Improvement in the Estimation of Inhaled Concentrations of Carbon Dioxide, Nitrogen Dioxide, and Nitric Oxide Using Physiological Responses and Power Spectral Density from an Astrapi Spectrum Analyzer
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Paradigm
2.2. Data Collection
2.3. Machine Learning Model Development
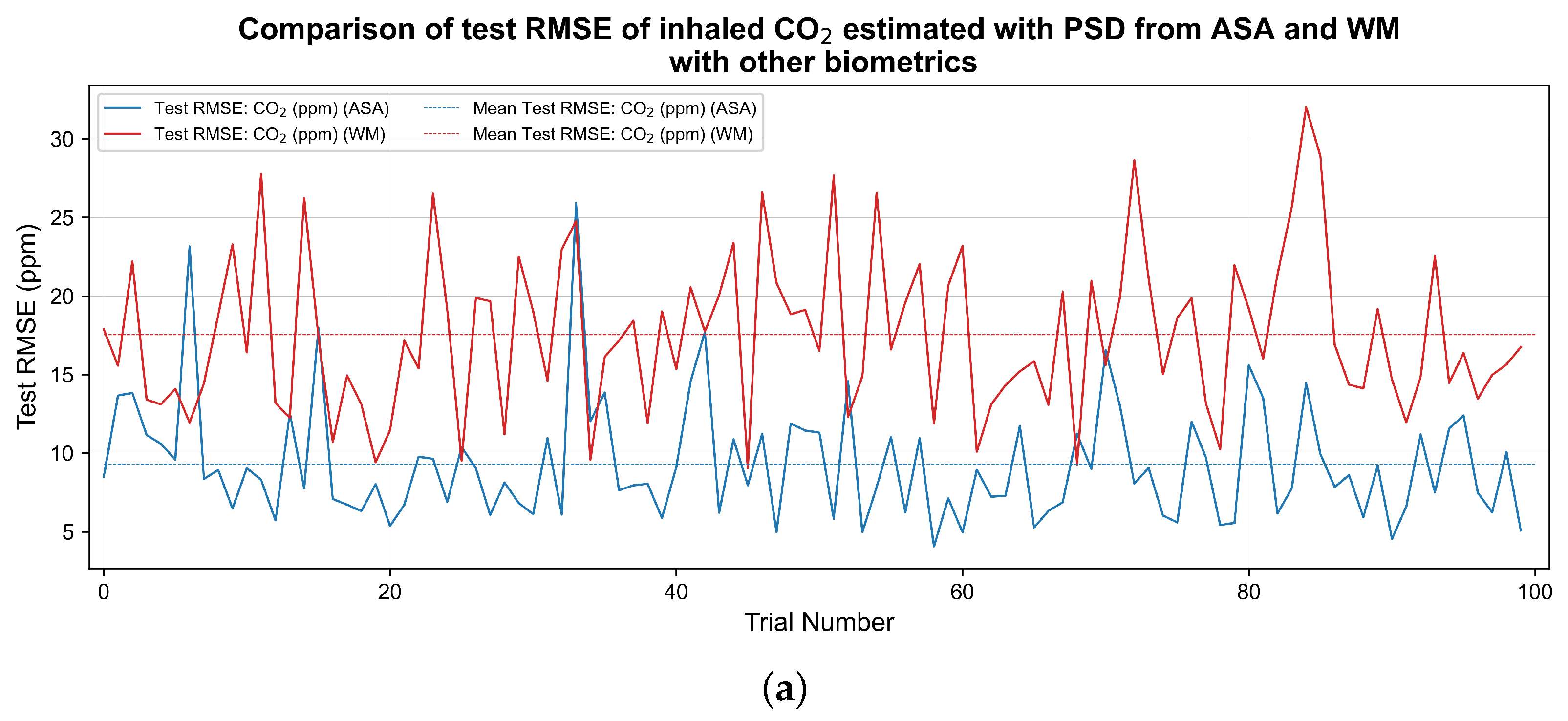
