Preformulation Studies of Novel Menthol Prodrugs with Antiparasitic Activity: Chemical Stability, In Silico, and In Vitro Permeability Assays
Abstract
:1. Introduction
2. Results
2.1. Stability Studies
2.1.1. Validation of GC Method
2.1.2. Identification of Degradation Compounds
2.1.3. Aqueous Stability
2.2. Permeability Studies
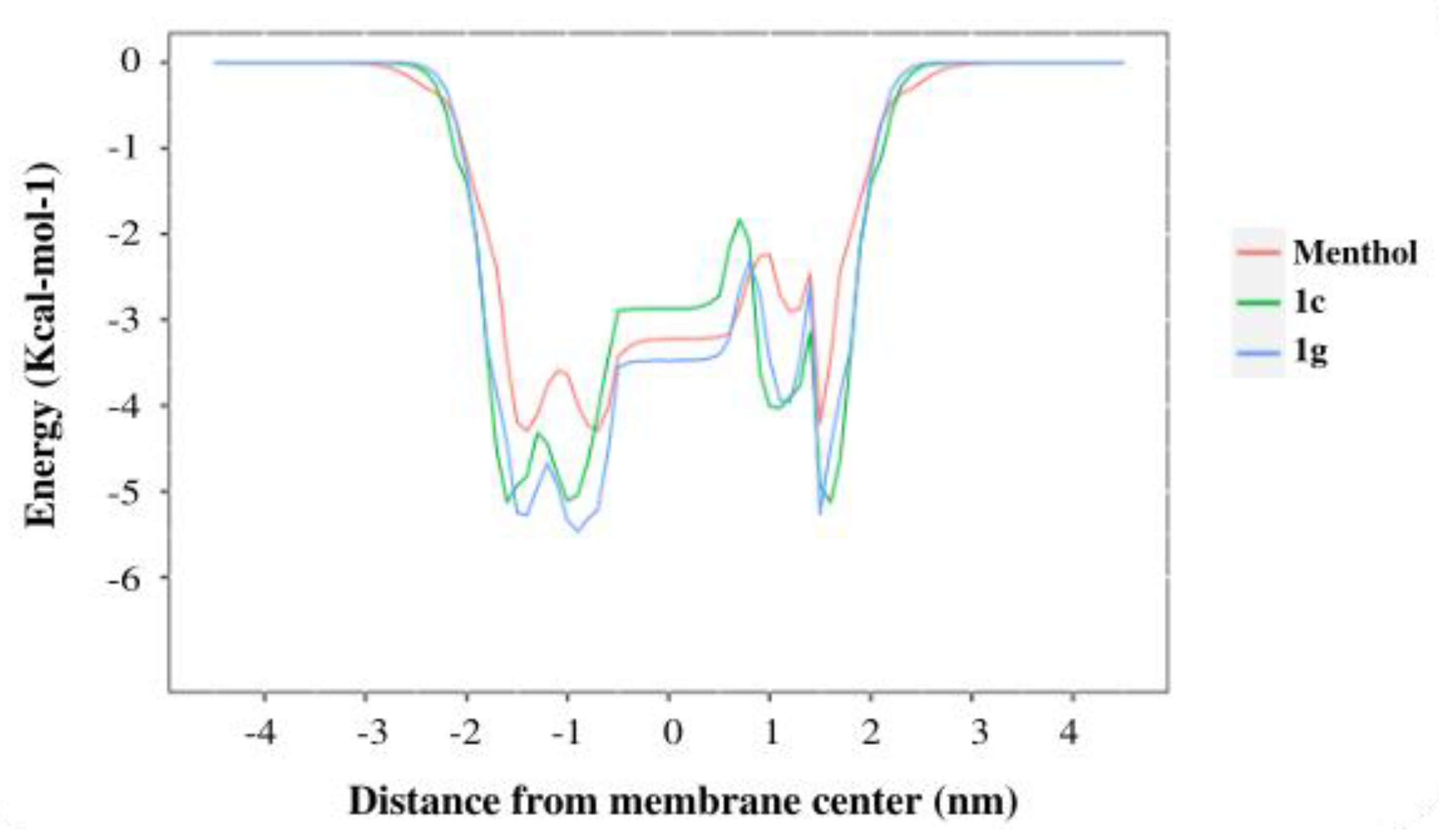
2.2.1. In Silico Assays
2.2.2. In Vitro Assays
3. Discussion
4. Materials and Methods
4.1. General Procedure for the Synthesis of Carbonates of Menthol
4.2. Stability Studies
4.2.1. GC Conditions
4.2.2. Validation of GC Method
4.2.3. Chemical Stability in Aqueous Buffers
4.3. Permeability Studies
4.3.1. In Silico Assays
4.3.2. In Vitro Assays
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto, G.B.; dos Reis Corrêa, A.; da Silva, G.N.C.; da Costa, J.S.; Figueiredo, P.L.B. Drug Development from Essential Oils: New Discoveries and Perspectives. In Drug Discovery and Design Using Natural Products; Cruz, J.N., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 79–101. ISBN 9783031352058. [Google Scholar]
- Boukhatem, M.N.; Setzer, W.N. Aromatic Herbs, Medicinal Plant-Derived Essential Oils, and Phytochemical Extracts as Potential Therapies for Coronaviruses: Future Perspectives. Plants 2020, 9, 800. [Google Scholar] [CrossRef]
- De Matos, S.P.; Teixeira, H.F.; de Lima, Á.A.N.; Veiga-Junior, V.F.; Koester, L.S. Essential Oils and Isolated Terpenes in Nanosystems Designed for Topical Administration: A Review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef]
- Jafri, H.; Ahmad, I. Thymus Vulgaris Essential Oil and Thymol Inhibit Biofilms and Interact Synergistically with Antifungal Drugs against Drug Resistant Strains of Candida Albicans and Candida Tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based. Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; Abd El-Hack, M.E. Using Essential Oils to Overcome Bacterial Biofilm Formation and Their Antimicrobial Resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef] [PubMed]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St Rose, T.; Puchalski, K.; Langland, J. Antimicrobial Activity of the Volatile Substances from Essential Oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular Mechanism of the Anti-Inflammatory Effects of Plant Essential Oils: A Systematic Review. J. Ethnopharmacol. 2023, 301, 115829. [Google Scholar] [CrossRef] [PubMed]
- Ben Ammar, R. Potential Effects of Geraniol on Cancer and Inflammation-Related Diseases: A Review of the Recent Research Findings. Molecules 2023, 28, 3669. [Google Scholar] [CrossRef]
- De Cássia da Silveira E Sá, R.; Andrade, L.N.; Dos Reis Barreto de Oliveira, R.; de Sousa, D.P. A Review on Anti-Inflammatory Activity of Phenylpropanoids Found in Essential Oils. Molecules 2014, 19, 1459–1480. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of Essential Oils in Food Safety: Antimicrobial and Antioxidant Applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; de Moraes, A.A.B.; da Costa, K.S.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; de Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential Oils as Anticancer Agents: Potential Role in Malignancies, Drug Delivery Mechanisms, and Immune System Enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants 2021, 11, 20. [Google Scholar] [CrossRef]
- De Mesquita, L.S.S.; Luz, T.R.S.A.; de Mesquita, J.W.C.; Coutinho, D.F.; do Amaral, F.M.M.; de Sousa Ribeiro, M.N.; Malik, S. Exploring the Anticancer Properties of Essential Oils from Family Lamiaceae. Food Rev. Int. 2019, 35, 105–131. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A Simple Monoterpene with Remarkable Biological Properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, S.; Kumar, A.; Kumar, P.; Narasimhan, B. Synthesis, Antimicrobial Evaluation, and QSAR Analysis of 2-Isopropyl-5-Methylcyclohexanol Derivatives. Med. Chem. Res. 2012, 21, 511–522. [Google Scholar] [CrossRef]
- Clemente, C.M.; Ravetti, S.; Allemandi, D.A.; Hergert, L.Y.; Pineda, T.; Robledo, S.M. Synthesis, In Vitro Antiprotozoal Activity and Cytotoxicity of New Thymol Carbonate Derivatives. ChemistrySelect 2021, 6, 6597–6600. [Google Scholar] [CrossRef]
