1. Introduction
Spinal cord-injured (SCI) patients are at a high risk of developing pressure injures (PI), with a reported prevalence of 34% for persons with quadriplegia and 47% for persons with paraplegia [
1]. The management of pressure injures in this vulnerable population is critical, as these wounds can significantly impact their quality of life. Surgical treatment is always considered in Grade II-IV PI, according to the American National Pressure Ulcer Advisory Panel (NPUAP) [
2]. However, before surgery, we must consider that SCI patients present specific features that may have a high impact on our results.
In addition, pressure injuries represent a major global health issue, posing challenges not only to the affected individuals but also to healthcare systems. The annual cost associated with treating pressure injuries can be staggering for their management and complications. Therefore, understanding the underlying mechanisms, risk factors, and effective management strategies for pressure injuries in SCI patients is essential [
3,
4].
In this paper, we want to review all the aspects that allow for a reduction in complications, and it is a narrative review and expert opinion based on plastic surgeons’ institutional experience of over 10 years at Montecatone Rehabilitation Institute, a Tertiary Rehabilitation Hospital for SCI in Italy. It is the main regional referral center for intensive rehabilitation of people with spinal cord injury and one of the three regional referral centers for severe acquired brain injuries. Furthermore, it is renowned at the national level; in fact, more than half of the patients come there from outside the Emilia-Romagna Region. The hospital also includes a Critical Care Unit, which allows very early rehabilitation care, immediately after an injury. Since 2010 Montecatone Rehabilitation Institute has been accredited by the Emilia Romagna Regional Health Service for 150 beds for inpatient care and 8 beds for day hospital, dedicated to people with SCI/D and/or severe acquired brain injuries. In 2011, a Plastic Surgeons Service dedicated to the treatment of PIs was implemented, and a specific protocol of treatment was initiated. This narrative review and expert opinion offers a critical analysis of tips to obtain better results in the treatment of PIs in SCI patients.
2. Authors’ Experience
Our experience is based on more than 10 years of treatment of PI in SCI patients at Montecatone Rehabilitation Institute, a tertiary rehabilitation hospital and spinal unit in Italy.
Since 2011, 520 SCI patients were treated at Montecatone Rehabilitation Institute for a PI. Over these years, our knowledge concerning surgery in SCI patients has largely improved, thanks to the experience and analysis of our results. In fact, several research studies were conducted to analyze the results of our surgical and rehabilitative protocols.
In 2017, we first published our results regarding the scarce usefulness of pre-operative swab cultures, with the results being predictive of the etiology of the infective process only in 25% of pressure injuries (PIs) [
5].
In 2023, we published a retrospective cohort study on a consecutive sample of adult individuals with SCI, who developed a PI after the first discharge from a spinal unit and who underwent flap surgery between July 2011 and January 2018 (378 patients, 434 surgical interventions, and 521 treated PIs) according to our surgical and rehabilitative protocol [
6]. One significant advancement in our approach has been the establishment of a longitudinal follow-up system, allowing us to monitor and evaluate patient outcomes over extended periods. This proactive monitoring has resulted in the early identification of complications, ultimately facilitating timely interventions. The overall incidence of complications was 17.5% (95% CI: 14.0–21.4%). The higher incidence was noted in cases of sacral PIs and in patients reconstructed with muscle flaps. Minor complications (Grade 1–2 Clavien–Dindo) were reported in 13.6% patients (95% CI: 10.5–17.2%), and major complications in 3.9% patients (95% CI: 2.3–6.2%). The average length of hospital stay was 55 days (42–150). After a mean follow-up of 21 months, we reported a recurrence rate of 1.4% (95% CI: 0.5–3.0%). Neither the level nor the cause of SCI, sex, or age were associated with the risk of complications or recurrence.
In 2024, we reported the result of another important study that changed our clinical practice: a retrospective study of 46 patients with SCI and spasticity treated for 54 PIs between October 2013 and March 2022 [
7]. It included 46 SCI patients affected by spasticity treated for 54 PIs. The overall incidence of complications was 48.1% (95% CI: 34.3–62.2%). Major complications were reported in 13% patients (95% CI: 5.4–24.9%), and minor complications in 30.7% patients (95% CI: 0.37–74%). The average length of hospital stay was 3.8 months (IQR: 2.0–6.1).
Furthermore, our research group proposed an algorithm for flap choice based on PI site [
8] and reported a case series of trochanteric PIs [
9].
