Abstract
A project to assess air pollution at the National Archeological Museum in Naples was carried out. The main goal of the project was to develop and test a reliable yet simple monitoring system to be adopted at the same time in several exposition rooms. Nitrogen dioxide, hydrogen chloride, nitrous acid, and sulphur dioxide were the chemical species addressed by the technique. Monitoring was simultaneously performed in five rooms, and pollutant concentrations were determined using two passive samplers. The sampling time was approximately one month per period. In addition to passive samplers, environmental data loggers were used to obtain temperature and relative humidity data. Results show high concentrations of nitrogen dioxide inside rooms, which were consistent with those found in outdoor environments and are close to the values calculated considering the air exchange rates, estimated through time gradients of ambient temperature. The minimum values were recorded in a basement room that had a low ventilation rate. The conversion of nitrogen dioxide to real surfaces produces nitric acid and nitrous acid. Large amounts of nitrous acid, up to 15 µg/m3, were found in exposition rooms, with maximum values in the basement room, where the air exchange rate is limited, and the surface-to-volume ratio is the highest among the monitored rooms. Data analysis demonstrated that the system could discriminate between nitrous acid and nitrogen dioxide. The results show that, for the first time, passive samplers can overcome the problem of mutual interference between nitrogen-containing species. Nitrates and nitrites found in the alkaline passive sampler were generally found not to be interfered by nitrogen dioxide. Nitric acid was also found in the gas phase, likely generated by dissociation of ammonium nitrate in particulate matter. Hydrogen chloride and sulphur dioxide were present at few µg/m3. Nitrous acid is the most relevant acidic species found indoors. The presence of pollutants was discussed in terms of the reliability of the analytical procedure and its significance for indoor air pollution.
1. Introduction
The protection of cultural heritage is one important priority for our society. It is a significant resource for economic growth in terms of both services offered via tourism and cultural exchange. In Italy alone, a report by the Italian National Statistical Institute [1] indicates that 4265 museums and similar institutions, public and private, were open or partially open in 2020, comprising 3337 museums, 295 archaeological areas, and 633 monuments or monumental complexes. There are also many structures that are not open to the public; these include cultural heritages that require appropriate protection [1]. Air pollution, is a main hazard for human health, the local and global environment, and materials, also poses a risk for the conservation of cultural heritage. Indeed, air pollution affects cultural heritage materials exhibited both outdoors and indoors, causing irreversible damages to the art-works [2].
The major polluting species that may be found inside museums and pose a risk to cultural materials are sulphur dioxide, nitrogen dioxide, nitrogen oxide, ozone, reduced sulphur gases such as hydrogen sulphide, and particulate matter [3]. To effectively protect art-works preserved in museums, it is therefore appropriate to develop monitoring activities with the aim of assessing the nature of contaminants and their concentration levels. This information, coupled with the evaluation of physical parameters such as temperature and relative humidity, can guide researchers toward more effectively preventing potential damage to art-works caused by exposure to these elements. Although no standard has been issued for the most common air pollutants of interest for cultural heritage conservation, some limits have been reported [2]. They range from practically zero to a few parts per billion. Therefore, assessing pollution, especially in indoor environments (museums, etc.) requires techniques that are sufficiently sensitive and reliable.
To fulfil this requirement, many measurement campaigns have been conducted in museums using analytical systems with different complexities [4,5,6,7,8]. However, these monitoring approaches have two limitations. First, techniques can be very expensive and require highly specialized personnel, leading to excessive expenses; in addition, they cannot be applied simultaneously in several rooms inside museums where air contaminants may show different concentrations. Second, most of these activities are usually conducted over a relatively short period of time. This does not allow data to be acquired over a wide time horizon, which may be relevant for fully understanding the evolution of air pollution [9,10]. Although the most important museums can afford and perform sophisticated campaigns for the characterization of atmospheric pollutants, the number of sites that require protection and monitoring is very high. Therefore, the complexity and the cost of monitoring excludes many important cultural sites from a proper evaluation of risks posed by air pollution.
A direct solicitation to develop a monitoring approach that is very simple, low-cost, and easily adaptable methodology that can be used simultaneously in several sites is discussed here. This approach offers significant opportunity for widespread applications as it is compatible with reduced budgets and can provide important and preliminary information for possible and desirable in-depth monitoring and control of atmospheric contaminants. The approach pursued in our strategy is very similar to that established by the EU Directive on ambient air quality 2024/2881 (European Union, 2024) [11], a revision of the former EU Directive 2008/50/CE, Art. 9, comma 3a, which allows indicative measurements or modelling applications to provide sufficient information for the assessment of air quality with regard to limit values, target values, critical levels, alert thresholds and information thresholds, as well as adequate information for the public, in addition to the information provided by the sampling points for fixed measurements. Indicative measurements provide data quality objectives that are less strict than those required for fixed measurements, which can then be used for preliminary assessment. This implies the use of less sophisticated systems than those required by the Directive for the areas in which monitoring with certified and an approved system is mandatory [12]. The preliminary assessment then provides data on which the control bodies may plan the final monitoring network in compliance with the Directive.
According to this approach, a simple system based on the use of passive samplers with the aim of monitoring the most important species that cause damage to cultural heritage was adopted. The system is based on the use of Analyst type passive samplers that have been developed for the measurement of acid gases [13] and nitrogen dioxide [14].
The use of passive sampler Analyst for monitoring the MANN (the National Archaeological Museum of Naples) museum is very effective, as it can be exposed for a long period due to its large capacity. This allows data to be averaged over a long period of time (in this case: 1 month), reducing the cost of long-term monitoring. The paper primarily addresses acidic species, which play an important role in the preservation of stone art-works. This is particularly true in museums housed in historical buildings, in which treating air is difficult for technical reasons. This paper contains important information about nitrous acid. As is well known, this acid is generated by the interaction of nitrogen dioxide with the surface, leaving nitric acid. This acid is highly corrosive. The method used for monitoring is designed to limit the cross-interference between the measurements of NO2 and HONO. This is quite evident from the data treatment. This issue is particularly important in indoor pollution. Now, experiments are underway to test this finding in the laboratory. The generation of a pure HONO gas mixture is the next objective needed to demonstrate our findings. Nitric acid is another important monitored species. As with HONO, the interaction of NO2 with the surfaces generates nitric acid that remains on the surface. Our data demonstrate that the source for this species is the dissociation of nitrate aerosols. Also, this result was obtained by data treatment, including those provided by the environmental dataloggers. Passive samplers used through the monitoring campaign consist of quartz and carbon paper active surfaces alkalinized with sodium carbonate and glycerin. The quartz active surface irreversibly adsorbs acids, while carbon paper collects nitrogen dioxide that is converted into nitrite (NO2− anion). Ions on both surfaces are then extracted with bi-distilled water and analyzed by Ion Chromatography (IC).
This configuration, integrated with an environmental datalogger for the measurement of temperature and relative humidity, have been used for a one year of monitoring in five rooms at one of the most important Italian museums: MANN. This demonstration project provided useful data related to the presence of pollutants and also gave important and sometimes unexpected results about the reliability of the suggested technique, especially when intended for the evaluation of nitrogen dioxide and nitrous acid.
It is important to explain why Ozone (O3) and solid particulate matter (PM10) were not measured in this work, even though these pollutants represent two target pollutant species indicated by the European EPA (https://www.eea.europa.eu/publications/europes-air-quality-status-2024, accessed on 6 June 2024, last modified 30 July 2024) and also by the Italian Environmental Agency (https://www.eea.europa.eu/en/topics/in-depth/air-pollution/air-pollution-country-fact-sheets-2024/italy-air-pollution-country-fact-sheet-2024, accessed on 10 December 2024, modified 18 December 2024).
The first reason we chose not to measure O3 is the low levels of O3 concentration in indoor museum environments. In fact, the literature [5] shows that the levels of O3 concentration (0.94 μg/m3 is average concentration recorded in Capodimonte’s museum, which is another museum located in Naples) are below the legal limit (which is 2 μg/m3, according to literature [5] and references cited therein] and come essentially from external pollution. Internal sources of O3 could be attributable to the organic material surfaces and wooden components; secondary reactions can occur on these which contribute to the production of O3 in indoor environments. In the case of MANN museum, there are fewer organic materials and wood surfaces because the sculptures are mostly in marble, so internal sources of O3 are negligible.
The second reason that O3 was not considered in this study is its negligible contribution to the damage and risk equation/R, formulated in the literature for stones (especially sandstones) as reported by [15] and very well described by Equation (S1) as shown on Supplementary Information Section, File S1. In this equation, the damage function R depends linearly on the concentration of acids (mainly nitric acid/HNO3 concentration/µg m−3, by a coefficient equal to 0.078 × [HNO3] × RH60, where RH60 is the measured relative humidity when it is 1 if RH > 60%, otherwise 0); 0.054 Rain [H+] and on the particulate matter (firstly PM10), where this latter component exhibits the smallest coefficient (as 0.0258·[PM10]) between that of nitric acid and acid rain, respectively. This equation model/R [15] does not consider the damaging effect caused by Ozone at all, showing that it is negligible (compared to the main effects provoked by acids) for art-works in museums. Furthermore, the contribution of O3 to the damage of metal surfaces is described by another equation, as reported in the literature [16]. However, even here, the coefficient of proportionality of O3 is significantly lower (0.20 × [O3]) than that of hydrogen sulphide (45.30 × [H2S]); sulphur dioxide (3.90 × [SO2]); nitrogen dioxide (1.46 × [NO2]); hydrochloric acid (4.81 × [HCl]); relative humidity (1.04 × RH); and temperature (0.79 T), towards metallic surfaces (as Ag, Cu, Zn, etc.; see Ref [16], and also the Equation (S2) and (S3) on Supplementary Information Section, File S1).
Also, several other pollutants, such as VOCs (Volatile Organic Compounds) are not described/included into R equation for stones [15] for the metallic damage equation [16] or the soiling equation [17] for PM10. For this purpose, and according to these literature (concerning the negligible damage effects of VOCs on art-works), the VOC pollutants have been not measured during this work.
