Abstract
Acute lymphoblastic leukemia is the most common cause of cancer-related death in children and represents a poor prognosis for patients in high-risk groups. Current treatment protocols are based on intensive polychemotherapy, which is associated with a significant toxicity profile. Due to their higher specificity and lower toxicity, immunotherapies based on monoclonal antibodies, in particular antibody–drug conjugates (ADCs), are revolutionizing cancer therapy. However, reports on the potential efficacy of ADC-targeted therapy in ALL and its subgroups are limited. Gene expression data suggest that potentially new ADC antigens are highly abundant in ALL subgroups and represent promising targets for cancer therapy. In addition, the PI3K/AKT and RAS/MAPK signaling pathways are often persistently activated in ALL and recent data showed that active feedback loops following inhibition of these pathways can lead to redundancy of cell surface receptors that can potentially serve as antigens for ADC treatment. Therefore, we provide here an overview of the most interesting receptors of the various ALL subgroups and discuss the influence that feedback loops of the PI3K/AKT and RAS/MAPK signaling pathways may have on increasing protein expression of the aforementioned receptors, which could lead to targeted combination therapy approaches in the future.
1. Introduction
- Antibody-Based Immunotherapies and Their Great but Underutilized Potential in ALL
1.1. Current Challenges in ALL Treatment
Despite improved overall prognosis, the treatment of ALL (acute lymphocytic leukemia) remains a serious clinical challenge, as 5-year and long-term survival rates decline with increasing age. While the survival rate in children (<15 years) is still comparatively high (90–95%), it drops significantly in young adults (15–39 years; approximately 70–80%) and especially in adults. Therefore, depending on the risk group, these patients only achieve a long-term survival rate of 40–70% [1,2,3,4,5].
Thereby, some subgroups are associated with a particularly poor prognosis, but each ALL subtype brings its own challenges (prognosis, risk of relapse, infestation of the central nervous system) [5]. For example, BCR-ABL1 (breakpoint cluster region/Abelson murine leukemia viral oncogene homolog 1)-positive ALL is present in 3% of pediatric B-ALL and 25% of adult B-ALL cases and is linked to poor outcome and a higher proportion of relapses. KMT2A (Histone-lysine N-methyltransferase 2A) gene rearrangements are found in 70% of infants and 15% of adults and confer a very poor prognosis. Moreover, therapy options for relapsed/refractory (r/r) T-cell-ALL (T-ALL) are limited [6]. In summary, both children and adults with a KMT2A-rearrangement, hypodiploidy, the presence of the BCR-ABL1 fusion gene, the Ph-like subtype (which has a similar genetic profile to the Ph-positive subtype without carrying the BCR-ABL1 fusion gene) and T-ALL frequently have a poor prognosis, thus requiring additional therapeutic options. However, other subtypes, such as the ETV6-RUNX1 subtype, also pose challenges, as up to 20% of patients may relapse [7]. Current ALL treatment protocols are essentially based on intensive polychemotherapy and associated with severe side effects, the occurrence of secondary tumors and r/r-disease.
1.2. Role of Antibody–Drug Conjugates
Compared to conventional chemotherapy, antibody-based therapy options (e.g., antibody–drug conjugates; ADCs) have a wider therapeutic window. ADCs combine a monoclonal antibody, a cytotoxic drug (payload) and a chemical linker to deliver chemotherapy specifically to cancer cells. In the treatment of B-ALL, standard chemotherapy leads to significantly worse results than the new immunotherapies and combinations. This has been proven by international, randomized studies [8,9]. Small-molecule inhibitors and humanized monoclonal antibodies directed against RTKs (receptor tyrosine kinases) are among the most effective targeted therapies in the clinic [10]. To date, immunotherapy approaches targeting the B cell antigen CD19 (blinatumomab), CD22 (inotuzumab ozogamicin) and CD20 (rituximab) were successfully included into treatment of B-ALL [11]. Of importance, immunotherapy may partly overcome high risk genotypes as highlighted by the imposing result of blinatumomab in KMT2A-r infants [12].
Up to now, 12 ADCs (tisotumab vedotin, loncastuximab tesirine, belantamab mafodotin, sacituzumab govitecan, trastuzumab deruxtecan, enfortumab vedotin, polatuzumab vedotin, inotuzumab ozogamicin, trastuzumab emtasine, brentuximab vedotin and gemtuzumab ozogamicin) have been approved for treatment of hematological and solid tumors, along with more than 150 novel ADCs in clinical development [13]. The CD22-directed ADC inotuzumab ozogamicin (InO) received approval for r/r B-ALL. However, not all patients benefit from InO therapy and therapy failure due to downregulation or alternative splicing of the CD22 antigen is commonly observed [14]. Reports on potential efficacy of ADC-targeted therapy in ALL and its subgroups beyond CD22-targeted therapy are limited [15]. CD19-targeted therapy is a promising approach, since CD19 is expressed on a variety of B cells and has a broader expression profile than that of other B-cell specific antigens like CD20 and CD22. However, the emergence of B-cell antigen-loss tumor escape variants (e.g., CD19) after treatment demonstrates that additional target antigens are required [16].
ADCs selectively deliver potent cytotoxic substances to tumor cells by binding to their target on the tumor cells. One of the key challenges for ADC design and clinical success is the selection of a suitable target for the specific tumor. Furthermore, the choice of the highly potent payload and the design of the linker for effective drug delivery must be carefully considered. The optimal target for ADC development should exhibit both high and uniform expression in tumor cells while precluding expression in normal and healthy cells [17]. However, ADC targets, which are currently under development, exhibit a broad expression profile in both tumor and normal cells. Some studies have already very successfully carried out the identification process of ADC targets for solid tumors [17,18,19]. The lack of validated suitable secondary target antigens for ALL is also a crucial reason for the failure of treatment of resistant cells. Therefore, the identification of a broad repertoire of diverse target antigens is essential.
1.3. Key Signaling Pathways and Feedback Loops
Here, we utilize transcriptomics, proteomics, immunohistochemistry and cell surface membrane data from publicly available datasets and databases for evidence-based filtering to identify ADC targets with improved tumor selectivity. This review aims to provide a more detailed characterization of the different genetic subtypes of ALL. To this end, we discuss subgroup-specific cell surface markers that may serve as novel targets for ADC treatment of ALL cells. In addition, we explain that PI3K/AKT and RAS/MAPK signaling pathways play an important role in the regulation of cell surface receptors and why combination therapy with PI3K/AKT (phosphoinositide 3-kinase/protein kinase B) and RAS/MAPK (rat sarcoma/mitogen-activated protein kinase) pathway inhibitors can improve patient response to ADC immunotherapy.
2. The AKT and MAPK Pathway—Important Intracellular Regulatory Nodes
2.1. The AKT Pathway
AKT (Protein kinase B) belongs to the superfamily of AGC kinases (cAMP dependent protein kinase A/G/C), which also includes protein kinase A and C (PKA and PKC). AKT was identified as a viral oncogene of the transforming retrovirus AKT8 and as a homolog of PKA and PKC [20,21]. In mammalian cells, three AKT isoforms, AKT1, AKT2 and AKT3, have been identified, which are encoded by three different genes with partially different phenotypes and functions [22,23]. AKT1-null mice show growth retardation and increased lethality [24]. AKT2 knockout mice show altered glucose metabolism and insulin-resistant diabetes [25]. In contrast, AKT3 knockout mice have reduced brain size, combined with smaller neuronal cells [26]. AKT isoform-specific knockdown leads to increased phosphorylation of the other remaining AKT isoforms [27].
The activation of the PI3K/AKT signaling pathway starts with the binding of a ligand to the RTK. After RTK activation, the PI3K recruits to the plasma membrane, interacts with the phosphotyrosine residues of the receptor via the SH2 (src homology 2) domain of its p85 subunit and undergoes a conformational change with activation of its catalytic p110 subunit. Subsequently, the p110 subunit converts PtdIns(4,5)P2 to PtdIns(3,4,5)P3. AKT can bind to PtdIns(3,4,5)P3 with its N-terminal PH (pleckstrin homology) domain and becomes phosphorylated after conformational change at threonine residue 308 by PDK (phosphoinositide-dependent protein kinase-1) and for full activation at serine residue 473 by mTORC2 (mechanistic target of rapamycin complex 2). Phosphorylation of AKT at serine residue 473 also correlates with AKT kinase activity [28]. AKT regulates numerous substrates and thereby inhibits apoptosis, increases proliferation and, through the activation of mTOR, increases translation. While AKT itself is a crucial regulator in translation, mTORC1 is often considered the most important protein mediating translation.
A recurrent point mutation in the PH domain (E17K) of AKT isoform 1 has been identified in different tumors and leads to a conformational change in the PH domain, which allows AKT to bind to the membrane even when no PtdIns(3,4,5)P3 is present there. This subsequently leads to constitutive activation of AKT. In an animal model, the AKT1 (E17K) mutation leads to leukemia development with a 60% probability [29]. Constitutive activation of AKT is observed in 87% of patients with T-ALL and in over 80% of patients with B-ALL [30,31,32]. In addition, it was shown that the overexpression of AKT2 correlates with the aggressiveness of cancer and a poor survival rate [33,34]. Capivasertib, a selective AKT inhibitor, was approved together with fulvestrant for patients with ER-positive and HER2-negative breast cancer whose tumors harbored PIK3CA (catalytic subunit alpha)/AKT1/PTEN (phosphatase and tensin homolog) alterations [35]. Recent data have shown that active feedback loops following inhibition of these pathways can lead to redundancy of cell surface receptors that can potentially serve as antigens for ADC treatment.
2.2. The MAPK Pathway
RAS belongs to the Ras superfamily, which includes over 150 small GTPases (guanosine triphosphateases) divided into five distinct subfamilies (RAS, RHO/RAC, RAN, RAB and ARF GTPases). Members of the RAS GTPases family encode different protein isoforms: HRAS, KRAS and NRAS with partially different transformation potential in different cellular contexts. In addition, KRAS4A and KRAS4B arise from alternative splicing of exon 4 of the KRAS locus [36,37]. The RAS proteins continuously cycle between active (GTP-bound) and inactive (GDP-bound) conformational states. To this end, RAS proteins interact with negative (RAS-GAP; GTPase-activating proteins) and positive (RAS-GEF; guanine nucleotide exchange factor) cellular regulators. Members of the RAS GTPase family play an important role in human malignancies by regulating cell growth, differentiation and survival through downstream signaling cascades, including the RAF/MEK/ERK (rapidly accelerated fibrosarcoma/mitogen-activated protein kinase/extracellular signal-related kinase) and PI3K/AKT pathways.
The importance of Ras signaling in tumorigenesis is underscored by the deregulation of many of its activator and effector pathways. Activation of RTKs by extracellular ligands leads to tyrosine phosphorylation of their cytoplasmic domains. These phosphorylation sites serve as a docking site for the SH2 domain-containing GRB2 (growth factor receptor-bound 2) adaptor protein. SOS (son of sevenless) can bind to GRB2 and then phosphorylate tyrosine-residues of the activated receptor. Subsequently, activated SOS promotes the exchange of GDP for GTP from RAS via its GEF activity. The active, GTP-bound RAS subsequently activates the RAF-MEK-ERK axis. ERK kinases can translocate to the nucleus and activate various transcription factors. Negative regulators of this pathway include members of the DUSP (dual specificity phosphatase), SPRY (sprouty) and SPRED (sprouty-related protein with EVH-1 domain) families, which are able to suppress this signaling. RAS family members are frequently activated by point mutations in human cancers, leading to constitutive activation of their downstream signaling pathways. Oncogenic mutations at position 12, 13 or 61 of RAS family member genes are among the most common genetic lesions in human cancers [38]. These mutations result in significant impairment of the GTPase activity of RAS proteins, locking them in a constitutively activated state, even in the absence of extracellular stimuli.
RAS mutations have been reported to make B-ALL cells more susceptible to relapse, as reflected by a higher relapse frequency. NRAS mutations, in particular, are overrepresented in children with ALL [39,40]. Overall, more than 50% of relapsed pediatric ALL patients have been shown to harbor mutations in the RAS pathway (KRAS, NRAS, NF1, EPOR) and recent studies on RAS mutations show a prevalence of 25.2% in newly diagnosed and 38.9% in relapsed children with ALL [40]. Several potent KRAS-G12C small-molecule inhibitors have been discovered, including AMG-510, MRTX849 and ARS-1620 [41,42,43]. However, NRAS-mutated cancer cells are resistant to KRAS-G12C-targeted inhibitors [42,43], highlighting the limitations and specificity of RAS isoform-targeted therapy. Furthermore, studies using MEK inhibitors (e.g., selumetinib) to treat leukemia patients with NRAS mutations do not lead to satisfactory responses due to the complex RAS downstream signaling and its compensatory effects [44].
The expression of the HRAS, NRAS and KRAS genes is nearly ubiquitous and largely conserved across species. However, there are specific differences in expression and activation status in certain tumor types and their developmental stages, often due to mutations, suggesting that the different RAS members have distinct biological characteristics with specific downstream signaling and transcriptional networks [45]. For example, NRAS mutations occur in a high percentage of acute leukemias, whereas HRAS and KRAS mutations are significantly less common [46,47,48]. Interestingly, several syndromes are associated with aberrant RAS signaling (such as Noonan or LEOPARD syndrome) [49,50]. This is frequently due to mutations in the PTPN11 gene, which encodes SHP2, a negative regulator of the RAS signaling pathway [49]. Moreover, patients with Noonan syndrome (NS) have a predisposition to leukemia and certain solid tumors [51] and would therefore also benefit from ADCs against targets upregulated by the constitutively activated RAS signaling pathway.
3. Feedback Signaling of the PI3K/AKT and RAS/MAPK Pathways Influence the Regulation of Cell Surface Markers
ALL risk groups are defined by alterations in tyrosine kinases and transcription factors, particularly those involved in lymphoid development. As a result, an aberrantly altered phosphorylation cascade or transcription leads to altered gene expression, including receptors.
Recent results show that the B-cell-specific transcription factor EBF1 (early B-cell factor1) is a key regulator of CD22 expression and thus influences the response to the CD22-specific ADC inotuzumab ozogamicin [52]. These risk subgroups often respond poorly to standard chemotherapy. While remission can usually be achieved in approximately 75% of cases after induction of chemotherapy, overall survival in all risk groups across ALL drops to 50% [53]. The identification of genetic subgroups in recent years has increased the likelihood of response to targeted therapy.
Activated cell surface receptors, for example, receptor tyrosine kinases (RTKs), recruit adaptor proteins to trigger a cascade of protein interactions between intracellular effectors, ultimately leading to altered gene expression as well as protein function and thus strongly influence critical cellular processes such as proliferation and survival. This downstream signaling includes effectors such as RAS/MAPK or PI3K/AKT pathway proteins. Genetic alterations of RTKs, such as gene rearrangements or amplifications, lead to constitutively active signaling of these pathways. Attempts to inhibit individual downstream molecules of these signaling pathways frequently result in complex feedback mechanisms that redirect cell signaling back to RTKs or result to cross talk between these pathways [10,54]. In particular, the PI3K/AKT and RAS/MAPK signaling pathways can compensate for each other in promoting cell proliferation and survival [55].
The PI3K/AKT/mTOR signaling pathway is constitutively activated in various solid cancers and the majority of hematological malignancies, including ALL [56]. Genetic alterations of RTKs (B-ALL: 4–10% and T-ALL: 2–23%) and aberrantly activated tyrosine kinases (BCR-ABL) are one of the main causes of constitutive activation of AKT. Despite the knowledge of the therapeutic significance of the AKT signaling pathway, monotherapy with inhibitors targeting various components of the PI3K/AKT/mTOR pathway was shown to be ineffective and often led to resistance [57,58]. Consequently, increased AKT activation in patients with pre-B-ALL correlates with poor response to chemotherapy and is also associated with poor overall survival [59]. For example, AKT is responsible for the development of therapy resistance in T-ALL cells to glucocorticoids [60]. Furthermore, imatinib-resistant Ph-positive ALL cells show upregulation of the PI3K/AKT/mTOR and RAS/MAPK signaling pathways. However, it was shown that inhibition of constitutively activated AKT helps to overcome chemotherapy resistance in cancer cells [61].
Activation of the PI3K/AKT signaling pathway in tumors is modulated by negative feedback, including mTORC1-mediated inhibition of upstream signaling proteins. In turn, AKT inhibition has been shown to induce the expression and activation of several receptor tyrosine kinases in a wide range of tumor types, including HER3 (human epidermal growth factor receptor 3), IGF-1R (insulin-like growth factor 1 receptor) and the insulin receptor [62,63]. The PI3K/AKT signaling pathway is a crucial regulator and orchestrates its downstream effectors FOXO (forkhead box class O), mTOR, GSK3 and S6K via phosphorylation, ultimately leading to a complex cascade of cellular events (Figure 1A). Inhibition of mTORC1 leads to inhibition of S6 kinase (S6K), a mediator of many transcriptional responses downstream of PI3K and AKT.
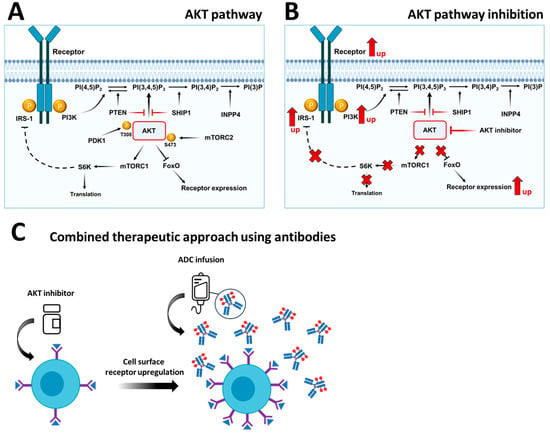
Figure 1.
Inhibitory and feedback mechanisms of the PI3K/AKT/mTOR signaling pathway. (A) AKT and mTOR are two important proteins that serve as hubs in the regulation of PI3K/AKT/mTOR signaling. mTOR regulates translation via S6 kinase and adaptor proteins such as IRS-1. AKT regulates receptor expression through transcription factors such as FOXO. (B) AKT inhibition leads to the loss of negative feedback in the pathway and thus to increased receptor expression. It also leads to the inhibition of mTOR. mTOR inhibition leads to the stabilization of IRS-1 and thus to increased receptor and PI3K activation. (C) Schematic representation of the combined therapeutic approach consisting of an AKT inhibitor that upregulates receptor expression on the cell surface and an antibody–drug conjugate that binds to the upregulated receptor and specifically transports the conjugated cytotoxin into the cell by subsequent endocytosis.
S6K-mediated negative IRS (insulin receptor substrate)-dependent feedback also increases the expression and activity of receptor tyrosine kinases and thus the PI3K/AKT signaling pathway [64]. Furthermore, AKT can directly regulate transcription factors, including the FOXO family members. Inhibition of AKT leads to a FOXO-dependent activation of receptor expression (Figure 1B). Consequently, inhibition of mTOR or AKT leads to increased AKT phosphorylation and upregulation of IRS-1 and FOXO, which subsequently leads to increased expression and activity of receptor tyrosine kinases in approximately 70% of primary AML cells [65]. Moreover, tumors in which AKT suppresses HER3 expression respond more effectively to combined inhibition of AKT and HER3 than to inhibition of either inhibitor alone [63]. In addition, HER2 (human epidermal growth factor receptor 2) inhibition (e.g., trastuzumab) abrogates PI3K/AKT signaling, thereby activating FOXO3A-mediated ERBB3 transcription. Elevated ERBB3 levels thus provide compensatory input for PI3K/AKT signaling and offer the potential to protect cancer cells from HER2-targeted therapies [66]. Inhibition of AKT or mTOR alone showed insufficient results to stop the growth of B-ALL cells [27].
The observed effects can be explained by a pronounced feedback mechanism and increased phosphorylation and activation of signaling molecules downstream of the receptor-PI3K-AKT-mTOR axis. However, the combination of mTOR and AKT inhibitors inhibits the phosphorylation of S6, as a readout signal for mTOR pathway activation, more effectively. In turn, the knockdown of AKT leads to the increase in the activity of several receptor tyrosine kinases in ALL, including ERBB2/3/4, EPHA1/2/3/4/5 (ephrin type-A receptor 1/2/3/4/5), EGFR (epidermal growth factor receptor), RET, MERTK, AXL, INSRR (insulin receptor related receptor), ROS1, TRKB (tropomyosin receptor kinase B), DDR1/2 (discoidin domain receptor 1/2), FGFR2/3 (fibroblast growth factor receptor 2/3) and VEGFR (vascular endothelial growth factor receptor) [27]. Overall, a combined therapeutic approach consisting of an AKT inhibitor that upregulates receptor expression on the cell surface and an antibody–drug conjugate that binds the upregulated receptor and specifically transports the conjugated cytotoxin into the cell represents a stringent therapy approach (Figure 1C).
The RAS/MAPK pathway represents a second important cellular signaling pathway that transduces signals from receptors into the nucleus and subsequently activates various transcription factors, including members of the FOXO family, SOX10 (SRY-related HMG-box 10), MYC or HIF1/2 (hypoxia inducible factor 1/2), that can alter receptor expression at the cell surface [67,68,69] (Figure 2A). Inhibition of MEK (e.g., by trametinib) leads to feedback activation of cell surface receptors, including the IGF1 receptor, which in turn leads to increased activation and signaling of RAS, PI3K and IRS proteins (Figure 2B) [70,71,72]. MEK or RAF inhibition has been shown to upregulate the expression of several RTKs by suppressing the transcription factor SOX10.
