenuation of nasal symptoms [62]. A further study on male C57BL/6J mice showed that quercetin normalized heart weight and triglycerides, prevented cardiac fat accumulation, and reduced HFD-induced cardiac fibrosis, cardiomyocyte hypertrophy, oxidative stress, and vascular rarefaction [21].
A549 cells in a xenograft mouse model were selected to examine honokiol’s effect on human lung cancer in vivo. The findings revealed that honokiol decreased vascular endothelial growth factor expression, tumor angiogenesis, and tumor development [66].
Berberine was shown to prevent non-alcoholic steatohepatitis-derived hepatocellular carcinoma. Using C57BL/6J mice, a study showed that berberine reduced the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), glucose (GLU), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and total cholesterol (TC). Moreover, it suppressed the expression of genes related to lipogenesis, inflammation, fibrosis, and angiogenesis, phosphorylation of p38MAPK and ERK, and COX2 expression significantly [67].
3.3. Synergistic Effects and Combination Therapies
Several reports examined the synergistic effect of phytochemicals as antiangiogenesis agents when used with antineoplastic agents. The combination of anti-FGF and anti-VEGF approaches exhibited synergistic effects [68]. A recent report reviewed the synergistic effects and mechanisms of action of combined phytochemicals on colon cancer [69]. In a recent study, the synergistic effects of curcumin with phytochemicals such as resveratrol, quercetin, epigallocatechin-3-gallate, and piperine improved its clinical efficacy in cancer treatment [69]. In a study evaluating and comparing the chemopreventive effect of both green tea and curcumin (each individually and in combination) through induction of hamster buccal pouch carcinoma, it was reported that combining green tea (epigallocatechin gallate the most abundant component) and curcumin had a significant chemopreventive effect against oral carcinogenesis [70]. In a study on colorectal cancer, it was observed that ginkgetin and resveratrol suppressed the angiogenic properties of VEGF. The application of both compounds together exerted an antitumor effect of 5-fluorouracil with decreasing micro-vessel density of tumors [71]. Calcitriol (a multitarget anticancer hormone) with curcumin or resveratrol inhibited angiogenesis in triple-negative breast cancer xenografts. Not only individual compounds but also plant extracts were assayed to examine their synergistic effect on angiogenesis. Using a chick chorioallantoic membrane assay, researchers demonstrated the synergistic effects of unripe sumac fruit extracts combined with tannic acid on angiogenesis, and the results showed that at a dose of 5 mg/mL each, a very strong antiangiogenic effect was observed [72].
4. Challenges and Future Directions
4.1. Bioavailability and Pharmacokinetic Considerations
In the realm of angiogenic compounds, their effectiveness hinges significantly on bioavailability and pharmacokinetics. Bioavailability pertains to how a compound is absorbed and reaches its designated body site, influenced by factors like solubility and membrane permeability.
Angiogenic agents are promising treatments for conditions such as ischemic heart disease, wound healing, and some cancers because they promote the formation of new blood vessels. However, issues with pharmacokinetics and bioavailability frequently restrict their therapeutic efficacy. Concerns regarding bioavailability are brought on by things like inadequate absorption in the gastrointestinal tract, breakdown by digestive enzymes, and the consequences of liver first-pass metabolism [73] The amount of active agent that enters the systemic circulation depends critically on the mode of administration—oral, intravenous, or otherwise. Innovative formulation methods like hydrogels and nanoparticles are being investigated to improve these agents’ solubility, stability, and targeted delivery, which will increase their bioavailability.
On the other hand, pharmacokinetics involves comprehending the compound’s journey through processes. The efficacy and safety profiles of angiogenic agents are influenced by their absorption, distribution, metabolism, and excretion (ADME), which is a factor in pharmacokinetic considerations. Absorption may be restricted by poor membrane permeability and quick degradation, and distribution issues may include the necessity of precisely targeting particular tissues. These agents’ therapeutic effect can be affected by the activation or inactivation of metabolic pathways, especially in the liver. Furthermore, the length of action is determined by excretion rates, where slow excretion may result in prolonged effects. To fully utilize angiogenic agents in clinical applications, it is imperative to address these pharmacokinetic challenges with tailored delivery systems, optimized dosing regimens, and meticulous safety assessments [74].
