1. Introduction
Plant-based bioactives, including polyphenols, flavonoids, organic acids, and tannins, exhibit noteworthy antioxidant properties that are beneficial for topical applications [
1]. They are generally favored in cosmeceuticals due to their antimicrobial, anti-inflammatory, and anti-aging activity with low toxicity and cost-effectiveness [
2,
3]. However, challenges arise from the high water content in fruit and vegetable extracts, which can lead to the degradation of these bioactives during conventional extraction methods, such as solvent extraction and hydroextraction, thereby compromising their stability and shelf life. This degradation represents a significant obstacle in developing long-lasting and effective products [
4,
5]. Consequently, advanced extraction techniques, encapsulation technologies, and preservation methods are critical for enhancing the stability and extending the shelf life of natural ingredient formulations, but are often complex and costly [
6,
7,
8]. There is an urgent need for an improved and enhanced natural industrial extraction process that is easy to operate and scale, economical, and restores all health-promoting potentials from natural resources.
Fermentation presents a promising bioprocess for extracting bioactives from fruits and vegetables. This biotechnological process leverages microorganisms to break down complex compounds, producing valuable metabolites as bioactive compounds. Unlike the conventional methods, fermentation retains essential nutrients, reduces toxic compounds, and generates new active ingredients that offer additional health benefits [
9]. Several studies have underscored the advantages of fermentation, particularly its impact on enhancing the antioxidant properties of fruits and vegetables [
10]. A recent research [
11] has shown that fermentation can enhance the release or conversion of bioactives into more active forms, largely due to the metabolic activities of microorganisms. These processes affect phenolic acids, flavonoids, tannins, and the release of antioxidant peptides, as well as changes in vitamin content and the production of exopolysaccharides [
12]. The positive relationship between fermentation and increased phytochemical activity is primarily attributed to microbial hydrolysis, which increases the concentration of phenolic compounds and flavonoids in fruits and vegetables. For example, apple cider vinegar is a fermented apple derivative that is extensively researched for its potential therapeutic properties [
13]. Fermentation enhances the safety, nutritional profile, stability, and shelf life of the extracts, making it a straightforward and environmentally sustainable practice for developing cosmeceuticals.
This study provides valuable insights into the development of topical formulations utilizing natural antioxidants extracted from fruits and vegetables such as banana, papaya, pomegranate, and beetroot through fermentation technology, using both single and mixed substrate strategies. Notably, the novel combinations of pomegranate with beetroot and papaya with banana explored in this research have not been previously reported for topical applications, highlighting the innovative potential of these formulations in skin and hair care. Addressing the industrial challenge of developing an efficient, cost-effective, and scalable extraction process, this work employs a Quality by Design (QbD) approach to optimize conditions for producing extracts rich in antioxidants, anti-inflammatory compounds, phenolic acids, and gallic acid. The proposed extraction method is compared to conventional processes to establish its advantages over traditional techniques commonly used in industrial manufacturing. This research culminates in the creation of unique, first-time formulations of FERMENZA for skin and hair care applications, evaluated against conventional formulations for phytochemical and microbiological activity to assess therapeutic efficacy. FERMENZA is the brand name chosen for the patented formulation that is derived from fermented ingredients. Additionally, the formulations utilize minimal excipients from the USFDA GRAS (Generally Regarded As Safe) list, ensuring reduced synthetic content while maintaining affordability.
2. Materials and Methods
2.1. Materials Required
Pomegranate (Punica granatum), Beetroot (Beta vulgaris), Banana (Musa acuminata and Musa balbisiana), and Papaya (Carica papaya) were purchased from a local market in Mohali, Punjab. Specialty chemicals such as 1,1-diphenyl-2-picrylhydrazyl (DPPH), sodium pentacyanonitrosylferrate(III) (commonly sodium nitroprusside), Griess reagent, and gallic acid were procured from local supplier of Sigma Aldrich (St. Louis, MO, USA). Folin–Ciocalteu phenol reagent (FCR), chloroform, methanol, ethanol, dichloromethane, n-hexane, sodium carbonate, aluminum chloride, potassium acetate, sodium sulphate, propylene glycol, glycerine, sodium benzoate, and hydroxyethylcellulose were procured from local supplier of Merck (Darmstadt, Germany). All chemicals, solvents, and buffers used in the work were of AR grade.
2.2. Conventional Methods of Extraction
Hydro distillation of 150 g paste of a fruit and vegetable combination mixture (1:1:1:1 ratio) was carried out for 5 h with 300 mL distilled water using a conventional steam distillation setup and Clevenger apparatus. The yield of extract is below 5% of the total volume. Solvent extraction by shake flask method with constant rotatory shaking (190 rpm) was also carried out using the same quantity of raw material and 300 mL of food-grade ethanol at 30 °C for 5 h. The yield of extract is around 15–20% in the solvent extraction process. Extracts were stored for further analyses and use.