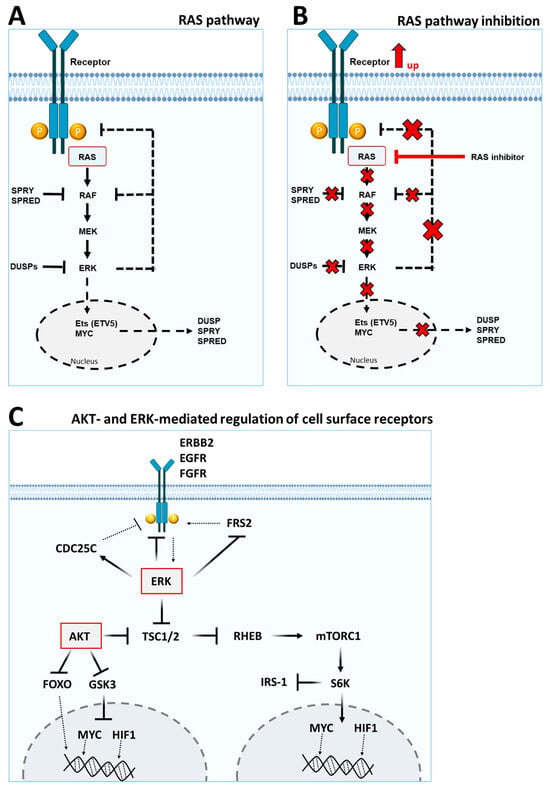
Figure 2.
Inhibitory and feedback mechanisms of the RAS/MAPK signaling pathway. (A) Receptor tyrosine kinases (RTKs) at the plasma membrane are activated by extracellular ligands. Binding of the ligand to the receptor facilitates the activation of the tyrosine kinase activity of the cytoplasmic domain of the receptor. The tyrosine phosphorylated receptor serves as docking site for the SH2-domain containing GRB2 adapter protein. SOS, in turn, can bind to GRB2 and becomes activated. Activated SOS, via its GEF activity, promotes the exchange of GDP for GTP from a member of the RAS subfamily. GTP bound and active RAS then activates RAF kinases. RAF kinases phosphorylate and activate MEK kinases. MEK kinases phosphorylate and activate ERK kinases. ERK kinases can translocate into the nucleus of the cell and can activate different transcription factors, which among others, upregulates its own negative regulators of the DUSP, SPRY and SPRED family. High ERK activity leads to feedback mechanism and in consequence to reduced expression and/or lower activity of the initiating RTK through various mechanisms. (B) RAS/MAPK pathway inhibition leads to the loss of negative feedback in the pathway and thus to increased receptor expression, which in turn can again activate the mitogen-activated protein kinase pathway. (C) AKT- and ERK-mediated regulation of cell surface receptors. AKT and ERK control cellular transcription and activation of cell surface receptors, in part, through MYC, HIF1 and FOXO. AKT and ERK phosphorylate and inhibit TSC1/2 thus activating mTORC1 by relieving inhibition of RHEB. The S6K is a downstream target of mTORC1 and can activate MYC and HIF1 or inhibit the receptor adaptor protein IRS-1. AKT can also inhibit FOXO, MYC and HIF1, partly by inhibiting GSK3. GSK3-mediated phosphorylation of MYC and HIF1 targets their proteasomal degradation. ERK can also influence the expression and activation of receptors directly by phosphorylation or by phosphorylation of mediators such as CDC25C and FRS2.
MYC, a transcriptional suppressor of ERBB2 and ERBB3, is stabilized by ERK-mediated phosphorylation. Inhibition of MEK, upstream of ERK, reduces ERK activity and leads to dephosphorylation and degradation of MYC in the nucleus. The lack of MYC suppression, in turn, leads to increased transcription of ERBB2 and ERBB3 and upregulation of their protein expression [73].
In addition, negative feedback can also be conveyed by ERK-mediated phosphorylation of inhibitory phosphorylation sites on EGFR (tyrosine 669) or ERBB2 (tyrosine 677) [74]. CDC25C (cell division cycle 25C) is a phosphatase that negatively regulates EGFR. ERK signaling activates CDC25C function, but CDC25C becomes inactive after BRAF inhibition [75].
FRS2 (fibroblast growth factor receptor substrate 2) is a membrane-anchored docking protein that links EGFR and FGFR receptors to the MAPK signaling cascade by recruiting Grb2/SOS. Upon receptor stimulation with FGF, FRS2 is phosphorylated not only at tyrosine residues but also at several threonine residues by ERK. Threonine phosphorylation of FRS2 is associated with reduced tyrosine phosphorylation of the docking protein and reduced Grb2 recruitment. Preventing threonine phosphorylation (e.g., by ERK inhibition) leads to enhanced tyrosine phosphorylation of FRS2 and increased MAPK stimulation [76].
It has been previously reported that inhibition of the RAS/MAPK signaling pathway can cause the upregulation and activation of several RTKs, including PDGFRB (platelet-derived growth factor receptor B), FGFR1, IGF1R, KDR (kinase insert domain receptor), AXL, ERBB2/3, EGFR and DDR1/2, as well as their ligands, in various tumor identities. Activation of these RTKs, in turn, is associated with increased AKT and ERK signaling [10,77,78]. The complex network of PI3K/AKT and RAS/MAPK signaling can mutually compensate for each other and promote cell proliferation and survival, in part through the same key downstream effectors, including mTOR/S6K/MYC and FOXO, as shown in Figure 2C. A common mechanism for tumors to evade RTK pathway inhibition is to activate and increase expression of alternative RTKs. For example,
EGFR inhibition upregulates PDGFRB, MET and AXL expression [79,80,81]. However, it is reported that not only RTKs are upregulated by inhibition of the PI3K/AKT and RAS/MAPK signaling pathways. There are several reports describing MYC-, FOXO-, or HIF1/2-dependent regulation of non-RTKs cell surface markers (e.g., CSPG4, GLUT1/3, NOTCH3, CD20, PTPRZ1, CD146; F3, CNR1, VIPR2) [82,83,84,85,86,87,88,89,90,91,92,93]. Thus, treatment of tumor cells with PI3K/AKT (Figure 1) or RAS/MAPK pathway (Figure 2) inhibitors may lead to upregulation of receptor tyrosine kinases or other cell surface markers proteins, improving accessibility to ADC immunotherapeutics. In the next section, we therefore discuss the identification of novel potential antibody targets.
4. Identification of ALL Subgroup-Specific Expression of Cell Surface Markers
The exponential growth of large transcriptome data opens a new dimension in molecular biology by analyzing the entire spectrum of RNA transcripts in a cell. Large transcriptome datasets from ALL samples from major studies [94,95,96] provide important information and have led to groundbreaking discoveries. To explore novel cell surface targets from publicly available transcriptome datasets of ALL patient samples, we first selected protein-coding genes using Ensembl BioMart (Ensembl release 115; EMBL-EBI, Hinxton, UK). In the next step, we selected plasma membrane genes using an annotation list consisting of datasets from “the in silico human surfaceome” database, the Human Protein Atlas (HPA; subcellular location: selected for plasma membrane and cell junctions), the Gene Ontology database (GO:0009986; cell surface) and an “immune inhibitory receptor” dataset by Sing and colleagues (Figure 3) [97,98,99,100,101,102].
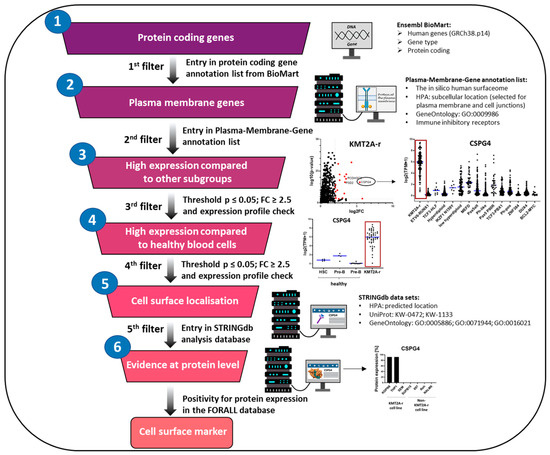
Figure 3.
Cell surface marker identification workflow. Workflow depicting approach used for identification of new targets for the treatment of ALL patients divided into different ALL subgroups. Starting from all protein-coding genes from patient material in the publicly available datasets, we first selected for genes located at the plasma membrane. In the next step, we selected these genes for each B-ALL subgroup based on significance, expression level and subgroup-specific expression profile. We then eliminated genes that exhibited high expression in hematopoietic stem cells and progenitor cells. Genes exhibiting a leukemia subgroup-specific profile were thoroughly examined for their localization on the outer cell membrane and thus their accessibility to immunotherapy. Genes that met these criteria were analyzed for their protein expression in corresponding leukemia cell databases. The three most promising candidates for each B-ALL subgroup and T-ALL cells were listed. HPA: human protein atlas; GO: gene ontology; UniProt: universal protein database; FC: fold change; STRINGdb: STRING database; FORALL: functional omics resource of acute lymphoblastic leukemia.
To increase the probability of true expression from background noise, we selected the receptors based on significance, expression level and subgroup-specific expression profile (Table S1). To minimize potential side effects of ADC targeting on healthy cells, we considered the removal of genes with high expression in hematopoietic stem cells and progenitor cells and thus prioritized cell surface targets with high expression on tumor cells.
The absence of targetable antigens on hematopoietic stem cells offers an advantage, as normal blood cells can be replenished from hematopoietic stem cells after transient ADC-induced depletion. Genes were carefully reviewed for their location on the outer cell membrane for their accessibility to immunotherapeutics using the STRING database analysis function and manual analysis of different datasets (UniProt keywords: KW-0472/membrane; UniProt keywords: KW-1133/transmembrane helix; Gene Ontology—cellular component: GO:0005886/plasma membrane; Gene Ontology—cellular component: GO:0071944/cell periphery; Gene Ontology—cellular component: GO:0016021/integral component of membrane and HPA—predicted location) [103] (Table S2) as well as prioritized based on their protein expression using immunohistochemical (IHC) data [104] (Table S3). The three most promising candidates for each B-ALL subgroup and T-ALL cells were listed (Table 1).
For each target, we then examined the literature evidence regarding its potential role in tumor biology by assigning it to the biologically important PI3K/AKT or RAS/MAPK signaling pathway and investigated whether antibodies had already been generated for each target (Table 2). For 12 of the 46 targets on our list, ADCs have already been tested preclinically or clinically, including ERBB2 and F3. This underscores the validity and potential of the depicted antigens in this review. In contrast, we introduce 34 additional targets that, to our knowledge, have not yet been used for ADC development.
However, there are some potential candidates for which an unconjugated antibody has already been generated. One potential target candidate in this list is the OSM receptor (oncostatin M), for which a fully human monoclonal antibody (vixarelimab) has already been developed. The antibody is already being used in clinical trials for the treatment of pruritus in prurigo nodularis [105]. Preclinical data show that overexpression of OSMR leads to adverse outcomes in a variety of tumor types [106,107]. In summary, these cell surface markers may have the potential to be developed into ADCs to target specific antigens for each ALL subgroup.

Table 1.
Information of new putative ADC target structures.
Table 1.
Information of new putative ADC target structures.
| Subtype | Gene 1 | Protein ID | Normal Tissue Expression | Pathway | Gene 2 | Protein ID | Normal Tissue Expression | Pathway | Gene 3 | Protein ID | Normal Tissue Expression | Pathway |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KMT2A-r | CSPG4 | CSPG-4 | Intestine | MAPK; AKT | PSD2 | PSD-2 | Brain | n.a. | PCDHGC3 | PCDH-gamma-C3 | Brain | MAPK |
| ETV6-RUNX1 | DSC3 | DSC-3 | Esophagus, Skin | MAPK; AKT | LRRC4B | NGL-3 | Brain, Cervix, Pituitary gland | MAPK; AKT | PTPRK | R-PTP-kappa | Low tissue specificity | n.a. |
| TCF3-HLF | CLSTN2 | CLSTN2 | Brain, Ovary | n.a. | LPAR1 | LPA-1 | Brain | MAPK; AKT | F3 | TF/CD142 | Low tissue specificity | MAPK; AKT |
| Hyperdiploid (HeH) | EFNB1 | Ephrin-B1 | Low tissue specificity | MAPK | IL3RA | IL-3R-alpha/CD123 | Low tissue specificity | MAPK; AKT | IL13RA1 | IL-13R-alpha/CD213a1 | Low tissue specificity | MAPK; AKT |
| IKZF1-N159Y | TRPC4 | TrpC4 | Endometrium, Seminal vesicle, Smooth muscle | MAPK; AKT | ATP1A2 | ATP1A2 | Brain, Skeletal muscle, Tongue | n.a. | CACNG4 | TARP gamma-4 | Brain | n.a. |
| Low hyperdiploid (L-HD) | PDGFRB | PDGF-R-beta/CD140B | Low tissue specificity | MAPK; AKT | SLC6A9 | GlyT-1 | Brain, Skin | n.a. | DDR2 | CD167b | Low tissue specificity | MAPK; AKT |
| MEF2D | PTPRZ1 | R-PTP-zeta | Brain | AKT | TNFRSF13B | CD267 | Intestine, Lymphoid tissue, Skeletal muscle | n.a. | DCHS2 | Cadherin-27 | Brain, Intestine | n.a. |
| PAX5alt | ERBB2 | HER2/CD340 | Low tissue specificity | MAPK; AKT | VIPR2 | VIP-R-2 | Low tissue specificity | MAPK; AKT | MS4A1 | CD20 | Lymphoid tissue | MAPK; AKT |
| Ph-like | CRLF2 | CRLF-2 | Bone marrow, Gallbladder, Lymphoid tissue | AKT | TTYH2 | hTTY2 | Brain | n.a. | CHRNA1 | CHRNA-1 | Skeletal muscle | n.a. |
| PAX5-P80R | MEGF10 | MEGF-10 | Brain, Retina | n.a. | TNFRSF13B | CD267 | Intestine, Lymphoid tissue, Skeletal muscle | n.a. | PDGFRB | PDGF-R-beta/CD140B | Low tissue specificity | MAPK; AKT |
| TCF3-PBX1 | LRRC15 | LRRC-15 | Lymphoid tissue, Skin | n.a. | ITGA8 | ITG-alpha-8 | Prostate | AKT | SLAMF1 | IPO-3/CD150 | Lymphoid tissue | AKT |
| Ph-pos | IL2RA | CD25 | Adipose tissue, Lymphoid tissue, Urinary bladder | MAPK; AKT | TSPAN15 | Tspan-15 | Low tissue specificity | MAPK | OSMR | IL31RB | Low tissue specificity | MAPK; AKT |
| ZNF384 | XKR3 | XKR-3 | Testis | n.a. | SLC2A3 | GLUT-3 | Bone marrow | MAPK; AKT | CD226 | DNAM-1/CD226 | Lymphoid tissue, Parathyroid gland | MAPK; AKT |
| DUX4 | PTPRM | R-PTP-mu | Low tissue specificity | MAPK; AKT | MCAM | MUC18/CD146 | Low tissue specificity | MAPK; AKT | CLEC12B | CLEC-12B | Bone marrow, Skin, Testis | MAPK; AKT |
| BCL2-MYC | CNR1 | CB-1 | Adipose tissue, Pituitary gland | MAPK; AKT | CD70 | CD70 | Lymphoid tissue | MAPK; AKT | BEST3 | Bestrophin-3 | Skeletal muscle, Tongue | MAPK |
| T-ALL | CD1B | CD1b | Lymphoid tissue | n.a. | NOTCH3 | Notch-3 | Low tissue specificity | MAPK; AKT | CCR9 | CCR-9/CD199 | Lymphoid tissue | MAPK; AKT |
n.a.: not available; normal tissue expression taken from HPA [97].

Table 2.
Antibody–drug conjugates for novel cell surface receptors in ALL.
Table 2.
Antibody–drug conjugates for novel cell surface receptors in ALL.
| Subtype | Gene 1 | ADC | Reference | Gene 2 | ADC | Reference | Gene 3 | ADC | Reference |
|---|---|---|---|---|---|---|---|---|---|
| KMT2A-r | CSPG4 | anti-CSPG4-(PDD); anti-CSPG4(scFv)-SNAP-AURIF | [108,109], experimental | PSD2 | No | PCDHGC3 | No | ||
| ETV6-RUNX1 | DSC3 | No | LRRC4B | No | PTPRK | No | |||
| TCF3-HLF | CLSTN2 | No | LPAR1 | No | F3 | Tisotumab vedotin | [110], approved | ||
| Hyperdiploid (HeH) | EFNB1 | No | IL3RA | BAY-943 | [111], experimental | IL13RA1 | No | ||
| IKZF1-N159Y | TRPC4 | No | ATP1A2 | No | CACNG4 | No | |||
| Low hyperdiploid (L-HD) | PDGFRB | No | SLC6A9 | No | DDR2 | No | |||
| MEF2D | PTPRZ1 | No | TNFRSF13B | anti-TNFRSF13B (Tabalumab)-MC-Vc-PAB-MMAE | CreativeBiolabs, CAT#ADC-W-1907 experimental | DCHS2 | No | ||
| PAX5alt | ERBB2 | Trastuzumab deruxtecan; Trastuzumab emtansine | [112,113], approved | VIPR2 | No | MS4A1 | MRG001 | [114], NCT05155839 (Phase 1) | |
| Ph-like | CRLF2 | No | TTYH2 | No | CHRNA1 | No | |||
| PAX5-P80R | MEGF10 | No | TNFRSF13B | anti-TNFRSF13B (Tabalumab)-MC-Vc-PAB-MMAE | CreativeBiolabs, CAT#ADC-W-1907 experimental | PDGFRB | No | ||
| TCF3-PBX1 | LRRC15 | ABBV-085 | [115], NCT02565758 (Phase 1) | ITGA8 | No | SLAMF1 | No | ||
| Ph-pos | IL2RA | Camidanlumab tesirine | [116], NCT04052997 (Phase 2) | TSPAN15 | No | OSMR | No | ||
| ZNF384 | XKR3 | No | SLC2A3 | anti-SLC3A2 ADC (19G4-MMAE); | [117], experimental | CD226 | No | ||
| DUX4 | PTPRM | No | MCAM | AMT-253 | [118], NCT06209580 (Phase 1/2) | CLEC12B | No | ||
| BCL2-MYC | CNR1 | No | CD70 | PRO1160; Vorsetuzumab mafodotin (SGN-75) | [119], NCT05721222 (Phase 1/2), NCT01015911 (Phase 1) | BEST3 | No | ||
| T-ALL | CD1B | No | NOTCH3 | PF-06650808 | [120], NCT02129205 (Phase 1) | CCR9 | No |
Trial identifier/NCT number from ClinicalTrials.gov; accessed on 27 September 2025.
5. Introduction of New ADC Target Genes
In this review, we highlight ALL subgroup-specific cell surface markers as potential candidates for immunotherapies. To identify novel ALL subgroup-specific candidates, we explored publicly available expression data and compared the gene expression of the different ALL subgroups (Table S1). The detailed functions of the different genes per subgroup, including ADC availability/development status (see also Table S4) and PI3K/AKT and RAS/MAPK pathway affiliation, are listed in the following section (see Figure 4 for overview).
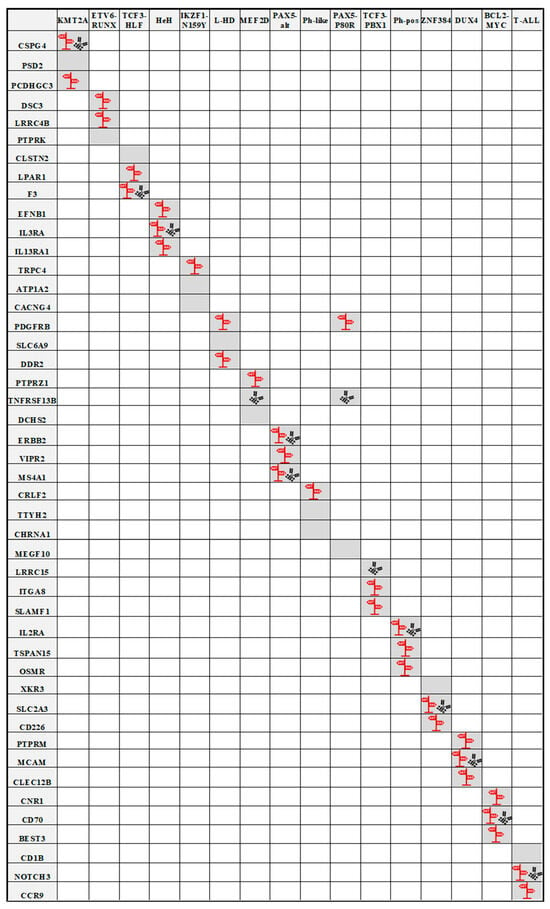
Figure 4.
Visual overview of ALL subgroup-specific cell surface markers as potential candidates for immunotherapies. The diagram shows the cell surface markers and the different ALL subgroups. The most relevant markers (according to their expression) in the respective subgroup are highlighted in gray. The antibody symbol (black) indicates all targets for which ADCs are already being used preclinically or clinically. The signpost symbol (red) indicates all targets for which there is evidence of involvement in the AKT or RAS signaling pathway.
5.1. KMT2A-r (CSPG4, PSD2, PCDHGC3)
CSPG4: Chondroitin sulfate proteoglycan 4 is a membrane-bound proteoglycan and was originally identified as a tumor antigene on the surface of melanoma cells with restricted distribution in normal tissue [121]. However, it is expressed on malignant cells, including cancer-initiating cells in various types of solid tumors. It was also shown that CSPG4 is aberrantly expressed on the leukemic blasts of a subset of AML (acute myeloid leukemia) and ALL patients, but absent from the surface of healthy hematopoietic cells [122]. In AML, CSPG4 expression has been shown to correlate with KMT2A rearrangement, but was not dependent on specific fusion partner genes of KMT2A [122]. However, CSPG4 expression was shown to correlate with the monocytic morphology status of blasts [122,123]. CSPG4-mediated activation of the FAK (focal adhesion kinase) integrin signaling pathway increases chemotherapy resistance through sustained activation of the AKT signaling pathway [124]. Furthermore, CSPG4 activates RAS/MAPK signaling via RTK-dependent and -independent mechanisms [125,126]. CSPG4 has been shown to play an important role in tumor cell growth, migration and metastasis [124,127]. An ADC targeting CSPG4 has been developed, containing a single-chain antibody fragment conjugated to auristatin F. This ADC was developed for triple-negative breast cancer [109]. Another ADC comprises an anti-CSPG4 antibody linked to pyrridinobenzodiazepine (PDD). Anti-CSPG4-PDD inhibited the growth and colony formation of CSPG4-expressing melanoma cells and induced apoptosis at low concentrations without off-target toxicity [108].
