Optimizing Inguinal Lymph Node Dissection for Penile Cancer: A Pathway to Improve Outcomes and Complications—A Narrative Review
Abstract
1. Introduction
2. Methods
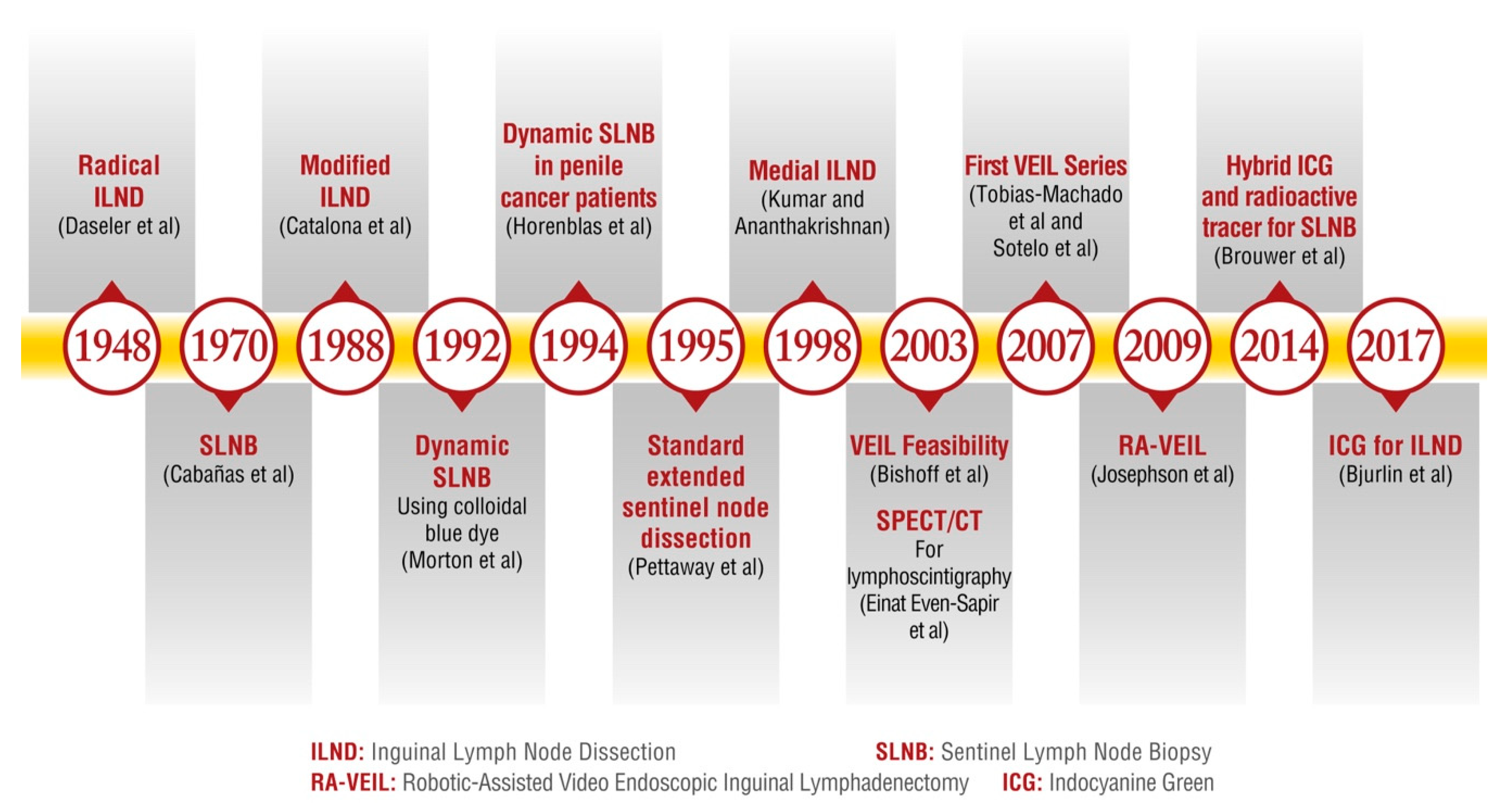
2.1. Evolution and Innovations in Lymph Node Management
| Approach | Minor Complication Rate (%) | Major Complication Rate (%) | Main Complications/Comments | References |
|---|---|---|---|---|
| Open | 35–66 | 13–17 | Wound complications alone up to 77%. High rates of wound infection, dehiscence, and lymphedema. | [49,50,51,52,53,54] |
| VEIL | 12–35 | 5–15 | Lower overall complication rates compared to open, particularly cutaneous complications. | [43,49,51,52,55] |
| RA-VEIL | 9–30 | 2–17 | Similar complication rate to VEIL. There is a lack of direct comparative studies between VEIL and RA-VEIL. | [49,51,52,54,55] |
| DSLNB | 16.4–26 | 1–1.4 | Operator-dependent. Steep learning curve. Good accuracy (87%) and minimal complications. | [26,34,35,56,57] |
2.2. Inguinal Lymph Node Dissection Complications
2.3. Lymphatic Complications
2.4. Cutaneous Complications
- Surgical incisions:
- 2.