The presence of full-time plastic surgeons dedicated to a spinal cord unit is not common, and based on plastic surgeons’ institutional experience of over 10 years in a spinal unit, we will offer expert opinions of the key aspects to consider for optimizing surgical outcomes.
3. Treatment Protocol: Multidisciplinary Approach
Not many studies have been published regarding a protocol for surgical management of PIs in SCI patients, and few studies are prospective and include a very limited sample of patients (37–39 PIs), while most of the studies are retrospective [
10,
11]. All the proposed protocols are based on a multidisciplinary approach, and most of them have a rehabilitative objective.
SCI patients represent a complex population with multiple features that must be taken into consideration. PIs are particularly frequent during the chronic phase of the SCI. In this phase, patients can experience complications related to the SCI affecting the vascular system, bowel, bladder, and nervous autonomic system [
12].
In our practice, the importance of integrating various specialty teams cannot be overstated, and the literature confirms that a multidisciplinary approach is fundamental to adequately evaluate and treat SCI patients. The multidisciplinary team should include a plastic surgeon, wound care nurse, physiotherapist, infectious disease specialist, and specialist in bowel management and neurogenic bladder. Additionally, some other specialists should be involved, if necessary, such as orthopedic surgeons, urologists, or general surgeons. This collaboration ensures comprehensive assessments that address all aspects of patient care. Furthermore, with the rise of telemedicine and digital health solutions, the incorporation of technology in the multidisciplinary approach has also gained prominence, improving the efficiency of patient management [
6,
13].
4. Indication for Surgery
Pressure injures classified as Grades I and II according to the NPUAP guidelines can often be managed effectively through non-surgical interventions. These early-stage wounds typically involve skin redness without the presence of open injuries or partial-thickness skin loss that does not expose underlying structures. Non-surgical management strategies emphasize the importance of preventive measures, such as optimizing patient positioning, implementing regular skin assessments, ensuring adequate nutrition, and utilizing specialized pressure-relieving devices. Furthermore, advanced wound care products, including hydrocolloid dressings and moisture-retentive therapies, can promote healing by maintaining a moist environment and protecting the wound from external contaminants [
14,
15]. By focusing on preventive and conservative care measures, many of these PIs can heal effectively, avoiding the need for surgical intervention altogether.
The indication for surgery in a PI is generally limited to Grade III and IV NPUAP injuries that have no potential of healing with advanced wound care treatments. Coordination with wound care nurses is mandatory to select patients properly and manage the pre-operative period, which should be limited.
In spinal cord injury patients, one key distinction in the management of pressure injures lies in the timing of intervention. Our research group considers surgery as an indication only in the chronic phase of spinal cord injury.
We typically do not consider surgical treatment as an indication for any PI that appeared during the acute phase of SCI because it is generally related more to the use of immobilizing devices for a prolonged period, intensive care unit admission, high injury severity score, mechanical ventilation, and intracranial pressure monitoring, rather than to behavioral and social factors. Performing surgical treatments on a PI during the acute phase can interfere with the rehabilitation programs and training, which should be prioritized. While surgery may sometimes be deemed necessary during this phase, careful consideration is essential, as surgery can often delay necessary rehabilitation efforts. In addition, in our experience, we generally observe a high rate of spontaneous healing of PIs during the acute phase, likely attributed to a reduced presence of comorbidities—complications that are typical of the chronic phase, such as vascular problems, disautonomy, and tissues atrophy.
We advise against planning debridement and reconstruction in acutely infected wounds; while debridement can be performed, reconstruction should be postponed until a less acute phase of infection to improve the success rate. In cases of acute infection, we prioritize debridement, biopsies for microbiological culture tests to guide appropriate antibiotic therapy, and implementation of adequate dressings.
We also consider surgery in cases of unstable scars consequent to previous surgery or healing by secondary intention that lead to the frequent recurrences of superficial wounds. These wounds, while not particularly severe, can necessitate frequent bed rest and dressing changes, thereby impeding the patient’s overall recovery and rehabilitation.
When evaluating surgical indications, it is critical to consider the individual profiles of patients. Factors such as age, overall health, comorbidities, lifestyle, and support systems can significantly influence surgical decisions. Engaging the patient in discussions about their treatment options, expectations, and potential risks is paramount. This approach not only empowers patients but can also improve adherence to post-operative care plans [
16].
