Practical Strategies to Predict, Avoid and Manage the Complications of Robotic-Assisted Partial Nephrectomy
Abstract
1. Introduction
2. Preoperative Considerations
2.1. Patient Selection and Planning
2.1.1. Imaging
- Renal vascular anatomy (number, location, branching);
- Tumour size, location (polar location, depth, relationship to hilum and collecting system), and margin/boundary (well defined, irregular, vascular invasion);
- Relationship of the kidney and the tumour to surrounding structures;
- Patient anatomical variations—spinal abnormalities, aberrant abdominal vasculature (such as superior mesenteric artery origin).
2.1.2. Three Dimensional (3D) Models
2.1.3. Nephrometry Scores
2.1.4. Transperitoneal Versus Retroperitoneal Approach
2.1.5. Prediction of Postoperative Complications
3. Procedural Considerations
3.1. Positioning
3.2. Kidney Exposure Complications
3.2.1. Vascular
3.2.2. Bowel
3.2.3. Spleen
3.2.4. Pleura
3.2.5. Pancreas
3.2.6. Liver
3.2.7. Lymphatic
4. Specific Complications to Robotic-Assisted Partial Nephrectomy
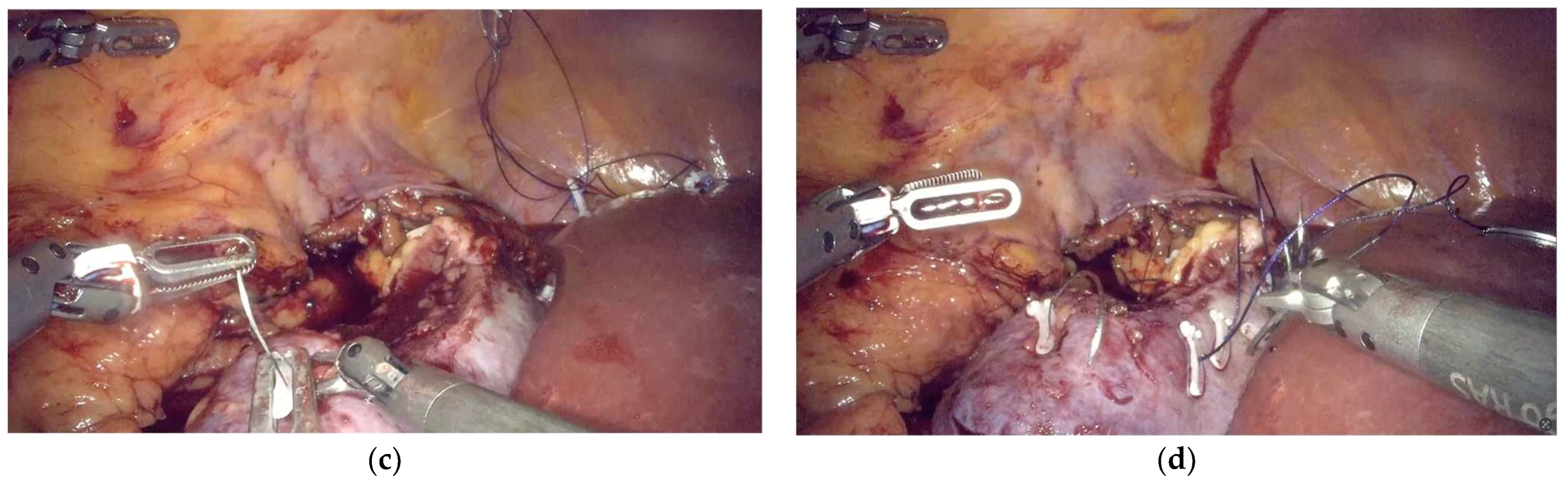
4.1. Bleeding
4.2. Urine Leak
4.3. Positive Surgical Margin
4.4. Renal Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.E.; You, J.H.; Kim, D.K.; Rha, K.H.; Lee, S.H. Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: A systematic review and meta-analysis. Eur. Urol. 2015, 67, 891–901. [Google Scholar] [CrossRef]
- Mottrie, A.; De Naeyer, G.; Schatteman, P.; Carpentier, P.; Sangalli, M.; Ficarra, V. Impact of the Learning Curve on Perioperative Outcomes in Patients Who Underwent Robotic Partial Nephrectomy for Parenchymal Renal Tumours. Eur. Urol. 2010, 58, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Schiavina, R.; Bianchi, L.; Borghesi, M.; Chessa, F.; Cercenelli, L.; Marcelli, E.; Brunocilla, E. Three-dimensional digital reconstruction of renal model to guide preoperative planning of robot-assisted partial nephrectomy. Int. J. Urol. 2019, 26, 931–932. [Google Scholar] [CrossRef]
- Bianchi, L.; Schiavina, R.; Bortolani, B.; Cercenelli, L.; Gaudiano, C.; Mottaran, A.; Droghetti, M.; Chessa, F.; Boschi, S.; Molinaroli, E.; et al. Novel Volumetric and Morphological Parameters Derived from Three-dimensional Virtual Modeling to Improve Comprehension of Tumor’s Anatomy in Patients with Renal Cancer. Eur. Urol. Focus 2022, 8, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Shirk, J.D.; Thiel, D.D.; Wallen, E.M.; Linehan, J.M.; White, W.M.; Badani, K.K.; Porter, J.R. Effect of 3-Dimensional Virtual Reality Models for Surgical Planning of Robotic-Assisted Partial Nephrectomy on Surgical Outcomes: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e1911598. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.; Khene, Z.E.; Prudhomme, T.; Boulenger de Hauteclocque, A.; Cornelis, F.H.; Percot, M.; Simeon, H.; Dupitout, L.; Bensadoun, H.; Capon, G.; et al. 3D-Image guided robotic-assisted partial nephrectomy: A multi-institutional propensity score-matched analysis (UroCCR study 51). World J. Urol. 2023, 41, 303–313. [Google Scholar] [CrossRef]