2.3. Preparation of Fermented Fruits and Vegetable Cider Extracts
The whole fruits and vegetables were washed properly using clean water. The fruit and vegetable combinations were chopped and ground into a uniform paste and subjected to fermentation with 50% substrate concentration. The fermentation experiments were carried out in aqueous medium using a fermentor/bioreactor and a shake flask, which was inoculated with 1% commercially available yeasts (Saccharomyces cerevisiae) powder. The yield of fermented extract is about 65–70% of the total volume.
2.4. Fermentation Process Optimization
The fermentation process was designed and optimized using the design of experiment or Quality by Design (QbD) approach to obtain an optimized, scalable, and economical extraction process to obtain the desired high-quality pure natural fermented extracts for skin and haircare products. To obtain pure antioxidant and gallic acid-rich fermented extract, the fermentation conditions were chosen using a central composite rotatable design (CCRD) using two parameters of extraction. Extraction temperatures (30, 35, 40 °C) and extraction times (48, 72, 96 h) were varied at three levels. The experimental domain of the two factors of CCRD consisted of 13 runs. The set of extracting conditions summarized in
Table 1 was designed by adopting the CCRD model through the statistical software Design Expert 7.0. For a given set of extracting conditions, three independent extraction trial runs were conducted in the laboratory as shown in
Table 1, except for the central point (72 h and 35 °C). The duplication of the central point was identified through five trials and was used to find the experimental error in the study. The fermented extracts obtained were gravimetrically weighed and successively stored well until further analyses and use.
2.5. Evaluation and Comparison of Phytochemical Properties of the Extracts
Initially, all four fruits and vegetables mentioned, pomegranate, beetroot, banana, and papaya, were chopped and ground to a paste in a 1:1:1:1 ratio, subject to various extraction processes, and the phytochemical assays were performed with four different extracts: fermented extract, hydrodistillation extract, solvent extract, and Clevenger extract. The following phytochemical assays were performed with the fermented extracts as well as conventional extracts according to Ghosh et al. [
14]. The total phenolic compounds were estimated using Folin-Ciocalteu reagent and expressed as mg gallic acid equivalent/g extract from the respective standard curves [
15]. All the experiments were performed in triplicate assays, and the measurement of absorbance was performed using a UV-Vis Spectrophotometer (Shimadzu Model UV-2450, Kyoto, Japan) throughout the studies.
2.5.1. DPPH Assay for Antioxidant Activity Measurement
The DPPH (2,2-diphenyl-1-picrylhydrazyl) assay is one of the most widely used methods for assessing the antioxidant capacity of various compounds, particularly plant-based bioactives. The assay is based on the reduction of the DPPH radical, a stable free radical with a characteristic deep purple color, which becomes colorless or pale yellow upon reduction by an antioxidant [
15]. The change in color corresponds to a decrease in absorbance at 517 nm, and this reduction is quantitatively measured to determine the scavenging ability of the antioxidant compound. The efficiency of antioxidants is expressed in terms of IC
50, which is the concentration of the antioxidant required to reduce the initial DPPH radical concentration by 50%. A lower IC
50 value indicates a higher antioxidant capacity [
16]. In this study, the antioxidant activity of four different extracts—fermented extract, hydodistillation extract, solvent extract, and Clevenger extract—was evaluated using the DPPH assay. The scavenging capacity of each extract was determined by measuring the decrease in absorbance at 517 nm, and the results were expressed as IC
50 values to compare their relative antioxidant potentials.
2.5.2. BHT Assay for Antioxidant Activity
The BHT (Butylated Hydroxytoluene) assay assesses the antioxidant potential of compounds by comparing their activity to that of BHT, which serves as a standard reference antioxidant. BHT, a phenolic compound, inhibits lipid peroxidation by donating hydrogen atoms to free radicals, thereby terminating radical chain reactions that cause oxidation [
17]. In the context of antioxidant activity assessment, the BHT assay is often employed to evaluate the efficiency of natural and synthetic antioxidants in stabilizing products that are susceptible to oxidative degradation [
18]. The results of antioxidant assays using BHT as a reference are expressed in terms of percentage inhibition, where the performance of the tested compound is compared to that of BHT [
18]. In this study, the free radical scavenging activity of the four different extracts—fermented extract, hydodistillation extract, solvent extract, and Clevenger extract was evaluated. The antioxidant efficiency of each extract was compared to BHT by determining their IC
50 values, providing a reference for evaluating the potency of natural antioxidants.
