Receptor-Targeted Nanomedicine for Cancer Therapy
Abstract
1. Introduction
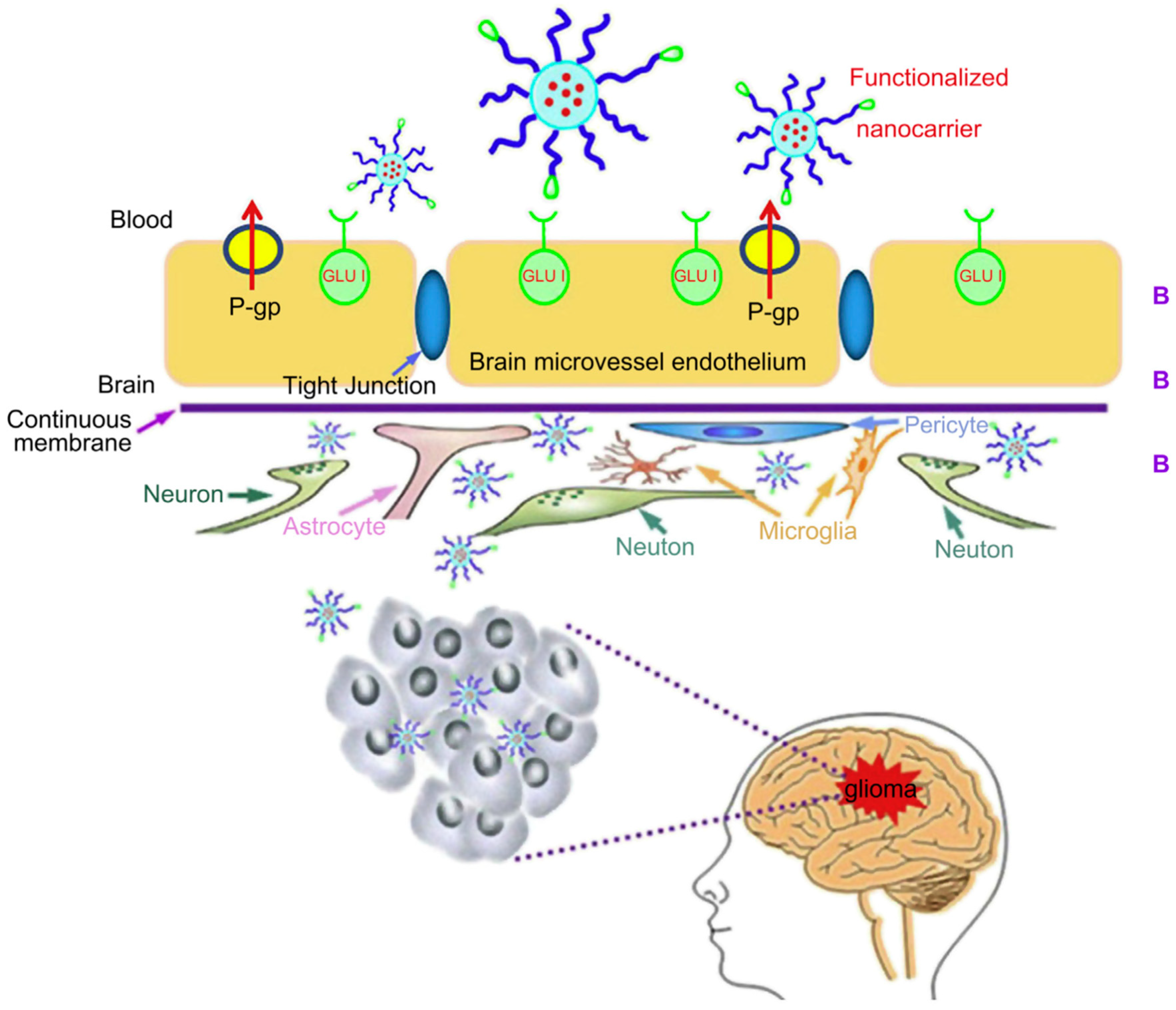
2. Receptors in Cancer
2.1. Epidermal Growth Factor Receptor
2.2. Folate Receptor
2.3. Transferrin Receptor
2.4. Integrins
2.5. Mucin-1
2.6. CD44
2.7. Hormone Receptors
2.8. Programmed Death-1 Receptor
2.9. Nicotinic Acetylcholine Receptors
| Receptor | Key Role in Cancer | Dysregulation Mechanisms | Contribution to Tumorigenesis | Predominant/Prevalent Cancer Types | Reference |
|---|---|---|---|---|---|
| Epidermal Growth Factor Receptor | Triggers uncontrolled cell growth via Ras/Raf/MAPK, PI3K/Akt, and JAK/STAT pathways. | Involves mutations (point mutations, exon deletions, insertions), overexpression, and amplification. | Activation, even without ligands, due to mutations is a hallmark in various cancers, driving tumorigenesis. |
| [143,144,145] |
| Folate Receptor | Upregulated in cancer, modulates Wnt/β-catenin, PI3K/Akt/mTOR, and Notch pathways. | Upregulation adapts to metabolic shifts, ensuring efficient extracellular folate uptake. | Enhances angiogenesis, providing a selective advantage in nutrient uptake for distant site survival and metastasis. |
| [146,147] |
| Transferrin Receptor | Overexpressed in cancers, meets iron demand, and influences DNA synthesis. | Hypoxia-induced upregulation via hypoxia-inducible factors (HIFs), a dual role in cancer progression. | Upregulation is linked to the tumor microenvironment, influencing processes like DNA synthesis and repair. |
| [148,149] |
| Integrins | Pivotal in cell adhesion, impacts migration, survival, and more. | Altered expression influences cancer cell behavior, including changes in migration, proliferation, and survival. | Drive angiogenesis by interacting with growth factor receptors, impacting tumor vasculature and angiogenic processes. |
| [150,151,152] |
| Mucin-1 | Acts as a guardian in healthy tissues and becomes an oncogene. | Serves as a proinflammatory agent, regulates apoptosis, and inhibits the immune response. | Influences cancer cell survival, migration, and chemoresistance; facilitates recruitment of inflammatory cells in the tumor microenvironment. |
| [153,154] |
| CD44 | Multifunctional receptor in physiological processes and cancer. | Impacts migration, invasion, and angiogenesis through interactions with ligands (HA, OPN, MMPs). | Considered a marker for cancer stem cells, regulates key proteins involved in osteoclast differentiation and tumor metastasis. |