- Subfascial space creation:
- 3.
- Attention to devitalized tissue:
- 4.
- Wound closure/care:
2.5. Infectious Complications
2.6. Nerve/Musculoskeletal Complications
2.7. Vascular Complications
3. Gaps in Knowledge and Potential Research Areas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ficarra, V.; Akduman, B.; Bouchot, O.; Palou, J.; Tobias-Machado, M. Prognostic factors in penile cancer. Urology 2010, 76 (Suppl. 2), S66–S73. [Google Scholar] [CrossRef] [PubMed]
- Pettaway, C.A.; Crook, J.M.; Pagliaro, L.C. Tumors of the Penis. In Campbell-Walsh-Wein “Urology”, 12th ed.; Partin, A.W., Dmochowski, R.R., Kavoussi, L.R., Peters, C.A., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 1742–1775. [Google Scholar]
- Ekstrom, T.; Edsmyr, F. Cancer of the penis; a clinical study of 229 cases. Acta. Chir. Scand. 1958, 115, 25–45. [Google Scholar] [PubMed]
- Beggs, J.H.; Spratt, J.S. Epidermoid Carcinoma of the penis. J. Urol. 1964, 91, 166–172. [Google Scholar] [CrossRef]
- Thomas, J.A.; Small, C.S. Carcinoma of the penis in Southern India. J. Urol. 1968, 100, 520–526. [Google Scholar] [CrossRef]
- Edwards, R.H.; Sawyers, J.L. The management of carcinoma of the penis. South. Med. J. 1968, 61, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, J.T.; Garlick, F.H.; Mammen, K.E. Results of Surgical Treatment of Carcinoma of the Penis. ANZ J. Surg. 1968, 41, 157–159. [Google Scholar] [CrossRef]
- Skinner, D.G.; Leadbetter, W.F.; Kelley, S.B. The surgical management of squamous cell carcinoma of the penis. J. Urol. 1972, 107, 273–277. [Google Scholar] [CrossRef]
- Cabanas, R.M. An approach for the treatment of penile carcinoma. Cancer 1977, 39, 456–466. [Google Scholar] [CrossRef]
- Fosså, S.D.; Hall, K.S.; Johannessen, N.B.; Urnes, T.; Kaalhus, O. Cancer of the penis. Experience at the Norwegian Radium Hospital 1974–1985. Eur. Urol. 1987, 13, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.; Morse, M.J.; Herr, H.W.; Sogani, P.C.; Whitmore, W.F. Penile cancer: Relation of extent of nodal metastasis to survival. J. Urol. 1987, 137, 880–882. [Google Scholar] [CrossRef] [PubMed]
- McDougal, W.S.; Kirchner, F.K.; Edwards, R.H.; Killion, L.T. Treatment of carcinoma of the penis: The case for primary lymphadenectomy. J. Urol. 1986, 136, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Teh, J.; Duncan, C.; Qu, L.; Guerra, G.; Narasimhan, V.; Pham, T.; Lawrentschuk, N. Inguinal lymph node dissection for penile cancer: A contemporary review. Transl. Androl. Urol. 2020, 9, 3210–3218. [Google Scholar] [CrossRef]
- Patel, K.N.; Bhirud, C.; Dipin, J.; Nandy, K.; Venugopal, V.; Salunke, A.; Pandya, S.J. A proposed Clino-radio-pathological Risk Scoring System (CRiSS) for prediction and management of inguinal lymph-nodes metastasis in squamous cell carcinoma of the penis. Surg. Oncol. 2021, 36, 147–152. [Google Scholar] [CrossRef]
- Daseler, E.H.; Anson, B.J.; Reimann, A.F. Radical excision of the inguinal and iliac lymph glands; a study based upon 450 anatomical dissections and upon supportive clinical observations. Surg. Gynecol. Obstet. 1948, 87, 679–694. [Google Scholar]