2.5.3. Nitric Oxide Scavenging Assay for Antioxidant Activity
The nitric oxide scavenging assay is based on the generation of nitric oxide from sodium pentacyanonitrosylferrate(III), commonly sodium nitroprusside, under physiological conditions, which reacts with oxygen to form nitrite ions. These nitrite ions can be quantified using the Griess reagent, which forms a colored azo dye upon reaction with nitrite. Antioxidants can reduce the formation of nitrite by scavenging NO radicals, and the degree of inhibition is measured spectrophotometrically at 540 nm [
19]. The percentage of nitric oxide scavenged by the antioxidant is calculated, and lower IC
50 values indicate stronger scavenging activity. The antioxidant activity of the four different extracts—fermented extract, hydrodistillation extract, solvent extract, and Clevenger extract—was evaluated using the nitric oxide (NO) scavenging assay. The ability of each extract to neutralize NO radicals was quantified by measuring the decrease in absorbance, and the results were expressed as IC
50 values, with lower values indicating greater NO scavenging potential.
2.6. Formulation of Final Finished Products with Fermented Extract and Conventional Extracts
The fermented and conventional extracts from pomegranate and beetroot combination are further developed into final products with brand name FERMENZA for personal and healthcare usages as disclosed in patent [
20] (Indian Patent No. 459674). A brief, simple method of preparation had been mentioned in detail in
Table 2. All the ingredients were weighed accurately and mixed uniformly. The aqueous base comprising water, propylene glycol, and glycerin was heated at 80 to 90 °C, and then, under stirring, the active fermented extracts or conventional extracts and sodium benzoate were added and mixed for 1 h for complete mixing. Propylene glycol and glycerine were heated to 80–90 °C to reduce their viscosity and enhance their miscibility, ensuring uniform mixing and the formation of a stable base formulation. Polyphenols are generally regarded as heat-labile compounds [
21], hence having negligible chances to alter the antioxidant activity of the four different extracts, namely fermented extract, hydrodistillation extract, solvent extract, and Clevenger extract, during this heat exposure for finished product formulation. The first time composition and method of preparation had been kept very simple for industrial scalability and economy. The efficiency of these combined extracts was surprisingly found to be very effective for health and personal care products because of the immense antioxidant potential of these extracts, as discussed in earlier sections. The formulation prepared was packed into bottles after cooling, which can be used as skin and hair care products, comprising but not limited to hair oil, hair tonics, hair serum, face serum, face toners, beard serum, beard oil, etc.
2.7. Evaluation and Comparison of Phytochemical Properties of the Finished Product
The phytochemical properties, such as radical scavenging activities, total phenolic contents, and reducing power of both the finished product formulated with fermented extract and conventional extracts, respectively, were further assessed and compared for their efficacies to be used as skin and hair care therapeutics. The assays were performed as per the methods described above in
Section 2.5.
2.8. Determination of Antimicrobial Activity of the Finished Product by Microbroth Dilution Method
The antimicrobial potency of the product formulated with fermented extract and conventional extract (from pomegranate and beetroot combination) with best phytochemical properties was determined from the minimum inhibitory concentration (MIC) values against five strains of microorganisms (
Escherichia coli ATCC 25922,
Staphylococcus aureus ATCC 25923,
Pseudomonas aeruginosa ATCC 27853,
Propionibacterium acne MTCC 1951 and
Malessezia furfur MTCC 1374) in accordance to the method of Ghosh et al. [
14], with little modifications. For MIC determination, Mueller-Hinton broth from HiMedia Laboratories (Thane, India) was used in the broth dilution method. The microwells were fitted with Muller-Hinton broth (100 μL), to which 100 μL extract was added and serially double diluted into eight microwells. 10 μL of microbial culture broth was subsequently added to each well of the 96-well microtiter plate. The microorganisms were incubated in a BOD incubator (REMI CI-6 Plus, Mumbai, India) at 37 °C for 24 h. The inhibitory effect of the extracts on the growth of the microorganisms was monitored by measuring the optical density at 620 nm in microtiter wells at 0 h and 24 h using a microplate reader (Micronaut System, Bruker Optics GmbH & Co. KG, Ettlingen, Germany).
2.9. Statistical Analysis
Data from the comparative study of phytochemical activities between bioactives extracted using fermentation and conventional extraction methods were statistically analyzed using appropriate methods to ensure robust interpretation of results. All experiments were performed in triplicate, and the results were expressed as mean ± standard deviation (SD). The statistical significance of differences between groups (fermentation vs. conventional extraction) was evaluated using one-way analysis of variance (ANOVA), which was chosen to determine if there were significant differences in antioxidant activity, total phenolic content, total flavonoid content, and other relevant phytochemical markers between the two extraction methods. A significance level of p < 0.05 was set as the threshold for statistical significance, indicating that differences observed between methods were not due to random chance.
3. Results and Discussion
3.1. Comparison of Radical Scavenging Activity with Different Extraction Procedures
In this study, the radical scavenging potential of the various extracts was evaluated using the IC
50 value, which represents the concentration of the extract required to inhibit 50% of free radicals. As depicted in
Figure 1, the amount of extract necessary to neutralize 50% of free radicals varied among the different extraction methods, showcasing the differential antioxidant capacities of the extracts. Notably, there is an inverse relationship between IC
50 values and scavenging activity—lower IC
50 values indicate higher antioxidant potential.
