Current Evidence of Natural Products against Overweight and Obesity: Molecular Targets and Mechanisms of Action
Abstract
1. Introduction
1.1. Main Categories of Secondary Metabolites
1.1.1. Polyphenols
1.1.2. Alkaloids
1.1.3. Saponins
1.1.4. Terpenes
1.2. Relationship between Lifestyle, Oxidative Stress, and Inflammation in Overweight and Obesity
2. Action Mechanisms of Phytochemicals against Overweight and Obesity
2.1. Lipase Inhibitors
2.2. Regulation of Adipogenesis
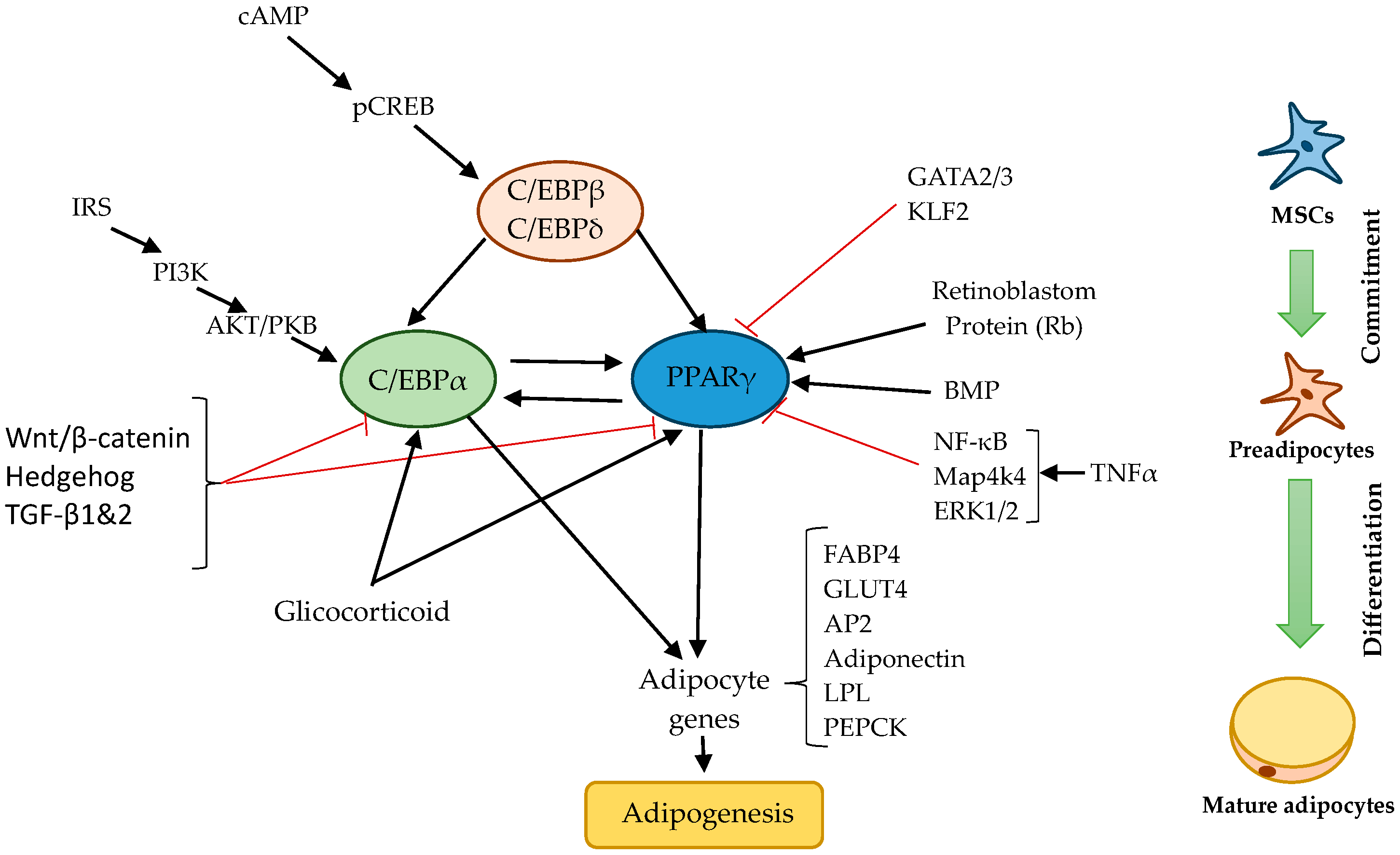
2.3. Thermogenesis
2.4. Appetite Suppressants
3. Polyphenols Modulating Overweight and Obesity
4. Alkaloids Modulating Overweight and Obesity
5. Saponins Modulating Overweight and Obesity
6. Terpenes Modulating Overweight and Obesity
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 January 2024).
- Centers for Disease Control and Prevention. Adult Obesity Facts. Available online: https://www.cdc.gov/obesity/data/adult.html (accessed on 10 January 2024).
- National Institute of Diabetes and Digestive and Kidney Diseases. Overweight and Obesity Statistics. Available online: https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity (accessed on 10 January 2024).