PSD2: Pleckstrin and Sec7 domain-containing 2 is a guanine nucleotide exchange factor for ADP-ribosylation factor 6 (ARF6) that regulates membrane trafficking of small G proteins [128]. The 5′ end of the PSD gene contains five CpG islands, so that its expression can be controlled by epigenetic modification. In addition, PSD mRNA correlates positively with its methylation status and in gastric cancer PSD was shown to be silenced by epigenetic modification. However, it has recently been implicated in the progression of various other cancers [129].
PCDHGC3: Protocadherin gamma-C3 is a putative calcium-dependent cell adhesion protein and member of the PCDH cluster. In cancer, clustered PCDHs are subject to epigenetic silencing through hypermethylation [130]. Hypermethylation and suppressed expression of PCDHGC3 were detected in 17 of 28 (~61%) carcinomas [131]. Interestingly, this epigenetic alteration was detected in primary tumors that developed metastases several years later. Downregulation of PCDHGC3 leads to metastasis-promoting capabilities by increasing cell proliferation, migration and invasion [132]. Knockout of PCDHGC3 leads to an increased migration rate of cerebral microvascular endothelial cells, mediated by RAS/MAPK, β-catenin/Wnt and mTOR signaling pathways [133]. Overexpression of PCDHGC3 in colon cancer cell lines resulted in reduced cell proliferation and colony-forming capacity [131]. Furthermore, PCDHGC3 is highly expressed in glioblastoma and its high expression is associated with longer progression-free survival of patients [134].
5.2. ETV6-RUNX1 (DSC3, LRRC4B, PTPRK)
DSC3: Desmocollin-3 belongs to the cadherin superfamily and is an integral component of the intercellular desmosome junctions. Methylation of DSC3 has been detected in 41% of colorectal cancer samples, which is associated with a poor prognosis. Furthermore, DSC3 has been reported to be hypomethylated in patients with ETV6-RUNX1 (ETS variant transcription factor 6/runt-related transcription factor 1) subtype compared to other B-ALL subtypes [135]. In the hyperdiploid B-ALL subtype (HeH), DSC3 was hypermethylated and downregulated compared to non-leukemic controls [136]. It has also been shown to be regulated by p53 [137]. DSC3, in turn, inhibits EGFR/MAPK and AKT signaling [138,139].
LRRC4B (NGL-3): Leucine-rich repeat protein C4 belongs to the leucine-rich repeat protein family and has been shown to be expressed in brain tissue [140]. It is a synaptic adhesion protein that regulates the formation of excitatory synapses. LRRC4 inhibits glioblastoma cell proliferation, migration, invasion and angiogenesis by downregulating the expression and response of pleiotropic cytokines. LRRC4 negatively regulates the ERK, AKT and STAT3 (signal transducer and activator of transcription 3) signaling pathways [141,142]. Furthermore, LRRC4 induces cell cycle delay in the late G1 phase by affecting the expression of various cell cycle-regulating proteins (e.g., p21 and p27). Overall, LRRC4 plays an important role in maintaining normal functions and suppressing tumorigenesis in the central nervous system [143].
PTPRK (R-PTP-kappa): Receptor-type tyrosine-protein phosphatase kappa belongs to the R2B family (PTPRT, PTPRM, PTPRK and PTPRU). The MAM domain of PTPRK contributes to the homophilic adhesion of PTPRK and PTPRM while preventing the interaction of PTPRK with PTPRM [144,145]. PTPRK is stabilized by transhomophilic interactions at cell–cell contact at the plasma membrane and regulates processes involving cell contact and adhesion, such as growth control, tumor invasion and metastasis. PTPRK acts as a negative regulator of EGFR signaling [146]. PTPRK was found methylated in 27 of 57 (47%) primary ALL samples. Methylation of the PTPRK promoter was associated with increased cell proliferation, decreased apoptosis and reduced overall survival [147]. Methylation was reversible with decitabine and histone deacetylase inhibitors [147]. Reduced PTPRK expression activates AKT, ERK1/2 and the β-catenin/Wnt signaling pathway and influences the stabilization of the E-cadherin complex at the cell membrane to promote cell–cell adhesion [147,148].
5.3. TCF-HLF (CLSTN2, LPAR1, F3)
CLSTN2: Calsyntenin-2 modulates calcium-mediated postsynaptic signaling. CLSTN2 showed strong expression in ALL cells with TCF3-HLF (transcription factor 3/hepatic leukemia factor) translocation compared to other ALL subtypes. After knockout of TCF3-HLF in HAL-01 cells, CLSTN2 was one of the significantly differentially expressed genes [149]. CLSTN2 was found hypermethylated in the hyperdiploid subtype of pediatric B-ALL cases [150].
LPAR1 (LPA-1): Lysophosphatidic acid receptor 1 is a G protein-coupled receptor for lysophosphatidic acid (LPA) binding. LPA and LPA receptors are integral components of signaling pathways involved in cell proliferation, migration, survival, vascular homeostasis, stromal remodeling, lymphocyte trafficking and immune regulation [151,152]. In accordance, LPAR1 has been shown to promote metastasis and tumor motility and abnormal LPAR1 expression has been observed in various cancers [151,153]. In B cells, LPA acts as a growth factor that promotes cell proliferation and inhibits apoptosis by triggering PLC (phospholipase C), RAS/MAPK and PI3K/AKT signaling [152,154]. In contrast, knockdown of LPAR1 reduced the expression levels of phosphorylated ERK, JNK (Jun N-terminal kinase) and AKT [155].
F3 (TF/CD142): F3/Tissue factor is a cell-associated receptor that initiates the blood coagulation cascade by forming a complex with circulating factor VII or VIIa. The [F3:VIIa] complex activates factors IX or X through specific, limited proteolysis. At the same time, F3 is also known as a mediator of intracellular signaling events that, as a cytokine-like receptor, can alter gene expression patterns and cell behavior. It regulates coagulation-independent processes such as tumor growth, angiogenesis and inflammatory regulation [156]. F3 upregulation is frequently observed on cancer cells as well as on tumor-associated endothelial cells or inflammatory cells [157,158]. In different cell types F3 can stimulate the transcription of VEGF (vascular endothelial growth factor A). Conversely, VEGF, a key factor in angiogenesis, can induce F3 expression in endothelial cells and further promote angiogenesis [159]. F3 regulates the RAS/MAPK and PI3K/AKT signaling pathways, as F3 knockdown reduced protein levels of phosphorylated ERK and AKT [160]. Tisotumab vedotin (Tivdak) is an approved antibody–drug conjugate for the treatment of adult patients with recurrent or metastatic cervical cancer whose disease progression occurs during or after chemotherapy. It consists of a fully human monoclonal antibody specific for F3 that is conjugated to monomethyl auristatin E (MMAE) [110]. Recent studies suggest that F3 is a useful surface target for the treatment of patients with triple-negative breast cancer [161].
5.4. Hyperdiploid (EFNB1, IL3RA, IL13RA)
EFNB1 (Ephrin-B1): EFNB1 represents a cell-surface transmembrane ligand for Eph receptors. Eph receptors constitute the largest known subfamily of receptor protein tyrosine kinases and are critical for migration and adhesion during neuronal, vascular and epithelial development [162]. Since both ephrin ligands and Eph receptors are membrane-bound proteins, binding and activation of intracellular Eph/ephrin signaling pathways can only occur via direct cell–cell interaction. EFNB1 has been reported to contribute to tumor progression in various cancers and high EFNB1 expression is significantly associated with a poor prognosis in human DLBCL (diffuse large B-cell lymphoma) and gliomas [163,164]. EFNB1 levels were significantly associated with the cell of origin in B cell neoplasms [163]. EFNB1 can activate mTOR, p38 MAPK and ERK signaling pathways in medulloblastoma [165].
IL3RA (CD123): The interleukin-3 receptor subunit alpha is a cytokine receptor that is overexpressed in various hematological malignancies, including AML and ALL. In detail, IL3RA is overexpressed in approximately 97% of Ph-positive and 86% of Ph-negative B-ALL cases. Within the Ph-negative cohort, IL3RA was frequently expressed in cases with Ph-like signature [166]. Furthermore, IL3RA expression in B-ALL correlated with poor overall survival and is associated with a high risk of relapse [167,168]. The high expression of IL3RA on leukemia cells and its low expression on normal hematopoietic stem cells make IL3RA an attractive target for therapy [169]. After IL-3 binding to IL3RA, IL-3R heterodimerization promotes the stable formation of the IL-3/IL-3R complex, leading to the activation of JAK (janus kinase)/STAT, RAS/MAPK and PI3K/AKT signaling [170,171,172]. IMGN632 is an IL3RA-targeting antibody–drug conjugate consisting of a humanized anti-IL3RA antibody coupled to a DNA-monoalkylating cytotoxic payload with limited toxicity in normal myeloid progenitor cells [173]. In addition, the ADC BAY-943 consists of a humanized anti-IL3RA antibody conjugated to a kinesin spindle protein inhibitor and demonstrates efficacy in preclinical models of hematological malignancies (e.g., AML) [111].
IL13RA1 (CD213a1): The interleukin-13 receptor subunit alpha-1 is a glycosylated protein that binds IL-13 with low affinity. However, in combination with IL4RA, it forms a functional IL-13 receptor for high-affinity binding of IL-13. On human B cells, IL13RA1 expression is modulated by CD40 ligands or surface immunoglobulins [174]. IL-13RA1 contains a proline-rich box-1 region that binds JAK1, JAK3 and TYK2 (tyrosine kinase 2) and in response to IL-13, receptor heterodimers induce phosphorylation of Janus tyrosine kinases [175,176]. IL-4, another cytokine that binds to the IL-13 receptor, has been shown to induce strong tyrosine phosphorylation of IRS-1/2 and increase RAS/MAPK and PI3K/AKT activity [177,178].
5.5. IKZF1-N159Y (TRPC4, ATP1A2, CACNG4)
TRPC4: The short transient receptor potential channel 4 forms a receptor-activated, non-selective, calcium-permeant cation channel and thus functions as a cell–cell contact-dependent endothelial calcium entry channel. The TRPC subfamily comprises seven members, TRPC1 to TRPC7. Heteromultimers can be formed between TRPC1, 4 and 5 on the one hand, and between TRPC3, 6 and 7 on the other [179]. After stimulation of G protein-coupled receptors or receptor tyrosine kinases, the PLC signaling pathway regulates the formation of TRPC4 and TRPC5 cation channels [180]. Knockdown of TRPC4 suppressed the activation of ERK and AKT signaling in endothelial cells [181].
ATP1A2: The sodium/potassium-transporting ATPase subunit alpha-2 belongs to the family of P-type cation-transporting ATPases and the subfamily of Na+/K+ ATPases. ATP1A2 is an integral membrane protein that catalyzes the hydrolysis of ATP and simultaneously facilitates the exchange of sodium and potassium ions across the plasma membrane. This creates the electrochemical gradient of sodium and potassium, which provides the energy for the active transport of various nutrients. Together with the small glycoprotein subunit beta, the large catalytic alpha-ATP1A2 subunit forms the enzyme. Mutations in this gene can lead to familial basilar or hemiplegic migraine, as well as to alternating hemiplegia of childhood [182].
CACNG4 (TARP gamma-4): The voltage-dependent calcium channel gamma-4 subunit regulates the activity of L-type calcium channels that contain CACNA1C as a pore-forming subunit. Downregulation of CACNG4 modulates the channels to maintain their active/open state, leading to higher intracellular calcium levels. CACNG4 thus acts by closing the channel pore and inhibiting calcium influx. Subsequently, CACNG4 alters calcium signaling and influences AKT signaling. CACNG4 expression is elevated in patients with breast cancer associated with poor prognosis [183]. CACNG4 was one of ten genes significantly overexpressed in erlotinib-resistant glioblastoma cells [184].
5.6. Low Hyperdiploid (PDGFRB, SLC6A9, DDR2)
PDGFRB (CD140B): Platelet-derived growth factor receptor beta is a tyrosine-protein kinase that acts as a cell-surface receptor for homodimeric PDGFB and PDGFD, as well as for heterodimers of PDGFA and PDGFB. PDGFRB possesses five extracellular immunoglobulin-like domains, a single membrane-spanning helix domain, an intracellular juxtamembrane domain, a tyrosine kinase domain and a carboxyl tail. Upon binding to the PDGF ligand, dimerization of the receptor releases inhibitory conformations through autophosphorylation of regulatory tyrosine residues. Activated PDGFRB regulates the PI3K/AKT and RAS/MAPK signaling pathways, including a negative feedback loop to PDGFRB, particularly in mTOR-activated cells [185,186]. PDGFRB plays an essential role in the regulation of embryonic development, cell proliferation, cell survival, cell differentiation and migration. PDGFRB gene fusions (e.g., with EBF1; early B cell factor 1) are frequently found in B-ALL cases with Ph-like subtype. PDGFRB fusion partners often contain dimerization domains to enhance the kinase activity of PDGFRB [187].
SLC6A9 (GlyT-1): The sodium- and chloride-dependent glycine transporter 1 inhibits glycine signaling by rapidly removing glycine from the synaptic cleft, as glycine acts as an inhibitory neurotransmitter in the central nervous system [188]. The SLC6A transporter has been reported to associate with the NMDA receptor [189]. To date, no specific role for SLC6A9 in hematological malignancies has been described. Therefore, further efforts should be made to further characterize this protein in the disease of ALL.
DDR2 (CD167b): Discoidin domain-containing receptors, including DDR1 and DDR2, form a unique class of receptor tyrosine kinases. They are activated by fibrillar collagens at the cell–matrix interface and are involved in the regulation of tissue remodeling and bone development [190,191]. Furthermore, DDR2 regulates extracellular matrix remodeling through upregulation of collagenases, cell differentiation, migration and proliferation. Inhibition of DDR2 (e.g., by dasatinib) has been shown to enhance tumor responses to anti-PD-1 immunotherapy [192]. Binding of collagen to DDR2 activates RAS/MAPK and PI3K/AKT signaling pathways as well as the transcription factor RUNX2 [193,194]. Furthermore, knockdown of AKT3 activates DDR kinases in bone-seeking breast cancer cells [195].
5.7. MEF2D (PTPRZ1, TNFRSF13B, DCHS2)
PTPRZ1 (R-PTP-zeta): Receptor-type tyrosine-protein phosphatase zeta belongs to the R5 receptor tyrosine phosphatase subfamily and negatively regulates the proliferation of oligodendrocyte precursor cells in the embryonic spinal cord. It is therefore required for the differentiation of precursor cells into mature, fully myelinating oligodendrocytes and PTPRZ1 expression is associated with cancer stem cell development in glioblastoma. In consequence, knockdown of PTPRZ altered the expression levels of the transcription factors SOX2 (SRY-box transcription factor 2) and POU3F2 (POU class 3 homeobox 2), which are responsible for stem cell development [196]. Previous studies showed that several members of the protein tyrosine phosphatase family, including PTPRZ1, were methylated and silenced in CLL (chronic lymphocytic leukemia) [197,198]. PTPRZ1 has been shown to negatively regulate AKT kinase activity in ovarian cells [199].
TNFRSF13B (TACI/CD267): TNFRSF13B encodes the tumor necrosis factor superfamily member 13B, a transmembrane receptor of lymphocytes that binds members of the tumor necrosis ligand family (APRIL/TNFSF13 and BAFF) and heparan sulfate chains [200]. Binding of BAFF and APRIL to the receptor activates TNFR-associated factors (TRAF 2, 5 and 6), which in turn activate NF-kB (nuclear factor kappa-light-chain-enhancer of activated B-cells), c-Jun and AP-1 (activator protein 1) [201,202]. On B cells, APRIL can bind to the receptors TNFRSF17 (BCMA) and TNFRSF13B, leading to the survival of plasma cells in the bone marrow. Moreover, CLL cells with high TNFRSF13B expression showed improved survival when cultured with BAFF and/or APRIL [203]. APRIL signaling via TNFRSF13B has also been shown to mediate immunosuppression by regulatory T cells in multiple myeloma [204]. Anti-TNFRSF13B (tabalumab)-MC-Vc-PAB-MMAE is a monoclonal anti-TNFRSF13B antibody conjugated to MMAE via an MC-Vc linker [205].
DCHS2 (CDH27/PCDH23): Protein dachsous homolog 2 is a large protein encoded in humans by the DCHS2 gene. It belongs to the calcium-dependent cell adhesion proteins and contains multiple cadherin domains. FAT4 (FAT atypical cadherin 4) in the stroma binds to DCHS1/2 to inhibit the self-renewal of nephron progenitor cells. DCHS2 and its paralog DCHS1 function partially redundantly to regulate the number of nephron progenitor cells [206]. Nothing is known about its role in leukemia, so far.
5.8. PAX5alt (ERBB2, VIPR2, MS4A1)
ERBB2 (HER2/CD340): The ErbB/HER family of receptor tyrosine kinases consists of four cell surface glycoproteins: ERBB1 (EGFR), ERBB2, ERBB3 and ERBB4 [207]. Ligands for HER2 have not yet been identified. ERBB2 is part of several cell surface receptor complexes and requires a coreceptor for ligand binding. HER2 can heterodimerize with any of the other three family members [208]. Dimerization leads to the autophosphorylation of tyrosine residues within the cytoplasmic domain of the receptors and initiates various signaling pathways, such as the RAS/MAPK and PI3K/AKT pathway. Signaling via the ERBB receptor family promotes cell proliferation and counteracts apoptosis. ERBB2 is expressed in approximately 30% of B-ALL cases and 56% of Ph (philadelphia)-positive ALL cases show elevated HER2 activity [209]. Furthermore, HER2 is one of the most highly phosphorylated tyrosine kinase receptors in AKT-knockdown cells [27]. Accordingly, ERBB2 overexpression leads to the activation of multiple signaling pathways, such as the PI3K/AKT and RAS/MAPK pathways [210,211]. Two HER2-targeting ADCs, trastuzumab emtansine (Kadcyla) and trastuzumab deruxtecan (Enhertu), are approved as second-line treatment after trastuzumab taxane-based therapy in patients with HER2-positive breast cancer [112,113].
VIPR2: Vasoactive intestinal peptide (VIP) receptor 2 is a G protein-coupled receptor linked to adenylyl cyclase via the Gαs protein. VIP stimulation leads to cAMP (cyclic adenosine monophosphate) production, activation of protein kinase A and ERK signaling [212]. VIPR2 also interacts with the Gαi and Gαq proteins to regulate PI3K/AKT and PKC signaling and VIPR2 signaling via the RAS/MAPK and PI3K/AKT pathways, regulates cyclin D1 levels to control cell proliferation and migration [213,214].
MS4A1 (CD20): The B lymphocyte antigen MS4A1 is a B lymphocyte-specific membrane protein involved in the regulation of cellular calcium influx, which is necessary for the development, differentiation and activation of B lymphocytes. It represents a storage-gated calcium channel component that promotes calcium influx after activation by the B cell receptor. MS4A1 is induced by CXCR4/SDF1 (C-X-C chemokine receptor type 4/stromal cell-derived factor 1) chemokine signaling. It consists of four hydrophobic transmembrane domains [215]. MS4A1 is expressed by the majority of B cells, starting with late pre-B lymphocytes, and is lost in terminally differentiated plasmablasts and plasma cells [216]. Thus, anti-CD20 monoclonal antibodies have revolutionized the treatment of lymphomas [217]. MS4A1 upregulation has been frequently observed in high-risk patients, patients with high minimal residual disease (MRD) at the end of the induction phase and in relapsed patients [218]. Immunotherapy with rituximab inhibits the constitutively activated survival signaling pathways ERK1/2, p38 MAPK, NF-kappaB and AKT [219]. MRG001 is an anti-MS4A1 antibody–drug conjugate for patients with r/r advanced non-Hodgkin lymphoma. It consists of a chimeric monoclonal anti-MS4A1 antibody conjugated to monomethyl auristatin E (MMAE) via a valine-citrulline linker. In preclinical studies, the ADC showed significant inhibition of tumor growth in rituximab-resistant non-Hodgkin lymphoma models [114].
5.9. Ph-like (CRLF2, TTYH2, CHRNA1)
CRLF2: Cytokine receptor-like factor 2 belongs to the type I cytokine receptor family and forms a functional complex with TSLP (thymic stromal lymphopoietin) and IL7R (interleukin 7 receptor), which can stimulate cell proliferation by activating the JAK/STAT signaling pathway. Thus, CRLF2 (cytokine receptor-like factor 2) is involved in the development of the hematopoietic system. In the Ph-like B-ALL subtype, CRLF2 was overexpressed in 75% of cases [220]. Patients with high-risk ALL with CRLF2 overexpression show high relapse rates and poor overall survival. Furthermore, chromosomal breaks and fusion of CRLF2 with IGH@ (immunoglobulin heavy locus) or the G protein-coupled purinergic receptor gene P2Y8 (P2Y receptor family member 8) have been demonstrated [221]. The fusion to both genes place CRLF2 under alternative transcriptional control, leading to CRLF2 overexpression [221]. Furthermore, CRLF2 alterations are strongly associated with JAK mutations [222]. In addition, increased activation of the AKT/mTOR/S6 axis can be observed in a large number of primary CRLF2-altered ALL samples [223].