- Clemente, C.M.; Pineda, T.; Yepes, L.M.; Upegui, Y.; Allemandi, D.A.; Robledo, S.M.; Ravetti, S. Eugenol Carbonate Activity against Plasmodium Falciparum, Leishmania Braziliensis, and Trypanosoma Cruzi. Arch. Pharm. 2022, 355, e2100432. [Google Scholar] [CrossRef] [PubMed]
- Clemente, C.M.; Robledo, S.M.; Ravetti, S. Menthol Carbonates as Potent Antiparasitic Agents: Synthesis and in Vitro Studies along with Computer-Aided Approaches. BMC Complement. Med. Ther. 2022, 22, 156. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, J.; Clemente, C.M.; Elissondo, N.; Gambino, G.; Ravetti, S.; Hergert, L.Y.; Palma, S.D.; Elissondo, M.C. Anti-Echinococcal Activity of Menthol and a Novel Prodrug, Menthol-Pentanol, against Echinococcus multilocularis. Acta Tropica 2020, 205, 105411. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, R.; Mazumder, A.; Salahuddin; Yadav, R.K.; Chauhan, B.; Abdulah, M.M. Camphor and Menthol as Anticancer Agents: Synthesis, Structure-Activity Relationship and Interaction with Cancer Cell Lines. Anticancer Agents Med. Chem. 2023, 23, 614–623. [Google Scholar] [PubMed]
- Szymczak, J.; Sobotta, L.; Dlugaszewska, J.; Kryjewski, M.; Mielcarek, J. Menthol Modified zinc(II) Phthalocyanine Regioisomers and Their Photoinduced Antimicrobial Activity against Staphylococcus Aureus. Dyes Pigm. 2021, 193, 109410. [Google Scholar] [CrossRef]
- Daryadel, S.; Atmaca, U.; Taslimi, P.; Gülçin, İ.; Çelik, M. Novel Sulfamate Derivatives of Menthol: Synthesis, Characterization, and Cholinesterases and Carbonic Anhydrase Enzymes Inhibition Properties. Arch. Pharm. 2018, 351, e1800209. [Google Scholar] [CrossRef]
- Ben Miri, Y.; Nouasri, A.; Herrera, M.; Djenane, D.; Ariño, A. Antifungal Activity of Menthol, Eugenol and Their Combination against Aspergillus Ochraceus and Aspergillus Niger In Vitro and in Stored Cereals. Foods 2023, 12, 2108. [Google Scholar] [CrossRef]