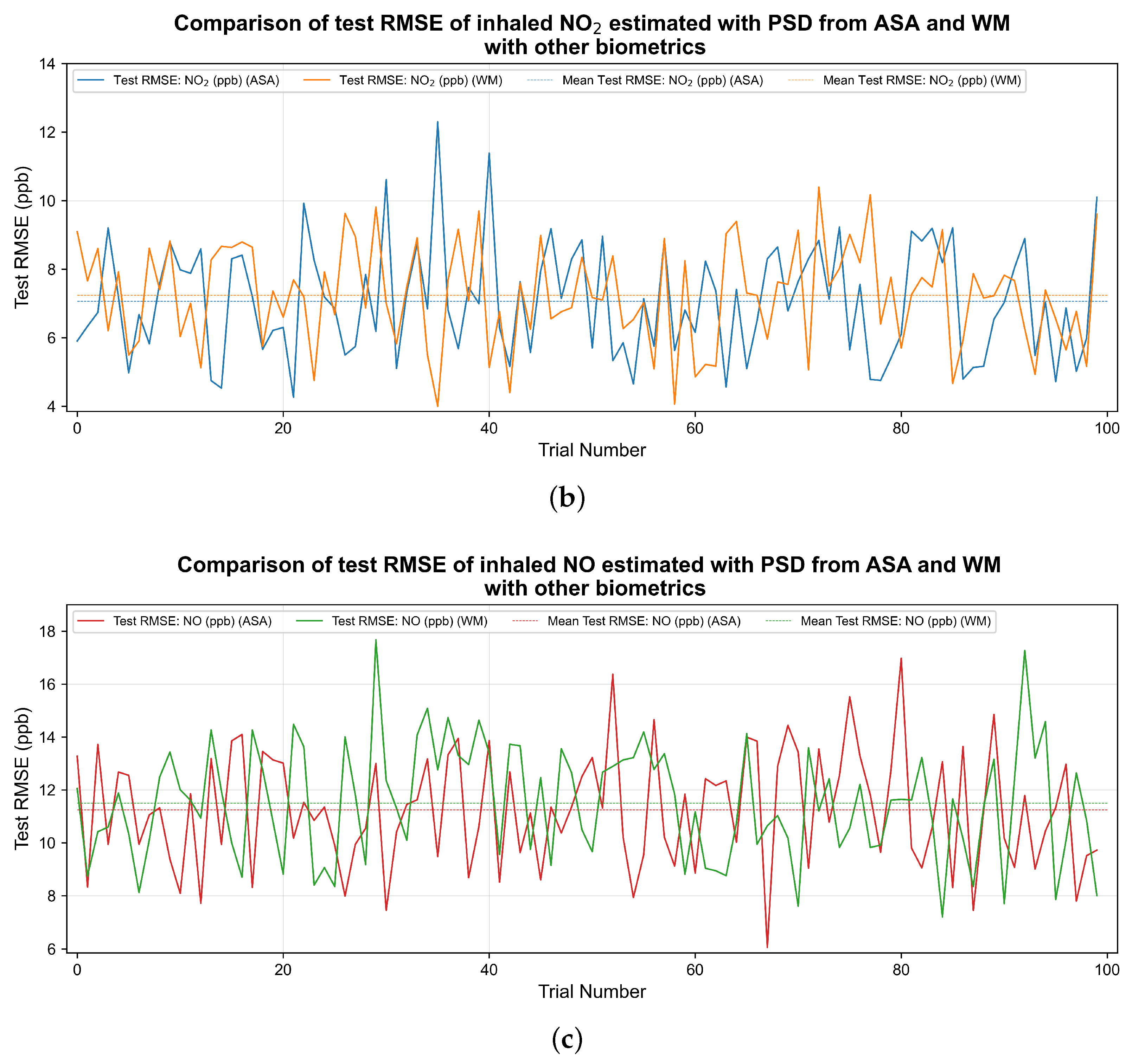
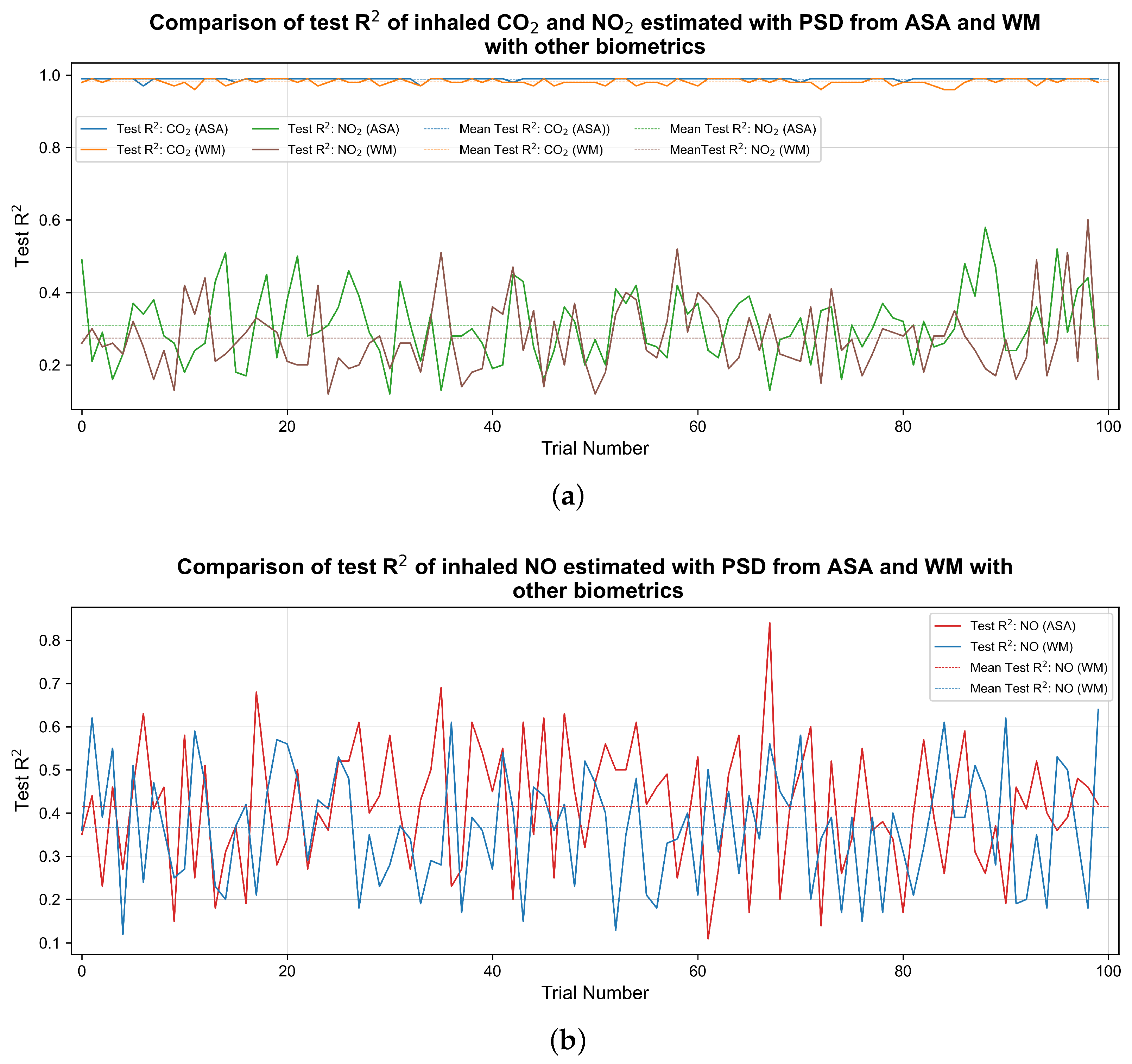
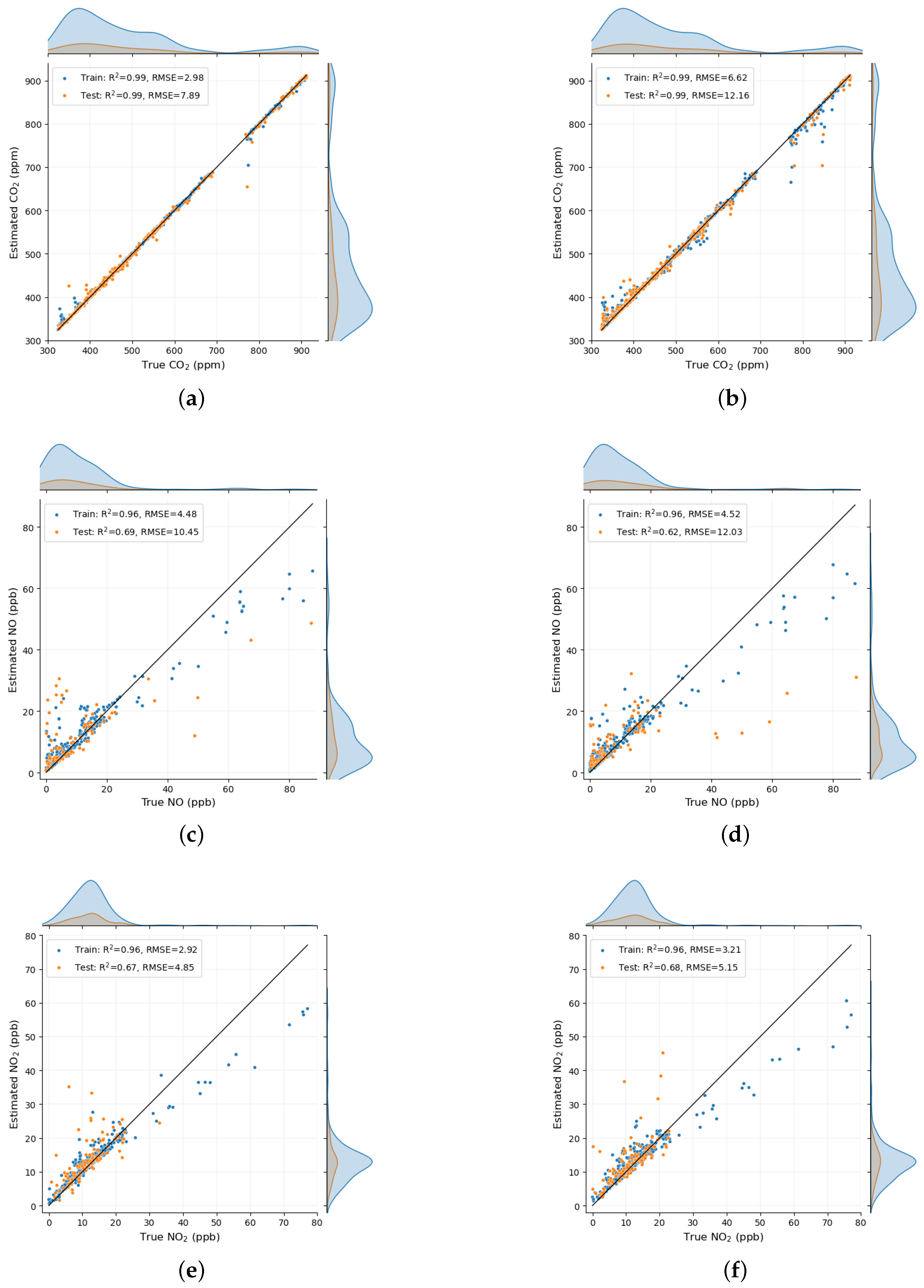
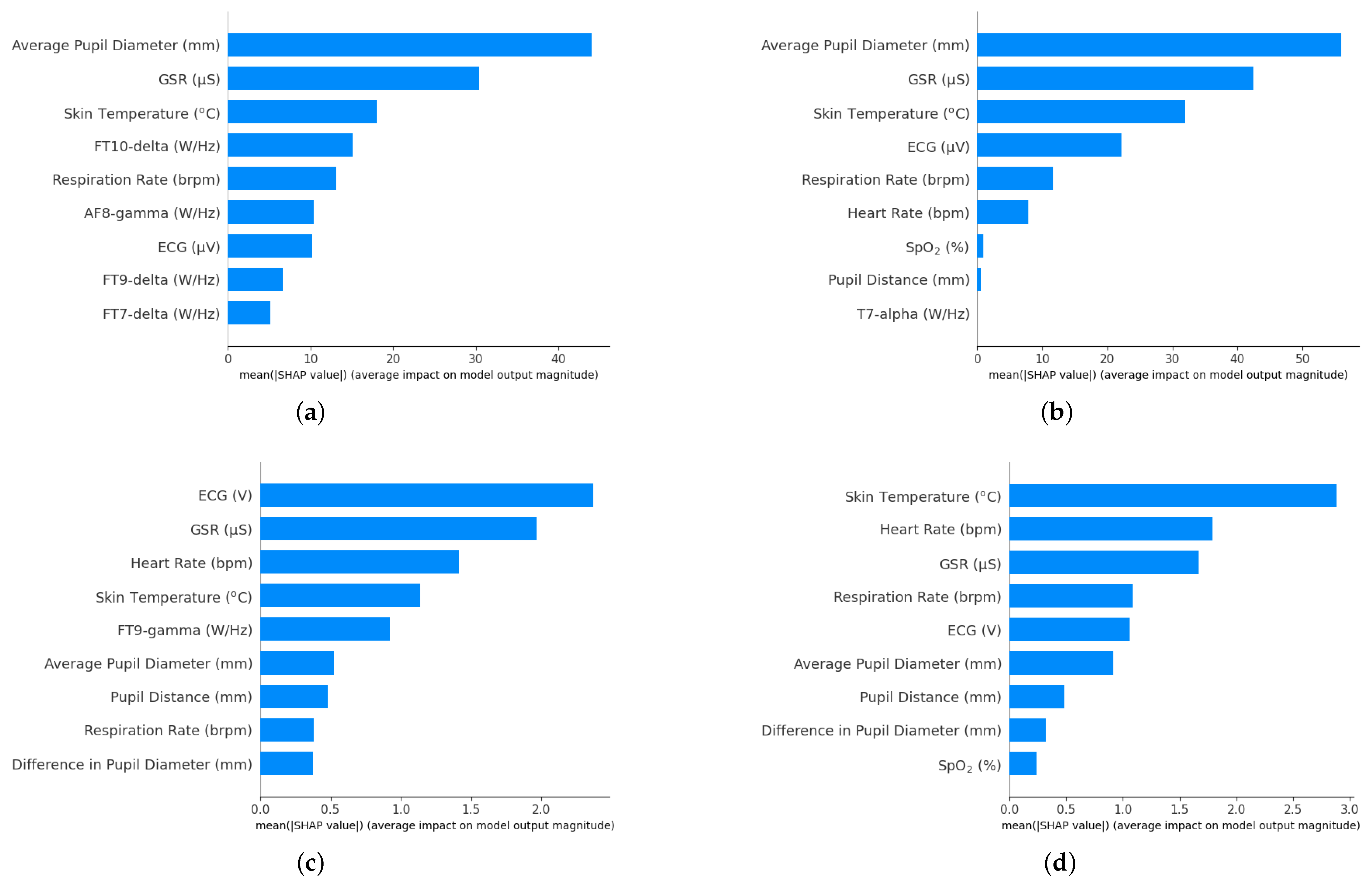
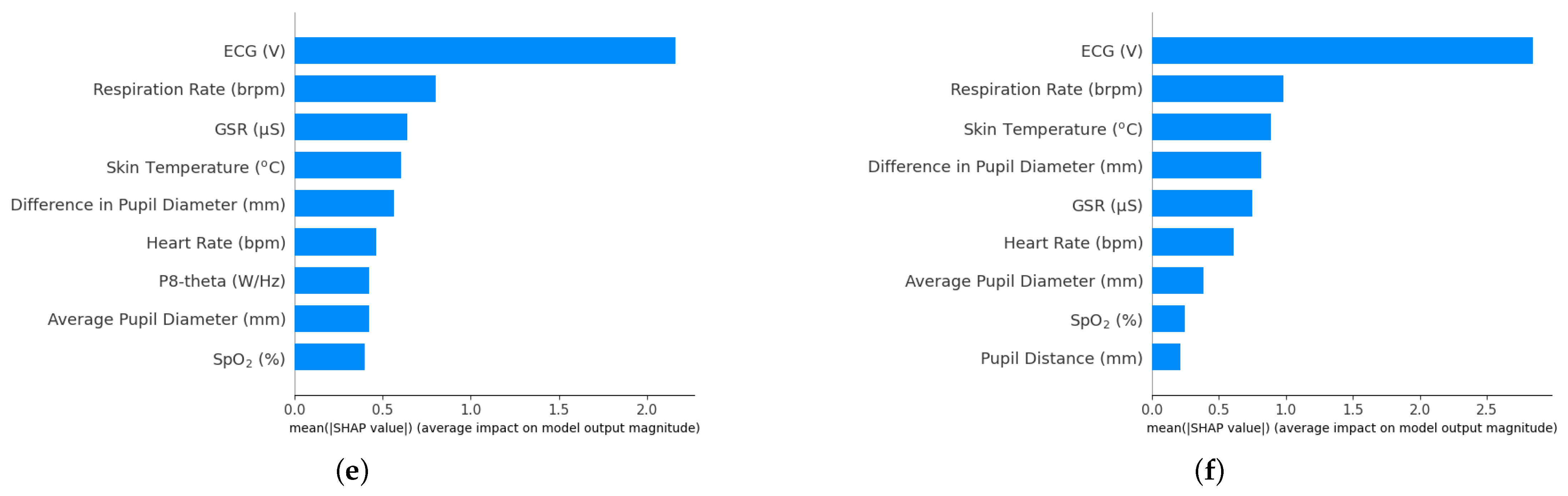
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| PM | Particulate Matter |
| EEG | Electroencephalogram |
| GSR | Galvanic Skin Response |
| Blood Oxygen Saturation | |
