Neem Oil: A Comprehensive Analysis of Quality and Identity Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Free Fatty Acids (FFAs) and Acidity Value
2.2.2. Peroxide Value (Acetic Acid–Isooctane Method) and Oil Stability Index
2.2.3. Calculated Iodine Value and Calculated Saponification Value of Fats and Oils
2.2.4. Chlorophyll Pigments and Total Carotenoids
2.2.5. Color of Fats and Oils—Automated Method
2.2.6. Lipid Classes
2.2.7. Fatty Acid Composition
2.2.8. Triacylglycerol Composition
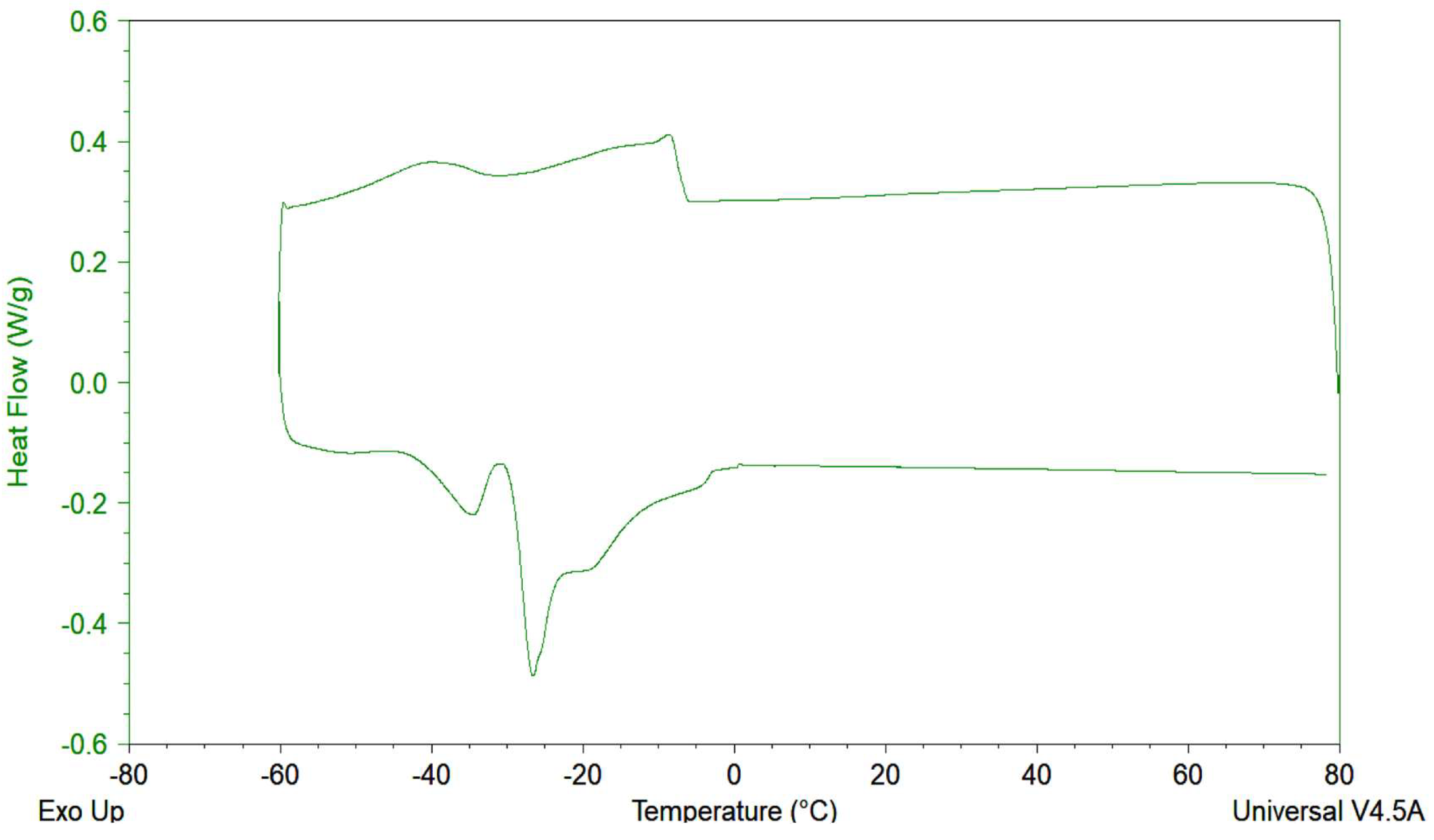
2.2.9. Thermal Behavior (Differential Scanning Calorimeter (DSC))
2.3. Statistical Analysis
3. Results and Discussion
Results of Two Determinations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhary, M.F.; Ashraf, A.; Waseem, M.; Hayat, S.; Nadeem, H.U.; Siddique, M.H.; Muzammil, A.; Afzal, M.; Muzammil, S. Neem oil. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 57–73. [Google Scholar] [CrossRef]
- Loganathan, T.; Barathinivas, A.; Soorya, C.; Balamurugan, S.; Nagajothi, T.G.; Ramya, S.; Jayakumararaj, R. Physicochemical, Druggable, ADMET Pharmacoinformatics and Therapeutic Potentials of Azadirachtin—A Prenol Lipid (Triterpenoid) from Seed Oil Extracts of Azadirachta indica A. Juss. J. Drug Deliv. Ther. 2021, 11, 33–46. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, N.; Devi, L.S.; Kumar, S.; Kamle, M.; Kumar, P.; Mukherjee, A. Neem oil and its nanoemulsion in sustainable food preservation and packaging: Current status and future prospects. J. Agric. Food Res. 2022, 7, 100254. [Google Scholar] [CrossRef]
- Achutha, S.; Nisha, S.K.; Pushpa, S.B.; Andrews, S. Antimicrobial polyvinyl alcohol/neem oil flexible film for food packaging applications. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Sanuja, S.; Agalya, A.; Umapathy, M. Synthesis and characterization of zinc oxide–neem oil–chitosan bionanocomposite for food packaging application. Int. J. Biol. Macromol. 2015, 74, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Chonhenchob, V.; Huang, C.; Suwanamornlert, P. Antifungal Activity of Propyl Disulfide from Neem (Azadirachta indica) in Vapor and Agar Diffusion Assays against Anthracnose Pathogens (Colletotrichum gloeosporioides and Colletotrichum acutatum) in Mango Fruit. Microorganisms 2021, 9, 839. [Google Scholar] [CrossRef]
