Abstract
Blending is a commonly utilized technique for enhancing the oxidative stability, nutritional quality, and physicochemical properties of vegetable oils. This study explored the potential of a vegetable oil blend consisting of common seed oils (sunflower, soybean, rapeseed, cottonseed, and corn oils), through partial least squares analysis, as a substitute for palm oil in the food preparation sector. Oxidative stability assays were conducted initially and after 14 and 28 days of incubation at 60 °C. These assays included radical inhibition activities between the optimal blended oil and palm oil through DPPH• inhibition activity and thermal stability via accelerated oxidation conditions with Rancimat (110 °C, 15 L/h) and conjugated diene and triene formation. The impact of each oil was assessed through correlation analyses and Pareto plots. The optimal blended oil consisted of soybean/sunflower/cottonseed/corn oils at a ratio of 2:1:4:4. It had an induction period (i.e., full rancidity) vastly enhanced to 5.38 h but was statistically significantly lower than the stable palm oil by ~50%. Prior to thermal incubation, the blended oil was more potent in inhibiting DPPH•, as it recorded 139.83 μmol of Trolox equivalents per kg of oil, ~53% more than palm oil. The conjugated diene and triene concentrations were similar for both oils at ~15 and ~7 mmol/kg oil, respectively. The Fourier-Transform Infrared spectra revealed the prevalence of cis fatty acids in the optimal oil blend and trans fatty acids in palm oil, indicating an enhancement in the nutritional quality of the vegetable oil blend. The results of the study could provide a nutritional oil blend that could be used as a substitute for palm oil in the food industry.
1. Introduction
Oils and fats are an important source of energy to humans, since they supply calories and provide the body with fat-soluble vitamins. Additionally, they serve as precursors to various hormones and supply essential fatty acids [1]. The fatty acid compositions of some commonly used vegetable oils vary significantly [2]. For instance, coconut and palm oils have a high content of saturated fats (i.e., ~90 and ~44%, respectively), whereas seed oils (i.e., corn, sunflower, or soybean oil) have mostly polyunsaturated fatty acids. There is a strong relation between the economic, organoleptic, and functional qualities of foods and food products and the types of vegetable oils or fats used [3]. Olive oil and other vegetable oils, such as mid-oleic sunflower oil and rapeseed oil containing cis-monounsaturated and polyunsaturated fatty acids, are known to provide health benefits to consumers, such as protection from cardiovascular diseases [4]. In fact, the monounsaturated-to-polyunsaturated fatty acid ratios of conventional vegetable oils such as soybean (1.5:3.8), rapeseed (9.7:3.9), sunflower (2.5:5.9), cottonseed (2.0:5.7), and corn (2.0:3.0) oils were previously documented [5]. In terms of thermal stability, it is widely known that the higher the amount of double bonds in a fatty acid, the greater the susceptibility to oxidation [6]. On the contrary, trans fatty acids shows exceptional thermal stability, but a high consumption rate could dysregulate biological pathways that affect cardiometabolic health [7].
The oil obtained from the palm tree (Elaeis guineensis) has gained popularity as a major edible oil in recent decades in the food industry. Palm oil has been used extensively in Western Europe as a substitute for partially hydrogenated oils in food applications given that it is solid at ambient temperature and its fractions provide a broad spectrum of melting profiles [8]. The high content of saturated fatty acids is a concern to human health when compared to other conventional vegetable oils. It should be noted that the yield of palm oil per year is the highest of any edible oil. However, palm oil is also a topic of debate, since it causes negative environmental issues such as the destruction of vast rainforests. A partial or total reduction of this oil may provide a long-term solution to deforestation while simultaneously having positive economic and environmental effects [9]. To that end, legislation in an increasing number of nations is imposing restrictions, which has led to a growing interest in finding alternatives to palm oil [10].
The blending of vegetable oils possessing distinct properties represents a straightforward approach to developing novel products that possess the intended sensory and oxidative characteristics [11]. A single vegetable oil may not meet consumer standards for oxidative stability, physical and chemical properties, or nutritional quality. The blending of oils alters their triacylglycerol profiles, which subsequently impacts various physical properties of the oils, including their density, viscosity, cloud point, solid fat content, and sensory quality [12]. Furthermore, oils having different amounts of unsaponifiable material, antioxidant compounds, and fatty acid compositions would fry differently and reduce hazardous compounds [13]. Bioactive compounds such as tocopherols, polyphenols, chlorophylls, and carotenoids could also contribute to the oxidative stability of oils, since they scavenge free radicals [6]. It was previously mentioned that the formation of hazardous polar compounds, such as triglyceride oligomers or diglycerides, during deep frying was more significantly influenced by the type of oil rather than by the type of food. Trans fatty acid synthesis is directly related to the oxidative stability of oils; thus, elements that speed up lipid oxidation will also raise the trans fatty acid content [14]. These reactions may significantly impair the quality of oils as the frying process progresses [15]. Therefore, by identifying the properties of oils and utilizing suitable oils for blending, it is possible to enhance the nutritional and sensory attributes of the product [16]. Given that the hydrogenation of fatty acids produces dangerous trans fatty acids and lowers the quality of the oil, specific oil blending could improve the oil stability under frying and replace these types of oils in the food industry [17].
Palm oil has been used for the frying process due to its high stability at high temperatures [18]. Several seed oils, produced from plants that can be cultivated in various climates worldwide, with high concentrations of polyunsaturated fatty acids, such as corn oil or sunflower oil, are also utilized for frying in the food sector [19,20]. To the best of the authors’ knowledge, studies examining the blending of vegetable oils using multiple different seed oils are limited. To that end, this study aimed to create a vegetable oil blend with five widely commercially available seed oils used in the food sector (i.e., sunflower, soybean, corn, rapeseed, and cottonseed oils) as a palm oil substitute through partial least squares. The nutritional quality and oxidative stability of the blend were compared to palm oil. The results of this study could indicate a suitable oil blend of high nutritional quality that could be widely used in the food industry.
