Abstract
Petroselinum crispum (Mill.) Fuss (Apiaceae), popularly known as parsley, is a ubiquitous aromatic herb used for culinary and medicinal purposes worldwide. In addition to the richness in nutrients, several bioactive secondary metabolites, especially the flavone apigenin and its glycosides, have been described in this species. Parsley has already been shown to have several health-promoting activities, such as antithrombotic, antihypertensive, and hypolipidemic properties. The multiple studies conducted in animal models so far suggest this species is a potential source of cardioprotective agents. This review provides up-to-date information and perspectives on the potential of parsley and its bioactive compounds for the development of nutraceutical products and drugs for promoting cardiovascular health. It includes not only a discussion of parsley’s reported bioactivities but also the knowledge of supplements and food additives developed as innovative parsley-based products.
1. Introduction
Cardiovascular diseases (CVD) are a group of disorders that impact the quality of life of millions of people around the world. Annually, CVD are responsible for 17.9 million deaths worldwide, constituting a major concern for global health [1]. Several causes are related to death from CVD, with an emphasis on ischemic heart disease, ischemic stroke, hemorrhagic stroke, hypertension, and peripheral arterial disease [1,2,3].
Some aromatic herbs used as food flavoring in various regions of the world are also known for their beneficial health properties [4,5,6,7,8]. These herbs, as functional foods and nutraceuticals, can exert a protective and therapeutic role against multiple diseases, such as cancer, viral diseases, and cardiometabolic pathologies. Their actions include, among others, strengthening the immunity defense against infectious agents, preventing cell damage, and modulating inflammation, hemostasis, and the metabolism of lipids and glucose [9,10,11,12]. Among these widely used herbs, we highlight basil (Ocimum basilicum L.; Lamiaceae), coriander (Coriandrum sativum L.; Apiaceae), oregano (Origanum vulgare L.; Lamiaceae), and parsley (Petroselinum crispum (Mill.) Fuss; Apiaceae), which are also popularly used as medicines against symptoms related to cardiovascular diseases. The ethnopharmacological use of these spices has encouraged research groups to investigate their alleged beneficial properties [13,14,15,16].
P. crispum (syn. Apium crispum Mill., A. petroselinum L., Carum petroselinum (L.) Benth. & Hook. f., Petroselinum hortense var. crispum L.H. Bailey, P. vulgare Lag., Selinum petroselinum (L.) E.H.L. Krause, and Wydleria portoricensis DC.) is an annual or biennial plant that is native to Europe and the Mediterranean regions but is widely spread across various regions of the world, where it is cultivated for its aromatic edible leaves. The triangular leaves are dark green, divided into curly or flat leaflets, mainly known as three varieties: Italian or flat-leaved parsley (P. crispum var. neapolitanum), curly-leaved parsley (P. crispum var. crispum), and Hamburg parsley (P. crispum var. tuberosum). The curly-leaf variety occurs more frequently. The inflorescences have umbels composed of greenish-yellow flowers. The great morphological variation of parsley is reflected in the large number of its cultivars [17,18,19].
Parsley’s aerial parts and fresh or dried leaves are used in a wide variety of culinary specialties. Parsley is commonly used as a condiment in sauces, salads, and as a garnish. Its use in soups, in addition to enhancing the flavor, contributes to less need for added salt. Parsley cultivars are known for their high vitamin contents, such as pantothenic acid (B5), nicotinamide (B3), riboflavin (B2), ascorbic acid (C), thiamine (B1), pyridoxine (B6), beta-carotene (pro-vitamin A), folic acid (B9), alpha-tocopherol (E), and minerals, essential oils, and various polyphenol compounds, mainly flavonoids [18,20]. Terpenes and phenylpropanoids are abundant in the oil extracted from parsley seeds, fruits, roots, and leaves. Myristicin and apiol, both belonging to the phenylpropanoid class and showing antioxidant activities, are the main components of parsley essential oil [17]. Its chemical composition is very variable and depends on several factors, such as the influence of the growing conditions, different cultivars and chemotypes, geographic regions, and the plant organ [21]. Such variation accounts for the difference in the aroma of the various cultivars of curly parsley and Italian parsley. The curly variety is generally less aromatic [6].
The World Health Organization (WHO) guidelines recommend reducing the intake of salt (sodium) in the diet, with the aim of contributing to better health. An excess of salt in the diet is associated with 1.89 million deaths each year [1]. A lesser amount of salt in food can be compensated for the addition of herbs and spices to provide greater flavor [5]. A greater awareness of the importance of diet in health should explain, at least in part, the greater consumption of herbs and spices and, consequently, the growth of the market. The global seasonings and spices market was valued in 2022 at 37.26 billion USD. The annual growth forecast is 5.6% between 2023 and 2030. This expansion is due, in part, to the fact that the medicinal benefits of spices and herbs are known to more and more users among the world population, leading to greater consumption [22]. The parsley market will grow at a compound annual growth rate (CAGR) of 4.84% between 2022 and 2027, and the size of the market is forecast to increase by 1.19 billion USD [23].
Medicinal plants continue to be the first therapeutic resource in many regions of the world where access to basic healthcare is precarious [24]. Several reviews cite traditional uses of parsley in folk medicine in many countries. The leaves and stems are used to treat menstrual problems, bladder inflammation, renal dysfunctions, prostate inflammation, edema, arthritis, and rheumatism, among other conditions [16,17,25]. Parsley is also used to combat diabetes and hypertension [26,27,28,29]. Scientific evidence confirms the popular use of parsley as a diuretic [30].
Recent review articles reveal an increase in the number of studies on cardioprotective natural products, which partly reflects the interest in the search for more natural therapeutic agents, especially those present in plants used in food preparations or with long-standing ethnomedicinal support and with less likelihood of adverse effects [11,31,32,33]. In this context, the therapeutic potential of parsley, as well as its application in food products, has been revealed in a growing number of articles. It is important to highlight that in the vast majority of these articles, the variety of parsley used (curly leaf or flat leaf) is not specified by the authors. Given the importance of knowledge regarding the chemical composition of parsley, its therapeutic profile for cardiovascular health, and the potential of this herb for applications in nutraceutical products, the lack of a review bringing together this accumulated information becomes an important topic for public health. Therefore, this review focuses on the state-of-the-art of cardiovascular-related activities (antithrombotic, antihypertensive, and lipid-lowering) that have been demonstrated for parsley extracts, with the aim of discussing the potential use of this medicinal herb as a source of nutraceutical and/or food preservative products.
The searches were carried out using the PubMed database and Google Scholar, from 1946 to October, 2023, using the keywords “parsley” OR “Petroselinum crispum” combined with “cardiovascular,” “anticoagulant,” “antiplatelet,” “antithrombotic,” “antihypertensive,” or “hypolipidemic.” Research articles reporting the antithrombotic, antihypertensive, and/or hypolipidemic activities of Petroselinum crispum were then selected for this review. In addition, to understand the current or potential use of this species as a nutraceutical, food additive, supplement, and preservative, a search and a brief analysis of patents from around the world was also conducted. The search was carried out by employing the Derwent Innovation database, using the title, abstract, and claim fields and “(Petroselinum or parsley) AND (nutraceutical* OR “food supplement*” OR “food additive*” OR preservative OR digestive)” as terms, combined also with the IPC code A23V, related to “Food compositions, function of food ingredients or processes for food or foodstuffs.” Only patents involving the use of parsley as a dietary supplement, food product, nutraceutical, or other comparable composition were detailed. Patents covering processing techniques, methods for manufacturing food, or those in which parsley was listed as one of many ingredients, but not in a significant amount, were eliminated from the analysis.
2. Chemical Composition of Parsley Extracts
The aerial parts, leaves, and seeds are the most-used parts of parsley. These are also the most investigated in studies of its biological activities. The majority of these studies were carried out using aqueous extracts, emulating the popular use of the plant, or extracts obtained using polar organic solvents (methanol and ethanol) [17]. This was also true for the studies related to cardiovascular health, as discussed in the next topic.
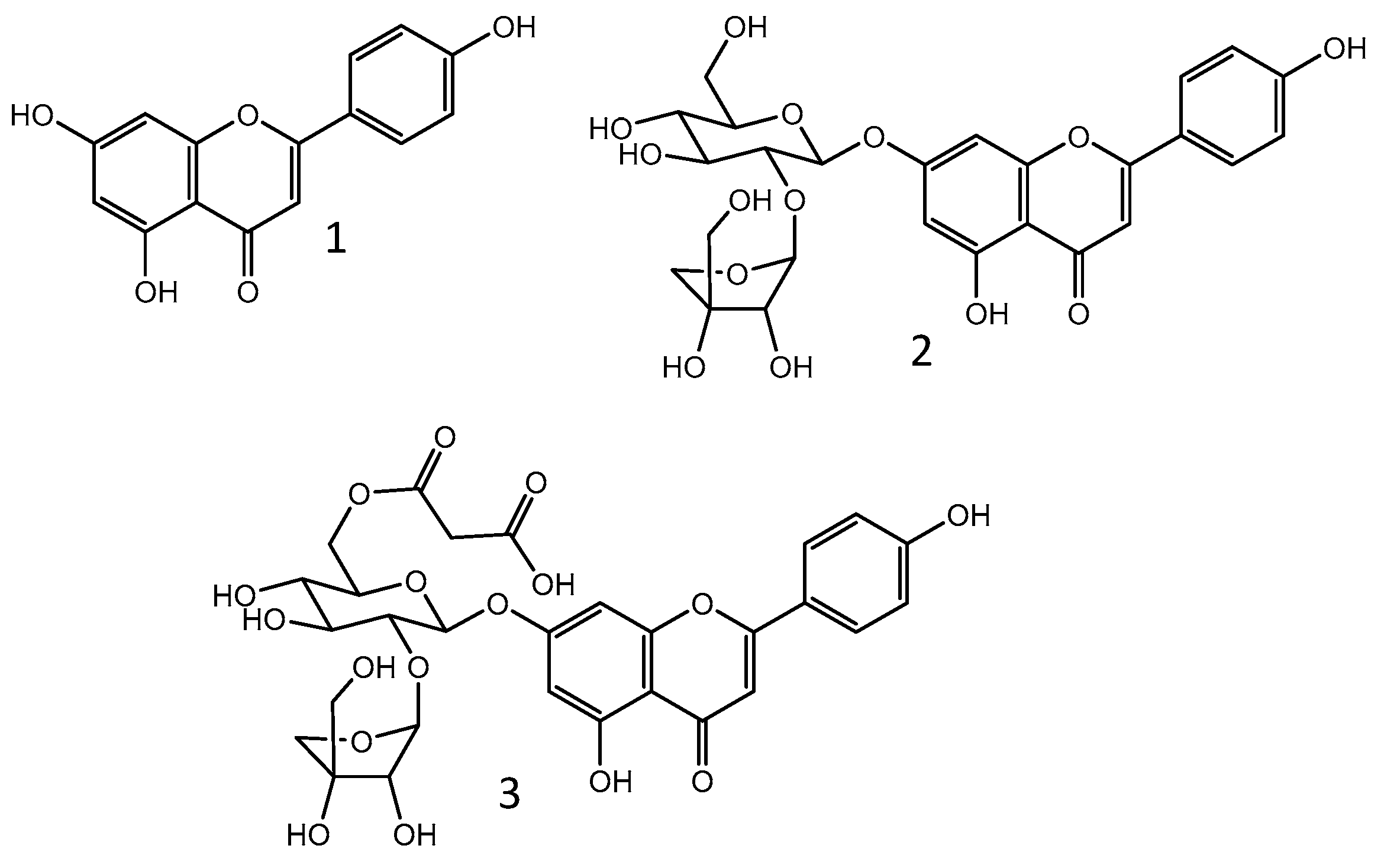
The chemical composition of polar extracts of parsley leaves and aerial parts has been extensively investigated. Among the reported compounds, flavonoids stand out. A study conducted using a hydrolyzed methanolic extract of parsley’s aerial parts revealed that its major aglycone is apigenin [34]. Apigenin (1) and its glycosides (Figure 1), especially apiin (apigenin-7-O-apioglucoside; 2) and malonylapiin (3), were confirmed as major compounds in polar extracts from aerial parts or leaves of parsley by multiple studies [35,36,37,38,39], including those carried out by our group [40,41]. Bioavailability studies with human volunteers evidenced that apigenin can be detected in plasma and urine after parsley ingestion [42,43]. Thus, it is possible to presume that apiin and other apigenin glycosides that are present in the plant are metabolized into apigenin. This is indeed the fate of most glycosylated flavonoids after oral intake [44]. A recent study showed that the ingestion of a leaf parsley infusion or of parsley leaf powder mixed in yogurt resulted in the detection of apigenin metabolites (apigenin-4′-glucuronide and apigenin-7-sulfate) in the plasma and urine of human volunteers. These metabolites are likely the outcome of apigenin release in the colon followed by phase II metabolism. The intake of pure apigenin mixed in hot water resulted in the same metabolites; however, its bioavailability was much lower in comparison with the flavone availability detected after parsley ingestion. This finding was attributed to the low hydrosolubility of apigenin, which hinders its absorption [45].
