Complications of Vertebroplasty in Adults: Incidence, Etiology, and Therapeutic Strategies—A Comprehensive, Systematic Literature Review
Abstract
1. Introduction
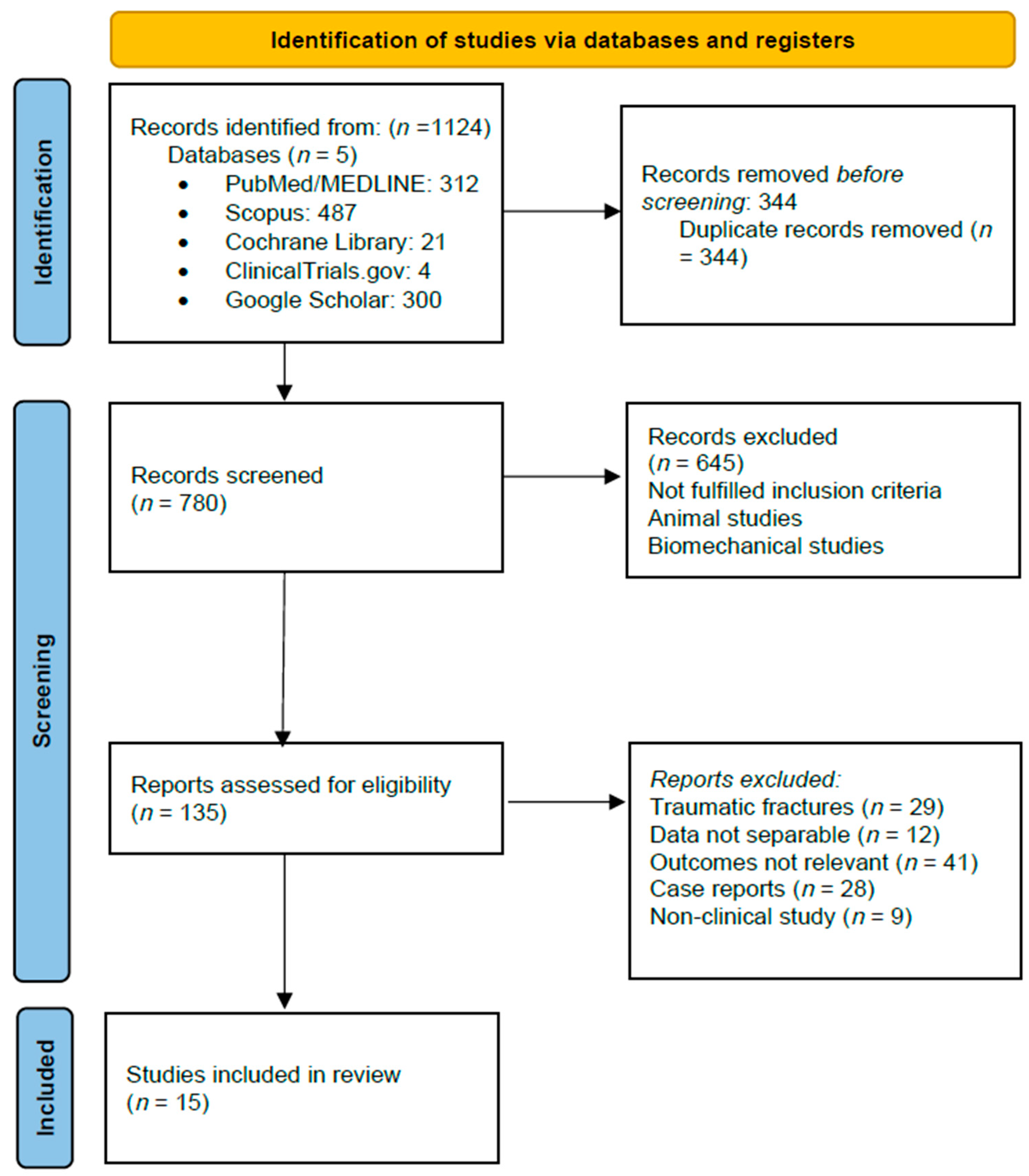
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction and Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Patient Population
3.3. Pathophysiology of Osteoporotic vs. Neoplastic Fractures
3.4. Outcomes
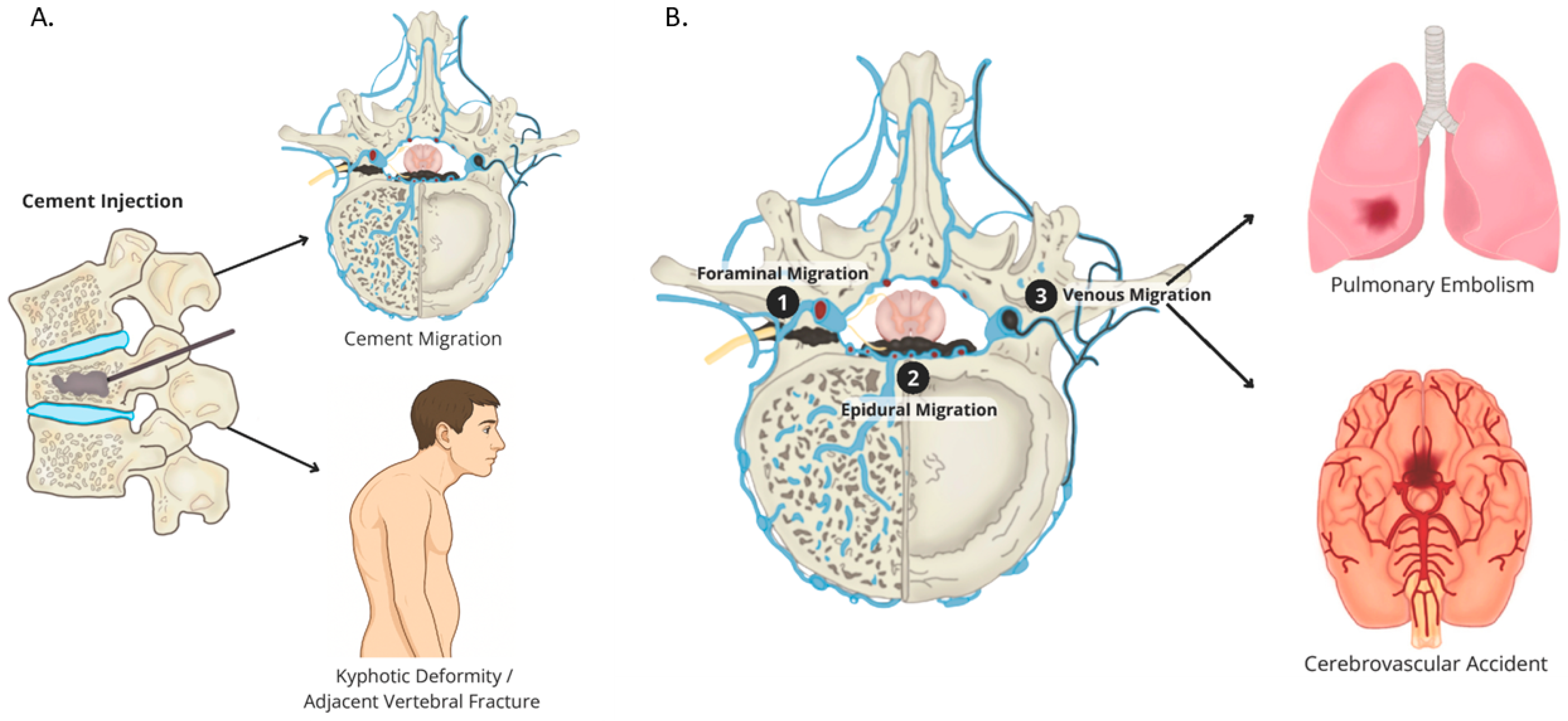
3.4.1. Cement Leakage
3.4.2. Pulmonary Cement Embolism (PCE)
3.4.3. Adjacent-Level Fractures
3.4.4. Infection (Spondylodiscitis)
3.4.5. Neurological Injury
4. Discussion
4.1. Clinical Decision-Making Framework
4.2. Complication Prevention and Technical Optimization
4.3. Management of Complications
Informed Consent and Risk Communication
4.4. Future Research Priorities
4.5. Limitations of Evidence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVP | Percutaneous Vertebroplasty |
| RCT | Randomized Controlled Trial |
| OVCF | Osteoporotic Vertebral Compression Fracture |
| PMMA | Polymethylmethacrylate |
| FDA | U.S. Food and Drug Administration |
| SCOPUS | A bibliographic database of peer-reviewed literature |
| MEDLINE | Medical Literature Analysis and Retrieval System Online |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| PCE | Pulmonary Cement Embolism |
| DOAC | Direct Oral Anticoagulant |
| UTI | Urinary Tract Infection |
| TB | Tuberculosis |
| MSSA | Methicillin-Sensitive Staphylococcus Aureus |
References
- Dong, Y.; Peng, R.; Kang, H.; Song, K.; Guo, Q.; Zhao, H.; Zhu, M.; Zhang, Y.; Guan, H.; Li, F. Global Incidence, Prevalence, and Disability of Vertebral Fractures: A Systematic Analysis of the Global Burden of Disease Study 2019. Spine J. 2022, 22, 857–868. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, S.; Lan, G.; Li, C.; Wei, J. Global, Regional, and National Burden of Fracture of Vertebral Column, 1990–2021: Analysis of Data from the Global Burden of Disease Study 2021. Front. Public Health 2025, 13, 1573888. [Google Scholar] [CrossRef]
- Curtis, J.R.; Taylor, A.J.; Matthews, R.S.; Ray, M.N.; Becker, D.J.; Gary, L.C.; Kilgore, M.L.; Morrisey, M.A.; Saag, K.G.; Warriner, A.; et al. “Pathologic” Fractures: Should These Be Included in Epidemiologic Studies of Osteoporotic Fractures? Osteoporos. Int. 2009, 20, 1969–1972. [Google Scholar] [CrossRef]
- Burton, A.W.; Rhines, L.D.; Mendel, E. Vertebroplasty and Kyphoplasty: A Comprehensive Review. Neurosurg. Focus. 2005, 18, e1. [Google Scholar] [CrossRef] [PubMed]