5. Pre-Operative Evaluations
Before surgery, it is essential to investigate the etiology of the PI to gain a comprehensive understanding of the patient’s condition. Correcting etiology can significantly influence post-surgical rehabilitation outcomes. As an example, following our surgical protocol published in 2023 [
6], we mandate that, prior to surgery, the patient is always evaluated by a physiotherapist to meticulously assess his/her rehabilitation and prevention needs. This step is vital, especially in the case of ischial injuries, where pressure mapping is performed to ascertain if the patient’s own cushion provides adequate pressure relief.
Comprehensive assessments should include the evaluations of mobility, range of motion, and spasticity levels. By identifying these factors early, we can tailor our surgical interventions and rehabilitation practices to each patient’s specific needs, ultimately enhancing recovery. Additionally, improper bowel and/or intestinal management may also be etiological factors for a PI and should be thoroughly evaluated and implemented before surgery to minimize the risk of post-operative complications and recurrences.
In cases where the PI is close to the anal sphincter, a protective colostomy may be considered as a temporary or permanent solution.
The pre-operative diagnosis of osteomyelitis remains a controversial topic within the surgical community. Although imaging techniques, such as CT scans and MRIs, have been proposed to diagnose osteomyelitis pre-operatively, their sensitivity may not be sufficiently reliable. Therefore, clinical judgment remains crucial in the decision-making process [
17].
Another notable aspect to consider is the reliance on the traditional collection of superficial swabs to define antibiotic therapy for PIs. In a previous study, we indicated that there is a poor concordance between superficial swabs and intra-operative specimen cultures (22%), yielding a sensitivity of 80% and a specificity of 54% [
5]. Therefore, we recommend prioritizing intra-operative specimen cultures when determining antibiotic therapy [
18].
Standard pre-operative examinations encompass comprehensive blood tests, including PCR, bleeding times, albumin, pre-albumin, and protein count, alongside ECG and X-rays.
Pre-operative protein supplementation may be needed based on laboratory results to optimize healing.
We advocate for the admission of the patient one day prior to surgery to conduct all the pre-operative evaluations in an inpatient setting to avoid discomfort, as a patient with a PI needs bed rest to mitigate pressure on the wound.
6. Spasticity Management
Regarding the use of muscle flaps in SCI patients, we must consider another fundamental aspect: more than 60–80% of SCI patients are affected by spasticity, especially in cases of higher levels of lesions [
19,
20]. Given the prevalent nature of spasticity in this patient population, understanding its impact on both surgical outcomes and rehabilitation is critical. Spasticity can complicate positioning, increase the likelihood of pressure damage, and interfere with the healing of existing injuries. In these patients, a muscle spasm after surgery can jeopardize the reconstruction success and lead to flap detachment from the surgical site.
To mitigate the risk in SCI patients affected by spasticity with an indication for muscle flaps, we prefer to administer botulinum toxin 10 days before surgery into the chosen muscle to reduce its activity and allow for better reconstructive outcomes [
6]. Typically, 100 units of botulinum toxin are injected into the target muscle under ultrasound guidance. This technique enables the targeted treatment of specific muscle groups while minimizing systematic effects. In our clinical experience, this practice largely reduces the rate of post-surgical complications associated with spasticity. In addition to botulinum toxin injections, multimodal strategies encompassing physical therapy and the use of orthotic devices can offer additional benefits. Utilizing functional electrical stimulation and other neuromuscular re-education techniques pre-operatively can help improve muscle tone and motor control, leading to better surgical outcomes [
21].
7. Surgical Treatment: Debridement
Debridement remains a cornerstone of surgical management for PIs. It involves the removal of necrotic tissue, foreign materials, and infected bone tissue to promote healing and prevent further complications. Specifically, debridement reduces microbial load and helps create a favorable environment for granulation tissue formation and epithelialization by removing barriers to healing. The pre-operative diagnosis of osteomyelitis is fraught with challenges; therefore, we recommend debriding all exposed bone tissue and sending it for pathological and microbiological analyses.
The application of a methyl blue solution during surgery aids in visualizing all exposed tissues, thereby guiding comprehensive debridement efforts. In our experience, most IV-degree PIs with bone exposure reveal infected bone upon the evaluation of intra-operative samples sent for microbiology and pathology, which emphasizes the necessity of thorough debridement [
6].
The most common ways to perform debridement are surgical and mechanical methods. They involve the use of sharp instruments to excise necrotic tissue and employ friction or irrigation to remove debris and devitalized tissue. This procedure requires additional attention to avoid damaging healthy tissue.