- Phunpee, S.; Saesoo, S.; Sramala, I.; Jarussophon, S.; Sajomsang, W.; Puttipipatkhachorn, S.; Soottitantawat, A.; Ruktanonchai, U.R. A Comparison of Eugenol and Menthol on Encapsulation Characteristics with Water-Soluble Quaternized β-Cyclodextrin Grafted Chitosan. Int. J. Biol. Macromol. 2016, 84, 472–480. [Google Scholar] [CrossRef]
- Dittert, L.W.; Higuchi, T. Rates of hydrolysis of carbamate and carbonate esters in alkaline solution. J. Pharm. Sci. 1963, 52, 852–857. [Google Scholar] [CrossRef]
- N’da, D.D.; Breytenbach, J.C. Synthesis of Methoxypoly(ethylene Glycol) Carbonate Prodrugs of Zidovudine and Penetration through Human Skin in Vitro. J. Pharm. Pharmacol. 2009, 61, 721–731. [Google Scholar] [CrossRef]
- Delrivo, A.; Aloisio, C.; Longhi, M.R.; Granero, G. Artificial Lipid Membrane Permeability Method for Predicting Intestinal Drug Transport: Probing the Determining Step in the Oral Absorption of Sulfadiazine; Influence of the Formation of Binary and Ternary Complexes with Cyclodextrins. AAPS PharmSciTech 2018, 19, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Zoratti, M. Prodrugs of Quercetin and Resveratrol: A Strategy under Development. Curr. Drug Metab. 2014, 15, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of Membrane Toxicity of Hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving Cellular Uptake of Therapeutic Entities through Interaction with Components of Cell Membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef]
- Lomize, A.L.; Hage, J.M.; Schnitzer, K.; Golobokov, K.; LaFaive, M.B.; Forsyth, A.C.; Pogozheva, I.D. PerMM: A Web Tool and Database for Analysis of Passive Membrane Permeability and Translocation Pathways of Bioactive Molecules. J. Chem. Inf. Model. 2019, 59, 3094–3099. [Google Scholar] [CrossRef]
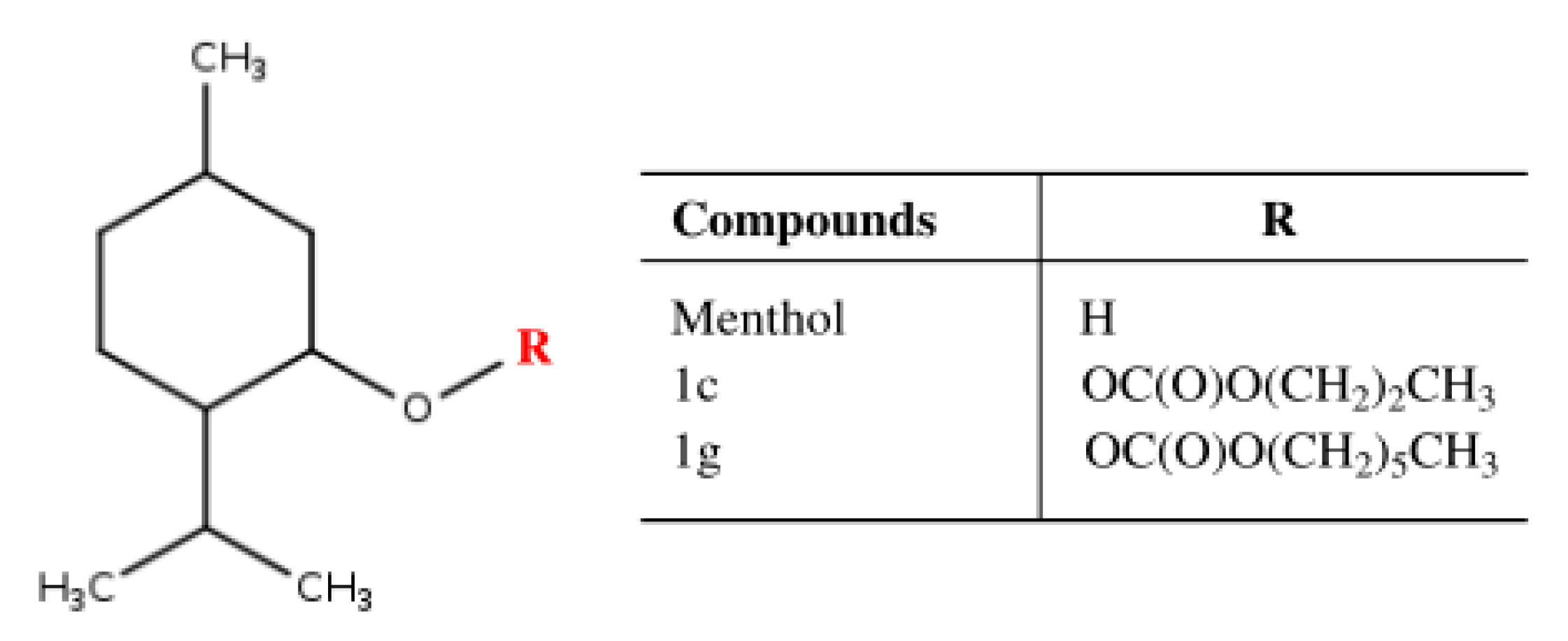

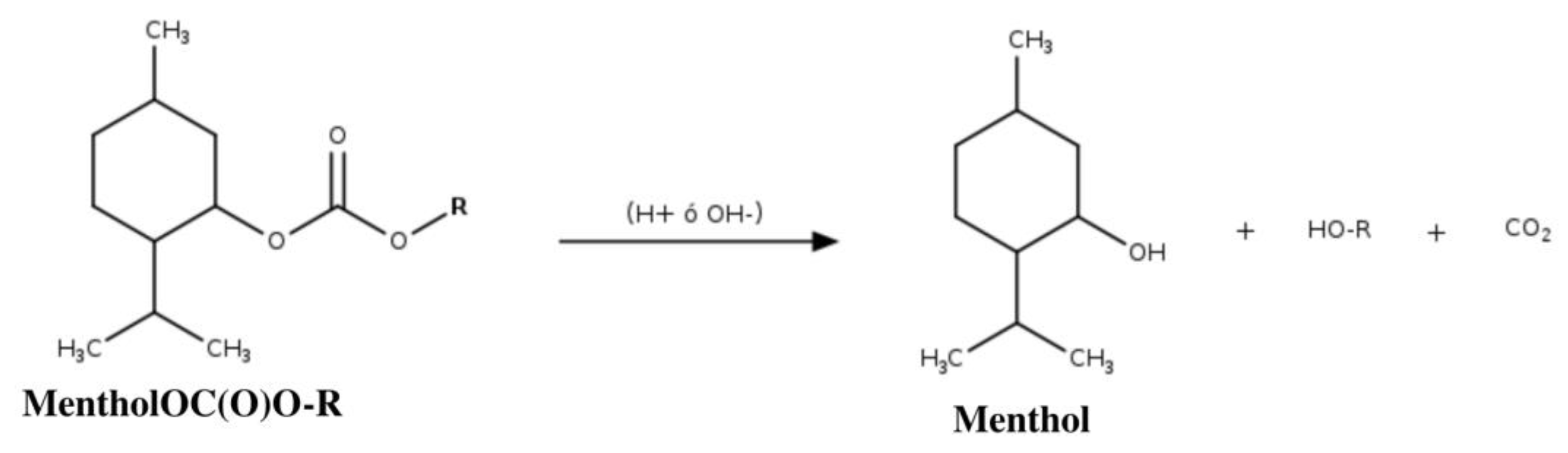
| Menthol Validation Parameters | ||
|---|---|---|
| y = ax + b | a ± (s t) | 1.8 × 104 ± (1.8 × 102) |
| b ± (s t) | 0.07 ± 0.01 | |
| r | 0.99 | |
| Limit of detection (LOD) | 3.9 × 10−6 | |