2. Materials and Methods
2.1. Reagents and Chemicals
Cyclohexane and DPPH• (2,2-diphenyl-1-picrylhydrazyl radical) were both purchased from Sigma-Aldrich (St. Louis, MA, USA). Ethyl acetate was bought from Carlo Erba (Vaul de Reuil, France). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was purchased from Glentham Life Sciences (Corsham, UK).
2.2. Vegetable Oil Sample Procurement
Fresh, refined vegetable oils, including soybean oil, sunflower oil, rapeseed oil, cottonseed oil, corn oil, and palm oil, were all purchased from a local market (Karditsa, Greece). All samples were stored in the refrigerator at a temperature < 4 °C. To achieve thermal equilibrium between them and room temperature, they were kept in the dark (20 °C) prior to analysis and were immediately analyzed after opening. Each prepared blend was homogenized by stirring for at least 20 min with a Heidolph magnetic stirrer (Heidolph Instruments GmbH & Co. KG, Schwabach, Germany).
2.3. Blended Vegetable Oil Mixture Preparations and Thermal Treatment
A diverse range of blends was produced by combining pure edible vegetable oil samples at different concentrations. The variables X1–X5 represent each vegetable oil, excluding palm oil, and their respective ratio is indicated in the Coded Variable Levels. Further details are available in Table 1 below. A quantity of 10 g of each oil blend and palm oil was placed in Duran bottles and was incubated at 60 °C for 28 days in a water bath in the dark, as per our previous study [21] and other similar approaches [22,23,24]. To perform the oil stability analyses, sampling was conducted before incubation and 14 and 28 days after incubation. To shed more light on the quality of the blended oils, pure substrate oils were also included in the study.

Table 1.
The actual and coded levels of the independent variables were used to optimize the best blend of vegetable oils using the Main Effects Screening design.
2.4. Main Effects Screening Design
Factorial experiments benefit substantially from orthogonal arrays with mixed levels when studying factors with different numbers of levels [25]. However, many mixed-level orthogonal arrays require a large number of experiments, which presents a major concern. By slightly easing the requirement of orthogonality, nearly orthogonal arrays reduce the number of experiments needed, making them a good fit for such situations [26]. To that end, the Main Effects Screening design within a Response Surface Methodology (RSM) was utilized to effectively explore the design space with fewer experiments in order to investigate the optimal ratios of the oil samples and to assess their antioxidant activities and thermal stabilities. To achieve this, the ratio of the oils was adjusted. The optimization method was accomplished using a Screening design of 25 design points with different compositions. The design also included five pure vegetable oils along with pure palm oil. Summary-of-fit tests, the analysis of variance (ANOVA), the overall model significance (R2, p-value), and the significance of the model (equations) coefficients were evaluated at a minimum level of 95%, with Equation (1) describing the polynomial plot:
where the independent variables are represented as Xi and Xj, and the predicted response variable is defined as Yk. The terms β0, βi, βii, and βij describe the model’s linear, quadratic, and interaction terms, represented by the intercept and regression coefficients, respectively.
2.5. Oil Quality Tests
2.5.1. Accelerated Oxidation through Rancimat Apparatus
The oxidation stability assessment was conducted using a Rancimat 743 apparatus from Metrohm LTD. (Herisau, Switzerland) and was similar to a previous approach [27]. The device was connected to 873 Biodiesel Rancimat software (version 1.00). The mass of the oil sample used was 3 ± 0.01 g, with the device being set to a temperature of 110 °C and an air flow rate of 15 L/h. The assessment was conducted on days 0, 14, and 28 of incubation. The beginning of oxidative degradation, or rancidity, was identified as the point of abrupt inflation in conductivity from the plotted graph.
2.5.2. Determination of Antiradical Activity (AAR)
The antiradical activity was evaluated using an established methodology [28]. Briefly, 1.0 g of oil was dissolved in 10 mL of ethyl acetate. Then, 50 μL of each oil sample was mixed with 1950 μL DPPH• solution (100 μM in ethyl acetate) and vigorously shaken for 10 s. The absorbance was immediately recorded at 515 nm after mixing (A515(i)) and after exactly 30 min (A515(f)). The % inhibition of DPPH• was calculated according to the following Equation (2):
The antiradical activity was calculated as μmol of Trolox equivalent antioxidant capacity (TEAC) per kilogram of oil (μmol TEAC/kg oil) through a calibration curve (50–500 μM Trolox).
The percentage of oxidative stability of oils (%OS) was calculated in both Rancimat induction period (IP) and DPPH values on days 0, 14, and 28 of incubation using the following equation:
2.5.3. Conjugated Diene and Triene Determination
Conjugated dienes (CDs) and conjugated trienes (CTs) were measured following our previously described method [29]. Cyclohexane was added to a 5 mL volumetric flask along with 0.01 g of oil sample. The concentrations of CDs were detected at 232 nm, while the concentrations of CTs were detected at 270 nm.
The absorbances at 232 nm and 270 nm are denoted as A232 and A270, respectively; ε is the molar absorptivity of linoleic acid hydroperoxide, which is 2.525 × 104 M−1 cm−1, and l is the path length of the cuvette (1 cm). To express the concentrations of CDs (CCD) and CTs (CCT) in mmol per kg of oil, the factor 5 × 103 accounts for the volume of the solvent (5 mL) used to dissolve the oil sample, and w is the weight of the oil sample in grams.
The percentage of oxidative power (%OP) of oils was calculated in both CD and CT values on days 0, 14, and 28 of incubation using the following equation:
2.6. AΤR–FTIR Spectroscopy Acquisition
The FTIR spectra were obtained using a Shimadzu Fourier-Transform Infrared Prestige21 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) equipped with an Attenuated Total Reflectance (ATR) accessory with a trough plate composed of ZnSe crystal. The instrument was equipped with a deuterated triglycine sulphate doped with an L-alanine (DLATGS) detector, a KBr beam splitter, high-energy throughput optical elements, and a bright ceramic light source. A total of 32 scans were taken from 0.8 mL of each oil sample, ranging from 4000 to 400 cm−1 at a resolution of 4 cm−1. The ATR crystal was thoroughly cleaned before and after the measurement with a tissue soaked in acetone to ensure that no oil residue was present. For each sample, all measurements were conducted in triplicate. The FTIR spectra of all samples were processed through Shimadzu IRsolution (version 1.60) software. The spectra of the samples were recorded for an analysis of the baseline and signal-to-noise ratios.