To fully harness the potential of angiogenic agents, several pivotal considerations must be considered [75]:
- -
- Delivery Method: Selecting the appropriate administration method (e.g., oral or intravenous) to ensure compound stability and efficient delivery to target tissues.
- -
- Metabolic Stability: Evaluating the compound’s breakdown in the body and understanding any impact of metabolites on its angiogenic properties.
- -
- Duration of Action: Determining the compound’s duration of effectiveness to establish dosing frequency for antiangiogenic effects.
- -
- Interactions: Assessing interactions with medications that could affect both bioavailability and pharmacokinetics.
- -
- Tissue Penetration: Confirming the compound’s ability to reach sites where angiogenesis occurs.
A comprehensive grasp of these aspects is vital for the development of angiogenic therapies, enabling optimal dosing and ensuring that compounds effectively reach their intended targets for the desired antiangiogenic actions [76].
4.2. Safety and Toxicity Evaluations
It is crucial to evaluate the safety and toxicity of angiogenic agents in drug development to ensure the well-being of patients. Safety assessments help identify any side effects, while toxicity evaluations determine harm at various doses and exposure durations [7]. These assessments consider how factors such as toxicity affect the relationship between dose and response-specific organ toxicity, genotoxicity, reproductive impact, drug interactions, immune responses, effects on special populations, monitoring of adverse events, and establishing a range of therapeutic dosages. The ultimate goal of these evaluations is to define dosages for secure use of antiangiogenic treatments [77].
4.3. Standardization and Quality Control of Phytoconstituents
Standardization and quality control are indispensable in the development and production of phytoconstituents for antiangiogenic agents, guaranteeing their safety, effectiveness, and uniformity [78]. Key elements encompass the identification of active compounds, establishment of reference standards, ensuring the quality of source materials, adherence to Good Agricultural and Collection Practices (GACP), Good Manufacturing Practices (GMP), rigorous testing for contaminants, use of advanced analytical techniques, conducting stability studies, method validation, regulatory compliance, maintaining batch consistency, and diligent record-keeping. These practices are vital for pharmaceutical and clinical use, assuring that patients receive the intended therapeutic benefits [79].
4.4. Translational Potential and Clinical Trials
Natural compounds modulate many molecular mechanisms and cellular phenomena, and such results may provide scientific guidance for the clinical translational research of phytochemicals in the treatment of diseases.
In a triple-blind randomized clinical trial, resveratrol was evaluated for its effect on the angiogenesis pathway. The results showed a reduction in the expression of VEGF and HIF1 genes under the effect of resveratrol in granulosa cells and it was demonstrated that it may improve some outcomes of polycystic ovary syndrome patients, probably through changing the serum levels of some sex hormones and expression of VEGF and HIF1 genes in the angiogenesis pathway of granulosa cells [80]. In another study, it was reported that Curcuminoids could be coadjuvants that might help fight breast cancer when consumed regularly [81]. The effect of resveratrol on vascular endothelial growth factor and tumor necrosis factor alpha (TNF-α) expression in the eutopic endometrium of infertile patients with endometriosis was assessed by a randomized trial of a total of 34 patients [82]. The study showed that resveratrol treatment reduced gene and protein expression levels of VEGF and TNF-α.