- Wespes, E.; Simon, J.; Schulman, C.C. Cabañas approach: Is Sentinel Node Biopsy Reliable for Staging Penile Carcinoma? Urology 1986, 28, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: Technique and preliminary results. J. Urol. 1988, 140, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Protzel, C.; Alcaraz, A.; Horenblas, S.; Pizzocaro, G.; Zlotta, A.; Hakenberg, O.W. Lymphadenectomy in the surgical management of penile cancer. Eur. Urol. 2009, 55, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- d’Ancona, C.A.L.; de Lucena, R.G.; Querne, F.A.d.O.; Martins, M.H.T.; Denardi, F.; Netto, N.R. Long-term followup of penile carcinoma treated with penectomy and bilateral modified inguinal lymphadenectomy. J. Urol. 2004, 172, 498–501; discussion 501. [Google Scholar] [CrossRef]
- Chipollini, J.; Tang, D.H.; Manimala, N.; Gilbert, S.M.; Pow-Sang, J.M.; Sexton, W.J.; Poch, M.A.; Spiess, P.E. Evaluating the accuracy of intraoperative frozen section during inguinal lymph node dissection in penile cancer. Urol. Oncol. 2018, 36, 14.e1–14.e5. [Google Scholar] [CrossRef]
- Kroon, B.K.; Lont, A.P.; Valdés Olmos, R.A.; Nieweg, O.E.; Horenblas, S. Morbidity of dynamic sentinel node biopsy in penile carcinoma. J. Urol. 2005, 173, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Spiess, P.E.; Hernandez, M.S.; Pettaway, C.A. Contemporary inguinal lymph node dissection: Minimizing complications. World J. Urol. 2009, 27, 205–212. [Google Scholar] [CrossRef]
- Morton, D.L.; Wen, D.R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch. Surg. 1992, 127, 392–399. [Google Scholar] [CrossRef]
- Alex, J.C.; Krag, D.N. Gamma-probe guided localization of lymph nodes. Surg. Oncol. 1993, 2, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Horenblas, S.; Jansen, L.; Meinhardt, W.; Hoefnagel, C.A.; De Jong, D.; Nieweg, O.E. Detection Of Occult Metastasis In Squamous Cell Carcinoma Of The Penis Using A Dynamic Sentinel Node Procedure. J. Urol. 2000, 163, 100–104. [Google Scholar] [CrossRef]
- Goyal, A.; Mansel, R.E. Current status of sentinel lymph node biopsy in solid malignancies. World J. Surg. Oncol. 2004, 2, 9. [Google Scholar] [CrossRef][Green Version]
- Lee, E.W.C.; Issa, A.; Oliveira, P.; Lau, M.; Sangar, V.; Parnham, A.; Fankhauser, C.D. High diagnostic accuracy of inguinal ultrasonography and fine-needle aspiration followed by dynamic sentinel lymph node biopsy in men with impalpable and palpable inguinal lymph nodes. BJU Int. 2022, 130, 331–336. [Google Scholar] [CrossRef]
- Pettaway, C.A.; Pisters, L.L.; Dinney, C.P.; Jularbal, F.; Swanson, D.A.; von Eschenbach, A.C.; Ayala, A. Sentinel lymph node dissection for penile carcinoma: The M. D. Anderson Cancer Center experience. J. Urol. 1995, 1154, 1999–2003. [Google Scholar] [CrossRef]
- Kumar, S.; Ananthakrishnan, N.; Prema, V. Predicting regional lymph node metastasis in carcinoma of the penis: A comparison between fine-needle aspiration cytology, sentinel lymph node biopsy and medial inguinal lymph node biopsy. Br. J. Urol. 1998, 81, 453–457. [Google Scholar] [CrossRef]