Figure 1.
Chemical structure of apigenin (1) and its glycosides apiin (2) and 6″-O-malonylapiin (3).
Despite the predominance of apigenin derivatives, other flavonoids can be found in polar extracts of parsley aerial parts and/or leaves, such as glycosides of luteolin, diosmetin, kaempferol, quercetin, and isorhamnetin. Phenolic acids, coumarins, and coumaric acid derivatives are also frequently reported [35,36,37,38,40,41,46]. Table 1 summarizes the secondary metabolites identified in these extracts.

Table 1.
Secondary metabolites reported in polar extracts 1 of parsley aerial parts and/or leaves 2.
It is also noteworthy that most studies do not take into consideration the parsley variety investigated, thus hindering comparisons among them. Interestingly, in a recent study, Liberal et al. [35] assessed the phenolic composition of hydroethanolic leaf extracts of different cultivars from three varieties of parsley (flat-leaved, curly-leaved, and turnip-rooted). The HPLC-MS/MS analysis revealed seven flavonoids in all the cultivars: three apigenin glycosides and four kaempferol glycosides. All of them, regardless of the variety, had apigenin-O-pentoside-O-hexoside, which likely corresponds to apiin, as the major detected phenolic. Moreover, significant quantitative variability was detected among the cultivars of each variety [35]. This aspect emphasizes the need to not only correctly identify the plant material under study and thereby apply it in dietary supplement compositions, but also to standardize cultivation and extraction procedures. Finally, this allows for homogeneity in the chemical composition of the plant material and hence its application [51].
The chemical composition of aqueous and hydroalcoholic preparations of parsley seeds have been much less investigated, despite their ethnomedicinal use and the several pharmacological studies that have been carried out. Early studies of parsley seeds revealed apiin as one of its constituents. The compound was extracted from defatted seeds with ethanol and then purified and crystalized [52]. Fixed and essential oils of parsley seeds have been thoroughly characterized. The fixed oil was found to have a substantial amount of petroselinic acid, while the essential oils have shown a high content of apiole and multiple other compounds in variable proportions, such as myristicin, β-phellandrene, and 1,3,8-ρ-menthatriene [53,54]. The components of these oils, however, are expected to represent only a minor fraction of the polar extracts due to their limited solubility in water and hydroalcoholic mixtures.
Therefore, most studies on the chemical composition of polar extracts of parsley leaves and aerial parts provide evidence that they are rich in flavonoids, particularly apigenin glycosides, which are likely their most prevalent chemicals and probable bioactive substances. The composition of seed polar extracts, however, has been understudied so far.
3. Parsley and Cardiovascular Health
Over the last twenty years, there has been a notable advance in the knowledge of the beneficial properties of parsley for cardiovascular health. These studies began with the investigation of the in vitro protective effects of parsley on thrombosis. Later, in vivo studies demonstrated the antithrombotic activity of parsley extracts. The cardioprotective profile of parsley was strengthened with proof of its antihypertensive effects in vivo. More recently, it was possible to show that parsley also contributes to improving lipid parameters, which are closely related to coronary heart disease and stroke. The studies reporting the antithrombotic, antihypertensive, and hypolipidemic activities of parsley are summarized in Table 2. They were conducted using the aerial parts, leaves, and seeds of parsley, and in most of them, aqueous extracts were employed. Additionally, more than half of these studies were based on animal models. This provides more robust evidence for the possible effects of parsley in the cardiovascular system, since in vitro activity does not always translate into in vivo effects. In the following subsections, these studies will be further discussed. Moreover, since apigenin and its derivatives seem to be major compounds in parsley, the cardiovascular-related activities of this flavone will be discussed alongside the effects reported for parsley extracts.

Table 2.
Cardiovascular health-related studies carried out using parsley extracts or fractions.
3.1. Antithrombotic Activity
Thrombosis consists of the formation of a blood clot within an arterial or venous vessel. The consequent limitation of blood flow can result in major life-threatening events such as myocardial infarction, ischemic stroke, and pulmonary embolism [60,61]. Free blood flow in vessels is maintained by a complex homeostasis in which many factors take part: platelets, endothelial cells, plasmatic proteins, coagulation factors, and cytokines, among others. Imbalances in these physiologic processes result in a higher risk of thrombosis [60]. The widespread nature and the high mortality and morbidity of thrombosis-related events make prevention strategies for thrombosis a major goal to reduce its global burden [61]. Furthermore, the pharmacological management of arterial and venous thrombotic conditions, which rely mainly on antiplatelet and anticoagulant agents, can be limited by side effects, such as an increased risk of bleeding [62,63]. Thus, the search for new therapeutic agents to prevent and/or treat thrombosis has recently attracted significant attention.
The first evidence we retrieved regarding the activity of parsley in the hemostatic system comes from an in vitro study (Table 2). Mekhfi et al. [55] demonstrated that an infusion from the aerial parts of parsley is able to inhibit thrombin and ADP-induced platelet aggregation (IC50 6.4 and 6.7 mg/mL, respectively). Later, interdisciplinary studies developed by the teams of Sônia S. Costa and Russolina B. Zingali showed the in vitro antiplatelet activity of a parsley (flat leaves variety) leaf decoction and two of its flavonoids: apigenin and comosiin (apigenin-7-O-glucoside), which had IC50 values of 1.8, 0.04 and 0.2 mg/mL, respectively, in an ADP-induced aggregation assay. The extract and flavonoids showed no anticoagulant effects, on the other hand, having not prolonged the clotting time in PT and aPTT assays, which are used to assess coagulation pathways [40]. In addition, a study showed that a fraction of aglycone flavonoids from parsley, among which apigenin and kaempferol were identified, evidenced in vitro inhibition of thrombin, ADP, and collagen-induced platelet aggregation, with IC50 values ranging from 0.28 to 0.08 mg/mL [57]. Therefore, flavonoids, especially apigenin, seem to have an important contribution to parsley’s in vitro antiaggregant effects.
The antiplatelet activity of parsley was later confirmed by in vivo studies. Gadi et al. [56] showed that a parsley leaf decoction orally administered to rats (3 g/kg) was able to reduce thrombin, ADP, and collagen-induced-platelet aggregation by approximately 20% 2 h after administration, while rats treated with aspirin showed an approximately 40% reduction in this parameters. The treatment with parsley also doubled the bleeding time of the rats, an effect attributed by the authors to its antiplatelet activity. The phytochemical composition of the extracts employed in this study was, however, not assessed.
Recently, the antithrombotic activity of a decoction from the aerial parts of the flat-leaved variety of parsley was evaluated in rats in the laboratories of Russolina B. Zingali and Sônia S. Costa [41]. This extract was chemically characterized by HPLC-DAD-MS/MS, having apiin (major phenolic substance) and coumaric acid derivatives as the main identified compounds. In a model of arterial thrombosis, oral administration of this extract at 15 and 25 mg/kg 60 min before thrombosis induction resulted in an increase in artery occlusion time by 150% and 240%, respectively. Assays conducted using blood collected from animals similarly treated (same doses, administration routes, and time before collection) revealed that the extract was able to increase the recalcification time of platelet-rich plasma (58.2% increase), but not of platelet-poor plasma, thus corroborating previous findings of the antiplatelet effects of parsley extracts. It is noteworthy that these effects were obtained using a much lower dose than those used in a study by Gadi et al. [56]. This can be attributed to differences in the chemical compositions of the extracts, resulting from distinct extraction processes (infusion vs decoction) and/or variations in the chemical composition of the plant itself. However, this earlier study provided no information regarding the parsley variety evaluated or on the chemical composition of the extract, which hinders comparisons.
The effects of parsley in a venous thrombosis model were also evaluated in the same study. The intravenous administration of the above-mentioned extract to rats at 25 mg/kg 5 min before thrombosis induction reduced thrombi formation by 98.2%, while the oral administration of 125 mg/kg 60 min before induction produced a reduction of 76.2%. Since coagulation plays a major role in venous thrombosis, the anticoagulant activity of the extract was also evaluated in blood collected from experimental animals similarly treated, using PT and aPTT assays. No anticoagulant activity was observed, confirming previous in vitro results [41]. It is noticeable that the effects of the extract on venous thrombosis were obtained using a five times higher oral dose than that in which arterial thrombi formation was prevented. In summary, this study evidenced the in vivo antithrombotic activity of parsley in both arterial and venous models. However, only antiplatelet activity was observed, with no alteration in the coagulation process. Platelets are known to play a prominent role in arterial thrombosis but are also recognized to take part in venous thrombosis [60,64]. Therefore, the antiplatelet activity of parsley extracts may contribute to the prevention of both types of thrombosis.
As suggested in some of the studies discussed above, apigenin and its derivatives, which are likely metabolized into apigenin, seem to be in some measure responsible for the antithrombotic activity of parsley. The effects of apigenin in platelet aggregation have been investigated in vitro. The antiplatelet activity of flavone seems to be related to its capacity to block thromboxane A2 (TxA2) receptors [65,66] and to interfere with thrombin intracellular signaling without interacting with thrombin receptors [67]. Interestingly, it also showed the ability to potentiate the antiaggregant effects of aspirin, as assessed in platelets exposed to aspirin in vivo and incubated with apigenin in vitro [65]. In a recent study, an apigenin-based formulation containing flavone esterified with docosahexaenoic acid (4′-DHA-apigenin) in olive oil evidenced more potent in vitro antiaggregant activity and better bioavailability than pure apigenin [68]. It would be interesting to prepare and evaluate the effects of apigenin-rich parsley-based formulations in future studies, as they may also present improved properties in comparison with isolated flavone and extracts not incorporated in a formulation.