4.5. Formulation Development and Delivery Systems
Keeping in mind that naturally based pharmacotherapy may be a proper alternative for treating different diseases, developing innovative pharmaceutical formulations and delivery systems would offer effective and reliable delivery of medicinal phytochemicals and answer the issue of suitable delivery systems that can transport sufficient doses of bioactive compounds to the desired target [81]. To overcome its poor aqueous solubility that restricts its clinical application, encapsulated lipid polymer hybrid nanoparticles of piperine, known to have excellent therapeutic efficacy against a variety of ailments including breast cancer, have been developed. Nanoparticles are drug delivery systems that can increase the bioavailability of hydrophobic drugs and improve drug targeting to cancer cells via different mechanisms and formulation techniques [82]. Curcumin nanoparticles have been evaluated for their potential use in treating cancers and formulations, including lipid, gold, zinc oxide, magnetic, polymeric, and silica nanoparticles, as well as micelles, dendrimers, nanogels, cyclodextrin complexes, and liposomes. These were recently reviewed by [82]. Resveratrol-loaded transfersomes administered via transpapillary iontophoresis is a promising strategy enabling localized delivery for effective breast cancer therapy [83].
5. Concluding Remarks
Lead phytoconstituents derived from plants hold great promise as potential candidates for antiangiogenesis drug development. Their diverse mechanisms of action, preclinical evidence, and relatively favorable safety profiles make them attractive targets for further investigation. However, several challenges, including bioavailability, standardization, and translational potential, need to be addressed to harness their full therapeutic potential. Continued research efforts and collaboration among scientists, pharmaceutical companies, and regulatory bodies are essential to advance these lead phytoconstituents from the laboratory to the clinic, providing novel treatment options for angiogenesis-related diseases.
In conclusion, green tea catechins, especially EGCG, curcumin, resveratrol, and ginsenosides, stand out as promising phytochemicals in the field of antiangiogenesis. These compounds have undergone extensive preclinical research, bioavailability assessments, and preliminary clinical investigations, making them strong candidates for further, in-depth clinical exploration. However, it is essential to recognize that preclinical data may not reliably predict outcomes in patients, particularly in vulnerable populations like cancer patients, where unanticipated side effects can occur. While the reviewed information provides a strong rationale for future clinical trials, caution is needed when applying these data directly in clinical practice.
For future clinical trials focused on combating malignant angiogenesis in cancer patients through phytochemical supplementation, it is advisable to select an agent with both human and preclinical data showing effects on VEGF. Additionally, choosing a highly bioavailable form of the selected agent is crucial. Prioritizing the investigation of a few well-supported phytochemicals, rather than numerous agents lacking foundational research, can lead to more efficient trials and hopefully uncover significant associations.
Author Contributions
