Major Vascular Injuries in Laparoscopic Urological Surgeries
Abstract
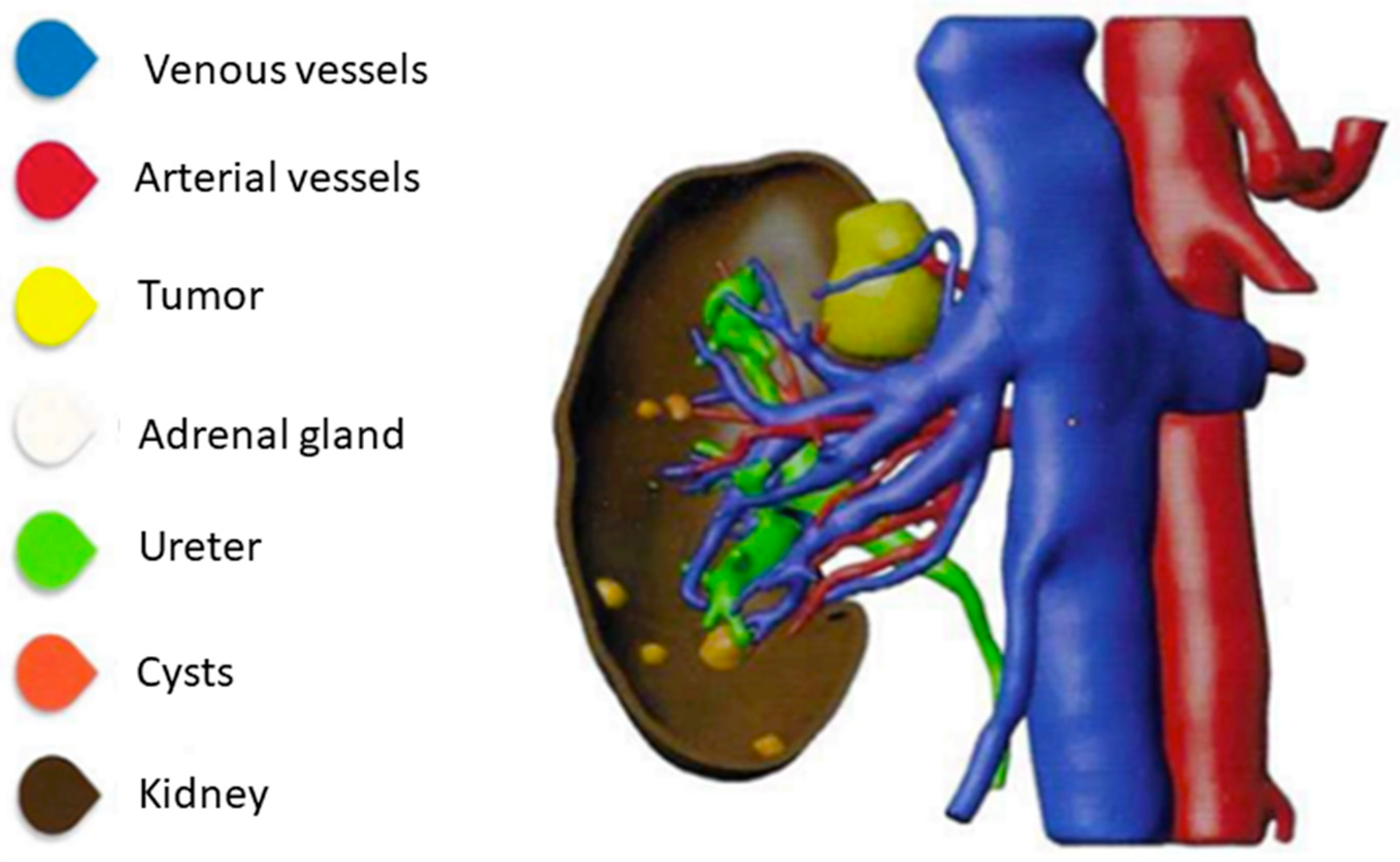
1. Introduction
2. Evidence Acquisition
3. Incidence and Risk Factors
4. Management Strategies
- Immediate Hemostasis: Identify and compress/clamp the bleeding site.