TTYH2 (hTTY2): Tweety family member 2 is a cell surface protein with five transmembrane regions and represents a Ca2+-activated chloride channel. TTYH2 gene expression is significantly upregulated in colon carcinomas and renal cell carcinomas [224]. The TTYH2 gene is thought to regulate both the proliferative and metastatic potential of colon carcinomas [225]. Furthermore, TTYH2 is among the genes whose gene expression profile was elevated in high-risk ALL cases and associated with poor outcome [226].
CHRNA1: Upon binding to acetylcholine, the acetylcholine receptor subunit alpha undergoes a comprehensive conformational change affecting all subunits and leading to the opening of an ion-conducting channel across the plasma membrane. In melanoma, CHRNA1 is highly expressed in metastatic samples compared to their primary tumor samples. CHRNA1 is strongly associated with CHRNB1 and CHRNG and together these genes are negatively involved in the survival of melanoma patients [227].
5.10. PAX5-P80R (MEGF10, PDGFRB, TNFRSF13B)
MEGF10: Multiple epidermal growth factor-like domain protein 10 is a membrane receptor involved in the phagocytosis of apoptotic cells by macrophages and astrocytes. MEGF10 recognizes C1q, an “eat-me” signal, on the surface of apoptotic cells [228]. MEGF10 interacts with ABCA1 (ATP-binding cassette transporter A1) in the process of phagocytosis. Moreover, MEGF10 belongs to a group of hypermethylated genes that were epigenetically repressed and showed significantly reduced expression in neuroblastoma cells. Low MEGF10 expression also correlates with reduced relapse-free survival. Knockdown of MEGF10 expression in neuroblastoma cells led to increased cell growth [229].
Information on PDGFRB and TNFRSF13B can be found in Section 5.6 or Section 5.7, respectively.
5.11. TCF-PBX1 (LRRC15, ITGA8, SLAMF1)
LRRC15: Leucine-rich repeat-containing protein 15 is involved in cell–cell and cell–matrix interactions and adheres to extracellular matrix components such as fibronectin and collagen [230]. LRRC15 exhibits high expression levels in solid tumors compared to normal tissue. High LRRC15 expression correlates with metastasis, poor response to chemotherapy and shortened overall survival in patients with osteosarcoma [231]. Furthermore, LRRC15 has been reported to be regulated by TGFB (transforming growth factor beta) [232]. ABBV-085 is a humanized anti-LRRC15 IgG1 antibody conjugated to the antimitotic MMAE payload via a protease-cleavable valine-citrulline linker [233]. In preclinical experiments it demonstrated efficacy in sarcomas and other advanced solid tumors [115].
ITGA8: The integrin family consists of cell adhesion receptors that are widely expressed in various cancer types. Integrins mediate tumor cell proliferation, migration and invasion [234]. Integrin alpha-8 interacts with FAK to activate the downstream PI3K/AKT/mTOR signaling pathway [235]. Recently, ITGA8 was observed to be highly expressed in multiple myeloma patients with early relapse [236].
SLAMF1 (IPO-3/CD150): The signaling lymphocytic activation molecule 1 belongs to the signaling lymphocytic activation molecule family. Like other receptors in this family, SLAMF1 is expressed in various hematopoietic cells and plays a role in the regulation and differentiation of immune cells. SLAM receptors are self-ligand receptors that are activated by homo- or heterotypic cell–cell interactions. SLAMF1 signaling leads to the activation of RAS/MAPK and PI3K/AKT signaling, thus influencing cytotoxicity, humoral immune responses, lymphocyte development, cell survival, cell growth and cell adhesion [237,238,239]. SLAMF receptors negatively regulate B cell receptor (BCR) signaling in CLL. Consequently, SLAMF1 was shown to be downregulated on CLL cells compared to their healthy counterparts [240].
5.12. Ph-pos (IL2RA, TSPAN15, OSMR)
IL2RA (CD25): The interleukin-2 receptor alpha subunit is one of three chains of the IL2 receptor and is involved in the regulation of immune tolerance by controlling the activity of regulatory T cells (TREGs). Accordingly, it is primarily expressed on T and NK cells and only the binding of IL-2 to the dimeric and trimeric IL-2Rs leads to downstream signaling via three main pathways: PI3K/AKT, JAK/STAT5 and RAS/MAPK [241,242]. However, IL2RA has also been found to be overexpressed in AML cells and has been associated with chemotherapy resistance and poor outcome [243]. ALL cells, particularly Ph-positive ALL cells, have been shown to aberrantly express IL2RA. While normal B cells have low levels of IL2RA, primary ALL cells and ALL cell lines highly overexpress IL2RA. High IL2RA expression levels were associated with poor clinical outcomes for patients with B-ALL, in contrast to favorable outcomes for lymphoma patients [244]. On B cells, IL2RA formed an inhibitory complex involving PKCδ as well as SHIP1 and SHP1 phosphatases for feedback control of oncogenic BCR signaling or its oncogenic mimics [245]. Silencing of IL2RA in primary ALL cells induces cell cycle arrest and apoptosis [246]. Furthermore, in Ph-positive ALL cells, aberrant DNA methylation patterns and gene expression profiles were shown to be centered around a cytokine network defined by high hypomethylation and strong overexpression of IL2RA [244]. Camidanlumab tesirine (ADCT-301) is an ADC consisting of a human anti-CD25 antibody conjugated to SG3199 via a cathepsin-cleavable valine-alanine linker and is used to treat AML and ALL cells [116].
TSPAN15: The tetraspanin family comprises 33 family members that interact with integrins, adhesion molecules and T cell receptors, forming dimers or heterodimers. Tetraspanin-15 regulates the maturation and transport of the transmembrane metalloprotease ADAM10 (ADAM metallopeptidase domain 10). Tspan15 is a marker for poor prognosis and is upregulated in certain cancers [247,248]. In a mouse model TSPAN15 promotes cancer progression [248]. Overexpression of TSPAN15 activates the PI3K/AKT and EGFR/MAPK signaling pathways, promoting tumor cell proliferation in hepatocellular carcinoma [247,249].
OSMR (IL31RB): The oncostatin M receptor belongs to the family of type I cytokine receptors and forms the IL31 receptor with IL31RA. Upon binding of IL31, the IL31 receptor complex activates STAT proteins. Oncostatin M (OSM) is an inflammatory cytokine of the interleukin-6 family produced by osteoblasts, bone marrow macrophages and neutrophils. OSM acts via a heterodimeric receptor, consisting of CD130 and the OSM receptor and induces the proliferation of hematopoietic stem and progenitor cells [250]. Thus, OSMR signaling can regulate tumor growth and metastasis and provide an advantage to cancer cells [107]. OSMR, as a member of the cytokine receptor family, is capable of activating signaling pathways such as JAK/STAT, RAS/MAPK and PI3K/AKT upon OSM ligand binding [106,251,252]. OSMR has been shown to be overexpressed in various cancer types and is associated with poor outcomes [106,107].
5.13. ZNF384 (XKR3, SLC2A3, CD226)
XKR3: XK-related protein 3 belongs to the XK family. XKR3 is a membrane transporter in the XK/Kell complex of the Kell blood group system. A NUP214-XKR3 fusion has been detected in a case of T-ALL [253]. But overall, not much is known about the functions of XKR33 in cancer.
SLC2A3 (GLUT3): Solute carrier family 2 member 3 represents a glucose transporter that mediates the uptake of various monosaccharides across the cell membrane. SLC2A3 is highly expressed in colorectal cancer (CRC) and negatively associated with disease progression in CRC patients. Mechanistically, low glucose status led to dramatic upregulation of SLC2A3 via the AMPK signaling pathway. Under glucose-limiting conditions, SLC2A3 accelerated CRC cell growth by upregulating glucose supply and promoting nucleotide synthesis. SLC2A3 has the highest affinity for glucose among all GLUT (glucose transporter) transporters and demonstrated a greater impact on cell growth than GLUT1 under glucose-limiting stress [254]. Following SLC2A3 silencing, the RAS/MAPK and PI3K/AKT signaling pathways were inactivated [255]. 19G4-MMAE is a SLC3A2-targeting antibody–drug conjugate that exhibits potent antitumor activity in head and neck squamous cell carcinoma with a favorable safety profile [117].
CD226 (DNAM-1): CD226 belongs to the immunoglobulin superfamily and functions as an adhesion molecule. It is involved in intercellular adhesion, lymphocyte signaling, cytotoxicity and lymphokine secretion mediated by cytotoxic T lymphocytes and NK cells. Upon binding to the NECTIN2 ligand, CD226 stimulates the activation and proliferation of CD8 T cells [256]. Another ligand of human CD226 is CD155, which is expressed at high levels in solid cancers and hematological malignancies [257]. CD226 influences intracellular metabolism and enhances the PI3K/AKT and RAS/MAPK signaling pathways [258].
5.14. DUX4 (PTPRM, MCAM, CLEC12B)
PTPRM (R-PTP-mu): Receptor-type tyrosine-protein phosphatase mu belongs to the R2B family (PTPRT, PTPRM, PTPRK and PTPRU). PTPRM is involved in cell–cell adhesion through homophilic interactions. PTPRM was found methylated in 36 of 57 (64%) primary ALL samples [147]. PTPRM expression positively correlates with the survival of breast cancer patients with BRCA mutation [144]. Knockdown of PTPRM in breast cancer cells also led to increased cell migration and invasion through regulation of the RAS/MAPK signaling pathway [145,259]. Furthermore, overexpression of PTPRM in cervical cancer has been shown to affect the SRC-AKT axis [144].
MCAM (MUC18/CD146): The melanoma cell adhesion molecule is a cell surface glycoprotein that plays a role in cell adhesion and the cohesion of the endothelial monolayer at intercellular junctions in vascular tissue. MCAM enables melanoma cells to interact with cellular elements of the vasculature, thus promoting hematogenous tumor spread. However, it has been found to be highly expressed in many tumors at every stage of cancer development and progression, particularly during metastasis. It serves as a receptor for various ligands, including growth factors and extracellular matrices such as galectins and laminins. As a coreceptor of VEGFR2 and PDGFRB, CD146 activates PI3K/AKT, RAS/MAPK and PLC to enhance pro-angiogenesis signaling, which promotes cell growth, proliferation, differentiation and survival [260,261]. The ADC AMT-253 consists of a humanized IgG1 antibody directed against MCAM conjugated to exatecan, a topoisomerase I inhibitor. AMT-253 was developed for the treatment of MCAM-positive solid tumors and was evaluated in a Phase I clinical trial [118].
CLEC12B: CLEC12B represents the C-Type lectin domain family 12 member B. It is an inhibitory cell surface receptor that protects target cells against lysis by natural killer cells. Thus, high expression of CLEC12B represents an important selective advantage for tumor cells. The extracellular domain of CLEC12B shows homology to the activating natural killer cell receptor NKG2D (natural killer group 2D). However, CLEC12B contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its intracellular domain and can counteract NKG2D-mediated signals [262]. CLEC12B negatively regulates PI3K/AKT and RAS/MAPK signaling by recruiting SHP1/2 phosphatases to its ITIM.
5.15. BCL2-MYC (CNR1, CD70, BEST3)
CNR1 (CB-1): Cannabinoid receptor 1 belongs to the group of G protein-coupled receptors and is part of the endocannabinoid system. Cannabinoid receptors 1 and 2 are overexpressed in various malignancies and cannabinoids have an important influence on the viability and growth of tumor cells. In particular, overexpression of CNR1 and 2 (cannabinoid receptor 1/2) has been observed in CLL compared to healthy B cells [263]. CNR1 promotes embryonic development via PI3K/AKT and RAS/MAPK signaling pathways [264].
CD70: CD70 is a type 2 transmembrane glycoprotein and belongs to the tumor necrosis factor (TNF) ligand family. CD70 is expressed on activated antigen-presenting cells, including B cells, and binds to CD27 on T lymphocytes to induce T cell activation and proliferation. Binding of CD27 to CD70 activates PI3K/AKT and RAS/MAPK signaling pathways in lymphocytes [265]. CD70 has been shown to be a promising target in AML due to its expression on AML blasts and leukemia stem cells and its lack of expression on normal hematopoietic stem cells [266]. Increased CD70 expression was observed in relapsed acute myeloid leukemia cells after treatment with hypomethylating agents [267]. Furthermore, high CD70 surface expression has been observed on ALL cells compared to healthy controls [268]. PRO1160 is a human monoclonal antibody specific for CD70 and conjugated to exatecan via a protease-cleavable linker. This ADC is highly effective in cell-based xenograft models of renal cell carcinoma, non-Hodgkin lymphoma and nasopharyngeal carcinoma [119].
BEST3 (Bestrophin-3): Bestrophin-3 belongs to the bestrophin family and forms calcium-sensitive chloride channels. Bestrophins are transmembrane proteins and have a homology region with a high content of aromatic residues. BEST3 is predominantly expressed in the brain and gastrointestinal tract [97,269]. ERK-dependent upregulation of bestrophin-3 in renal epithelial cells has been shown to be part of an adaptive response to counteract endoplasmic reticulum stress-induced cell death [270].
5.16. T-ALL (CD1B, NOTCH3, CCR9)
CD1B: The T cell surface glycoprotein CD1B is an antigen-presenting protein that binds self and exogenous lipid and glycolipid antigens and presents them to T cell receptors on natural killer T cells [271]. Accordingly, CD1 is expressed on the surface of numerous different human antigen-presenting cells. Human CD1 molecules are divided into group 1 (CD1A and CD1C) and group 2 (CD1D). CD1B belongs to group 1 of the CD1 family of transmembrane glycoproteins. CD1B-autoreactive T cells recognize phospholipid antigens and contribute to antitumor immunity against CD1B-positive T cell lymphoma [272].
NOTCH3: The neurogenic locus notch homolog protein 3 acts as a receptor for the membrane-bound ligands Jagged1, Jagged2 and Delta1 on juxtaposing cells to regulate cell fate determination following conformational changes. Upon ligand binding, protease-mediated cleavage of the NOTCH receptor by the gamma-secretase complex and ADAM metalloproteases leads to release of the intracellular Notch domain (NICD) that translocates to the nucleus. NICD forms a transcriptional activator complex with RBPJ/RBPSUH and activates genes for differentiation, proliferation and apoptosis [273,274]. Furthermore, NOTCH3 has been shown to contribute to the growth of T-cell leukemia by regulating the unfolded protein response [275]. Activating NOTCH3 mutations were recently identified in T-ALL and are detectable even in the absence of activated NOTCH1 [276]. NOTCH3 activates downstream RAS/MAPK and AKT/mTOR signaling [277,278]. PF-06650808 is an anti-NOTCH3 antibody–drug conjugate that delivers an auristatin-based cytotoxic payload to target cells. In a phase I trial for treatment of patients with breast cancer and other advanced solid tumors, PF-06650808 demonstrated a manageable safety profile and antitumor activity [120].
CCR9 (CD199): The C-C chemokine receptor type 9 is a 7-pass transmembrane G-coupled receptor for the ligand CCL25 [279]. CCR9 is expressed in more than 70% of T-ALL cases, including more than 85% of cases of r/r disease and only on a small proportion (<5%) of normal T cells [280]. In cancer cells, CCR9/CCL25 upregulates AKT and ERK1/2 levels to exert antiapoptotic effects and chemoresistance [281,282,283]. CCL25/CCR9, as a member of CC chemokines and their receptors, is known to be involved in chemotactic cells, lymphocyte development, survival, proliferation and migration [284].
6. Discussion
The biological importance of an antibody target is underscored by its overexpression in cancer cells, its restricted expression in healthy and normal tissues and its key role in disease development. In particular, increased expression of the target on various other blood cell types can limit ADC accumulation at target tumor sites [285]. The results in this review are intended to guide the development of novel ADCs against surface targets, which, based on these data, appear to be of utmost importance for each individual ALL subgroup.
Of the novel targets described here, 12 ADC targets have already been evaluated in clinical trials (e.g., ERBB2 and F3) or are in preclinical testing, including NOTCH3 and IL3RA antibodies, demonstrating the validity of this approach. Nevertheless, further investigations are necessary to evaluate the internalization potential of these novel targets. For this purpose, a high-affinity monoclonal antibody must be developed, if none already exists. Furthermore, the choice of the highly potent payload (e.g., microtubule inhibitors, DNA-damaging agents or topoisomerase inhibitors), the drug-to-antibody ratio (DAR), and the design of the linker for effective drug delivery (cleavable or non-cleavable) must be carefully considered. In particular, the intracellular transport of an ADC plays an important role in drug discovery. Acid-soluble linkers are released in the early endosomes, while proteolytic linkers rely on the presence of proteases (e.g., cathepsin B) in the late endosomes or lysosomes [286].
However, resistance to various chemotherapeutic agents has been shown to be mediated by mutation or altered expression of the drug target topoisomerase, overexpression of drug transporter (ATP-binding cassette transporter such as MDR1 or MRP1), altered apoptotic mechanisms or signaling pathways as well as defects in ADC trafficking pathways (e.g., clathrin- or caveolin-dependent endocytosis) [287]. In particular, ATP-binding cassette (ABC) transporters can export the common payloads used in ADCs (MMAE, MMAF or calicheamicin) from the cell and mediate resistance.
ALL subgroups such as KMT2A-r, TCF3-HLF and hypodiploid karyotypes define rare subgroups with highly drug-resistant disease [288]. Heterogeneity within the tumor and the adaptive mechanisms that help cells survive under changing physiological conditions complicate the effective treatment of cancer with targeted therapies. Often, drug tolerance in these cells is driven by overexpression and increased receptor activity of redundant receptors.
However, protein degradation and methylation can influence transcriptomic results and can lead to reduced protein expression of these targets. One subgroup known for extensive methylation is the KMT2A-r subgroup. In these cases, a combinatorial therapy approach including DNA methyltransferase inhibitors (e.g., decitabine) along with ADCs may improve treatment. For example, it has already been shown that the expression of the established melanoma antigen CSPG4 can be induced in ovarian cancer cells by the hypomethylating activity of the FDA-approved drug decitabine. Treatment of CSPG4-negative SKOV-3 ovarian cancer cells with decitabine resulted in upregulation of CSPG4 in the majority of decitabine-treated SKOV-3 cells [289].
Of the genes discussed in this work, 12/46 (~26%) genes are known to be aberrantly methylated (CSPG4, PSD2, PCDHGC3, DSC3, PTPRK, CLSTN2, ERBB2, PTPRZ1, MEGF10, IL2RA, PTPRM and CD70), 33/46 (~72%) genes play a role in at least one pathway of the PI3K/AKT and RAS/MAPK signaling pathways (see Table 1), 3/46 (~7%) genes are RTKs (DDR2, PDGFRB and ERBB2), 43/46 (~93%) genes are non-RTK cell surface markers and 13/46 (~28%) genes are regulated by at least one member of the FOXO, HIF or MYC family (CSPG4, CD1B, CD70, NOTCH3, CD20, CD226, PTPRZ1, CNR1, GLUT3, CD146, F3, DSC3 and VIPR2). For 12 of the 46 targets on our list, ADCs have already been tested preclinically or clinically (Table 2: CSPG4, F3/TF, IL3RA, TNFRSF13B, ERBB2/HER2, MS4A1/CD20, LRRC15, IL2RA/CD25, SLC2A3, MCAM, CD70 and NOTCH3). In addition, 13 of the 46 cell surface proteins are clinically relevant as they are already being investigated in clinical trials for the treatment of cancer (Table S4: CSPG4, PDGFRB, PTPRZ1, ERBB2/HER2, CRLF2, LRRC15, IL2RA/CD25, IL3RA, CD70, NOTCH3, F3/TF, DDR2 and MS4A1/CD20) [ClinicalTrials.gov; accessed on 27 September 2025]. In consequence, we are introducing 30 new targets that, to our knowledge, have not previously been used for ADC development or investigated in clinical trials for cancer treatment.
The identification of new cell surface markers that could serve as specific targets for targeted immunotherapy represents a further step in the development of new therapeutics for the treatment of leukemia. Testing the antileukemic efficacy of novel or approved ADCs in primary ALL samples to anticipate direct effects of antibody-based immunotherapy in a preclinically relevant manner can be performed by ex vivo drug response profiling (DRP). To examine if treatment with ADCs is effective in ALL patient samples or in cell lines, samples can be measured in high throughput in a 384-well format, as illustrated in Figure 5. For DRP, primary human leukemic cells can be co-cultured on hTERT (human telomerase catalytic subunit gene)-immortalized bone marrow stroma cells under serum-free conditions. After 72 h of incubation with the ADC, cellular viability can be measured by automated microscopy-based image analysis (live-cell imaging). The use of the antibody–drug conjugate inotuzumab ozogamicin for DRP screening of patient samples has been shown before [290], but DRP data for other promising ADCs for ALL treatment is missing so far. However, the development and establishment of an ADC involves several steps beyond design, internalization and high-throughput DRP. Nonspecific cytotoxicity must also be evaluated, for example, by additionally saturating the antigen binding site with an uncoupled antibody prior to ADC application. The ADC should then be tested in vivo for antitumor activity, distribution, half-life and stability in the organism as well as for toxicity.
Figure 5.
Setup of the drug response profiling platform. Drug profiling can be performed on ALL cells (patient material or cell lines) in co-culture with human bone marrow–derived stroma cells. Viability (based on intact DNA content) can be analyzed after 72 h of treatment with ADC compounds by quantifying viable ALL cells and furthermore to generate dose–response curves (IC50). This platform enables the identification of drug-response phenotypes in individual ALL patients, providing an additional layer of information to facilitate individual treatment approaches.
The expression of target antigens is often modulated according to the mutational profile of the tumor cells [291]. RAS mutations have a major impact on key cellular signaling pathways, which in turn can affect the protein expression of important surface markers. For example, it has already been shown that BRAF mutations lead to altered expression levels of NECTIN4, a target molecule used to treat thyroid cancer with the approved antibody enfortumab vedotin [19].