2.7. Statistical Analysis
The samples were measured in triplicate for each assay, and the results are shown as means of triplicate determinations. A one-way analysis of variance (ANOVA) test was used to determine the statistical significance of the differences between mean values; a multiple comparisons test (Tukey HSD) was used to determine the statistical significance at p < 0.05. Using JMP® Pro 16 software (SAS, Cary, NC, USA), the experimental design for the Response Surface Methodology and all related statistics was completed. Pareto plot analysis, partial least squares (PLS) analysis, multiple factor analysis (MFA), and multivariate correlation analysis (MCA) were all performed using the same software.
3. Results and Discussion
Oil blending is a traditionally employed technique to modify fatty acid profiles while improving the nutritional quality in oils. Blended oils with better properties and increased oxidative stability are a common product of the food industry. De Marco et al. [30] studied the possibility of preparing a binary mixture of sunflower and palm oils in various ratios, creating a mixture richer in tocopherols and UFAs, which would retain its liquid form under ambient conditions and be oxidative and more stable in deep frying conditions. However, they used only one oil to partially substitute palm oil. A similar approach was conducted by Siddique et al. [31], who blended rapeseed, soybean, and sunflower oils with palm oil in order to achieve a stable blend with a low melting point. Several studies also include the use of unconventional oils (i.e., black cumin oil, coriander oil, or pomegranate seed oil) to improve the nutritional quality of vegetable oils [19,32,33]. In our study, we opted to use several cheap and conventional vegetable oils with a wide range of MUFAs (~19–62%) and PUFAs (~30–56%) in order to enhance the nutritional quality of the blend and SFAs (~8–28%) to maintain thermal stability. We aimed to examine the potential oxidation-resistant capacity of the optimum blended vegetable oil sample in comparison to palm oil, which has considerably higher long-term oxidative stability [34]. The specific oils were chosen as alternatives to palm oil, since they are the most prevalent oils produced in the European Union [22].
3.1. Alterations in Antiradical Activity and Oxidative Stability
The Trolox-equivalent antiradical activity (TEAC) and oxidative stability were assessed before and after incubation to determine whether alterations in the oxidative condition of each sample affected their radical-inhibiting capacity. To that end, sampling was conducted initially and after 14 and 28 days following thermal incubation at 60 °C. The results of the Rancimat procedure and DPPH• inhibition activity are shown in Table 2. It was observed that the induction period ranged from 1.12–10.8 h in unexposed oil samples. Design point 7, which consisted of sunflower, cottonseed, rapeseed, and corn oils in high proportions, had the lowest value at the induction period but showed moderately high (67.9%) oxidative stability, whereas palm oil had the highest value, indicating high oxidation resistance. It was revealed that a high content of both sunflower and rapeseed oils was not preferable in the blended oil (vide infra). However, design point 17, mainly consisting of rapeseed oil, showed the highest oxidative stability of 87.1%. Despite the low pre-incubation resistance of 2.69 h at 60 °C, it appeared to resist oxidation, demanding 2.34 h for full oxidation after 28 days. Regarding DPPH• inhibition activity, it ranged from 66.51 to 179.07 μmol TEAC/kg of oil. The lowest value was measured in palm oil, whereas design point 13 had the highest value. By the end of the incubation period, the radical inhibition ability decreased in all samples, as expected. However, the decrease ratio was different for each sample, indicating that bioactive compounds naturally present in vegetable oils could explain their inherent radical-scavenging potential [35].

Table 2.
Experimental findings for the five independent variables under investigation and the dependent variable response in % oxidative stability (%OS) using the Rancimat and DPPH methods during storage at 60 °C for 28 days; pure substrate oils (26–30) are also presented.
3.2. Generation of Oxidation By-Products Assessment
To simulate long-term stability, the conjugated dienes and trienes were measured until day 28 of incubation. Monitoring the CD values helps assess the extent of lipid oxidation and rancidity. CTs are less stable than CDs and could decompose after a certain period when compared to CDs. The results from the CD and CT values along with the % oxidative power (%OP) are shown in detail in Table 3. It can be observed that CDs ranged from 6.77 to 22.38 mmol/kg oil, whereas CTs ranged from 1.28 to 8.48 mmol/kg oil. It should be highlighted that lower CD values indicate better oxidative stability. In addition, a low value of %OP possibly suggested preferable properties, but negative values were not included in the RSM, as a decrease from initial CT values signifies the presence of oxidation products and the degradation of unsaturated fatty acids [36]. Interestingly, it was observed that sunflower oil (i.e., design point 27) had the least resistance to oxidation, while design point 3 showed the highest protective effect from oxidation. This was probably caused by the chemical composition of the blended oil when compared to sole sunflower oil. Blending vegetable oils resulted in a protective effect, achieving lower CD and CT values also from palm oil. Similar results were obtained by Ramroudi et al. [12], where sunflower, sesame, and corn oils and their blends were exposed to deep frying for 3 days. It was observed that corn oil was the most stable oil, with the blends also achieving promising results.

Table 3.
Experimental findings for the five independent variables under investigation and the dependent variable response in % oxidative power (%OP) by the conjugated diene (CD) and triene (CT) values during storage at 60 °C for 28 days; pure substrate oils (26–30) are also presented.
