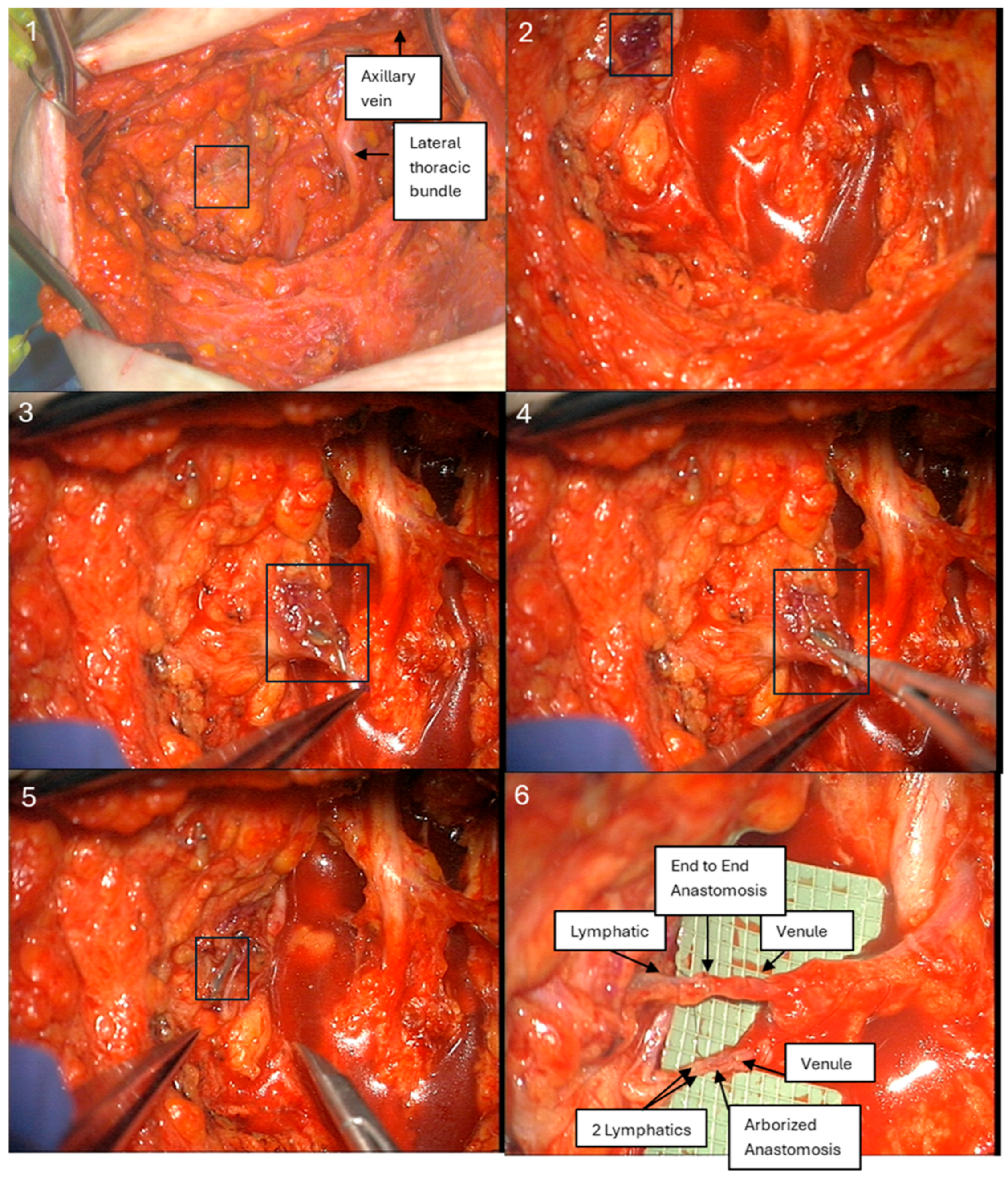
1. Introduction
Breast cancer-related lymphedema (BCRL) remains a consequential morbidity after sentinel lymph node biopsy (SLNB) and, more commonly, axillary lymph node dissection (ALND), affecting up to one-third of patients. The physical, psychosocial, and economic burdens are substantial, with pain, functional limitation, recurrent infections, and long-term care costs [
1,
2,
3]. Without a cure, prevention has become a priority. Immediate lymphatic reconstruction (ILR)—performing a lymphaticovenous bypass (LVB) in the axilla at the time of ALND—has emerged as a promising preventive strategy, with the contemporary series suggesting a reduction in BCRL incidence to approximately 10% [
1].