Always considering this R damage equation [15], it is clear that the contribution of particulate matter (with respect to Ozone) is present but always at lower levels if compared with those reproduced for acids and acid rain (which have the greatest impact on cultural heritage). Therefore, the particulate matter has also not been measured in this study, but future studies may foresee monitoring campaigns that also include sampling of the particulate matter and the estimation in the R function of its contribution according to the proportionality coefficient equal to 0.0258·[PM10], which is lower than that of inorganic acids and acid rains [15].
Furthermore, it is worth adding that particulate matter contributes to the damage of historical surfaces more with the “Soiling effect” than with the “Etching effect”; this latter mainly related to the acids, described above by the R equation [15]. For the soiling effect, there is a “Square Root Law”, which explains this darkening effect, as cited in the literature [17], and also highlighted by the Equation (S4) on Supplementary Information Section, File S1. The square root function is mathematically slower as a law that correlates certain quantities, including the concentration of pollutants, compared to a linear function (as with the R equation for etching effects provoked by the gaseous acid pollutants, reported previously), and this implies that the action of a “soiling” particulate matter will be secondary to a corrosion effect (mainly provoked by HNO3 and acid water/rain). This means that corrosion/etching phenomena is far superior to the typical soiling effect of the particulate matter for marble sculptures and cultural heritage.
It should be added that the contribution of PM10 to the damage equations towards metal surfaces is negligible, as shown by the equation in the literature [16]. Data of particulate matter would be interesting if they included the chemical speciation.
Finally, the reasons above and those supported by the literature [15,16,17] consistently justify the choice adopted in this work not to measure O3, VOCs, and PM10. We also did not consider the data concerning relative humidity (RH) because it is negligible according to the R equation damages (mainly due to the acid etching effects [15]), metal surface equation damages [16] and soiling events (for PM10 solid matter component) that were reported above).
We believe that the results presented in this paper and other information can be used to predict the evolution of pollutants in the museum, especially according to the previous damage equations (cited above [15,16,17]) and briefly reported on in the Supplementary Information Section, File S1. This is certainly true over a long-term time basis (monthly). Briefly, we can easily model the daily evolution of nitrogen dioxide according to synoptic meteorological conditions (as reported later in the full text). By knowing the air exchange rate, we may apply Equation (2) (in the full text) to estimate the indoor concentration. Thus, it is possible to evaluate damage through the deposition velocity of acidic species (which are responsible for most of the etching and oxidation events on stones and metallic surfaces, respectively). We preliminarily estimated the damage. However, these data require further evaluation and will be published as soon as they are complete.
2. Material and Methods
2.1. Monitoring Sites and Monitoring Program
The National Archaeological Museum of Naples (MANN) is an Italian museum extending over an exhibition area of 12,650 m2, and it is considered to be one of the most important archaeological museums in the world. The main exhibitions include Roman sculptures from the Farnese collection, a Pompeian collection that includes many finds from the area of Pompeii and other locations near the Vesuvius volcano [18], and an important Egyptian collection (https://www.electa.it/en/product/guide-to-the-egyptian-collection-in-the-mann/, accessed on 8 October 2016). The museum is located in the central area of Naples in a historical building with nearby streets characterized by very heavy traffic, causing significant atmospheric pollution episodes. A monitoring station near the museum consistently measures high levels of pollutants, especially nitrogen dioxide (https://www.arpacampania.it/bollettini, accessed on 16 April 2025). The museum is extended over three floors and a basement. Figure 1 shows the museum site maps of the ground and underground floors, as well as the monitoring site locations.
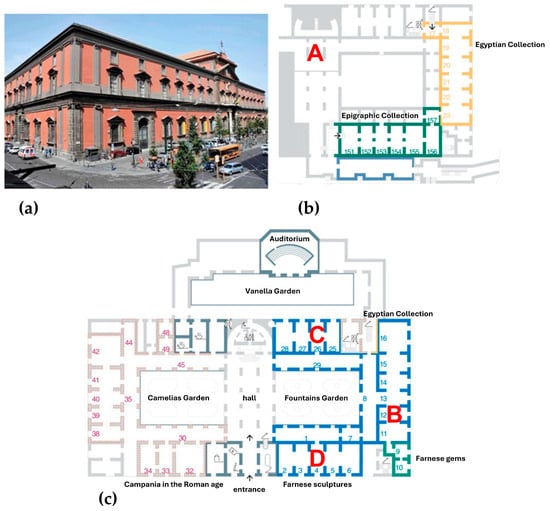
Figure 1.
(a): External view of the museum building. (b): Underground map with monitoring site A in room 45 (Named Cavaiuole) that represents the underground deposit of the statues, not exhibited in the Museum. (c): Ground floor map with sampling sites B that is the room 12, where the Farnese collection is exhibited; C is the room 26 where the Farnesina collection is shown; D is the room 4 where the Tyrannicides are exhibited; and E is the outdoor restoration laboratories of marble sculptures.
In this demonstration project, the locations were mostly placed on the ground floor as that level is the most exposed to air pollutants. It includes showrooms and deposits that comprise statues and marbles which are very sensitive to air pollutants of acidic nature [19,20]. Site E is still located on the ground floor but in a room (restoration laboratories) near the central garden, which is directly exposed to external air during working periods. Site A is underground, where many stone and marble art-works that are not yet being exhibited to the public are stored.
Measurements were carried out in these sites for 9 periods, shown in Table 1.

Table 1.
Sampling periods at MANN with start and end days.
As mentioned before, the average exposure of the samplers for the first eight periods was approximately one month. The ninth period had duration of approximately four months. The last period was extended for a longer time since it is known that the passive samplers used are characterized by a high capacity, and therefore, the results were used to evaluate their suitability for a longer period of time. Auto-consistency tests have been proved in [14]. The overall sampling period lasted approximately one year.
2.2. Passive Samplers and Exposition Shield
Analyst® passive samplers have long been used for the monitoring of air pollutants [13]. The basic design of this kind of passive sampler is shown in Figure 2.
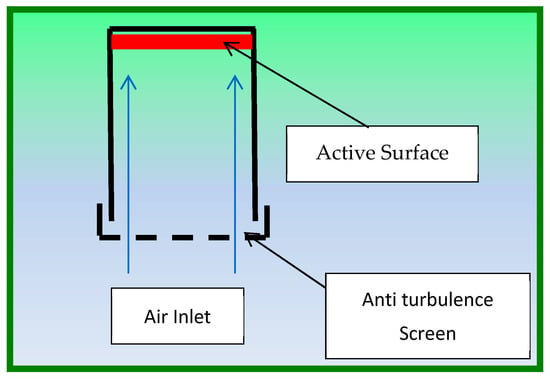
Figure 2.
Scheme of the Analyst passive sampler.
It is characterized by extensive laboratory evaluation and has high capacity, allowing sampling over extended periods of time (up to several months). The design also includes inlet diffusive screens made of steel and polyester to reduce air turbulence, which is known to affect the diffusion mass flow. For this monitoring campaign, a three-place exposition shield was used. Two supports were used to accommodate two passive samplers which monitored acids and nitrogen dioxide. The third support accommodates a data logger for temperature/relative humidity monitoring (see Section 2.6). The shield includes a front steel net that protects the passive samplers from dust and insects and provides a first dumping of air turbulence. This is furtherly reduced by the inlet screens on the passive samplers.
The shields were fixed to the walls inside the rooms where monitoring was performed. They were placed at a height of 3 m in rooms B, C, and D, while in rooms A and E, they were operating at approximately 2 m. The shields are characterized by an insignificant visual impact, which is essential for exposition rooms. An example of the exposition in room C is given in Figure 3.

Figure 3.
The exposure shield for two passive samplers and one environmental datalogger is shown in room C.
2.3. Passive Sampler for Acids
Acids are directly adsorbed onto the active surface of quartz filters (Whatman QM-A) coated with an alkaline solution (1.8% Na2CO3 and 1.8% glycerin in water/ethanol 60/40). This solution is also used for the preparation of passive samplers intended for NO2 (see Section 2.4). Such an active surface is able to collect inorganic and organic acids with high efficiency and retention. After the sampling step, passive samplers were extracted with water and analyzed by ion-chromatography (IC). The apparent flow rates used for the calculations of concentration from IC data are shown in Table 2.

Table 2.
Apparent flow rates used for the calculations of the gas phase concentration for the investigated pollutants. Flow rates have been provided by the manufacturer.
These data were used to calculate the gas phase concentration by the concentration of relevant ions extracted from the filter. Since laboratory and field blanks approach zero, the minimum detectable amount of most acids is less than 0.2 ppb for a monthly sampling time. For nitrous and nitric acids, this amount corresponds to less than 0.4 µg/m3 and 0.6 µg/m3, respectively. Both the Field and Laboratory control sample values are summarized on Table 3.

Table 3.
Limit of Detection (L.O.D.) * for field and laboratory sample controls.
Table 3.
Limit of Detection (L.O.D.) * for field and laboratory sample controls.
| Pollutants | L.O.D./µg m−3 Laboratory Control | L.O.D./µg m−3 Field Control | Sampling Time |
|---|---|---|---|
| Nitric Acid HNO3 | 0.22 ± 0.01 | 0.60 ± 0.02 | Monthly |
| Hydrogen Chloride HCl | 0.20 ± 0.02 | 0.53 ± 0.01 | Monthly |
| Nitrous Acid HNO2 | 0.08 ± 0.005 | 0.40 ± 0.01 | Monthly |
| Formic Acid | 0.14 ± 0.01 | 0.50 ± 0.03 | Monthly |
| Acetic Acid | 0.12 ± 0.005 | 0.49 ± 0.005 | Monthly |
* LOD (the Detection Limit) is quantified by measuring and reading 10 blanks/controls (and is reported as the average value); LOQ (limit of quantification) is the first calibration line point (as reported in Table 4).

Table 4.