- Cavka, M.; Delimar, D.; Rezan, R.; Zigman, T.; Duric, K.S.; Cimic, M.; Dumic-Cule, I.; Prutki, M. Complications of Percutaneous Vertebroplasty: A Pictorial Review. Medicina 2023, 59, 1536. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Hatten, H.P.J.; Linovitz, R.; Tahernia, A.D.; Schaufele, M.K.; McCollom, V.; Gilula, L.; Maurer, P.; Benyamin, R.; Mathis, J.M.; et al. A Prospective Randomized FDA-IDE Trial Comparing Cortoss With PMMA for Vertebroplasty: A Comparative Effectiveness Research Study With 24-Month Follow-Up. Spine 2012, 37, 544. [Google Scholar] [CrossRef] [PubMed]
- Cosar, M.; Sasani, M.; Oktenoglu, T.; Kaner, T.; Ercelen, O.; Kose, K.C.; Ozer, A.F. The Major Complications of Transpedicular Vertebroplasty. J. Neurosurg. Spine 2009, 11, 607–613. [Google Scholar] [CrossRef]
- Layton, K.F.; Thielen, K.R.; Koch, C.A.; Luetmer, P.H.; Lane, J.I.; Wald, J.T.; Kallmes, D.F. Vertebroplasty, First 1000 Levels of a Single Center: Evaluation of the Outcomes and Complications. AJNR Am. J. Neuroradiol. 2007, 28, 683–689. [Google Scholar]
- Lee, M.J.; Dumonski, M.; Cahill, P.; Stanley, T.; Park, D.; Singh, K. Percutaneous Treatment of Vertebral Compression Fractures: A Meta-Analysis of Complications. Spine 2009, 34, 1228–1232. [Google Scholar] [CrossRef]
- Rahimi, B.; Boroofeh, B.; Dinparastisaleh, R.; Nazifi, H. Cement Pulmonary Embolism after Percutaneous Vertebroplasty in a Patient with Cushing’s Syndrome: A Case Report. Respir. Med. Case Rep. 2018, 25, 78–85. [Google Scholar] [CrossRef]
- Liao, J.-C.; Lai, P.-L.; Chen, L.-H.; Niu, C.-C. Surgical Outcomes of Infectious Spondylitis after Vertebroplasty, and Comparisons between Pyogenic and Tuberculosis. BMC Infect. Dis. 2018, 18, 555. [Google Scholar] [CrossRef]
- Anselmetti, G.C.; Marcia, S.; Saba, L.; Muto, M.; Bonaldi, G.; Carpeggiani, P.; Marini, S.; Manca, A.; Masala, S. Percutaneous Vertebroplasty: Multi-Centric Results from EVEREST Experience in Large Cohort of Patients. Eur. J. Radiol. 2012, 81, 4083–4086. [Google Scholar] [CrossRef]
- Hsieh, M.-K.; Kao, F.-C.; Chiu, P.-Y.; Chen, L.-H.; Yu, C.-W.; Niu, C.-C.; Lai, P.-L.; Tsai, T.-T. Risk Factors of Neurological Deficit and Pulmonary Cement Embolism after Percutaneous Vertebroplasty. J. Orthop. Surg. Res. 2019, 14, 406. [Google Scholar] [CrossRef]
- Abdelrahman, H.; Siam, A.E.; Shawky, A.; Ezzati, A.; Boehm, H. Infection after Vertebroplasty or Kyphoplasty. A Series of Nine Cases and Review of Literature. Spine J. 2013, 13, 1809–1817. [Google Scholar] [CrossRef]
- Kim, H.-J.; Zuckerman, S.L.; Cerpa, M.; Yeom, J.S.; Lehman, R.A.; Lenke, L.G. Incidence and Risk Factors for Complications and Mortality After Vertebroplasty or Kyphoplasty in the Osteoporotic Vertebral Compression Fracture-Analysis of 1,932 Cases From the American College of Surgeons National Surgical Quality Improvement. Global Spine J. 2022, 12, 1125–1134. [Google Scholar] [CrossRef]