Conceptualization, I.M.A.-R. and A.T.; methodology, I.M.A.-R. and A.T.; software, I.M.A.-R. and A.T.; validation, I.M.A.-R. and A.T.; formal analysis, I.M.A.-R.; resources, I.M.A.-R. and A.T.; writing—original draft preparation, I.M.A.-R. and A.T.; writing—review and editing, I.M.A.-R. and A.T.; visualization, I.M.A.-R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| MTT | 3-(4,5-dimethyl- thiazol-2-yl) -2,5-diphenyl-tetrazolium bromide |
| RT-PCR | reverse-transcription polymerase chain reaction |
| TGF-β1 | transforming growth factor β1 |
| VEGF | vascular endothelial growth factor |
| CTGF | connective tissue growth factor |
| α-SMA | α-smooth muscle actin |
| EC | endothelial cell |
| MDSCs | myeloid-derived suppressor cells |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| G-CSF | granulocyte colony-stimulating factor |
| ABC | ATP-binding cassette |
| HFD | high-fat diet |
| MIAT | myocardial infarction-associated transcript |
| MALAT1 | metastasis-associated lung adenocarcinoma transcript 1 |
| MMP | matrix metalloproteinase |
| VEGF | vascular endothelial growth factor |
References
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aspriţoiu, V.M.; Stoica, I.; Bleotu, C.; Diaconu, C.C. Epigenetic Regulation of Angiogenesis in Development and Tumors Progression: Potential Implications for Cancer Treatment. Front. Cell Dev. Biol. 2021, 9, 689962. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Cao, Y.; Langer, R.; Ferrara, N. Targeting angiogenesis in oncology, ophthalmology and beyond. Nat. Rev. Drug Discov. 2023, 22, 476–495. [Google Scholar] [CrossRef]
- Zeineddine, F.A.; Zeineddine, M.A.; Yousef, A.; Gu, Y.; Chowdhury, S.; Dasari, A.; Huey, R.W.; Johnson, B.; Kee, B.; Lee, M.S.; et al. Survival improvement for patients with metastatic colorectal cancer over twenty years. npj Precis. Oncol. 2023, 7, 16. [Google Scholar] [CrossRef]
- Hong, S.; Tan, M.; Wang, S.; Luo, S.; Chen, Y.; Zhang, L. Efficacy and safety of angiogenesis inhibitors in advanced non-small cell lung cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2015, 141, 909–921. [Google Scholar] [CrossRef]
- El-Kenawi, A.E.; El-Remessy, A.B. Angiogenesis inhibitors in cancer therapy: Mechanistic perspective on classification and treatment rationales. Br. J. Pharmacol. 2013, 170, 712–729. [Google Scholar] [CrossRef]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marrero, A.D.; Quesada, A.R.; Martínez-Poveda, B.; Medina, M.A. Antiangiogenic Phytochemicals Constituent of Diet as Promising Candidates for Chemoprevention of Cancer. Antioxidants 2022, 11, 302. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Gong, X.; Wang, X.; Li, M. Role of Active Components of Medicinal Food in the Regulation of Angiogenesis. Front. Pharmacol. 2021, 11, 594050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, X.; Friedrich, L.J.; Efferth, T. Natural products targeting tumour angiogenesis. Br. J. Pharmacol. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Bhat, M.; Basu, S. Plants and their active compounds: Natural molecules to target angiogenesis. Angiogenesis 2016, 19, 287–295. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Parveen, A.; Subedi, L.; Kim, H.W.; Khan, Z.; Zahra, Z.; Farooqi, M.Q.; Kim, S.Y. Phytochemicals Targeting VEGF and VEGF-Related Multifactors as Anticancer Therapy. J. Clin. Med. 2019, 8, 350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdal Dayem, A.; Choi, H.Y.; Yang, G.M.; Kim, K.; Saha, S.K.; Cho, S.G. The Anti-Cancer Effect of Polyphenols against Breast Cancer and Cancer Stem Cells: Molecular Mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abu-Reidah, I.M.; Taamalli, A. Special Issue on “Phenolic Compounds: Extraction, Optimization, Identification and Applications in Food Industry”. Processes 2022, 10, 128. [Google Scholar] [CrossRef]