- Visual Optimization: Clear the surgical field to assess the injury.
- Resuscitation Preparedness: Mobilize resources for fluid/blood product administration.
- Pneumoperitoneum Adjustment: Temporarily increase pressure to reduce venous bleeding.
- Trocar Placement: Insert additional ports to facilitate repair.
- Vascular Exposure: Dissect and isolate the injured vessel.
- Definitive Repair: Suture or clamp the bleeding source.
- Temporary Occlusion: Apply bulldog clamps if needed.
- Conversion to Open Surgery: Proceed if laparoscopic repair is inadequate.
5. Prevention
6. Recommendations for Training
- -
- Three-dimensional simulation for preoperative rehearsal.
- -
- Structured mentorship in high-risk procedures.
- -
- Competency-based assessments prior to independent practice [27].
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.-H.; Chung, H.-J.; Lin, A.T.; Chang, Y.-H.; Huang, W.J.; Hsu, Y.-S.; Chang, S.-C.; Chen, K.-K. Complications of Pure Transperitoneal Laparoscopic Surgery in Urology: The Taipei Veterans General Hospital Experience. J. Chin. Med. Assoc. 2007, 70, 481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, J.; Zeng, Z.; Cao, R.; Hu, J. Intraoperative serious complications of laparoscopic urological surgeries: A single institute experience of 4,380 procedures. Int. Braz. J. Urol. 2019, 45, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Furriel, F.T.G.; Laguna, M.P.; Figueiredo, A.J.C.; Nunes, P.T.C.; Rassweiler, J.J. Training of European urology residents in laparoscopy: Results of a pan-European survey. BJU Int. 2013, 112, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Guillonneau, B.; Abbou, C.C.; Doublet, J.D.; Gaston, R.; Janetschek, G.; Mandressi, A.; Rassweiler, J.J.; Vallancien, G. Proposal for a ‘European Scoring System for Laparoscopic Operations in Urology’. Eur. Urol. 2001, 40, 2–6; discussion 7. [Google Scholar] [CrossRef]
- Ghavamian, R. Complications of Laparoscopic and Robotic Urologic Surgery; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Simforoosh, N.; Basiri, A.; Ziaee, S.-A.; Tabibi, A.; Nouralizadeh, A.; Radfar, M.H.; Sarhangnejad, R.; Mirsadeghi, A. Major vascular injury in laparoscopic urology. JSLS J. Soc. Laparoendosc. Surg. 2014, 18, e2014-00283. [Google Scholar] [CrossRef]
- Gaur, D.D. Retroperitoneal and pelvic extraperitoneal laparoscopy: An international perspective. Urology 1998, 52, 566–571. [Google Scholar] [CrossRef]
- Makai, G.; Isaacson, K. Complications of Gynecologic Laparoscopy. Clin. Obstet. Gynecol. 2009, 52, 401. [Google Scholar] [CrossRef]
- Saidi, M.H.; Vancaillie, T.G.; White, A.J.; Sadler, R.K.; Akright, B.D.; Farhart, S.A. Complications of major operative laparoscopy. A review of 452 cases. J. Reprod. Med. 1996, 41, 471–476. [Google Scholar]
- McDonald, P.T.; Rich, N.M.; Collins, G.J.; Andersen, C.A.; Kozloff, L. Vascular trauma secondary to diagnostic and therapeutic procedures: Laparoscopy. Am. J. Surg. 1978, 135, 651–655. [Google Scholar] [CrossRef]
- Nordestgaard, A.G.; Bodily, K.C.; Osborne, R.W., Jr.; Buttorff, J.D. Major vascular injuries during laparoscopic procedures. Am. J. Surg. 1995, 169, 543–545. [Google Scholar] [CrossRef]
- Vilos, G.; Ternamian, A.; Dempster, J.; Lefebvre, G.; Allaire, C.; Arneja, J.; Birch, C.; Dempsey, T.; Laberge, P.Y.; Leduc, D.; et al. Laparoscopic Entry: A Review of Techniques, Technologies, and Complications. J. Obstet. Gynaecol. Can. 2007, 29, 433–447. [Google Scholar] [CrossRef]