- Pledger, S.L.; Ahmadizar, F. Gene-environment interactions and the effect on obesity risk in low and middle-income countries: A scoping review. Front. Endocrinol. 2023, 14, 1230445. [Google Scholar] [CrossRef] [PubMed]
- Withrow, D.; Alter, D.A. The economic burden of obesity worldwide: A systematic review of the direct costs of obesity. Obes. Rev. 2011, 12, 131–141. [Google Scholar] [CrossRef]
- González-Castejón, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D. Pharmacological management of obesity: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Schindler, C.; Engeli, S. Modern pharmacological treatment of obese patients. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018819897527. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Ellwood, L.; Green, H.; Fernandez, R. Pharmacological Treatment for Obesity in Adults: An Umbrella Review. Ann. Pharmacother. 2020, 54, 106002801989891. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic. Prescription Weight-Loss Drugs. Available online: https://www.mayoclinic.org/healthy-lifestyle/weight-loss/in-depth/weight-loss-drugs/art-20044832 (accessed on 20 December 2023).
- Li, X.; Zheng, L.; Zhang, B.; Deng, Z.Y.; Luo, T. The Structure Basis of Phytochemicals as Metabolic Signals for Combating Obesity. Front. Nutr. 2022, 9, 913883. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23, 3–16. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Barquera, S.; Barata Cavalcanti, O.; Ralston, J. The Global Pandemic of Overweight and Obesity. In Handbook of Global Health; Kickbusch, I., Ganten, D., Moeti, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 739–773. [Google Scholar]
- Ahmad, B.; Friar, E.P.; Vohra, M.S.; Garrett, M.D.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Mechanisms of action for the anti-obesogenic activities of phytochemicals. Phytochemistry 2020, 180, 112513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, D.D.; Lakhawat, S.S.; Yasmeen, N.; Pandey, A.; Singla, R.K. Biogenic Phytochemicals Modulating Obesity: From Molecular Mechanism to Preventive and Therapeutic Approaches. Evid.-Based Complement. Altern. Med. 2022, 2022, 6852276. [Google Scholar] [CrossRef]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Leyva-López, N.; Vazquez-Olivo, G.; Heredia, J.B. Oregano as a potential source of antidiabetic agents. J. Food Biochem. 2022, 46, e14388. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Molath, A.; Choksi, H.; Kumar, S.; Mehra, R. Classifications of polyphenols and their potential application in human health and diseases. Int. J. Physiol. Nutr. Phys. Educ. 2021, 6, 293–301. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Kukula-Koch, W.; Widelski, J. Alkaloids; Academic Press: Cambridge, MA, USA, 2017; pp. 163–198. [Google Scholar]
- Abookleesh, F.L.; Al-Anzi, B.S.; Ullah, A. Potential Antiviral Action of Alkaloids. Molecules 2022, 27, 903. [Google Scholar] [CrossRef]
- Roberts, M.F. Alkaloids: Biochemistry, Ecology, and Medicinal Applications; Springer US: New York, NY, USA, 2013. [Google Scholar]
- Aguilar Salguero, S.A. Relación de la estructura de las saponinas con sus aplicaciones, una revisión actualizada; UCE: Quito, Ecuador, 2022. [Google Scholar]
- Brahmkshatriya, P.P.; Brahmkshatriya, P.S. Terpenes: Chemistry, Biological Role, and Therapeutic Applications. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2665–2691. [Google Scholar]
- Roba, K. The role of terpene (secondary metabolite). Nat. Prod. Chem. Res. 2020, 9, 1000. [Google Scholar]