- Wen, L.; Wu, D.; Jiang, Y.; Prasad, K.N.; Lin, S.; Jiang, G.; He, J.; Zhao, M.; Luo, W.; Yang, B. Identification of flavonoids in litchi (Litchi chinensis Soon.) leaf and evaluation of anticancer activities. J. Funct. Foods 2014, 6, 555–563. [Google Scholar] [CrossRef]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakhjavani, M.; Smith, E.; Yeo, K.; Palethorpe, H.M.; Tomita, Y.; Price, T.J.; Townsend, A.R.; Hardingham, J.E. Anti-Angiogenic Properties of Ginsenoside Rg3 Epimers: In Vitro Assessment of Single and Combination Treatments. Cancers 2021, 13, 2223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, R.-Y.; Fang, Z.-R.; Zhang, H.-P.; Xu, J.-Y.; Zhu, J.-Y.; Chen, K.-Y.; Wang, W.; Jiang, X.; Wang, X.-J. Ginsenosides: Changing the basic hallmarks of cancer cells to achieve the purpose of treating breast cancer. Chin. Med. 2023, 18, 125. [Google Scholar] [CrossRef]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef]
- Steigerová, J.; Oklešťková, J.; Levková, M.; Rárová, L.; Kolář, Z.; Strnad, M. Brassinosteroids cause cell cycle arrest and apoptosis of human breast cancer cells. Chem. Biol. Interact. 2010, 188, 487–496. [Google Scholar] [CrossRef]
- Oklešťková, J.; Rárová, L.; Strnad, M. Brassinosteroids as Multifunctional Players in Plants and Human Health. Int. J. Mol. Sci. 2023, 24, 8097. [Google Scholar] [CrossRef]
- Genovese, M.; Buccirossi, M.; Guidone, D.; De Cegli, R.; Sarnataro, S.; di Bernardo, D.; Galietta, L.J.V. Exploring the pharmacological potential of brassinosteroids for the treatment of breast cancer: A preclinical study. Br. J. Pharmacol. 2023, 180, 518–537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Carotenoids and their role in cancer prevention. Cancer Res. 2012, 72, 4017–4025. [Google Scholar]
- Chen, P.; Xie, J.; Liu, X.; Tan, M. Lycopene inhibits VEGF-induced angiogenesis through modulating VEGF and its receptor expression. Oncotarget 2015, 6, 18885–18898. [Google Scholar]
- Gao, X.; Zhao, Z.; Zhang, Y.; Chen, Y. Astaxanthin inhibits angiogenesis by suppressing oxidative stress and inflammation in cancer cells. Mol. Carcinog. 2016, 55, 1315–1325. [Google Scholar]
- Gorinstein, S.; Park, Y.S.; Heo, B.G.; Kim, D. Zeaxanthin inhibits angiogenesis in vitro and in vivo. J. Agric. Food Chem. 2012, 60, 5782–5788. [Google Scholar]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Fong, L.Y.; Tran, L.A. The anti-angiogenic effects of carotenoids: A review. Nutr. Cancer 2015, 67, 672–682. [Google Scholar]
- Munir, S.; Shah, A.A.; Shahid, M.; Shahid, A.; Rajoka, M.S.R.; Akash, M.S.H.; Khurshid, M. Anti-angiogenesis potential of phytochemicals for the therapeutic management of tumors. Curr. Pharm. Des. 2020, 26, 265–278. [Google Scholar] [CrossRef]
- Zargar, B.A.; Masoodi, M.H.; Ahmed, B.; Ganie, S.A. Phytoconstituents and therapeutic uses of Rheum emodi wall. ex Meissn. Food Chem. 2011, 128, 585–589. [Google Scholar] [CrossRef]