In addition, advanced technologies have emerged in wound debridement procedures. For instance, the use of ultrasonic and laser-assisted devices can aid surgeons in achieving more precise debridement, ultimately facilitating better wound healing outcomes and reducing recovery times [
22,
23].
8. Surgical Treatment: Flap Selection
Surgical procedures need to take into consideration the high risk of recurrence; for this reason, the better reconstructive choice is, in our opinion, the simplest effective method. In cases of ischial or sacral PIs, we predominantly prefer local fasciocutaneous flaps. Specifically, when addressing sacral injuries, flaps are harvested unilaterally to preserve the contralateral side as a donor site, enhancing surgical options for any future complications or recurrences. Designing flaps tailored to the patient’s individual anatomy and the specific characteristics of the wound is crucial for success. Surgeons must carefully consider the vascular supply and tension dynamics involved in each case. To optimize flap viability, we assess the incidence of spasticity and adjust flap designs accordingly. Strategies may include the incorporation of additional skin or muscle from adjacent areas to adequately cover the defect while minimizing tension. In the case of trochanteric injuries, we usually perform muscle or musculocutaneous flaps [
24]. This decision is based on the frequent need to perform the wide debridement of the great trochanter and, often, the femoralis head. After the debridement in trochanteric injuries, we administer topical negative pressure therapy for a period of one month to reduce the dead space before performing a muscle flap to adequately fill it, reducing the risk of hematoma or seroma formation. In certain cases, multiple muscle flaps are necessary to reconstruct larger defects [
25].
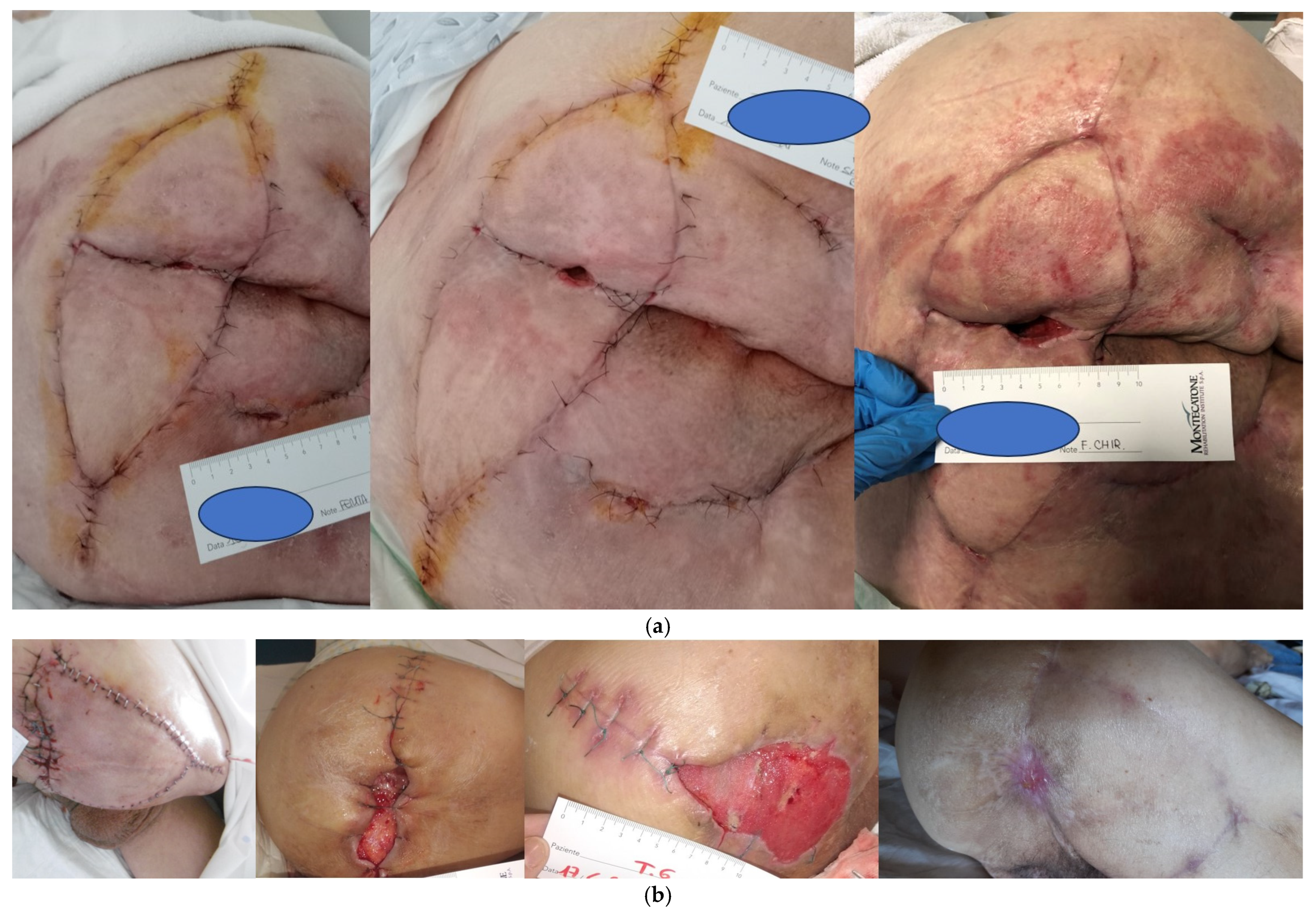
The use of V-Y random advancement flaps is frequently described in the reconstruction after pressure injures’ debridement. On the other hand, a V-Y flap is not sufficiently safe in SCI patients in our experience. The first factor to consider is spasticity, which is present in more than 60% of SCI patients. Muscle spasms can cause the detachment of the proximal aspect of the flap at different planes, leading to deep wound dehiscence (
Figure 1a,b). Spasticity must be considered, and the flap must be designed to prevent tension with spasms [
18]. Another aspect is represented by vascular dysfunction after SCI [