3.3. Impact of Variables on Oxidative Stability Assays through Pareto Plot Analysis
Pareto plots are a valuable tool in statistical analysis, particularly in the field of quality control and improvement [37]. They help in identifying the most significant factors in a dataset, which in this context, are the transformed estimates for the %OS and %OP in various assays. In Figure 1, the reference gold line (or gold reference rectangle) mentioned is crucial, as it denotes the level of significance, commonly set at p < 0.05, which helps in distinguishing between statistically significant effects and those that are not. This differentiation is essential for making informed decisions based on the data. For instance, in the Rancimat and DPPH assays, which measure the %OS, and the CD and CT assays, which assess the %OP, the Pareto plot can visually communicate which factors have the most substantial impact on the outcomes and should therefore be prioritized for optimization or further investigation. The use of such plots simplifies complex data and aids in focusing efforts where they can be most effective, ensuring resources are not wasted on insignificant variables [38]. Regarding the variables examined through the Pareto plot, it was observed that the X2 and X3 variables had a positive impact on oxidative stability under accelerated conditions using the Rancimat apparatus. As for DPPH• inhibition activity, it was concluded that corn oil highly contributed to the antioxidant activity of blended vegetable oils. This trend was previously noticed, as pure corn oil had the highest antioxidant activity of all pure substrate oils. In addition, the specific oil kept the highest inhibition activity after 28 days of incubation. On the other hand, it was observed that the variables X2 and X4 (i.e., sunflower and cottonseed oils) had a negative impact on the CD values, indicating that their blend produced high amounts of primary oxidation products. This may also explain the negative effect on the formation of conjugated trienes.

Figure 1.
Pareto plots of transformed estimates for oxidative stability (%OS) and oxidative power (%OP) in various assays. Plot (A) is Rancimat, and plot (B) is DPPH assays, which measure %OS; plot (C) is the CD assay, and plot (D) is the CT assay, which assess %OP. A gold reference rectangle is drawn on the plot to indicate the significance level (p < 0.05).
3.4. Multiple Factor Analysis (MFA)
A method that expands the Principal Components Analysis to datasets with several variables assessed on the same items is Multiple Factor Analysis (MFA). When the variables are transformed into orthogonal factors, it becomes possible to compare the variables. These elements show how things are similar and different according to the variable’s evaluations. To investigate the connections between the measured variables, we employed MFA, the outcomes of which are shown in Figure 2. The factor scores for each measurement variable on the first two dimensions are also shown. These dimensions account for 30 and 20.6% of the total variance, respectively. The analyzed variables (X1–X5) are also shown in the figure, with their levels indicated. As a result of their connection in the factor space, the plot shows blocks of elements that are familiar (or similar). It was observed that the position of pure oil samples varied significantly. Samples X1 and X4 were positioned nearby, indicating a similar trend between the two oils in the investigated assays. For instance, a negative correlation of both oil samples was observed with %OS from the accelerated oxidation process using Rancimat at 0 and 14 d. On the other hand, a positive correlation between sample X5 (i.e., corn oil) and DPPH• was observed, an outcome that could be attributed to the chemical composition of the specific oil sample.
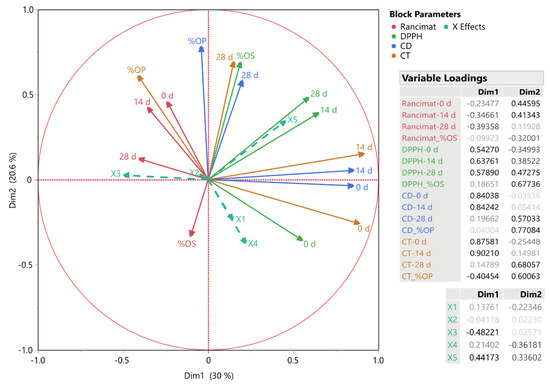
Figure 2.
Multiple factor analysis for the measured variables; the plot displays the factor scores of each variable. Inset tables include variable loadings and colors representing the variables’ block parameters.
A measure of the contribution of each set of variables to each dimension, block partial inertia, is shown in Figure 3. The block partial inertia is multiplied by the eigenvalue and divided by 100 to calculate it. This facilitates the evaluation of the extent to which each block contributes to the overall structure of the data. Dimension 1 accounts for 30% of the total variance within the dataset, representing the most significant pattern or trend. Conversely, Dimension 2 explains 20.6% of the variance, capturing the second most significant pattern, which is orthogonal to the first dimension. The inset tables display the variable loadings, which represent the correlations between the variables and the components, as well as the contribution of each original parameter to the principal component. The dots of varying sizes represent the significance of the data points. Larger dots signify higher values or greater importance, while smaller dots denote lower values or lesser importance. The relative relevance of the variables in each set is shown by the colors in both Figure 2 and Figure 3, which correspond to the block parameters.

Figure 3.
Multiple factor analysis for the measured variables; the plot displays the factor scores of each variable and shows the block partial inertias. The inset table includes block partial inertias and colors representing the variables’ block parameters.
3.5. Multivariate Correlation Analysis (MCA)
To further understand the relationship between the variables, an MCA was also conducted. The ability to measure the degree of positive or negative correlation between the variables is the primary benefit of this analysis compared to the previous. The following caption indicates that the color map used in this context uses a color scale to represent correlation values ranging from −1 to 1. Figure 4 depicts the findings from this analysis. An expected trend included the positive correlation with CD and CT values. A positive correlation (>0.6) in CD and CT values at 0 days was observed, whereas a strong positive correlation (>0.8) was confirmed at 14 days. Interestingly, no strong negative correlation between each variable was observed. Regarding thermal–oxidative stability and bioactive compound correlation, it should be noted that Rancimat and DPPH variables did not have a strong positive correlation. This probably means that the observed antiradical activity was due to bioactive compounds rather than the fatty acid composition. However, the oxidation resistance observed in Rancimat may not be attributed to the presence of antioxidant compounds but rather to the structural characteristics of fatty acids. This is because the chemical bonds within trans fatty acids show greater oxidative stability compared to the corresponding cis bonds [39].
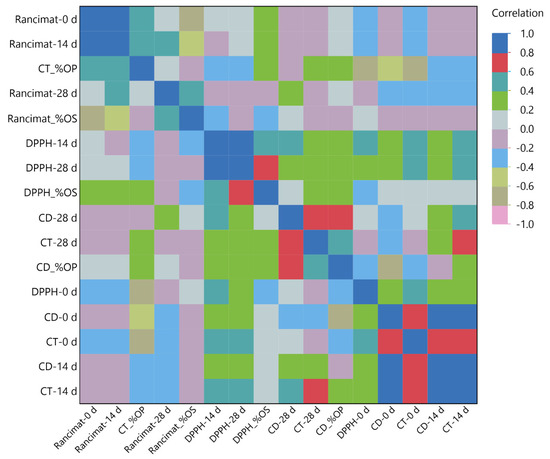
Figure 4.