- Dell’Oglio, P.; de Vries, H.M.; Mazzone, E.; KleinJan, G.H.; Donswijk, M.L.; van der Poel, H.G.; Horenblas, S.; van Leeuwen, F.W.B.; Brouwer, O.R. Hybrid Indocyanine Green-99mTc-nanocolloid for Single-photon Emission Computed Tomography and Combined Radio- and Fluorescence-guided Sentinel Node Biopsy in Penile Cancer: Results of 740 Inguinal Basins Assessed at a Single Institution. Eur. Urol. 2020, 78, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, S.; Botero Fonnegra, C.; Reyes, A.; Hui, V.W. Developments in the Use of Indocyanine Green (ICG) Fluorescence in Colorectal Surgery. J. Clin. Med. 2024, 13, 4003. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Van Den Berg, N.S.; Mathéron, H.M.; Van Der Poel, H.G.; Van Rhijn, B.W.; Bex, A.; Van Tinteren, H.; Olmos, R.A.V.; Van Leeuwen, F.W.B.; Horenblas, S. A Hybrid Radioactive and Fluorescent Tracer for Sentinel Node Biopsy in Penile Carcinoma as a Potential Replacement for Blue Dye. Eur. Urol. 2014, 65, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Wever, L.; de Vries, H.M.; Dell’Oglio, P.; van der Poel, H.G.; Donswijk, M.L.; Sikorska, K.; Leeuwen, F.W.B.; Horenblas, S.; Brouwer, O.R. Incidence and risk factor analysis of complications after sentinel node biopsy for penile cancer. BJU Int. 2022, 130, 486–495. [Google Scholar] [CrossRef]
- Akrida, I.; Michalopoulos, N.V.; Lagadinou, M.; Papadoliopoulou, M.; Maroulis, I.; Mulita, F. An updated review on the emerging role of indocyanine green (ICG) as a sentinel lymph node tracer in breast cancer. Cancers 2023, 15, 5755. [Google Scholar] [CrossRef]
- Wever, L.; de Vries, H.M.; van der Poel, H.; van Leeuwen, F.; Horenblas, S.; Brouwer, O. Minimally invasive evaluation of the clinically negative inguinal node in penile cancer: Dynamic sentinel node biopsy. Urol. Oncol. 2022, 40, 209–214. [Google Scholar] [CrossRef]
- Bjurlin, M.A.; Zhao, L.C.; Kenigsberg, A.P.; Mass, A.Y.; Taneja, S.S.; Huang, W.C. Novel Use of Fluorescence Lymphangiography During Robotic Groin Dissection for Penile Cancer. Urology 2017, 107, 267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, P.; Xie, Y.; Xu, R.; Li, Y.; Yao, K.; Liu, J.; Yan, B.; Jiang, S.; Liu, Q.; Chen, Q.; et al. Efficacy of indocyanine green fluorescence-guided inguinal lymph node dissection for penile cancer: A randomised trial. BJU Int. 2024, 133, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Bishoff, J.T.; Basler, J.W.; Teichman, J.M.; Thompson, I.M. Endoscopic subcutaneous modified inguinal lymph node dissection (ESMIL) for squamous cell carcinoma of the penis. In Journal of Urology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; p. 78. [Google Scholar]
- Tobias-Machado, M.; Tavares, A.; Ornellas, A.A.; Molina, W.R.; Juliano, R.V.; Wroclawski, E.R. Video endoscopic inguinal lymphadenectomy: A new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J. Urol. 2007, 177, 953–957; discussion 958. [Google Scholar] [CrossRef]
- Josephson, D.Y.; Jacobsohn, K.M.; Link, B.A.; Wilson, T.G. Robotic-assisted endoscopic inguinal lymphadenectomy. Urology 2009, 73, 167–170; discussion 170–171. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT01526486?cond=penile%20cancer&intr=Lymphadenectomy&rank=7 (accessed on 13 January 2025).