- Del Serrone, P.; Toniolo, C.; Nicoletti, M. Neem (Azadirachta indica A. Juss) Oil: A Natural Preservative to Control Meat Spoilage. Foods 2015, 4, 3–14. [Google Scholar] [CrossRef]
- Islas, J.F.; Acosta, E.; G-Buentello, Z.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.; Moreno-Cuevas, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods 2020, 74, 104171. [Google Scholar] [CrossRef]
- Gopinath, H.; Karthikeyan, K. Neem in Dermatology. Indian J. Dermatol. 2021, 66, 706. [Google Scholar] [CrossRef]
- Cabral, S.M.; Silva, F.L.D.N.; Rodrigues, P.J.; Rambo, M.K.D.; Santos, J.R.; Chaves, M.H.; Pinto, B.F. Extraction and chemical characterization of neem seed oil (Azadirachta indica). J. Biotechnol. Biodivers. 2023, 11, 170–181. [Google Scholar] [CrossRef]
- Ghosh, S.; Mali, S.N.; Bhowmick, D.; Pratap, A.P. Neem oil as natural pesticide: Pseudo ternary diagram and computational study. J. Indian Chem. Soc. 2021, 98, 100088. [Google Scholar] [CrossRef]
- Adusei, S.; Azupio, S. Neem: A Novel Biocide for Pest and Disease Control of Plants. J. Chem. 2022, 2022, 6778554. [Google Scholar] [CrossRef]
- Saha, J.; Ara, M.R.; Pranta, A.D.; Hossain, S. Antimicrobial and antioxidant functionalization of cellulosic fabric via mushroom and neem oil treatment: A step toward sustainable textiles. J. Vinyl. Addit. Technol. 2024, 30, 1659–1671. [Google Scholar] [CrossRef]
- Benelli, G.; Canale, A.; Toniolo, C.; Higuchi, A.; Murugan, K.; Pavela, R.; Nicoletti, M. Neem (Azadirachta indica): Towards the ideal insecticide? Nat. Prod. Res. 2017, 31, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Ratna, K.N.P.; Afifah, F.N.; Kartika, T.; Prianto, A.H. Neem Oil (Azadirachta indica A. juss) as a Potential Natural Active Compound in Cosmetic Properties Title. In Biomass-Based Cosmetics; Springer: Singapore, 2024; pp. 325–350. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 6th ed.; AOCS: Urbana, IL, USA, 2009. [Google Scholar]
- Dobarganes, M.C.; Velasco, J.; Dieffenbacher, A. Determination of polar compounds, polymerized and oxidized triacylglycerols, and diacylglycerols in oils and fats: Results of collaborative studies and the standardized method (Technical report). Pure Appl. Chem. 2000, 72, 1563–1575. [Google Scholar] [CrossRef]
- Hartman, L.; Lago, R.C.A. Rapid preparation of fatty acid methyl esters from lipids. Lab. Pract. 1973, 22, 475–494. [Google Scholar] [PubMed]
- Filho, N.R.A.; Mendes, O.L.; Lanças, F.M. Computer prediction of triacylglycerol composition of vegetable oils by HRGC. Chromatographia 1995, 40, 557–562. [Google Scholar] [CrossRef]