With the help of recent cadaveric studies by Suami et al., we now know that there are distinct lymphosomes draining distinct regions of the arm [
4,
5]. It has been theorized that disruption to the upper lymphatic drainage (identified as the primary drainage source of the extremity) is responsible for lymphedema. Axillary reverse mapping (ARM) leverages this anatomic framework to identify and, when oncologically appropriate, preserve upper-extremity lymphatics while maintaining comprehensive oncologic clearance [
6,
7].
ARM delineates upper-extremity lymphatic drainage to the axilla versus breast lymphatic drainage to mitigate BCRL of the extremity. Lymphatic visualization rates are variable, depending on the physicochemical properties of the tracer and the physics of detection. Indocyanine green (ICG) rapidly binds albumin/lipoproteins; its near-infrared (NIR) fluorescence can be detected several millimeters beneath the skin, permitting real-time visualization of both superficial and modestly deep lymphatic channels [
8,
9]. In contrast, blue dyes (isosulfan and methylene blue) are visible only under direct line-of-sight, favor superficial structures, and differ in protein binding: isosulfan blue binds strongly to tissue proteins and may be retained within the vessel wall, whereas methylene blue is smaller, binds weakly, and diffuses more readily into surrounding tissues—features that can lower apparent channel conspicuity or lead to rapid washout [
9,
10]. Technetium-99 m radiocolloids (commonly used for sentinel node mapping) provide high node detectability with a gamma probe, but offer limited spatial resolution for delineating channels, and do not furnish continuous, real-time optical guidance. Beyond tracer chemistry, injection site and depth (distal vs. proximal; intradermal vs. subdermal), lymph flow dynamics, adiposity, and overlying tissue thickness further modulate signal strength [
11,
12].
Multiple techniques have been proposed, utilizing single versus multiple dyes, as well as a combination of dye and technetium radioisotopes. However, lymphatic visualization rates remain low. These methods show variable reliability and accuracy. Reported detection rates for blue dye are 61–71% and lymphatic visualization is unreliable, with additional risks of skin staining and hypersensitivity reactions [
9,
10]. Historically performed with blue dye, ARM suffers from variable detection and unreliable vessel visualization; blue dye alone yields nodal identification rates as low as 39–90% and can cause skin staining or reactions. As successful ILR relies heavily on the precise intraoperative identification of lymphatic vessels and appropriate recipient veins, an optimal dye technique for visualization of the arm’s lymphatic channels remains unestablished [
2].
To address these gaps, we propose a simultaneous dual-tracer ARM strategy that differentially labels lymphosomes by injection territory—ICG distally (hand/forearm) and isosulfan blue (ISB) proximally (upper arm)—during ALND. The principal methodological innovation of this study is the concurrent administration of both tracers; prior investigations have generally used single-dye protocols, injecting either ICG or a blue dye in isolation. While both dyes are routinely used in their respective settings, there have been no reports of using them concurrently to differentiate lymphatic flow from the distal (hand and forearm) and proximal (upper arm) lymphosomes of the same extremity.
We evaluate whether distal and proximal lymphatic systems converge on shared axillary targets or preferentially follow distinct drainage routes, and we quantify visualization and concordance rates under dual-dye mapping. By improving intraoperative differentiation of afferent channels, this approach aims to enhance ILR planning and, ultimately, reduce the risk of BCRL.
2. Results
Seventy-one breast cancer patients undergoing ILR following axillary lymph node dissection were reviewed; however, fifty-one patients met the criteria for the dual-dye technique. Twenty were excluded, as they had become clinically node negative following neoadjuvant chemotherapy and avoided an ALND after obtaining a complete pathological response. Channels were visualized in 50/51 (98%) axillae. Among patients with visualized channels, ICG was identified in 38 (74.5%), ISB dye in 6 (11.7%), and both dyes in 6 (11.7%); one case had no visualization with either dye (
Table 1). Mean age and body mass index (BMI) did not differ between the ICG and ISB groups (age 57.3 ± 12.3 vs. 63.2 ± 16.2 years,
p = 0.447; BMI 30.8 ± 8.2 vs. 28.4 ± 8.5 kg/m
2,
p = 0.640). Comorbidities were similar across groups (hypertension 37.1% vs. 50.0%,
p = 0.878; diabetes 20.0% vs. 0%,
p = 0.266). Tumor histology (invasive ductal: 74.3% vs. 100.0%,
p = 0.562), grade, clinical T and N stage, receptor status, and receipt of chemotherapy did not differ significantly between groups. A higher proportion of patients in the ICG group underwent a modified radical mastectomy (MRM) compared to the ISB group (65.7% vs. 33.3%,
p = 0.041), while breast-conserving surgery trended higher in the ISB group (11.4% vs. 50.0%,
p = 0.061). Most patients received adjuvant radiotherapy (≥89.5%). Sentinel lymph node biopsy (SLNB) and completion ALND rates were comparable between groups (SLNB 48.6% vs. 33.3%,
p = 0.576; ALND 82.9% vs. 100.0%,
p = 0.843).