The fermented extract demonstrated the most potent antioxidant activity with an IC50 value of 0.77 ± 0.03 mg/mL, significantly outperforming the solvent and steam-distilled extracts, which had IC50 values of 2.49 ± 0.01 mg/mL and 4.11 ± 0.03 mg/mL, respectively. This suggests that the fermentation process may enhance the release or bioavailability of phytochemicals with antioxidant properties. On the other hand, the Clevenger extract exhibited the weakest antioxidant activity, with the highest IC50 value of 5.81 ± 0.04 mg/mL, indicating a lower efficiency in scavenging free radicals. The IC50 values for NO scavenging were in the range of 1.12 ± 0.03 to 10.29 ± 0.03 mg/mL. The fermented extracts showed good activity compared to steam distillate extracts, Clevenger extracts, and solvent extracts.
These findings are consistent with previous research, which has shown that fermentation can enhance the bioactivity of plant extracts by breaking down complex molecules into more readily available forms [
22,
23]. The comparatively lower activity of solvent and steam-distilled extracts may be attributed to the limited ability of these methods to extract certain bioactive compounds that are more efficiently released during fermentation. Additionally, the Clevenger method, primarily focused on volatile oil extraction, may not efficiently capture non-volatile antioxidant components [
24].
This comparative study underscores the potential of fermented extracts as a superior source of antioxidants, which could be beneficial in the formulation of cosmeceutical products aimed at protecting the skin from oxidative stress and aging. Further research into the specific phytochemicals responsible for this enhanced activity in fermented extracts could provide deeper insights into their applications in skincare formulations.
3.2. Comparison of Total Phenolic Contents and Reducing Power of the Extracts from Different Extraction Procedures
The total phenolic content (TPC) of the various extracts, expressed in terms of gallic acid equivalent (GAE), was a key metric for evaluating the phytochemical activities of the bio-actives in this study. Phenolic compounds are well known for their potent antioxidant properties, making them important candidates for cosmeceutical formulations. Among the extracts, the fermented samples exhibited the highest TPC at 2.43 ± 0.03 mg GAE/g, significantly surpassing the solvent extracts (0.34 ± 0.01 mg GAE/g) and the Clevenger extracts, which showed the lowest phenolic content at 0.05 ± 0.001 mg GAE/g.
The total phenolic content of distilled extracts is also lower than solvent extracts, which is 0.21 ± 0.01 mg GAE/g. This notable difference suggests that fermentation plays a critical role in enhancing the phenolic content of the extract.
The comparative analysis of phytochemical activity between the various extracts from the mixed substrate revealed notable differences. While the Clevenger extract predominantly captured volatile compounds and, hence, provided the lowest yield, the distillation extract exhibited a broader range of phytochemicals, including non-volatile antioxidants, and provided a better yield of TPC. The superiority of the fermented extract in terms of TPC is consistent with previous studies, indicating that fermentation can improve the release and bioavailability of phenolic compounds. Fermentation processes often involve microbial enzymes that break down plant cell walls, thereby facilitating the release of bound phenolics, which are otherwise not extractable through conventional methods [
25,
26]. This highlights the influence of the extraction technique on the yield and quality of bioactives. This may explain why the solvent and Clevenger extracts, which rely on more traditional extraction techniques, demonstrated much lower TPC values, as shown in
Figure 1.
In addition to TPC, the antioxidant potential of the extracts was further evaluated by measuring their reducing power against the standard antioxidant butylated hydroxytoluene (BHT). Once again, the fermented extract exhibited the highest reducing power, suggesting that the increased phenolic content translates into enhanced antioxidant capacity. The solvent extracts demonstrated moderate reducing power, while the Clevenger extract, consistent with its low phenolic content, showed the least effectiveness.
These findings highlight the advantages of fermentation as a superior extraction method, not only in terms of yielding higher concentrations of bioactive phenolic compounds but also in enhancing the overall antioxidant potential of the extract. As phenolic compounds play a critical role in mitigating oxidative stress and protecting the skin from environmental damage, the fermented extract shows considerable promise as a potent cosmeceutical ingredient. This aligns with earlier research, which reported a correlation between high phenolic content and improved antioxidant activity in fermented extracts [
27,
28].