- Margue, G.; Bernhard, J.-C.; Giai, J.; Bouzit, A.; Ricard, S.; Jaffredo, M.; Guillaume, B.; Jambon, E.; Fiard, G.; Bigot, P.; et al. Clinical Trial Protocol for ACCURATE: A CCafU-UroCCR Randomized Trial: Three-dimensional Image-guided Robot-assisted Partial Nephrectomy for Renal Complex Tumor (UroCCR 99). Eur. Urol. Oncol. 2025, in press. [Google Scholar] [CrossRef]
- Schiavina, R.; Novara, G.; Borghesi, M.; Ficarra, V.; Ahlawat, R.; Moon, D.A.; Porpiglia, F.; Challacombe, B.J.; Dasgupta, P.; Brunocilla, E.; et al. PADUA and R.E.N.A.L. nephrometry scores correlate with perioperative outcomes of robot-assisted partial nephrectomy: Analysis of the Vattikuti Global Quality Initiative in Robotic Urologic Surgery (GQI-RUS) database. BJU Int. 2017, 119, 456–463. [Google Scholar] [CrossRef]
- Hew, M.N.; Baseskioglu, B.; Barwari, K.; Axwijk, P.H.; Can, C.; Horenblas, S.; Bex, A.; Rosette, J.J.; Pes, M.P. Critical appraisal of the PADUA classification and assessment of the R.E.N.A.L. nephrometry score in patients undergoing partial nephrectomy. J. Urol. 2011, 186, 42–46. [Google Scholar] [CrossRef]
- Kutikov, A.; Uzzo, R.G. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Secco, S.; Macchi, V.; Porzionato, A.; De Caro, R.; Artibani, W. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur. Urol. 2009, 56, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Hallett, A.; Patki, P.; Patel, N.; Barber, N.J.; Sullivan, M.; Thilagarajah, R. Robot-assisted partial nephrectomy: A comparison of the transperitoneal and retroperitoneal approaches. J. Endourol. 2013, 27, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Campi, R.; Schiavina, R.; Amparore, D.; Antonelli, A.; Artibani, W.; Barale, M.; Bertini, R.; Borghesi, M.; Bove, P.; et al. Nomogram for predicting the likelihood of postoperative surgical complications in patients treated with partial nephrectomy: A prospective multicentre observational study (the RECORd 2 project). BJU Int. 2019, 124, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Khene, Z.E.; Peyronnet, B.; Kocher, N.J.; Robyak, H.; Robert, C.; Pradere, B.; Oger, E.; Kammerer-Jacquet, S.-F.; Verhoest, G.; Rioux-Leclercq, N.; et al. Predicting morbidity after robotic partial nephrectomy: The effect of tumor, environment, and patient-related factors. Urol. Oncol. Semin. Orig. Investig. 2018, 36, e319–e338. [Google Scholar] [CrossRef]
- Bauman, T.M.; Potretzke, A.M.; Vetter, J.M.; Bhayani, S.B.; Figenshau, R.S. Cerebrovascular Disease and Chronic Obstructive Pulmonary Disease Increase Risk of Complications with Robotic Partial Nephrectomy. J. Endourol. 2015, 30, 293–299. [Google Scholar] [CrossRef]