Across the cohort, 115 ARM channels were identified (mean 2.4 per axilla). By channel phenotype, 82 (71.3%) were ICG-fluorescent only, 12 (10.4%) were ISB-stained only, 17 (14.7%) demonstrated both dyes, and 4 (3.5%) were negative to both. Nearly all patients had at least one channel identified (50/51), and only one patient had no visualized channel (
Table 2).
When the cohort was stratified by whether lymphatics were visualized by a given dye, the number of nodes removed did not differ for ICG-positive vs. ICG-negative axillae (13.31 ± 8.29 vs. 15.33 ± 4.13 nodes; Mann–Whitney
p = 0.184). For ISB (blue) visualization, the comparison trended toward more nodes being removed when ISB-positive lymphatics were present (16.30 ± 6.15 vs. 12.72 ± 8.18 nodes;
p = 0.069). Visualization of both dyes was not associated with differences in the nodes removed (17.75 ± 8.96 vs. 13.15 ± 7.67 nodes;
p = 0.309). Segmental versus total mastectomy, use of neoadjuvant chemotherapy, and the distribution of SLNB/ALND within these strata are summarized in
Table 3.
Among patients with channels visualized by ICG (
n = 38), ILR most commonly used an end-to-end configuration (23/38, 60.5%), followed by arborized/intussusception (13/38, 34.2%), and end-to-side (2/38, 5.2%). In the ISB group (
n = 6), end-to-end predominated (5/6, 83.3%), with arborized in 1/6 (16.7%), and no end-to-side anastomoses. In the group with both dyes (
n = 6), arborized/intussusception was most frequent (5/6, 83.3%), with 1/6 (16.7%) end-to-end. Early postoperative lymphedema was observed in 4/38 (10.5%) in the ICG group, 1/6 (16.7%) in the ISB group, and 1/6 (16.7%) in the both-dye group (
Table 4).
In this dual-dye ARM series, ICG provided the dominant signal for arm-draining lymphatic channel identification at the time of ALND, both by patient-level visualization rates and by the proportion of channels identified. Baseline clinicopathologic features were largely balanced between dye groups, with the exception of mastectomy type. Visualization with ISB dye showed a non-significant trend toward a greater number of nodes retrieved, while combined-dye visualization did not affect the nodal yield. ILR configuration varied by dye phenotype, with a higher use of arborized anastomoses when both dyes were present, and early lymphedema rates ranged from 10 to 17% across groups (
Figure 1). These data support the feasibility of a standardized dual-dye workflow and underscore the practical value of ICG-guided mapping for efficient channel identification and ILR performance.
3. Discussion
This retrospective review of a dual-dye axillary reverse mapping (ARM) technique during axillary lymph node dissection (ALND)—using indocyanine green (ICG) and isosulfan blue (ISB)—demonstrated marked differences in lymphatic channel identification by dye. Among 51 patients undergoing immediate lymphatic reconstruction (ILR), ICG fluorescence yielded a high ARM channel detection rate (86%), whereas ISB identified channels in 25% (
Figure 2). Concurrent visualization with both tracers occurred in only 11.7% of channels. Overall, stained lymphatics were seen in 50 of 51 patients (98%); only one patient had no axillary visualization.
Given the well-recognized morbidity of ALND—particularly upper-limb lymphedema with attendant functional limitation, pain, recurrent infection, and diminished quality of life—these findings have direct clinical relevance. They refine our understanding of upper-extremity lymphatic anatomy and its implications for breast cancer-related lymphedema (BCRL) [
1,
2].