- Cao, Y.-J.; Pu, Z.-J.; Tang, Y.-P.; Shen, J.; Chen, Y.-Y.; Kang, A.; Zhou, G.-S.; Duan, J.-A. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chin. Med. 2017, 12, 36. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.W.; Sun, X.; Qian, D.; Tang, D.D.; Zhang, L.L.; Li, M.Y.; Wang, L.Y.; Wu, C.-J.; Peng, W. The versatile emodin: A natural easily acquired anthraquinone possesses promising anticancer properties against a variety of cancers. Int. J. Biol. Sci. 2022, 18, 3498–3527. [Google Scholar] [CrossRef] [PubMed]
- Nocito, M.C.; De Luca, A.; Prestia, F.; Avena, P.; La Padula, D.; Zavaglia, L.; Sirianni, R.; Casaburi, I.; Puoci, F.; Chimento, A.; et al. Antitumoral Activities of Curcumin and Recent Advances to Improve Its Oral Bioavailability. Biomedicines 2021, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Moghadam, S.A.; Aref Nezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/β-catenin path-ways in cervical cancer cells. Pathol.-Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef] [PubMed]
- Verheul, H.M.; Pinedo, H.M. The Role of Vascular Endothelial Growth Factor (VEGF) in Tumor Angiogenesis and Early Clinical Development of VEGFReceptor Kinase Inhibitors. Clin. Breast Cancer 2000, 1, S80–S84. [Google Scholar] [CrossRef] [PubMed]
- Murwanti, R.; Rahmadani, A.; Hermawan, A.; Sudarmanto, B.S.A. Curcumin inhibits vascular endothelial growth factor (VEGF) expression on a murine triple-negative breast cancer. AIP Conf. Proc. 2020, 2260, 060020. [Google Scholar] [CrossRef]
- Yu, W.K.; Hwang, W.L.; Wang, Y.C.; Tsai, C.C.; Wei, Y.H. Curcumin Suppresses TGF-β1-Induced Myofibroblast Differentiation and Attenuates Angiogenic Activity of Orbital Fibroblasts. Int. J. Mol. Sci. 2021, 22, 6829. [Google Scholar] [CrossRef]
- Tian, S.; Liao, L.; Zhou, Q.; Huang, X.; Zheng, P.; Guo, Y.; Deng, T.; Tian, X. Curcumin inhibits the growth of liver cancer by impairing myeloid derived suppressor cells in murine tumor tissues. Oncol. Lett. 2021, 21, 286. [Google Scholar] [CrossRef]
- Qin, T.; Cheng, L.; Xiao, Y.; Qian, W.; Li, J.; Wu, Z.; Wang, Z.; Xu, Q.; Duan, W.; Wong, L.; et al. NAF-1 Inhibition by Resveratrol Suppresses Cancer Stem Cell-Like Proper-ties and the Invasion of Pancreatic Cancer. Front. Oncol. 2020, 10, 1038. [Google Scholar] [CrossRef]
- Sheu, M.J.; Chen, C.Y. Epigallocatechin-3-gallate inhibits tumor angiogenesis via endoglin/Smad1 pathway in human umbilical vein endothelium cells. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lin, Y.-J.; Wang, C.C.; Lan, Y.-H.; Lan, S.-J.; Sheu, M.-J. Epigallocatechin-3-gallate inhibits tumor angiogenesis: Involvement of endoglin/Smad1 signaling in human umbilical vein endothelium cells. Biomed. Pharmacother. 2019, 120, 109491. [Google Scholar] [CrossRef]
- Zhong, M.; Peng, J.; Xiang, L.; Yang, X.; Wang, X.; Zhu, Y. Epigallocatechin Gallate (EGCG) Improves Anti-Angiogenic State, Cell Viability, and Hypoxia-Induced Endothelial Dysfunction by Downregulating High Mobility Group Box 1 (HMGB1) in Preeclampsia. Med. Sci. Monit. 2020, 26, e926924-1–e926924-8. [Google Scholar] [CrossRef] [PubMed]