26]. This problem can cause a hematoma days after surgery with flap detachment and fistula formation. The vascular supply of the flap in SCI patients is not the same as that present in other patients due to hypotension. The descending pathways are disrupted in SCI, which results in sympathetic hypoactivity and thus hypotension and a loss of regular adaptability of blood pressure; these findings increase the risk of lower vascular supply to a flap. In our experience, it is preferable to harvest the shortest required flap to prevent distal flap necrosis. Finally, longer scars, such as those present in a V-Y flap, may represent a problem in the case of recurrence, limiting the possibility to harvest other flaps. A bilateral V-Y myocutaneous flap to reconstruct a sacral PI spoils both sides of donor sites, and in cases of failure or recurrence, this will represent a real challenge. For this reason, a unilateral fasciocutaneous flap should be considered as the first option for sacral PIs [
27]. In 2016, Mahmoud et al. [
28] published their experience on 15 pelvic pressure injuries repaired with the V–Y advancement flaps. Small series have also been published by Akan and Chen [
29,
30]. Many of the reported cases are bilateral flaps, and authors have reported the possibility to readvance the flap in the case of recurrence. Unfortunately, it is difficult to support our position to avoid V-Y advancement, especially bilateral V-Y advancement in the sacral region, because of the tendency to report only successful small series, with results comparable to other flaps reported in the literature. In our experience, long-term recurrences in patients with previous bilateral V-Y advancement flaps are the only indication for microsurgical flaps for pressure injuries: once you see the first patient with a wide recurrence on previous bilateral V-Y flaps, you understand this position. Readvancement is possible only for the minor loss of substance, and the presence of extensive scars determines the impossibility of planning fasciocutaneous flaps or perforator flaps. A perforator flap could probably be harvested and rotated as a propeller flap, but the flap design is conditioned by the scars, making donor site closure impossible by first intention, and skin grafts for the donor site closure are a poor option in a load area. Furthermore, often, the advancement of V-Y flaps is performed as musculocutaneous flap advancement, making it impossible to plan a rotation of a gluteus maximum muscular flap as a rescue plan. Reported V-Y small series do not take into consideration long-term wide recurrence in operated patients; therefore, they do not address this issue; on the other hand, studies to support our statements are difficult to find because we do not perform V-Y flaps and can only collect data for the limited number of patients that are referred to our center for recurrence after surgery elsewhere. All these aspects, which are specific to SCI patients, require a lot of attention during the reconstruction planning to reduce complication rates. In conclusion, the surgeon’s expertise plays a vital role in flap selection and execution. Familiarity with various flap techniques, including perforator flaps, propeller flaps, and free flaps, allows for customizable solutions that can be adapted to individual patient needs.