ARM effectiveness is reflected in both detection performance and safety. In this cohort, ICG was more consistently identified in the axilla than ISB. Mechanistically, ICG’s protein binding increases intralymphatic persistence and detectability with near-infrared imaging, while ISB lacks comparable fluorescence and may disperse more rapidly into surrounding tissues; blue dye also requires direct line-of-sight to a superficially stained vessel, whereas near-infrared ICG can be seen through ~5–10 mm of tissue/fat [
3,
8,
13].
In this study, stained lymphatics were visualized in 50/51 individuals (98% of the study group). Only one participant had no visualization of stained lymphatics in the axilla. We observed that ICG was more reliably identified in the axilla compared to ISB. The inherent properties of the dyes may play a role, as ICG binds specifically to lymphatic channels and resists washout, making it more persistent and detectable with near-infrared imaging systems, whereas ISB may disperse more rapidly in surrounding tissues. These results compare similarly to those in the literature. Wu et al. reported higher nodal detection with ICG than methylene blue (87.2% vs. 52.5%), and fewer dye-related issues [
9]. In a previous study conducted by Nos et al. [
12], the LAD mapping rate was 91%, with positive identification in 21 of 23 individuals, with an ARM technique combining injections of radioactive isotope and blue dye [
9]. In a larger study, Tummel et al. [
13] identified stained arm nodes and lymphatics in 153 of 213 individuals (71.8%) who underwent ARM [
1].
The principal methodological innovation of this study is the simultaneous administration of two tracers. In contrast, prior investigations employed single-dye protocols, injecting either indocyanine green (ICG) or methylene blue (MB) in isolation [
2,
8,
9,
10,
13,
14]. Interestingly, in our cohort, both dyes were identified in the axilla in only 11.7% of cases, suggesting anatomical variation, with distal and proximal extremity injections likely following different lymphatic pathways. This finding supports the concept of overlapping or converging lymphosomes within the upper extremity. However, the partial overlap also implies the presence of distinct drainage routes for different regions of the arm.
Our dissection also demonstrated that not all proximal (inner upper arm) lymphatics drained to the axilla. In some cases, the dye failed to appear in the axilla, suggesting the existence of alternative drainage routes. These may represent protective collateral pathways or lymphosomes that bypass axillary nodes, possibly to supraclavicular, deltopectoral, or internal mammary nodes [
4,
5]. These alternative routes may serve as compensatory pathways that modulate lymphedema severity.
No adverse events were observed with ICG in this series. Blue dyes used for lymphatic mapping are generally safe for subcutaneous injection, although transient skin tattooing can occur; selecting the inner upper arm as an injection site may mitigate cosmetic concerns.
Taken together, these data support a more nuanced view of upper-extremity lymphatic drainage in the setting of ALND. Both regional anatomic variability and tracer-specific properties influence mapping outcomes, reinforcing the value of individualized, fluorescence-guided strategies—particularly for patients at elevated risk of BCRL. Further prospective work delineating alternate drainage routes and standardizing dual-dye protocols may yield practical predictors of dermal backflow and inform operative decision-making and postoperative surveillance.
4. Methods
4.1. Study Design
A retrospective review of patients who underwent planned ILR following ALND for breast cancer between June 2021 and June 2023 was conducted. All patients who underwent ARM with our dual-dye technique were identified and included, while patients with incomplete records or aborted ILR due to complete pathologic response on intraoperative SLNB were excluded. Institutional review board approval was obtained (protocol number 20010040).
4.2. Dual-Dye Technique
At our center, the oncologic care plan was determined by a multidisciplinary team, including one breast surgical oncologist and a plastic surgeon (D.L.C). Patients scheduled to undergo possible or definite ALND (defined as clinical node positive disease, proven by core-needle biopsy or 3 or more positive nodes from SLNB) were referred to our lymphedema specialty clinic to discuss possible ILR. All these patients received neoadjuvant chemotherapy, and nodal involvement was re-evaluated at the time of surgery. If residual disease was found upon the frozen section pathology, patients underwent ALND and subsequently ILR.
Prior to the start of the oncologic case, patients received intradermal injections comprising 0.25 mL of 2% FITC (AK-FLUOR 10%; Akorn Inc, Lake Forrest, IL, USA) mixed with albumin in four sites of the ipsilateral hand and wrist—two injections into the dorsal hand (first and third webspaces) and two into the volar wrist at the radial and ulnar aspects of the distal volar forearm (10 cm apart), to visualize distal lymphatics for ILR. Concurrently, the patients received intradermal ISB injections (3 cc isosulfan blue) circumferentially in the medial intermuscular groove to visualize proximal lymphatics for ARM, shown in
Figure 3. Dye injection in the upper part of the arm was selected in concordance with anatomic studies, which demonstrated this location as the site where nearly all arm lymphatics aggregate, and injection in this area enables rapid migration of the dye to the axilla.