| Limit of quantification (LOQ) | 9.4 × 10−3 | |
| Recovery (% REC) | 100.0; 102.7; 100.5 | |
| Precision (RSD) | 1.4; 3.7 | |
| Retention time (tR) | Menthol | 6.07 |
| 1c | 8.57 | |
| 1g | 12.64 | |
| Compound | pH 1.2 | pH 5.8 | pH 7.4 | |||
|---|---|---|---|---|---|---|
| k (min−1) | t1/2 (min) | k (h−1) | t1/2 (h) | k (h−1) | t1/2 (h) | |
| 1c | 0.007 | 99.00 | 0.007 | 99.00 | 0.010 | 69.31 |
| 1g | 0.007 | 99.00 | 0.007 | 115.52 | 0.010 | 69.31 |
| Compounds | Free Energy of Binding (DOPC) |
|---|---|
| Menthol | −4.32 |
| 1c | −5.13 |
| 1g | −5.41 |
| Compound | cLogP | Papp (10−4) (cm.s−1) | Jss (10−5) (mg.s−1) |
|---|---|---|---|
| Menthol | 3.33 | 2.75 ± 0.59 | 3.96 ± 0.85 |
| 1c | 4.68 | 3.50 ± 1.66 | 1.23 ± 0.61 |
| 1g | 6.25 | 5.85 ± 4.15 | 2.99 ± 2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente, C.M.; Onnainty, R.; Usseglio, N.; Granero, G.E.; Ravetti, S. Preformulation Studies of Novel Menthol Prodrugs with Antiparasitic Activity: Chemical Stability, In Silico, and In Vitro Permeability Assays. Drugs Drug Candidates 2023, 2, 770-780. https://doi.org/10.3390/ddc2030038
Clemente CM, Onnainty R, Usseglio N, Granero GE, Ravetti S. Preformulation Studies of Novel Menthol Prodrugs with Antiparasitic Activity: Chemical Stability, In Silico, and In Vitro Permeability Assays. Drugs and Drug Candidates. 2023; 2(3):770-780. https://doi.org/10.3390/ddc2030038
Chicago/Turabian StyleClemente, Camila M., Renée Onnainty, Nadina Usseglio, Gladys E. Granero, and Soledad Ravetti. 2023. "Preformulation Studies of Novel Menthol Prodrugs with Antiparasitic Activity: Chemical Stability, In Silico, and In Vitro Permeability Assays" Drugs and Drug Candidates 2, no. 3: 770-780. https://doi.org/10.3390/ddc2030038
APA StyleClemente, C. M., Onnainty, R., Usseglio, N., Granero, G. E., & Ravetti, S. (2023). Preformulation Studies of Novel Menthol Prodrugs with Antiparasitic Activity: Chemical Stability, In Silico, and In Vitro Permeability Assays. Drugs and Drug Candidates, 2(3), 770-780. https://doi.org/10.3390/ddc2030038