- Mabou, F.D.; Yossa, I.B.N. TERPENES: Structural classification and biological activities. IOSR J. Pharm. Biol. Sci. 2021, 16, 2319–7676. [Google Scholar]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Biobaku, F.; Ghanim, H.; Batra, M.; Dandona, P. Macronutrient-Mediated Inflammation and Oxidative Stress: Relevance to Insulin Resistance, Obesity, and Atherogenesis. J. Clin. Endocrinol. Metab. 2019, 104, 6118–6128. [Google Scholar] [CrossRef]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Reductive Stress in Inflammation-Associated Diseases and the Pro-Oxidant Effect of Antioxidant Agents. Int. J. Mol. Sci. 2017, 18, 2098. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Sikaris, K.A. The clinical biochemistry of obesity. Clin. Biochem. Rev. 2004, 25, 165. [Google Scholar]
- Boix-Castejón, M.; Herranz-López, M.; Pérez Gago, A.; Olivares-Vicente, M.; Caturla, N.; Roche, E.; Micol, V. Hibiscus and lemon verbena polyphenols modulate appetite-related biomarkers in overweight subjects: A randomized controlled trial. Food Funct. 2018, 9, 3173–3184. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Olivares-Vicente, M.; Boix-Castejón, M.; Caturla, N.; Roche, E.; Micol, V. Differential effects of a combination of Hibiscus sabdariffa and Lippia citriodora polyphenols in overweight/obese subjects: A randomized controlled trial. Sci. Rep. 2019, 9, 2999. [Google Scholar] [CrossRef]
- López-Tenorio, I.I.; Domínguez-López, A.; Miliar-García, Á.; Escalona-Cardoso, G.N.; Real-Sandoval, S.A.; Gómez-Alcalá, A.; Jaramillo-Flores, M.E. Modulation of the mRNA of the Nlrp3 inflammasome by Morin and PUFAs in an obesity model induced by a high-fat diet. Food Res. Int. 2020, 137, 109706. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, S.; Lambert, J.D. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. Eur. J. Nutr. 2014, 53, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.N.; Xiang, J.Z.; Qi, Z.; Du, M. Plant extracts in prevention of obesity. Crit. Rev. Food Sci. Nutr. 2022, 62, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Yu, H.; Gao, L.; Lu, Y.-T.; Xu, Z.; Liu, H.; Gu, L.-Q.; Ye, J.-M.; Huang, Z.-S. Natural alkaloid bouchardatine ameliorates metabolic disorders in high-fat diet-fed mice by stimulating the sirtuin 1/liver kinase B-1/AMPK axis. Br. J. Pharmacol. 2017, 174, 2457–2470. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Lian, C.-F.; Sun, Q.-W.; Wang, T.-T.; Liu, Y.-Y.; Ye, J.; Gao, L.-L.; Yang, Y.-F.; Liu, S.-N.; Shen, Z.-F.; et al. Ramulus Mori (Sangzhi) Alkaloids Alleviate High-Fat Diet-Induced Obesity and Nonalcoholic Fatty Liver Disease in Mice. Antioxidants 2022, 11, 905. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis 2015, 243, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Woo, S.L.; Guo, X.; Li, H.; Zheng, J.; Botchlett, R.; Liu, M.; Pei, Y.; Xu, H.; Cai, Y.; et al. Berberine Ameliorates Hepatic Steatosis and Suppresses Liver and Adipose Tissue Inflammation in Mice with Diet-induced Obesity. Sci. Rep. 2016, 6, 22612. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, X.; Feng, X.; Liu, X.; Deng, C.; Hu, C.H. Berberine Alleviates Olanzapine-Induced Adipogenesis via the AMPKα-SREBP Pathway in 3T3-L1 Cells. Int. J. Mol. Sci. 2016, 17, 1865. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, Y. Berberine alleviates hepatic lipid accumulation by increasing ABCA1 through the protein kinase C δ pathway. Biochem. Biophys. Res. Commun. 2018, 498, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Ma, S.R.; Zuo, Z.Y.; Wu, Y.B.; Kong, W.J.; Wang, A.P.; Jiang, J.D. Berberine inhibits adipocyte differentiation, proliferation and adiposity through down-regulating galectin-3. Sci. Rep. 2019, 9, 13415. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, T.; Ma, G.; Zheng, L.; Jiang, X.; Yang, F.; Wang, Z.; Li, N.; He, Z.; Song, X.; et al. Berberine modulates deacetylation of PPARγ to promote adipose tissue remodeling and thermogenesis via AMPK/SIRT1 pathway. Int. J. Biol. Sci. 2021, 17, 3173–3187. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hou, T.; Gao, Z.; Guo, X.; Wang, C.; Wang, J.; Liu, Y.; Liang, X. Discovery of eight alkaloids with D1 and D2 antagonist activity in leaves of Nelumbo nucifera Gaertn. Using FLIPR assays. J. Ethnopharmacol. 2021, 278, 114335. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Xia, J.; Xu, J.-f.; Chen, L.; Yang, Y.; Wu, J.-J.; Tang, F.; Ao, H.; Peng, C. Nuciferine, an active ingredient derived from lotus leaf, lights up the way for the potential treatment of obesity and obesity-related diseases. Pharmacol. Res. 2022, 175, 106002. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, C.; Liu, M.; Chen, L.; Zhu, Y.; Gao, W.; Du, X.; Song, Y.; Li, X.; Liu, G. Nuciferine ameliorates nonesterified fatty acid-induced bovine mammary epithelial cell lipid accumulation, apoptosis, and impaired migration via activating LKB1/AMPK signaling pathway. J. Agric. Food Chem. 2022, 71, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.-T.; Liao, J.-B.; Yang, Z.-X.; Cui, H.-T.; Zhang, Z.-Y.; Wen, W.-B.; Wang, H.-W. Effect of nuciferine on gut microbiota and inflammatory response in obese model mice. China J. Chin. Mater. Medica 2021, 46, 2104–2111. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, T.; Zou, L.; Gong, Y.; Wu, B.; Yi, Z.; Jia, T.; Zhao, S.; Shi, L.; Li, L.; et al. Evodiamine Attenuates P2X(7)-Mediated Inflammatory Injury of Human Umbilical Vein Endothelial Cells Exposed to High Free Fatty Acids. Oxid. Med. Cell. Longev. 2018, 2018, 5082817. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Huang, K.; Zhou, J. Hepatic AMP Kinase as a Potential Target for Treating Nonalcoholic Fatty Liver Disease: Evidence from Studies of Natural Products. Curr. Med. Chem. 2018, 25, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Lee, H.S.; Han, H.-K.; Choi, C.-I. Saikosaponin A and D Inhibit Adipogenesis via the AMPK and MAPK Signaling Pathways in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2021, 22, 11409. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Khan, M.Z.; Shin, J.H.; Shin, T.S.; Lee, Y.B.; Kim, M.Y.; Kim, J.D. Pharmacological Approaches to Attenuate Inflammation and Obesity with Natural Products Formulations by Regulating the Associated Promoting Molecular Signaling Pathways. BioMed Res. Int. 2021, 2021, 2521273. [Google Scholar] [CrossRef]
- Wang, F.; Park, J.-S.; Ma, Y.; Ma, H.; Lee, Y.-J.; Lee, G.-R.; Yoo, H.-S.; Hong, J.-T.; Roh, Y.-S. Ginseng Saponin Enriched in Rh1 and Rg2 Ameliorates Nonalcoholic Fatty Liver Disease by Inhibiting Inflammasome Activation. Nutrients 2021, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-T.; Nakamura, Y.; Akasaka, T.; Katakura, Y.; Tanaka, Y.; Shirouchi, B.; Jiang, Z.; Yuan, X.; Sato, M. Soyasaponin ameliorates obesity and reduces hepatic triacylglycerol accumulation by suppressing lipogenesis in high-fat diet-fed mice. J. Food Sci. 2021, 86, 2103–2117. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lim, S.M.; Jung, J.I.; Kim, S.M.; Lee, J.K.; Kim, Y.H.; Cha, K.M.; Oh, T.K.; Moon, J.M.; Kim, T.Y.; et al. Gynostemma Pentaphyllum Extract Ameliorates High-Fat Diet-Induced Obesity in C57BL/6N Mice by Upregulating SIRT1. Nutrients 2019, 11, 2475. [Google Scholar] [CrossRef] [PubMed]