- Venmans, A.; Klazen, C.A.H.; Lohle, P.N.M.; van Rooij, W.J.; Verhaar, H.J.J.; de Vries, J.; Mali, W.P.T.M. Percutaneous Vertebroplasty and Pulmonary Cement Embolism: Results from VERTOS II. AJNR Am. J. Neuroradiol. 2010, 31, 1451–1453. [Google Scholar] [CrossRef] [PubMed]
- Luetmer, M.T.; Bartholmai, B.J.; Rad, A.E.; Kallmes, D.F. Asymptomatic and Unrecognized Cement Pulmonary Embolism Commonly Occurs with Vertebroplasty. AJNR Am. J. Neuroradiol. 2011, 32, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Li, L.; Song, J. Delayed Neurological Deficits Caused by Cement Extravasation Following Vertebroplasty: A Case Report. J. Int. Med. Res. 2021, 49, 03000605211019664. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Cui, L.; Chen, X.; Liu, Y. Risk Factors for Cement Leakage in Percutaneous Vertebroplasty for Osteoporotic Vertebral Compression Fractures: An Analysis of 1456 Vertebrae Augmented by Low-Viscosity Bone Cement. Spine (Phila Pa 1976) 2021, 46, 216–222. [Google Scholar] [CrossRef]
- Kasó, G.; Horváth, Z.; Szenohradszky, K.; Sándor, J.; Dóczi, T. Comparison of CT Characteristics of Extravertebral Cement Leakages after Vertebroplasty Performed by Different Navigation and Injection Techniques. Acta Neurochir. 2008, 150, 677–683; discussion 683. [Google Scholar] [CrossRef]
- Baek, I.-H.; Park, H.-Y.; Kim, K.-W.; Jang, T.-Y.; Lee, J.-S. Paraplegia Due to Intradural Cement Leakage after Vertebroplasty: A Case Report and Literature Review. BMC Musculoskelet. Disord. 2021, 22, 741. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, A.; Yoshiura, T.; Yamashita, K.; Kamano, H.; Honda, H. Ultrashort TE MRI: Usefulness after Percutaneous Vertebroplasty. AJR Am. J. Roentgenol. 2010, 195, W365–W368. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zong, M.; Wang, W.-T.; Xu, L.; Cao, D.; Zou, Y.-F. Analysis of Risk Factors Causing Short-Term Cement Leakages and Long-Term Complications after Percutaneous Kyphoplasty for Osteoporotic Vertebral Compression Fractures. Acta Radiol. 2018, 59, 577–585. [Google Scholar] [CrossRef]
- Barakat, A.S.; Owais, T.; Alhashash, M.; Shousha, M.; El Saghir, H.; Lauer, B.; Boehm, H. Presentation and Management of Symptomatic Central Bone Cement Embolization. Eur. Spine J. 2018, 27, 2584–2592. [Google Scholar] [CrossRef]
- Stricker, K.; Orler, R.; Yen, K.; Takala, J.; Luginbühl, M. Severe Hypercapnia Due to Pulmonary Embolism of Polymethylmethacrylate during Vertebroplasty. Anesth. Analg. 2004, 98, 1184–1186. [Google Scholar] [CrossRef]