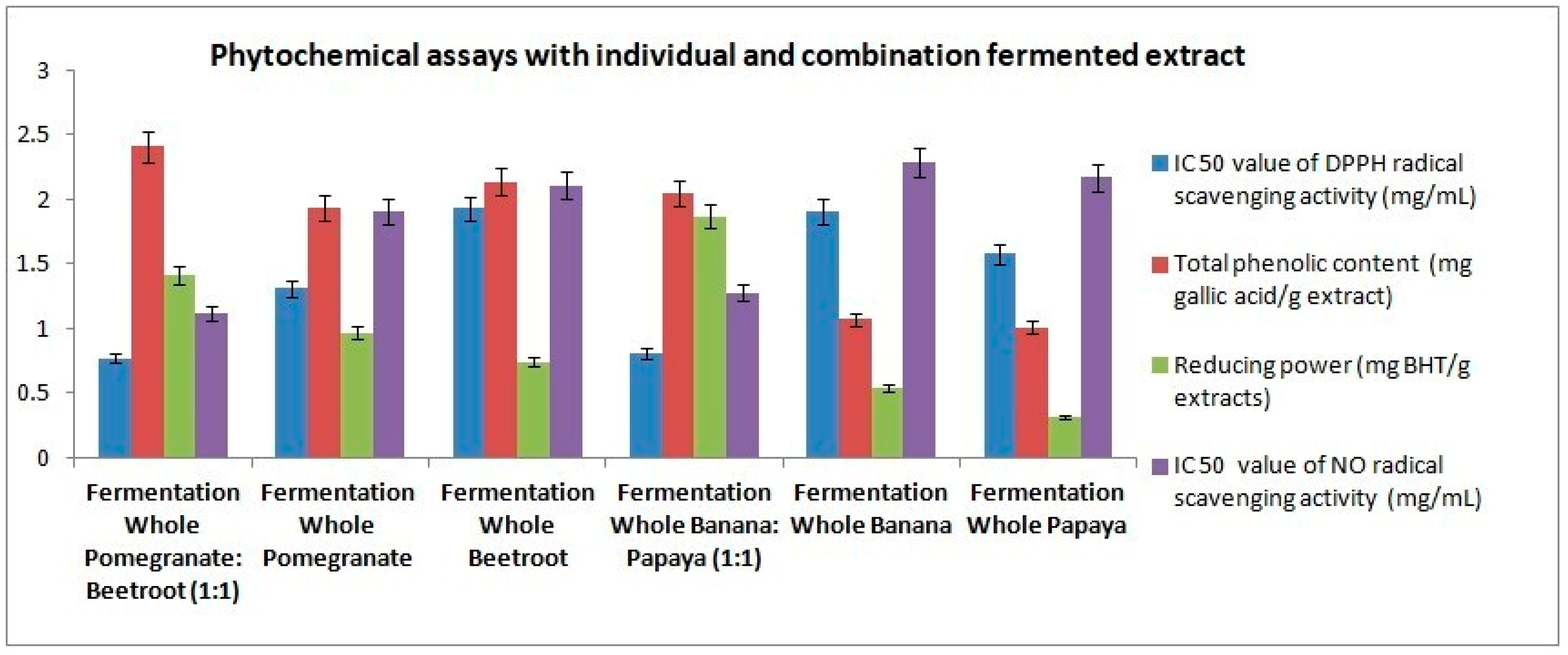
3.3. Synergistic Effect of Mixed Substrate Fermentation on Phytochemical Activities
The synergistic effects of mixed fruit fermentation on phytochemical activity were clearly demonstrated in this study, particularly with respect to DPPH and nitric oxide (NO) radical scavenging, and BHT reducing power. In this study, the fermented pomegranate and beetroot extract demonstrated the highest antioxidant activity with an IC
50 value of 0.77 ± 0.03 mg/mL, in comparison with individual fermented pomegranate and fermented beetroot extracts at 1.31 ± 0.02 mg/mL and 1.93 ± 0.01 mg/mL, respectively. Similarly, the fermented banana and papaya extracts demonstrated the highest antioxidant activity with an IC
50 value of 0.81 ± 0.01 mg/mL, when compared with individual fermented banana and fermented papaya extracts at 1.91 ± 0.03 mg/mL and 1.58 ± 0.02 mg/mL, respectively. The IC
50 values for NO scavenging were in the range of 1.12 ± 0.03 to 2.29 ± 0.03 mg/mL, where the mixed fruit fermented extract also showed the maximum activity towards NO radical scavenging. The mixed fruit fermentation process resulted in a significantly higher TPC compared to individually fermented extract samples, indicating enhanced bioavailability and release of phenolic compounds during fermentation. This is likely due to the synergistic enzymatic activity of the microorganisms involved in fermentation, which significantly enhances and liberates more bound phenolic compounds, increasing the overall phenolic content of the mixed fermented extract [
29]. The increased TPC, in turn, correlated with superior antioxidant activities, as measured by BHT reduction assays. The fermented banana and papaya mixed fermented extracts showed the highest BHT reduction activity of 1.87 ± 0.01 mg BHT/g of extract, suggesting that the combined effects of different fruit phenolics may have created a synergistic interaction that enhanced their radical neutralizing capacity. The mixed fruit fermentation approach amplified this activity, likely due to the diverse range of bioactive compounds working together to neutralize free radicals [
30].
Thus, the overall improvements in TPC, DPPH, and NO scavenging, as well as BHT reducing power, as represented in
Figure 2, underline the value of mixed fruit fermentation in boosting the phytochemical efficacy of cosmeceutical ingredients. These results support previous findings that fermentation of plant-based materials can significantly improve the bioactivity and therapeutic potential of phytochemicals [
30]. This makes fermented mixed fruit extracts a promising option for inclusion in skincare formulations aimed at protecting against oxidative damage and inflammation.