Multivariate correlation analysis of measured variable parameters.
3.6. Optimal Blended Vegetable Oil Composition
An inexpensive technique is the use of conventional oils in mixtures [40]. Blending vegetable oils has become a widely accepted practice world widely. For instance, combinations of sunflower oil and canola oil or palm oil [30,41], hydrogenated soybean oil and corn oil, or high-oleic sunflower oil and soybean oil are some examples [42,43]. To meet industrial and consumer demands, it has also been acceptable to mix conventional edible oil (i.e., sunflower or corn oil) with uncommon oils like rice bran oil. [40]. The nutritional quality and stability of oils can be enhanced by blending different vegetable oils, which changes the fatty acid composition and increases the quantities of natural bioactive compounds in the blends [44].
The impact of the examined oil combinations (variables X1, X2, X3, X4, and X5) was explored using a PLS analysis. Visualizing the impact of each vegetable oil on both oxidative stability and inhibitory activity testing, Figure 5A shows the application of the PLS technique to generate a correlation loading plot. The impact of each oil on thermal stability and antiradical activity was revealed in Figure 5B. An increased variable significance for the projection (VIP) factor, particularly beyond 0.8, suggests that this variable makes a larger contribution. According to the results, variables X1, X2, and X3 were observed to have a negative impact on both the inhibition activity and oxidative stability in ascending concentrations. To this end, it was considered preferable for the three specific oils to be present in a low ratio. On the other hand, it was observed that variables X4 and X5 (i.e., cottonseed and corn oils) highly affected the oxidative stability through accelerated oxidation and DPPH• inhibition activity. As such, an increased content of variables X4 and X5 was found preferable to enhance the mentioned activities. It was previously shown that the X5 variable had the highest %OS in the DPPH• assay. A possible explanation for this trend could lie mostly in the fatty acid composition, as previously revealed using MCA. Regarding the exclusion of rapeseed oil, despite the higher monounsaturated fatty acid content than polyunsaturated fatty acids [5], this ratio may not fully compensate for the high content of carotenoids, which are also vulnerable to oxidation [45]. As such, corn and cottonseed oils were shown to have the ideal composition of fatty acids and bioactive compounds to create a nutritious and thermal stable oil blend.
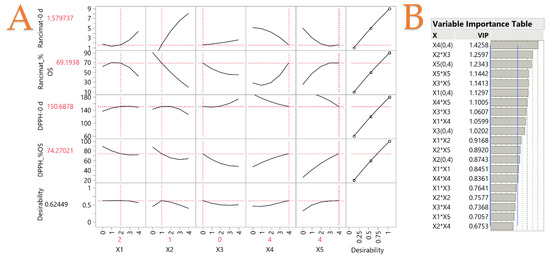
Figure 5.
The PLS prediction profiler of each variable and the desirability function for optimizing blended vegetable oils are shown in plot (A), while the Variable Importance Plot (VIP) option graph with the VIP values for each predictor variable is shown in the plot (B) table. A blue dashed line in the VIT at 0.8 indicates the significance level of each variable.
Consequently, the optimum sample underwent the same assays as palm oil and was compared, the ratio and results of which are shown in Table 4. Figure 6 illustrates the relationship between the induction period and temperature through a polynomial regression fit. This graphical representation is crucial for understanding the kinetics of the reaction under study. The curve obtained from the polynomial regression provides insights into the rate at which the induction period changes with the temperature, offering valuable data for predictive modeling and analysis. Low-temperature (90–110 °C) incubation declares palm oil as more resistant to oxidation. Since saturated fatty acids are more resistant to oxidation than polyunsaturated fatty acids [46,47], this outcome is anticipated. On the contrary, they show a similar pattern at temperatures > 120 °C, providing further evidence that their stability is comparable in this kind of exposure. Furthermore, the inhibition activity was significantly higher (p < 0.05) for the optimal compared to the palm oil by ~40%, rendering it healthier when consumed raw. This outcome could be attributed to the prevalent polyunsaturated fatty acids found in the optimal oil sample. However, the presence of these fatty acids led to higher CD values by 17% before the accelerated oxidation process, since they are more easily oxidized than saturated fatty acids. The oil blending effect led to an induction period until full rancidity at 5.38 h, which was not enough to match the value of palm oil (10.31 h).

Table 4.
Comparison of palm oil and the optimal sample (X1: 2, X2: 1, X3: 0, X4: 4, and X5: 4) in the various analyses.

Figure 6.
The relationship between the induction period and temperature through a polynomial regression fit.
Table 5 presents the statistical analysis results conducted to examine the relationship between the induction period and temperature. A polynomial regression fit was employed, providing a detailed view of how temperature variations can influence the induction phase. The data indicate a significant correlation between these variables, suggesting that the temperature plays a crucial role in the stability of these oils during the induction period, especially that above 120 °C. Figure 6 indicates a fit for both samples, with the optimal one also having a lower value in the root mean square error (RMSE). It was also observed that the palm oil curve had a steeper slope than the blended oil.

Table 5.
Statistical analysis between the induction period and temperature for both palm oil and optimal sample.
3.7. ATR–FTIR Spectra Analysis
ATR–FTIR spectroscopy is well known as a quick, easy, and inexpensive way to measure qualitative and quantitative data from fatty acids [48]. A further comparison in the chemical compositions of the two oil samples was conducted through ATR–FTIR analysis, the stacked spectra of which are illustrated in Figure 7. The major wavenumbers in the ATR–FTIR spectra of the two oils typically correspond to the functional groups that are most abundant or reactive in the samples (Table 6), providing valuable insights on sample composition and aiding in more accurate analysis and quality control in the food industry.

Figure 7.
Stacked ATR–FTIR spectra of palm oil (orange line) and the optimal sample (green line). Blue values indicate the major wavenumbers in specific functional groups.