- Sotelo, R.; Sánchez-Salas, R.; Carmona, O.; Garcia, A.; Mariano, M.; Neiva, G.; Trujillo, G.; Novoa, J.; Cornejo, F.; Finelli, A. Endoscopic lymphadenectomy for penile carcinoma. J. Endourol. 2007, 21, 364–367; discussion 367. [Google Scholar] [CrossRef]
- Greco, I.; Fernandez-Pello, S.; Sakalis, V.I.; Barreto, L.; Albersen, M.; Ayres, B.; Lopes, T.A.; Campi, R.; Crook, J.; Perdomo, H.A.G.; et al. Systematic Review and Meta-analysis of Minimally Invasive Procedures for Surgical Inguinal Nodal Staging in Penile Carcinoma. Eur. Urol. Focus 2023, 10, 567–580. [Google Scholar] [CrossRef]
- Gopman, J.M.; Djajadiningrat, R.S.; Baumgarten, A.S.; Espiritu, P.N.; Horenblas, S.; Zhu, Y.; Protzel, C.; Pow-Sang, J.M.; Kim, T.; Sexton, W.J.; et al. Predicting postoperative complications of inguinal lymph node dissection for penile cancer in an international multicentre cohort. BJU Int. 2015, 116, 196–201. [Google Scholar] [CrossRef]
- Gómez-Ferrer, A.; Collado, A.; Ramírez, M.; Domínguez, J.; Casanova, J.; Mir, C.; Wong, A.; Marenco, J.L.; Nagore, E.; Soriano, V.; et al. A single-center comparison of our initial experiences in treating penile and urethral cancer with video-endoscopic inguinal lymphadenectomy (VEIL) and later experiences in melanoma cases. Front. Surg. 2022, 9, 870857. [Google Scholar] [CrossRef]
- Muñoz Guillermo, V.; Rosino Sánchez, A.; Rivero Guerra, Á.; Barceló Bayonas, I.; Pardo Martínez, A.; Jiménez Peralta, D.; George, C.C.; Pietricica, B.N.; Morejón, E.I.; de Abia, F.I.; et al. Video endoscopic inguinal lymphadenectomy in penile cancer: Systematic review. Arch. Esp. Urol. 2019, 72, 992–999. [Google Scholar]
- Chua, K.J.; Balraj, V.; Patel, H.V.; Srivastava, A.; Doppalapudi, S.K.; Elsamra, S.E.; Jang, T.L.; Singer, E.A.; Ghodoussipour, S.B. Wound complication rates after inguinal lymph node dissection: Contemporary analysis of the NSQIP database. J. Am. Coll. Surg. 2023, 236, 18–25. [Google Scholar] [CrossRef]
- Jeanne-Julien, A.; Bouchot, O.; De Vergie, S.; Branchereau, J.; Perrouin-Verbe, M.-A.; Rigaud, J. Morbidity and risk factors for complications of inguinal lymph node dissection in penile cancer. World J. Urol. 2022, 41, 109–118. [Google Scholar] [CrossRef] [PubMed]
- La-Touche, S.; Ayres, B.; Lam, W.; Alnajjar, H.M.; Perry, M.; Watkin, N. Trial of ligation versus coagulation of lymphatics in dinamic inguinal sentinel lymph node biopsy for staging of squamous cell carcinoma of the penis. Ann. R. Coll. Surg. Engl. 2012, 94, 344–346. [Google Scholar] [CrossRef]
- Greene, A.K.; Goss, J.A. Diagnosis and staging of lymphedema. Semin. Plast. Surg. 2018, 32, 12–16. [Google Scholar] [CrossRef]
- Heyns, C.F.; Fleshner, N.; Sangar, V.; Schlenker, B.; Yuvaraja, T.B.; Van Poppel, H. Management of the Lymph Nodes in Penile Cancer. Urology 2010, 76, S43–S57. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zheng, L.; Li, Y.; Gan, L.; Wang, Z.; Zeng, Z.; Meng, C.; Li, K.; Ma, J.; Wang, D.; et al. Comparing the safety and effectiveness of minimally invasive surgery and open inguinal lymph node dissection in penile cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2024, 50, 108553. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, Z.; Tan, Q.; Hu, X.; Liu, Y.; Wei, L.; Deng, C.; Zhou, S.; Yang, N.; Duan, G.; et al. Comparison of antegrade robotic assisted vs. laparoscopic inguinal lymphadenectomy for penile cancer. BMC Surg. 2023, 23, 55. [Google Scholar] [CrossRef]