- Paulucci, D.J.; Abaza, R.; Eun, D.D.; Hemal, A.K.; Badani, K.K. Robot-assisted partial nephrectomy: Continued refinement of outcomes beyond the initial learning curve. BJU Int. 2017, 119, 748–754. [Google Scholar] [CrossRef]
- Larcher, A.; Muttin, F.; Peyronnet, B.; De Naeyer, G.; Khene, Z.-E.; Dell’Oglio, P.; Ferreiro, C.; Schatteman, P.; Capitanio, U.; D’Hondt, F.; et al. The Learning Curve for Robot-assisted Partial Nephrectomy: Impact of Surgical Experience on Perioperative Outcomes. Eur. Urol. 2019, 75, 253–256. [Google Scholar] [CrossRef]
- Antonelli, A.; Allinovi, M.; Cocci, A.; Russo, G.I.; Schiavina, R.; Rocco, B.; Giovannalberto, P.; Celia, A.; Galfano, A.; Varca, V.; et al. The Predictive Role of Biomarkers for the Detection of Acute Kidney Injury After Partial or Radical Nephrectomy: A Systematic Review of the Literature. Eur. Urol. Focus 2020, 6, 344–353. [Google Scholar] [CrossRef]
- Lane, B.R.; Babitz, S.K.; Vlasakova, K.; Wong, A.; Noyes, S.L.; Boshoven, W.; Grady, P.; Zimmerman, C.; Engerman, S.; Gebben, M.; et al. Evaluation of Urinary Renal Biomarkers for Early Prediction of Acute Kidney Injury Following Partial Nephrectomy: A Feasibility Study. Eur. Urol. Focus 2020, 6, 1240–1247. [Google Scholar] [CrossRef]
- Chen, J.; Lin, J.; Lin, C. Serum and urinary biomarkers for predicting acute kidney injury after partial nephrectomy. Clin. Investig. Med. 2015, 38, E82–E89. [Google Scholar] [CrossRef]
- Mills, J.T.; Burris, M.B.; Warburton, D.J.; Conaway, M.R.; Schenkman, N.S.; Krupski, T.L. Positioning injuries associated with robotic assisted urological surgery. J. Urol. 2013, 190, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Deane, L.A.; Lee, H.J.; Box, G.N.; Abraham, J.B.; Abdelshehid, C.S.; Elchico, E.R.; Alipanah, R.; Borin, J.F.; Johnson, R.W.; Jackson, D.J.; et al. Third place: Flank position is associated with higher skin-to-surface interface pressures in men versus women: Implications for laparoscopic renal surgery and the risk of rhabdomyolysis. J. Endourol. 2008, 22, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Pariser, J.J.; Pearce, S.M.; Patel, S.G.; Anderson, B.B.; Packiam, V.T.; Shalhav, A.L.; Bales, G.T.; Smith, N.D. Rhabdomyolysis After Major Urologic Surgery: Epidemiology, Risk Factors, and Outcomes. Urology 2015, 85, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Saada, L.; Sapre, N.; Challacombe, B.J. Patient Positioning for Renal Surgery. In Robotic Urologic Surgery; Wiklund, P., Mottrie, A., Gundeti, M.S., Patel, V., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 441–446. [Google Scholar]
- Reisiger, K.E.; Landman, J.; Kibel, A.; Clayman, R.V. Laparoscopic renal surgery and the risk of rhabdomyolysis: Diagnosis and treatment. Urology 2005, 66, 29–35. [Google Scholar] [CrossRef]
- Pareek, G.; Hedican, S.P.; Gee, J.R.; Bruskewitz, R.C.; Nakada, S.Y. Meta-analysis of the complications of laparoscopic renal surgery: Comparison of procedures and techniques. J. Urol. 2006, 175, 1208–1213. [Google Scholar] [CrossRef]