| PSD | Power Spectral Density |
| ASA | Astrapi Spectrum Analyzer |
| WM | Welch Method |
| RMSE | Root Mean Square Error |
| USEPA | United States Environmental Protection Agency |
| NAAQS | National Ambient Air Quality Standards |
| FT | Fourier Transform |
| ECG | Electrocardiogram |
| GPS | Global Positioning System |
Appendix A
| Pollutant | Set of n_estimators | Set of max_features | Folds for Cross-Validation | Total Number of Training | Optimized Parameter |
|---|---|---|---|---|---|
| 80, 90, 100, 110, 120 | 250, 275, 300, 325 | 3 | 60 | 80, 250 | |
| NO | 80, 90, 100, 110, 120 | 250, 275, 300, 325 | 3 | 60 | 90, 275 |
| 80, 90, 100, 110, 120 | 250, 275, 300, 325 | 3 | 60 | 80, 250 |
| Pollutant | Set of n_estimators | Set of max_features | Folds for Cross-Validation | Total Number of Training | Optimized Parameter |
|---|---|---|---|---|---|
| 80, 90, 100, 110, 120 | 250, 275, 300, 325 | 3 | 60 | 110, 250 | |
| NO | 80, 90, 100, 110, 120 | 250, 275, 300, 325 | 3 | 60 | 120, 275 |
| 80, 90, 100, 110, 120 | 250, 275, 300, 325 | 3 | 60 | 110, 300 |
References
- WHO. What Are the WHO Air Quality Guidelines? 2024. Available online: https://www.who.int/news-room/feature-stories/detail/what-are-the-who-air-quality-guidelines (accessed on 12 November 2024).
- Environmenal Protection Agency. Reviewing National Ambient Air Quality Standards (NAAQS): Scientific and Technical Information. 2024. Available online: https://www.epa.gov/naaqs (accessed on 12 November 2024).