Table 6.
Major wavenumbers in the ATR–FTIR spectra of palm oil and the optimal sample.
The spectra show the characteristic bands of the fatty acids in the two oil samples. Firstly, it can be seen that from ~2800–2900 cm−1 are the characteristic symmetric and non-symmetric C–H bond vibrations of the aliphatic part of the fatty acids. It was clearly shown that the two bands at 2922 and 2853 cm−1 were more intense in palm oil, as they refer to the bonds between C and H in saturated fatty acids. This finding was expected, as palm oil has a greater amount of saturated fatty acids that significantly contribute to its thermal stability. It should be noted that the difference in the specific bands between the two oils was not significant, meaning that the optimum blend also possesses important oxidative stability. On the other hand, however, at 3006 cm−1 the stretching symmetric vibration of the cis double bonds is distinguishable, whereas in the optimal sample, the absorbance is higher, demonstrating the existence of more cis double bonds due to the presence of more unsaturated fatty acids. This result is supported by the absorptions at 912 and 721 cm−1, where rocking and bending vibrations of –HC=CH– are highlighted, with the absorptions being higher in the optimal sample by up to 40% compared to palm oil. The bands referring to the double bonds between C (i.e., 912, and 3006 cm−1) revealed that the optimum sample had the highest amount of unsaturated fatty acids, which have health-promoting benefits. Similar results were found in a study by Poiana et al. [49], who investigated olive oil adulterations with other edible oils. Finally, a band in 1745 cm−1 was observed at similar absorbances in both oil samples, which indicates the presence of an ester carbonyl functional group.
3.8. Limitations and Future Perspectives
The objective of this study was to conduct an initial investigation to identify the most suitable combination of seed oils with a balanced ratio of fatty acids and bioactive compounds, thereby classifying it as a highly nutritious oil. Furthermore, this blended oil would demonstrate comparable oxidative stability to palm oil, potentially leading to a gradual decrease in the reliance on palm oil and its detrimental impact on the natural environment. Nevertheless, it is important to emphasize that future research necessitates the evaluation of additional bioactive compounds, such as tocopherols, carotenoids, and sterols, to elucidate the impacts of these compounds. Finally, for a better understanding of the oxidative stability of oils at high temperatures, a future study could also compare the optimal blend with palm oil at deep-frying temperatures.
4. Conclusions
To summarize, the global reliance on deep-fried foods necessitates the development of oils of higher nutritional quality. Palm oil is inadequate to meet this demand, as it has plenty of saturated fatty acids. In fact, it may contribute to severe health complications by producing hazardous oxidation by-products. On the other hand, the wide range of monounsaturated and polyunsaturated fatty acids could provide a healthier alternative to several sectors of the food industry. Furthermore, considering that oilseeds could be cultivated in different climates worldwide and produced on a large scale, it could lead to the substitution of palm oil, providing a sustainable way to reduce the detrimental environmental effects of deforestation. A novel blended oil consisting of common vegetable oils such as soybean, sunflower, cottonseed, and corn oils in a 2:1:4:4 ratio, respectively, was prepared and compared to palm oil through several assays (i.e., accelerated oxidation conditions through Rancimat, antiradical inhibition activity, and the measurement of oxidation by-products). The high thermal stability of palm oil due to saturated fatty acids was unmatched by the blended oil; however, its high nutritional quality was revealed through its high antioxidant capacity (~40% higher). This finding was also supported by ATR–FTIR spectra, which revealed the high content of trans fatty acids in palm oil and cis fatty acids in blended oil, rendering it more suitable for consumption. This work could provide the basis for the large-scale production of antioxidant-rich nutritional oil blends using common vegetable oils with substantial oxidative stability as a substitute for palm oil in the food industry.
Author Contributions
Conceptualization, V.A., T.C. and S.I.L.; methodology, V.A., T.C. and S.I.L.; software, V.A.; validation, V.A., T.C., D.K., E.B. and S.I.L.; formal analysis, V.A. and T.C.; investigation, V.A. and T.C.; resources, S.I.L.; data curation, V.A., T.C. and S.I.L.; writing—original draft preparation, V.A. and D.K.; writing—review and editing, V.A., T.C., D.K., E.B. and S.I.L.; visualization, V.A. and T.C.; supervision, S.I.L.; project administration, S.I.L.; funding acquisition, S.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dorni, C.; Sharma, P.; Saikia, G.; Longvah, T. Fatty Acid Profile of Edible Oils and Fats Consumed in India. Food Chem. 2018, 238, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, A.; Lokesh, B.R. Interesterified Coconut Oil Blends with Groundnut Oil or Olive Oil Exhibit Greater Hypocholesterolemic Effects Compared with Their Respective Physical Blends in Rats. Nutr. Res. 2007, 27, 580–586. [Google Scholar] [CrossRef]