Analytical parameters quantified in IC analytical method LOD (the Detection Limit) is quantified by measuring and reading 10 blanks (and is reported as the average value); LOQ (limit of quantification) is the first calibration line point.
Table 4.
Analytical parameters quantified in IC analytical method LOD (the Detection Limit) is quantified by measuring and reading 10 blanks (and is reported as the average value); LOQ (limit of quantification) is the first calibration line point.
| Ions | LOD (ppm) | LOQ (ppm) | Φ (mL min−1) | LOD (µg/m3) | LOQ (µg/m3) |
|---|---|---|---|---|---|
| Chloride/Cl− (from HCl) | 0.01 | 0.1 | 8.1 | 0.14 | 1.48 |
| Nitrite/NO2− (from NO2) | 0.02 | 0.2 | 12.3 | 0.18 | 1.8 |
| Nitrite/NO2− (from HNO2) | 0.02 | 0.2 | 9.1 | 0.25 | 2.5 |
| Nitrate//NO3− (from HNO3) | 0.02 | 0.5 | 10.5 | 0.22 | 5.5 |
| Sulphate/SO42− (from SO2) | 0.045 | 0.5 | 9.9 | 0.52 | 5.2 |
Earlier experimental data [13] confirmed that the reproducibility of this type of passive sampler is within 10–15%. This good result is also due to the positive effects of the inlet steel screens that smooth out any air turbulence at the inlet.
Data from passive samplers intended for acids also include organic acids. However, formic acid was not measured because it was emitted by the plastic shields. Acetic acid was always found below the minimum detectable concentration.
One important point to be considered concerns the possibility that nitrogen dioxide interferes with the substrate of the active surface intended for the collection of acids, yielding nitrite and nitrate ions according to the following reaction:
2NO2 + H2O → HONO + HNO3
This reaction generates nitrite and nitrate ions that are retained on the filter, then interfere with the same ions formed by nitrous and nitric acid, respectively. Such interference can be very high if the concentration of nitrogen dioxide largely exceeds those of the two acids.
Reaction occurs on all surfaces, and its extent depends on many variables, such as the type of surface, pH, and relative humidity. Thus, the extent to which nitrogen dioxide is converted into nitric and nitrous acid is difficult to estimate a priori. However, as will be shown in the Section 4, this reaction is practically not occurring on the active surfaces of passive samplers; thus, the devices can measure acidic species with sufficient reliability without the interference of nitrogen dioxide. The starting point is that, in a case where the reaction (Equation (1)) is occurring on the filter, the resulting nitrite and nitrate ions ware irreversibly adsorbed on the surface, yielding a 1:1 ratio. Experimental values of this ratio are never close to 1:1.
Quality control was carried out by analyzing field blanks and replicates of passive samplers used in the monitoring campaign. For each monitoring period, one sampling site was supplemented by a pair of replicates and one field blank, i.e., a passive sampler not exposed. The variability found between replicates was on average less than 6–7%
It is worth observing that the monitoring campaign required just 10 passive samplers per period and then 10 analyses per month. This, in turn, positively reflects the low cost of the simultaneous monitoring activity for the selected five rooms.
2.4. Passive Sampler for Nitrogen Dioxide
Most passive samplers intended for monitoring nitrogen dioxide use of triethanolamine (TEA) as an active surface. TEA converts nitrogen dioxide into nitrite ions that can be measured by ion chromatography. Although a number of experimental campaigns have been carried out [21], data shows an evident poor reliability in terms of accuracy and precision. The reason for deviations may be that TEA is not a perfect sink for nitrogen dioxide, as was previously demonstrated by using a diffusion denuder [22]. Moreover, TEA collects nitrous acid. This species is present in indoor environments at high concentrations, often higher than those experienced outside and having the same order of magnitude as nitrogen dioxide levels [23]. Since nitrous acid is also converted into nitrite ion, it may cause a 100% interference. The effect of nitrous acid has been largely overlooked since most applications of nitrogen dioxide passive samplers were carried out in ambient atmosphere, where the presence of nitrous acid was negligible.
The sampler used for collecting nitrogen dioxide in this campaign was based on an active surface prepared by impregnating a 20-mm carbon paper disk (Envint srl, Montopoli di Sabina, Italy) with the solution described in Section 2.3. This procedure follows the method developed in [24]. As shown in previous studies, alkaline carbon directly converts nitrogen dioxide into nitrite ions that can be analyzed by IC. The conversion of NO2 into nitrite ion was also confirmed by Raman spectroscopy [25]. The apparent flow rate for this type of passive sampler for NO2 is 12.3 ± 0.7 mL min−1.
After sampling, the filter disc is removed from the passive sampler, extracted with 5 mL of water for approximately 1 h, and analyzed by ion chromatography (Dionex ICS-1000, Thermo Scientific, Waltham, MA, USA). The procedure is such that, for an exposition of 1 month, the minimum detectable concentration is less than 0.4 µg/m3.
Unfortunately, this kind of passive sampler for nitrogen dioxide was not fully exploited in the field. An interesting report [21] concluded that it can be used for the monitoring of nitrogen dioxide without further details.
The carbon filter paper treatment ensures that the blanks are practically zero, and zero is the field blank. The stability of nitrites on the filter is good, even in the presence of a huge amount of ozone, which could oxidize nitrites into nitrates, causing negative deviations. However, the alkaline carbon paper also adsorbs many other acidic substances. Nitrous acid (HONO) is very likely to be included, yielding nitrites and potentially interfering 100% with the measurement of nitrogen dioxide.
The problem of HONO interference has been discussed in several papers, especially those in which triethanolamine was used [26], but it was considered unimportant because the ambient concentrations of HONO are much lower than those of NO2. Unfortunately, for indoor environments, this is not always the case. Some devices were also developed to reduce this interference. A paper that specifically addressed this problem [27] described a device based on three-layer active source. This device is not simple to prepare and use, so its use has been limited to a few applications in archives and libraries.
Our measurements in MANN demonstrate that HONO is present at concentrations comparable to, and often larger than that of NO2. As will be shown later, data demonstrate that the interference of HONO with the measurement of NO2 is not significant, and that the passive samplers for nitrogen dioxide are free from interference due to nitrous acid. This is one of the major technical findings for this monitoring campaign.
2.5. Analysis of the Samples
Analysis of the samples was performed by ion chromatography (IC). The IC apparatus is the model ICS 1000 instrument, having a Dionex Ion pack 12° as the chromatographic column. This IC apparatus is equipped with the suppressor aers 500 carbonate 4 mm. The chromatographic separation is carried out isocratically (and not in gradient mode). After exposure, the passive samplers were extracted with 5-mL water and analyzed using an ion chromatograph Dionex ICS-1000. The analytical procedure ensures a limit of detection (LOD) and a limit of quantification (LOQ) in ppm, shown in Table 4.
Both passive samplers were analyzed using the same analytical procedure. Corrections for blanks were not significant, and the reproducibility of collocated samplers showed a standard deviation of 7%.
It is worth mentioning that, by using the apparent flow rate of pollutants into the passive sampler and for an exposition time of one month (30 days), it is possible to convert LOD and LOQ in ppm into concentrations in µg/m3. From the same table, it is possible to observe that, for most of the species of interest, the minimum detectable concentration was well below 1 µg/m3 while reliable quantifications are above a few µg/m3. The analysis of both passive samplers used in this monitoring campaign used the same procedure. This also reduced the effort required to improve the quality of the data and, clearly, reduces the cost of the monitoring campaign.
2.6. Data Logger
Data loggers used for this campaign are based on the Honeywell HumidIcon™ Digital Humidity/Temperature sensor HIH8120 (Honeywell, Industrial Automation 2425 South 21st Street, Phoenix, AZ 85034, USA).
This sensor ensures an accuracy level of ±2.0%RH (Relative Humidity) and a temperature accuracy level of ±0.5 °C; thus, it is very suitable for indoor measurements. The sensor is within a small plastic box that fits the clips used to hold the passive samplers, and can then be placed into the same exposition shield. The data logger is battery operated and programmed to select the sampling frequency and to set up the internal clock. The sampling frequency can be selected from 1 to 60 min. For the specific application, the sampling frequency was 15 min, along which the dataloggers collect one measurement per minute and then average the data. Thus, the data logger records 4 average temperature and humidity data points every hour. At the end of the sampling period programmed for passive samplers, the data loggers were removed and connected to a personal computer from which an Excel file is extracted. After sampling and reading the output files, the data-loggers can be used repeatedly.
2.7. Outdoor Pollution Data
Monitoring of atmospheric pollution in the city of Naples is continuously carried out by the technical authority in charge, in this case, the Regional Agency for Environmental Protection of Campania ARPAC, (https://www.arpacampania.it/, accessed on 23 December 2014).
The Agency manages a monitoring network in compliance with European Union Directives. Data are regularly published at (https://www.arpacampania.it/bollettini, accessed on 16 April 2025). Our interest was in the station “Museo” which is located near the MANN museum. This station provides an hourly average of several pollutants, including nitrogen dioxide. Meteorological data were provided by the station “Osservatorio” in Naples.
3. Results
3.1. Results of the Monitoring Campaign (NO2)
The results from the monitoring campaign are shown in the next Table 5, where data from nitrogen dioxide (expressed in µg/m3) are reported for each period and for each sampling site (room). As mentioned previously, the data were deduced by assuming that the nitrites in the individual alkaline carbon passive samplers are due to the absorption of NO2 only.

Table 5.
Nitrogen Dioxide concentrations (µg/m3) in the sampling sites averaged over the sampling periods.
Data shows that the average pollutant concentrations in Room A are much lower than those in the other rooms. This result is understandable because site A is located in the basement, and intrusion of the outside air is strongly limited.
This is clearly shown in Figure 4, where temperature data from rooms A, B, and E during the four days of sampling period 5 are reported, and while the day/night modulation is high for room E (~5–6 °C), the modulation is much less for room B (about 1–1.5 °C) and close to zero for room A. As mentioned before, room E is the restoration room; it has a glass door that opens several times during the workday (about 09:00 AM to 05:00 PM) to allow workers and visitors to enter and exit. Therefore, considering the temperature trends in the exposition rooms, site A shows the minimum intrusion.