| [155,156,157] |
| Hormone Receptors | Distinct roles in hormone-responsive cancers. | The interplay between estrogen and progesterone receptors synergistically augments cellular proliferation. | Androgen receptor activation propels prostate cancer progression; mutations may lead to resistance to androgen deprivation therapy. |
| [158,159] |
| PD-1 Receptor | Operates as a modulator of T-cell activation, and immune checkpoint. | Engagement by cancer cells initiates inhibitory signals, fostering an immunosuppressive microenvironment. | Prolonged engagement leads to T cell exhaustion, contributing to unabated tumor growth, metastasis, and resistance to immune-mediated destruction. |
| [160,161,162] |
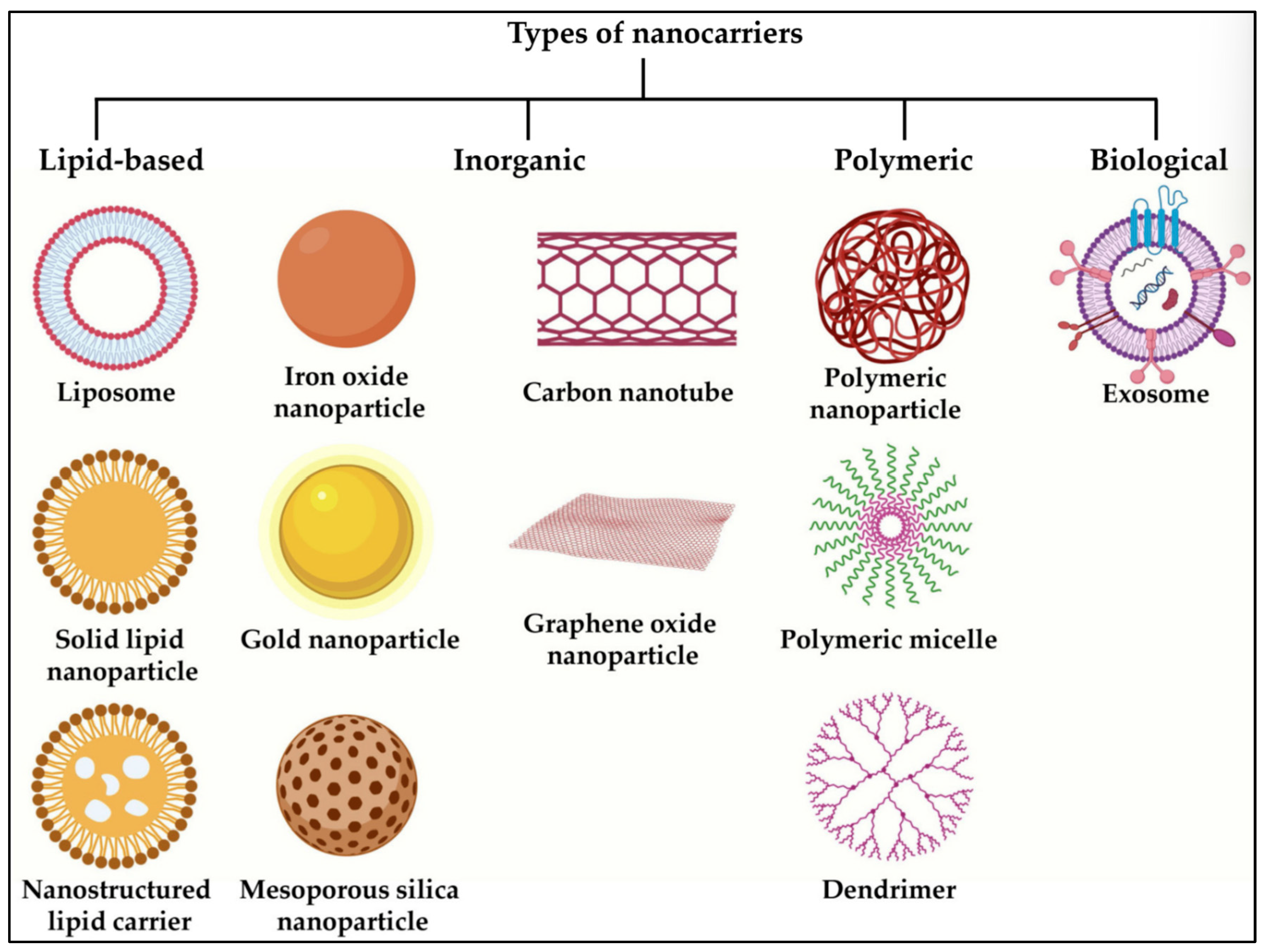
3. Common Nanocarrier Types
3.1. Lipidic
3.1.1. Liposomes
3.1.2. Solid Lipid Nanoparticle
3.1.3. Phytosomes
3.1.4. Nanostructured Lipid Carrier
3.2. Polymeric
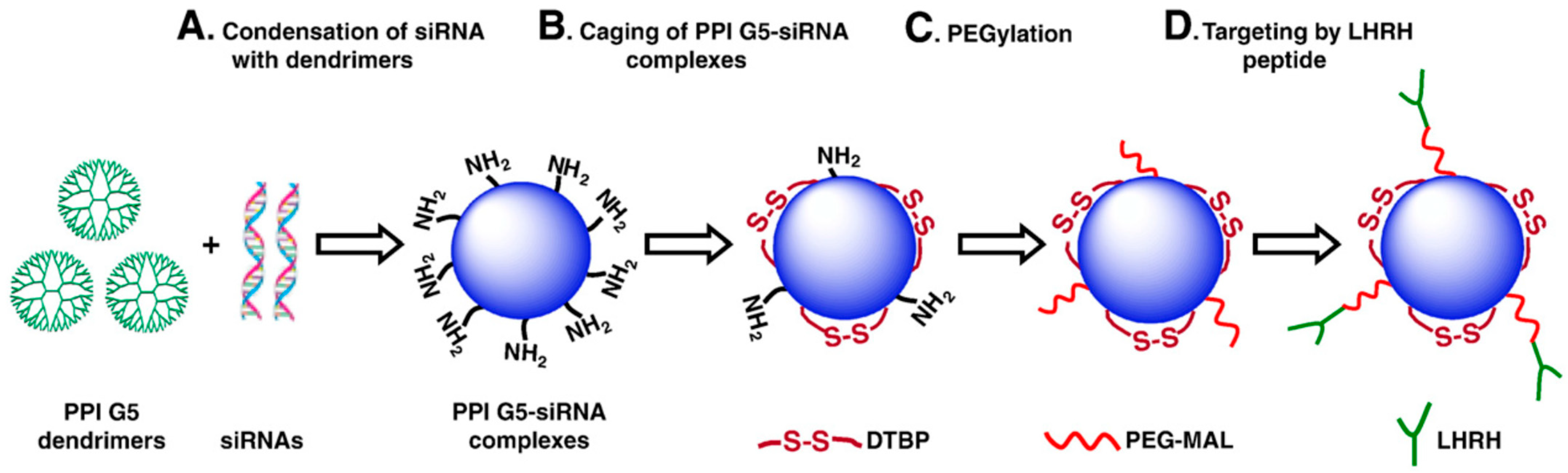
3.2.1. Dendrimer
3.2.2. Polymeric Micelle
3.2.3. Polymer Drug Conjugate
3.3. Inorganic
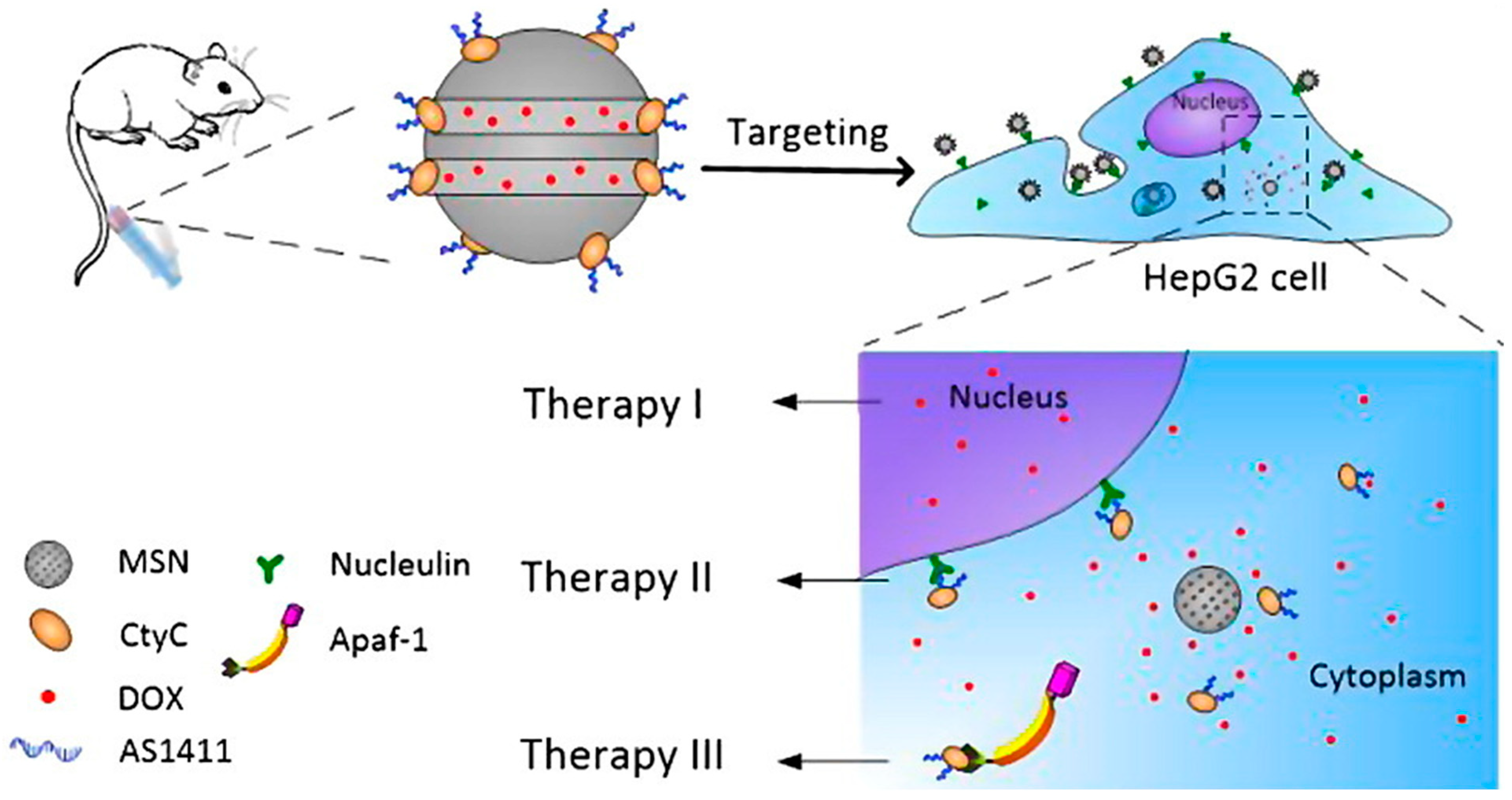
3.3.1. Silica Nanoparticle
3.3.2. Silver Nanoparticles
3.3.3. Gold Nanoparticles
4. Targeting Strategies
4.1. Small Molecules
4.2. Peptides
4.3. Aptamers
4.4. Proteins
4.5. Antibodies
5. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greish, K. Enhanced Permeability and Retention of Macromolecular Drugs in Solid Tumors: A Royal Gate for Targeted Anticancer Nanomedicines. J. Drug Target. 2007, 15, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Salave, S.; Longare, S.; Agarwal, R.; Kalia, K.; Benival, D. Nanotherapeutics in Tumour Microenvironment for Cancer Therapy. Nanosci. Nanotechnol.-Asia 2021, 12, e080921196283. [Google Scholar] [CrossRef]
- Rana, D.; Salave, S.; Perla, A.; Nadkarni, A.; Kolhe, S.; Jindal, A.B.; Mandoli, A.; Dwivedi, P.; Benival, D. Bugs as Drugs: Understanding the Linkage between Gut Microbiota and Cancer Treatment. Curr. Drug Targets 2022, 23, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rana, D.; Pande, S.; Salave, S.; Giri, J.; Benival, D.; Kommineni, N. “Bioinspired” Membrane-Coated Nanosystems in Cancer Theranostics: A Comprehensive Review. Pharmaceutics 2023, 15, 1677. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, T.G.; Salave, S.; Shinde, T.; Srikanth, I.; Gyanani, V.; Haley, J.C.; Jain, A. Understanding the Role of Endothelial Cells in Brain Tumor Formation and Metastasis: A Proposition to Be Explored for Better Therapy. J. Natl. Cancer Cent. 2023, 3, 222–235. [Google Scholar] [CrossRef]