- Sun, H.-B.; Jing, X.-S.; Shan, J.-L.; Bao, L.; Wang, D.-C.; Tang, H. Risk Factors for Pulmonary Cement Embolism Associated with Percutaneous Vertebral Augmentation: A Systematic Review and Meta-Analysis. Int. J. Surg. 2022, 101, 106632. [Google Scholar] [CrossRef]
- Dargelos-Descoubez, M.; Martin, F.; Frampas, E.; Perret, C.; David, A.; Volpi, S. Progression toward Vertebral Collapse of Vertebral Metastases Treated with Percutaneous Vertebroplasty: Rate and Risk Factors. J. Vasc. Interv. Radiol. 2024, 35, 59–68. [Google Scholar] [CrossRef]
- Mazzantini, M.; Figliomeni, A.; Bottai, V.; Manca, M.L.; Puglioli, M.; Di Munno, O.; Mosca, M. High Rate of Vertebral Refracture after Vertebroplasty in Patients Taking Glucocorticoids: A Prospective Two-Year Study. Clin. Exp. Rheumatol. 2020, 38, 649–653. [Google Scholar]
- Park, S.; Sik Choi, S.; Kim, H.; Yoon Byun, S.; Lee, C.H. Risk Factors for New Vertebral Compression Fracture after Vertebroplasty and Efficacy of Osteoporosis Treatment: A STROBE-Compliant Retrospective Study. Medicine 2023, 102, e35042. [Google Scholar] [CrossRef]
- Zhai, G.; Li, A.; Liu, B.; Lv, D.; Zhang, J.; Sheng, W.; Yang, G.; Gao, Y. A Meta-Analysis of the Secondary Fractures for Osteoporotic Vertebral Compression Fractures after Percutaneous Vertebroplasty. Medicine 2021, 100, e25396. [Google Scholar] [CrossRef]
- Nieuwenhuijse, M.J.; Putter, H.; van Erkel, A.R.; Dijkstra, P.D.S. New Vertebral Fractures after Percutaneous Vertebroplasty for Painful Osteoporotic Vertebral Compression Fractures: A Clustered Analysis and the Relevance of Intradiskal Cement Leakage. Radiology 2013, 266, 862–870. [Google Scholar] [CrossRef]
- Marselou, E.; Kelekis, A.; Dimitriadis, Z.; Koumantakis, G.A. Risk Factors for Refracture or New Vertebral Compression Fractures after Percutaneous Vertebroplasty: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2025, 36, 1297–1311. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wu, B.-S.; Hsu, M.-C.; Yao, Y.-C.; Lin, H.-H.; Chou, P.-H.; Wang, S.-T.; Chang, M.-C.; Hsiung, W.; Wang, C.-Y.; Chen, K.-J. Efficiency and Safety of Repeated Vertebroplasty for Adjacent Segment Fractures. J. Clin. Med. 2024, 14, 166. [Google Scholar] [CrossRef]
- Lamy, O.; Uebelhart, B.; Aubry-Rozier, B. Risks and Benefits of Percutaneous Vertebroplasty or Kyphoplasty in the Management of Osteoporotic Vertebral Fractures. Osteoporos. Int. 2014, 25, 807–819. [Google Scholar] [CrossRef]