3.4. Optimization of Fermentation Process Conditions
3.4.1. Central Composite Rotatable Design Model Fit
In this study, the extraction process was optimized to improve the yield of GA and TPC in the pomegranate-beetroot combined fermented extract. The fermented extracts thus obtained were further assayed for BHT reducing power and antioxidant activities (DPPH and nitric oxide (NO) radical scavenging). The study was conducted using central composite rotatable design (CCRD) methodology. The selection of the optimal extraction conditions was guided by the highest-order polynomial models, ensuring that additional terms were significant and non-aliased based on the sequential sum of squares. A quadratic polynomial model was chosen, as recommended by the software, and it was well-suited for the two independent parameters and response variables under study.
The significance of this quadratic model was evaluated through analysis of variance (ANOVA), which revealed that high F-values and low
p-values for each term indicated a strong influence on the corresponding response variables. The second-order polynomial model for GA and TPC yields demonstrated statistical significance (
p < 0.05), with
p-values of less than 0.001 for the responses (
Table 3 and
Table 4). Additionally, the coefficient of determination (R
2) values for GA and TPC yield were 0.9666 and 0.9491, respectively, indicating a good fit of the quadratic model to the experimental data. The R
2, defined as the ratio of explained variation to total variation, is a measure of model fit, with values closer to 1 indicating a better fit [
14,
31].
The
p-values served as a tool to assess the significance of each coefficient, revealing the interaction patterns between the extraction variables. Smaller
p-values indicated more significant coefficients, as observed in
Table 3 and
Table 4. Both linear and quadratic terms for the extraction parameters (extraction temperature and extraction time) significantly affected GA and TPC yield (
p < 0.05). Furthermore, the interaction between extraction temperature and time had a significant influence on BHT reducing power, DPPH, and NO radical scavenging activities (
Figure 3). Among the two extraction parameters, extraction time played a more crucial role in the extraction of phenolic compounds from the pomegranate-beetroot ferment, followed by extraction temperature. These results highlight the importance of optimizing extraction time and temperature to maximize the yield of bioactive compounds in pomegranate-beetroot fermented extracts. The use of a well-fitted quadratic model provided a reliable predictive framework for identifying the ideal extraction conditions and enhancing the functional properties of the fermented extract.
3.4.2. Analysis of Response Surfaces
The relationship between extraction temperature and extraction time on the yield of gallic acid (GA) and total phenolic content (TPC) is presented in response surface plots (
Figure 3a,b). Both variables, temperature and time, showed significant linear and quadratic effects on GA and TPC yields (
p < 0.05), as detailed in
Table 3 and
Table 4. Specifically, increasing the extraction temperature up to 35 °C resulted in a marked increase in GA and TPC in the fermented extracts, followed by a slight decrease at higher temperatures. This suggests that moderate heating enhances phenolic extraction, but higher temperatures may induce the degradation of phenolic antioxidants. The mild heating at 35 °C likely softens plant tissues, disrupts cell wall integrity, and hydrolyzes the bonds between bound phenolics (e.g., phenol-protein or phenol-polysaccharide), improving phenolic solubility and facilitating their release into the fermentation medium.
At the optimal temperature of 35 °C, the highest phenolic content was achieved with a shorter extraction time of 72 h. This indicates that prolonged extraction may diminish the advantages of moderate heating by promoting oxidation or degradation of phenolic compounds, thus reducing the yield of GA and TPC. From the combined results shown in
Figure 3a,b, it is evident that extraction temperature and time are the most crucial factors influencing phenolic compound extraction from fermented extracts. These variables also significantly affect BHT reducing power and antioxidant activities (DPPH and NO radical scavenging), as shown in
Figure 3c–e. The modulation of phenolic compounds under these optimal conditions may contribute to enhanced phytochemical activity.
Optimizing both extraction temperature and time is important not only for maximizing phenolic compound yield but also for minimizing the energy costs associated with the process. The results indicate that an extraction conducted at a moderate temperature of 35 °C for a relatively short duration of 72 h was sufficient to achieve the maximum concentration of phenolic compounds (
Table 1). Furthermore, this condition minimizes the risk of degradation of phenolics that are sensitive to heat and light, preserving the bioactive compounds in the fermented extract.
3.4.3. Verification Experiment
The optimal extraction conditions were determined based on the regression analysis of the actual versus predicted data, and from the response surface plots (
Figure 3) according to the methodology described by Montgommery [
31]. The model predicted the highest yield of GA and TPC at an extraction temperature of 35 °C and an extraction time of 72 h. Verification experiments, conducted in triplicate under the predicted conditions, showed that the experimental values were in close agreement with the predicted values, confirming the accuracy of the models used. At the optimized extraction temperature of 35 °C and an extraction time of 72 h, the yields of GA and TPC were 90.7 ± 0.1 ppm and 2.43 ± 0.01 mg GA equivalent/g of fermented extract, respectively. Additionally, the antioxidant activity of the extract was assessed, with the radical scavenging activity recorded as 0.76 ± 0.05 mg/mL (IC
50 value for DPPH) and 1.11 ± 0.05 mg/mL (IC
50 value for NO). The reducing power of the extract was measured at 1.49 ± 0.01 mg BHT equivalent/g of fermented extract.