- Desai, N.; Katare, P.; Makwana, V.; Salave, S.; Vora, L.K.; Giri, J. Tumor-Derived Systems as Novel Biomedical Tools—Turning the Enemy into an Ally. Biomater. Res. 2023, 27, 113. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Cao, Y.; Xu, M.; Han, X.; Xiong, H.; Gao, Y.; Xu, B.; Zhu, G.; Li, J. Cancer Nanomedicine: Emerging Strategies and Therapeutic Potentials. Molecules 2023, 28, 5145. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Greish, K. Enhanced Permeability and Retention (EPR) Effect for Anticancer Nanomedicine Drug Targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Chen, Z.G.; Shin, D.M. Advances of Cancer Therapy by Nanotechnology. Cancer Res. Treat. 2009, 41, 1–11. [Google Scholar] [CrossRef]
- Alexis, F.; Rhee, J.-W.; Richie, J.P.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. New Frontiers in Nanotechnology for Cancer Treatment. Urol. Oncol. Semin. Orig. Investig. 2008, 26, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The Big Picture on Nanomedicine: The State of Investigational and Approved Nanomedicine Products. Nanomedicine 2013, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Quirk, T. There’s Plenty of Room at the Bottom. Australas. Biotechnol. 2006, 16, 36. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Pardhe, R.; Bule, P.; Benival, D. Unravelling Micro and Nano Vesicular System in Intranasal Drug Delivery for Epilepsy. Pharm. Nanotechnol. 2022, 10, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.; Salave, S.; Rana, D.; Benival, D. Development and In-Vitro Evaluation of Dexamethasone Enriched Nanoemulsion for Ophthalmic Indication. Drug Deliv. Lett. 2023, 13, 196–212. [Google Scholar] [CrossRef]
- Rana, D.; Gupta, R.; Bharathi, K.; Pardhe, R.; Jain, N.K.; Salave, S.; Prasad, R.; Benival, D.; Kommineni, N. Porous Silica Nanoparticles for Targeted Bio-Imaging and Drug Delivery Applications. In Nanomaterials in Healthcare; CRC Press: Boca Raton, FL, USA, 2023; pp. 133–154. [Google Scholar]