- Kumar, A.; Upadhyay, N.; Padghan, P.V.; Gandhi, K.; Lal, D.; Sharma, V. Detection of Vegetable Oil and Animal Depot Fat Adulteration in Anhydrous Milk Fat (Ghee) Using Fatty Acid Composition. MOJ Food Process. Technol. 2015, 1, 46–54. [Google Scholar] [CrossRef]
- Joris, P.J.; Mensink, R.P. Role of Cis-Monounsaturated Fatty Acids in the Prevention of Coronary Heart Disease. Curr. Atheroscler. Rep. 2016, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review. Molecules 2023, 28, 6393. [Google Scholar] [CrossRef] [PubMed]
- Lužaić, T.; Kravić, S.; Stojanović, Z.; Grahovac, N.; Jocić, S.; Cvejić, S.; Pezo, L.; Romanić, R. Investigation of Oxidative Characteristics, Fatty Acid Composition and Bioactive Compounds Content in Cold Pressed Oils of Sunflower Grown in Serbia and Argentina. Heliyon 2023, 9, e18201. [Google Scholar] [CrossRef] [PubMed]
- Oteng, A.-B.; Kersten, S. Mechanisms of Action of Trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Boateng, L.; Ansong, R.; Owusu, W.B.; Steiner-Asiedu, M. Coconut Oil and Palm Oil’s Role in Nutrition, Health and National Development: A Review. Ghana Med. J. 2016, 50, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Vijay, V.; Pimm, S.L.; Jenkins, C.N.; Smith, S.J. The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss. PLoS ONE 2016, 11, e0159668. [Google Scholar] [CrossRef]
- Absalome, M.A.; Massara, C.-C.; Alexandre, A.A.; Gervais, K.; Chantal, G.G.-A.; Ferdinand, D.; Rhedoor, A.J.; Coulibaly, I.; George, T.G.; Brigitte, T.; et al. Biochemical Properties, Nutritional Values, Health Benefits and Sustainability of Palm Oil. Biochimie 2020, 178, 81–95. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Vegetable Oil Blending: A Review of Physicochemical, Nutritional and Health Effects. Trends Food Sci. Technol. 2016, 57, 52–58. [Google Scholar] [CrossRef]
- Ramroudi, F.; Yasini Ardakani, S.A.; Dehghani-tafti, A.; Khalili Sadrabad, E. Investigation of the Physicochemical Properties of Vegetable Oils Blended with Sesame Oil and Their Oxidative Stability during Frying. Int. J. Food Sci. 2022, 2022, 3165512. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Rashid, R. Influence of Food Type, Oil Type and Frying Frequency on the Formation of Trans-Fatty Acids during Repetitive Deep-Frying. Food Control 2023, 147, 109557. [Google Scholar] [CrossRef]
- Li, X.; Wu, G.; Yang, F.; Meng, L.; Huang, J.; Zhang, H.; Jin, Q.; Wang, X. Influence of Fried Food and Oil Type on the Distribution of Polar Compounds in Discarded Oil during Restaurant Deep Frying. Food Chem. 2019, 272, 12–17. [Google Scholar] [CrossRef]
- Sadoudi, R.; Ammouche, A.; Ali Ahmed, D. Thermal Oxidative Alteration of Sunflower Oil. Afr. J. Food Sci. 2014, 8, 116–121. [Google Scholar] [CrossRef][Green Version]
- Sharma, K.; Kumar, M.; Lorenzo, J.M.; Guleria, S.; Saxena, S. Manoeuvring the Physicochemical and Nutritional Properties of Vegetable Oils through Blending. J. Am. Oil Chem. Soc. 2023, 100, 5–24. [Google Scholar] [CrossRef]
- Zbikowska, A.; Onacik-Gür, S.; Kowalska, M.; Zbikowska, K.; Feszterová, M. Trends in Fat Modifications Enabling Alternative Partially Hydrogenated Fat Products Proposed for Advanced Application. Gels 2023, 9, 453. [Google Scholar] [CrossRef]
- Matthäus, B. Use of Palm Oil for Frying in Comparison with Other High-stability Oils. Eur. J. Lipid Sci. Technol. 2007, 109, 400–409. [Google Scholar] [CrossRef]
- Mohamed, K.M.; Elsanhoty, R.M.; Hassanien, M.F.R. Improving Thermal Stability of High Linoleic Corn Oil by Blending with Black Cumin and Coriander Oils. Int. J. Food Prop. 2014, 17, 500–510. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Oilseed Crop Sunflower (Helianthus annuus) as a Source of Food: Nutritional and Health Benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef]
- Kalompatsios, D.; Athanasiadis, V.; Chatzimitakos, T.; Palaiogiannis, D.; Lalas, S.I.; Makris, D.P. Sustainable Exploitation of Waste Orange Peels: Enrichment of Commercial Seed Oils and the Effect on Their Oxidative Stability. Waste 2023, 1, 761–774. [Google Scholar] [CrossRef]
- Pokhrel, K.; Kouřimská, L.; Rudolf, O.; Tilami, S.K. Oxidative Stability of Crude Oils Relative to Tocol Content from Eight Oat Cultivars: Comparing the Schaal Oven and Rancimat Tests. J. Food Compos. Anal. 2024, 126, 105918. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Zu, Y.; Chen, X.; Wang, F.; Liu, F. Oxidative Stability of Sunflower Oil Supplemented with Carnosic Acid Compared with Synthetic Antioxidants during Accelerated Storage. Food Chem. 2010, 118, 656–662. [Google Scholar] [CrossRef]
- Silva, L.; Pinto, J.; Carrola, J.; Paiva-Martins, F. Oxidative Stability of Olive Oil after Food Processing and Comparison with Other Vegetable Oils. Food Chem. 2010, 121, 1177–1187. [Google Scholar] [CrossRef]
- Yilmaz, C.; Krishna, A.S.; Memon, A.; Porter, A.; Schmidt, D.C.; Gokhale, A.; Natarajan, B. Main Effects Screening: A Distributed Continuous Quality Assurance Process for Monitoring Performance Degradation in Evolving Software Systems. In Proceedings of the 27th International Conference on Software Engineering—ICSE ’05, St. Louis, MO, USA, 15–21 May 2005; ACM Press: New York, NY, USA, 2005; p. 293. [Google Scholar]