9. Post-Operative Management: What Is the Best Setting
Upon the completion of surgery, the patient lays in bed for 3 weeks, never bearing any weight on the sutures. During these three weeks, a meticulous follow-up by a plastic surgeon is crucial; the surgeon applies dressings on the surgical site at least 3 times a week to identify and promptly treat any instances of bleeding, collections, or minor dehiscence that may arise and need re-suturing. The patient is non-sensitive in the area and can often be treated at the bedside.
Compression therapy can be an invaluable tool during the post-operative phase. The use of compression devices or bandages can improve venous return and reduce edema, which can facilitate healing and minimize complications [
31].
Drains are typically removed after 5 days. Stitches are removed after 3 weeks, and subsequently, the patient is usually transferred to a rehabilitation ward, where the sitting position is allowed for 1 h daily for 1 week and progressively increased by 1 h daily each week.
The results of biopsies sent intra-operatively for microbiology and pathology determine the type of antibiotics to administer and how long to continue them. After 3 weeks of bed rest, 2 additional weeks are usually needed to complete intravenous therapy and rehabilitation efforts for the sitting position, correct passages, and preventive strategies.
After discharge, follow-up visits are planned after 3, 6, and 12 months to assess the quality of the reconstruction and to educate the patient with further instructions. The sitting position is generally increased up to 6 h after 6 months. Lower leg physiotherapy and swimming pool activities are allowed after 6 months. After 12 months, the sitting time can be considered unlimited, but our suggestion is not to sit for more than 4 h consecutively and to rest in bed for 2 h after 4 sitting hours. The use of a WC is allowed after 12 months. We must consider that the WC represents an important cause of PIs. SCI patients often stay more than an hour on the WC to evacuate, and, during this time, their whole weight is pressing on their ischial region. Another risk is represented by the passage to the WC, which is a frequent cause of trauma. Sport practice is also allowed after 6–12 months, depending on the kind of sport and the related risk of high pressure and rubbing. For example, basketball or archery may represent a high risk of rubbing due to the frequent position changes on the wheelchair.
A crucial aspect of the post-operative period is also represented by the education about prevention strategies. Patients and caregivers should receive hands-on training in repositioning techniques, skin care, and the importance of regular assessments. A focus on personal responsibility for their care can empower patients and improve long-term outcomes [
32].
10. Complication Management
A meticulous follow-up of the wound during the first three post-operative weeks by a fully trained plastic surgeon is fundamental to detect and promptly intervene in any complications, such as bleeding, hematomas, collections, and dehiscence that require re-suturing; the surgeon needs to dress the patient at least 3 times a week, and as the patient lacks sensitivity in the area, most minor complications can be treated at the bedside, avoiding prolonged healing times.
It is essential to recognize that complication rates can vary significantly across different studies and populations [
33]. Factors such as baseline patient health, wound severity, and surgical techniques play a significant role in determining outcomes [
34]. The literature indicates the presence of heterogeneous outcomes; minor complications, that do not require reintervention, range from 6 to 37%, while major complications that require reintervention, range from 6 to 14% (
Table 1). In our experience, we witnessed lower rates, probably attributed to the completeness of our protocol that takes into consideration all the specific aspects of the SCI patients. Higher complication rates are reported in cases of sacral injuries and with muscular/musculocutaneous flaps. Sacral injuries may require less bone debridement compared to the other sites, and during the post-operative period, there is a higher risk of friction during bed mobilization. Regarding the type of reconstruction, muscle flaps present a higher risk of seroma formation [
35] and dehiscence due to spasticity [
20]. Another aspect to consider is the recurrence rate, which is reported to be a mean of 19% (range: 5–31%) in the literature [
36,
37,
38,
39,
40].
Various factors can influence complication rates, such as low serum albumin levels (<3.5 g/dL) and being over- or underweight [
9]; therefore, a nutritional status assessment and, if needed, corrective measures prior to surgery must be performed to optimize outcomes.
11. Recurrence
In accordance with other authors, we believe that a rehabilitation team involved in the whole treatment of SCI patients plays a pivotal role in reducing the risk of recurrences [
41]. A multidisciplinary approach is crucial for the treatment of PIs in SCI patients to improve outcomes, reducing complication rates and recurrences.