3.2. Antihypertensive Activity
Hypertension, defined as arterial blood pressure values of 140/90 mmHg or higher, is the most common risk factor for cardiovascular diseases (e.g., stroke and heart failure) and also has the strongest evidence for causality [69,70,71]. According to WHO estimates, approximately 1.3 billion people between 30 and 79 years of age have hypertension, among which less than half are aware of their condition and receive proper treatment [70]. Many antihypertensive drugs are available, such as angiotensin-converting enzyme inhibitors, diuretics, and calcium channel blockers [72]. Nevertheless, lifestyle interventions, particularly at the nutritional level, play a prominent role in the prevention and management of hypertension [71].
The effects of parsley on arterial blood pressure were assessed in two in vivo studies (Table 2). Campos et al. [58] evaluated the outcomes of an oral single dose (1 mL) of a parsley seed infusion in rats. The extract had a hypotensive effect (arterial pressure values approximately 20% lower than the control group) and also increased their urinary flow and sodium and potassium excretion. Hypertension is related to the retention of sodium and consequently water; therefore, diuretic drugs are used for its management [73]. Seeds and leaves of parsley have evidenced diuretic activities in previous studies as well [30,74]. Likely, this activity is at least partially related to the hypotensive effects of the plant.
A more recent study evaluated the effects of a parsley leaf decoction that was orally administered to normotensive and N(ω)-nitro-L-arginine methyl ester-induced (L-NAME-induced) hypertensive rats in a single dose or once daily for seven days (sub-chronic test) [28]. In the normotensive rats, a single dose (160 mg/kg) had no significant effects on blood pressure. The hypertensive rats, on the other hand, showed an important reduction in both their systolic and diastolic pressure, resulting in values similar to those of the normotensive rats, an effect comparable to the positive control drug (Lasilix®). The repeated oral administration of parsley extract (160 mg/kg) showed similar results in the hypertensive rats. In this case, however, a reduction of approximately 15–20% was also observed for the blood pressure of the normotensive animals. The study also reported the in vitro effects of the extract in rat aortic rings, having observed a vasorelaxant activity through the blockage of calcium channels. The authors stated that this mechanism led to the hypotensive effects that were revealed; however, this was not confirmed in vivo. The possible contribution of diuretic activity, as previously mentioned, can also play a role in the hypotensive effects of parsley leaves and should be considered for further investigation as well.
The chemical compositions of the parsley extracts in the above-mentioned studies were not accessed. Nevertheless, since aqueous extracts from the leaves and seeds presented hypotensive activities, it is possible to infer they shared the same bioactive substances. Indeed, apiin has been shown to be present in both the leaves and seeds of parsley (see Section 2). However, it is not impossible that different bioactive substances are responsible for these effects. The correlation between the chemical composition and the hypotensive activity of extracts from different plant parts should be an object of future studies to clarify this issue.
Apigenin may also play a role in the antihypertensive activity of parsley. This flavone showed in vitro vasorelaxant activity in rat aortic rings [75]. An in vivo study employing a renovascular hypertension mouse model showed that treatment with apigenin administered orally (50–100 mg/kg) for 4 weeks resulted in a reduction in blood pressure and an improvement in cardiac hypertrophy and abnormal glucolipid metabolism, which were consequences of the adaptative response to heart pressure overload. The mechanism of these effects seems to be related to the downregulation of HIF-1α expression. This hypoxia-inducible factor is overexpressed in the cardiac hypertrophy process, which in turn alters the expression of peroxisome proliferator-activated receptors (PPARα and PPARγ). The expression levels of the latter were also regulated by apigenin treatment [76].
In another in vivo study, the effects of a much lower oral apigenin dose (1.44 mg/kg; 6 weeks) were evaluated in L-NAME-induced hypertensive rats [77]. In this model, which was also used to evaluate the effects of parsley [28], hypertension is induced via nitric oxide (NO) deficiency, an event that is observed in endothelial dysfunction [78]. The animals that received apigenin had a lower blood pressure, better vasodilatory response, lower sodium retention, and less renal and cardiac damage than the control group, an effect probably related to an increased production of NO [77]. The same researchers carried out a similar study using a different hypertension model (spontaneous hypertensive rats) and obtained markedly different results. In this model, in which hypertension is a result of elevated peripheral vascular resistance and not NO deficiency, apigenin (1.44 mg/kg; 6 weeks) orally administered produced no significant reduction in blood pressure. However, it presented moderate benefits, such as improving aortic vascular relaxation and reducing vascular abnormalities. These contrasting results provide evidence that the effects of apigenin in attenuating hypertension and improving vascular function are more efficient in cases of NO deficiency, since this flavone seems to induce NO production [79]. It would be interesting to evaluate the effects of parsley extracts in this hypertension model as well. If apigenin and its derivatives are the main compounds responsible for parsley bioactivity, similar results can be expected. On the other hand, since other phenolics can take part in this activity, their possible role could also be assessed in the case of divergent results.
3.3. Hypolipidemic Activity
Dyslipidemias consist of a range of lipid abnormalities, including high plasmatic concentrations of total cholesterol, LDL-cholesterol, and/or triglycerides, as well as low HDL-cholesterol levels [80,81]. They represent a major risk factor for coronary heart disease and stroke. In 2019, it was estimated that 44% of global deaths caused by ischemic heart disease were related to high LDL-cholesterol levels [80]. Management of dyslipidemias includes lifestyle modifications and often lipid-lowering drugs, mainly statins [80,81]. Nutraceuticals such as plant sterols and soluble fibers can also improve lipid profiles, representing an additional tool in the management and prevention of dyslipidemias [82].
Two in vivo studies have evidenced the hypolipidemic potential of parsley extracts (Table 2). A dry methanolic seed extract resuspended in water with the surfactant Tween 80 orally administered for 8 weeks to rats with a hypercholesterolemic diet had remarkable effects on their lipid levels. As expected, the cholesterol-enriched diet induced an increase in their total cholesterol, triglycerides, LDL, and VLDL-cholesterol, as well as a reduction in their HDL cholesterol. The deterioration of the lipid profile was, nevertheless, partially prevented by the treatment with parsley seed extract. The treated animals also exhibited an improvement in their liver function, resulting in less damage and inflammation in the hepatic tissues that were histologically evaluated as well as lower activities of liver enzymes—alanine transaminase (ALT), aspartate aminotransferase (ASP), and alkaline phosphatase (ALP)—which were also affected by the hypercholesterolemic diet. An additional effect of the parsley seed extract was cardioprotection; the treated animals showed lower levels of lactate dehydrogenase, an enzyme related to myocardial damage, and less detrimental effects on heart tissue in comparison with the control group, whose myocardial muscles showed marked degeneration [59].
In another study, the effects of an aqueous extract of parsley leaves were evaluated in normal and streptozotocin-induced diabetic rats [29]. Diabetes is often associated with dyslipidemias, which increase the risk of atherosclerotic cardiovascular disease among diabetic patients [83]. In the study mentioned above, the diabetic rats presented higher levels of total cholesterol, triglycerides, and LDL-cholesterol, as well as lower levels of HDL-cholesterol in comparison with the non-diabetic rats. The non-diabetic rats treated with parsley leaf extract (2 g/kg) for 45 days presented a slight reduction in their total cholesterol and triglycerides, while the effects on the diabetic rats were outstanding: their lipid levels were normalized by parsley treatment, and the results were more pronounced than those obtained using gliclazide, an antidiabetic drug used as positive control [29]. As the authors did not assess the glycemic levels of the treated animals, we cannot know if the effects of the parley on the lipid levels were associated with a reduction in glycemia, as it likely occurred in the group treated with gliclazide. Therefore, it is not possible to infer whether the observed effects were secondary to better glycemic control, resulting in an improvement in the metabolic imbalances that typically result from uncontrolled diabetes, or if the parsley leaf extract acted on lipid levels through direct mechanisms. It is actually possible that both occurred; parley is known to possess antihyperglycemic activity [26,27,28,29], as previously mentioned, and as evidenced by the study discussed above, parsley seed extract can also improve lipid profiles in a non-diabetes model. If confirmed, dual antihyperglycemic and hypolipidemic action would be particularly interesting for type 2 diabetic subjects, as most of them have lipid abnormalities, despite good glycemic control [84]. However, the studies reported so far were conducted using different plant parts (leaves and seeds) and different solvents for extraction. Thus, the differences in the chemical composition of the evaluated extracts, even though some common substances may have occurred (Section 2), do not allow further assumptions to be made. This issue should be clarified by more studies on the effects of chemically characterized parsley extracts in diabetic and non-diabetic animal models.
The hypolipidemic effects of parsley might also be partially attributed to apigenin and its derivatives. This flavone has evidenced similar effects in animal models with diet-induced metabolic disorders. High-fat diet-induced obese mice whose food was supplemented with 0.005% w/w apigenin for 16 weeks showed lower plasmatic levels of total cholesterol, free fatty acids, and apolipoprotein B (a component of lipoprotein particles) in comparison with the control group. Apigenin also improved glucose tolerance and hepatic function in treated animals [85]. Similarly, rats with high-fructose diet-induced metabolic syndrome fed apigenin (2.6 mmol/kg diet) for 13 weeks showed markedly lower levels of total cholesterol, LDL-cholesterol, and free fatty acids, as well as higher levels of HDL-cholesterol and improved glucose metabolism [86]. Moreover, a study carried out using hamsters kept on a high-cholesterol diet showed that treatment with apigenin for nine weeks (60 and 300 ppm diet) resulted not only in lower non-HDL-cholesterol levels but also a 30% reduction in aorta plaque formation in comparison with the control group [87].
An additional study conducted experiments differently: instead of apigenin administration simultaneously with a high-fat or high-fructose diet, the authors treated rats already submitted to a 12-week high-fat diet with different doses of apigenin (20, 40, and 80 mg/kg) for six weeks. The flavone produced a dose-dependent reduction in the plasmatic levels of total cholesterol, triglycerides, and LDL-cholesterol, as well as an increase in HDL cholesterol. The effects produced by the highest dose (80 mg/kg) were comparable to those exerted by the positive control drug (simvastatin) [88]. These results evidence that apigenin could both prevent and treat hyperlipidemia.
All these reports highlight the beneficial effects of parsley extracts on cardiovascular disorders, which are supported by in vivo research. Despite being studied for its antithrombotic, antihypertensive, and hypolipidemic properties, all of which are possibly attributable to apigenin derivatives, most studies do not identify the bioactive compounds in parsley that are responsible for these activities.
4. Toxicity of Parsley
The use of aromatic herbs and derived products, though widespread, should not be regarded as devoid of risk. Their safety and toxicity should be properly taken into account [89]. The safety profile of parsley extracts has been assessed in some in vitro and in vivo studies.
A hydromethanolic extract of parsley leaves at concentrations ranging from 3 to 25 µg/mL evidenced low cytotoxicity to neuroblastoma (SKN-BE(2)C) and hepatoblastoma (HepG2) cell lines [90]. In another study, a similar extract evidenced cytotoxicity to cancerous (A375; melanoma) and non-cancerous (CV1-P; fibroblasts) cells, but only at the highest concentration evaluated (2 mg/mL). This effect was attributed to the pro-oxidant activity of the extract against proteins and DNA [91]. Tang et al. [92] assessed the effects of parsley aerial parts that were sequentially extracted using increasing polarity solvents in cancer cell lines (MCF-7, MDA-MB-231, and HT-29). In this study, only dichloromethane extracts exhibited important cytotoxicity at 500 µg/mL, while ethyl acetate, methanol, and aqueous extracts inhibited cell viability by less than 20% at the same concentration [92]. More recently, the cytotoxicity of an aqueous leaf extract of parsley was evaluated using Saccharomyces cerevisiae cells. This parsley extract was not able to significantly affect cell survival at the tested concentrations (0.1 and 0.2 mg/mL) [38]. Since the extract types and concentration ranges were different, direct comparison among these studies is not possible. It is worth noting, however, that these two in vitro antithrombotic studies (Table 1; Section 3) were carried out using high concentrations of aqueous leaf extracts of parsley (both applied concentrations higher than 1.5 mg/mL). Despite the lack of cytotoxicity data for these aqueous extracts in this concentration range, it is plausible to assume that they could have cytotoxic effects. More investigations are necessary to clarify this.