- Campos, R. Experimental Methodology. In Fat Crystal Networks; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Ismaila, S.S.; Sani, Y.; Sani, A.A.; Yakasai, S.M.; Momoh, H.; Mohammed, S.E. Determination of fatty acids and physicochemical properties of neem (Azadrachta indica L.) seed oil extracts. Dutse J. Pure Appl. Sci. 2022, 8, 149–160. [Google Scholar] [CrossRef]
- Djibril, D.; Mamadou, F.; Gérard, V.; Geuye, M.-D.C.; Oumar, S.; Luc, R. Physical characteristics, Chemical composition and Distribution of constituents of the Neem seeds (Azadirachta indica A. Juss) collected in Senegal. Res. J. Chem. Sci. 2015, 5, 52–58. [Google Scholar]
- Mulla, M.; Ahmed, J.; Alagarsamy, S.; Habeebullah, S.F.K. Utilization of novel and rapid techniques for characterization of neem Azadirachta indica seed oil and palm oil blends. Int. J. Food Eng. 2020, 16, 20200047. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; de Barros Vilas Boas, E.V.; Riul, T.R.; Pantoja, L.; Marinho, H.A.; dos Santos, A.S. Influence of the extraction method and storage time on the physicochemical properties and carotenoid levels of pequi (Caryocar brasiliense Camb.) oil. Food Sci. Technol. 2012, 32, 386–392. [Google Scholar] [CrossRef]
- O’Brien, R.D. Fats and Oils; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Shahidi, F. (Ed.) Bailey’s Industrial Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Brazil. Diretoria Colegiada da Agência Nacional de Vigilância Sanitária. Instrução Normativa IN N° 87, de 15 de Março de 2021. Estabelece a Lista de Espécies Vegetais Autorizadas, as Designações, a Composição de Ácidos Graxos e os Valores Máximos de Acidez e de Índice de Peróxidos para Óleos e Gorduras Vegetais; Diário Oficial da União: Brasília, Brazil, 2021; p. 10. [Google Scholar]
- Jessinta, S.; Azhari, H.N.; Saiful, N.T. Impact of geographic variation on physicochemical properties of neem (Azadirachta indica) seed oil. Int. J. Pharm. Sci. Res. 2014, 5, 4406. [Google Scholar]
- Nahak, G.; Sahu, R. Evaluation of antioxidant activity of flower and seed oil of Azadirachta indica A. juss. J. Appl. Nat. Sci. 2011, 3, 78–81. [Google Scholar] [CrossRef]