- Azevedo, J.L.M.C.; Azevedo, O.C.; Miyahira, S.A.; Miguel, G.P.; Becker, O.M., Jr.; Hypólito, O.H.M.; Machado, A.C.C.G.; Cardia, W.; Yamaguchi, G.A.; Godinho, L.; et al. Injuries caused by Veress needle insertion for creation of pneumoperitoneum: A systematic literature review. Surg. Endosc. 2009, 23, 1428–1432. [Google Scholar] [CrossRef]
- Castillo, O.A.; Peacock, L.; Vitagliano, G.; Pinto, I.; Portalier, P. Laparoscopic repair of an iliac artery injury during radical cystoprostatectomy. Surg. Laparosc. Endosc. Percutan Tech. 2008, 18, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Roviaro, G.C.; Varoli, F.; Saguatti, L.; Vergani, C.; Maciocco, M.; Scarduelli, A. Major vascular injuries in laparoscopic surgery. Surg. Endosc. Other Interv. Tech. 2002, 16, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Sandadi, S.; Johannigman, J.A.; Wong, V.L.; Blebea, J.; Altose, M.D.; Hurd, W.W. Recognition and management of major vessel injury during laparoscopy. J. Minim. Invasive Gynecol. 2010, 17, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Asfour, V.; Smythe, E.; Attia, R. Vascular injury at laparoscopy: A guide to management. J. Obstet. Gynaecol. 2018, 38, 598–606. [Google Scholar] [CrossRef]
- Cheng, S.; Zheng, Q.; Xu, L.; Zhao, W.; Li, G.; Ding, G. Management of major vascular injury in laparoscopic urology. Laparosc. Endosc. Robot. Surg. 2020, 3, 107–110. [Google Scholar] [CrossRef]
- Dunne, N.; Booth, M.I.; Dehn, T.C.B. Establishing pneumoperitoneum: Verres or Hasson? The debate continues. Ann. R. Coll. Surg. Engl. 2011, 93, 22–24. [Google Scholar] [CrossRef]
- Lafullarde, T.; Van Hee, R.; Gys, T. A safe and simple method for routine open access in laparoscopic procedures. Surg. Endosc. 1999, 13, 769–772. [Google Scholar] [CrossRef]
- Jafari, M.D.; Pigazzi, A. Techniques for laparoscopic repair of major intraoperative vascular injury: Case reports and review of literature. Surg. Endosc. 2013, 27, 3021–3027. [Google Scholar] [CrossRef]
- Li, P.; Mao, D.; Zhou, J.; Sun, H. Management of inferior vena cava injury and secondary thrombosis after percutaneous nephrolithotomy: A case report. J. Int. Med. Res. 2021, 49, 3000605211058868. [Google Scholar] [CrossRef]
- Rogers, C.G.; Singh, A.; Blatt, A.M.; Linehan, W.M.; Pinto, P.A. Robotic partial nephrectomy for complex renal tumors: Surgical technique. Eur. Urol. 2008, 53, 514–521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gill, I.S.; Kavoussi, L.R.; Lane, B.R.; Blute, M.L.; Babineau, D.; Colombo, J.R.; Frank, I.; Permpongkosol, S.; Weight, C.J.; Kaouk, J.H.; et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J. Urol. 2007, 178, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Simhan, J.; Smaldone, M.C.; Tsai, K.J.; Li, T.; Reyes, J.M.; Canter, D.; Kutikov, A.; Chen, D.Y.; Greenberg, R.E.; Uzzo, R.G.; et al. Perioperative outcomes of robotic and open partial nephrectomy for moderately and highly complex renal lesions. J. Urol. 2012, 187, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Najib, I.D.; Wang, Q.; Wang, S. Two-Dimensional Versus Three-Dimensional Laparoscopic Systems in Urology: A Systematic Review and Meta-Analysis. J. Endourol. 2018, 32, 781–790. [Google Scholar] [CrossRef]