- Khanna, A.J.; Reinhardt, M.K.; Togawa, D.; Lieberman, I.H. Functional Outcomes of Kyphoplasty for the Treatment of Osteoporotic and Osteolytic Vertebral Compression Fractures. Osteoporos. Int. 2006, 17, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Essibayi, M.A.; Mortezaei, A.; Azzam, A.Y.; Bangash, A.H.; Eraghi, M.M.; Fluss, R.; Brook, A.; Altschul, D.J.; Yassari, R.; Chandra, R.V.; et al. Risk of Adjacent Level Fracture after Percutaneous Vertebroplasty and Kyphoplasty vs Natural History for the Management of Osteoporotic Vertebral Compression Fractures: A Network Meta-Analysis of Randomized Controlled Trials. Eur. Radiol. 2024, 34, 7185–7196. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga-García, J.P.; Leon-Aldana, S.; Herrera, D. Spondylodiscitis: A Comprehensive Review of Diagnostic Challenges, Microbial Etiology, and Management Strategies. SN Compr. Clin. Med. 2025, 7, 92. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Y.; Wang, J.; Wang, X.; Lü, G.; Kuang, L.; Li, J. Spinal Infection after Vertebral Augmentation: A Covert Complication with Serious Havoc. Arch. Orthop. Trauma. Surg. 2024, 144, 1461–1471. [Google Scholar] [CrossRef]
- Patel, A.A.; Vaccaro, A.R.; Martyak, G.G.; Harrop, J.S.; Albert, T.J.; Ludwig, S.C.; Youssef, J.A.; Gelb, D.E.; Mathews, H.H.; Chapman, J.R.; et al. Neurologic Deficit Following Percutaneous Vertebral Stabilization. Spine (Phila Pa 1976) 2007, 32, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Shen, W.-C.; Lo, Y.-C.; Liu, Y.-J.; Yu, T.-C.; Chen, I.-H.; Chung, H.-W. Recurrent Pain After Percutaneous Vertebroplasty. Am. J. Roentgenol. 2010, 194, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.D.; Jensen, M.E.; Hirsch, J.A.; McGraw, J.K.; Barr, R.M.; Brook, A.L.; Meyers, P.M.; Munk, P.L.; Murphy, K.J.; O’Toole, J.E.; et al. Position Statement on Percutaneous Vertebral Augmentation: A Consensus Statement Developed by the Society of Interventional Radiology (SIR), American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS), American College of Radiology (ACR), American Society of Neuroradiology (ASNR), American Society of Spine Radiology (ASSR), Canadian Interventional Radiology Association (CIRA), and the Society of NeuroInterventional Surgery (SNIS). J. Vasc. Interv. Radiol. 2014, 25, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Corcos, G.; Dbjay, J.; Mastier, C.; Leon, S.; Auperin, A.; De Baere, T.; Deschamps, F. Cement Leakage in Percutaneous Vertebroplasty for Spinal Metastases: A Retrospective Evaluation of Incidence and Risk Factors. Spine (Phila Pa 1976) 2014, 39, E332–E338. [Google Scholar] [CrossRef]
- Ebeling, P.R.; Akesson, K.; Bauer, D.C.; Buchbinder, R.; Eastell, R.; Fink, H.A.; Giangregorio, L.; Guanabens, N.; Kado, D.; Kallmes, D.; et al. The Efficacy and Safety of Vertebral Augmentation: A Second ASBMR Task Force Report. J. Bone Miner. Res. 2019, 34, 3–21. [Google Scholar] [CrossRef]