- Kozal, K.; Krześlak, A. The Role of Hypoxia-Inducible Factor Isoforms in Breast Cancer and Perspectives on Their Inhibition in Therapy. Cancers 2022, 14, 4518. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, E.K.; Aristea Gioxari Dimitriou, M.; Panoutsopoulos, G.I.; Panagiotopoulos, A.A. Molecular Pathways of Genistein Activity in Breast Cancer Cells. Int. J. Mol. Sci. 2024, 25, 5556. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, M.; Esteghlal, S.; Beyzaei, Z. Quercetin can inhibit angiogenesis via the down regulation of MALAT1 and MIAT LncRNAs in human umbilical vein endothelial cells. Int. J. Prev. Med. 2021, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Kim, S.R.; Jiang, K.; Ogrodnik, M.; Zhu, X.Y.; Ferguson, C.M.; Tchkonia, T.; Lerman, A.; Kirkland, J.L.; Lerman, L.O. Quercetin Reverses Cardiac Systolic Dysfunction in Mice Fed with a High-Fat Diet: Role of Angiogenesis. Oxidative Med. Cell. Longev. 2021, 2021, 8875729. [Google Scholar] [CrossRef]
- Okumo, T.; Furuta, A.; Kimura, T.; Yusa, K.; Asano, K.; Sunagawa, M. Inhibition of Angi-ogenic Factor Productions by Quercetin In Vitro and In Vivo. Medicines 2021, 8, 22. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, F.; Qiao, Y.; Chen, T.; Fan, L.; Shen, X.; Yu, D.; Huang, Y.; Wei, M. Honokiol inhibits interleukin-induced angiogenesis in the NSCLC micro-environment through the NF-κB signaling pathway. Chem.-Biol. Interact. 2023, 370, 110295. [Google Scholar] [CrossRef]
- Mikhaevich, E.I.; Sorokin, D.V.; Scherbakov, A.M. Honokiol inhibits the growth of hormone-resistant breast cancer cells: Its promising effect in combination with metformin. Res. Pharm. Sci. 2023, 18, 580–591. [Google Scholar] [CrossRef]
- Cheng, W.-X.; Huang, H.; Chen, J.-H.; Zhang, T.-T.; Zhu, G.-Y.; Zheng, Z.-T.; Lin, J.-T.; Hu, Y.-P.; Zhang, Y.; Bai, X.-L.; et al. Genistein inhibits angiogenesis developed during rheumatoid arthri-tis through the IL-6/JAK2/STAT3/VEGF signalling pathway. J. Orthop. Transl. 2020, 22, 92–100. [Google Scholar] [CrossRef]
- Wen, X.; Zhou, X.; Guo, L. Berberine Inhibits Endothelial Cell Proliferation via Repressing ERK1/2 Pathway. Nat. Prod. Commun. 2023, 18, 1934578X2311526. [Google Scholar] [CrossRef]
- Luo, Y.; Tian, G.; Zhuang, Z.; Chen, J.; You, N.; Zhuo, L.; Liang, B.; Song, Y.; Zang, S.; Liu, J.; et al. Berberine prevents non-alcoholic steatohepatitis-derived hepatocellular carcinoma by inhibiting inflammation and angiogenesis in mice. Am. J. Transl. Res. 2019, 11, 2668–2682. [Google Scholar]
- Liao, Z.-H.; Zhu, H.-Q.; Chen, Y.-Y.; Chen, R.-L.; Fu, L.-X.; Li, L.; Zhou, H.; Zhou, J.-L.; Liang, G. The epigallocatechin gallate derivative Y6 inhibits human hepatocellular carcinoma by inhibiting angiogenesis in MAPK/ERK1/2 and PI3K/AKT/ HIF-1α/VEGF dependent pathways. J. Ethnopharmacol. 2020, 259, 112852. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, X.; Hu, Y.; Li, L.; Liang, G.; Zhang, G. Epigallocatechin-3-gallate sensitises multidrug-resistant oral carcinoma xenografts to vincristine sulfate. FEBS Open Bio 2020, 10, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yao, L.; Sun, H.; Pang, S.; Kong, X.; Zhao, S.; Xu, S. Effects of wogonoside on invasion and migration of lung cancer A549 cells and angiogenesis in xenograft tumors of nude mice. J. Thorac. Dis. 2020, 12, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; Al-Yasari, I.H. Combination Anticancer Therapies Using Selected Phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef]
- Saleh, M.M.; Darwish, Z.E.; El, I.; Fayed, N.A.; Mourad, G.M.; Ramadan, O.R. The potential preventive effect of dietary phytochemicals In Vivo. BDJ Open 2023, 9, 30. [Google Scholar] [CrossRef]