Parsley seed extracts obtained using different solvents have also exhibited cytotoxicity in cell lineages. An ethanolic seed extract decreased the viability of HepG2 hepatocarcinoma cells at concentrations higher than 100 µg/mL [93]. Interestingly, a less polar extract, obtained using chloroform, was cytotoxic to this cell line in the same concentration range [94]. Moreover, an extract obtained using ethanol was cytotoxic to breast cancer cells MCF-7 at concentrations higher than 50 µg/mL [95]. These studies did not evaluate the cytotoxicity of seed extracts in non-cancer cell lines, which would clarify whether the observed cytotoxic effects were selective to cancer cells. On the other hand, an aqueous seed extract of parsley at up to 2 mg/mL evidenced no effects on the viability of Vero cells, a non-tumoral kidney cell lineage [96].
In vitro toxicity studies do not take into account the metabolism of the chemical components of the tested extracts, which may modify the observed effects. Therefore, the results of these studies, especially in tumor cell lines, cannot be extrapolated to in vivo models. As far as we know, there are limited data for parsley’s in vivo toxicity. Awe et al. [97] investigated the effects of a parsley leaf ethanolic extract on the liver and kidney, as well as some biochemical and hematological parameters in rats. The animals received an oral daily dose of the extract (10, 100, and 1000 mg/kg) for eight weeks. None of the doses produced significant changes in their body weight, total plasma count, serum albumin, and hematological values. However, the animals that received 1000 mg/kg of the extract showed higher levels of alanine aminotransferase (ALT) and blood urea nitrogen (BUN), which evidences liver and kidney damage. This was confirmed via a histopathological analysis, which evidenced inflammation and necrosis in these organs [97]. In another study, rats orally treated for four weeks with 500 and 1000 mg/kg of a parsley aerial part hydroethanolic extract evidenced no signs of toxicity. In contrast to the previous study, the treatment produced no liver histopathological changes and no significant differences in the plasmatic levels of hepatic enzymes, urea, and creatinine [98]. Differences in the extract’s chemical composition and treatment duration would likely explain these disparities.
The in vivo toxicological studies mentioned above were all carried out using alcoholic or hydroalcoholic extracts, although homemade parsley preparations have been made using water to obtain teas and decoctions in folk medicine. Most of the in vivo studies discussed in Section 3 used aqueous extracts, which are more in line with popular tradition. However, to our knowledge, the toxicity of these extracts has not been evaluated in animal models. On the other hand, there have been risk assessments regarding the intake of teas [99] and food supplements [100] containing parsley based on exposure to alkenylbenzenes, such as apiol and myristicin, which can be carcinogenic and genotoxic [99]. A UPLC analysis was used to determine the levels of alkenylbenzenes in teas and supplements commercialized in the Netherlands. The amount of these compounds in each product was used to calculate their margin of exposure (MOE), a ratio that considers epidemiological or experimental data on carcinogenesis and the estimated daily intake of the substances. These studies indicated that consuming parsley-containing teas or supplements for a short period (a few weeks) is likely to pose a low risk; however, it may be an issue for long-term use [99,100]. The consumption of parsley may also be a concern during pregnancy, as the plant is reported to be applied in infusions used to induce abortions [101].
The data discussed in the present topic evidence knowledge gaps regarding the toxicity of parsley, especially regarding its aqueous extracts. The potential risks of intake during pregnancy and lactation should be investigated as well. It would also be important to identify the substances responsible for any potential toxic effects. Alkenylbenzenes, as mentioned, are a concern, but it is important to assess the risk of other parsley compounds as well to gain a complete understanding of this matter.
5. New Parsley-Based Nutraceuticals and Food Products: Cultivation Conditions, Applications, Patents, and Technological Aspects
The health-promoting action of parsley extracts and their constituents in different cardiovascular conditions, combined with the fact that parsley is a culinary herb, has prompted studies aimed at the potential application of parsley in the development of dietary supplements and fortified food products in recent years. In addition, several patents focused on the use of this herb, alone or in combination with other herbs as food supplements, preservatives, or related products, have been filed around the world.
Conventionally, parsley is used as a fresh culinary herb, being produced under environmental or in greenhouse conditions and, more recently, also hydroponically [102]. Due to the commercial importance of this herb, its producers urge the development of “improved production recipes” that take several environmental parameters into account in order to produce better-quality herbs that are also enriched with bioactive molecules. In this sense, the recent modulation of light conditions can be cited [102]. For example, when evaluating the effects of different light qualities on parsley microgreens, Samuolienė et al. [103] observed that the application of red light at 665 and 638 nm during its cultivation improved the β-carotene content and DPPH antioxidant activity of this herb [104]. In another study, a treatment with blue light (16%) increased the total tocopherol content of parsley microgreens, while a higher percentage of blue light (33%) resulted in a higher content of chlorophylls a and b, carotenoids, α- and β-carotenes, lutein, violaxanthin, and zeathin [103]. Also studying parsley microgreens, Carillo et al. [105] showed that a mixture of red, green, yellow, and blue light resulted in higher amounts of secondary metabolites, especially the total polyphenol content and the amounts of apigenin derivatives. Both light and the application of other conditions have been described as tools for improving the nutraceutical value of parsley leaves. The application of Streptomyces fulvissimus (strain AtB-42) and Trichoderma harzianum (strain T22) microorganisms in a consortium under field conditions increased the content of some metabolites, including the carotenoid capsanthone [49].
Parsley is often freshly applied to food products, in certain cases to limit cholesterol oxidation caused by the cooking process, due the high content of phenolic compounds and significant antioxidant activity of parsley leaves. In this sense, the addition of fresh parsley (4% w/w) in grilled sardines was found to effectively reduce cholesterol oxide formation in the final product [106]. A similar result was observed for omelets, for which the potential application of fresh parsley (at 0.75% w/w) as a natural inhibitor of cholesterol and lipid oxidation was described [107]. The authors observed the thermo-degradation of parsley phenolic compounds after cooking, with the preparation using air-frying conditions being the best one for preserving these metabolites.
Powdered parsley leaves have been proposed as a functional ingredient for the fortification of wheat-based products as well [108,109,110]. Despite their important role as an energy source in human nutrition, these products are poor in bioactive compounds, and their fortification, especially with phenolic-rich ingredients, is therefore envisaged [108,109]. As an example of this application, Sęczyk et al. [108] evaluated the effects of dried-parsley supplementation on the nutraceutical and nutritional properties of wheat pasta. The parsley leaves employed were rich in apigenin, diosmetin, and isorhamnetin derivatives, as well as catechin, and most of them remained bio-accessible after simulated digestion. Pasta fortified with 4% parsley leaf powder, after simulated digestion, was characterized by 67% more phenolic compounds and a significantly higher antioxidant activity, as evidenced by a 146% increase in antiradical activity (ABTS assay) and a 220% increase in reducing power (FRAP assay). The supplementation did not affect the starch digestibility, but it did reduce the protein digestibility, likely due to the interaction between phenolic compounds and proteins, including digestive enzymes [108].
In a similar study, the supplementation of wheat pasta with parsley leaf powder (2.5–10%) resulted in a concentration-dependent increase in its phenolic content and antioxidant activity. The authors also investigated the impacts of this supplementation on the cooking quality and texture of the pasta. It resulted in color alteration, a reduction in its cooking time and hardness, as well as an increase in its adhesiveness and water absorption capacity. Most of these effects may have been associated with changes in the pasta composition and microstructure, such as an increased fiber content and reduced gluten proportion. Despite these differences, when their organoleptic properties were evaluated, the supplemented pastas were considered acceptable overall by the evaluators. The pasta fortified with 5% parsley was considered the best compromise between nutraceutical quality and physical/organoleptic characteristics [110].
Also, regarding wheat-derived products, parsley powdered leaves were evaluated as an additive for bread. Breads prepared using flour containing 1–5% parsley were investigated for their chemical composition and physical and sensorial properties [109]. The parsley-supplemented breads had higher mineral and fiber contents but were similar to the control bread in fat and protein contents. The supplementation resulted in a decrease in the bread volume and an increase in the crumb moisture. Similar to what was observed for the parsley-fortified pasta, these differences were likely associated with gluten dilution and an increased fiber content, which, in this particular case, would cause a reduction in the dough’s capacity to retain carbon dioxide and an increased ability to retain water, respectively. The greenness and redness of the crumbs were also altered by parsley supplementation due to the influence of the plant pigments; however, no significant changes in the crumb texture were observed. When evaluated for sensory properties, the breads with up to 3% parsley were considered overall acceptable, while higher parsley proportions resulted in lower scores, particularly for their smell and taste. Furthermore, the supplementation resulted in breads with a higher phenolic contents and antioxidant activities. Particularly, the bread with 3% parsley had approximately two times more phenolic compounds [109].
All these studies evidence that parsley can be an interesting supplement for pasta and bread. It is also a source of bio-accessible phenolics, resulting in products that, regardless of some property changes, have an adequate sensory acceptability. However, although there is interest in and potential for the application of P. crispum leaves for the supplementation of food products [108,109,110], more research and the development of commercial food supplements using this herb is needed, providing a niche to be explored.
In fact, conventional spice powders may impose issues that hinder their utilization, which could explain the low current use of parsley as a food supplement and similar products. One of the issues that can be mentioned is the drying process, a key step for food product preservation and application [111]. The drying process, however, can cause a loss of volatiles and pigment degradation, thus impairing color and flavor. Moreover, endogenous enzymes are only partially inactivated and may recover activity once the product is rehydrated. Considering this, some studies are proposing better techniques to dry parsley material or alternatives for its use in food products.
Regarding the drying process, Mouhoubi et al. [112] recently showed that the use of a microwave at 100 W is the best way to dry parsley leaves without substantial modifications in their polyphenol content or antioxidant activity when compared with a ventilated oven under three temperatures (40, 80 and 120 °C) and a microwave at 500 or 1000 W. In fact, the use of a microwave for drying parsley has been already evaluated in a previous study [113].
Some other food products containing parsley leaves have also been investigated. Kaiser et al. [114] proposed paste-like parsley products as an alternative to powdered parsley. The authors evaluated different production processes: mincing fresh parsley followed by a heating step (80–100 °C) and steam or water-blanching (80–100 °C) of the plant material followed by mincing. The water bleaching process was considered to be the most suitable, since the resulting paste-like product had a brighter color and a higher pigment retention. At 90 and 100 °C (but not 80 °C), a complete inactivation of peroxidase and polyphenol oxidase was observed. This process also enhanced the antioxidant activity of the samples in spite of a decrease in their phenolic contents [114]. The chemical composition of the paste-like products obtained via the bleaching processes was further evaluated by the authors. They observed that short-time (1 min) steam and water bleaching had minimal effects on the phenolic composition, except for apiin, which exhibited a reduced content. Thus, they recommend blanching for the production of parsley paste-like products [36]. These paste-like products may indeed confer advantages for the industrial application of parsley in the production of food and nutraceutical products, providing more chemical stability and microbiological safety for the raw plant material. However, the effects of the production process on the phenolic composition of the plant should be further evaluated regarding their possible impacts on its biological activities.