- Dunne, N.; Booth, M.I.; Dehn, T.C. Establishing pneumoperitoneum: Verres or Hasson? The debate continues. Ann. R. Coll. Surg. Engl. 2011, 93, 22–24. [Google Scholar] [CrossRef]
- Meraney, A.M.; Samee, A.A.; Gill, I.S. Vascular and bowel complications during retroperitoneal laparoscopic surgery. J. Urol. 2002, 168, 1941–1944. [Google Scholar] [CrossRef]
- van der Voort, M.; Heijnsdijk, E.A.; Gouma, D.J. Bowel injury as a complication of laparoscopy. Br. J. Surg. 2004, 91, 1253–1258. [Google Scholar] [CrossRef]
- Canby-Hagino, E.D.; Morey, A.F.; Jatoi, I.; Perahia, B.; Bishoff, J.T. Fibrin sealant treatment of splenic injury during open and laparoscopic left radical nephrectomy. J. Urol. 2000, 164, 2004–2005. [Google Scholar] [CrossRef]
- Rosevear, H.M.; Montgomery, J.S.; Roberts, W.W.; Wolf, J.S., Jr. Characterization and management of postoperative hemorrhage following upper retroperitoneal laparoscopic surgery. J. Urol. 2006, 176, 1458–1462. [Google Scholar] [CrossRef]
- Del Pizzo, J.J.; Jacobs, S.C.; Bishoff, J.T.; Kavoussi, L.R.; Jarrett, T.W. Pleural injury during laparoscopic renal surgery: Early recognition and management. J. Urol. 2003, 169, 41–44. [Google Scholar] [CrossRef]
- Varkarakis, I.M.; Allaf, M.E.; Bhayani, S.B.; Inagaki, T.; Su, L.M.; Kavoussi, L.R.; Jarrett, T.W. Pancreatic injuries during laparoscopic urologic surgery. Urology 2004, 64, 1089–1093. [Google Scholar] [CrossRef]
- Sawaya, D.E., Jr.; Johnson, L.W.; Sittig, K.; McDonald, J.C.; Zibari, G.B. Iatrogenic and noniatrogenic extrahepatic biliary tract injuries: A multi-institutional review. Am. Surg. 2001, 67, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Shafizadeh, S.F.; Daily, P.P.; Baliga, P.; Rogers, J.; Baillie, G.M.; Rajagopolan, P.R.; Chavin, K.D. Chylous ascites secondary to laparoscopic donor nephrectomy. Urology 2002, 60, 345. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Yoo, E.S.; Kim, T.H.; Kwon, T.G. Chylous ascites as a complication of laparoscopic nephrectomy. J. Urol. 2010, 184, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Maurice, M.J.; Caputo, P.A.; Nelson, R.J.; Kara, Ö.; Malkoç, E.; Kaouk, J.H. Predicting complications in partial nephrectomy for T1a tumours: Does approach matter? BJU Int. 2016, 118, 940–945. [Google Scholar] [CrossRef]
- Bertolo, R.; Campi, R.; Mir, M.C.; Klatte, T.; Kriegmair, M.C.; Salagierski, M.; Ouzaid, I.; Capitanio, U. Systematic Review and Pooled Analysis of the Impact of Renorrhaphy Techniques on Renal Functional Outcome After Partial Nephrectomy. Eur. Urol. Oncol. 2019, 2, 572–575. [Google Scholar] [CrossRef]
- Bertolo, R.; Campi, R.; Klatte, T.; Kriegmair, M.C.; Mir, M.C.; Ouzaid, I.; Salagierski, M.; Bhayani, S.; Gill, I.; Kaouk, J.; et al. Suture techniques during laparoscopic and robot-assisted partial nephrectomy: A systematic review and quantitative synthesis of peri-operative outcomes. BJU Int. 2019, 123, 923–946. [Google Scholar] [CrossRef]