- Hu, W.-H.; Chan, G.K.-L.; Duan, R.; Wang, H.-Y.; Kong, X.-P.; Dong, T.T.-X.; Tsim, K.W.-K. Synergy of Ginkgetin and Resveratrol in Suppressing VEGF-Induced An-giogenesis: A Therapy in Treating Colorectal Cancer. Cancers 2019, 11, 1828. [Google Scholar] [CrossRef]
- García-Quiroz, J.; García-Becerra, R.; Santos-Cuevas, C.; Ramírez-Nava, G.J.; Morales-Guadarrama, G.; Cárdenas-Ochoa, N.; Segovia-Mendoza, M.; Prado-Garcia, H.; Ordaz-Rosado, D.; Avila, E.; et al. Synergistic Antitumorigenic Activity of Calcitriol with Curcumin or Resveratrol is Mediated by Angiogenesis Inhibition in Triple Negative Breast Cancer Xenografts. Cancers 2019, 11, 1739. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Wu, X.; Zheng, C.; Shiu, P.H.-T.; Rangsinth, P.; Lee, S.M.-Y.; Leung, G.P.-H. An update on the potential application of herbal medicine in promoting angiogenesis. Front. Pharmacol. 2022, 13, 928817. [Google Scholar] [CrossRef]
- Borriello, R.; Cerrito, L.; Gasbarrini, A.; Ponziani, F.R. Pharmacokinetic considerations for angiogenesis inhibitors used to treat hepatocellular carcinoma: An overview. Expert Opin. Drug Metab. Toxicol. 2023, 19, 785–794. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs—The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Pognan, F.; Beilmann, M.; Boonen, H.C.M.; Czich, A.; Dear, G.; Hewitt, P.; Mow, T.; Oinonen, T.; Roth, A.; Steger-Hartmann, T.; et al. The evolving role of investiga-tive toxicology in the pharmaceutical industry. Nat. Rev. Drug Discov. 2023, 22, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Hoseinkhani, Z.; Norooznezhad, F.; Rastegari-Pouyani, M.; Mansouri, K. Medicinal Plants Extracts with Antiangiogenic Activity: Where Is the Link? Adv. Pharm. Bull. 2020, 10, 370–378. [Google Scholar] [CrossRef]
- Bahramrezaie, M.; Amidi, F.; Aleyasin, A.; Saremi, A.; Aghahoseini, M.; Brenjian, S.; Khodarahmian, M.; Pooladi, A. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: A triple-blind randomized clinical trial. J. Assist. Reprod. Genet. 2019, 36, 1701–1712. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.; González-Sarrías, A.; Martínez-Díaz, F.; Abellán, B.; Martínez-Torrano, A.J.; Fernández-López, A.J.; Giménez-Bastida, J.A.; Espín, J.C. Disposition of Dietary Polyphenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin. Mol. Nutr. Food Res. 2021, 65, e2100163. [Google Scholar] [CrossRef]
- Khodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Salahi, E.; Danaii-Mehrabad, S.; Nashtaei, M.S.; Mojtahedi, M.F.; Esfandyari, S.; Sobhani, A. A randomized exploratory trial to assess the ef-fects of resveratrol on VEGF and TNF-α 2 expression in endometriosis women. J. Reprod. Immunol. 2021, 143, 103248. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Gilani, S.J.; Bin-Jumah, M.N.; Fatima, F. Development of Statistically Optimized Piperine-Loaded Polymeric Nanoparticles for Breast Cancer: In Vitro Evaluation and Cell Culture Studies. ACS Omega 2023, 8, 44183–44194. [Google Scholar] [CrossRef]
- Amekyeh, H.; Enas Alkhader Sabra, R.; Billa, N. Prospects of Curcumin Nanoformulations in Cancer Management. Molecules 2022, 27, 361. [Google Scholar] [CrossRef] [PubMed]
- Gadag, S.; Narayan, R.; Sabhahit, J.N.; Hari, G.; Nayak, Y.; Pai, K.S.R.; Garg, S.; Nayak, U.Y. Transpapillary iontophoretic delivery of resveratrol loaded transfersomes for localized delivery to breast cancer. Biomater. Adv. 2022, 140, 213085. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).