- Hutter, H.P.; Haluza, D.; Piegler, K.; Hohenblum, P.; Fröhlich, M.; Scharf, S.; Uhl, M.; Damberger, B.; Tappler, P.; Kundi, M.; et al. Semivolatile compounds in schools and their influence on cognitive performance of children. Int. J. Occup. Med. Environ. Health 2013, 26, 628–635. [Google Scholar]
- Satish, U.; Mendell, M.J.; Shekhar, K.; Hotchi, T.; Sullivan, D.; Streufert, S.; Fisk, W.J. Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance. Environ. Health Perspect. 2012, 120, 1671–1677. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Luo, G.; Sun, J.; Zhao, C.; Xie, J.; Liu, J.; Zhang, N. Effects of exposure to carbon dioxide and human bioeffluents on sleep quality and physiological responses. Build. Environ. 2023, 238, 110382. [Google Scholar] [CrossRef]
- Zhang, X.; Wargocki, P.; Lian, Z. Physiological responses during exposure to carbon dioxide and bioeffluents at levels typically occurring indoors. Indoor Air 2017, 27, 65–77. [Google Scholar] [PubMed]
- Jin, R.N.; Inada, H.; Négyesi, J.; Ito, D.; Nagatomi, R. Carbon dioxide effects on daytime sleepiness and EEG signal: A combinational approach using classical frequentist and Bayesian analyses. Indoor Air 2022, 32, e13055. [Google Scholar]
- Xu, F.; Uh, J.; Brier, M.R.; John Hart, J.; Yezhuvath, U.S.; Gu, H.; Yang, Y.; Lu, H. The Influence of Carbon Dioxide on Brain Activity and Metabolism in Conscious Humans. J. Cereb. Blood Flow Metab. 2011, 31, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gillespie-Bennett, J.; Pierse, N.; Wickens, K.; Crane, J.; Howden-Chapman, P. The respiratory health effects of nitrogen dioxide in children with asthma. Eur. Respir. J. 2011, 38, 303–309. [Google Scholar] [CrossRef]
- Cibella, F.; Cuttitta, G.; Della Maggiore, R.; Ruggieri, S.; Panunzi, S.; De Gaetano, A.; Bucchieri, S.; Drago, G.; Melis, M.R.; La Grutta, S.; et al. Effect of indoor nitrogen dioxide on lung function in urban environment. Environ. Res. 2015, 138, 8–16. [Google Scholar] [CrossRef]
- Latza, U.; Gerdes, S.; Baur, X. Effects of nitrogen dioxide on human health: Systematic review of experimental and epidemiological studies conducted between 2002 and 2006. Int. J. Hyg. Environ. Health 2009, 212, 271–287. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Wang, M.; Qian, Y.; Steenland, K.; Caudle, W.M.; Liu, Y.; Sarnat, J.; Papatheodorou, S.; Shi, L. Long-term exposure to nitrogen dioxide and mortality: A systematic review and meta-analysis. Sci. Total Environ. 2021, 776, 145968. [Google Scholar] [CrossRef]
- Crüts, B.; van Etten, L.; Törnqvist, H.; Blomberg, A.; Sandström, T.; Mills, N.L.; Borm, P.J. Exposure to diesel exhaust induces changes in EEG in human volunteers. Part. Fibre Toxicol. 2008, 5, 4. [Google Scholar]
- Witek, J.; Lakhkar, A.D. Nitric Oxide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Weinberger, B. The Toxicology of Inhaled Nitric Oxide. Toxicol. Sci. 2001, 59, 5–16. [Google Scholar] [CrossRef]
- Miller, O.; Celermajer, D.; Deanfield, J.; Macrae, D. Guidelines for the safe administration of inhaled nitric oxide. Arch. Dis. Child. Fetal Neonatal Ed. 1994, 70, F47. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Lary, D.J.; Wijeratne, L.O.; Fernando, B.; Lary, T.; Lary, M.; Sadler, J.; Sridhar, A.; Waczak, J.; Aker, A.; et al. Decoding physical and cognitive impacts of particulate matter concentrations at ultra-fine scales. Sensors 2022, 22, 4240. [Google Scholar] [CrossRef]