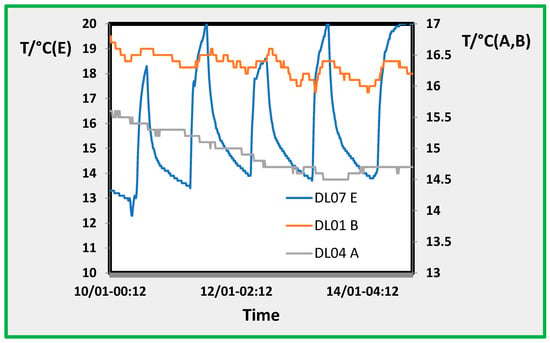
Figure 4.
Temperature trend in the three rooms A, B, and E. Sampling periods: from Jan 10th through 14 January 2022.
The relationship between outdoor and indoor concentrations is also shown in Table 5. Indoor averages ranged from 11 to 25 µg/m3, whereas the average outdoor concentrations during the sampling periods ranged from 37 to 47 µg/m3. The relationship between indoor and outdoor pollution by NO2 is shown in Figure 5.
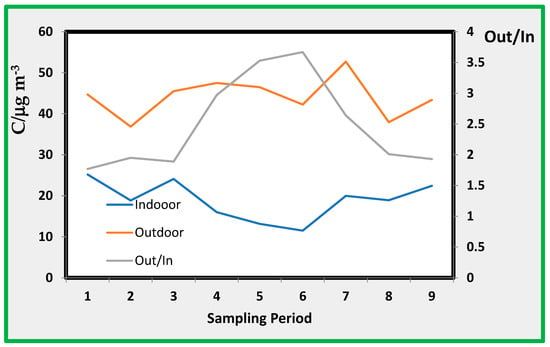
Figure 5.
Indoor (In) and outdoor (Out) nitrogen dioxide average concentration (C, µg/m3) along with sampling periods and ratio.
Outdoor values are fairly constant along the sampling periods, while indoor data show minimum values during periods from 4 to 7, i.e., during winter. In fact, in this period, windows and doors are usually closed, limiting air intrusion from outside. However, as Table 5 shows, room E has a similar indoor/outdoor ratio of the other rooms, even though is the most exposed to external air, Figure 5 shows.
3.2. Time Evolution of Outdoor Air Pollution
In order to explain this apparent contradiction, Figure 6 shows an example of the time evolution of NO2 pollution in the city of Naples, as recorded by the monitoring station near the museum on 15 January 2022, when high NO2 concentrations were observed. A typical trend was an increase in nitrogen dioxide concentration in the morning, coinciding with an increase in vehicular traffic, reaching a high value of more than 100 µg/m3. In late morning, atmospheric turbulence was experienced: air from the free troposphere diluted the ground air, and the concentration of air was drastically reduced. In the late afternoon, the atmospheric stability was restored, and the pollutant concentration increased again to high values. Overnight, the reduction in traffic emissions decreased the pollutant concentration. Atmospheric turbulence developing in the afternoon were also responsible for the fumigation of ozone, which reaches maximum values. Therefore, even though room E was exposed to outdoor air, the outdoor atmosphere was depleted of nitrogen dioxide. It is worth stressing that the turbulence period in which air pollution is relatively low is about 3–4 h in winter and about 7 h in summer.
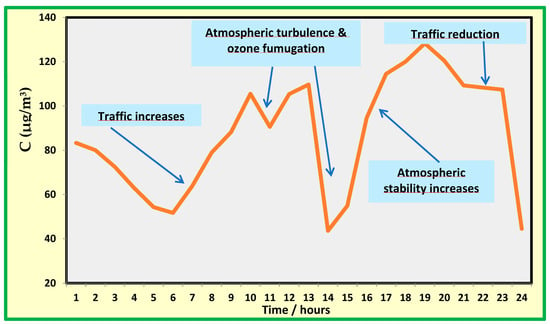
Figure 6.
Time trend of nitrogen oxide (NO) in a specific day in winter.
The behavior shown in Figure 6 is typical of periods in which synoptic meteorological situation is dominated by high pressure. The air advected from aloft from late morning to late afternoon, as said, is rich in ozone that shows maximum values during the clean air window (see Figure 7).
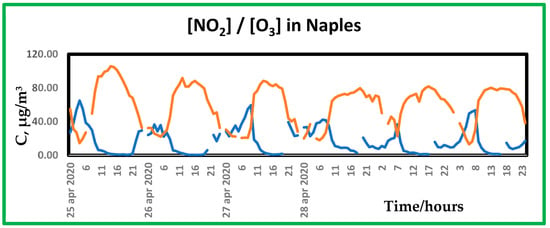
Figure 7.
Time trend of nitrogen dioxide (●) and ozone (●) concentration ratio as recorded in the monitoring station near the museum for the week starting on 25 April 2022.
Ozone fumigation led to ground concentrations up to 100 µg/m3 (about 50 ppb) and, in some instances, even more. As is well known, ozone is another polluting species that may impact several types of materials. Indoor concentrations of ozone are usually low and were not recorded during this campaign. Since most of the art-works preserved in the museum consist of stone and marble sculptures, the effect of ozone is not very significant. On the contrary, ozone produces well-documented damages on other art-works, such as paints [28,29,30]. According to the preliminary nature of the monitoring campaign, ozone is one of the best candidates for a future monitoring protocol.
From the data shown in Figure 6 and Figure 7, it is possible to understand the evolution of HONO in the outdoor environment. Nitrogen dioxide starts to increase in concentration at approximately 07:00 in the morning. However, at that time, the ozone concentration was low or near zero because it had been consumed in the boundary layer. In any case, the solar radiation intensity in the photodissociation range useful for ozone (wavelength between 240 and 320 nm) was not sufficient to promote the formation of the OH• radicals necessary to start photochemical processes. However, OH• radicals are easily provided by HONO, which is formed on real surfaces (see Equation (1)) [31]. This explains the formation of NO2 in the morning by NO oxidation. In this study, no outdoor HONO measurements were carried out.
3.3. Other Indoor Pollutants
As said before, the experimental apparatus adopted for sampling in the museum provides information about the presence of indoor HONO. This species was collected on alkaline quartz fiber filters and detected as nitrite. The results for HONO during the sampling campaigns are shown in the following Figure 8a, which shows the averages for sites B, C, and D, compared with the average NO2 concentrations.
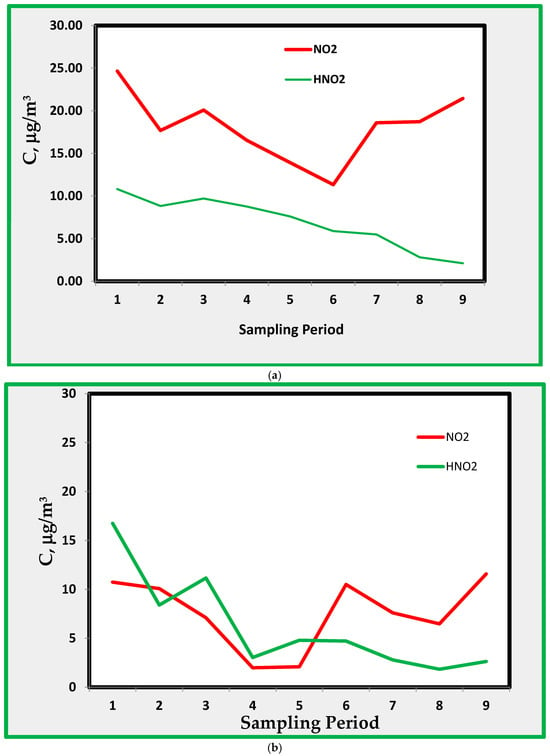
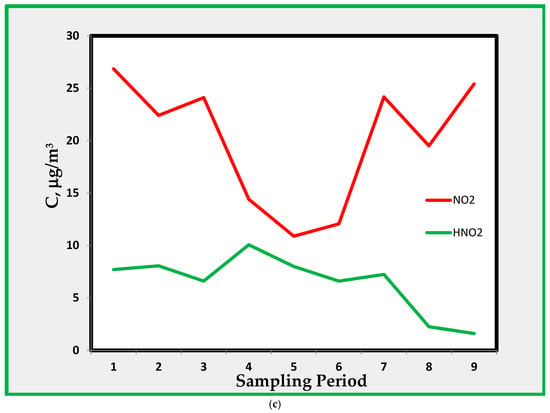
Figure 8.
(a). Average concentrations profiles for NO2 and HONO in B, C, and D rooms. The sampling period is shown in Table 1. (b). Nitrogen dioxide and nitrous acid in room A. (c). Nitrogen dioxide and nitrous acid in room E.
The amount of HONO found in the three rooms is about 1/3 to 1/10 that of nitrogen dioxide. No clear correlation is between the two pollutants along the sampling period. The same graphs for rooms A and E are shown below in Figure 8b,c, respectively.
While room E shows about the same level as the average values (Figure 8a), data in room A show that the amount of HONO and that of NO2 are more or less the same and, in some instances, the concentration of HONO is higher than that of NO2.
We also noted a clear decreasing trend of HNO2, but we did not have a clear explanation for this finding; there are several hypotheses. For instance, the literature [32] declares that nitrous acid can photolyze (from the early hours of the morning) and generate OH• radicals and nitrogen monoxide, NO. The latter is in photostationary equilibrium with NO2, which, in the presence of humidity, generates HNO3 and HONO, which adsorbs on surfaces of historical-artistic interest and cultural heritage [33]. Furthermore, in the presence of VOCs, it could undergo transformation reactions that generate organic nitro compounds, such as nitrosamines and peroxyacetyl nitrates (i.e., PAN) [34]. All these possible events represent reasonable mechanisms that help explain the trend observed in the exhibition rooms regarding the uniform decrease (even if very slight) of the nitrous acid/HONO concentration profiles. In this study (MANN museum environmental monitoring), we need to evaluate and perform other additional measurements to corroborate the experimental data/results with more solid evidence. It would be appropriate to investigate all the mechanisms involved in HONO production [35], including the heterogeneous chemical reactions which involve NO2 (adsorbed on historical surfaces and art-works located into indoor environment) with water (RH%), and the heterogeneous NO3− (nitrate anion) dissociation, which occurs on particulate matter (mainly PM2.5 and PM10) surfaces.