Surgery for breast cancer, either modified radical mastectomy or wide local excision of the lesion, was then performed, followed by ALND (~20 min after dye injection). The presence of blue dye in transected lymphatic channels was recorded by the breast surgeon during SLNB and ALND. Any ARM node that appeared suspicious (rounded, firm, ≥10 mm, located in areas enriched for crossover) underwent FNAC and/or excision—there was no routine preservation of suspicious nodes. Pathology results of blue-dye-stained nodes were recorded.
Following completion of ALND, transected lymphatics and adjacent axillary vein branches amenable to LVB were explored at the same incision site as the axillary dissection, using an operating microscope. ARM lymphatics were visualized as either blue lymphatics or fluorescent during exploration by detecting “hot spots” of leakage, using a near-infrared imaging system (SPY-PHI, Stryker Corporation, Kalamazoo, MI, USA) (
Figure 4). LVB was performed by the senior author (D.L.C.) using a surgical microscope (Pentero 800, Carl Zeiss AG, Oberkochen, Germany) with 10-0 nylon sutures (Ethicon, Somerville, NJ, USA) in either an end-to-end or arborized fashion. Intraoperative number, location, and diameter measurements were recorded for all visualized lymphatic vessels stained with either blue dye alone, ICG, or both.
4.3. Follow-Up
Patients were monitored in our lymphedema specialty clinic in 1–3–6–12 month intervals for a minimum of 12 months. The incidence of lymphedema was recorded by the query of subjective symptoms, conservative management use, survey of the Lymphedema Life Impact Scale, and a bioimpedance spectroscopy (defined as a change greater than 10 in BIS score during the follow-up period).
4.4. Data Analysis
Demographic data were extracted, including age at the time of surgery, gender, and race. Medical records were reviewed to determine oncologic history: type of oncologic resection, chemotherapy, radiation, number of lymph nodes removed, pathology, and incidence of lymphedema. Secondary outcomes included: dye injection site, number of lymphatic channels visualized, channel diameter (mm), time-to-first channel, coordinates relative to fixed landmarks, ILR configuration, and pathologic findings.
For each axilla, our primary outcome included the rate of visualization of lymphatic channels by each dye (ISB, ICG, or both). Secondary outcomes included: (1) channel count; (2) caliber; (3) clustered vs. solitary; (4) coordinates referenced to fixed landmarks, as demonstrated in
Figure 5 (horizontal distance from pectoralis border/axillary vein; vertical distance from intercostal brachial nerve); and (5) nearby venules suitable for anastomosis.
Descriptive statistics included comparisons of channel visualization rates to historical single-dye controls as well as proportion of lymphatic channels visualized between the two injection dyes via t-tests/Mann–Whitney U, as well as comparisons between channel identification by dye group (age, BMI, neoadjuvant therapy, nodal stage) via Freeman–Halton (MC) or Kruskal–Wallis tests.
5. Conclusions
This study demonstrates that proximal and distal upper extremity lymphatics exhibit both overlapping and distinct drainage patterns, with ICG more reliably reaching the axilla than ISB. The presence of both dyes in the axilla in some cases suggests a shared lymphatic pathway, while the absence of ISB dye in others indicates alternative drainage routes. These findings underscore the complexity of upper extremity lymphatic anatomy and support the need for individualized lymphatic mapping to better guide surgical decision-making and lymphedema prevention.
Limitations
This study is limited by its retrospective design, modest sample size, and early follow-up, which may underestimate late-onset BCRL. The dual-tracer approach also introduces potential sources of bias (e.g., injection timing, tissue dye kinetics, and partial mixing in the axilla). While our oncologic safeguards mirror those in published series, broader validation—including standardized FNAC algorithms, systematic mapping of crossover zones, and prospective adjudication of BCRL using volumetric and bioimpedance endpoints—is needed. Furthermore, although the primary outcome of this study was not to evaluate the efficacy of immediate lymphatic reconstruction, our reported lymphedema rates may be under-representative, due to the short follow-up time. As lymphedema typically presents one to two years after oncologic surgery and treatment, future studies with longer follow-up time are essential.