3.5. Antimicrobial Activity of the Finished Product Formulated with Fermented and Conventional Extract
In this study, antimicrobial activity was assessed by determining minimum inhibitory concentration (MIC) values for pomegranate and beetroot extracts, similar to methods applied in previous research [
14] (
Table 5). Propylene glycol served as a negative control, while ampicillin and ketoconazole were used as positive controls for antibacterial and antifungal activity, respectively. The MIC values for the solvent extracts of pomegranate and beetroot against
Escherichia coli,
Staphylococcus aureus,
Pseudomonas aeruginosa,
Propionibacterium acnes, and
Malassezia furfur were 1.2 mg/mL, 1.0 mg/mL, 1.5 mg/mL, 2.0 mg/mL, and 2.8 mg/mL, respectively, demonstrating the highest antibacterial activity among the conventional extracts tested. In comparison, the Clevenger extract exhibited lower inhibitory effects, with MIC values of 1.5 mg/mL for
E. coli, 1.8 mg/mL for
S. aureus, 2.0 mg/mL for
P. aeruginosa, 2.4 mg/mL for
P. acnes, and 3.2 mg/mL for
M. furfur. The steam-distilled extract demonstrated the lowest inhibitory activity, with MIC values of 2.5 mg/mL, 4.0 mg/mL, 3.2 mg/mL, 6.3 mg/mL, and 7.5 mg/mL for the same organisms, as shown in
Table 5. Notably, the fermented extract exhibited the highest antimicrobial activity, likely due to its increased antioxidant content, with MIC values against
E. coli,
S. aureus,
P. aeruginosa,
P. acnes, and
M. furfur recorded at 0.60 mg/mL, 0.55 mg/mL, 0.25 mg/mL, 0.50 mg/mL, and 0.40 mg/mL, respectively. These findings align with prior research data on pomegranate’s potent antibacterial properties [
32,
33].
In comparison, the Clevenger and steam-distilled extracts displayed comparatively lower inhibition, with the fermented extract achieving the strongest inhibition, likely due to higher antioxidant content. Studies on plant extracts, particularly pomegranate, have similarly shown potent inhibition across several pathogens when evaluated through MIC methods, attributed to the plant’s phenolic content and bioactive compounds, which effectively disrupt microbial cell functions [
34].
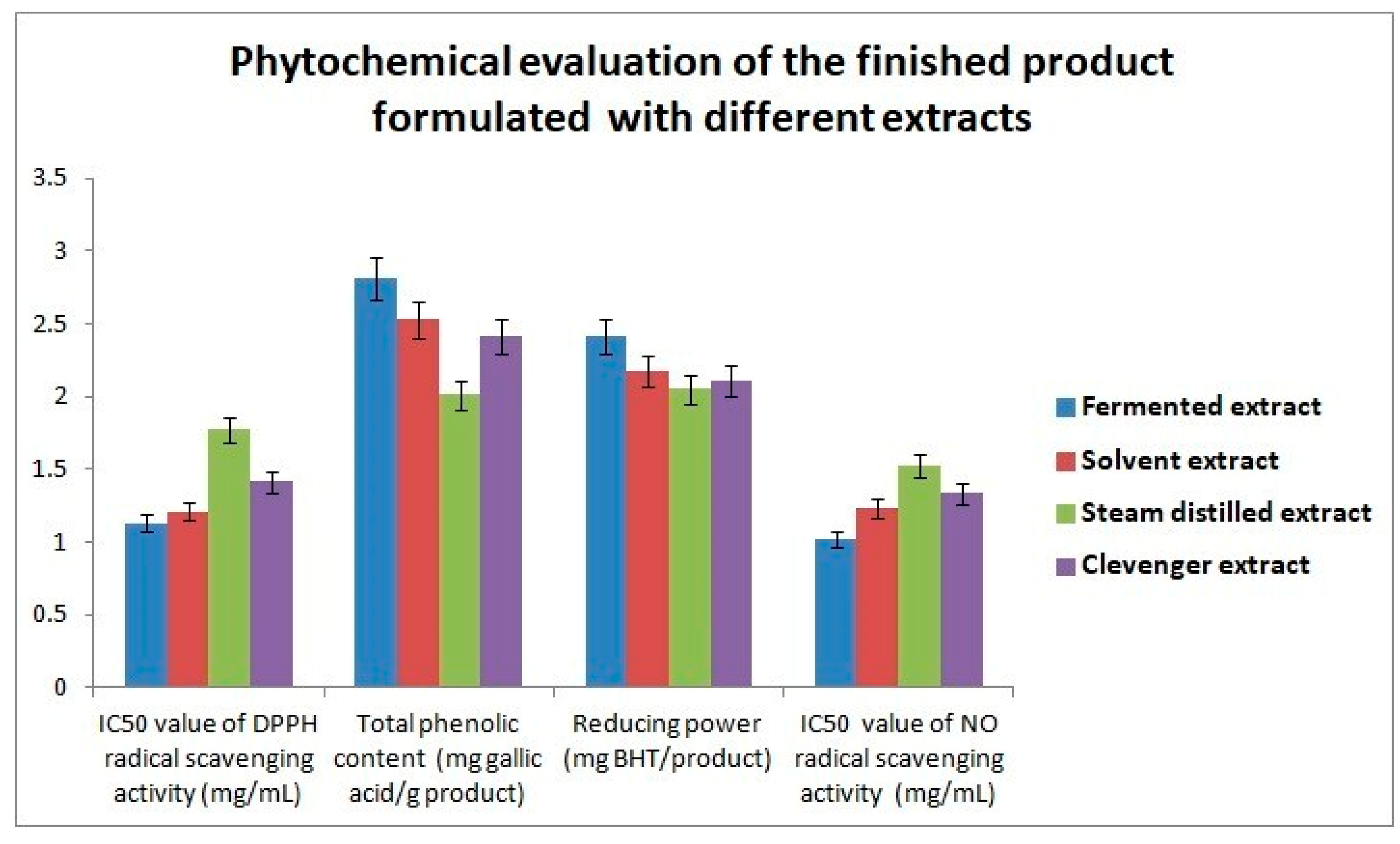
3.6. Phytochemical Evaluation of the Finished Product Comprising Conventional and Fermented Extracts
The phytochemical properties of the final formulation FERMENZA, incorporating both fermented and conventional extracts, were analyzed for potential cosmeceutical development. Notably, the fermented formulation demonstrated the highest antioxidant activity, with an IC
50 value of 1.13 ± 0.02 mg/mL, significantly surpassing the solvent, steam-distilled, and Clevenger extracts, which exhibited IC
50 values of 1.21 ± 0.01 mg/mL, 1.77 ± 0.02 mg/mL, and 1.41 ± 0.02 mg/mL, respectively. Studies have shown that fermentation can enhance antioxidant and bioactive compound efficacy, contributing to stronger antioxidant potential [