- Peet, D.J.; Turley, S.D.; Ma, W.; Janowski, B.A.; Lobaccaro, J.-M.A.; Hammer, R.E.; Mangelsdorf, D.J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell 1998, 93, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Pachura, N.; Kupczyński, R.; Lewandowska, K.; Włodarczyk, M.; Klemens, M.; Kuropka, P.; Nowaczyk, R.; Krzystek-Korpacka, M.; Bednarz-Misa, I.; Sozański, T.; et al. Biochemical and Molecular Investigation of the Effect of Saponins and Terpenoids Derived from Leaves of Ilex aquifolium on Lipid Metabolism of Obese Zucker Rats. Molecules 2022, 27, 3376. [Google Scholar] [CrossRef]
- Lahrita, L.; Moriai, K.; Iwata, R.; Itoh, K.; Kato, E. Quassinoids in Brucea javanica are Potent Stimulators of Lipolysis in Adipocytes. Fitoterapia 2019, 137, 104250. [Google Scholar] [CrossRef] [PubMed]
- Tammam, M.A.; Aly, O.; Pereira, F.; Mahdy, A.; El-Demerdash, A. Unveiling the Potential of Marine-Derived Diterpenes from the order Alcyonacea as Promising Anti-obesity Agents. Curr. Res. Biotechnol. 2024, 7, 100175. [Google Scholar] [CrossRef]
- Rahim, A.T.M.A.; Takahashi, Y.; Yamaki, K. Mode of pancreatic lipase inhibition activity in vitro by some flavonoids and non-flavonoid polyphenols. Food Res. Int. 2015, 75, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Ràfols, E.M. Tejido adiposo: Heterogeneidad celular y diversidad funcional. Endocrinol. Y Nutr. 2014, 61, 100–112. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From Stem Cell to Adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Nic-Can, G.I.; Rodas-Junco, B.A.; Carrillo-Cocom, L.M.; Zepeda-Pedreguera, A.; Peñaloza-Cuevas, R.; Aguilar-Ayala, F.J.; Rojas-Herrera, R.A. Epigenetic Regulation of Adipogenic Differentiation by Histone Lysine Demethylation. Int. J. Mol. Sci. 2019, 20, 3918. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, D.; Zeng, R.; Xian, T.; Lu, Y.; Zeng, G.; Sun, Z.; Huang, B.; Huang, Q. Epigallocatechin-3-gallate inhibits adipogenesis through down-regulation of PPARγ and FAS expression mediated by PI3K-AKT signaling in 3T3-L1 cells. Eur. J. Pharmacol. 2017, 795, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Balaji, M.; Ganjayi, M.S.; Hanuma Kumar, G.E.N.; Parim, B.N.; Mopuri, R.; Dasari, S. A review on possible therapeutic targets to contain obesity: The role of phytochemicals. Obes. Res. Clin. Pract. 2016, 10, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Khalilpourfarshbafi, M.; Gholami, K.; Murugan, D.D.; Abdul Sattar, M.Z.; Abdullah, N.A. Differential effects of dietary flavonoids on adipogenesis. Eur. J. Nutr. 2019, 58, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Li, Y.; Shu, T.; Wang, J. Cytokines and inflammation in adipogenesis: An updated review. Front. Med. 2019, 13, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.H.; Ka, S.; Kim, A.Y.; Kim, J.B. Regulation of Adipocyte Differentiation via MicroRNAs. Endocrinol. Metab. 2014, 29, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.E.; O’Rahilly, S.; Rochford, J.J. Adipogenesis at a glance. J. Cell Sci. 2011, 124, 2681–2686. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Virtue, S.; Goie, J.Y.G.; Png, C.W.; Guo, J.; Li, Y.; Jiao, H.; Chua, Y.L.; Campbell, M.; Moreno-Navarrete, J.M.; et al. Regulation of adipogenic differentiation and adipose tissue inflammation by interferon regulatory factor 3. Cell Death Differ. 2021, 28, 3022–3035. [Google Scholar] [CrossRef] [PubMed]