In this study, we suggest clinically validated and novel immunotherapy targets against malignant cells of the different ALL subgroups. In addition, we describe a new strategy to overcome resistance of non-responsive ALL cells by combining RAS/MAPK or PI3K/AKT pathway inhibitors with ALL subgroup-specific ADCs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/lymphatics3040033/s1, Table S1: Gene expression of novel cell surface structures in ALL subgroups; Table S2: Annotation of genes depending on their location according to occurrence in specified datasets; Table S3: Protein expression of novel cell surface structures in ALL subgroups; Table S4: Targets in clinical trials.
Author Contributions
Conceptualization, P.A.H.E.; Methodology, P.A.H.E.; Software, P.A.H.E.; Formal Analysis, P.A.H.E.; Investigation, P.A.H.E.; Data curation, P.A.H.E.; Writing—Original Draft Preparation, P.A.H.E.; Writing—Review and Editing, P.A.H.E., C.R.; Visualization, P.A.H.E.; Supervision, P.A.H.E.; Project Administration, P.A.H.E. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
RAS/RAF/MEK/ERK, rat sarcoma/rapidly accelerated fibrosarcoma/mitogen-activated protein kinase/extracellular signal-related kinase; GEF, guanine nucleotide exchange factor; PI3-kinase, phosphoinositide 3-kinase/PI3K; AKT, protein kinase B/PKB; mTOR, mechanistic target of rapamycin; PTEN, phosphatase and tensin homolog; ADC, Antibody–drug Conjugate; DRP, Drug Response Profiling; ALL, Acute lymphoblastic leukemia; RTK, Receptor Tyrosine Kinase.
References
- Pui, C.H.; Yang, J.J.; Bhakta, N.; Rodriguez-Galindo, C. Global efforts toward the cure of childhood acute lymphoblastic leukaemia. Lancet Child Adolesc. Health 2018, 2, 440–454, Erratum in Lancet Child Adolesc. Health 2018, 2, e25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berkman, A.M.; Andersen, C.R.; Cuglievan, B.; McCall, D.C.; Lupo, P.J.; Parsons, S.K.; DiNardo, C.D.; Short, N.J.; Jain, N.; Kadia, T.M.; et al. Long-Term Outcomes among Adolescent and Young Adult Survivors of Acute Leukemia: A Surveillance, Epidemiology, and End Results Analysis. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1176–1184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rheingold, S.R.; Bhojwani, D.; Ji, L.; Xu, X.; Devidas, M.; Kairalla, J.A.; Shago, M.; Heerema, N.A.; Carroll, A.J.; Breidenbach, H.; et al. Determinants of survival after first relapse of acute lymphoblastic leukemia: A Children’s Oncology Group study. Leukemia 2024, 38, 2382–2394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luskin, M.R. Acute lymphoblastic leukemia in older adults: Curtain call for conventional chemotherapy? Hematol. Am. Soc. Hematol. Educ. Program 2021, 2021, 7–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kantarjian, H.; Jabbour, E. Adult Acute Lymphoblastic Leukemia: 2025 Update on Diagnosis, Therapy, and Monitoring. Am. J. Hematol. 2025, 100, 1205–1231. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, H.H. Targeted treatment of T-cell acute lymphoblastic leukemia: Latest updates from the 2022 ASH Annual Meeting. Exp. Hematol. Oncol. 2023, 12, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuster, L.; Grausenburger, R.; Fuka, G.; Kaindl, U.; Krapf, G.; Inthal, A.; Mann, G.; Kauer, M.; Rainer, J.; Kofler, R.; et al. ETV6/RUNX1-positive relapses evolve from an ancestral clone and frequently acquire deletions of genes implicated in glucocorticoid signaling. Blood 2011, 117, 2658–2667. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Martinelli, G.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.; Wang, K.; Wang, T.; et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2016, 375, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Tomuleasa, C.; Tigu, A.B.; Munteanu, R.; Moldovan, C.S.; Kegyes, D.; Onaciu, A.; Gulei, D.; Ghiaur, G.; Einsele, H.; Croce, C.M. Therapeutic advances of targeting receptor tyrosine kinases in cancer. Signal Transduct. Target. Ther. 2024, 9, 201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gore, L.; Locatelli, F.; Zugmaier, G.; Handgretinger, R.; O’bRien, M.M.; Bader, P.; Bhojwani, D.; Schlegel, P.-G.; Tuglus, C.A.; von Stackelberg, A. Survival after blinatumomab treatment in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Blood Cancer J. 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van der Sluis, I.M.; de Lorenzo, P.; Kotecha, R.S.; Attarbaschi, A.; Escherich, G.; Nysom, K.; Stary, J.; Ferster, A.; Brethon, B.; Locatelli, F.; et al. Blinatumomab Added to Chemotherapy in Infant Lymphoblastic Leukemia. N. Engl. J. Med. 2023, 388, 1572–1581. [Google Scholar] [CrossRef]
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Gillespie, E.; Naqvi, A.S.; Hayer, K.E.; Ang, Z.; Torres-Diz, M.; Quesnel-Vallières, M.; Hottman, D.A.; Bagashev, A.; Chukinas, J.; et al. Modulation of CD22 Protein Expression in Childhood Leukemia by Pervasive Splicing Aberrations: Implications for CD22-Directed Immunotherapies. Blood Cancer Discov. 2022, 3, 103–115. [Google Scholar] [CrossRef]
- Talleur, A.C.; Pui, C.H.; Karol, S.E. What is Next in Pediatric B-cell Precursor Acute Lymphoblastic Leukemia. Lymphatics 2023, 1, 34–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Y.; Zhao, F. Strategies to overcome tumour relapse caused by antigen escape after CAR T therapy. Mol. Cancer 2025, 24, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bosi, C.; Bartha, Á.; Galbardi, B.; Notini, G.; Naldini, M.M.; Licata, L.; Viale, G.; Mariani, M.; Pistilli, B.; Ali, H.R.; et al. Pan-cancer analysis of antibody-drug conjugate targets and putative predictors of treatment response. Eur. J. Cancer 2023, 195, 113379. [Google Scholar] [CrossRef] [PubMed]
- Razzaghdoust, A.; Rahmatizadeh, S.; Mofid, B.; Muhammadnejad, S.; Parvin, M.; Torbati, P.M.; Basiri, A. Data-Driven Discovery of Molecular Targets for Antibody-Drug Conjugates in Cancer Treatment. BioMed. Res. Int. 2021, 2021, 2670573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kathad, U.; Biyani, N.; Peru YColón De Portugal, R.L.; Zhou, J.; Kochat, H.; Bhatia, K. Expanding the repertoire of Antibody Drug Conjugate (ADC) targets with improved tumor selectivity and range of potent payloads through in-silico analysis. PLoS ONE 2024, 19, e0308604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, J.; Weiskirchen, R. What Does the “AKT” Stand for in the Name “AKT Kinase”? Some Historical Comments. Front. Oncol. 2020, 10, 1329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hassan, D.; Menges, C.W.; Testa, J.R.; Bellacosa, A. AKT kinases as therapeutic targets. J. Exp. Clin. Cancer Res. 2024, 43, 313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corre, E.; Soum, C.; Pfeifer, R.; Bessière, C.; Dailhau, S.; Marbœuf, C.; Meggetto, F.; Touriol, C.; Récher, C.; Bousquet, M.; et al. Differential prognostic values of the three AKT isoforms in acute myeloid leukemia. Sci. Rep. 2024, 14, 7070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geißert, R.; Lammert, A.; Wirth, S.; Hönig, R.; Lohfink, D.; Unger, M.; Pek, D.; Schlüter, K.; Scheftschik, T.; Smit, D.J.; et al. K-Ras(V12) differentially affects the three Akt isoforms in lung and pancreatic carcinoma cells and upregulates E-cadherin and NCAM via Akt3. Cell Commun. Signal. 2024, 22, 85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, H.; Thorvaldsen, J.L.; Chu, Q.; Feng, F.; Birnbaum, M.J. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 2001, 276, 38349–38352. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Mu, J.; Kim, J.K.; Thorvaldsen, J.L.; Chu, Q.; Crenshaw EB3rd Kaestner, K.H.; Bartolomei, M.S.; Shulman, G.I.; Birnbaum, M.J. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 2001, 292, 1728–1731. [Google Scholar] [CrossRef]
- Tschopp, O.; Yang, Z.Z.; Brodbeck, D.; Dummler, B.A.; Hemmings-Mieszczak, M.; Watanabe, T.; Michaelis, T.; Frahm, J.; Hemmings, B.A. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 2005, 132, 2943–2954. [Google Scholar] [CrossRef]
- Ehm, P.; Grottke, A.; Bettin, B.; Jücker, M. Investigation of the function of the PI3-Kinase / AKT signaling pathway for leukemogenesis and therapy of acute childhood lymphoblastic leukemia (ALL). Cell Signal. 2022, 93, 110301. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, K.; Oeck, S.; Malewicz, N.M.; Jendrossek, V. New Insights into Protein Kinase B/Akt Signaling: Role of Localized Akt Activation and Compartment-Specific Target Proteins for the Cellular Radiation Response. Cancers 2018, 10, 78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carpten, J.D.; Faber, A.L.; Horn, C.; Donoho, G.P.; Briggs, S.L.; Robbins, C.M.; Hostetter, G.; Boguslawski, S.; Moses, T.Y.; Savage, S.; et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 2007, 448, 439–444. [Google Scholar] [CrossRef]
- Silva, A.; Yunes, J.A.; Cardoso, B.A.; Martins, L.R.; Jotta, P.Y.; Abecasis, M.; Nowill, A.E.; Leslie, N.R.; Cardoso, A.A.; Barata, J.T. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J. Clin. Investig. 2008, 118, 3762–3774. [Google Scholar] [CrossRef]
- Gomes, A.M.; Soares, M.V.; Ribeiro, P.; Caldas, J.; Póvoa, V.; Martins, L.R.; Melão, A.; Serra-Caetano, A.; de Sousa, A.B.; Lacerda, J.F.; et al. Adult B-cell acute lymphoblastic leukemia cells display decreased PTEN activity and constitutive hyperactivation of PI3K/Akt pathway despite high PTEN protein levels. Haematologica 2014, 99, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Ehm, P.A.H.; Horn, S.; Hoffer, K.; Kriegs, M.; Horn, M.; Giehler, S.; Nalaskowski, M.; Rehbach, C.; Horstmann, M.A.; Jücker, M. Ikaros sets the threshold for negative B-cell selection by regulation of the signaling strength of the AKT pathway. Cell Commun. Signal. 2024, 22, 360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riggio, M.; Perrone, M.C.; Polo, M.L.; Rodriguez, M.J.; May, M.; Abba, M.; Lanari, C.; Novaro, V. AKT1 and AKT2 isoforms play distinct roles during breast cancer progression through the regulation of specific downstream proteins. Sci. Rep. 2017, 7, 44244. [Google Scholar] [CrossRef]
- Wang, L.; Ji, X.B.; Wang, L.H.; Qiu, J.G.; Zhou, F.M.; Liu, W.J.; Wan, D.D.; Lin, M.C.; Liu, L.Z.; Zhang, J.Y.; et al. Regulation of MicroRNA-497-Targeting AKT2 Influences Tumor Growth and Chemoresistance to Cisplatin in Lung Cancer. Front. Cell Dev. Biol. 2020, 8, 840, Erratum in Front. Cell Dev. Biol. 2022, 10, 1008088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dilawari, A.; Buturla, J.; Osgood, C.; Gao, X.; Chen, W.; Ricks, T.K.; Schaefer, T.; Avasarala, S.; Reyes Turcu, F.; Pathak, A.; et al. US Food and Drug Administration Approval Summary: Capivasertib With Fulvestrant for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Locally Advanced or Metastatic Breast Cancer With PIK3CA/AKT1/PTEN Alterations. J. Clin. Oncol. 2024, 42, 4103–4113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whitley, M.J.; Tran, T.H.; Rigby, M.; Yi, M.; Dharmaiah, S.; Waybright, T.J.; Ramakrishnan, N.; Perkins, S.; Taylor, T.; Messing, S.; et al. Comparative analysis of KRAS4a and KRAS4b splice variants reveals distinctive structural and functional properties. Sci. Adv. 2024, 10, eadj4137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nuevo-Tapioles, C.; Philips, M.R. The role of KRAS splice variants in cancer biology. Front. Cell Dev. Biol. 2022, 10, 1033348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holderfield, M.; Lee, B.J.; Jiang, J.; Tomlinson, A.; Seamon, K.J.; Mira, A.; Patrucco, E.; Goodhart, G.; Dilly, J.; Gindin, Y.; et al. Concurrent inhibition of oncogenic and wild-type RAS-GTP for cancer therapy. Nature 2024, 629, 919–926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irving, J.; Matheson, E.; Minto, L.; Blair, H.; Case, M.; Halsey, C.; Swidenbank, I.; Ponthan, F.; Kirschner-Schwabe, R.; Groeneveld-Krentz, S.; et al. Ras pathway mutations are prevalent in relapsed childhood acute lymphoblastic leukemia and confer sensitivity to MEK inhibition. Blood 2014, 124, 3420–3430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qian, J.; Li, Z.; Pei, K.; Li, Z.; Li, C.; Yan, M.; Qian, M.; Song, Y.; Zhang, H.; He, Y. Effects of NRAS Mutations on Leukemogenesis and Targeting of Children With Acute Lymphoblastic Leukemia. Front. Cell Dev. Biol. 2022, 10, 712484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janes, M.R.; Zhang, J.; Li, L.S.; Hansen, R.; Peters, U.; Guo, X.; Chen, Y.; Babbar, A.; Firdaus, S.J.; Darjania, L.; et al. Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor. Cell 2018, 172, 578–589.e17. [Google Scholar] [CrossRef] [PubMed]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Hallin, J.; Engstrom, L.D.; Hargis, L.; Calinisan, A.; Aranda, R.; Briere, D.M.; Sudhakar, N.; Bowcut, V.; Baer, B.R.; Ballard, J.A.; et al. The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020, 10, 54–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, N.; Curran, E.; Iyengar, N.M.; Diaz-Flores, E.; Kunnavakkam, R.; Popplewell, L.; Kirschbaum, M.H.; Karrison, T.; Erba, H.P.; Green, M.; et al. Phase II study of the oral MEK inhibitor selumetinib in advanced acute myelogenous leukemia: A University of Chicago phase II consortium trial. Clin. Cancer Res. 2014, 20, 490–498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mozzarelli, A.M.; Simanshu, D.K.; Castel, P. Functional and structural insights into RAS effector proteins. Mol. Cell 2024, 84, 2807–2821, Erratum in Mol. Cell 2024, 84, 3163–3164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, R.; Shuldiner, E.G.; Kelly, M.; Murray, C.W.; Hebert, J.D.; Andrejka, L.; Tsai, M.K.; Hughes, N.W.; Parker, M.I.; Cai, H.; et al. Multiplexed screens identify RAS paralogues HRAS and NRAS as suppressors of KRAS-driven lung cancer growth. Nat. Cell Biol. 2023, 25, 159–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alawieh, D.; Cysique-Foinlan, L.; Willekens, C.; Renneville, A. RAS mutations in myeloid malignancies: Revisiting old questions with novel insights and therapeutic perspectives. Blood Cancer J. 2024, 14, 72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Wu, Z.; Li, T.; Li, Y.; Wang, W.; Hao, Q.; Xie, X.; Wan, D.; Jiang, Z.; Wang, C.; et al. Mutational spectrum and prognosis in NRAS-mutated acute myeloid leukemia. Sci. Rep. 2020, 10, 12152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gelb, B.D.; Tartaglia, M. Noonan syndrome and related disorders: Dysregulated RAS-mitogen activated protein kinase signal transduction. Hum. Mol. Genet. 2006, 15 (Suppl. S2), R220–R226. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Niihori, T.; Banjo, T.; Okamoto, N.; Mizuno, S.; Kurosawa, K.; Ogata, T.; Takada, F.; Yano, M.; Ando, T.; et al. Gain-of-function mutations in RIT1 cause Noonan syndrome, a RAS/MAPK pathway syndrome. Am. J. Hum. Genet. 2013, 93, 173–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jongmans, M.C.; van der Burgt, I.; Hoogerbrugge, P.M.; Noordam, K.; Yntema, H.G.; Nillesen, W.M.; Kuiper, R.P.; Ligtenberg, M.J.; van Kessel, A.G.; van Krieken, J.H.; et al. Cancer risk in patients with Noonan syndrome carrying a PTPN11 mutation. Eur. J. Hum. Genet. 2011, 19, 870–874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escherich, C.S.; Li, Z.; Barnett, K.R.; Li, Y.; Walker, M.; Yoshimura, S.; Yang, W.; Huang, X.; Yu, J.; Stock, W.; et al. Differentiation-dependent EBF1 activity determines CD22 transcription and leukemia sensitivity to inotuzumab ozogamicin. Blood 2025, 146, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Gökbuget, N.; Kneba, M.; Raff, T.; Trautmann, H.; Bartram, C.-R.; Arnold, R.; Fietkau, R.; Freund, M.; Ganser, A.; Ludwig, W.-D.; et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood 2012, 120, 1868–1876. [Google Scholar] [CrossRef]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell-cell communication: New insights and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tóthová, Z.; Šemeláková, M.; Solárová, Z.; Tomc, J.; Debeljak, N.; Solár, P. The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization. Int. J. Mol. Sci. 2021, 22, 7682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef]
- van der Ploeg, P.; Uittenboogaard, A.; Thijs, A.M.J.; Westgeest, H.M.; Boere, I.A.; Lambrechts, S.; van de Stolpe, A.; Bekkers, R.L.M.; Piek, J.M.J. The effectiveness of monotherapy with PI3K/AKT/mTOR pathway inhibitors in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2021, 163, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Browne, I.M.; Okines, A.F.C. Resistance to Targeted Inhibitors of the PI3K/AKT/mTOR Pathway in Advanced Oestrogen-Receptor-Positive Breast Cancer. Cancers 2024, 16, 2259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lill, C.B.; Fitter, S.; Zannettino, A.C.W.; Vandyke, K.; Noll, J.E. Molecular and cellular mechanisms of chemoresistance in paediatric pre-B cell acute lymphoblastic leukaemia. Cancer Metastasis Rev. 2024, 43, 1385–1399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pourhassan, H.; Murphy, L.; Aldoss, I. Glucocorticoid Therapy in Acute Lymphoblastic Leukemia: Navigating Short-Term and Long-Term Effects and Optimal Regimen Selection. Curr. Hematol. Malig. Rep. 2024, 19, 175–185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jabbarzadeh Kaboli, P.; Salimian, F.; Aghapour, S.; Xiang, S.; Zhao, Q.; Li, M.; Wu, X.; Du, F.; Zhao, Y.; Shen, J.; et al. Akt-targeted therapy as a promising strategy to overcome drug resistance in breast cancer—A comprehensive review from chemotherapy to immunotherapy. Pharmacol. Res. 2020, 156, 104806. [Google Scholar] [CrossRef] [PubMed]
- Tanno, S.; Tanno, S.; Mitsuuchi, Y.; Altomare, D.A.; Xiao, G.H.; Testa, J.R. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001, 61, 589–593. [Google Scholar]
- Chandarlapaty, S.; Sawai, A.; Scaltriti, M.; Rodrik-Outmezguine, V.; Grbovic-Huezo, O.; Serra, V.; Majumder, P.K.; Baselga, J.; Rosen, N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 2011, 19, 58–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamburini, J.; Chapuis, N.; Bardet, V.; Park, S.; Sujobert, P.; Willems, L.; Ifrah, N.; Dreyfus, F.; Mayeux, P.; Lacombe, C.; et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: Rationale for therapeutic inhibition of both pathways. Blood 2008, 111, 379–382. [Google Scholar] [CrossRef]
- Bertacchini, J.; Heidari, N.; Mediani, L.; Capitani, S.; Shahjahani, M.; Ahmadzadeh, A.; Saki, N. Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cell. Mol. Life Sci. 2015, 72, 2337–2347. [Google Scholar] [CrossRef]