It is important to mention that the application of new technologies and formulations can also be an alternative to be explored. Herbal supplements can be formulated into different delivery systems, which are able to enhance the stability, solubility, and bioavailability of their active compounds [115]. Helmy et al. [116] evaluated the properties of silver nanoparticles synthesized using parsley extract. Silver nanoparticles are known to exert multiple biological activities and can be applied in the preparation of food, drugs, and cosmetics [116,117]. In this study, silver nanoparticles prepared using a parsley aqueous extract in combination with corn silk and gum arabic extracts showed greater antioxidant, antimicrobial, and anti-inflammatory activities than any of the plants crude extracts, alone or combined [116]. Thus, silver nanoparticles can be considered to be a potentially useful system for parsley-based nutraceutical formulations.
Regarding the technological development of products based on parsley, the search for patents resulted in 186 documents containing the terms of interest. However, after reading the abstracts and specific claims of the patents, only 46 of the documents were selected. These documents consisted of food supplements, nutraceuticals, additives, and recipes with added parsley.
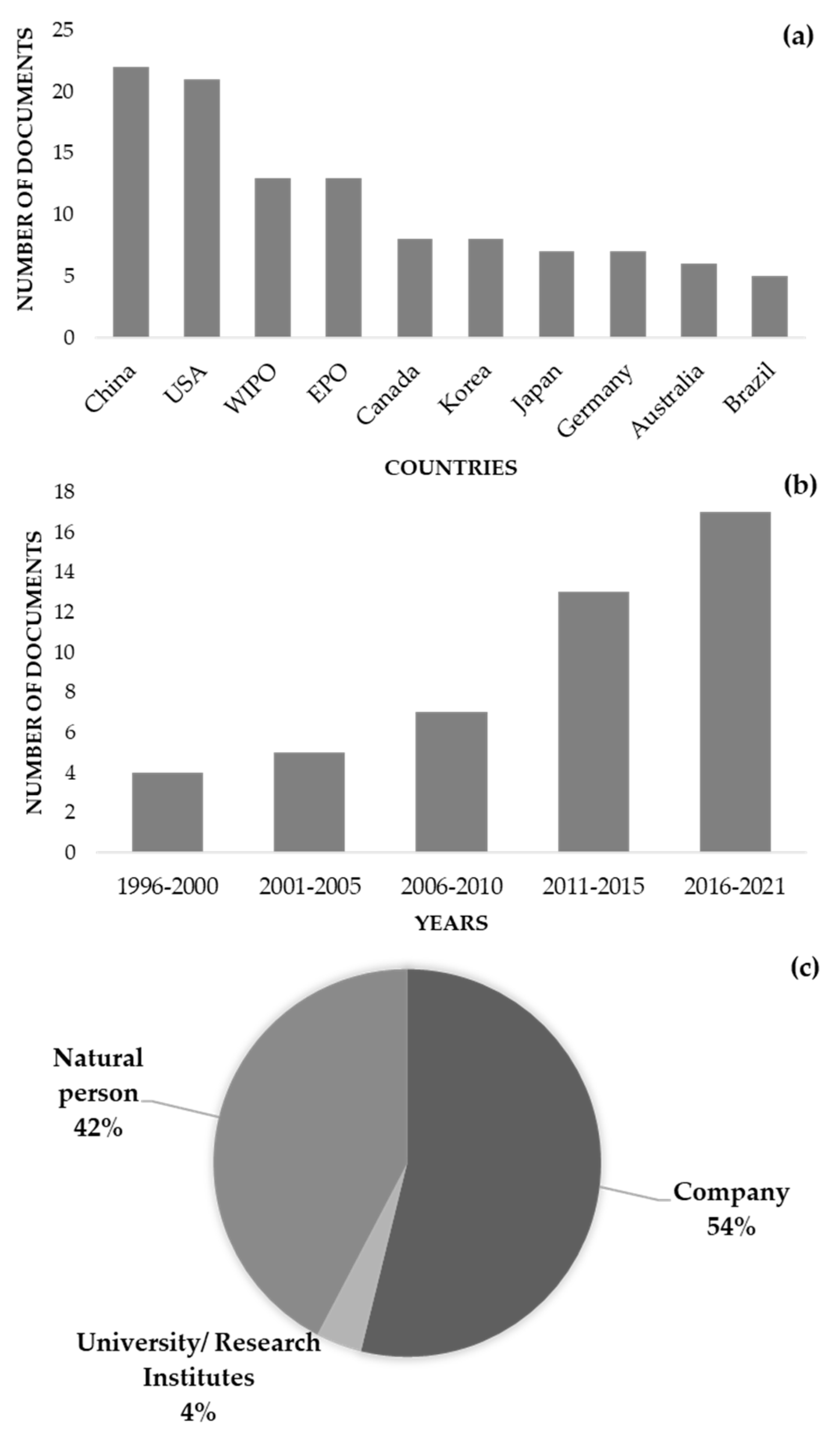
Most of these 46 documents were deposited in China, followed by the United States, the World Intellectual Property Organization (WIPO), and the European Patent Office (EPO) (Figure 2a). In fact, most of the patents were from China, following a global tendency for many other areas [118]. These documents were related to the use of parsley in several food preparations, including pasta, fish, and shrimp, among others. The documents’ publication dates indicated a trend toward more patent deposits in the previous ten to fifteen years (Figure 2b), with firms and natural persons making up most of the assignees (Figure 2c).
Figure 2.
Details of the 46 patents identified using the Derwent Innovation database, using the title, abstract, and claim fields and the following strategy: (Petroselinum OR parsley) AND (nutraceutical* OR “food supplement*” OR “food additive*” OR preservative)” and the IPC code A23V. (a) Countries of deposit; (b) publication date; (c) assignees details.
After carefully checking all 46 documents, it was observed that 13 of the patents (Table 3) adequately described and focused on compositions using parsley as an ingredient for applications in food supplements, dietary supplements, nutraceutical products, functional foods, or food additives.

Table 3.
Details of the selected patents using parsley in food supplements, additives, or nutraceutical compositions.
In most of these documents, which were generally deposited by companies, parsley is used in association with other herbs. Only two of the documents included the possibility of using parsley as the only herb (EP1616489 and EP1602364). Few documents are currently active, with most of them being inactive, suspended, canceled, refused, or expired. Moreover, few are under evaluation (not granted). Regarding the type of products developed, most of the technologies described are for compositions, but extracts and microcapsules also appear as formulations. These different compositions have applications as several food products, especially as food or dietary supplements (6) and as functional foods or nutraceuticals (4). However, other applications in the food sector were also described; for example, as food emulsifiers or additives (Table 3).
Several of the documents do not mention the part of parsley used in the composition, while the documents that detail this aspect mostly employ leaves (Table 3). It is interesting to observe that some other plant parts are also considered, such as fruits and roots, as well as the entire plant. Although the leaves, fruits, roots, or the entire plant can be used as fresh or dry materials, most of the documents are focused on plant extracts. This could be explained by the fact that extracts are better-suited for use in plant-based formulations. Using plant extracts allows for greater quality control, larger-scale and regulated production, better transportation and storage, and the avoidance of mold and other microbial contamination [51].
Among the 46 documents analyzed, we found a recent patent (WO22103901) describing the use of apigenin isolated from parsley, an important bioactive compound, as a constituent of a nutritional supplement with nutraceutical applications for the support and protection of the immune system. In fact, there is a product related to this patent being sold online under the commercial name STEMRCM®, produced by the company STEMSATION, the assignee of the patent [119].
The results of the patent analysis show that, since 1996, there has been an increased interest in the use of parsley in food products, some of them generically attributed to health improvement. Innovations in the use of parsley in foodstuff have been increasing, but most of these patents are especially focused on the application of this plant species in food preparations (such as dishes) much more than in finished goods (for instance, nutraceutical formulations). Considering the overall importance of parsley, especially in cardiovascular conditions, the development of parsley-based food products is a market niche to be explored and developed by the food industry.
6. Conclusions and Perspectives
The role of parsley—including its aerial parts, leaves, and seeds—in diseases that are directly linked to cardiovascular problems is undeniable. The pharmacological data available today encourage a deeper study of the effects of parsley formulations with a view of the global assessment of parameters such as glycemia, lipidemia, hemostasis, and hypertension. The various results of preclinical studies carried out using parsley represent an important step towards the technological development of products based on this herb for improving conditions that impact cardiovascular health. However, the development of a plant-based therapeutic product depends on several factors, such as access to the starting plant material, the definition of the chemical composition of the plant extract, the choice of solvent and method used in the plant extraction, the chemical markers of the plant extract, the toxicity of the plant, the efficacy of the plant preparation, and bioavailability, among others.
Parsley occupies a prominent place in the global seasonings and spices market and is therefore an easily accessible plant material. The various data on the best conditions for growing parsley reflect the biotechnological advances that have been achieved in recent years, as were presented in this review.
The full identification of parsley, including the specification of the variety used, in studies is very important information, but it is rarely mentioned in the studies that were discussed. The variability of the chemical composition of an extract, which depends on several factors, can modify the expected biological response, efficacy, toxicity, and bioavailability, affecting the reproducibility and interpretation of the results. This impacting factor can be overcome by standardizing the desired extract based on a given variety of parsley. The available data on the chemical composition of the aerial parts of the two most-used parsley varieties (flat-leaf parsley and curly-leaf parsley) point to apiin as the main flavonoid. Therefore, this flavonoid could be considered as a chemical marker for parsley-based formulations.
The consistent data on the bioavailability of parsley from different studies, revealing the presence of metabolites of apigenin (aglycone) in the plasma and urine of volunteers, is an advantage for the development of products based on this herb. It is known that apiin and other flavonoids derived from the apigenin skeleton occurring in parsley are metabolized into derivatives of this aglycone. Therefore, apigenin could be considered as a biomarker of parsley.
Despite a number of in vivo studies demonstrating the beneficial effects of parsley extracts in various pharmacological models, there are no reports on the isolation and identification of the chemical substances that are directly involved in their proven biological activities. Considering that plant extracts are typically multicomponent mixtures of active, partially active, and inactive substances, and that a given pharmacological activity is often not exerted on a single target, it becomes very complex to isolate, identify, and attribute this activity to a single chemical substance. In the specific case of parsley aqueous extracts, would the substances responsible for these hypolipidemic, antihypertensive, and antithrombotic activities be the same? The studies presented here do not answer these questions. This is an important gap that needs to be addressed. Additional efforts are needed to identify these therapeutic substances in parsley extracts. Their identification will allow for the study of their mechanism of action, and an evaluation of additional parameters, in addition to enabling the standardization of parsley extracts based on these bioactive compounds.
Another important gap is the absence of toxicological evaluation studies of the parsley aqueous extracts whose pharmacological activities were discussed in the present review. This step needs to be taken to outline the safety margins of their formulations, deepening current knowledge about their possible undesirable side effects.
The efforts that have been made aiming at the production of parsley-based food supplements demonstrate the potential of this herb in the development of formulations that contribute to the preservation of health. Despite the existence of several patents focused on parsley, few of them describe the use of the plant in formulations, and none of them are applied for the prevention of cardiovascular conditions. These patents demonstrate that technological products based on parsley can be interesting, encouraging new investment opportunities with new approaches to this culinary and medicinal herb.