- Linda, C.; Urarat, N.; Rawiwun, K.; Sudarat, H.; Wanwisa, S. Inhibitory Effect of Carallia Brachiata Extract Through Regulation of Adipogenesis Pathways in 3T3-L1 Cells. Pharmacogn. J. 2022, 14, 655–660. [Google Scholar] [CrossRef]
- Audano, M.; Pedretti, S.; Caruso, D.; Crestani, M.; De Fabiani, E.; Mitro, N. Regulatory mechanisms of the early phase of white adipocyte differentiation: An overview. Cell. Mol. Life Sci. 2022, 79, 139. [Google Scholar] [CrossRef] [PubMed]
- Pant, R.; Firmal, P.; Shah, V.K.; Alam, A.; Chattopadhyay, S. Epigenetic Regulation of Adipogenesis in Development of Metabolic Syndrome. Front. Cell Dev. Biol. 2020, 8, 619888. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Zhu, X.; Maretich, P.; Chen, Y. Combating Obesity With Thermogenic Fat: Current Challenges and Advancements. Front. Endocrinol. 2020, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, X.; Chen, Y. Recruitment of Thermogenic Fat: Trigger of Fat Burning. Front. Endocrinol. 2021, 12, 696505. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Kim, M.-S. Molecular Mechanisms of Appetite Regulation. Diabetes Metab. J. 2012, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M.; Elkhayat, E.S.; El Dine, R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef]
- Hussain, S.S.; Bloom, S.R. The regulation of food intake by the gut-brain axis: Implications for obesity. Int. J. Obes. 2013, 37, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural Products with Anti-obesity Effects and Different Mechanisms of Action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Tamtaji, O.R.; Lankarani, K.B.; Akbari, M.; Dadgostar, E.; Dabbaghmanesh, M.H.; Kolahdooz, F.; Shamshirian, A.; Momen-Heravi, M.; Asemi, Z. The effects of resveratrol intake on weight loss: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 375–390. [Google Scholar] [CrossRef]
- Hillsley, A.; Chin, V.; Li, A.; McLachlan, C.S. Resveratrol for Weight Loss in Obesity: An Assessment of Randomized Control Trial Designs in ClinicalTrials.gov. Nutrients 2022, 14, 1424. [Google Scholar] [CrossRef]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; López-Martínez, L.X.; Contreras-Angulo, L.A.; Elizalde-Romero, C.A.; Heredia, J.B. Plant Alkaloids: Structures and Bioactive Properties. In Plant-Derived Bioactives: Chemistry and Mode of Action; Swamy, M.K., Ed.; Springer Singapore: Singapore, 2020; pp. 85–117. [Google Scholar]
- Sun, S.; Yang, Y.; Xiong, R.; Ni, Y.; Ma, X.; Hou, M.; Chen, L.; Xu, Z.; Chen, L.; Ji, M. Oral berberine ameliorates high-fat diet-induced obesity by activating TAS2Rs in tuft and endocrine cells in the gut. Life Sci. 2022, 311, 121141. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Singh, S.; Sharma, N.; Zahoor, I.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Najmi, A.; Bungau, S. Expatiating the Pharmacological and Nanotechnological Aspects of the Alkaloidal Drug Berberine: Current and Future Trends. Molecules 2022, 27, 3705. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, Z.; Zhao, X.; Liao, C.; Quan, J.; Bode, A.M.; Cao, Y.; Luo, X. Natural alkaloid and polyphenol compounds targeting lipid metabolism: Treatment implications in metabolic diseases. Eur. J. Pharmacol. 2020, 870, 172922. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Geng, C.A.; Huang, X.Y.; Ma, Y.B.; Zheng, X.H.; Yang, T.H.; Chen, X.L.; Yin, X.J.; Zhang, X.M.; Chen, J.J. Bioassay-guided isolation of saikosaponins with agonistic activity on 5-hydroxytryptamine 2C receptor from Bupleurum chinense and their potential use for the treatment of obesity. Chin. J. Nat. Med. 2017, 15, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, M.; Jin, Z.; Yaqoob, S.; Zheng, M.; Cai, D.; Liu, J.; Guo, S. Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct. 2019, 10, 3603–3614. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Huh, T.-L.; Kim, S.-Y.; Oh, M.-R.; Tirupathi Pichiah, P.B.; Chae, S.-W.; Cha, Y.-S. Antiobesity effect of Gynostemma pentaphyllum extract (actiponin): A randomized, double-blind, placebo-controlled trial. Obesity 2014, 22, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, W.; Zhang, Y.; Di, L.; Shan, J. A review of saponin intervention in metabolic syndrome suggests further study on intestinal microbiota. Pharmacol. Res. 2020, 160, 105088. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Mubarak, M.S. Anti-obesity Effect of Plant Diterpenes and their Derivatives: A Review. Phytotherapy Res. 2020, 34, 1216–1225. [Google Scholar] [CrossRef]
- Goto, T.; Takahashi, N.; Hirai, S.; Kawada, T. Various Terpenoids Derived from Herbal and Dietary Plants Function as PPAR Modulators and Regulate Carbohydrate and Lipid Metabolism. PPAR Res. 2010, 2010, 483958. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, I.D.; Angdisen, J.; Moran, E.; Schulman, I.G. Differential Regulation of Gene Expression by LXRs in Response to Macrophage Cholesterol Loading. Mol. Endocrinol. 2013, 27, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.; Kumar, A.; Chahal, J.; Dalal, S.; Kataria, S.K. A Review on Obesity Management through Natural Compounds and a Green Nanomedicine-Based Approach. Molecules 2021, 26, 3278. [Google Scholar] [CrossRef] [PubMed]
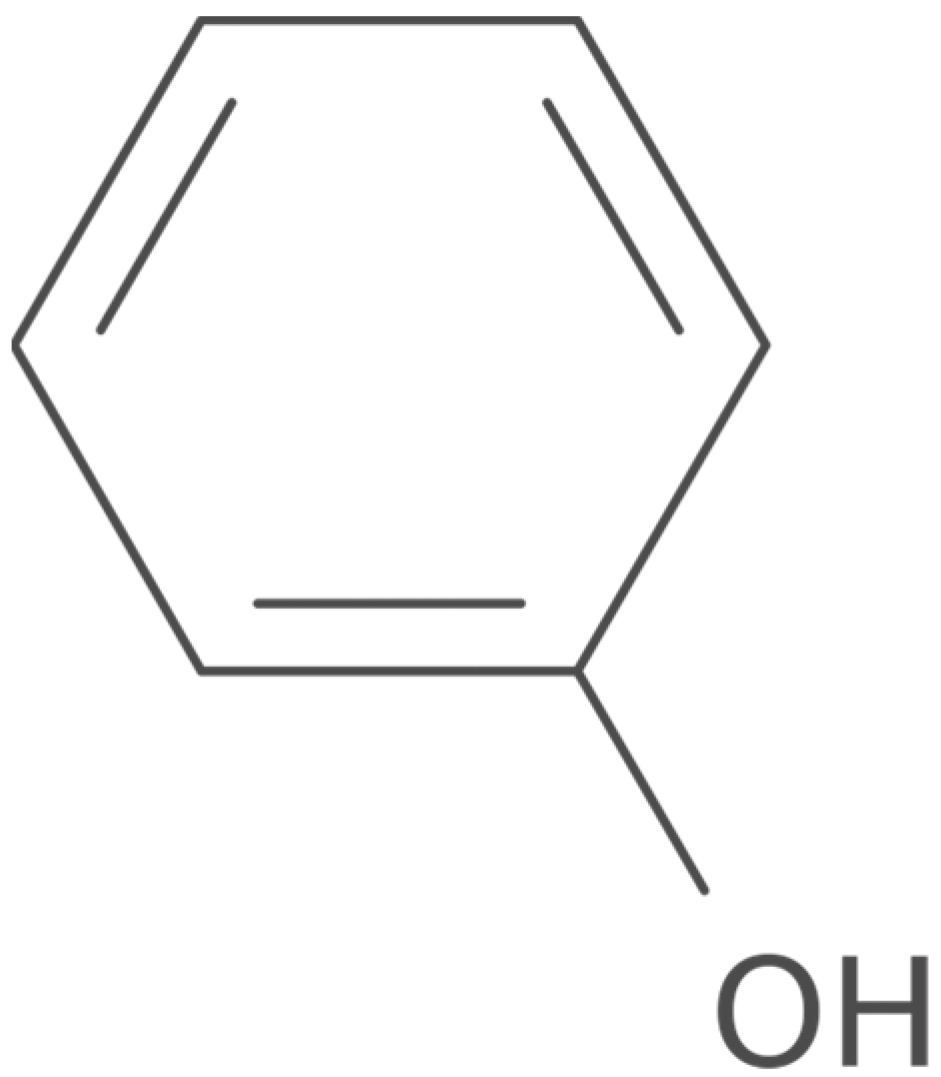
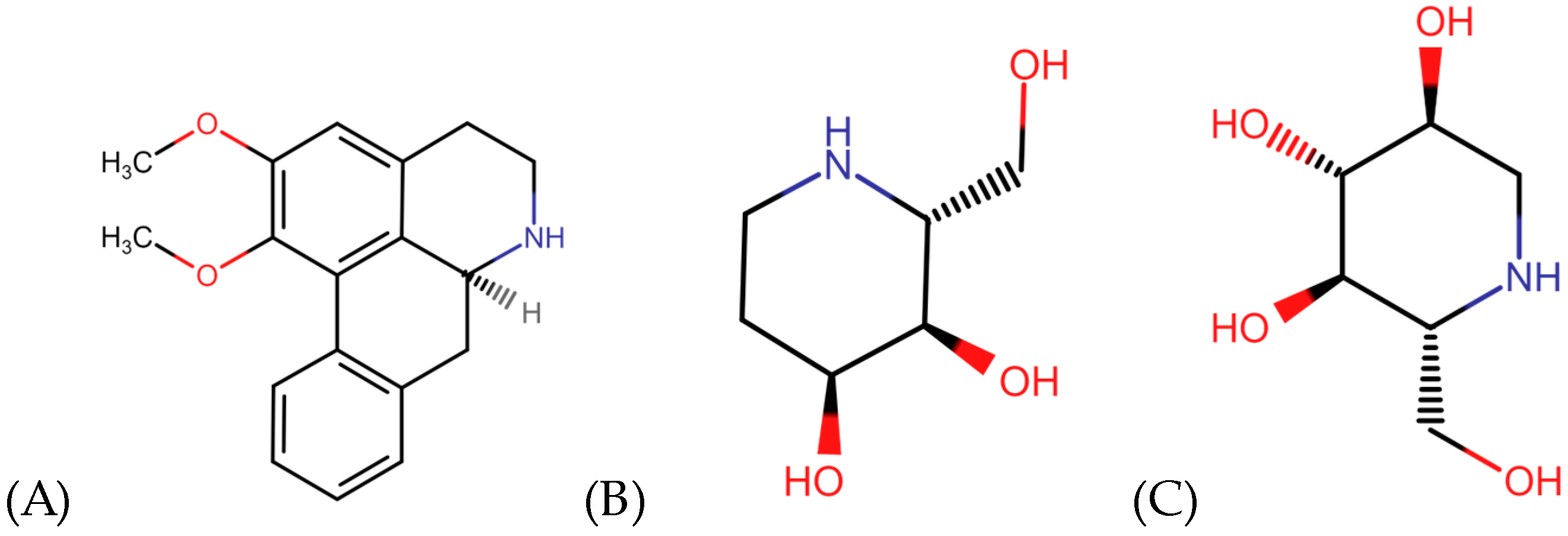

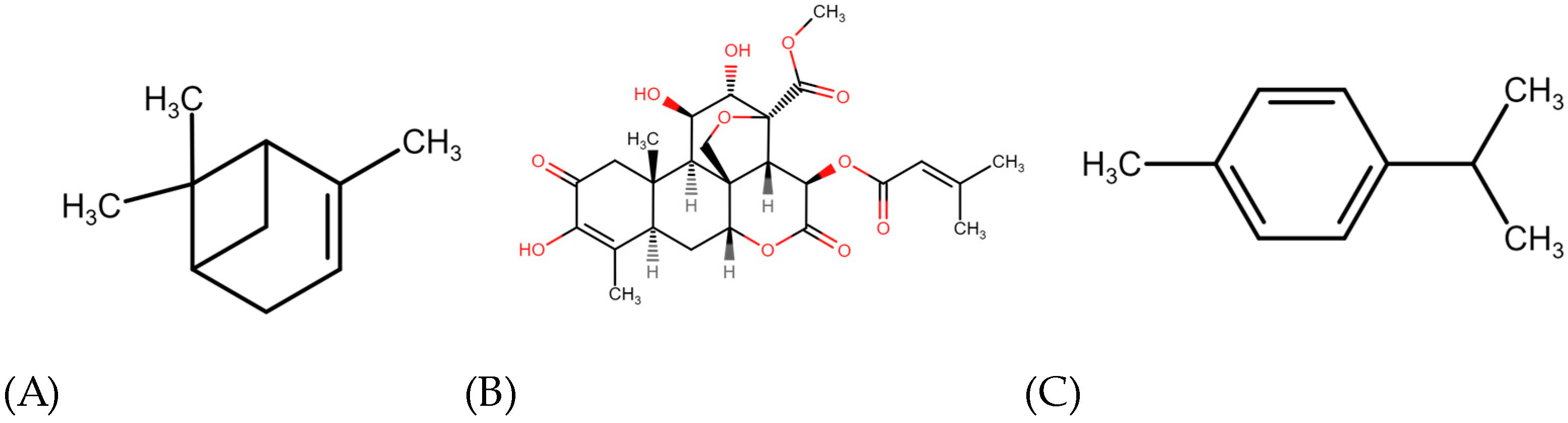
| Category | Compound(s) | Effect | Reference |
|---|---|---|---|
| Polyphenols | Polyphenols extracted from Lippia citriodora and Hibiscus sabdariffa | Modulation of AMP-activated protein kinases. Decrease body fat, blood pressure, and heart rate. Modulate anorexigenic hormones like glucagon-like peptide-1 and ghrelin, an orexigenic hormone. | [35] |
| Verbascoside, delphinidin-3-O-sambudioside, cyanidin-3-O-samudioside, and isover-bascoside | Mediation of leptin levels, regulation of satiety, and activation of AMPK. | [36] | |
| Morin | Decreases levels of triacylglycerides, increases HDL, and decreases LDL levels. Downregulation of the expression of the inflammasome biomarker Nlrp3 mRNA in high-fat-fed Wistar rats. | [37] | |
| Cocoa polyphenols | Decrease plasmatic levels of proinflammatory biomarkers IL-6 and MCP-1 and increase levels of adiponectin. | [38] | |
| Curcumin | Modulates SIRT1/AMPKα/FOXO1 and UPC1 in bovine adipocytes. | [39] | |
| Alkaloids | Bouchardatine | Facilitates activation of AMPK by increasing sirtuin 1 (SIRT1) activity, reduces lipid accumulation inside the cells, and activates the SIRT1-LKB1-AMPK pathway. | [40] |
| 1-deoxynojirimycin, 1,4-dideoxy-1,4-imino-D-arabinitol, and fagomin | Reduce expression of lipid uptake genes, proinflammatory genes, and interferon alpha-inducible protein 27-like protein 2A. Increase mRNA levels of AdipoR1 and AdipoR2. | [41] | |
| Berberine | Increases fatty acid oxidation by activating AMPK and reduces the phosphorylation state of JNK1 and the mRNA levels of proinflammatory cytokines. Alleviates hepatic lipid accumulation and inhibits adipocyte differentiation, proliferation, and adiposity, all through downregulating galectin-3. Activates the energy metabolic sensing pathway AMPK/SIRT1 axis, increasing PPARγ deacetylation. Via signaling the TAS2Rs pathway, upregulates release of GLP-1. | [42,43,44,45,46,47] | |