- Singh, A.; Jaipuria, J.; Goel, A.; Shah, S.; Bhardwaj, R.; Baidya, S.; Jain, J.; Jain, C.; Rawal, S. Comparing Outcomes of Robotic and Open Inguinal Lymph Node Dissection in Patients with Carcinoma of the Penis. J. Urol. 2018, 199, 1518–1525. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Christopoulos, P.; Shilito, S.; Gall, Z.; Murby, B.; Ashworth, D.; Taylor, B.; Carrington, B.; Shanks, J.; Clarke, N.; et al. Dynamic sentinel lymph node biopsy for penile cancer: A comparison between 1- and 2-day protocols. BJU Int. 2016, 117, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Fallara, G.; Pozzi, E.; Cakir, O.O.; Tandogdu, Z.; Castiglione, F.; Salonia, A.; Alnajjar, H.M.; Muneer, A. Diagnostic Accuracy of Dynamic Sentinel Lymph Node Biopsy for Penile Cancer: A Systematic Review and Meta-analysis–ClinicalKey. Eur. Urol. Focus 2023, 9, 500–512. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Medina, L.G.; Sayegh, A.S.; La Riva, A.; Perez, L.C.; Eppler, M.B.; Gill, I.; Sotelo, R. Assessment and reporting of perioperative adverse events and complications in patients undergoing inguinal lymphadenectomy for melanoma, vulvar cancer, and penile cancer: A systematic review and meta-analysis. World J. Surg. 2023, 47, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Golder, H.; Casanova, D.; Papalois, V. Evaluation of the usefulness of the Clavien-Dindo classification of surgical complications. Cirugía Española (Engl. Ed.) 2023, 101, 637–642. [Google Scholar] [CrossRef]
- Sotelo, R.; Sayegh, A.S.; Medina, L.G.; Perez, L.C.; La Riva, A.; Eppler, M.B.; Gaona, J.; Tobias-Machado, M.; Spiess, P.E.; Pettaway, C.A.; et al. Complications and adverse events in lymphadenectomy of the inguinal area: Worldwide expert consensus. BJS Open 2024, 8, zrae056. [Google Scholar] [CrossRef] [PubMed]
- Faut, M.; Heidema, R.M.; Hoekstra, H.J.; van Ginkel, R.J.; Been, S.L.B.; Kruijff, S.; Van Leeuwen, B.L. Morbidity after inguinal lymph node dissections: It is time for a change. Ann. Surg. Oncol. 2017, 24, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Gerken, A.L.H.; Herrle, F.; Jakob, J.; Weiß, C.; Rahbari, N.N.; Nowak, K.; Karthein, C.; Hohenberger, P.; Weitz, J.; Reißfelder, C.; et al. Definition and severity grading of postoperative lymphatic leakage following inguinal lymph node dissection. Langenbecks Arch. Surg. 2020, 405, 697–704. [Google Scholar] [CrossRef]
- Sood, A.; Rudzinski, J.K.; Spiess, P.E.; Pettaway, C.A. The acute complications after surgery for penile carcinoma and strategies for their management: A systematic review of the literature. Semin. Oncol. Nurs. 2022, 38, 151285. [Google Scholar] [CrossRef]
- Gupta, M.K.; Patel, A.P.; Master, V.A. Technical considerations to minimize complications of inguinal lymph node dissection. Transl. Androl. Urol. 2017, 6, 820–825. [Google Scholar] [CrossRef]
- Zhang, X.; Sheng, X.; Niu, J.; Li, H.; Li, D.; Tang, L.; Li, Q.; Li, Q. Sparing of saphenous vein during inguinal lymphadenectomy for vulval malignancies. Gynecol. Oncol. 2007, 105, 722–726. [Google Scholar] [CrossRef]
- Zhang, S.H.; Sood, A.K.; Sorosky, J.I.; Anderson, B.; Buller, R.E. Preservation of the saphenous vein during inguinal lymphadenectomy decreases morbidity in patients with carcinoma of the vulva. Cancer 2000, 89, 1520–1525. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, H.; Liu, L.; Chen, Z.; Chen, J.; Qi, L.; Zu, X. Saphenous vein sparing during laparoscopic bilateral inguinal lymphadenectomy for penile carcinoma patients. Int. Urol. Nephrol. 2016, 48, 363–366. [Google Scholar] [CrossRef]
- Papanikolaou, A.; Minger, E.; Pais, M.-A.; Constantinescu, M.; Olariu, R.; Grobbelaar, A.; Lese, I. Management of postoperative seroma: Recommendations based on a 12-year retrospective study. J. Clin. Med. 2022, 11, 5062. [Google Scholar] [CrossRef]