Several teams of researchers around the world have demonstrated the beneficial effects of parsley in diseases related to cardiovascular disorders in animal models, revealing the potential of this herb in dyslipidemia, hypertension, and hemostasis disorders. Would parsley-based formulations be able to reduce cholesterol in patients with hyperlipidemia? Would these formulations be able to normalize blood pressure in hypertensive patients or even reduce the possibility of thrombus formation in patients with hemostasis disorders? In order to answer these questions and achieve progress in this area, clinical trials are necessary to evaluate the effects that were previously observed for parsley aqueous extracts in animal models. However, this stage is challenging because it depends on efforts to resolve the gaps and other concerns mentioned above.
In summary, some important challenges have already been overcome, as shown here. Therefore, based on this current state of knowledge, parsley can be considered as a strong candidate for the development of herbal formulations for cardiovascular health promotion.
Author Contributions
Conceptualization, L.M.C., L.B.d.S.N. and S.S.C.; data curation, L.M.C. and L.B.d.S.N.; writing—review and editing, L.M.C., L.B.d.S.N. and S.S.C.; supervision, S.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This review generated no new data. All the consulted data are available in the cited literature.
Acknowledgments
The authors express their gratitude to the ISI Biossintéticos e Fibras from SENAI CETIQT (Brazil) for allowing the use of their patent search database.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization Cardiovascular Diseases (CVDs)—Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 15 October 2023).
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Li, H.; Ghorbani, S.; Ling, C.-C.; Yong, V.W.; Xue, M. The Extracellular Matrix as Modifier of Neuroinflammation and Recovery in Ischemic Stroke and Intracerebral Hemorrhage. Neurobiol. Dis. 2023, 186, 106282. [Google Scholar] [CrossRef]
- Leja, K.B.; Czaczyk, K. The Industrial Potential of Herbs and Spices: A Mini Review. Acta Sci. Pol. Technol. Aliment. 2016, 15, 353–368. [Google Scholar] [CrossRef]
- Gupta, K.; Testa, H.; Greenwood, T.; Kostek, M.; Haushalter, K.; Kris-Etherton, P.M.; Petersen, K.S. The Effect of Herbs and Spices on Risk Factors for Cardiometabolic Diseases: A Review of Human Clinical Trials. Nutr. Rev. 2022, 80, 400–427. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, S.; Eltablawy, N.A.; Hamed, M.S. Parsley: A Review of Habitat, Phytochemistry, Ethnopharmacology and Biological Activities. J. Med. Plants Stud. 2016, 9, 49–55. [Google Scholar]
- Craig, W.J. Health-Promoting Properties of Common Herbs. Am. J. Clin. Nutr. 1999, 70, 491S–499S. [Google Scholar] [CrossRef] [PubMed]
- Albadwawi, M.A.O.K.; Ahmed, Z.F.R.; Kurup, S.S.; Alyafei, M.A.; Jaleel, A. A Comparative Evaluation of Aquaponic and Soil Systems on Yield and Antioxidant Levels in Basil, an Important Food Plant in Lamiaceae. Agronomy 2022, 12, 3007. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Ichwan, S.J.A.; Soundharrajan, I.; Govindan, N. Nutraceuticals as Potential Therapeutic Agents for Colon Cancer: A Review. Acta Pharm. Sin. B 2014, 4, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Roviello, G.N. Exploring the Protective Effect of Food Drugs against Viral Diseases: Interaction of Functional Food Ingredients and SARS-CoV-2, Influenza Virus, and HSV. Life 2023, 13, 402. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Izzo, C.; Forte, M.; Sommella, E.; Di Pietro, P.; Venturini, E.; Ciccarelli, M.; Galasso, G.; Rubattu, S.; Campiglia, P.; et al. A Novel Promising Frontier for Human Health: The Beneficial Effects of Nutraceuticals in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 8706. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Freedman, J.E. Review: Nutriceuticals as Antithrombotic Agents. Cardiovasc. Ther. 2010, 28, 227–235. [Google Scholar] [CrossRef]
- Tohti, I.; Tursun, M.; Umar, A.; Turdi, S.; Imin, H.; Moore, N. Aqueous Extracts of Ocimum basilicum L. (sweet basil) Decrease Platelet Aggregation Induced by ADP and Thrombin in Vitro and Rats Arterio–Venous Shunt Thrombosis in Vivo. Thromb. Res. 2006, 118, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Mahleyuddin, N.N.; Moshawih, S.; Ming, L.C.; Zulkifly, H.H.; Kifli, N.; Loy, M.J.; Sarker, M.M.R.; Al-Worafi, Y.M.; Goh, B.H.; Thuraisingam, S.; et al. Coriandrum Sativum L.: A Review on Ethnopharmacology, Phytochemistry, and Cardiovascular Benefits. Molecules 2021, 27, 209. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Berkay Yılmaz, Y.; Antika, G.; Salehi, B.; Tumer, T.B.; Kulandaisamy Venil, C.; Das, G.; Patra, J.K.; Karazhan, N.; Akram, M.; et al. Phytochemical Constituents, Biological Activities, and Health-promoting Effects of the Genus Origanum. Phytother. Res. 2021, 35, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, E.S.; Aishwaya, J. Nutraceuticals Potential of Petroselinum crispum: A Review. J. Complement. Med. Altern. Healthc. 2018, 7, 555707. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A Review of Ethnopharmacology, Phytochemistry and Biological Activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef]
- Dobričević, N.; Šic Žlabur, J.; Voća, S.; Pliestić, S.; Galić, A.; Delić, A.; Fabek Uher, S. Bioactive Compounds Content and Nutritional Potential of Different Parsley Parts (Petroselinum crispum Mill.). J. Cent. Eur. Agric. 2019, 20, 900–910. [Google Scholar] [CrossRef]
- Missouri Botanical Garden—Petroselinum crispum. Available online: https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=276060 (accessed on 18 November 2023).
- Santos, J.; Herrero, M.; Mendiola, J.A.; Oliva-Teles, M.T.; Ibáñez, E.; Delerue-Matos, C.; Oliveira, M.B.P.P. Fresh-Cut Aromatic Herbs: Nutritional Quality Stability during Shelf-Life. LWT—Food Sci. Technol. 2014, 59, 101–107. [Google Scholar] [CrossRef]
- dos Santos Franciscato, L.M.S.; Mendes, S.S.; Frederico, C.; Goncalves, J.E.; Faria, M.G.I.; Gazim, Z.C.; Ruiz, S.P. Parsley (Petroselinum crispum): Chemical Composition and Antibacterial Activity of Essential Oil from Organic against Foodborne Pathogens. Aust. J. Crop Sci. 2022, 16, 605–611. [Google Scholar] [CrossRef]
- Seasoning & Spices Market Size, Share & Trends Analysis Report By Product (Spices, Herbs, Salt & Salts Substitutes) Report ID: GVR-2-68038-639-4. Available online: https://www.grandviewresearch.com/industry-analysis/seasonings-spices-market (accessed on 23 September 2023).
- Parsley Market by Product, Distribution Channel, and Geography—Forecast and Analysis 2023–2027. Available online: https://www.technavio.com/report/parsley-market-industry-analysis (accessed on 23 September 2023).
- Patrício, K.P.; Minato, A.C.D.S.; Brolio, A.F.; Lopes, M.A.; Barros, G.R.D.; Moraes, V.; Barbosa, G.C. O Uso de Plantas Medicinais Na Atenção Primária à Saúde: Revisão Integrativa. Ciênc. Saúde Coletiva 2022, 27, 677–686. [Google Scholar] [CrossRef]
- Noureddine, B.; Mostafa, E.; Mandal, S.C. Ethnobotanical, Pharmacological, Phytochemical, and Clinical Investigations on Moroccan Medicinal Plants Traditionally Used for the Management of Renal Dysfunctions. J. Ethnopharmacol. 2022, 292, 115178. [Google Scholar] [CrossRef]
- Ziyyat, A.; Legssyer, A.; Mekhfi, H.; Dassouli, A.; Serhrouchni, M.; Benjelloun, W. Phytotherapy of Hypertension and Diabetes in Oriental Morocco. J. Ethnopharmacol. 1997, 58, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Mootoosamy, A.; Fawzi Mahomoodally, M. Ethnomedicinal Application of Native Remedies Used against Diabetes and Related Complications in Mauritius. J. Ethnopharmacol. 2014, 151, 413–444. [Google Scholar] [CrossRef]
- Ajebli, M.; Eddouks, M. Antihypertensive Activity of Petroselinum crispum through Inhibition of Vascular Calcium Channels in Rats. J. Ethnopharmacol. 2019, 242, 112039. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.A.; Eltablawy, N.A.; Hamed, M.S. The Ameliorative Effect of Petroselinum crispum (Parsley) on Some Diabetes Complications. J. Med. Plants Stud. 2015, 3, 92–100. [Google Scholar]
- Kreydiyyeh, S.I.; Usta, J. Diuretic Effect and Mechanism of Action of Parsley. J. Ethnopharmacol. 2002, 79, 353–357. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, T.; Zhang, W.; Zhao, Z.; Sun, J. Natural Drugs as a Treatment Strategy for Cardiovascular Disease through the Regulation of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 5430407. [Google Scholar] [CrossRef] [PubMed]
- Soltani, D.; Azizi, B.; Rahimi, R.; Talasaz, A.H.; Rezaeizadeh, H.; Vasheghani-Farahani, A. Mechanism-Based Targeting of Cardiac Arrhythmias by Phytochemicals and Medicinal Herbs: A Comprehensive Review of Preclinical and Clinical Evidence. Front. Cardiovasc. Med. 2022, 9, 990063. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Li, C.; Lu, L.; Zhang, Q.; Zhu, R.; Wang, W. A Review of Chinese Herbal Medicine for the Treatment of Chronic Heart Failure. Curr. Pharm. Des. 2018, 23, 5115–5124. [Google Scholar] [CrossRef]