Figure 9 shows the average concentration levels for sulphur dioxide at the B, C, and D sampling sites.
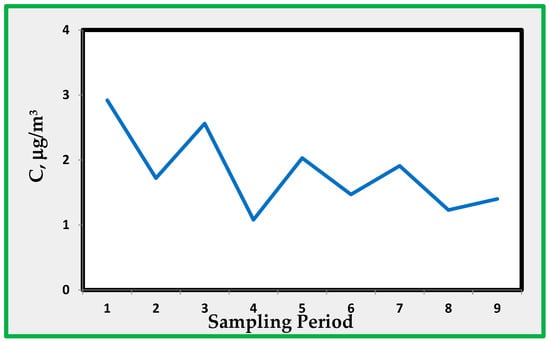
Figure 9.
Sulphur dioxide average concentrations in rooms B, C, and D.
The average concentrations are within a few µg/m3 and do not show a definite trend. The average concentration in the site A, like NO2, is less than the average of the other four sampling sites. This indicates that sulphur dioxide is coming from air outside the museum. Sulphur dioxide levels measured by the Naples monitoring network approach values that are near the minimum detectable concentration. Therefore, no direct comparison between the two sets of data is possible. The amount of SO2 is within the range 1–3 µg/m3. Figure 10 shows the measured concentrations of HNO3 as detected through the alkaline quartz passive sampler.
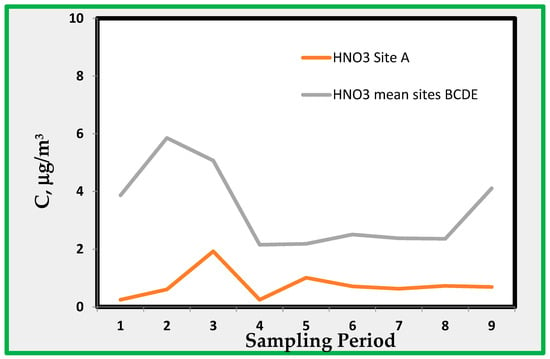
Figure 10.
Nitric acid found indoor as average of rooms B, C, D, and E, and that of room A.
Figure 10 shows the concentrations found in site A with the average concentrations found for the other four sites (B, C, D, and E). As expected, the concentrations found at site A were much lower because of the limited air exchange with the ambient atmosphere and because of the high deposition velocity for this species [36]. Even for HNO3, no data are available for external ambient air. However, the data seem quite reasonable and coherent with those for NO2.
Figure 11 shows the concentration trend of Hydrogen chloride that is monitored by chloride ions found on the fiber glass sampler.
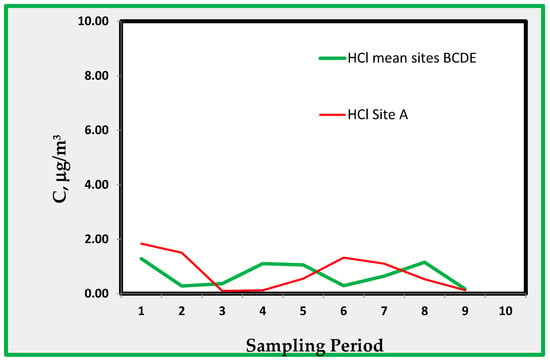
Figure 11.
Hydrogen Chloride found indoors as an average of rooms B, C, D, and E, and that of room A.
Hydrogen chloride shows very low concentration when compared to those recorded for nitric acid. These measurements are based on the amount of chloride ions found in the glass fiber filter, so it is expected that their affect is somewhat uncertain given the ubiquitous presence of chloride ions. We included room E in the averages for strong acids only (Nitric acid/HNO3 and Hydrogen chloride/HCl) to have a general picture of strong acids exposition in the museum. Figure 10 and Figure 11 show that the pollution concentrations are much lower in room A.
4. Discussion
This section is dedicated to a discussion of the results and has been organized into sub-paragraphs, each concerning the individual measured species. Emphasis is placed on nitrogen-containing compounds because they are in relatively high concentrations, causing exposed art-work to rapidly deteriorate.
Dataloggers are essential for monitoring the evolution of temperature and relative humidity in the exposition rooms. However, they are also valuable because they provide data to estimate the evolution of pollutants entering the museum (Ci) from outside (Co). The indoor/outdoor concentration ratio can be expressed by the following Equation (2):
where
Air-exchange rate, usually expressed as h−1, i.e., exchanges per hours.
Volume of the room (m3).
Surface area of the room (m2).
Deposition velocity of the pollutant (mass of the pollutant deposed on a unit surface per unit time).
By knowing the deposition velocity of an individual pollutant and the geometrical characteristics of the exposition rooms (V and S), it is possible to estimate the concentration ratio (indoor to outdoor) if the air exchange rates a is known. As is well known, many pollutants show a time trend that can be followed using Equation (2). This is, for instance, the case of ozone that shows a time trend defined by a vertical process occurring in the atmosphere, causing the fumigation of this pollutant from the free troposphere to the ground (see Figure 7).
In order to estimate Ci, it is necessary to know the term a, air exchange rate. This can be achieved using various techniques [37,38,39]. However, most of these experimental methods are expensive, time consuming, and not simple to implement. According to our main goal, which is to obtain preliminary information on the museum atmospheric environment with simple methods that can be reproduced in many locations, we attempted to estimate the air exchange rate by the difference in indoor–outdoor temperatures. In fact, the exposition rooms of MANN are not conditioned. The size of the rooms is such that the visitor effect can be neglected, and the low surface-to-volume ratio is such that the thermal effects of the walls can also be neglected.
In this hypothesis, the indoor air temperature Ti can be expressed as a function of the outdoor temperature To, according to Equation (3):
where dTi is the increase (or decrease) in indoor temperature caused by ventilation of a volume of air dV. To and Ti are the outdoor and indoor temperatures, respectively. Unfortunately, this equation cannot be integrated directly because the variables involved in it are both time dependent. The indoor temperature in an unconditioned room, such as those of the MANN museum, will be just the low-pass-filtered outdoor temperature. Therefore, in a steady state, the indoor temperature will be the same as the outdoor temperature. However, the thermal capacity of the surfaces and the poor mixing of outdoor air are such that strong deviations from this simple model can be expected.
Our simple approach starts by assuming that the time trend of indoor temperature follows the external temperature, and that the maximum value of room temperature can be used in Equation (3). It is therefore possible to estimate, with the said uncertainties, the value of dV, and then the value of a.
Several calculations conducted throughout the monitoring periods, showed that the term dV/V is about 0.02, with little difference between the exposition rooms and monitoring periods. For instance, in room B (dimensions 36 × 18 × 7 m), the volume is about 4500 m3, while the total surface area is 2000 m2. The ratio S/V = 2000/4500 = 0.44 m−1.
For the term deposition velocity, it is possible to determine the average deposition velocity of all surfaces in a room from Equation (3), as well as the so-called ‘surface removal rate’ Vd (S/V). This rate is directly comparable to the air exchange rate. The deposition velocity of a particular pollutant varies within different material types and under different conditions, such as changing relative humidity [40]. A study of museum buildings showed a surface removal rate of 0.4 h−1 for a large and open gallery [41]. A surface removal rate of 0.4 h−1 means a deposition velocity of approximately 1 m h−1.
When adapting these data to Equation (3) and using the value of deposition velocity ranging from 1 to 0.1 h−1, then the estimated Ci/Co ranges from 0.043 to 0.31. The ratio between the NO2 concentrations found in the outdoor and indoor ranges is between 0.5 and 0.3, which fits the expected concentration ratios calculated by our simple model.
4.1. Nitrogen Dioxide (NO2)
The average concentrations of the NO2 pollutant reported in Table 4 appear to be relatively constant, except for the data from room A. This means that the exposure to this pollutant in rooms B, C, and E are approximately the same. This could not be consistent with the observation that data from site E should be higher because this room is more exposed to external air. However, it should be considered that the room is mostly affected by external air during the workday; as shown in Figure 6 and Figure 7, external air pollution is modulated in such a way that during the workday, the concentration of pollutants (except ozone) in the external air is low.
The concentration of nitrogen dioxide in the external air is well over the standard fixed by the European Union for the protection of public health, at a yearly average of 20 μg/m3 and at a daily average of 50 μg/m3 [11,42].
The ratio between the concentrations found in the outdoor and indoor range was between 2 and 3.5. This result is consistent with similar data found in different museums and other indoor environments where no internal sources of NOx were present [43]. The highest ratios between outdoor and indoor data were found for periods 4 to 7, i.e., in autumn–winter, when most of the atmospheric stability processes are likely to occur and when intrusion from external air is limited by windows and doors closed during this period of the year.
The relatively high levels of air pollutants found inside the museum are associated with the evolution of pollution in the outside environment. As mentioned before, the area around the MANN museum is characterized by intense emissions, especially by vehicles, which cause high concentrations of primary NOx pollutants. These, in turn, when irradiated by sunlight, generate many photochemical pollutants, leading to high concentrations of nitrogen dioxide (as seen in Figure 5).
In outdoor air, Nitrogen Dioxide concentrations start to increase at approximately 6–7 AM, reaching a level of more than 100 µg/m3. In the late morning through the early afternoon, the concentration drops to low levels, increasing once again in the late afternoon, when it reaches values of more than 120 µg/m3. This behavior is very similar to that observed in many locations and can be easily explained by considering the time development of the boundary layer. Overnight, the layer is quite stable, and in the early morning, primary pollution increases because of intense traffic. Solar irradiation in the morning provides heating of the ground, resulting in turbulence that causes ground air to mix with air in the free troposphere. At this time, ozone concentrations increase, whereas nitrogen dioxide concentration decreases because of mixing with relatively clean air advected from the free troposphere. In the late afternoon, solar radiation decreases, and a ground-based mixed layer is again established.