- Garrett, J.T.; Olivares, M.G.; Rinehart, C.; Granja-Ingram, N.D.; Sánchez, V.; Chakrabarty, A.; Dave, B.; Cook, R.S.; Pao, W.; McKinely, E.; et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc. Natl. Acad. Sci. USA 2011, 108, 5021–5026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tandon, A.; Kuriappan, J.A.; Dubey, V. Translocation Tales: Unraveling the MYC Deregulation in Burkitt Lymphoma for Innovative Therapeutic Strategies. Lymphatics 2023, 1, 97–117. [Google Scholar] [CrossRef]
- Mlakar, V.; Morel, E.; Mlakar, S.J.; Ansari, M.; Gumy-Pause, F. A review of the biological and clinical implications of RAS-MAPK pathway alterations in neuroblastoma. J. Exp. Clin. Cancer Res. 2021, 40, 189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Dong, Q.; Cui, Y. Synergistic inhibition of MEK and reciprocal feedback networks for targeted intervention in malignancy. Cancer Biol. Med. 2019, 16, 415–434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Houde, N.; Beuret, L.; Bonaud, A.; Fortier-Beaulieu, S.P.; Truchon-Landry, K.; Aoidi, R.; Pic, É.; Alouche, N.; Rondeau, V.; Schlecht-Louf, G.; et al. Fine-tuning of MEK signaling is pivotal for limiting B and T cell activation. Cell Rep. 2022, 38, 110223. [Google Scholar] [CrossRef] [PubMed]
- Kun, E.; Tsang, Y.T.M.; Ng, C.W.; Gershenson, D.M.; Wong, K.K. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat. Rev. 2021, 92, 102137. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Garg, S.; Danesh, A.; Bruce, J.; Shaw, P.; Tan, Q.; Quevedo, R.; Braunstein, M.; Oza, A.M.; Pugh, T.; et al. Heterogeneous alteration of the ERBB3-MYC axis associated with MEK inhibitor resistance in a KRAS-mutated low-grade serous ovarian cancer patient. Cold Spring Harb. Mol. Case Stud. 2019, 5, a004341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawasaki, Y.; Sakimura, A.; Park, C.M.; Tomaru, R.; Tanaka, T.; Ozawa, T.; Zhou, Y.; Narita, K.; Kishi, H.; Muraguchi, A.; et al. Feedback control of ErbB2 via ERK-mediated phosphorylation of a conserved threonine in the juxtamembrane domain. Sci. Rep. 2016, 6, 31502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, K.; Zheng, M.; Lu, R.; Du, J.; Zhao, Q.; Li, Z.; Li, Y.; Zhang, S. The role of CDC25C in cell cycle regulation and clinical cancer therapy: A systematic review. Cancer Cell Int. 2020, 20, 213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lax, I.; Wong, A.; Lamothe, B.; Lee, A.; Frost, A.; Hawes, J.; Schlessinger, J. The docking protein FRS2alpha controls a MAP kinase-mediated negative feedback mechanism for signaling by FGF receptors. Mol. Cell 2002, 10, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, Y. Receptor tyrosine kinases: Biological functions and anticancer targeted therapy. MedComm 2023, 4, e446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kiyatkin, A.; van Alderwerelt van Rosenburgh, I.K.; Klein, D.E.; Lemmon, M.A. Kinetics of receptor tyrosine kinase activation define ERK signaling dynamics. Sci. Signal. 2020, 13, eaaz5267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akhavan, D.; Pourzia, A.L.; Nourian, A.A.; Williams, K.J.; Nathanson, D.; Babic, I.; Villa, G.R.; Tanaka, K.; Nael, A.; Yang, H.; et al. De-repression of PDGFRβ transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov. 2013, 3, 534–547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benedettini, E.; Sholl, L.M.; Peyton, M.; Reilly, J.; Ware, C.; Davis, L.; Vena, N.; Bailey, D.; Yeap, B.Y.; Fiorentino, M.; et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am. J. Pathol. 2010, 177, 415–423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyer, A.S.; Miller, M.A.; Gertler, F.B.; Lauffenburger, D.A. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci. Signal. 2013, 6, ra66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boewe, A.S.; Wrublewsky, S.; Hoppstädter, J.; Götz, C.; Kiemer, A.K.; Menger, M.D.; Laschke, M.W.; Ampofo, E. C-Myc/H19/miR-29b axis downregulates nerve/glial (NG)2 expression in glioblastoma multiforme. Mol. Ther. Nucleic Acids 2024, 35, 102120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osthus, R.C.; Shim, H.; Kim, S.; Li, Q.; Reddy, R.; Mukherjee, M.; Xu, Y.; Wonsey, D.; Lee, L.A.; Dang, C.V. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 2000, 275, 21797–21800. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.B.; Berger, P.L.; Ljungman, M.; Miranti, C.K. Human prostate luminal cell differentiation requires NOTCH3 induction by p38-MAPK and MYC. J. Cell Sci. 2017, 130, 1952–1964. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Davis, D.A.; Veeranna, R.P.; Haque, M.; Yarchoan, R. Characterization of the activation of protein tyrosine phosphatase, receptor-type, Z polypeptide 1 (PTPRZ1) by hypoxia inducible factor-2 alpha. PLoS ONE 2010, 5, e9641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, J.; Li, J.; Zhang, S.; Xu, X.; Zheng, M.; Jiang, G.; Li, F. IGF-1 induces hypoxia-inducible factor 1α-mediated GLUT3 expression through PI3K/Akt/mTOR dependent pathways in PC12 cells. Brain Res. 2012, 1430, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Gao, Q.; Yin, C.; Zou, M.; Lu, K.; Liu, W.; Zhu, Y.; Zhang, M.; Cheng, R. The CD146-HIF-1α axis regulates epithelial cell migration and alveolar maturation in a mouse model of bronchopulmonary dysplasia. Lab. Investig. 2022, 102, 794–804. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Lin, S.; Shang, J.; Liu, J.; Li, J.; Yuan, S.; Zhang, L. Early growth response gene-1 and hypoxia-inducible factor-1α affect tumor metastasis via regulation of tissue factor. Acta Oncol. 2013, 52, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Evens, A.M.; Bhalla, S.; Singh, A.; James, C.; Yang, S.; Prachand, S.; Dokic, D.; Gascoyne, R.D.; Winter, J.N.; et al. Hypoxia Inducible Factor 1á (HIF-1á) Regulates CD20 Expression in Lymphoma Cells: Possible Implications for Rituximab Based Therapy in Diffuse Large B Cell Lymphoma (DLBCL). Blood 2009, 114, 1698. [Google Scholar] [CrossRef]
- Marshall, A.D.; Lagutina, I.; Grosveld, G.C. PAX3-FOXO1 induces cannabinoid receptor 1 to enhance cell invasion and metastasis. Cancer Res. 2011, 71, 7471–7480. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Liu, Z.; Wei, Q.; Yu, D.; Zhao, M.; Zhang, X.; Gao, X.; Fan, Z.; Wang, S. FOXO1 orchestrates the intestinal homeostasis via neuronal signaling in group 3 innate lymphoid cells. J. Exp. Med. 2023, 220, e20230133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gopinath, S.D.; Webb, A.E.; Brunet, A.; Rando, T.A. FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem Cell Rep. 2014, 2, 414–426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pyrzynska, B.; Dwojak, M.; Zerrouqi, A.; Morlino, G.; Zapala, P.; Miazek, N.; Zagozdzon, A.; Bojarczuk, K.; Bobrowicz, M.; Siernicka, M.; et al. FOXO1 promotes resistance of non-Hodgkin lymphomas to anti-CD20-based therapy. Oncoimmunology 2018, 7, e1423183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gu, Z.; Churchman, M.L.; Roberts, K.G.; Moore, I.; Zhou, X.; Nakitandwe, J.; Hagiwara, K.; Pelletier, S.; Gingras, S.; Berns, H.; et al. PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat. Genet. 2019, 51, 296–307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, Y.T.; Zhang, F.; Fang, H.; Li, J.F.; Lu, G.; Jiang, L.; Chen, B.; Mao, D.D.; Liu, Y.F.; Wang, J.; et al. Transcriptome-wide subtyping of pediatric and adult T cell acute lymphoblastic leukemia in an international study of 707 cases. Proc. Natl. Acad. Sci. USA 2022, 119, e2120787119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beder, T.; Hansen, B.T.; Hartmann, A.M.; Zimmermann, J.; Amelunxen, E.; Wolgast, N.; Walter, W.; Zaliova, M.; Antić, Ž.; Chouvarine, P.; et al. The Gene Expression Classifier ALLCatchR Identifies B-cell Precursor ALL Subtypes and Underlying Developmental Trajectories Across Age. Hemasphere 2023, 7, e939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uhlen, M.; Karlsson, M.J.; Zhong, W.; Tebani, A.; Pou, C.; Mikes, J.; Lakshmikanth, T.; Forsström, B.; Edfors, F.; Odeberg, J.; et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science 2019, 366, eaax9198. [Google Scholar] [CrossRef] [PubMed]
- Bausch-Fluck, D.; Goldmann, U.; Müller, S.; van Oostrum, M.; Müller, M.; Schubert, O.T.; Wollscheid, B. The in silico human surfaceome. Proc. Natl. Acad. Sci. USA 2018, 115, E10988–E10997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gene Ontology Consortium. Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, A.; Miranda Bedate, A.; von Richthofen, H.J.; Vijver, S.V.; van der Vlist, M.; Kuhn, R.; Yermanos, A.; Kuball, J.J.; Kesmir, C.; Pascoal Ramos, M.I.; et al. A novel bioinformatics pipeline for the identification of immune inhibitory receptors as potential therapeutic targets. Elife 2024, 13, RP92870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szklarczyk, D.; Nastou, K.; Koutrouli, M.; Kirsch, R.; Mehryary, F.; Hachilif, R.; Hu, D.; Peluso, M.E.; Huang, Q.; Fang, T.; et al. The STRING database in 2025, protein networks with directionality of regulation. Nucleic Acids Res. 2025, 53, D730–D737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leo, I.R.; Aswad, L.; Stahl, M.; Kunold, E.; Post, F.; Erkers, T.; Struyf, N.; Mermelekas, G.; Joshi, R.N.; Gracia-Villacampa, E.; et al. Integrative multi-omics and drug response profiling of childhood acute lymphoblastic leukemia cell lines. Nat. Commun. 2022, 13, 1691. [Google Scholar] [CrossRef]
- Sofen, H.; Bissonnette, R.; Yosipovitch, G.; Silverberg, J.I.; Tyring, S.; Loo, W.J.; Zook, M.; Lee, M.; Zou, L.; Jiang, G.L.; et al. Efficacy and safety of vixarelimab, a human monoclonal oncostatin M receptor β antibody, in moderate-to-severe prurigo nodularis: A randomised, double-blind, placebo-controlled, phase 2a study. EClinicalMedicine 2023, 57, 101826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geethadevi, A.; Nair, A.; Parashar, D.; Ku, Z.; Xiong, W.; Deng, H.; Li, Y.; George, J.; McAllister, D.M.; Sun, Y.; et al. Oncostatin M Receptor-Targeted Antibodies Suppress STAT3 Signaling and Inhibit Ovarian Cancer Growth. Cancer Res. 2021, 81, 5336–5352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, B.Y.; Hogg, E.K.J.; Below, C.R.; Kononov, A.; Blanco-Gomez, A.; Heider, F.; Xu, J.; Hutton, C.; Zhang, X.; Scheidt, T.; et al. Heterocellular OSM-OSMR signalling reprograms fibroblasts to promote pancreatic cancer growth and metastasis. Nat. Commun. 2021, 12, 7336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoffmann, R.M.; Crescioli, S.; Mele, S.; Sachouli, E.; Cheung, A.; Chui, C.K.; Andriollo, P.; Jackson, P.J.M.; Lacy, K.E.; Spicer, J.F.; et al. A Novel Antibody-Drug Conjugate (ADC) Delivering a DNA Mono-Alkylating Payload to Chondroitin Sulfate Proteoglycan (CSPG4)-Expressing Melanoma. Cancers 2020, 12, 1029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mungra, N.; Biteghe, F.A.N.; Malindi, Z.; Huysamen, A.M.; Karaan, M.; Hardcastle, N.S.; Bunjun, R.; Chetty, S.; Naran, K.; Lang, D.; et al. CSPG4 as a target for the specific killing of triple-negative breast cancer cells by a recombinant SNAP-tag-based antibody-auristatin F drug conjugate. J. Cancer Res. Clin. Oncol. 2023, 149, 12203–12225. [Google Scholar] [CrossRef]
- Breij, E.C.; de Goeij, B.E.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 2014, 74, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, D.; Stelte-Ludwig, B.; Lerchen, H.G.; Wengner, A.M.; Ahsen, O.V.; Buchmann, P.; Märsch, S.; Mahlert, C.; Greven, S.; Dietz, L.; et al. IL3RA-Targeting Antibody-Drug Conjugate BAY-943 with a Kinesin Spindle Protein Inhibitor Payload Shows Efficacy in Preclinical Models of Hematologic Malignancies. Cancers 2020, 12, 3464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Guo, Y.; Wang, Z.; Wu, M.; Peng, W.; Sun, L.; Sun, J.; Li, M.; Zhu, J. A Dose Escalation Phase Ia Study of Anti-CD20 Antibody Drug Conjugate, MRG001 in Relapsed/Refractory Advanced Non-Hodgkin Lymphom. Blood 2021, 138 (Suppl. S1), 2490. [Google Scholar] [CrossRef]
- Demetri, G.D.; Luke, J.J.; Hollebecque, A.; Powderly, J.D., 2nd; Spira, A.I.; Subbiah, V.; Naumovski, L.; Chen, C.; Fang, H.; Lai, D.W.; et al. First-in-Human Phase I Study of ABBV-085, an Antibody-Drug Conjugate Targeting LRRC15, in Sarcomas and Other Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3556–3566. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.D.; Atallah, E.; Rizzieri, D.; Walter, R.B.; Chung, K.Y.; Spira, A.; Stock, W.; Tallman, M.S.; Cruz, H.G.; Boni, J.; et al. Camidanlumab tesirine, an antibody-drug conjugate, in relapsed/refractory CD25-positive acute myeloid leukemia or acute lymphoblastic leukemia: A phase I study. Leuk. Res. 2020, 95, 106385. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, Z.; Li, M.; Yang, N.; Lu, H.; Zhang, Z.; Dong, Y.; Chen, Y.; Zhu, Z.; Tong, A.; et al. A novel SLC3A2-targeting antibody-drug conjugate exerts potent antitumor efficacy in head and neck squamous cell cancer. Transl. Oncol. 2024, 45, 101981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, J.; Yan, J.; Huang, Y.; Guo, J.; Kong, Y.; Meng, X.; Liu, S.-H. Abstract 739, AMT-253, a first-in-class MUC18-targeting antibody-drug conjugate, for the treatment of MUC18-positive solid tumors. Cancer Res. 2024, 84 (Suppl. S6), 739. [Google Scholar] [CrossRef]
- Jonasch, E.; Call, J.; Kambhampati, S.; Caimi, P.; Curti, B.; Diefenbach, C.; Heath, E.; Park, S.; Kornblum, N.; Li, W.; et al. Phase 1/2 study of PRO1160, a CD70-directed antibody-drug conjugate, in patients with advanced solid tumors and hematologic malignancies. Oncologist 2023, 28, S14–S15. [Google Scholar] [CrossRef]
- Rosen, L.S.; Wesolowski, R.; Baffa, R.; Liao, K.H.; Hua, S.Y.; Gibson, B.L.; Pirie-Shepherd, S.; Tolcher, A.W. A phase I, dose-escalation study of PF-06650808, an anti-Notch3 antibody-drug conjugate, in patients with breast cancer and other advanced solid tumors. Investig. New Drugs 2020, 38, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, J.; Grandits, M.; Palhares, L.C.G.F.; Mele, S.; Nakamura, M.; López-Abente, J.; Crescioli, S.; Laddach, R.; Romero-Clavijo, P.; Cheung, A.; et al. Anti-cancer pro-inflammatory effects of an IgE antibody targeting the melanoma-associated antigen chondroitin sulfate proteoglycan 4. Nat. Commun. 2023, 14, 2192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoffmeister, L.M.; Orhan, E.; Walter, C.; Niktoreh, N.; Hanenberg, H.; von Neuhoff, N.; Reinhardt, D.; Schneider, M. Impact of KMT2A Rearrangement and CSPG4 Expression in Pediatric Acute Myeloid Leukemia. Cancers 2021, 13, 4817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, F.O.; Rauch, C.; Williams, D.E.; March, C.J.; Arthur, D.; Hilden, J.; Lampkin, B.C.; Buckley, J.D.; Buckley, C.V.; Woods, W.G.; et al. The human homologue of rat NG2, a chondroitin sulfate proteoglycan, is not expressed on the cell surface of normal hematopoietic cells but is expressed by acute myeloid leukemia blasts from poor-prognosis patients with abnormalities of chromosome band 11q23. Blood 1996, 87, 1123–1133. [Google Scholar] [CrossRef]
- Chen, X.; Habib, S.; Alexandru, M.; Chauhan, J.; Evan, T.; Troka, J.M.; Rahimi, A.; Esapa, B.; Tull, T.J.; Ng, W.Z.; et al. Chondroitin Sulfate Proteoglycan 4 (CSPG4) as an Emerging Target for Immunotherapy to Treat Melanoma. Cancers 2024, 16, 3260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, K.; Yong, J.; Zauner, R.; Wally, V.; Whitelock, J.; Sajinovic, M.; Kopecki, Z.; Liang, K.; Scott, K.F.; Mellick, A.S. Chondroitin Sulfate Proteoglycan 4 as a Marker for Aggressive Squamous Cell Carcinoma. Cancers 2022, 14, 5564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurokawa, T.; Imai, K. Chondroitin sulfate proteoglycan 4, An attractive target for antibody-based immunotherapy. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2024, 100, 293–308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ilieva, K.M.; Cheung, A.; Mele, S.; Chiaruttini, G.; Crescioli, S.; Griffin, M.; Nakamura, M.; Spicer, J.F.; Tsoka, S.; Lacy, K.E.; et al. Chondroitin Sulfate Proteoglycan 4 and Its Potential As an Antibody Immunotherapy Target across Different Tumor Types. Front. Immunol. 2018, 8, 1911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saegusa, S.; Fukaya, M.; Kakegawa, W.; Tanaka, M.; Katsumata, O.; Sugawara, T.; Hara, Y.; Itakura, M.; Okubo, T.; Sato, T.; et al. Mice lacking EFA6C/Psd2, a guanine nucleotide exchange factor for Arf6, exhibit lower Purkinje cell synaptic density but normal cerebellar motor functions. PLoS ONE 2019, 14, e0216960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, D.; Guo, Y.; Tang, P.; Li, H.; Chen, L. Arf6 as a therapeutic target: Structure, mechanism, and inhibitors. Acta Pharm. Sin. B. 2023, 13, 4089–4104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vega-Benedetti, A.F.; Loi, E.; Moi, L.; Blois, S.; Fadda, A.; Antonelli, M.; Arcella, A.; Badiali, M.; Giangaspero, F.; Morra, I.; et al. Clustered protocadherins methylation alterations in cancer. Clin. Epigenetics 2019, 11, 100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dallosso, A.R.; Øster, B.; Greenhough, A.; Thorsen, K.; Curry, T.J.; Owen, C.; Hancock, A.L.; Szemes, M.; Paraskeva, C.; Frank, M.; et al. Long-range epigenetic silencing of chromosome 5q31 protocadherins is involved in early and late stages of colorectal tumorigenesis through modulation of oncogenic pathways. Oncogene 2012, 31, 4409–4419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernardo-Castineira, C.; Valdés, N.; Celada, L.; Martinez, A.S.J.; Sáenz-de-Santa-María, I.; Bayón, G.F.; Fernández, A.F.; Sierra, M.I.; Fraga, M.F.; Astudillo, A.; et al. Epigenetic Deregulation of Protocadherin PCDHGC3 in Pheochromocytomas/Paragangliomas Associated With SDHB Mutations. J. Clin. Endocrinol. Metab. 2019, 104, 5673–5692. [Google Scholar] [CrossRef] [PubMed]