35]. Additionally, the nitric oxide (NO) scavenging activity for the fermented formulation showed an IC
50 value of 1.02 ± 0.03 mg/mL, which was more effective than the conventional extracts, with IC
50 values ranging from 1.23 ± 0.01 to 1.52 ± 0.03 mg/mL. This finding is in line with previous studies that observed enhanced NO scavenging abilities in extracts with higher phenolic content and fermentation-derived bioactives [
36].
Total phenolic content (TPC) further supported these observations as depicted in
Figure 4. The fermented samples exhibited a high TPC of 2.81 ± 0.03 mg GAE/g, significantly exceeding that of solvent extracts (2.53 ± 0.07 mg GAE/g) and the steam-distilled extract (2.01 ± 0.001 mg GAE/g). Consistent with the literature, elevated TPC levels correlated with enhanced antioxidant activities measured by butylated hydroxytoluene (BHT) reduction assays, where the fermented formulation achieved the highest BHT reduction activity of 2.41 ± 0.01 mg BHT/g [
37].
Fermented product formulations like FERMENZA could highlight the potential mechanisms, such as the enhancement of antioxidant defense systems to reduce oxidative stress in skin and scalp, and antimicrobial action to maintain a healthy microbiome balance.
4. Conclusions
In conclusion, the study highlights that fermentation significantly enhances the bioactive properties of natural extracts, such as antioxidants, phenolics, and antimicrobial efficacy, making fermented cosmetics more effective than conventional natural cosmetics. This positions FERMENZA® as a cutting-edge formulation in the cosmeceutical industry. Through the application of a Quality by Design (QbD) approach, the study identifies optimal fermentation conditions (e.g., 35 °C and 72 h) to maximize the yield of active compounds like gallic acid and phenolics. This ensures higher efficacy while maintaining affordability, making the process suitable for large-scale, cost-effective production. Additionally, the formulations created herein, designed for skin and hair care, were developed with minimal use of synthetic chemicals, adhering to GRAS (Generally Recognized As Safe) standards to enhance safety, cost-effectiveness, and ecological impact. The patented formulation process enhances the phytochemical richness and antimicrobial properties of FERMENZA®. Its efficacy against pathogens such as M. furfur and P. acne underscores its potential for developing effective skincare and haircare solutions, setting it apart as a scientifically validated cosmeceutical development innovation. Overall, these formulations provide a promising alternative to synthetic products, contributing valuable insights to the cosmeceutical industry’s drive for more sustainable, natural products.