- Salave, S.; Rana, D.; Vitore, J.; Jain, A. Functionalized Carbon Nanotubes for Cell Tracking. In Functionalized Carbon Nanotubes for Biomedical Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2023; pp. 319–338. [Google Scholar] [CrossRef]
- Khunt, D.; Prajapati, B.G.; Prajapti, M.; Misra, M.; Salave, S.; Patel, J.K.; Patelfor, R.J. Drug Delivery by Micro, Nanoemulsions in Tuberculosis. In Tubercular Drug Delivery Systems: Advances in Treatment of Infectious Diseases; Springer International Publishing: Cham, Switzerland, 2023; pp. 173–188. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Salave, S.; Rawat, G.; Benival, D. Nanomedicines for the Treatment of Systemic Candidiasis. AAPS Adv. Pharm. Sci. Ser. 2023, 56, 95–124. [Google Scholar] [CrossRef]
- Khunt, D.; Salave, S.; Rana, D.; Benival, D.; Gayakvad, B.; Prajapati, B.G. Nose to Brain Delivery for the Treatment of Alzheimer’s Disease. In Alzheimer’s Disease and Advanced Drug Delivery Strategies; Academic Press: Cambridge, MA, USA, 2024; pp. 61–71. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted Therapy in Chronic Diseases Using Nanomaterial-Based Drug Delivery Vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef]
- Giri, P.M.; Banerjee, A.; Layek, B. A Recent Review on Cancer Nanomedicine. Cancers 2023, 15, 2256. [Google Scholar] [CrossRef]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef]
- Tong, R.; Kohane, D.S. New Strategies in Cancer Nanomedicine. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.; Avasthi, A.; Paez-Muñoz, J.M.; Pernia Leal, M.; Garcia-Martin, M.L. Passive Targeting of High-Grade Gliomas: Via the EPR Effect: A Closed Path for Metallic Nanoparticles? Biomater. Sci. 2021, 9, 7984–7995. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer Active Targeting by Nanoparticles: A Comprehensive Review of Literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Enhanced Permeability and Retention Effect Based Nanomedicine, a Solution for Cancer. World J. Pharmacol. 2015, 4, 168. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and Challenges towards Targeted Delivery of Cancer Therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Antignani, A.; Chun, E.; Ho, H.; Bilotta, M.T.; Qiu, R.; Sarnvosky, R.; Fitzgerald, D.J. Targeting Receptors on Cancer Cells with Protein Toxins. Biomolecules 2020, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer Nanotechnology: The Impact of Passive and Active Targeting in the Era of Modern Cancer Biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Wieduwilt, M.J.; Moasser, M.M. The Epidermal Growth Factor Receptor Family: Biology Driving Targeted Therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef]