- Gabbert, L.; Dilling, C.; Meybohm, P.; Burek, M. Deletion of Protocadherin Gamma C3 Induces Phenotypic and Functional Changes in Brain Microvascular Endothelial Cells In Vitro. Front. Pharmacol. 2020, 11, 590144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feldheim, J.; Wend, D.; Lauer, M.J.; Monoranu, C.M.; Glas, M.; Kleinschnitz, C.; Ernestus, R.I.; Braunger, B.M.; Meybohm, P.; Hagemann, C.; et al. Protocadherin Gamma C3 (PCDHGC3) Is Strongly Expressed in Glioblastoma and Its High Expression Is Associated with Longer Progression-Free Survival of Patients. Int. J. Mol. Sci. 2022, 23, 8101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Busche, S.; Ge, B.; Vidal, R.; Spinella, J.F.; Saillour, V.; Richer, C.; Healy, J.; Chen, S.H.; Droit, A.; Sinnett, D.; et al. Integration of high-resolution methylome and transcriptome analyses to dissect epigenomic changes in childhood acute lymphoblastic leukemia. Cancer Res. 2013, 73, 4323–4336. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, Z.; Morenos, L.; Mechinaud, F.; Ashley, D.M.; Craig, J.M.; Sexton-Oates, A.; Halemba, M.S.; Parkinson-Bates, M.; Ng, J.; Morrison, D.; et al. Epigenetic deregulation in pediatric acute lymphoblastic leukemia. Epigenetics 2014, 9, 459–467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, T.; Chen, Y.; Yang, L.; Knösel, T.; Zöller, K.; Huber, O.; Petersen, I. DSC3 expression is regulated by p53, and methylation of DSC3 DNA is a prognostic marker in human colorectal cancer. Br. J. Cancer 2011, 104, 1013–1019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, T.; Chen, Y.; Yang, L.; Knösel, T.; Huber, O.; Pacyna-Gengelbach, M.; Petersen, I. The p53 target gene desmocollin 3 acts as a novel tumor suppressor through inhibiting EGFR/ERK pathway in human lung cancer. Carcinogenesis 2012, 33, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Yang, L.; Ma, Y.; Petersen, I.; Chen, Y. Desmocollin 3 has a tumor suppressive activity through inhibition of AKT pathway in colorectal cancer. Exp. Cell Res. 2019, 378, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Y.; Ren, X.; Li, D.; Fu, H.; Liu, C.; Zhou, W.; Liu, Q.; Liu, Q.; Wu, M. Leucine-rich repeat containing 4 act as an autophagy inhibitor that restores sensitivity of glioblastoma to temozolomide. Oncogene 2020, 39, 4551–4566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.; Huang, C.; Gan, K.; Huang, H.; Chen, Q.; Ouyang, J.; Tang, Y.; Li, X.; Yang, Y.; Zhou, H.; et al. LRRC4, a putative tumor suppressor gene, requires a functional leucine-rich repeat cassette domain to inhibit proliferation of glioma cells in vitro by modulating the extracellular signal-regulated kinase/protein kinase B/nuclear factor-kappaB pathway. Mol. Biol. Cell 2006, 17, 3534–3542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.; Huang, C.; Li, X.; Li, X.; Gan, K.; Chen, Q.; Tang, Y.; Tang, K.; Shen, S.; Li, G. LRRC4 inhibits glioblastoma cell proliferation, migration, and angiogenesis by downregulating pleiotropic cytokine expression and responses. J. Cell. Physiol. 2008, 214, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Ren, X.; Fu, H.; Li, D.; Chen, X.; Zu, X.; Liu, Q.; Wu, M. LRRC4 mediates the formation of circular RNA CD44 to inhibitGBM cell proliferation. Mol. Ther. Nucleic Acids 2021, 26, 473–487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, Z.; Wang, T.; Cao, X.; Sun, M.; Qu, Y. The role of receptor-type protein tyrosine phosphatases in cancer. Precis. Med. Sci. 2023, 12, 48–57. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, T.; Wang, A.Q.; Chen, X.A.; Zhang, R.Z.; Wang, X.C.; Lv, C.Y.; Pan, R.L.; Wang, O.C.; Lu, X.C. Protein tyrosine phosphatase receptor type kappa (PTPRK) revisited: Evolving insights into structure, function, and pathology. J. Transl. Med. 2025, 23, 534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, K.A.; Wojdyla, K.; Lai, T.; Mulholland, K.E.; Aldaz Casanova, S.; Antrobus, R.; Andrews, S.R.; Biggins, L.; Mahler-Araujo, B.; Barton, P.R.; et al. The receptor protein tyrosine phosphatase PTPRK promotes intestinal repair and catalysis-independent tumour suppression. J. Cell Sci. 2024, 137, jcs261914. [Google Scholar] [CrossRef]
- Stevenson, W.S.; Best, O.G.; Przybylla, A.; Chen, Q.; Singh, N.; Koleth, M.; Pierce, S.; Kennedy, T.; Tong, W.; Kuang, S.Q.; et al. DNA methylation of membrane-bound tyrosine phosphatase genes in acute lymphoblastic leukaemia. Leukemia 2014, 28, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Novellino, L.; De Filippo, A.; Deho, P.; Perrone, F.; Pilotti, S.; Parmiani, G.; Castelli, C. PTPRK negatively regulates transcriptional activity of wild type and mutated oncogenic beta-catenin and affects membrane distribution of beta-catenin/E-cadherin complexes in cancer cells. Cell Signal. 2008, 20, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Priebe, V.; Drakul, A.; Zeyen, S.; Bornhauser, B.; Santoro, R.; Bourquin, J.P. P331, The Oncogenic Transcription Factor Tcf3, :Hlf Marks a Distinct Enhancer-Promoter Interaction Network in T(17;19) Positive Childhood Acute Lymphoblastic Leukemia. Hemasphere 2023, 7, e88138e3. [Google Scholar] [CrossRef] [PubMed Central]
- Navarrete-Meneses, M.D.P.; Pérez-Vera, P. Alteraciones epigenéticas en leucemia linfoblástica aguda [Epigenetic alterations in acute lymphoblastic leukemia]. Bol. Med. Hosp. Infant. Mex. 2017, 74, 243–264. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, Y.C.; Chen, C.C. Lysophosphatidic Acid Receptor Antagonists and Cancer: The Current Trends, Clinical Implications, and Trials. Cells 2021, 10, 1629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geraldo, L.H.M.; Spohr, T.C.L.S.; Amaral, R.F.D.; Fonseca, A.C.C.D.; Garcia, C.; Mendes, F.A.; Freitas, C.; dosSantos, M.F.; Lima, F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Signal Transduct. Target. Ther. 2021, 6, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bokaii Hosseini, Z.; Rajabi, F.; Morovatshoar, R.; Ashrafpour, M.; Behboodi, P.; Zareie, D.; Natami, M. Downregulation of LPAR1 Promotes Invasive Behavior in Papillary Thyroid Carcinoma Cells. Cancer Inform. 2024, 23, 11769351241277012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, X.; Haney, N.; Kropp, D.; Kabore, A.F.; Johnston, J.B.; Gibson, S.B. Lysophosphatidic acid (LPA) protects primary chronic lymphocytic leukemia cells from apoptosis through LPA receptor activation of the anti-apoptotic protein AKT/PKB. J. Biol. Chem. 2005, 280, 9498–9508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, G.H.; Cheon, J.; Kim, D.; Jun, H.S. Lysophosphatidic Acid Promotes Epithelial-Mesenchymal Transition in Kidney Epithelial Cells via the LPAR1/MAPK-AKT/KLF5 Signaling Pathway in Diabetic Nephropathy. Int. J. Mol. Sci. 2022, 23, 10497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Cao, D.; Zheng, X.; Wang, G.; Liu, M. Tissue factor as a new target for tumor therapy-killing two birds with one stone: A narrative review. Ann. Transl. Med. 2022, 10, 1250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ren, Z.; Xue, Y.; Liu, L.; Zhang, X.; Pei, J.; Zhang, Y.; Wang, Y.; Yu, K. Tissue factor overexpression in triple-negative breast cancer promotes immune evasion by impeding T-cell infiltration and effector function. Cancer Lett. 2023, 565, 216221. [Google Scholar] [CrossRef] [PubMed]
- Koizume, S.; Miyagi, Y. Tissue factor in cancer-associated thromboembolism: Possible mechanisms and clinical applications. Br. J. Cancer 2022, 127, 2099–2107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mechtcheriakova, D.; Wlachos, A.; Holzmüller, H.; Binder, B.R.; Hofer, E. Vascular endothelial cell growth factor-induced tissue factor expression in endothelial cells is mediated by EGR-1. Blood 1999, 93, 3811–3823. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Wei, M.N.; Huang, J.R.; Zhang, Z.J.; Zhang, W.J.; Jiang, Q.W.; Yang, Y.; Wang, H.Y.; Jin, H.L.; Wang, K.; et al. Targeting TF-AKT/ERK-EGFR Pathway Suppresses the Growth of Hepatocellular Carcinoma. Front. Oncol. 2019, 9, 150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Z. Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer. Sci. Rep. 2020, 10, 2815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, X.; Yang, Y.; Tang, J.; Xiang, J. Ephs in cancer progression: Complexity and context-dependent nature in signaling, angiogenesis and immunity. Cell Commun. Signal. 2024, 22, 299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Zhang, C.; Deng, M.; Jiang, Y.; He, Z.; Qian, H. EFNB1 levels determine distinct drug response patterns guiding precision therapy for B-cell neoplasms. iScience 2023, 27, 108667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Y.; Shi, J. EFNB1 drives glioma progression and shapes the immune microenvironment: A potential prognostic biomarker. Discov. Oncol. 2025, 16, 249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatzikalil, E.; Stergiou, I.E.; Papadakos, S.P.; Konstantinidis, I.; Theocharis, S. The Clinical Relevance of the EPH/Ephrin Signaling Pathway in Pediatric Solid and Hematologic Malignancies. Int. J. Mol. Sci. 2024, 25, 3834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mekkaoui, L.; Rassart, C.; Rozen, L.; Janssens, A.; Bron, D.; Ferster, A.; Cantinieaux, B. Use of CD 123 Expression on Blasts from AML, ALL and RAEB As Minimal Residual Disease Marker. Blood 2015, 126, 5402. [Google Scholar] [CrossRef]
- Liu, J.; Tan, X.; Ma, Y.Y.; Liu, Y.; Gao, L.; Gao, L.; Kong, P.; Peng, X.G.; Zhang, X.; Zhang, C. Study on the Prognostic Value of Aberrant Antigen in Patients With Acute B Lymphocytic Leukemia. Clin. Lymphoma Myeloma Leuk. 2019, 19, e349–e358. [Google Scholar] [CrossRef] [PubMed]
- Al-Mudallal, S.S.; Shaker, H.; Dede, H. Assessment of Expression and Prognostic Significance of Interleukin 3 Receptor Alpha Subunit (CD123) in Childhood Acute Lymphoblastic Leukemia. Int. J. Med. Res. Prof. 2016, 2, 247–254. [Google Scholar]
- Pelosi, E.; Castelli, G.; Testa, U. CD123 a Therapeutic Target for Acute Myeloid Leukemia and Blastic Plasmocytoid Dendritic Neoplasm. Int. J. Mol. Sci. 2023, 24, 2718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hercus, T.R.; Kan, W.L.T.; Broughton, S.E.; Tvorogov, D.; Ramshaw, H.S.; Sandow, J.J.; Nero, T.L.; Dhagat, U.; Thompson, E.J.; Shing, K.S.C.T.; et al. Role of the β Common (βc) Family of Cytokines in Health and Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a028514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hara, T.; Miyajima, A. Function and signal transduction mediated by the interleukin 3 receptor system in hematopoiesis. Stem Cells 1996, 14, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Songyang, Z.; Baltimore, D.; Cantley, L.C.; Kaplan, D.R.; Franke, T.F. Interleukin 3-dependent survival by the Akt protein kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 11345–11350. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.; Jones, G.E.; Adams, S.; Harvey, L.; Audette, C.A.; Wilhelm, A.; Bai, C.; Rui, L.; Laleau, R.; Liu, F.; et al. A CD123-targeting antibody-drug conjugate, IMGN632, designed to eradicate AML while sparing normal bone marrow cells. Blood Adv. 2018, 2, 848–858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogata, H.; Ford, D.; Kouttab, N.; King, T.C.; Vita, N.; Minty, A.; Stoeckler, J.; Morgan, D.; Girasole, C.; Morgan, J.W.; et al. Regulation of interleukin-13 receptor constituents on mature human B lymphocytes. J. Biol. Chem. 1998, 273, 9864–9871. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, J.; Cheng, K.; Lu, Q.; Zhao, R.; Wang, S.; Zhang, Q.; Ge, L.; Pan, J.; Song, G.; et al. IL13Rα1 prevents a castration resistant phenotype of prostate cancer by targeting hexokinase 2 for ubiquitin-mediated degradation. Cancer Biol. Med. 2021, 19, 1008–1028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Olde Heuvel, F.; Rehman, R.; Aousji, O.; Froehlich, A.; Li, Z.; Jark, R.; Zhang, W.; Conquest, A.; Woelfle, S.; et al. Interleukin-13 and its receptor are synaptic proteins involved in plasticity and neuroprotection. Nat. Commun. 2023, 14, 200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prokopchuk, O.; Liu, Y.; Henne-Bruns, D.; Kornmann, M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: Evidence for autocrine and paracrine actions. Br. J. Cancer 2005, 92, 921–928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, J.; Song, X.; Traub, B.; Luxenhofer, M.; Kornmann, M. Involvement of IL-4, IL-13 and Their Receptors in Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 2998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kollewe, A.; Schwarz, Y.; Oleinikov, K.; Raza, A.; Haupt, A.; Wartenberg, P.; Wyatt, A.; Boehm, U.; Ectors, F.; Bildl, W.; et al. Subunit composition, molecular environment, and activation of native TRPC channels encoded by their interactomes. Neuron 2022, 110, 4162–4175.e7. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, J.; Park, C.H.; Jeong, B.; So, I. Direct modulation of TRPC ion channels by Gα proteins. Front. Physiol. 2024, 15, 1362987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, H.B.; Jun, H.O.; Kim, J.H.; Fruttiger, M.; Kim, J.H. Suppression of transient receptor potential canonical channel 4 inhibits vascular endothelial growth factor-induced retinal neovascularization. Cell Calcium 2015, 57, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Prontera, P.; Sarchielli, P.; Tonelli, A.; Bassi, M.T.; Cupini, L.M.; Caproni, S.; Siliquini, S.; Donti, E.; Calabresi, P. A novel ATP1A2 gene mutation in familial hemiplegic migraine and epilepsy. Cephalalgia 2014, 34, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, N.; Carmine-Simmen, K.; Nair, R.; Wang, C.; Moghadas-Jafari, S.; Blaser, H.; Tran-Thanh, D.; Wang, D.; Wang, P.; Wang, J.; et al. Amplification of a calcium channel subunit CACNG4 increases breast cancer metastasis. EBioMedicine 2020, 52, 102646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halatsch, M.E.; Löw, S.; Mursch, K.; Hielscher, T.; Schmidt, U.; Unterberg, A.; Vougioukas, V.I.; Feuerhake, F. Candidate genes for sensitivity and resistance of human glioblastoma multiforme cell lines to erlotinib. Lab. Investig. J. Neurosurg. 2009, 111, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Gu, F.M.; Wang, Z.; Jiang, J.H.; Yao, L.Q.; Tan, C.J.; Huang, X.Y.; Ke, A.W.; Dai, Z.; Fan, J.; et al. Activation of PI3K/AKT and MAPK pathway through a PDGFRβ-dependent feedback loop is involved in rapamycin resistance in hepatocellular carcinoma. PLoS ONE 2012, 7, e33379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Bajraszewski, N.; Wu, E.; Wang, H.; Moseman, A.P.; Dabora, S.L.; Griffin, J.D.; Kwiatkowski, D.J. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J. Clin. Investig. 2007, 117, 730–738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyazaki, B.; Ueno, T.; Sugiyama, M.; Kojima, S.; Arakawa, A.; Tao, K.; Tanimura, K.; Shiraishi, K.; Yagishita, S.; Kohsaka, S.; et al. Promoter swapping of truncated PDGFRB drives Ph-like acute lymphoblastic leukemia. npj Precis. Oncol. 2023, 7, 132. [Google Scholar] [CrossRef]
- Wang, X.; Yue, M.; Cheung, J.P.Y.; Cheung, P.W.H.; Fan, Y.; Wu, M.; Wang, X.; Zhao, S.; Khanshour, A.M.; Rios, J.J.; et al. Impaired glycine neurotransmission causes adolescent idiopathic scoliosis. J. Clin. Investig. 2024, 134, e168783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raiteri, L.; Raiteri, M. Functional ‘glial’ GLYT1 glycine transporters expressed in neurons. J. Neurochem. 2010, 114, 647–653. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Feng, T.; Xie, Y.; Swamiappan, S.; Zhou, Y.; Zhou, Y.; Zhou, H.; Peng, X. Recent Advances in the Development and Application of Cell-Loaded Collagen Scaffolds. Int. J. Mol. Sci. 2025, 26, 4009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trono, P.; Ottavi, F.; Rosano’, L. Novel insights into the role of Discoidin domain receptor 2 (DDR2) in cancer progression: A new avenue of therapeutic intervention. Matrix Biol. 2024, 125, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.M.; Lee, F.Y.F.; Jones, R.T.; Kimball, A.K.; Saravia, E.; Graziano, R.F.; Coleman, B.; Menard, K.; Yan, J.; Michaud, E.; et al. Targeting DDR2 enhances tumor response to anti-PD-1 immunotherapy. Sci. Adv. 2019, 5, eaav2437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ren, T.; Zhang, W.; Liu, X.; Zhao, H.; Zhang, J.; Zhang, J.; Li, X.; Zhang, Y.; Bu, X.; Shi, M.; et al. Discoidin domain receptor 2 (DDR2) promotes breast cancer cell metastasis and the mechanism implicates epithelial mesenchymal transition programme under hypoxia. J. Pathol. 2014, 234, 526–537. [Google Scholar] [CrossRef]
- Vessella, T.; Xiang, S.; Xiao, C.; Stilwell, M.; Fok, J.; Shohet, J.; Rozen, E.; Zhou, H.S.; Wen, Q. DDR2 signaling and mechanosensing orchestrate neuroblastoma cell fate through different transcriptome mechanisms. FEBS Open Bio. 2024, 14, 867–882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hinz, N.; Baranowsky, A.; Horn, M.; Kriegs, M.; Sibbertsen, F.; Smit, D.J.; Clezardin, P.; Lange, T.; Schinke, T.; Jücker, M. Knockdown of AKT3 Activates HER2 and DDR Kinases in Bone-Seeking Breast Cancer Cells, Promotes Metastasis In Vivo and Attenuates the TGFβ/CTGF Axis. Cells 2021, 10, 430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujikawa, A.; Sugawara, H.; Tanaka, T.; Matsumoto, M.; Kuboyama, K.; Suzuki, R.; Tanga, N.; Ogata, A.; Masumura, M.; Noda, M. Targeting PTPRZ inhibits stem cell-like properties and tumorigenicity in glioblastoma cells. Sci. Rep. 2017, 7, 5609. [Google Scholar] [CrossRef]
- Rush, L.J.; Raval, A.; Funchain, P.; Johnson, A.J.; Smith, L.; Lucas, D.M.; Bembea, M.; Liu, T.H.; Heerema, N.A.; Rassenti, L.; et al. Epigenetic profiling in chronic lymphocytic leukemia reveals novel methylation targets. Cancer Res. 2004, 64, 2424–2433. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, T.; Majumder, S.; Kutay, H.; Smith, D.S.; Neuberg, D.S.; Lucas, D.M.; Byrd, J.C.; Grever, M.; Jacob, S.T. Methylation and silencing of protein tyrosine phosphatase receptor type O in chronic lymphocytic leukemia. Clin. Cancer Res. 2007, 13, 3174–3181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Papadimitriou, E.; Kanellopoulou, V.K. Protein Tyrosine Phosphatase Receptor Zeta 1 as a Potential Target in Cancer Therapy and Diagnosis. Int. J. Mol. Sci. 2023, 24, 8093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muto, M.; Suzuki, H.; Suzuki, Y. New Insights and Future Perspectives of APRIL in IgA Nephropathy. Int. J. Mol. Sci. 2024, 25, 10340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albini, A.; Di Paola, L.; Mei, G.; Baci, D.; Fusco, N.; Corso, G.; Noonan, D. Inflammation and cancer cell survival: TRAF2 as a key player. Cell Death Dis. 2025, 16, 292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cascalho, M.; Platt, J.L. TNFRSF13B Diversification Fueled by B Cell Responses to Environmental Challenges—A Hypothesis. Front. Immunol. 2021, 12, 634544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mamara, A.; Germenis, A.E.; Kompoti, M.; Palassopoulou, M.; Mandala, E.; Banti, A.; Giannakoulas, N.; Speletas, M. TACI expression and signaling in chronic lymphocytic leukemia. J. Immunol. Res. 2015, 2015, 478753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tai, Y.T.; Lin, L.; Xing, L.; Cho, S.-F.; Yu, T.; Acharya, C.; Wen, K.; Hsieh, P.A.; Dulos, J.; van Elsas, A.; et al. APRIL signaling via TACI mediates immunosuppression by T regulatory cells in multiple myeloma: Therapeutic implications. Leukemia 2019, 33, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Creative-Bioloabs: Anti-TNFRSF13B (Tabalumab)-MC-Vc-PAB-MMAE.; CAT#ADC-W-1907. Available online: https://www.creative-biolabs.com/adc/tabalumab-mc-vc-pab-mmae-adc-2188.htm (accessed on 4 June 2025).