- Atta, N.M.; Ismaiel, G.H.; Hashish, A.E.M.S.; Mohamed, E.S. Physical and chemical characteristics of neem oils extracted from seed, whole fruit and flesh. Egypt. J. Agric. Res. 2015, 93, 887–899. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Tańska, M.; Ogrodowska, D. Phenolic compounds in plant oils: A review of composition, analytical methods, and effect on oxidative stability. Trends Food Sci. Technol. 2021, 113, 110–138. [Google Scholar] [CrossRef]
- Sarkar, S.; Singh, R.P.; Bhattacharya, G. Exploring the role of Azadirachta indica (neem) and its active compounds in the regulation of biological pathways: An update on molecular approach. 3 Biotech 2021, 11, 178. [Google Scholar] [CrossRef]
- Awasthi, R.; Shikha, D. Solvent Extraction of Neem Oil from Neem Seed for Development of Ecofriendly Pesticides. Int. J. Trend Sci. Res. Dev. 2019, 3, 119–122. [Google Scholar] [CrossRef]
- Venugopalan, V.K.; Gopakumar, L.R.; Kumaran, A.K.; Chatterjee, N.S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D.J.; Nagarajarao, R.C. Encapsulation and protection of omega-3-rich fish oils using food-grade delivery systems. Foods 2021, 10, 1566. [Google Scholar] [CrossRef]
- DeMan, J.M. Empirical relationships between iodine value and polyunsaturated fatty acid content of fats and oils. J. Am. Oil Chem. Soc. 1977, 54, 314–318. [Google Scholar] [CrossRef]
- Takić, M.; Ranković, S.; Girek, Z.; Pavlović, S.; Jovanović, P.; Jovanović, V.; Šarac, I. Current Insights into the Effects of Dietary α-Linolenic Acid Focusing on Alterations of Polyunsaturated Fatty Acid Profiles in Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 4909. [Google Scholar] [CrossRef]
| Properties | Neem Oil |
|---|---|
| Free fatty acids (% oleic acid) | 2.0 ± 0.02 |
| Acidity value (mg KOH/g) | 3.9 ± 0.04 |
| Peroxide value (mEq/1000 g) | 3.2 ± 0.1 |
| Oxidation induction period (h) | - |
| Calculated iodine value (g I2/100 g) | 116 ± 12 |
| Calculated saponification value (mg KOH/g) | 198 ± 8 |
| R color | 5.1 ± 0.1 |
| Y color | 70 ± 0.0 |
| B color | 0.0 ± 0.0 |
| N color | 1.1 ± 0.1 |
| Carotenoids (mg carotenoids/kg oil) | - |
| Chlorophyll (mg pheophytin/kg oil) | - |
| Triacylglycerols (% TAG) | 98 ± 0.01 |
| Diacylglycerols (% DAG) | 2.0 ± 0.01 |
| Monoacylglycerols + Free fatty acids (% MAG + AGL) | 0.1 ± 0.00 |
| Name/Fatty Acids | % (m/m) |
|---|---|
| Palmitic (C16:0) | 12 ± 0.00 |
| Stearic (C18:0) | 4.1 ± 0.00 |
| Oleic (C18:1) | 28 ± 0.01 |
| Linoleic (C18:2) | 46 ± 0.00 |
| Linolenic (C18:3) | 5.5 ± 0.00 |
| Σ saturated fatty acids (SFAs) | 16 ± 0.00 |