Effective strategies for preventing recurrence include the development of individualized maintenance plans that focus on continuous education, behavioral modifications, and adherence to pressure relief protocols. Cohesive effort from all healthcare providers, patients, and family members must foster an understanding of pressure sore prevention. Continued engagement with patients and caregivers post-discharge can significantly impact recurrence rates [
42]. Regular follow-up appointments, whether in person or via telemedicine, allow for the ongoing assessments of skin integrity and adherence to preventive strategies. Intervention plans can be adjusted based on patient needs and progress. One essential element of preventive strategies incorporates computerized pressure mapping to detect and correct asymmetries in sitting positions. Various models are now available that allow clinicians to visualize pressure distribution in real-time, making it easier to tailor interventions to individual patient requirements [
43].
12. Conclusions
Treating a pressure sore in a spinal cord injury patient represents a challenge due to specific aspects related to the pathology. A multidisciplinary approach is fundamental to consider all these aspects and to adequately treat them to improve the outcome. During the hospital stay, all the aspects related to the patient’s daily life must be considered to find every possible risk for PIs and to adequately implement strategies to correct them. Surgery is a frequent solution for PIs, but it must take into consideration all the specific aspects of SCI patients, such as the bed rest positioning after surgery and the rehabilitation to achieve better passages and the sitting position. Education of the patient is vital to mitigate future recurrence risks. A structured follow-up plan extending at least 1 year is essential to monitor healing and prevent the occurrence of new pressure injures.
In summary, the management of pressure injures in spinal cord-injured patients necessitates a holistic, multidisciplinary strategy that encompasses surgical, medical, and rehabilitative approaches. Through ongoing education, shared insights, and comprehensive care planning, we can reduce the burden of pressure injures and significantly enhance the lives of those living with spinal cord injuries.
We must underline that the better approach to pressure injures is the prevention. In our institution, patients are trained on preventive strategies, and at discharge, they receive a booklet that underlines all the preventive measures to reduce the risk of pressure injures.
The use of pressure relief devices is useful to reduce pressure in the body areas at risk and to reduce the intensity or duration of the pressure of body weight or other shear forces on a given skin area. Support surfaces should be selected on an individual basis, depending on the needs of the person, in terms of pressure redistribution and other therapeutic functions.
Depending on the support surface, a change in position/posture is performed approximately every four hours, and this favors a lower exposure of the areas of the body at risk.
Decompression maneuvers are advised; when the patient is sitting in a wheelchair, they must frequently change their position with the help of support surfaces such as armrests and perform lateral inclinations to decompress the ischial area. This rule does not apply to everyone, but must be related to the degree of autonomy of the person and based on the type of cushion.
Moreover, patients are advised not to change the height of the footrests on their own; if they are too high, the weight bears on their bottom, and if they are too low, the weight bears on their thighs.
Each device can be functional for a person or appropriate for a situation, but all must ensure that the skin remains dry. If this does not happen, an evaluation by your doctor/nurse is necessary to check if the device is used correctly or another device should be considered.
Maintaining proper protein and calorie intake and proper hydration is essential for the prevention of pressure injuries as dehydration and malnutrition weaken the skin and are proven risk factors for the formation of pressure injuries.
The most important rule to follow for the prevention of pressure injuries is the daily inspection of the skin, especially the areas at risk. The inspection should be performed using a mirror for the areas that are more difficult to see. The signs of risk are persistent redness or dark areas along with a bony prominence and the presence of unusual subcutaneous compactness or blisters, pustules, crusts, and skin wounds. If one of these signs appear, bed rest is recommended, and an appointment should be fixed to check the lesion.
13. Future Directions
As we extend our understanding and refine our protocols, there is a pressing need to implement and investigate standardized management protocols for complications, including pressure injures, to further enhance the overall success rate of surgical interventions and rehabilitation strategies.
Future directions should focus on optimizing patient-centered care that emphasizes rehabilitation and prevention. Establishing protocols that support the integration of physical therapy and advanced wound care technologies can lead to enhanced recovery outcomes. The recognition of the significant role psychological factors play in patient adherence also warrants attention.
Emerging innovations in wound healing technology, such as bioengineered skin substitutes and advanced dressings with antimicrobial properties, offer new avenues for improving outcomes. Research into modalities such as stem cell therapy and growth factors shows promise in accelerating wound healing and minimizing complications.
The future may hold significant potential in telehealth and remote monitoring technologies to facilitate follow-up care for SCI patients. This approach could enhance accessibility to specialized services, enabling patients to receive timely support while managing their condition at home. Virtual healthcare platforms can also serve as valuable resources for ongoing education regarding pressure ulcer prevention.