- Bagherie-Lachidan, M.; Reginensi, A.; Pan, Q.; Zaveri, H.P.; Scott, D.A.; Blencowe, B.J.; Helmbacher, F.; McNeill, H. Stromal Fat4 acts non-autonomously with Dchs1/2 to restrict the nephron progenitor pool. Development 2015, 142, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.K.; Keck, J.M.; Eide, C.A.; Bottomly, D.; Traer, E.; Tyner, J.W.; McWeeney, S.K.; Tognon, C.E.; Druker, B.J. ERBB2/HER2 mutations are transforming and therapeutically targetable in leukemia. Leukemia 2020, 34, 2798–2804. [Google Scholar] [CrossRef]
- Giacoletto, C.J.; Valente, L.J.; Brown, L.; Patterson, S.; Gokhale, R.; Mockus, S.M.; Grody, W.W.; Deng, H.W.; Rotter, J.I.; Schiller, M.R. New Gain-of-Function Mutations Prioritize Mechanisms of HER2 Activation. medRxiv 2025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irwin, M.E.; Nelson, L.D.; Santiago-O’Farrill, J.M.; Knouse, P.D.; Miller, C.P.; Palla, S.L.; Siwak, D.R.; Mills, G.B.; Estrov, Z.; Li, S.; et al. Small molecule ErbB inhibitors decrease proliferative signaling and promote apoptosis in philadelphia chromosome-positive acute lymphoblastic leukemia. PLoS ONE 2013, 8, e70608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grant, S.; Qiao, L.; Dent, P. Roles of ERBB family receptor tyrosine kinases, and downstream signaling pathways, in the control of cell growth and survival. Front. Biosci. 2002, 7, d376–d389. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Wang, J.; Shen, S.; Ni, X.; Gong, Z.; Zheng, B.; Sun, W.; Suo, T.; Liu, H.; Ni, X.; et al. ERBB2 S310F mutation independently activates PI3K/AKT and MAPK pathways through homodimers to contribute gallbladder carcinoma growth. Med. Oncol. 2022, 39, 64. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.; Yamasaka, M.; Ozasa, K.; Sakamoto, K.; Hayata-Takano, A.; Nakazawa, T.; Hashimoto, H.; Waschek, J.A.; Ago, Y. Vasoactive intestinal peptide-VIPR2 signaling regulates tumor cell migration. Front. Oncol. 2022, 12, 852358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asano, S.; Ono, A.; Baba, K.; Uehara, T.; Sakamoto, K.; Hayata-Takano, A.; Nakazawa, T.; Yanamoto, S.; Tanimoto, K.; Hashimoto, H.; et al. Blockade of vasoactive intestinal peptide receptor 2 (VIPR2) signaling suppresses cyclin D1-dependent cell-cycle progression in MCF-7 cells. J. Pharmacol. Sci. 2024, 154, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Eon Kuek, L.; Leffler, M.; Mackay, G.A.; Hulett, M.D. The MS4A family: Counting past 1, 2 and 3. Immunol. Cell Biol. 2016, 94, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Pavlasova, G.; Mraz, M. The regulation and function of CD20, an “enigma” of B-cell biology and targeted therapy. Haematologica 2020, 105, 1494–1506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahadevia, H.; Ananthamurugan, M.; Shah, K.; Desai, A.; Shrestha, A. A Review of Anti-CD20 Antibodies in the Management of B-Cell Lymphomas. Lymphatics 2024, 2, 10–24. [Google Scholar] [CrossRef]
- Dworzak, M.N.; Schumich, A.; Printz, D.; Pötschger, U.; Husak, Z.; Attarbaschi, A.; Basso, G.; Gaipa, G.; Ratei, R.; Mann, G.; et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: Setting the stage for anti-CD20 directed immunotherapy. Blood 2008, 112, 3982–3988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonavida, B. ‘Rituximab-induced inhibition of antiapoptotic cell survival pathways: Implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions’. Oncogene 2007, 26, 3629–3636. [Google Scholar] [CrossRef]
- Sanchez, R.; Ribera, J.; Morgades, M.; Ayala, R.; Onecha, E.; Ruiz-Heredia, Y.; Juárez-Rufián, A.; de Nicolás, R.; Sánchez-Pina, J.; Vives, S.; et al. A novel targeted RNA-Seq panel identifies a subset of adult patients with acute lymphoblastic leukemia with BCR-ABL1-like characteristics. Blood Cancer J. 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.J.; Capasso, M.; Vater, I.; Akasaka, T.; Bernard, O.A.; Calasanz, M.J.; Chandrasekaran, T.; Chapiro, E.; Gesk, S.; Griffiths, M.; et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood 2009, 114, 2688–2698. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.; Chu, A.; Hurtado, R.; Tirado, C.A. Integrative Insights into Philadelphia-like B-Cell Acute Lymphoblastic Leukemia: A Genetic and Molecular Landscape. Diagnostics 2025, 15, 385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tasian, S.K.; Doral, M.Y.; Borowitz, M.J.; Wood, B.L.; Chen, I.M.; Harvey, R.C.; Gastier-Foster, J.M.; Willman, C.L.; Hunger, S.P.; Mullighan, C.G.; et al. Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia. Blood 2012, 120, 833–842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toiyama, Y.; Mizoguchi, A.; Kimura, K.; Hiro, J.; Inoue, Y.; Tutumi, T.; Miki, C.; Kusunoki, M. TTYH2, a human homologue of the Drosophila melanogaster gene tweety, is up-regulated in colon carcinoma and involved in cell proliferation and cell aggregation. World J. Gastroenterol. 2007, 13, 2717–2721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leung, H.H.; Mansour, C.; Rousseau, M.; Nakhla, A.; Kiselyov, K.; Venkatachalam, K.; Wong, C.O. Drosophila tweety facilitates autophagy to regulate mitochondrial homeostasis and bioenergetics in Glia. Glia 2024, 72, 433–451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, H.; Chen, I.M.; Wilson, C.S.; Bedrick, E.J.; Harvey, R.C.; Atlas, S.R.; Devidas, M.; Mullighan, C.G.; Wang, X.; Murphy, M.; et al. Gene expression classifiers for relapse-free survival and minimal residual disease improve risk classification and outcome prediction in pediatric B-precursor acute lymphoblastic leukemia. Blood 2010, 115, 1394–1405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bakr, M.N.; Takahashi, H.; Kikuchi, Y. CHRNA1 and its correlated-myogenesis/cell cycle genes are prognosis-related markers of metastatic melanoma. Biochem. Biophys. Rep. 2023, 33, 101425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.Y.; Kim, H.; Chung, W.S.; Park, H. Selective regulation of corticostriatal synapses by astrocytic phagocytosis. Nat. Commun. 2025, 16, 2504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charlet, J.; Tomari, A.; Dallosso, A.R.; Szemes, M.; Kaselova, M.; Curry, T.J.; Almutairi, B.; Etchevers, H.C.; McConville, C.; Malik, K.T.; et al. Genome-wide DNA methylation analysis identifies MEGF10 as a novel epigenetically repressed candidate tumor suppressor gene in neuroblastoma. Mol. Carcinog. 2017, 56, 1290–1301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, X.; Liu, Y.; Hu, Y.; Su, F.; Wang, Z.; Chen, Y.; Zhuang, Z. Leucine rich repeat containing 15 promotes triple-negative breast cancer proliferation and invasion via the ITGB1/FAK/PI3K signalling pathway. Sci. Rep. 2025, 15, 14535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, J.; Dean, D.; Wei, R.; Hornicek, F.J.; Ulmert, D.; Duan, Z. Expression and clinical implications of leucine-rich repeat containing 15 (LRRC15) in osteosarcoma. J. Orthop. Res. 2020, 38, 2362–2372. [Google Scholar] [CrossRef] [PubMed]
- Storey, C.M.; Cheng, M.; Altai, M.; Park, J.E.; Tran, J.; Lueong, S.S.; Thorek, D.; Mao, L.; Zedan, W.; Yuen, C.; et al. Key Regulatory Elements of the TGFβ-LRRC15 Axis Predict Disease Progression and Immunotherapy Resistance Across Cancer Types. bioRxiv 2024. [Google Scholar] [CrossRef]
- Purcell, J.W.; Tanlimco, S.G.; Hickson, J.; Fox, M.; Sho, M.; Durkin, L.; Uziel, T.; Powers, R.; Foster, K.; McGonigal, T.; et al. LRRC15 Is a Novel Mesenchymal Protein and Stromal Target for Antibody-Drug Conjugates. Cancer Res. 2018, 78, 4059–4072. [Google Scholar] [CrossRef] [PubMed]
- Dalpati, N.; Rai, S.K.; Sharma, P.; Sarangi, P.P. Integrins and integrin-driven secretory pathways as multi-dimensional regulators of tumor-associated macrophage recruitment and reprogramming in tumor microenvironment. Matrix Biol. 2025, 135, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ji, W.; Cheng, X.; Lv, L.; Yu, X.; Wang, N.; Li, M.; Hu, T.; Shi, Z. Revealing a Novel Methylated Integrin Alpha-8 Related to Extracellular Matrix and Anoikis Resistance Using Proteomic Analysis in the Immune Microenvironment of Lung Adenocarcinoma. Mol. Biotechnol. 2025, 67, 1137–1155. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Koh, Y.; Park, H.; Kim, D.Y.; Kim, D.C.; Byun, J.M.; Lee, H.J.; Yoon, S.S. Highly Expressed Integrin-α8 Induces Epithelial to Mesenchymal Transition-Like Features in Multiple Myeloma with Early Relapse. Mol. Cells 2016, 39, 898–908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yurchenko, M.Y.; Kovalevska, L.M.; Shlapatska, L.M.; Berdova, G.G.; Clark, E.A.; Sidorenko, S.P. CD150 regulates JNK1/2 activation in normal and Hodgkin’s lymphoma B cells. Immunol. Cell Biol. 2010, 88, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Mikhalap, S.V.; Shlapatska, L.M.; Yurchenko, O.V.; Yurchenko, M.Y.; Berdova, G.G.; Nichols, K.E.; Clark, E.A.; Sidorenko, S.P. The adaptor protein SH2D1A regulates signaling through CD150 (SLAM) in B cells. Blood 2004, 104, 4063–4070. [Google Scholar] [CrossRef] [PubMed]
- Gordiienko, I.; Shlapatska, L.; Kholodniuk, V.; Sklyarenko, L.; Gluzman, D.F.; Clark, E.A.; Sidorenko, S.P. The interplay of CD150 and CD180 receptor pathways contribute to the pathobiology of chronic lymphocytic leukemia B cells by selective inhibition of Akt and MAPK signaling. PLoS ONE 2017, 12, e0185940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- von Wenserski, L.; Schultheiß, C.; Bolz, S.; Schliffke, S.; Simnica, D.; Willscher, E.; Gerull, H.; Wolters-Eisfeld, G.; Riecken, K.; Fehse, B.; et al. SLAMF receptors negatively regulate B cell receptor signaling in chronic lymphocytic leukemia via recruitment of prohibitin-2. Leukemia 2021, 35, 1073–1086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Peerlings, D.; Mimpen, M.; Damoiseaux, J. The IL-2—IL-2 receptor pathway: Key to understanding multiple sclerosis. J. Transl. Autoimmun. 2021, 4, 100123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, C.H.; Schlerka, A.; Grandits, A.M.; Koller, E.; van der Kouwe, E.; Vassiliou, G.S.; Staber, P.B.; Heller, G.; Wieser, R. IL2RA Promotes Aggressiveness and Stem Cell-Related Properties of Acute Myeloid Leukemia. Cancer Res. 2020, 80, 4527–4539. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Brennan, S.; Milne, T.A.; Chen, W.Y.; Li, Y.; Hurtz, C.; Kweon, S.M.; Zickl, L.; Shojaee, S.; Neuberg, D.; et al. Integrative epigenomic analysis identifies biomarkers and therapeutic targets in adult B-acute lymphoblastic leukemia. Cancer Discov. 2012, 2, 1004–1023. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Robinson, M.E.; Sun, R.; Kume, K.; Ma, N.; Cosgun, K.N.; Chan, L.N.; Leveille, E.; Geng, H.; Vykunta, V.S.; et al. Dynamic phosphatase-recruitment controls B-cell selection and oncogenic signaling. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carrà, G.; Cartellà, A.; Maffeo, B.; Circosta, P.; Cignetti, A.; Pautasso, M.; Familiari, U.; Volante, M.; Dragani, M.E.E.; Cambrin, G.R.; et al. Interleukin-2 Receptor Alpha Chain, Also Called CD25, Is a Potential Target in Acute Lymphoblastic Leukemia. Blood 2020, 136 (Suppl. S1), 11–12. [Google Scholar] [CrossRef]
- Sidahmed-Adrar, N.; Ottavi, J.F.; Benzoubir, N.; Ait Saadi, T.; Bou Saleh, M.; Mauduit, P.; Guettier, C.; Desterke, C.; Le Naour, F. Tspan15 Is a New Stemness-Related Marker in Hepatocellular Carcinoma. Proteomics 2019, 19, e1900025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Z.; Li, L.; Qin, Y.R.; Liu, H.; Jiang, C.; Zeng, T.T.; Li, M.Q.; Xie, D.; Li, Y.; et al. TSPAN15 interacts with BTRC to promote oesophageal squamous cell carcinoma metastasis via activating NF-κB signaling. Nat. Commun. 2018, 9, 1423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Song, Q.; Shang, K.; Li, Y.; Jiang, L.; Yang, L. Tspan protein family: Focusing on the occurrence, progression, and treatment of cancer. Cell Death Discov. 2024, 10, 187. [Google Scholar] [CrossRef]
- Sims, N.A.; Lévesque, J.P. Oncostatin M: Dual Regulator of the Skeletal and Hematopoietic Systems. Curr. Osteoporos. Rep. 2024, 22, 80–95. [Google Scholar] [CrossRef]
- Lantieri, F.; Bachetti, T. OSM/OSMR and Interleukin 6 Family Cytokines in Physiological and Pathological Condition. Int. J. Mol. Sci. 2022, 23, 11096. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polak, K.L.; Tamagno, I.; Parameswaran, N.; Smigiel, J.; Chan, E.R.; Yuan, X.; Rios, B.; Jackson, M.W. Oncostatin-M and OSM-Receptor Feed-Forward Activation of MAPK Induces Separable Stem-like and Mesenchymal Programs. Mol. Cancer Res. 2023, 21, 975–990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, M.H.; Yang, Q.M. NUP214 fusion genes in acute leukemia (Review). Oncol. Lett. 2014, 8, 959–962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, W.; Xu, Y.; Mo, S.; Li, Q.; Yu, J.; Wang, R.; Ma, Y.; Ni, Y.; Xiang, W.; Han, L.; et al. GLUT3 induced by AMPK/CREB1 axis is key for withstanding energy stress and augments the efficacy of current colorectal cancer therapies. Signal Transduct. Target. Ther. 2020, 5, 177. [Google Scholar] [CrossRef]
- Yan, B.; Li, X.; Peng, M.; Zuo, Y.; Wang, Y.; Liu, P.; Ren, W.; Jin, X. The YTHDC1/GLUT3/RNF183 axis forms a positive feedback loop that modulates glucose metabolism and bladder cancer progression. Exp. Mol. Med. 2023, 55, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Sim, H.I.; Yun, B.; Park, Y.; Jin, H.S. Revisiting T-cell adhesion molecules as potential targets for cancer immunotherapy: CD226 and CD2. Exp. Mol. Med. 2024, 56, 2113–2126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Z.; Qi, G.; Miller, J.S.; Zheng, S.G. CD226, An Emerging Role in Immunologic Diseases. Front. Cell Dev. Biol. 2020, 8, 564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakano, Y.; Sakano, K.; Hurrell, B.P.; Helou, D.G.; Shafiei-Jahani, P.; Kazemi, M.H.; Li, X.; Shen, S.; Hilser, J.R.; Hartiala, J.A.; et al. Blocking CD226 regulates type 2 innate lymphoid cell effector function and alleviates airway hyperreactivity. J. Allergy Clin. Immunol. 2024, 153, 1406–1422.e6. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, C.; Liao, Y.; Liu, J.; Huang, J.; Xia, M.; Chen, M.; Tan, H.; He, W.; Xu, M.; et al. High expression of PTPRM predicts poor prognosis and promotes tumor growth and lymph node metastasis in cervical cancer. Cell Death Dis. 2020, 11, 687. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Zhang, N.; Du, X.; Xu, G.; Yan, X. CD146, from a melanoma cell adhesion molecule to a signaling receptor. Signal Transduct. Target. Ther. 2020, 5, 148. [Google Scholar] [CrossRef]
- Joshkon, A.; Heim, X.; Dubrou, C.; Bachelier, R.; Traboulsi, W.; Stalin, J.; Fayyad-Kazan, H.; Badran, B.; Foucault-Bertaud, A.; Leroyer, A.S.; et al. Role of CD146 (MCAM) in Physiological and Pathological Angiogenesis-Contribution of New Antibodies for Therapy. Biomedicines 2020, 8, 633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malamud, M.; Brown, G.D. The Dectin-1 and Dectin-2 clusters: C-type lectin receptors with fundamental roles in immunity. EMBO Rep. 2024, 25, 5239–5264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freund, P.; Porpaczy, E.A.; Le, T.; Gruber, M.; Pausz, C.; Staber, P.; Jäger, U.; Vanura, K. Cannabinoid Receptors Are Overexpressed in CLL but of Limited Potential for Therapeutic Exploitation. PLoS ONE 2016, 11, e0156693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopez-Cardona, A.P.; Pérez-Cerezales, S.; Fernández-González, R.; Laguna-Barraza, R.; Pericuesta, E.; Agirregoitia, N.; Gutiérrez-Adán, A.; Agirregoitia, E. CB1 cannabinoid receptor drives oocyte maturation and embryo development via PI3K/Akt and MAPK pathways. FASEB J. 2017, 31, 3372–3382. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, C.; Ochsenbein, A.F. Immune checkpoints regulate acute myeloid leukemia stem cells. Leukemia 2025, 39, 1277–1293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sauer, T.; Parikh, K.; Sharma, S.; Omer, B.; Sedloev, D.; Chen, Q.; Angenendt, L.; Schliemann, C.; Schmitt, M.; Müller-Tidow, C.; et al. CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity. Blood 2021, 138, 318–330. [Google Scholar] [CrossRef]
- Marques-Piubelli, M.L.; Kumar, B.; Basar, R.; Panowski, S.; Srinivasan, S.; Norwood, K.; Prashad, S.; Szenes, V.; Balakumaran, A.; Arandhya, A.; et al. Increased expression of CD70 in relapsed acute myeloid leukemia after hypomethylating agents. Virchows Arch. 2024, 485, 937–941. [Google Scholar] [CrossRef]
- Sun, Q.; Zabrocki, P.; Seyfried, F.; de Haard, H.; Kronnie, G.; Debatin, K.M.; Hinrich Meyer, L. P317, Anti-Leukemia Activity of Cd70-Directed Immunotherapy in B Cell Precursor Acute Lymphoblastic Leukemia. Hemasphere 2023, 7, e6339407. [Google Scholar] [CrossRef] [PubMed Central]
- Sawada, H.; Daugherty, A. BEST3-Mediated MEKK2/3 Activation: A Novel Therapeutic Target in Aortopathies. Circulation 2023, 148, 607–609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, W.K.; Chakraborty, P.K.; Roussa, E.; Wolff, N.A.; Thévenod, F. ERK1/2-dependent bestrophin-3 expression prevents ER-stress-induced cell death in renal epithelial cells by reducing CHOP. Biochim. Biophys. Acta 2012, 1823, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shahine, A.; Cheng, T.Y.; Chen, Y.L.; Ng, S.W.; Balaji, G.R.; Farquhar, R.; Gras, S.; Hardman, C.S.; Altman, J.D.; et al. CD1 lipidomes reveal lipid-binding motifs and size-based antigen-display mechanisms. Cell 2023, 186, 4583–4596.e13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bagchi, S.; Li, S.; Wang, C.R. CD1b-autoreactive T cells recognize phospholipid antigens and contribute to antitumor immunity against a CD1b+ T cell lymphoma. Oncoimmunology 2016, 5, e1213932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonzalez, R.; Reinberg, D. The Notch pathway: A guardian of cell fate during neurogenesis. Curr. Opin. Cell Biol. 2025, 95, 102543. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lanzas, R.; Barclay, J.; Hardas, A.; Kalampalika, F.; Jiménez-Pompa, A.; Gallipoli, P.; Ganuza, M. A CADASIL NOTCH3 mutation leads to clonal hematopoiesis and expansion of Dnmt3a-R878H hematopoietic clones. Leukemia 2025, 39, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Giuli, M.V.; Diluvio, G.; Giuliani, E.; Franciosa, G.; Di Magno, L.; Pignataro, M.G.; Tottone, L.; Nicoletti, C.; Besharat, Z.M.; Peruzzi, G.; et al. Notch3 contributes to T-cell leukemia growth via regulation of the unfolded protein response. Oncogenesis 2020, 9, 93. [Google Scholar] [CrossRef]
- Lu, J.; Xue, X.; Wang, H.; Hao, Y.; Yang, Q. Notch1 activation and inhibition in T-cell acute lymphoblastic leukemia subtypes. Exp. Hematol. 2025, 148, 104771. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Y.; Xin, S.; Jin, L.; Cao, Q.; Wang, H.; Wang, K.; Liu, X.; Tang, C.; Li, W.; et al. NOTCH3 promotes docetaxel resistance of prostate cancer cells through regulating TUBB3 and MAPK signaling pathway. Cancer Sci. 2024, 115, 412–426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.; Ge, H.; Shen, C.; Hu, D.; Zhao, X.; Qin, R.; Wang, Y. NOTCH3 promotes malignant progression of bladder cancer by directly regulating SPP1 and activating PI3K/AKT pathway. Cell Death Dis. 2024, 15, 840. [Google Scholar] [CrossRef]
- Gong, X.; Wang, X.; Jin, J.; Gong, Z. The novel functions of chemokines in lung cancer progression. Front. Immunol. 2025, 16, 1607225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maciocia, P.M.; Wawrzyniecka, P.A.; Maciocia, N.C.; Burley, A.; Karpanasamy, T.; Devereaux, S.; Hoekx, M.; O’Connor, D.; Leon, T.; Rapoz-D’Silva, T.; et al. Anti-CCR9 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia. Blood 2022, 140, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Qiao, X.; Fang, Y.; Guo, R.; Bai, P.; Liu, S.; Li, T.; Jiang, Y.; Wei, S.; Na, Z.; et al. Epigenetics-targeted drugs: Current paradigms and future challenges. Signal Transduct. Target. Ther. 2024, 9, 332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson-Holiday, C.; Singh, R.; Johnson, E.L.; Grizzle, W.E.; Lillard, J.W., Jr.; Singh, S. CCR9-CCL25 interactions promote cisplatin resistance in breast cancer cell through Akt activation in a PI3K-dependent and FAK-independent fashion. World J. Surg. Oncol. 2011, 9, 46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, E.L.; Singh, R.; Johnson-Holiday, C.M.; Grizzle, W.E.; Partridge, E.E.; Lillard, J.W., Jr.; Singh, S. CCR9 interactions support ovarian cancer cell survival and resistance to cisplatin-induced apoptosis in a PI3K-dependent and FAK-independent fashion. J. Ovarian Res. 2010, 3, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tu, Z.; Xiao, R.; Xiong, J.; Tembo, K.M.; Deng, X.; Xiong, M.; Liu, P.; Wang, M.; Zhang, Q. CCR9 in cancer: Oncogenic role and therapeutic targeting. J. Hematol. Oncol. 2016, 9, 10. [Google Scholar] [CrossRef]
- Fang, J.; Guo, L.; Zhang, Y.; Guo, Q.; Wang, M.; Wang, X. The target atlas for antibody-drug conjugates across solid cancers. Cancer Gene Ther. 2024, 31, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Tumey, L.N. An Overview of the Current ADC Discovery Landscape. Methods Mol. Biol. 2020, 2078, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Buongervino, S.; Lane, M.V.; Garrigan, E.; Zhelev, D.V.; Dimitrov, D.S.; Bosse, K.R. Antibody-Drug Conjugate Efficacy in Neuroblastoma: Role of Payload, Resistance Mechanisms, Target Density, and Antibody Internalization. Mol. Cancer Ther. 2021, 20, 2228–2239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.H.R.; Li, Z.; Tai, S.T.; Oh, B.L.Z.; Yeoh, A.E.J. Genetic Alterations in Childhood Acute Lymphoblastic Leukemia: Interactions with Clinical Features and Treatment Response. Cancers 2021, 13, 4068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrer, D.C.; Schenkel, C.; Berking, C.; Herr, W.; Abken, H.; Dörrie, J.; Schaft, N. Decitabine-Mediated Upregulation of CSPG4 in Ovarian Carcinoma Cells Enables Targeting by CSPG4-Specific CAR-T Cells. Cancers 2022, 14, 5033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshimura, S.; Li, Z.; Gocho, Y.; Yang, W.; Crews, K.R.; Lee, S.H.R.; Roberts, K.G.; Mullighan, C.G.; Relling, M.V.; Yu, J.; et al. Impact of Age on Pharmacogenomics and Treatment Outcomes of B-Cell Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2024, 42, 3478–3490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Apavaloaei, A.; Zhao, Q.; Hesnard, L.; Cahuzac, M.; Durette, C.; Larouche, J.D.; Hardy, M.P.; Vincent, K.; Brochu, S.; Laverdure, J.P.; et al. Tumor antigens preferentially derive from unmutated genomic sequences in melanoma and non-small cell lung cancer. Nat. Cancer 2025, 6, 1419–1437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).