In this residual layer, the reactions of radicals are very active, oxidizing nitrogen oxides into nitrogen dioxide. Overnight, these reactions are quenched, and the decrease in emission decreases the nitrogen dioxide concentration to low values. In conclusion, two peaks were experienced: one in the morning and the other in the afternoon, with a minimum in the early afternoon. In most cases, the daily maxima occur in the evening, whereas in some cases, they are recorded in the morning. Such general behavior is reproduced throughout the year, although in summer, due to the more intense solar radiation, the window for clean air advected from the free troposphere is much wider and can be as large as 7 h, instead of 3–4 h in winter.
4.2. Sulphur Dioxide (SO2)
Sulphur dioxide has long been considered an important pollutant in museums. Figure 9 shoes that the concentration levels are in the range 1–3 µg/m3 and do not show a definite trend. The average concentration at site A, similar to NO2, was less than the average of the other four sampling sites. This is a clear indication that sulphur dioxide is coming from the air outside the museum. Sulphur dioxide is fairly correlated with nitrogen dioxide (Rsquared = 0.77), demonstrating that both of them are coming from intrusion by external air.
Sulphur dioxide levels measured by the public Naples monitoring network are approaching values near the minimum detectable concentration. Therefore, no direct comparison between the two sets of data is possible.
4.3. Hydrogen Chloride (HCl)
Acids measured using the suggested technique include hydrogen chloride and nitric acid. The latter is reported in Figure 10, which shows the average values found in the data from locations B through D. Data from site A do not show a definite difference from those from other sites. For HCl, (Figure 11) the observed concentrations are about 1 µg/m3. This amount is very close to the minimum detectable concentration and is derived from chloride ions extracted from the quartz alkaline passive sampler. As is well known, contamination may play an important role in the analysis of chlorides; thus, the reported data are not of sufficient reliability for further discussion. However, the data show that HCl does not reach significantly high values; therefore, it does not imply an excessive risk.
4.4. Nitric Acid (HNO3)
Nitric acid shows relatively high concentrations of up to 6 µg/m3. This species is generated by the reaction of nitrogen dioxide with OH• radicals (according to Equation (4)):
NO2 + OH• → HNO3
Under ambient conditions characterized by intense photochemical activity, the amount of both NO2 and OH• radicals in the atmosphere are expected to be high; therefore, the formation of nitric acid is highly probable. It is worth mentioning that nitric acid in ambient air reacts immediately with ammonia, generating ammonium nitrate in particulate matter according to the reversible reaction (Equation (5)):
HNO3 + NH3 ↔ (NH4)NO3
From Figure 10, it appears that HNO3, as expected, shows the highest concentrations during sampling carried out in summer. During this period of the year, the temperature and humidity are such that ammonium nitrate (NH4NO3) may dissociate back into HNO3, NH3, and evaporate [44]. Thus, nitric acid inside the museum may be generated by particulate matter entering the museum from outside. Ammonium nitrate may migrate inside the rooms where thermodynamic conditions for dissociation may be present, i.e., low humidity and high temperature. In order to show some data about the occurrence of thermodynamic conditions leading to the formation of nitric acid from particulate nitrate, we used data from the environmental data loggers. By adapting the formulas already developed in reference [44] for the calculation of the deliquescence humidity (RHD) and the dissociation constant Kn [45] expressed in ppb2, it is possible to estimate the concentrations of nitric acid:
ln Kn = 84.6 − 24220/T − 6.1ln (T/298)
ln (RHD) = 723.7/T + 1.7037
The evaluation of RHD (Deliquescence Relative Humidity) is the premise for applying relationships/Equations (6) and (7), according to the literature [46]. If the ambient humidity is higher than the RDH, ammonium nitrate is in the deliquescent liquid phase, and thus, it is not dissociated. From the data concerning T and RH obtained using data loggers installed in the sampling rooms, Table 6 can be compiled. This shows that the calculated concentration of HNO3 in equilibrium with ammonia is approximately 2 ppb in winter, reaching 8 ppb in summer. Because the range of observed concentrations is between approximately 6 and less than 2 µg/m3, these data are consistent with the calculated [HNO3]Equation

Table 6.
Average Temperature, Relative Humidity, dissociation constant Kn and the Equilibrium concentration of HNO3 ([HNO3]eq).
Therefore, the dissociation of ammonium nitrate particulate matter may bring a relatively high amount of this acid inside the museum rooms. It is worth stressing that particulate nitrate does not need to be formed locally because it can be transported over long distances [47].
The evaluation of nitric acid merits additional comments. This species was evaluated using nitrate ions extracted from an alkaline quartz passive sampler. Simultaneously, the alkalinized carbon paper sampler intended for NO2 was also in operation; therefore, it could be expected that the nitrates found in both samplers are the same. However, the behavior of the samplers with respect to nitric acid was completely different. The average values in the quartz filter show a mean value of 2.14 against 1.94 from the carbon paper. The standard deviation of the first set was 2.14, whereas 1.11 was the value observed for the second. This means that nitrate on carbon filter does not show significant variation. For instance, there were no significant differences between the data collected in room A and those collected in other rooms.
One hypothesis could be the oxidation of collected nitrites by ozone. However, this is in contrast with experimental data that exclude the collected nitrites from being oxidized to nitrate by ozone [24] and, in addition, the amount of indoor ozone is expected to be very low. In another paper, it was shown that the adsorption of peroxy-organic nitrates on alkaline carbon surfaces also yields nitrates [48]. Because the concentration of these species is very low, at least compared with that of nitric acid, this source is to be considered not significant. Thus, the presence of nitrates on the carbon paper sampler has not yet been explained and merits further investigation.
4.5. Nitrous Acid (HONO)
Nitrous acid is a weak acid (Ka = 4.0 10−4) that, at least in principle, moderately impacts the conservation of art-works. However, its presence in the indoor environment is clear evidence of the occurrence of reaction (Equation (1)) that, with nitrous acid, also generates nitric acid, a strong and oxidant acid that has a definite impact on the conservation of exposed materials. In addition, HONO is important for human health because it may generate nitroso-amines [49]. Therefore, investigations into the presence of HONO in the MANN museum are considered of high interest. The sources of indoor HONO, ref. [50] can be essentially caused by:
- −
- The reaction (Equation (1)) occurring on the surfaces followed by HONO desorption, or
- −
- The intrusion of HONO from the external atmosphere.
The extent of reaction (1) depends on the surface, ambient temperature, and relative humidity. Additionally, the reaction is favored by the surface-to-volume ratio (S/V), which, in ambient air, depends on the height of the mixed layer. After the conversion of NO2, HNO3 is retained on the surfaces, and HONO can be desorbed into the atmosphere. During atmospheric stability conditions, where the surface-to-volume ratio of the boundary layer is high, the formation of HONO leads to noticeable concentrations in ambient air that can reach several pbbs [51].
Consequently, during the early mornings, relevant amounts of HONO may be formed, and thus, photolysis into OH• radicals may occur. If high concentrations of nitrogen oxides are present, high amounts of nitrogen dioxide may also be generated by reaction radicals. This important aspect should be properly considered in future monitoring initiatives.
Using the same reaction mechanism (Equation (1)), we can postulate the presence of HONO inside museum rooms. Since the formation of HONO depends upon the surfaces and the S/V ratio [51,52], it is first useful to look into these ratios for the rooms selected as monitoring sites. The amount of HONO, as shown in Figure 3 and Figure 4, ranges from a few through 15 µg/m3 and is only weakly correlated with NO2 and showing concentrations ranging from 10 to 25 µg/m3. The amount of HONO decreased during the last period of monitoring, exhibiting maximum values during the first three periods. This is probably due to an increase in ambient temperature causing a more efficient desorption of HONO formed on surfaces.
If we consider HONO found in room A, characterized by a higher S/V ratio, then the amount of HONO is similar to that of NO2 and sometimes higher, as shown in Figure 4. This confirms the hypothesis that the HONO found in the museum can be attributed mostly to the indoor formation through Equation (1) on the museum surfaces.
An important point to consider is the possible interference of HONO in the measurement of NO2. In principle, alkaline carbon paper can quantitatively collect nitrogen dioxide and nitrous acid. However, the active surface of the passive sampler intended for collecting acids ultimately collected HONO. In this hypothesis, the true concentration of nitrogen dioxide [NO2]0 is a value lying between [NO2]1 and [NO2]2, where the former is the value from the carbon paper filter and the second is the difference between the value [NO2]1 and the concentration of HONO derived from the quartz alkaline filter:
[NO2]1 > [NO2]0 > [NO2]2 = [NO2]1 − [HONO]
Data relevant to rooms B, C, and D show that the term [NO2]2 is constantly less than [NO2]1. However, data from room A, which is characterized by a higher [HONO]/[NO2] ratio, show that the difference is often less than zero. This means that the interference of the HONO is very low. Data of the nitrite/nitrate molar ratios in acid passive samplers are reported in Table 7.

Table 7.
The nitrite/nitrate molar ratios in acid passive samplers.
As expected, the mean molar ratio is always greater than 1, especially in room A, demonstrating that nitrites and nitrates in the acid filter are not significantly generated by the disproportion of nitrogen dioxide.
This result (nitrous acid collected on the alkaline filter and not on the carbon filter) is not fully unexpected because several acid species, such as formic acid, are not collected by the alkalinized carbon paper filter. As mentioned before, formic acid was not detected in this study because it was emitted by the plastic shield used and then collected in high amounts by the quartz alkaline filter. Both passive samplers were exposed in the same container; however, no formate anion (HCO2−) were found in the carbon paper filter. To provide some context, the equivalent formic acid concentrations found in the glass fiber filter were in the range 60–80 µg/m3, while the amount in the alkaline carbon paper was always zero.