- Bertolo, R.; Ditonno, F.; Veccia, A.; De Marco, V.; Migliorini, F.; Porcaro, A.B.; Rizzetto, R.; Cerruto, M.A.; Autorino, R.; Antonelli, A. Single-layer versus double-layer renorrhaphy technique during robot-assisted partial nephrectomy: Impact on perioperative outcomes, complications, and functional outcomes. Minerva Urol. Nephrol. 2024, 76, 176–184. [Google Scholar] [CrossRef]
- Arora, S.; Bronkema, C.; Porter, J.R.; Mottrie, A.; Dasgupta, P.; Challacombe, B.; Rha, K.H.; Ahlawat, R.K.; Capitanio, U.; Yuvaraja, T.B.; et al. Omission of Cortical Renorrhaphy During Robotic Partial Nephrectomy: A Vattikuti Collective Quality Initiative Database Analysis. Urology 2020, 146, 125–132. [Google Scholar] [CrossRef]
- Moore, C.J.; Rozen, S.M.; Fishman, E.K. Two cases of pseudoaneurysm of the renal artery following laparoscopic partial nephrectomy for renal cell carcinoma: CT angiographic evaluation. Emerg. Radiol. 2004, 10, 193–196. [Google Scholar] [CrossRef]
- Potretzke, A.M.; Knight, B.A.; Zargar, H.; Kaouk, J.H.; Barod, R.; Rogers, C.G.; Mass, A.; Stifelman, M.D.; Johnson, M.H.; Allaf, M.E.; et al. Urinary fistula after robot-assisted partial nephrectomy: A multicentre analysis of 1 791 patients. BJU Int. 2016, 117, 131–137. [Google Scholar] [CrossRef]
- Inoue, R.; Isoyama, N.; Ozawa, S.; Kobayashi, K.; Yamamoto, Y.; Yano, S.; Hirata, H.; Matsumoto, H.; Matsuyama, H. Endoscopic laser treatment for urine leakage caused by an isolated calyx after robot-assisted partial nephrectomy. IJU Case Rep. 2021, 4, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Dibitetto, F.; Russo, P.; Marino, F.; Ragonese, M.; Nigro, D.; Foschi, N. Staghorn Caliceal Hem-o-lok Stone: A Long-Term Complication of Robotic Partial Nephrectomy—A Case Report and Literature Review. Urol. Int. 2024, 108, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Bic, A.; Mazeaud, C.; Salleron, J.; Bannay, A.; Balkau, B.; Larose, C.; Hubert, J.; Eschwège, P. Complications after partial nephrectomy: Robotics overcomes open surgery and laparoscopy: The PMSI French national database. BMC Urol. 2023, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Bravi, C.A.; Larcher, A.; Capitanio, U.; Mari, A.; Antonelli, A.; Artibani, W.; Barale, M.; Bertini, R.; Bove, P.; Brunocilla, E.; et al. Perioperative Outcomes of Open, Laparoscopic, and Robotic Partial Nephrectomy: A Prospective Multicenter Observational Study (The RECORd 2 Project). Eur. Urol. Focus 2021, 7, 390–396. [Google Scholar] [CrossRef]
- Peyronnet, B.; Seisen, T.; Oger, E.; Vaessen, C.; Grassano, Y.; Benoit, T.; Carrouget, J.; Pradère, B.; Khene, Z.; Giwerc, A.; et al. Comparison of 1800 Robotic and Open Partial Nephrectomies for Renal Tumors. Ann. Surg. Oncol. 2016, 23, 4277–4283. [Google Scholar] [CrossRef]