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative Analysis of Flavonols, Flavones, and Flavanones in Fruits, Vegetables and Beverages by High-Performance Liquid Chromatography with Photo-Diode Array and Mass Spectrometric Detection. J. Chromatogr. A 1998, 799, 101–110. [Google Scholar] [CrossRef]
- Liberal, Â.; Fernandes, Â.; Polyzos, N.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Petrović, J.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Bioactive Properties and Phenolic Compound Profiles of Turnip-Rooted, Plain-Leafed and Curly-Leafed Parsley Cultivars. Molecules 2020, 25, 5606. [Google Scholar] [CrossRef]
- Kaiser, A.; Carle, R.; Kammerer, D.R. Effects of Blanching on Polyphenol Stability of Innovative Paste-like Parsley (Petroselinum crispum (Mill.) Nym Ex A. W. Hill) and Marjoram (Origanum majorana L.) Products. Food Chem. 2013, 138, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Luthria, D.L.; Mukhopadhyay, S.; Kwansa, A.L. A Systematic Approach for Extraction of Phenolic Compounds Using Parsley (Petroselinum crispum) Flakes as a Model Substrate. J. Sci. Food Agric. 2006, 86, 1350–1358. [Google Scholar] [CrossRef]
- Epifanio, N.M.D.M.; Cavalcanti, L.R.I.; Dos Santos, K.F.; Duarte, P.S.C.; Kachlicki, P.; Ożarowski, M.; Riger, C.J.; Chaves, D.S.D.A. Chemical Characterization and in Vivo Antioxidant Activity of Parsley (Petroselinum crispum) Aqueous Extract. Food Funct. 2020, 11, 5346–5356. [Google Scholar] [CrossRef] [PubMed]
- Lechtenberg, M.; Zumdick, S.; Engelshowe, R.; Gerhards, C.; Schmidt, T.; Hensel, A. Evaluation of Analytical Markers Characterising Different Drying Methods of Parsley Leaves (Petroselinum crispum L.). Planta Med. 2006, 72, s-2006-950027. [Google Scholar] [CrossRef]
- Chaves, D.S.A.; Frattani, F.S.; Assafim, M.; de Almeida, A.P.; Zingali, R.B.; Costa, S.S. Phenolic Chemical Composition of Petroselinum crispum Extract and Its Effect on Haemostasis. Nat. Prod. Commun. 2011, 6, 1934578X1100600. [Google Scholar] [CrossRef]
- Frattani, F.S.; Assafim, M.; Casanova, L.M.; de Souza, J.E.; Chaves, D.S.D.A.; Costa, S.S.; Zingali, R.B. Oral Treatment with a Chemically Characterized Parsley (Petroselinum crispum Var. neapolitanum Danert) Aqueous Extract Reduces Thrombi Formation in Rats. J. Tradit. Complement. Med. 2021, 11, 287–291. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Young, J.F.; Daneshvar, B.; Lauridsen, S.T.; Knuthsen, P.; Sandström, B.; Dragsted, L.O. Effect of Parsley (Petroselinum crispum) Intake on Urinary Apigenin Excretion, Blood Antioxidant Enzymes and Biomarkers for Oxidative Stress in Human Subjects. Br. J. Nutr. 1999, 81, 447–455. [Google Scholar] [CrossRef]
- Meyer, H.; Bolarinwa, A.; Wolfram, G.; Linseisen, J. Bioavailability of Apigenin from Apiin-Rich Parsley in Humans. Ann. Nutr. Metab. 2006, 50, 167–172. [Google Scholar] [CrossRef]
- Naeem, A.; Ming, Y.; Pengyi, H.; Jie, K.Y.; Yali, L.; Haiyan, Z.; Shuai, X.; Wenjing, L.; Ling, W.; Xia, Z.M.; et al. The Fate of Flavonoids after Oral Administration: A Comprehensive Overview of Its Bioavailability. Crit. Rev. Food Sci. Nutr. 2022, 62, 6169–6186. [Google Scholar] [CrossRef]
- Borges, G.; Fong, R.Y.; Ensunsa, J.L.; Kimball, J.; Medici, V.; Ottaviani, J.I.; Crozier, A. Absorption, Distribution, Metabolism and Excretion of Apigenin and Its Glycosides in Healthy Male Adults. Free Radic. Biol. Med. 2022, 185, 90–96. [Google Scholar] [CrossRef]
- Proz, M.D.L.Á.; Da Silva, M.A.S.; Rodrigues, E.; Bender, R.J.; Rios, A.D.O. Effects of Indoor, Greenhouse, and Field Cultivation on Bioactive Compounds from Parsley and Basil. J. Sci. Food Agric. 2021, 101, 6320–6330. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Uemura, T.; Shimoda, H.; Kishi, A.; Kawahara, Y.; Matsuda, H. Medicinal Foodstuffs. XVIII. Phytoestrogens from the Aerial Part of Petroselinum crispum MILL. (PARSLEY) and Structures of 6”-Acetylapiin and a New Monoterpene Glycoside, Petroside. Chem. Pharm. Bull. 2000, 48, 1039–1044. [Google Scholar] [CrossRef]
- Sbai, H.; Saad, I.; Ghezal, N.; Greca, M.D.; Haouala, R. Bioactive Compounds Isolated from Petroselinum crispum L. Leaves Using Bioguided Fractionation. Ind. Crops Prod. 2016, 89, 207–214. [Google Scholar] [CrossRef]
- Staropoli, A.; Vassetti, A.; Salvatore, M.M.; Andolfi, A.; Prigigallo, M.I.; Bubici, G.; Scagliola, M.; Salerno, P.; Vinale, F. Improvement of Nutraceutical Value of Parsley Leaves (Petroselinum crispum) upon Field Applications of Beneficial Microorganisms. Horticulturae 2021, 7, 281. [Google Scholar] [CrossRef]
- Derouich, M.; Bouhlali, E.D.T.; Bammou, M.; Hmidani, A.; Sellam, K.; Alem, C. Bioactive Compounds and Antioxidant, Antiperoxidative, and Antihemolytic Properties Investigation of Three Apiaceae Species Grown in the Southeast of Morocco. Scientifica 2020, 2020, 3971041. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, M.A.; Prado, J.M. Natural Product Extraction: Principles and Applications; The Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- Gupta, S.R.; Sechsadri, F.A.S. A Study of Apiin from the Parsley Seeds and Plant. Proc. Indian Acad. Sci.—Sect. A 1952, 35, 242–248. [Google Scholar] [CrossRef]
- Piras, A.; Porcedda, S.; Falconieri, D.; Fais, A.; Era, B.; Carta, G.; Rosa, A. Supercritical Extraction of Volatile and Fixed Oils from Petroselinum crispum L. Seeds: Chemical Composition and Biological Activity. Nat. Prod. Res. 2022, 36, 1883–1888. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Saleh, A.M.; Mahmood, A.A.R.; Khalaf, M.A.; Calva, J.; Loyola-Gonzales, E.; Tataje-Napuri, F.E.; Chávez, H.; Almeida-Galindo, J.S.; Chavez-Espinoza, J.H.; et al. The Essential Oil of Petroselinum crispum (Mill) Fuss Seeds from Peru: Phytotoxic Activity and In Silico Evaluation on the Target Enzyme of the Glyphosate Herbicide. Plants 2023, 12, 2288. [Google Scholar] [CrossRef] [PubMed]
- Mekhfi, H.; Haouari, M.E.; Legssyer, A.; Bnouham, M.; Aziz, M.; Atmani, F.; Remmal, A.; Ziyyat, A. Platelet Anti-Aggregant Property of Some Moroccan Medicinal Plants. J. Ethnopharmacol. 2004, 94, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Gadi, D.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Legssyer, A.; Legrand, C.; Lafeve, F.F.; Mekhfi, H. Parsley Extract Inhibits in Vitro and Ex Vivo Platelet Aggregation and Prolongs Bleeding Time in Rats. J. Ethnopharmacol. 2009, 125, 170–174. [Google Scholar] [CrossRef]
- Gadi, D.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Legssyer, A.; Bruel, A.; Berrabah, M.; Legrand, C.; Fauvel-Lafeve, F.; Mekhfi, H. Flavonoids Purified from Parsley Inhibit Human Blood Platelet Aggregation and Adhesion to Collagen under Flow. J. Complement. Integr. Med. 2012, 9. [Google Scholar] [CrossRef]
- Campos, K.E.D.; Balbi, A.P.C.; Alves, M.J.Q.D.F. Diuretic and Hipotensive Activity of Aqueous Extract of Parsley Seeds (Petroselinum sativum Hoffm.) in Rats. Rev. Bras. Farmacogn. 2009, 19, 41–45. [Google Scholar] [CrossRef][Green Version]
- El Rabey, H.A.; Al-Seeni, M.N.; Al-Ghamdi, H.B. Comparison between the Hypolipidemic Activity of Parsley and Carob in Hypercholesterolemic Male Rats. BioMed Res. Int. 2017, 2017, 3098745. [Google Scholar] [CrossRef]
- Ashorobi, D.; Ameer, M.A.; Fernandez, R. Thrombosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Delluc, A.; Lacut, K.; Rodger, M.A. Arterial and Venous Thrombosis: What’s the Link? A Narrative Review. Thromb. Res. 2020, 191, 97–102. [Google Scholar] [CrossRef]
- Thachil, J. Deep Vein Thrombosis. Hematology 2014, 19, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Favaloro, E. Venous and Arterial Thromboses: Two Sides of the Same Coin? Semin. Thromb. Hemost. 2018, 44, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Núñez, L.; Lozano, M.L.; Palomo, M.; Martínez, C.; Vicente, V.; Castillo, J.; Benavente-García, O.; Diaz-Ricart, M.; Escolar, G.; Rivera, J. Apigenin Inhibits Platelet Adhesion and Thrombus Formation and Synergizes with Aspirin in the Suppression of the Arachidonic Acid Pathway. J. Agric. Food Chem. 2008, 56, 2970–2976. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.A.; Lozano, M.L.; Castillo, J.; Benavente-García, O.; Vicente, V.; Rivera, J. Flavonoids Inhibit Platelet Function through Binding to the Thromboxane A2 Receptor. J. Thromb. Haemost. 2005, 3, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Núñez, L.; Rivera, J.; Guerrero, J.; Martínez, C.; Vicente, V.; Lozano, M. Differential Effects of Quercetin, Apigenin and Genistein on Signalling Pathways of Protease-Activated Receptors PAR1 and PAR4 in Platelets: Flavonoids Inhibit PAR1 and PAR4 Signalling Cascades. Br. J. Pharmacol. 2009, 158, 1548–1556. [Google Scholar] [CrossRef]
- Tsiailanis, A.D.; Tellis, C.C.; Papakyriakopoulou, P.; Kostagianni, A.D.; Gkalpinos, V.; Chatzigiannis, C.M.; Kostomitsopoulos, N.; Valsami, G.; Tselepis, A.D.; Tzakos, A.G. Development of a Novel Apigenin Dosage Form as a Substitute for the Modern Triple Antithrombotic Regimen. Molecules 2023, 28, 2311. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- World Health Organization Hypertension—Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 4 September 2023).