- Zhan, Y.; Jiang, J.; Liao, H.; Tan, H.; Yang, K. Risk Factors for Cement Leakage After Vertebroplasty or Kyphoplasty: A Meta-Analysis of Published Evidence. World Neurosurg. 2017, 101, 633–642. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Jiang, H.; Lai, Z.; Wu, F.; Pan, Y.; Liu, Z. An Updated Comparison of High- and Low-Viscosity Cement Vertebroplasty in the Treatment of Osteoporotic Thoracolumbar Vertebral Compression Fractures: A Retrospective Cohort Study. Int. J. Surg. 2017, 43, 126–130. [Google Scholar] [CrossRef]
- Reyad, R.M.; Ghobrial, H.Z.; Hakim, S.M.; Hashem, R.H.; Elsaman, A.; Shaaban, M.H. Thick Cement Usage in Percutaneous Vertebroplasty for Malignant Vertebral Fractures at High Risk for Cement Leakage. Diagn. Interv. Imaging 2017, 98, 721–728. [Google Scholar] [CrossRef]
- Wucherer, P.; Stefan, P.; Abhari, K.; Fallavollita, P.; Weigl, M.; Lazarovici, M.; Winkler, A.; Weidert, S.; Peters, T.; de Ribaupierre, S.; et al. Vertebroplasty Performance on Simulator for 19 Surgeons Using Hierarchical Task Analysis. IEEE Trans. Med. Imaging 2015, 34, 1730–1737. [Google Scholar] [CrossRef]
- Hollensteiner, M.; Botzenmayer, M.; Fürst, D.; Winkler, M.; Augat, P.; Sandriesser, S.; Schrödl, F.; Esterer, B.; Gabauer, S.; Püschel, K.; et al. Characterization of Polyurethane-Based Synthetic Vertebrae for Spinal Cement Augmentation Training. J. Mater. Sci. Mater. Med. 2018, 29, 153. [Google Scholar] [CrossRef]
- Chandra, R.V.; Meyers, P.M.; Hirsch, J.A.; Abruzzo, T.; Eskey, C.J.; Hussain, M.S.; Lee, S.-K.; Narayanan, S.; Bulsara, K.R.; Gandhi, C.D.; et al. Vertebral Augmentation: Report of the Standards and Guidelines Committee of the Society of NeuroInterventional Surgery. J. Neurointerv Surg. 2014, 6, 7–15. [Google Scholar] [CrossRef]
- Tanigawa, N.; Kariya, S.; Komemushi, A.; Nakatani, M.; Yagi, R.; Kohzai, M.; Sawada, S. Percutaneous Vertebroplasty for Osteoporotic Compression Fractures: Long-Term Evaluation of the Technical and Clinical Outcomes. AJR Am. J. Roentgenol. 2011, 196, 1415–1418. [Google Scholar] [CrossRef]
| Author and Year | Country | Design/Evidence Level * | Patients (Levels) | % OVCF | Cement Leak% | Neuro% | PCE% | Infection% | Adjacent Fx% | 30-Day Mortality% |
|---|---|---|---|---|---|---|---|---|---|---|
| Anselmetti 2012 [12] | Italy | Prospective III | 4.547 (13.437) | 73 | 20.5 | 0 | NR | NR | 13 | NR |
| Hsieh 2019 [13] | Taiwan | Retro IV | 3.175 (3.812) | 100 | 45 † | 0.13 | 0.28 | 0 | NR | 0 |
| Liao 2018 [11] | Taiwan | Retro IV | 5.749 | 100 | NR | 0.13 ‡ | NR | 0.32 | NR | 0.1 § |
| Abdelrahman 2013 [14] | Germany | Retro IV | 1.307 | 100 | NR | NR | NR | 0.46 | NR | NR |