This means that this acid is not adsorbed by the carbon filter. Activated carbon is very effective at removing formic and acetic acids [53,54]. The removal of HONO may follow the same basic mechanism. This hypothesis is now being thoroughly tested through direct laboratory and experimental tests.
5. Conclusions
The goal of a simple low-cost monitoring activity and preliminary assessment was fully achieved at the MANN museum in Naples. In five rooms, it was possible to monitor many air pollutants, most of which are crucial for the conservation of exposed art-work objects. Coupling the observations with passive samplers and environmental dataloggers, a complete picture of indoor environment in the museum can be achieved with minimum resources.
The results show that pollutants found in the museum indoor environment often exceeded the maximum recommended concentrations for protecting art-work objects. Nitrogen dioxide ranged from 10 to 25 µg/m3, i.e., from 2 to 3.5 less than the amount found outdoors, and sulphur dioxide was found in the range 1–3 µg/m3. The source of nitrogen dioxide is the intense vehicular traffic in the streets around the building hosting the museum, which releases nitrogen oxides that are converted to nitrogen dioxide.
In addition to nitrogen dioxide, high concentrations of nitrous acid were found. This species is generated on surfaces by the reaction of nitrogen dioxide with water; in fact, the highest amounts were found in rooms where the surface-to-volume ratio is the highest. We were able to demonstrate that the use of alkali impregnated carbon paper for the monitoring of nitrogen dioxide is not significantly affected by the presence of HONO. Nitrous acid is, in turn, efficiently measured with the alkali-impregnated quartz filter.
The presence of HONO is of great concern because its formation also implies the formation of nitric acid, an oxidant and corrosive species. HONO may also come directly from ambient air. In fact, the nitrogen dioxide peaks are associated with very shallow mixed layers, where the formation of HONO is highly probable. Here, in ambient air, the ratio of the surface to the volume of ambient air is small; thus, the formation is highly enhanced. Moreover, the presence of HONO in the atmosphere triggers early morning photochemical processes, increasing the amount of nitrogen dioxide in the atmosphere. The formation of HONO can be partially controlled using appropriate materials applied to the walls.
Data on gas-phase nitric acid showed maximum concentrations during summer sampling. Because the formation in the ambient atmosphere is the direct oxidation of nitrogen dioxide by hydroxyl radicals, nitric acid can be transported from ambient air to the museum either as nitric acid or as ammonium nitrate. The concentration of this species can be as high as 6 µg/m3. This corresponds to a noticeable mass flow rate for deposition on surfaces.
The sources of nitric acid inside the museum could also be due to the dissociation of particulate ammonium nitrate. Such a reaction, according to the indoor thermodynamic conditions, is highly probable to explain the excess of nitric acid in the exposition rooms. For this reason, a campaign about the content of ammonium nitrate in the gas phase is solicited. Sulphur dioxide data are consistent with the average data found outdoors. However, the concentration of this species appears to be low, compared to nitrogen dioxide.
More attention should be paid to the presence of other sulphur species (H2S, H2SO4, etc.) in the museum. The experiments carried out inside the MANN offer very clear ideas about the temporal and spatial distribution of the pollutants and experiments that are recommended to clarify the many aspects discussed in this paper.
The simplicity and low cost involved in this activity, compared with the reliability and amount of gained information, make this campaign an example that can be easily replaced in other museums, where such information is not yet available. Our preliminary assessment could be a good example of how to address the problem of indoor museum contamination by external pollution and to evaluate, even as an early approximation, the risk of exposed art-work objects in thousands of museums and sites where art-work objects are preserved, but where environmental pollution is not considered.
The full evaluation of pollutants is recommended after this preliminary assessment. Priority should be given to particulate matter that should be monitored not only in terms of number or mass concentration but also in terms of basic chemical composition, especially nitrate and ammonium. In addition, it is recommended to include ozone and hydrogen sulphide in future monitoring campaigns, which are additional important pollutants for the conservation of cultural heritage. These species are also efficiently monitored by passive samplers, maintaining the simplicity and low cost, which were the basic objectives of this project.
Finally, it is permissible to have neglected the measurements of O3, PM10 and PM2.5, VOCs, and the graphs of relative humidity/RH (which have not been reported, unlike those of temperature plot), in accordance with the premise/introduction of the work which considers the contributions of these pollutants in museums to be negligible to the damage equations cited in references [15,16,17] and reproduced in the Supplementary Information Section of this work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/air3020012/s1, File S1: The equation damages related to the gaseous pollutants and particulate matter on different Cultural Heritage surfaces [55].
Author Contributions
F.V.: Study conceptualization and design. Supervision. Funding acquisition. I.A.: Writing. Formal analysis. Data curation. Funding acquisition. I.C.: Data curation and design. Supervision. C.Z.: Formal analysis, Supervision. A.M.: Writing. Supervision. C.B.: Technical assistance. A.N.: Editing, supervision, technical assistance. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant for “Economic valorisation of patents in favour of micro, small and medium-sized enterprises”, called “Brevetti +” CUP: C85F20000440008), co-financed by the Ministry of Economic Development. The work was also supported by MANN through the “Eco-Valor” project (Eco-sustainable project for Conservation and Valorization of color traces on Marble sculptures) POC to the PON “Culture and Development” FESR 2014-2020—Delibera CIPE n°45 of 10 August 2016. Project_ CUP F69D13000410001—CIG Z153147204. MANN Energy efficiency works of the Museum. Applied research and cognitive investigation with non-invasive techniques; and Tor Vergata brevetti POC-TV.B.POC according to the proof of concepts of the Italian Minister of Economic Development, “Tor Vergata brevetti Poc—TV.B. Poc”, with CUP code C86I20000130004, admitted to financing with communication from the National Agency for Investment Attraction and Business Development S.p.A.—Invitalia prot. no. 0139954 of 09/18/2020 and subsequent financing granting act prot. no. 0175114 of 11/10/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
Author Dr. Ivo Allegrini was employed by the company Envint Srl (Monopoli di Sabina, Rieti, Italy). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- ISTAT. Statistical Report on Museums in Italy, 2020; ISTAT: Chicago, IL, USA, 2020. [Google Scholar]
- Grzywacz, C.M. Monitoring for Gaseous Pollutants in Museum Environments, 2nd ed.; Getty Publications: Los Angeles, CA, USA, 2006; Volume 1, ISBN 978-0-89236-851-8. [Google Scholar]
- Ryhl-Svendsen, M. Indoor air pollution in museums: Prediction models and control strategies. Stud. Conserv. 2006, 51, 27. [Google Scholar] [CrossRef]
- Fabbri, B. Science and Conservation for Museum Collections; Kermes Quaderni; Nardini Editore: Florence, Italy, 2012; ISBN 978-0-89236-851-8. [Google Scholar]
- Chianese, E.; Riccio, A.; Duro, I.; Trifuoggi, M.; Iovino, P.; Capasso, S.; Barone, G. Measurements for indoor air quality assessment at the Capodimonte Museum in Naples, Italy. Int. J. Environ. Res. 2012, 6, 509. [Google Scholar]
- Kraševec, I.; Markelj, J.; Elnaggar, A.; Cigić, I.K. Indoor air pollutants and their seasonal monitoring in European museums. Herit. Sci. 2024, 12, 50. [Google Scholar] [CrossRef]
- D’Alvia, L.; Palermo, E.; Rossi, S.; Delprete, Z. Validation of a low-cost wireless sensor’s node for museum environmental monitoring. Acta IMEKO 2017, 6, 45. [Google Scholar] [CrossRef]
- Orawsk, L.; Thai, P.K.; Xiaoting, L.; Akwasi, A.-S.; Godwin, A.; Alena, B.; Bedini, A.; Fahe, C.; Bryce, C.; Dunbabin, M.; et al. Applications of low-cost sensing technologies for air quality monitoring and exposure assessment: How far have they gone? Environ. Int. 2018, 116, 286. [Google Scholar] [CrossRef]
- Askari, H.S.; Hijleh, B.A. Review of museums’ indoor environment conditions studies and guidelines and their impact on the museums’ artifacts and energy consumption. Build. Environ. 2018, 143, 186. [Google Scholar] [CrossRef]
- Hisham, E.; Sura, M.; Karenm, F.; Inji, K.; Brett, M.D. The regulations and reality of indoor environmental standards for objects and visitors in museums. Renew. Sustain. Energy Rev. 2021, 152, 111653. [Google Scholar] [CrossRef]
- European Union. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe; Official Journal of the European Union: Luxembourg, 2008; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202402881 (accessed on 11 June 2008).