- Rosenkranz, A.A.; Slastnikova, T.A. Epidermal Growth Factor Receptor: Key to Selective Intracellular Delivery. Biochemistry 2020, 85, 967–993. [Google Scholar] [CrossRef]
- Jorissen, R. Epidermal Growth Factor Receptor: Mechanisms of Activation and Signalling. Exp. Cell Res. 2003, 284, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, F.; Forrester, S.J.; Eguchi, S.; Zhang, M.-Z.; Harris, R.C. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 2016, 96, 1025–1069. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging Functions of the EGFR in Cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Peng, D.; Liang, P.; Zhong, C.; Xu, P.; He, Y.; Luo, Y.; Wang, X.; Liu, A.; Zeng, Z. Effect of EGFR Amplification on the Prognosis of EGFR-Mutated Advanced Non–Small-Cell Lung Cancer Patients: A Prospective Observational Study. BMC Cancer 2022, 22, 1323. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Zhang, H.; Ye, J.; Kan, M.; Liu, F.; Wang, T.; Deng, J.; Tan, Y.; He, L.; Liu, Y. Hypermethylated Epidermal Growth Factor Receptor (EGFR) Promoter Is Associated with Gastric Cancer. Sci. Rep. 2015, 5, 10154. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, B.; Sun, Z. Spectrum of EGFR Aberrations and Potential Clinical Implications: Insights from Integrative Pan-cancer Analysis. Cancer Commun. 2020, 40, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lei, L.; Wang, W.; Lin, L.; Zhu, Y.; Wang, H.; Miao, L.; Wang, L.; Zhuang, W.; Fang, M.; et al. Molecular Characteristics and Clinical Outcomes of EGFR Exon 19 C-Helix Deletion in Non–Small Cell Lung Cancer and Response to EGFR TKIs. Transl. Oncol. 2020, 13, 100791. [Google Scholar] [CrossRef]
- Hou, J.; Li, H.; Ma, S.; He, Z.; Yang, S.; Hao, L.; Zhou, H.; Zhang, Z.; Han, J.; Wang, L.; et al. EGFR Exon 20 Insertion Mutations in Advanced Non-Small-Cell Lung Cancer: Current Status and Perspectives. Biomark. Res. 2022, 10, 21. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Z.; Xu, C.; Zhang, X.; Yan, H.; Su, J.; Yang, J.; Xie, Z.; Guo, W.; Li, F.; et al. Intratumoral Heterogeneity of EGFR-Activating Mutations in Advanced NSCLC Patients at the Single-Cell Level. BMC Cancer 2019, 19, 369. [Google Scholar] [CrossRef]