- Lekivetz, R.; Sitter, R.; Bingham, D.; Hamada, M.S.; Moore, L.M.; Wendelberger, J.R. On Algorithms for Obtaining Orthogonal and Near-Orthogonal Arrays for Main-Effects Screening. J. Qual. Technol. 2015, 47, 2–13. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Palaiogiannis, D.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Evaluation of the Efficacy and Synergistic Effect of α- and δ-Tocopherol as Natural Antioxidants in the Stabilization of Sunflower Oil and Olive Pomace Oil during Storage Conditions. Int. J. Mol. Sci. 2023, 24, 1113. [Google Scholar] [CrossRef]
- Kalompatsios, D.; Palaiogiannis, D.; Makris, D.P. Optimized Production of a Hesperidin-Enriched Extract with Enhanced Antioxidant Activity from Waste Orange Peels Using a Glycerol/Sodium Butyrate Deep Eutectic Solvent. Horticulturae 2024, 10, 208. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Utilization of Blackthorn Plums (Prunus spinosa) and Sweet Cherry (Prunus avium) Kernel Oil: Assessment of Chemical Composition, Antioxidant Activity, and Oxidative Stability. Biomass 2024, 4, 49–64. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Parisini, C.; Battimo, I.; Falco, S.; Sacchi, R. Frying Performance of a Sunflower/Palm Oil Blend in Comparison with Pure Palm Oil. Eur. J. Lipid Sci. Technol. 2007, 109, 237–246. [Google Scholar] [CrossRef]
- Siddique, B.M.; Ahmad, A.; Ibrahim, M.H.; Hena, S.; Rafatullah, M.; Mohd Omar, A.K. Physico-Chemical Properties of Blends of Palm Olein with Other Vegetable Oils. Grasas Aceites 2010, 61, 423–429. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Wahdan, K.M.M. Blending of Corn Oil with Black Cumin (Nigella sativa) and Coriander (Coriandrum sativum) Seed Oils: Impact on Functionality, Stability and Radical Scavenging Activity. Food Chem. 2012, 132, 873–879. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Blending of Sunflower Oil with Pomegranate Seed Oil from Blanched Seeds: Impact on Functionality, Oxidative Stability, and Antioxidant Properties. Processes 2021, 9, 635. [Google Scholar] [CrossRef]
- Mitrea, L.; Teleky, B.-E.; Leopold, L.-F.; Nemes, S.-A.; Plamada, D.; Dulf, F.V.; Pop, I.-D.; Vodnar, D.C. The Physicochemical Properties of Five Vegetable Oils Exposed at High Temperature for a Short-Time-Interval. J. Food Compos. Anal. 2022, 106, 104305. [Google Scholar] [CrossRef]
- Castelo-Branco, V.N.; Santana, I.; Di-Sarli, V.O.; Freitas, S.P.; Torres, A.G. Antioxidant Capacity Is a Surrogate Measure of the Quality and Stability of Vegetable Oils. Eur. J. Lipid Sci. Technol. 2016, 118, 224–235. [Google Scholar] [CrossRef]
- Nayak, P.K.; Dash, U.; Rayaguru, K.; Krishnan, K.R. Physio-Chemical Changes During Repeated Frying of Cooked Oil: A Review. J. Food Biochem. 2016, 40, 371–390. [Google Scholar] [CrossRef]
- Smith, H.D.; Megahed, F.M.; Jones-Farmer, L.A.; Clark, M. Using Visual Data Mining to Enhance the Simple Tools in Statistical Process Control: A Case Study. Qual. Reliab. Eng. Int. 2014, 30, 905–917. [Google Scholar] [CrossRef]
- Abraham, Y.; Zhang, X.; Parker, C.N. Multiparametric Analysis of Screening Data: Growing Beyond the Single Dimension to Infinity and Beyond. J. Biomol. Screen. 2014, 19, 628–639. [Google Scholar] [CrossRef]
- Brühl, L. Fatty Acid Alterations in Oils and Fats during Heating and Frying. Eur. J. Lipid Sci. Technol. 2014, 116, 707–715. [Google Scholar] [CrossRef]
- Choudhary, M.; Grover, K.; Kaur, G. Development of Rice Bran Oil Blends for Quality Improvement. Food Chem. 2015, 173, 770–777. [Google Scholar] [CrossRef]
- Farag, R.S.; El-Agaimy, M.A.S.; Hakeem, B.S.A.E. Effects of Mixing Canola and Palm Oils with Sunflower Oil on the Formation of Trans Fatty Acids during Frying. Food Nutr. Sci. 2010, 1, 24–29. [Google Scholar] [CrossRef]
- Abdulkarim, S.M.; Myat, M.W.; Ghazali, H.M.; Roselina, K.; Abbas, K.A. Sensory and Physicochemical Qualities of Palm Olein and Sesame Seed Oil Blends during Frying of Banana Chips. J. Agric. Sci. 2010, 2, 18–29. [Google Scholar] [CrossRef]
- Naghshineh, M.; Ariffin, A.A.; Ghazali, H.M.; Mirhosseini, H.; Mohammad, A.S. Effect of Saturated/Unsaturated Fatty Acid Ratio on Physicochemical Properties of Palm Olein–Olive Oil Blend. J. Am. Oil Chem. Soc. 2010, 87, 255–262. [Google Scholar] [CrossRef]
- Aladedunye, F.; Przybylski, R. Frying Stability of High Oleic Sunflower Oils as Affected by Composition of Tocopherol Isomers and Linoleic Acid Content. Food Chem. 2013, 141, 2373–2378. [Google Scholar] [CrossRef]
- Sumara, A.; Stachniuk, A.; Olech, M.; Nowak, R.; Montowska, M.; Fornal, E. Identification of Sunflower, Rapeseed, Flaxseed and Sesame Seed Oil Metabolomic Markers as a Potential Tool for Oil Authentication and Detecting Adulterations. PLoS ONE 2023, 18, e0284599. [Google Scholar] [CrossRef]
- Yang, K.-M.; Chiang, P.-Y. Variation Quality and Kinetic Parameter of Commercial N-3 PUFA-Rich Oil during Oxidation via Rancimat. Mar. Drugs 2017, 15, 97. [Google Scholar] [CrossRef]
- Xu, Z.; Ye, Z.; Li, Y.; Li, J.; Liu, Y. Comparative Study of the Oxidation Stability of High Oleic Oils and Palm Oil during Thermal Treatment. J. Oleo Sci. 2020, 69, 573–584. [Google Scholar] [CrossRef]
- Sherazi, S.T.H.; Arain, S.; Mahesar, S.A.; Bhanger, M.I.; Khaskheli, A.R. Erucic Acid Evaluation in Rapeseed and Canola Oil by Fourier Transform-Infrared Spectroscopy. Eur. J. Lipid Sci. Technol. 2013, 115, 535–540. [Google Scholar] [CrossRef]
- Poiana, M.-A.; Alexa, E.; Munteanu, M.-F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR Spectroscopy to Detect the Changes in Extra Virgin Olive Oil by Adulteration Withsoybean Oil and High Temperature Heat Treatment. Open Chem. 2015, 13, 000010151520150110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).