| Kim 2022 [15] | USA | Database IV | 1.932 | 100 | NR | 0 | 1.3 | 0.2 | NR | 2.1 |
| Venmans 2010 [16] | Neth. | Prospective III | 97 (121) | 100 | 60 † | 1 | 26 | 0 | NR | 0 |
| Luetmer 2011 [17] | USA | Retro IV | 244 | 100 | NR | 0 | 9.4 (0.4 symp) | 0 | NR | 0 |
| Author and Year | Country | Design/Evidence Level * | Patients (levels) | Indication | Cement Leak% | Neuro% | PCE% | Infection% | Adjacent Fx% |
|---|---|---|---|---|---|---|---|---|---|
| Cavka 2023 [5] | Croatia | Retro IV | 189 (218) | 75% OVCF/25% pathol. | 35 | 0.5 | 0.5 | 0.5 | NR |
| Anselmetti 2012 [12] | Italy | Prospective III | 128 | 100% myeloma | 35 † | 0 | 0.8 | 1.6 | NR |
| Khanna 2016 [36] | USA | Retro IV | 350 | 100% metastatic | NR | 0.8 | 0.3 | 0.3 | 15 |
| Essibayi 2024 [37] | Multi | SR/meta (RCTs) | 2050 | 100% OVCF | <1 (symp) | 0 | <0.1 | 0 | 12–20 |
| Clinical Scenario | Risk Factors | Predominant Complication Pattern | Preferred Augmentation Strategy | Procedural Safeguards | Post-Procedure Surveillance |
|---|---|---|---|---|---|
| A. Low-risk primary OVCF | Intact posterior cortex, BMD > −2.5, no anticoagulation, ASA I–II | Radiographic paravertebral/intradiscal leaks (mostly asymptomatic) | Standard PVP, unilateral transpedicular, 4–5 mL high-viscosity PMMA | Continuous biplanar fluoro, stop ≤ 5 mm from posterior wall, single level per session | Out-patient; tele-visit at 2 weeks; no routine CT |
| B. Severe osteoporosis or steroid use (“cascade” phenotype) | ≥2 prior VCF, T-score < −3.0, chronic glucocorticoids | Higher adjacent-level fracture rate (13–20%/year) | Balloon kyphoplasty or low-pressure cavity creation; consider prophylactic augmentation of cephalad level | Vertebral body stent or double balloon to avoid disk leak; cement ≤ 6 mL; initiate anabolic therapy post-op | Standing radiograph and serum Ca/Vit-D at 3 months |
| C. Lytic metastasis without posterior wall breach | Single vertebra, mild cortical erosion, ECOG 0–1 | Venous (Type B/S) leaks; asymptomatic PCE (≤10%) | High-viscosity “doughy” cement PVP, CT-guided cannula placement | Test injection with contrast; inject < 10 psi; vascular plug in azygos if large channel | Chest X-ray in recovery; oncology f/u in 1 month |
| D. Lytic metastasis with posterior wall destruction/epidural tumor | Spinal canal encroachment, >50% cortical loss, ECOG > 1 | Symptomatic cord/root compression; central PCE; infection (up to 1%) | Stage 1: radiofrequency ablation + cavity creation; Stage 2: kyphoplasty with viscous cement < 4 mL | CT navigation; neuromonitoring; prophylactic IVC filter for multi-level procedures | In-hospital neuro checks q2 h × 24 h; MRI if any deficit |