- Tanaka, H.; Watanabe, T. Mechanisms Underlying Recurrent Genomic Amplification in Human Cancers. Trends Cancer 2020, 6, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Alam, S.; Shamsi, A.; Adnan, M.; Elasbali, A.M.; Al-Soud, W.A.; Alreshidi, M.; Hawsawi, Y.M.; Tippana, A.; Pasupuleti, V.R.; et al. Bax/Bcl-2 Cascade Is Regulated by the EGFR Pathway: Therapeutic Targeting of Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 869672. [Google Scholar] [CrossRef]
- Okamoto, K.; Okamoto, I.; Okamoto, W.; Tanaka, K.; Takezawa, K.; Kuwata, K.; Yamaguchi, H.; Nishio, K.; Nakagawa, K. Role of Survivin in EGFR Inhibitor–Induced Apoptosis in Non–Small Cell Lung Cancers Positive for EGFR Mutations. Cancer Res. 2010, 70, 10402–10410. [Google Scholar] [CrossRef] [PubMed]
- Jansen, G.; Peters, G.J. Novel Insights in Folate Receptors and Transporters: Implications for Disease and Treatment of Immune Diseases and Cancer. Pteridines 2015, 26, 41–53. [Google Scholar] [CrossRef]
- Zhao, R.; Diop-Bove, N.; Visentin, M.; Goldman, I.D. Mechanisms of Membrane Transport of Folates into Cells and Across Epithelia. Annu. Rev. Nutr. 2011, 31, 177–201. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.; Wallace-Povirk, A.; Ning, C.; Frühauf, J.; Tong, N.; Gangjee, A.; Matherly, L.H.; Hou, Z. Folate Transporter Dynamics and Therapy with Classic and Tumor-Targeted Antifolates. Sci. Rep. 2021, 11, 6389. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, B.; Bizzoni, C.; Jansen, G.; Leamon, C.P.; Peters, G.J.; Low, P.S.; Matherly, L.H.; Figini, M. Folate Receptors and Transporters: Biological Role and Diagnostic/Therapeutic Targets in Cancer and Other Diseases. J. Exp. Clin. Cancer Res. 2019, 38, 125. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Putt, K.S.; Singhal, S.; Han, H.; Visscher, D.W.; Murphy, L.M.; Low, P.S. Assessment of Folate Receptor Alpha and Beta Expression in Selection of Lung and Pancreatic Cancer Patients for Receptor Targeted Therapies. Oncotarget 2018, 9, 4485–4495. [Google Scholar] [CrossRef]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA Methylation: A Review of Molecular Mechanisms and the Evidence for Folate’s Role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- Naninck, E.F.G.; Stijger, P.C.; Brouwer-Brolsma, E.M. The Importance of Maternal Folate Status for Brain Development and Function of Offspring. Adv. Nutr. 2019, 10, 502–519. [Google Scholar] [CrossRef] [PubMed]