- Pouwer, A.W.; Hinten, F.; van der Velden, J.; Smolders, R.G.V.; Slangen, B.F.M.; Zijlmans, H.J.M.A.A.; IntHout, J.; van der Zee, A.G.J.; Boll, D.; Gaarenstroom, K.N.; et al. Volume-controlled versus short drainage after inguinofemoral lymphadenectomy in vulvar cancer patients: A Dutch nationwide prospective study. Gynecol. Oncol. 2017, 146, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.; Kim, J.; Ratnam, L.; Itkin, M. Lymphatic intervention, the frontline of modern lymphatic medicine: Part II. Classification and treatment of the lymphatic disorders. Korean J. Radiol. 2023, 24, 109–132. [Google Scholar] [CrossRef]
- Ciudad, P.; Sabbagh, M.D.; Agko, M.; Huang, T.C.T.; Manrique, O.J.; Carmen Román, L.; Reynaga, C.; Delgado, R.; Maruccia, M.; Chen, H.-C. Surgical management of lower extremity lymphedema: A comprehensive review. Indian J. Plast. Surg. 2019, 52, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Hu, X.; Ren, S.; Liao, D.; Yang, Z.; Liu, Y.; Lia, T.; Wu, K.; Xiong, S.; Yang, W.; et al. Comparison of different surgical methods and strategies for inguinal lymph node dissection in patients with penile cancer. Sci. Rep. 2022, 12, 2560. [Google Scholar] [CrossRef] [PubMed]
- Wevers, K.P.; Poos, H.P.A.M.; Van Ginkel, R.J.; Van Etten, B.; Hoekstra, H.J. Early mobilization after ilio-inguinal lymph node dissection for melanoma does not increase the wound complication rate. Eur. J. Surg. Oncol. 2013, 39, 185–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sudhir, R.; Krishnappa, R.S.; Khanna, S.; Sekon, R.; Koul, R. Video endoscopic inguinal lymphadenectomy (VEIL): Minimally invasive radical inguinal lymphadenectomy technique. Indian J. Surg. Oncol. 2012, 3, 257–261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kandasamy, S.G.; Chandran, K.R.; Pooleri, G.K. Minimal invasive approaches in lymph node management of carcinoma of penis: A review. Indian J. Urol. 2022, 38, 15–21. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Thomsen, J.B.; Sørensen, J.A. Prophylactic incisional negative pressure wound therapy shows promising results in prevention of wound complications following inguinal lymph node dissection for Melanoma: A retrospective case-control series. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1178–1183. [Google Scholar] [CrossRef]
- Asciutto, K.C.; Acosta, S.; Borgfeldt, C. Negative pressure wound therapy (NPWT) in groin wounds after lymphadenectomy in vulvar cancer patients. Vivo 2020, 34, 3511–3517. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.D.; Manna, B. Wound dehiscence. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Petroze, R.T.; Holton, L. Complications of inguinal lymphadenectomy. Surg. Technol. 2020, 52, 270–279. [Google Scholar]
- Dargan, D.; Hindocha, S.; Hadlett, M.; Wright, R.; Beck, D.; McConville, S.; Hartley-Large, D.; Mortimer, K.; Brackley, P. Groin dissections in skin cancer: Effect of a change in prophylactic antibiotic protocol. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Bychkov, Y.; Mutua, D.; Bhullar, R. A Review of Lithotomy Position-Related Intraoperative Peripheral Nerve Injury and Preventative Measures. ASA Monit. 2022, 86, 39–40. [Google Scholar] [CrossRef]
- Yıkılmaz, T.N.; Öztürk, E.; Hamidi, N.; Başar, H.; Yaman, Ö. Management of obturator nevre injury during pelvic lymph node dissection. Turk. J. Urol. 2019, 45, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, L.; Tempelhoff, G.-F.V.; Kirkpatrick, C.; Schneider, D.M.; Hommel, G.; Pollow, K. Comparison of unfractionated versus low molecular weight heparin for deep vein thrombosis prophylaxis during breast and pelvic cancer surgery: Efficacy, safety, and follow-up. Clin. Appl. Thromb. Hemost. 1998, 4, 268–273. [Google Scholar] [CrossRef]
- Saluja, M.; Gilling, P. Venous thromboembolism prophylaxis in urology: A review. Int. J. Urol. 2017, 24, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Uroweb–European Association of Urology. Uroweb.org. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 21 October 2024).