- Ruwali, S.; Talebi, S.; Fernando, A.; Wijeratne, L.O.; Waczak, J.; Dewage, P.M.; Lary, D.J.; Sadler, J.; Lary, T.; Lary, M.; et al. Quantifying Inhaled Concentrations of Particulate Matter, Carbon Dioxide, Nitrogen Dioxide, and Nitric Oxide Using Observed Biometric Responses with Machine Learning. BioMedInformatics 2024, 4, 1019–1046. [Google Scholar] [CrossRef]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Acharya, J.N.; Hani, A.J.; Cheek, J.; Thirumala, P.; Tsuchida, T.N. American clinical neurophysiology society guideline 2: Guidelines for standard electrode position nomenclature. Neurodiagnostic J. 2016, 56, 245–252. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Grinsztajn, L.; Oyallon, E.; Varoquaux, G. Why do tree-based models still outperform deep learning on typical tabular data? In Proceedings of the Thirty-sixth Conference on Neural Information Processing Systems Datasets and Benchmarks Track, New Orleans, LA, USA, 28 November–9 December 2022. [Google Scholar]
- Shwartz-Ziv, R.; Armon, A. Tabular Data: Deep Learning is Not All You Need. arXiv 2021, arXiv:2106.03253. [Google Scholar]
- Lundberg, S.M.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the Advances in Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; Guyon, I., Luxburg, U.V., Bengio, S., Wallach, H., Fergus, R., Vishwanathan, S., Garnett, R., Eds.; Curran Associates, Inc.: Brooklyn, NY, USA, 2017; Volume 30. [Google Scholar]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 2522–5839. [Google Scholar]



| Biometric Variable | Units |
|---|---|
| Electrical activities in brain using EEG | Volt (V) |
| Electrical activities in heart using ECG | Volt (V) |
| GSR | MicroSiemens (Siemens) |
| Percentage (%) | |
| Respiration rate | Breathing rate per minute (brpm) |
| Skin temperature | |
| Heart rate | Beats per minute (bpm) |
| Pupil diameter of each of the eyes | Millimeter (mm) |
| Distance between pupils | Millimeter (mm) |
| Pollutant | Total Number of Biometrics | Days of Data Collection | Number of Trials | Data Records in Each Trial | Total Number of Data Records |
|---|---|---|---|---|---|
| 328 | 9 June, 10 June | 4 | 710, 696, 673, 238 | 2317 | |
| 328 | 26 May, 9 June, 10 June | 6 | 136, 23, 126, 120, 132, 45 | 582 | |
| NO | 328 | 26 May, 9 June, 10 June | 6 | 81, 15, 96, 88, 98, 32 | 410 |
| Pollutant | Average Test (PSD by WM) | Average Test (PSD from ASA) | Average Test RMSE (PSD by WM) | Average Test RMSE (PSD from ASA) |
|---|---|---|---|---|
| 0.98 | 0.98 | 17.55 ppm | 9.28 ppm | |
| NO | 0.36 | 0.41 | 11.50 ppb | 11.24 ppb |
| 0.27 | 0.30 | 7.23 ppb | 7.06 ppb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruwali, S.; Prothero, J.; Bhatt, T.; Talebi, S.; Fernando, A.; Wijeratne, L.; Waczak, J.; Dewage, P.M.H.; Lary, T.; Lary, M.; et al. Improvement in the Estimation of Inhaled Concentrations of Carbon Dioxide, Nitrogen Dioxide, and Nitric Oxide Using Physiological Responses and Power Spectral Density from an Astrapi Spectrum Analyzer. Air 2025, 3, 11. https://doi.org/10.3390/air3020011
Ruwali S, Prothero J, Bhatt T, Talebi S, Fernando A, Wijeratne L, Waczak J, Dewage PMH, Lary T, Lary M, et al. Improvement in the Estimation of Inhaled Concentrations of Carbon Dioxide, Nitrogen Dioxide, and Nitric Oxide Using Physiological Responses and Power Spectral Density from an Astrapi Spectrum Analyzer. Air. 2025; 3(2):11. https://doi.org/10.3390/air3020011
Chicago/Turabian StyleRuwali, Shisir, Jerrold Prothero, Tanay Bhatt, Shawhin Talebi, Ashen Fernando, Lakitha Wijeratne, John Waczak, Prabuddha M. H. Dewage, Tatiana Lary, Matthew Lary, and et al. 2025. "Improvement in the Estimation of Inhaled Concentrations of Carbon Dioxide, Nitrogen Dioxide, and Nitric Oxide Using Physiological Responses and Power Spectral Density from an Astrapi Spectrum Analyzer" Air 3, no. 2: 11. https://doi.org/10.3390/air3020011
APA StyleRuwali, S., Prothero, J., Bhatt, T., Talebi, S., Fernando, A., Wijeratne, L., Waczak, J., Dewage, P. M. H., Lary, T., Lary, M., Aker, A., & Lary, D. (2025). Improvement in the Estimation of Inhaled Concentrations of Carbon Dioxide, Nitrogen Dioxide, and Nitric Oxide Using Physiological Responses and Power Spectral Density from an Astrapi Spectrum Analyzer. Air, 3(2), 11. https://doi.org/10.3390/air3020011