- Tan, J.-L.; Frydenberg, M.; Grummet, J.; Hanegbi, U.; Snow, R.; Mann, S.; Begashaw, K.; Moon, D. Comparison of perioperative, renal and oncologic outcomes in robotic-assisted versus open partial nephrectomy. ANZ J. Surg. 2018, 88, E194–E199. [Google Scholar] [CrossRef]
- Grimm, M.O.; Bedke, J.; Nyarangi-Dix, J.; Khoder, W.; Foller, S.; Sommerfeld, H.J.; Giessing, M.; Heck, M.; Meißner, W.; Slee, A.; et al. Open versus robotic-assisted partial nephrectomy in patients with intermediate/high-complexity kidney tumours: Final results of the randomised, controlled, open-label, multicentre trial OpeRa. Ann. Oncol. 2025, 36, 988–998. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, J.; Ma, X.; Du, Q.; Gong, H.; Zhang, X. Robotic and open partial nephrectomy for complex renal tumors: A matched-pair comparison with a long-term follow-up. World J. Urol. 2017, 35, 73–80. [Google Scholar] [CrossRef]
- Furukawa, J.; Kanayama, H.; Azuma, H.; Inoue, K.; Kobayashi, Y.; Kashiwagi, A.; Segawa, T.; Takahashi, Y.; Horie, S.; Ogawa, O.; et al. ‘Trifecta’ outcomes of robot-assisted partial nephrectomy: A large Japanese multicenter study. Int. J. Clin. Oncol. 2020, 25, 347–353. [Google Scholar] [CrossRef]
- Connor, J.; Doppalapudi, S.K.; Wajswol, E.; Ragam, R.; Press, B.; Luu, T.; Koster, H.; Tamang, T.-L.; Ahmed, M.; Lovallo, G.; et al. Postoperative Complications After Robotic Partial Nephrectomy. J. Endourol. 2020, 34, 42–47. [Google Scholar] [CrossRef]
- Yossepowitch, O.; Thompson, R.H.; Leibovich, B.C.; Eggener, S.E.; Pettus, J.A.; Kwon, E.D.; Herr, H.W.; Blute, M.L.; Russo, P. Positive surgical margins at partial nephrectomy: Predictors and oncological outcomes. J. Urol. 2008, 179, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.H.; Moreira, D.M.; Okhunov, Z.; Patel, V.R.; Chopra, S.; Razmaria, A.A.; Alom, M.; George, A.K.; Yaskiv, O.; Schwartz, M.J.; et al. Positive Surgical Margins Increase Risk of Recurrence after Partial Nephrectomy for High Risk Renal Tumors. J. Urol. 2016, 196, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Blute, M.L.; Ficarra, V.; Gill, I.S.; Kutikov, A.; Porpiglia, F.; Rogers, C.; Touijer, K.A.; Van Poppel, H.; Thompson, R.H. Renal Ischemia and Function After Partial Nephrectomy: A Collaborative Review of the Literature. Eur. Urol. 2015, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Parekh, D.J.; Weinberg, J.M.; Ercole, B.; Torkko, K.C.; Hilton, W.; Bennett, M.; Devarajan, P.; Venkatachalam, M.A. Tolerance of the human kidney to isolated controlled ischemia. J. Am. Soc. Nephrol. 2013, 24, 506–517. [Google Scholar] [CrossRef]
- Bjurlin, M.A.; Gan, M.; McClintock, T.R.; Volpe, A.; Borofsky, M.S.; Mottrie, A.; Stifelman, M.D. Near-infrared fluorescence imaging: Emerging applications in robotic upper urinary tract surgery. Eur. Urol. 2014, 65, 793–801. [Google Scholar] [CrossRef]