- Gerke, M.B.; Jansen, C.S.; Bilen, M.A. Circulating Tumor DNA in Genitourinary Cancers: Detection, Prognostics, and Therapeutic Implications. Cancers 2024, 16, 2280. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Moon, S.C.; Shumaker, L.A.; Patel, S.; Galgano, S.J.; Ferguson, J.E.; Basu, A.; Peyton, C.C. Utility of Tumor-Informed Circulating Tumor DNA for Monitoring Treatment Response in Nonmetastatic, Locally Advanced Penile and Primary Urethral Cancers. JCO Precis. Oncol. 2025, 9, e2500045. [Google Scholar] [CrossRef] [PubMed]
| Literature Gaps/Potential Research Areas | Implications |
|---|---|
| Lack of use and validation of predictive models for predicting lymph node metastasis. | Potential overtreatment for patients undergoing ILND. |
| Inconsistent reporting of ILND complications across studies. | Decrease the validity and overall strength of meta-analyses and/or other comparisons across studies. |
| Lack of use and validation of an ILND complication classification system. | Misclassification and inconsistent reporting. |
| Lack of consensus regarding the optimal ILND template and the postoperative pathway. | Variability in surgical practice, overtreatment or undertreatment, and inconsistent patient outcomes. |
| Lack of guidelines for the management of some ILND complications. | Variability in complication management leading to inconsistencies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eskenazi, F.; Medina, L.G.; Soto Suarez, R.; Fumero, L.; Lusinchi Delfino, A.C.; Patel, K.; Tobias Machado, M.; Lee, R.; Sotelo, R. Optimizing Inguinal Lymph Node Dissection for Penile Cancer: A Pathway to Improve Outcomes and Complications—A Narrative Review. Complications 2025, 2, 20. https://doi.org/10.3390/complications2030020
Eskenazi F, Medina LG, Soto Suarez R, Fumero L, Lusinchi Delfino AC, Patel K, Tobias Machado M, Lee R, Sotelo R. Optimizing Inguinal Lymph Node Dissection for Penile Cancer: A Pathway to Improve Outcomes and Complications—A Narrative Review. Complications. 2025; 2(3):20. https://doi.org/10.3390/complications2030020
Chicago/Turabian StyleEskenazi, Federico, Luis G. Medina, Roberto Soto Suarez, Laura Fumero, Alegría C. Lusinchi Delfino, Keval Patel, Marcos Tobias Machado, Randall Lee, and Rene Sotelo. 2025. "Optimizing Inguinal Lymph Node Dissection for Penile Cancer: A Pathway to Improve Outcomes and Complications—A Narrative Review" Complications 2, no. 3: 20. https://doi.org/10.3390/complications2030020
APA StyleEskenazi, F., Medina, L. G., Soto Suarez, R., Fumero, L., Lusinchi Delfino, A. C., Patel, K., Tobias Machado, M., Lee, R., & Sotelo, R. (2025). Optimizing Inguinal Lymph Node Dissection for Penile Cancer: A Pathway to Improve Outcomes and Complications—A Narrative Review. Complications, 2(3), 20. https://doi.org/10.3390/complications2030020