| E. Coagulopathy/DOAC therapy | INR > 1.4, platelets < 100 K, DOAC < 12 h from last dose | Epidural hematoma; large venous cement run-off | Delay procedure until parameters corrected; if urgent, perform kyphoplasty with viscoelastic cement | Reversal agents; limit balloon pressure; meticulous hemostasis at trocar site | CBC and neuro exam at 6 h; low-threshold MRI |
| F. Latent or active infection risk (diabetes, recent UTI, endemic TB) | CRP > 10 mg/L, WBC > 10 K, PPD +/IGRA + | Pyogenic or tuberculous spondylodiscitis (0.3–0.5%, mortality 17%) | Postpone until sepsis ruled out; if imperative, antibiotic-loaded cement (gentamicin 1 g/40 g PMMA) | Full surgical prep; new sterile needle for each pedicle; single-shot cefazolin + vancomycin | CRP/ESR at 2 and 6 weeks; MRI if pain recurs |
| G. Multilevel (>3) osteoporotic fractures | Frailty, restrictive lung disease, prone intolerance | Cumulative cement volume → higher PCE (up to 26%) | Two-stage PVP (max 3 levels/session); total cement ≤ 15 mL/48 h | Low-flow injectors; oxygen saturation monitoring; consider prone ventilation break | Chest CT only if O2 sat < 94%; repeat DXA 6 mo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuluaga-Garcia, J.P.; Sierra, M.A.; Call-Orellana, F.A.; Herrera, D.; Andrade-Almeida, R.A.; Ravindran, P.K.; Ramirez-Ferrer, E. Complications of Vertebroplasty in Adults: Incidence, Etiology, and Therapeutic Strategies—A Comprehensive, Systematic Literature Review. Complications 2025, 2, 22. https://doi.org/10.3390/complications2030022
Zuluaga-Garcia JP, Sierra MA, Call-Orellana FA, Herrera D, Andrade-Almeida RA, Ravindran PK, Ramirez-Ferrer E. Complications of Vertebroplasty in Adults: Incidence, Etiology, and Therapeutic Strategies—A Comprehensive, Systematic Literature Review. Complications. 2025; 2(3):22. https://doi.org/10.3390/complications2030022
Chicago/Turabian StyleZuluaga-Garcia, Juan Pablo, Maria Alejandra Sierra, Francisco Alfredo Call-Orellana, David Herrera, Romulo A. Andrade-Almeida, Pawan Kishore Ravindran, and Esteban Ramirez-Ferrer. 2025. "Complications of Vertebroplasty in Adults: Incidence, Etiology, and Therapeutic Strategies—A Comprehensive, Systematic Literature Review" Complications 2, no. 3: 22. https://doi.org/10.3390/complications2030022
APA StyleZuluaga-Garcia, J. P., Sierra, M. A., Call-Orellana, F. A., Herrera, D., Andrade-Almeida, R. A., Ravindran, P. K., & Ramirez-Ferrer, E. (2025). Complications of Vertebroplasty in Adults: Incidence, Etiology, and Therapeutic Strategies—A Comprehensive, Systematic Literature Review. Complications, 2(3), 22. https://doi.org/10.3390/complications2030022