- Ozemek, C.; Laddu, D.R.; Arena, R.; Lavie, C.J. The Role of Diet for Prevention and Management of Hypertension. Curr. Opin. Cardiol. 2018, 33, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.R.; McCormack, T.; Constanti, M.; McManus, R.J. Diagnosis and Management of Hypertension in Adults: NICE Guideline Update 2019. Br. J. Gen. Pract. 2020, 70, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M.; Bakris, G.; Williams, B. Redefining Diuretics Use in Hypertension: Why Select a Thiazide-like Diuretic? J. Hypertens. 2019, 37, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Vranješ, M.; Popović, B.M.; Štajner, D.; Ivetić, V.; Mandić, A.; Vranješ, D. Effects of Bearberry, Parsley and Corn Silk Extracts on Diuresis, Electrolytes Composition, Antioxidant Capacity and Histopathological Features in Mice Kidneys. J. Funct. Foods 2016, 21, 272–282. [Google Scholar] [CrossRef]
- Je, H.D.; Kim, H.-D.; La, H.-O. The Inhibitory Effect of Apigenin on the Agonist-Induced Regulation of Vascular Contractility via Calcium Desensitization-Related Pathways. Biomol. Ther. 2014, 22, 100–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Z.-Y.; Gao, T.; Huang, Y.; Xue, J.; Xie, M.-L. Apigenin Ameliorates Hypertension-Induced Cardiac Hypertrophy and down-Regulates Cardiac Hypoxia Inducible Factor-Lα in Rats. Food Funct. 2016, 7, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.; Romecín, P.; Atucha, N.; O’Valle, F.; Castillo, J.; Ortiz, M.; García-Estañ, J. Beneficial Effects of Different Flavonoids on Vascular and Renal Function in L-NAME Hypertensive Rats. Nutrients 2018, 10, 484. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Paredes, M.; Romecín, P.; Atucha, N.; O’Valle, F.; Castillo, J.; Ortiz, M.; García-Estañ, J. Moderate Effect of Flavonoids on Vascular and Renal Function in Spontaneously Hypertensive Rats. Nutrients 2018, 10, 1107. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global Epidemiology of Dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Kopin, L.; Lowenstein, C.J. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Vrablik, M.; Al Rasadi, K.; Banach, M.; Toth, P.P.; Rizzo, M. Nutraceuticals in the Management of Dyslipidemia: Which, When, and for Whom? Could Nutraceuticals Help Low-Risk Individuals with Non-Optimal Lipid Levels? Curr. Atheroscler. Rep. 2021, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Bahiru, E.; Hsiao, R.; Phillipson, D.; Watson, K.E. Mechanisms and Treatment of Dyslipidemia in Diabetes. Curr. Cardiol. Rep. 2021, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Dyslipidemia in Diabetes; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Jung, U.; Cho, Y.-Y.; Choi, M.-S. Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients 2016, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, L.; Cheng, Q.; Ji, B.; Yang, M.; Sanidad, K.Z.; Wang, C.; Zhou, F. Structurally Different Flavonoid Subclasses Attenuate High-Fat and High-Fructose Diet Induced Metabolic Syndrome in Rats. J. Agric. Food Chem. 2018, 66, 12412–12420. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Tan, Y.Q.; Lin, S.; Leung, L.K. Co-Administrating Apigenin in a High-Cholesterol Diet Prevents Hypercholesterolaemia in Golden Hamsters. J. Pharm. Pharmacol. 2018, 70, 1253–1261. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Y.; Du, C.; Wang, L.; Xiao, Y. Effects of Apigenin on the Expression of LOX-1, Bcl-2, and Bax in Hyperlipidemia Rats. Chem. Biodivers. 2021, 18, e2100049. [Google Scholar] [CrossRef]
- Kharchoufa, L.; Bouhrim, M.; Bencheikh, N.; Addi, M.; Hano, C.; Mechchate, H.; Elachouri, M. Potential Toxicity of Medicinal Plants Inventoried in Northeastern Morocco: An Ethnobotanical Approach. Plants 2021, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, N.; Scognamiglio, M.; Pacifico, S.; Mekhoukhe, A.; Madani, K.; Fiorentino, A.; Monaco, P. 1 H NMR Based Metabolic Profiling of Eleven Algerian Aromatic Plants and Evaluation of Their Antioxidant and Cytotoxic Properties. Food Res. Int. 2015, 76, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Lantto, T.A.; Raasmaja, A.; Hiltunen, R. Antioxidant, pro-Oxidant and Cytotoxic Properties of Parsley. Food Funct. 2011, 2, 328. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.L.; Rajarajeswaran, J.; Fung, S.; Kanthimathi, M. Petroselinum crispum Has Antioxidant Properties, Protects against DNA Damage and Inhibits Proliferation and Migration of Cancer Cells. J. Sci. Food Agric. 2015, 95, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Farshori, N.N.; Al-Sheddi, E.S.; Al-Oqail, M.M.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Cytotoxicity Assessments of Portulaca Oleracea and Petroselinum Sativum Seed Extracts on Human Hepatocellular Carcinoma Cells (HepG2). Asian Pac. J. Cancer Prev. 2014, 15, 6633–6638. [Google Scholar] [CrossRef] [PubMed]
- Al-Oqail, M.M.; Farshori, N.N.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Siddiqui, M.A.; Al-Khedhairy, A.A. Petroselinum Sativum Protects HepG2 Cells from Cytotoxicity and Oxidative Stress Induced by Hydrogen Peroxide. Mol. Biol. Rep. 2020, 47, 2771–2780. [Google Scholar] [CrossRef] [PubMed]
- Farshori, N.N.; Al-Sheddi, E.S.; Al-Oqail, M.M.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Anticancer Activity of Petroselinum Sativum Seed Extracts on MCF-7 Human Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2013, 14, 5719–5723. [Google Scholar] [CrossRef]
- Abu-Serie, M.M.; Habashy, N.H.; Maher, A.M. In Vitro Anti-Nephrotoxic Potential of Ammi Visnaga, Petroselinum crispum, Hordeum Vulgare, and Cymbopogon Schoenanthus Seed or Leaf Extracts by Suppressing the Necrotic Mediators, Oxidative Stress and Inflammation. BMC Complement. Altern. Med. 2019, 19, 149. [Google Scholar] [CrossRef]
- Awe, E.O.; Banjoko, S.O. Biochemical and Haematological Assessment of Toxic Effects of the Leaf Ethanol Extract of Petroselinum crispum (Mill) Nyman Ex A.W. Hill (Parsley) in Rats. BMC Complement. Altern. Med. 2013, 13, 75. [Google Scholar] [CrossRef]
- Slighoua, M.; Mahdi, I.; Amrati, F.E.-Z.; Di Cristo, F.; Amaghnouje, A.; Grafov, A.; Boucetta, N.; Bari, A.; Bousta, D. Assessment of in Vivo Estrogenic and Anti-Inflammatory Activities of the Hydro-Ethanolic Extract and Polyphenolic Fraction of Parsley (Petroselinum Sativum Hoffm.). J. Ethnopharmacol. 2021, 265, 113290. [Google Scholar] [CrossRef]
- Alajlouni, A.M.; Al-Malahmeh, A.J.; Isnaeni, F.N.; Wesseling, S.; Vervoort, J.; Rietjens, I.M.C.M. Level of Alkenylbenzenes in Parsley and Dill Based Teas and Associated Risk Assessment Using the Margin of Exposure Approach. J. Agric. Food Chem. 2016, 64, 8640–8646. [Google Scholar] [CrossRef]
- Alajlouni, A.M.; Al-Malahmeh, A.J.; Wesseling, S.; Kalli, M.; Vervoort, J.; Rietjens, I.M.C.M. Risk Assessment of Combined Exposure to Alkenylbenzenes through Consumption of Plant Food Supplements Containing Parsley and Dill. Food Addit. Contam. Part A 2017, 34, 2201–2211. [Google Scholar] [CrossRef]
- Ciganda, C.; Laborde, A. Herbal Infusions Used for Induced Abortion: ARTICLE. J. Toxicol. Clin. Toxicol. 2003, 41, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.J.; Lopez, R.G. Modeling Growth and Development of Hydroponically Grown Dill, Parsley, and Watercress in Response to Photosynthetic Daily Light Integral and Mean Daily Temperature. PLoS ONE 2021, 16, e0248662. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue Light Dosage Affects Carotenoids and Tocopherols in Microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Jankauskienė, J.; Sakalauskienė, S.; Duchovskis, P. Red Light-Dose or Wavelength-Dependent Photoresponse of Antioxidants in Herb Microgreens. PLoS ONE 2016, 11, e0163405. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; El-Nakhel, C.; De Micco, V.; Giordano, M.; Pannico, A.; De Pascale, S.; Graziani, G.; Ritieni, A.; Soteriou, G.A.; Kyriacou, M.C.; et al. Morpho-Metric and Specialized Metabolites Modulation of Parsley Microgreens through Selective LED Wavebands. Agronomy 2022, 12, 1502. [Google Scholar] [CrossRef]
- Ferreira, F.S.; De Oliveira, V.S.; Chávez, D.W.H.; Chaves, D.S.; Riger, C.J.; Sawaya, A.C.H.F.; Guizellini, G.M.; Sampaio, G.R.; Torres, E.A.F.D.S.; Saldanha, T. Bioactive Compounds of Parsley (Petroselinum crispum), Chives (Allium schoenoprasum L.) and Their Mixture (Brazilian cheiro-verde) as Promising Antioxidant and Anti-Cholesterol Oxidation Agents in a Food System. Food Res. Int. 2022, 151, 110864. [Google Scholar] [CrossRef]
- De Oliveira, V.S.; Chávez, D.W.H.; Paiva, P.R.F.; Gamallo, O.D.; Castro, R.N.; Sawaya, A.C.H.F.; Sampaio, G.R.; Torres, E.A.F.D.S.; Saldanha, T. Parsley (Petroselinum crispum Mill.): A Source of Bioactive Compounds as a Domestic Strategy to Minimize Cholesterol Oxidation during the Thermal Preparation of Omelets. Food Res. Int. 2022, 156, 111199. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U.; Luty, M.; Czyż, J. Effect of Fortification with Parsley (Petroselinum crispum Mill.) Leaves on the Nutraceutical and Nutritional Quality of Wheat Pasta. Food Chem. 2016, 190, 419–428. [Google Scholar] [CrossRef]
- Dziki, D.; Hassoon, W.H.; Biernacka, B.; Gawlik-Dziki, U. Dried and Powdered Leaves of Parsley as a Functional Additive to Wheat Bread. Appl. Sci. 2022, 12, 7930. [Google Scholar] [CrossRef]
- Bouasla, A.; Gassi, H.E.; Lisiecka, K.; Wójtowicz, A. Application of Parsley Leaf Powder as Functional Ingredient in Fortified Wheat Pasta: Nutraceutical, Physical and Organoleptic Characteristics. Int. Agrophysics 2022, 36, 37–45. [Google Scholar] [CrossRef]
- Hnin, K.K.; Zhang, M.; Ju, R.; Wang, B. A Novel Infrared Pulse-Spouted Freeze Drying on the Drying Kinetics, Energy Consumption and Quality of Edible Rose Flowers. LWT 2021, 136, 110318. [Google Scholar] [CrossRef]
- Mouhoubi, K.; Boulekbache-Makhlouf, L.; Madani, K.; Palatzidi, A.; Perez-Jimenez, J.; Mateos-Aparicio, I.; Garcia-Alonso, A. Phenolic Compounds and Antioxidant Activity Are Differentially Affected by Drying Processes in Celery, Coriander and Parsley Leaves. Int. J. Food Sci. Technol. 2022, 57, 3467–3476. [Google Scholar] [CrossRef]
- Kamel, S.M. Effect of Microwave Treatments on Some Bioactive Compounds of Parsley (Petroselinum crispum) and Dill (Anethum graveolens) Leaves. J. Food Process. Technol. 2013, 4, 1000233. [Google Scholar] [CrossRef]
- Kaiser, A.; Brinkmann, M.; Carle, R.; Kammerer, D.R. Influence of Thermal Treatment on Color, Enzyme Activities, and Antioxidant Capacity of Innovative Pastelike Parsley Products. J. Agric. Food Chem. 2012, 60, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef] [PubMed]
- Helmy, A.; El-Shazly, M.; Seleem, A.; Abdelmohsen, U.; Salem, M.A.; Samir, A.; Rabeh, M.; Elshamy, A.; Singab, A.N.B. The Synergistic Effect of Biosynthesized Silver Nanoparticles from a Combined Extract of Parsley, Corn Silk, and Gum Arabic: In Vivo Antioxidant, Anti-Inflammatory and Antimicrobial Activities. Mater. Res. Express 2020, 7, 025002. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-Spectrum Bioactivities of Silver Nanoparticles: The Emerging Trends and Future Prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef]
- World Intellectual Property Organization (WIPO) World Intellectual Property Indicators Report: Record Number of Patent Applications Filed Worldwide in 2022. Available online: https://www.wipo.int/pressroom/en/articles/2023/article_0013.html#:~:text=Registrations%20in%20force%20in%20China,)%20and%20Japan%20(270%2C073) (accessed on 8 December 2023).
- Optimal Health StemSation StemRCM. Available online: https://optimal-health.uk/stemsation-stemrcm (accessed on 8 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).