- Liwinski, T.; Lang, U.E. Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review. Nutrients 2023, 15, 3859. [Google Scholar] [CrossRef] [PubMed]
- Nong, S.; Han, X.; Xiang, Y.; Qian, Y.; Wei, Y.; Zhang, T.; Tian, K.; Shen, K.; Yang, J.; Ma, X. Metabolic Reprogramming in Cancer: Mechanisms and Therapeutics. MedComm 2023, 4, e218. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Zheng, W.; Duan, B.; Zhang, Q.; Ouyang, L.; Peng, W.; Qian, F.; Wang, Y.; Huang, S. Vitamin D-Induced Vitamin D Receptor Expression Induces Tamoxifen Sensitivity in MCF-7 Stem Cells via Suppression of Wnt/β-Catenin Signaling. Biosci. Rep. 2018, 38, BSR20180595. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Cong, G.; Tian, Z.; Ren, D.; Wilson, J.X.; Huang, G. Effects of Folate on Notch Signaling and Cell Proliferation in Neural Stem Cells of Neonatal Rats In Vitro. J. Nutr. Sci. Vitaminol. 2008, 54, 353–356. [Google Scholar] [CrossRef][Green Version]
- Daniels, T.R.; Delgado, T.; Rodriguez, J.A.; Helguera, G.; Penichet, M.L. The Transferrin Receptor Part I: Biology and Targeting with Cytotoxic Antibodies for the Treatment of Cancer. Clin. Immunol. 2006, 121, 144–158. [Google Scholar] [CrossRef]
- Mackenzie, E.L.; Iwasaki, K.; Tsuji, Y. Intracellular Iron Transport and Storage: From Molecular Mechanisms to Health Implications. Antioxid. Redox Signal. 2008, 10, 997–1030. [Google Scholar] [CrossRef] [PubMed]
- Kleven, M.D.; Jue, S.; Enns, C.A. Transferrin Receptors TfR1 and TfR2 Bind Transferrin through Differing Mechanisms. Biochemistry 2018, 57, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Ponka, P.; Lok, C.N. The Transferrin Receptor: Role in Health and Disease. Int. J. Biochem. Cell Biol. 1999, 31, 1111–1137. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, A.; Oliviero, I.; Deaglio, S.; Mariani, G.; Biffoni, M.; Sposi, N.M.; Malavasi, F.; Peschle, C.; Testa, U. Transferrin Receptor 2 Is Frequently Expressed in Human Cancer Cell Lines. Blood Cells Mol. Dis. 2007, 39, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, T.; Wu, J.; Wang, Y.; Hong, Y.; Zhou, H. Transferrin Receptor-Involved HIF-1 Signaling Pathway in Cervical Cancer. Cancer Gene Ther. 2019, 26, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Arima, Y.; Midorikawa, K.; Kobayashi, H.; Oikawa, S.; Zhao, W.; Zhang, Z.; Takeuchi, K.; Murata, M. Knockdown of TFRC Suppressed the Progression of Nasopharyngeal Carcinoma by Downregulating the PI3K/Akt/MTOR Pathway. Cancer Cell Int. 2023, 23, 185. [Google Scholar] [CrossRef]