- Nihan, C.; Matthew, F.; Birgit, F. Use of Low-Cost Air Quality Monitoring Devices for Assessment of Road Transport Related Emissions. Transp. Res. Procedia 2019, 41, 762. [Google Scholar] [CrossRef]
- De Santis, F.; Fino, A.; Vazzana, C.; Allegrini, I. Monitoring of atmospheric pollutants by passive sampling for the protection of historic buildings and monuments. Ann. Chim. 2001, 91, 759–765. [Google Scholar] [PubMed]
- De Santis, F.; Allegrini, I.; Fazio, M.C.; Pasella, D.; Piredda, R. Development of a passive sampling technique for the determination of nitrogen dioxide and sulphur dioxide in ambient air. Anal. Chim. Acta 1997, 346, 127. [Google Scholar] [CrossRef]
- Kucera, V.; Tidblad, J.; Kreislova, K.; Knotkova, D.; Faller, M.; Reiss, D.; Snethlage, R.; Yates, T.; Henriksen, J.; Schreiner, M. UN/ECE ICP materials dose-response functions for the multi-pollutant situation. Water Air Soil Pollut. Focus 2007, 7, 249. [Google Scholar] [CrossRef]
- Thickett, D.; Chisholm, R.; Lankester, P. Development of damage functions for copper, silver and enamels on copper. In Proceedings of the Climate for Collections, Standards and Uncertainties, Munich, Germany, 7–9 November 2012; Doerner Institut: Munich, Germany; Archetype Publications: London, UK, 2013; p. 325. [Google Scholar]
- Watt, J.; Jarrett, D.; Hamilton, R. Dose-Response Functions for the Soiling of Heritage Materials Due to Air Pollution Exposure. Sci. Total Environ. 2008, 400, 415. [Google Scholar] [CrossRef]
- Guidobaldi, M.P. Nuove ricerche archeologiche nell’area vesuviana (scavi 2003–2006). In Proceedings of the Atti del Convegno Internazionale, Rome, Italy, 1–3 February 2007; ISBN 9788882654795. [Google Scholar]
- Ruffolo, S.A.; La Russa, M.F.; Rovella, N.; Ricca, M. The Impact of Air Pollution on Stone Materials. Environments 2023, 10, 119. [Google Scholar] [CrossRef]
- Biscontin, G.; Diana, S.; Fassina, V.; Marabelli, M. The influence of atmospheric pollutants on the deterioration of mural paintings in the Scrovegni Chapel in Padua. Stud. Conserv. 1980, 25, 18–21. [Google Scholar] [CrossRef]
- Hafkenscheid, T.; Fromage-Mariette, A.; Goelen, E.; Hangartner, M.; Pfeffer, U.; Plaisance, H.; de Santis, F.; Saunders, K.; Swaans, W.; Tang, Y.S.; et al. Review of the application of diffusive samplers in the European Union for the monitoring of nitrogen dioxide in ambient air. JRC Sci. Tech. Rep. 2009, JRC51106, 1–79. [Google Scholar]
- Vichi, F.; De Santis, F. Triethanolamine (TEA) as a coating for collecting NO2 by using annular diffusion denuders. Environ. Technol. 2012, 33, 1065. [Google Scholar] [CrossRef]
- Liu, C.; Liang, L.; Xu, W.; Ma, Q. A review of indoor nitrous acid (HONO) pollution: Measurement techniques, pollution characteristics, sources, and sinks. Sci. Total Environ. 2024, 921, 171100. [Google Scholar] [CrossRef]
- De Santis, F.; Dogeroglu, T.; Fino, A.; Menichelli, S.; Vazzana, C.; Allegrini, I. Laboratory development and field evaluation of a new diffusive sampler to collect nitrogen oxides in the ambient air. Anal. Bioanal. Chem. 2002, 373, 901. [Google Scholar] [CrossRef]
- Valentini, F.; Allegrini, I. The conversion of NO2 into nitrite ion (NO2−): A Raman spectroscopy evidence. Anal. Methods 2025. submitted. [Google Scholar]
- Spicer, C.W.; Billick, I.H.; Yanagisawa, Y. Nitrous acid interference with passive NO2 measurement methods and the impact on indoor NO2 data. Indoor Air 2001, 11, 156. [Google Scholar] [CrossRef]
- Vichi, F.; Maskova, L.; Frattoni, M.; Imperiali, A.; Smolik, J. Simultaneous measurement of nitrous acid, nitric acid, and nitrogen dioxide by means of a novel multipollutant diffusive sampler in libraries and archives. Herit. Sci. 2016, 4, 4. [Google Scholar] [CrossRef]
- Shaver, C.; Cass, G.R.; Druzik, J.R. Ozone and the deterioration of works of art. Environ. Sci. Technol. 1983, 17, 748. [Google Scholar] [CrossRef][Green Version]
- Salmon, L.; Cass, G.R.; Bruckman, K.; Haber, J. Ozone exposure inside museums in the historic central district of Krakow, Poland. Atmos. Environ. 2000, 34, 3823. [Google Scholar] [CrossRef]
- Sarkhosh, M.; Atafar, Z. Atmospheric pollutants: Exposure assessment and material damage. In Air Pollution, Air Quality, and Climate Change; Elsevier: Amsterdam, The Netherlands, 2025; pp. 83–102. ISBN 9780443238161. [Google Scholar] [CrossRef]
- Zhang, W.; Tong, S.; Lin, D.; Li, F.; Zhang, X.; Wang, L.; Ji, D.; Tang, G.; Liu, Z.; Hu, B.; et al. Atmospheric chemistry of nitrous acid and its effects on hydroxyl radical and ozone at the urban area of Beijing in early spring 2021. Environ. Pollut. 2023, 316, 120710. [Google Scholar] [CrossRef]
- Alicke, B.; Platt, U.; Stutz, J. Impact of nitrous acid photolysis on the total hydroxyl radical budget during the Limitation of Oxidant Production/Pianura Padana Ozone Production study in Milan. J. Geophys. Res. 2002, 107, 8196. [Google Scholar] [CrossRef]
- Indarto, A. Heterogeneous reactions of HONO formation from NO2 and HNO3: A review. Res. Chem. Intermed. 2011, 38, 1029. [Google Scholar] [CrossRef]
- Qiao, X.; Sun, M.; Wang, Y.; Zhang, D.; Zhang, R.; Zhao, B.; Zhang, J. Strong relations of peroxyacetyl nitrate (PAN) formation to alkene and nitrous acid during various episodes. Environ. Pollut. 2023, 326, 121465. [Google Scholar] [CrossRef]
- Grassian, V.H. Heterogeneous uptake and reaction of nitrogen oxides and volatile organic compounds on the surface of atmospheric particles including oxides, carbonates, soot and mineral dust: Implications for the chemical balance of the troposphere. Int. Rev. Phys. Chem. 2001, 20, 467. [Google Scholar] [CrossRef]
- Pryor, S.C.; Klemm, O. Experimentally derived estimates of nitric acid dry deposition velocity and viscous sub-layer resistance at a conifer forest. Atmos. Environ. 2004, 38, 2769. [Google Scholar] [CrossRef]
- Biorling, M.; Hjulstad, G. Air exchange rate and internal air flows in a ventilated museum building. E3S Web Conf. 2021, 246, 01003. [Google Scholar] [CrossRef]
- Shinohara, N.; Kataoka, T.; Takamine, K.; Butsugan, M.; Nishijima, H.; Gamo, M. Modified Perfluorocarbon Tracer Method for Measuring Effective Multizone Air Exchange Rates. Int. J. Environ. Res. Public Health 2010, 7, 3348. [Google Scholar] [CrossRef] [PubMed]
- Thickett, D.; David, F.; Luxford, N. Air exchange rate: The dominant parameter for preventive conservation? Conservator 2005, 29, 19. [Google Scholar] [CrossRef]
- Grøntoft, T.; Raychaudhuri, M.R. Compilation of tables of surface deposition velocities for O3, NO2 and SO2 to a range of indoor surfaces. Atmos. Environ. 2003, 38, 533. [Google Scholar] [CrossRef]
- Blades, N.; Cassar, M.; Oreszczyn, T.; Croxford, B. Preventive conservation strategies for sustainable urban pollution control in museums. Stud. Conserv. 2000, 45, 24–28. [Google Scholar] [CrossRef]
- European Union. Available online: https://www.eea.europa.eu/data-and-maps/figures/nitrogen-dioxide-annual-limit-values-for-the-protection-of-human-health (accessed on 22 August 2014).
- Grøntoft, T. The influence of photochemistry on outdoor to indoor NO2 in some European museums. Indoor Air 2022, 32, e12999. [Google Scholar] [CrossRef]
- Stelson, A.; Seinfeld, J.H. Relative humidity and temperature dependence of the ammonium nitrate dissociation constant. Atmos. Environ. 1982, 16, 983. [Google Scholar] [CrossRef]
- Stephen, H.; Stephen, T. (Eds.) Solubilities of Inorganic and Organic Compounds; Macmillan: New York, NY, USA, 1963; Volume 1, Part 1; p. 217. [Google Scholar]
- Nazaroff, W.W.; Weschler, C.J. Indoor acids and bases. Indoor Air 2020, 30, 559. [Google Scholar] [CrossRef]
- Xin, K.; Chen, J.; Soyol-Erdene, T. Formation mechanism and source apportionment of nitrate in atmospheric aerosols. APN Sci. Bull. 2023, 13, 102. [Google Scholar] [CrossRef]
- Barber, V.P.; Le Mar, L.N.; Li, Y.; Zheng, J.W.; Keutsh, F.N.; Kroll, J.H. Enhanced Organic Nitrate Formation from Peroxy Radicals in the Condensed Phase. Environ. Sci. Technol. Lett. 2024, 11, 975. [Google Scholar] [CrossRef]
- Lifang, Z.; Yuan, W.; Zhao, W.; Yang, B.; Jiao, X.; Zhou, L.; Long, S.; Xu, J.; Huang, W.; Liu, C.; et al. Formation of Nitrosamines from the Heterogeneous Reaction of Nitrous Acid and Organic Amines in Indoor Environments. Environ. Sci. Technol. 2024, 58, 18881. [Google Scholar] [CrossRef]
- Spataro, F.; Ianniello, A. Sources of atmospheric nitrous acid: State of the science, current research needs, and future prospects. J. Air Waste Manag. Assoc. 2014, 64, 1232. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Zeng, J.; Mekic, M.; Yu, Z.; Zhou, W.; Gligorovski, S. Assessing indoor gas phase oxidation capacity through real-time measurements of HONO, and NOx in Guangzhou, China. Environ. Sci. Process. Impacts 2019, 21, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Febo, A.; Perrino, C.; Allegrini, I. Measurement of nitrous acid in Milan, Italy, by DOAS and diffusion denuders. Atmos. Environ. 1996, 30, 3599. [Google Scholar] [CrossRef]
- Grøntoft, T.; Lankester, P.; Thickett, D. Reduction of acidic pollutant gases inside showcases by the use of activated carbon adsorbers. e-Preserv. Sci. 2015, 12, 28. [Google Scholar]
- Cruz, A.J.; Pires, J.; Carvalho, A.P.; De Carvalho, M.B. Comparison of adsorbent materials for acetic acid removal in showcases. J. Cult. Herit. 2008, 9, 244. [Google Scholar] [CrossRef]
- Thickett, D. Practical Use of Damage Functions for Environmental Preventive Conservation and Sustainability—Examples from Naturally Ventilated Buildings. Heritage 2023, 6, 2633–2649. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).