| Alkaloids extracted from Nelumbo nucifera Gaertn. | Antagonize dopamine receptors D1 and D2. Reduce blood lipid and glucose values and ameliorate lipid accumulation, apoptosis, and impaired migration through the activation of the LKB1/AMPK signaling pathway. Downregulate expression of proinflammatory genes IL-6, IL-1β, and TNF-α. | [48,49,50,51] | |
| Evodiamine | Lowers oxidative stress and inflammation caused by free fatty acids by inhibiting enhanced expression of P2X7 and its dependent TNF-α expression and ERK 1/2 phosphorylation. | [52] | |
| Betaine | Activates AMPK and downregulates SREBP-1c, enhancing lipid metabolism. | [53] | |
| Saponins | Saikosaponin A and saikosaponin D | Suppress expression of adipogenic genes PPARγ, C/EBPα, SREBP-1c, and adiponectin. Downregulate expression of lipogenic genes FABP4, FAS, and LPL. | [54] |
| Saponins from Stevia rebaudiana | Inhibit expression of PPARγ, C/EBPα, aP2, and SREBP-1c. | [55] | |
| Ginsenoside Rg2 | Modulates the expression of inflammatory cytokines ARG1, CCL2, and IL-1β. | [56] | |
| Soy saponins | Downregulate adipogenesis, significantly downregulating SREBP-1c and FAS. | [57] | |
| Saponins from Gynostemma pentaphyllum | Decrease the expression of AP2 and SIRT1. Increase the expression of CPT1 and HSL. | [58] | |
| Terpenes | Terpenes from Ilex aquifolium | Significantly increase the expression of the LXR1 gene. | [59] |
| Terpenoids from I. aquifolium | Reduce the accumulation of lipids in the liver. Anti-inflammatory and antioxidant effects. | [60] | |
| Brucein A-C, brusatol, bruceantinol, hydroxybrucein A, and yadanzioside B | Exert pro-lipolytic activity in the cells. | [61] | |
| Terpenes from Sarcophyton glaucum | Reduce levels of fetuin A, fetuin B, and PTP1Β, while adropin and omentin are increased. Increase the expression of PPARγ-α. | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elizalde-Romero, C.A.; Leyva-López, N.; Contreras-Angulo, L.A.; Cabanillas Ponce de-León, R.; Rodriguez-Anaya, L.Z.; León-Félix, J.; Heredia, J.B.; Beltrán-Ontiveros, S.A.; Gutiérrez-Grijalva, E.P. Current Evidence of Natural Products against Overweight and Obesity: Molecular Targets and Mechanisms of Action. Receptors 2024, 3, 362-379. https://doi.org/10.3390/receptors3030017
Elizalde-Romero CA, Leyva-López N, Contreras-Angulo LA, Cabanillas Ponce de-León R, Rodriguez-Anaya LZ, León-Félix J, Heredia JB, Beltrán-Ontiveros SA, Gutiérrez-Grijalva EP. Current Evidence of Natural Products against Overweight and Obesity: Molecular Targets and Mechanisms of Action. Receptors. 2024; 3(3):362-379. https://doi.org/10.3390/receptors3030017
Chicago/Turabian StyleElizalde-Romero, Cristina Alicia, Nayely Leyva-López, Laura Aracely Contreras-Angulo, Rigoberto Cabanillas Ponce de-León, Libia Zulema Rodriguez-Anaya, Josefina León-Félix, J. Basilio Heredia, Saul Armando Beltrán-Ontiveros, and Erick Paul Gutiérrez-Grijalva. 2024. "Current Evidence of Natural Products against Overweight and Obesity: Molecular Targets and Mechanisms of Action" Receptors 3, no. 3: 362-379. https://doi.org/10.3390/receptors3030017
APA StyleElizalde-Romero, C. A., Leyva-López, N., Contreras-Angulo, L. A., Cabanillas Ponce de-León, R., Rodriguez-Anaya, L. Z., León-Félix, J., Heredia, J. B., Beltrán-Ontiveros, S. A., & Gutiérrez-Grijalva, E. P. (2024). Current Evidence of Natural Products against Overweight and Obesity: Molecular Targets and Mechanisms of Action. Receptors, 3(3), 362-379. https://doi.org/10.3390/receptors3030017