- Piramide, F.; Kowalewski, K.-F.; Cacciamani, G.; Belenchon, I.R.; Taratkin, M.; Carbonara, U.; Marchioni, M.; De Groote, R.; Knipper, S.; Pecoraro, A.; et al. Three-dimensional Model-assisted Minimally Invasive Partial Nephrectomy: A Systematic Review with Meta-analysis of Comparative Studies. Eur. Urol. Oncol. 2022, 5, 640–650. [Google Scholar] [CrossRef]
- Checcucci, E.; Amparore, D.; Fiori, C.; Manfredi, M.; Ivano, M.; Di Dio, M.; Niculescu, G.; Piramide, F.; Cattaneo, G.; Piazzolla, P.; et al. 3D imaging applications for robotic urologic surgery: An ESUT YAUWP review. World J. Urol. 2020, 38, 869–881. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Okhunov, Z.; Meneses, A.D.; Rodriguez-Socarras, M.E.; Rivas, J.G.; Porpiglia, F.; Liatsikos, E.; Veneziano, D. Impact of Three-dimensional Printing in Urology: State of the Art and Future Perspectives. A Systematic Review by ESUT-YAUWP Group. Eur. Urol. 2019, 76, 209–221. [Google Scholar] [CrossRef]
- Marconi, S.; Negrello, E.; Mauri, V.; Pugliese, L.; Peri, A.; Argenti, F.; Auricchio, F.; Pietrabissa, A. Toward the improvement of 3D-printed vessels’ anatomical models for robotic surgery training. Int. J. Artif. Organs 2019, 42, 558–565. [Google Scholar] [CrossRef]
- Hyde, E.R.; Berger, L.U.; Ramachandran, N.; Hughes-Hallett, A.; Pavithran, N.P.; Tran, M.G.B.; Ourselin, S.; Bex, A.; Mumtaz, F.H. Interactive virtual 3D models of renal cancer patient anatomies alter partial nephrectomy surgical planning decisions and increase surgeon confidence compared to volume-rendered images. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 723–732. [Google Scholar] [CrossRef]
- Porpiglia, F.; Fiori, C.; Checcucci, E.; Amparore, D.; Bertolo, R. Hyperaccuracy Three-dimensional Reconstruction Is Able to Maximize the Efficacy of Selective Clamping During Robot-assisted Partial Nephrectomy for Complex Renal Masses. Eur. Urol. 2018, 74, 651–660. [Google Scholar] [CrossRef]
- Kang, S.K.; Zhang, A.; Pandharipande, P.V.; Chandarana, H.; Braithwaite, R.S.; Littenberg, B. DWI for Renal Mass Characterization: Systematic Review and Meta-Analysis of Diagnostic Test Performance. AJR Am. J. Roentgenol. 2015, 205, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Sighinolfi, M.C.; Menezes, A.D.; Patel, V.; Moschovas, M.; Assumma, S.; Calcagnile, T.; Panio, E.; Sangalli, M.; Turri, F.; Sarchi, L.; et al. Three-Dimensional Customized Imaging Reconstruction for Urological Surgery: Diffusion and Role in Real-Life Practice from an International Survey. J. Pers. Med. 2023, 13, 1435. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalba Bachur, R.; Villoldo, G. Major Vascular Injuries in Laparoscopic Urological Surgeries. Complications 2025, 2, 18. https://doi.org/10.3390/complications2030018
Villalba Bachur R, Villoldo G. Major Vascular Injuries in Laparoscopic Urological Surgeries. Complications. 2025; 2(3):18. https://doi.org/10.3390/complications2030018
Chicago/Turabian StyleVillalba Bachur, Roberto, and Gustavo Villoldo. 2025. "Major Vascular Injuries in Laparoscopic Urological Surgeries" Complications 2, no. 3: 18. https://doi.org/10.3390/complications2030018
APA StyleVillalba Bachur, R., & Villoldo, G. (2025). Major Vascular Injuries in Laparoscopic Urological Surgeries. Complications, 2(3), 18. https://doi.org/10.3390/complications2030018