| Σ monounsaturated fatty acids (MUFAs) | 28 ± 0.01 |
| Σ polyunsaturated fatty acids (PUFAs) | 51 ± 0.01 |
| Carbon Number | Triacylglycerol | % (m/m) (2) |
|---|---|---|
| C50 | POP | 1.7 ± 0.1 |
| PLP | 3.3 ± 0.03 | |
| C52 | SOP | 1.1 ± 0.1 |
| POO | 4.9 ± 0.3 | |
| SLP | 1.6 ± 0.1 | |
| PLO | 12 ± 0.2 | |
| PLnO | 2.7 ± 0.2 | |
| PLL | 12 ± 0.2 | |
| PLnL | 2.7 ± 0.1 | |
| C54 | SOO | 1.5 ± 0.1 |
| SLO | 4.7 ± 0.1 | |
| OOO | 3.0 ± 0.1 | |
| OLO | 12 ± 0.3 | |
| SLL | 1.2 ± 0.1 | |
| OLnO | 1.5 ± 0.4 | |
| OLL | 15 ± 0.6 | |
| OLnL | 4.1 ± 0.2 | |
| LLL | 12 ± 0.1 | |
| LLnL | 3.1 ± 0.04 | |
| Class | Triacylglycerol | % |
| SSS (trisaturated) | --- | 0.0 ± 0.0 |
| SSU (monounsaturated) | POP, PLP, SOP, SLP | 7.8 ± 0.1 |
| SUU (diunsaturated) | POO, PLO, PLnO, PLL, PLnL, SOO, SLO, SLL | 41 ± 0.4 |
| UUU (triunsaturated) | OOO, OLO, OLnO, OLL, OLnL, LLL, LLnL | 51 ± 0.3 |
| Name/Fatty Acids | Cabral et al. [10] | Djibril et al. [20] | Mulla et al. [21] | Awashi and Shikha [30] |
|---|---|---|---|---|
| Palmitic (C16:0) | 18.7 | 15.6 ± 0.3 | 16.9 ± 0.1 | 17.9 |
| Stearic (C 18:0) | 16.7 | 18.7 ± 0.5 | 21.3 ± 0.1 | 19.4 |
| Oleic (C18:1) | 43.5 | 41.9 ± 0.7 | 44.9 ± 0.1 | 49.9 |
| Linoleic (C18:2) | 19.1 | 19.6 ± 0.4 | 14.8 ± 0.01 | 12.8 |
| Linolenic (C18:3) | 0.3 | 0.5 ± 0.01 | 0.3 ± 0.0 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, V.E.d.S.; Bócoli, P.F.J.; Iamarino, J.G.; Grimaldi, R.; Ribeiro, A.P.B.; Marangoni Júnior, L. Neem Oil: A Comprehensive Analysis of Quality and Identity Parameters. Lipidology 2025, 2, 9. https://doi.org/10.3390/lipidology2020009
Gomes VEdS, Bócoli PFJ, Iamarino JG, Grimaldi R, Ribeiro APB, Marangoni Júnior L. Neem Oil: A Comprehensive Analysis of Quality and Identity Parameters. Lipidology. 2025; 2(2):9. https://doi.org/10.3390/lipidology2020009
Chicago/Turabian StyleGomes, Vitor Emanuel de Souza, Paula Fernanda Janetti Bócoli, Julia Guirardello Iamarino, Renato Grimaldi, Ana Paula Badan Ribeiro, and Luís Marangoni Júnior. 2025. "Neem Oil: A Comprehensive Analysis of Quality and Identity Parameters" Lipidology 2, no. 2: 9. https://doi.org/10.3390/lipidology2020009
APA StyleGomes, V. E. d. S., Bócoli, P. F. J., Iamarino, J. G., Grimaldi, R., Ribeiro, A. P. B., & Marangoni Júnior, L. (2025). Neem Oil: A Comprehensive Analysis of Quality and Identity Parameters. Lipidology, 2(2), 9. https://doi.org/10.3390/lipidology2020009