- Klatte, T.; Ficarra, V.; Gratzke, C.; Kaouk, J.; Kutikov, A.; Macchi, V.; Mottrie, A.; Porpiglia, F.; Porter, J.; Rogers, C.G.; et al. A Literature Review of Renal Surgical Anatomy and Surgical Strategies for Partial Nephrectomy. Eur. Urol. 2015, 68, 980–992. [Google Scholar] [CrossRef]
- Gill, I.S.; Eisenberg, M.S.; Aron, M.; Berger, A.; Ukimura, O.; Patil, M.B.; Campese, V.; Thangathurai, D.; Desai, M.M. “Zero ischemia” partial nephrectomy: Novel laparoscopic and robotic technique. Eur. Urol. 2011, 59, 128–134. [Google Scholar] [CrossRef]
- Rogers, C.G.; Ghani, K.R.; Kumar, R.K.; Jeong, W.; Menon, M. Robotic Partial Nephrectomy with Cold Ischemia and On-clamp Tumor Extraction: Recapitulating the Open Approach. Eur. Urol. 2013, 63, 573–578. [Google Scholar] [CrossRef]

| Type of Injury | n | % |
|---|---|---|
| Hollow viscous | 2 | 0.3 |
| Pleura | 1 | 0.2 |
| Vascular | 2 | 0.3 |
| Adjacent organ | 4 | 0.7 |
| Intra-operative transfusion | 4 | 0.7 |
| Reference | Design, Location | Number of Patients | Haemorrhage | Pseudoaneurysm/ AVF | Urine Leak | PSM |
|---|---|---|---|---|---|---|
| Bic 2023 [46] | Multicentre case series, France | 1900 | 88 (4.6) | 3 (0.16) | 7 (0.37) | NR |
| Bravi 2021 [47] | Multicentre case series, Italy | 789 | 17 (2.2) | NR | 1 (0.13) | 41 (5) |
| Peyronnet 2016 [48] | Multicentre case series, France | 937 | 64 (6.9) | NR | 19 (2) | 48 (5.2) |
| Tan 2018 [49] | Multicentre case series, Australia | 145 | 4 (2.8) | 3 (2) | NR | 5 (3.5) |
| Grimm 2025 [50] | Prospective RCT, Germany | 123 | 2 (2) | 3 (3) | 4 (4) | 11 (10) |
| Wang 2017 [51] | Multicentre case series, China | 190 | 6 (3.2) | 2 (1) | 4 (2.1) | 3 (1.6) |
| Furukawa 2020 [52] | Multicentre case series, Japan | 804 | NR | 33 (4.1) | 2 (0.2) | 8 (1.1) |
| Connor 2020 [53] | Single-centre case series, USA | 395 | NR | 9 (2.3) | 1 (0.25) | NR |
| Ramirez 2016 [37] | Single centre case series, USA | 545 | 23 (4.2) | 0 (0) | 1 (0.8) | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepherd, A.R.H.; Challacombe, B.J. Practical Strategies to Predict, Avoid and Manage the Complications of Robotic-Assisted Partial Nephrectomy. Complications 2025, 2, 21. https://doi.org/10.3390/complications2030021
Shepherd ARH, Challacombe BJ. Practical Strategies to Predict, Avoid and Manage the Complications of Robotic-Assisted Partial Nephrectomy. Complications. 2025; 2(3):21. https://doi.org/10.3390/complications2030021
Chicago/Turabian StyleShepherd, Andrew R. H., and Benjamin J. Challacombe. 2025. "Practical Strategies to Predict, Avoid and Manage the Complications of Robotic-Assisted Partial Nephrectomy" Complications 2, no. 3: 21. https://doi.org/10.3390/complications2030021
APA StyleShepherd, A. R. H., & Challacombe, B. J. (2025). Practical Strategies to Predict, Avoid and Manage the Complications of Robotic-Assisted Partial Nephrectomy. Complications, 2(3), 21. https://doi.org/10.3390/complications2030021