- Jung, M.; Mertens, C.; Tomat, E.; Brüne, B. Iron as a Central Player and Promising Target in Cancer Progression. Int. J. Mol. Sci. 2019, 20, 273. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Yang, Q.; Huang, X. Src Regulates Tyr20 Phosphorylation of Transferrin Receptor-1 and Potentiates Breast Cancer Cell Survival. J. Biol. Chem. 2011, 286, 35708–35715. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Hwang, S.; Seong, R.H. Transferrin Receptor Regulates Pancreatic Cancer Growth by Modulating Mitochondrial Respiration and ROS Generation. Biochem. Biophys. Res. Commun. 2016, 471, 373–379. [Google Scholar] [CrossRef]
- Wan, Q.; Liao, Z.; Rao, Y.; Yang, C.; Ji, J.; Chen, X.; Su, J. Transferrin Receptor 1-Associated Iron Accumulation and Oxidative Stress Provides a Way for Grass Carp to Fight against Reovirus Infection. Int. J. Mol. Sci. 2019, 20, 5857. [Google Scholar] [CrossRef]
- Bayeva, M.; Khechaduri, A.; Puig, S.; Chang, H.-C.; Patial, S.; Blackshear, P.J.; Ardehali, H. MTOR Regulates Cellular Iron Homeostasis through Tristetraprolin. Cell Metab. 2012, 16, 645–657. [Google Scholar] [CrossRef]
- O’Donnell, K.A.; Yu, D.; Zeller, K.I.; Kim, J.; Racke, F.; Thomas-Tikhonenko, A.; Dang, C.V. Activation of Transferrin Receptor 1 by C-Myc Enhances Cellular Proliferation and Tumorigenesis. Mol. Cell Biol. 2006, 26, 2373–2386. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Kadry, Y.A.; Calderwood, D.A. Structural and Signaling Functions of Integrins. Biochim. Biophys. Acta (BBA)—Biomembr. 2020, 1862, 183206. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Kukkurainen, S.; Hytönen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Reif, S.; Lang, A.; Lindquist, J.N.; Yata, Y.; Gäbele, E.; Scanga, A.; Brenner, D.A.; Rippe, R.A. The Role of Focal Adhesion Kinase-Phosphatidylinositol 3-Kinase-Akt Signaling in Hepatic Stellate Cell Proliferation and Type I Collagen Expression. J. Biol. Chem. 2003, 278, 8083–8090. [Google Scholar] [CrossRef]
- Yee, K.L.; Weaver, V.M.; Hammer, D.A. Integrin-Mediated Signalling through the MAP-Kinase Pathway. IET Syst. Biol. 2008, 2, 8–15. [Google Scholar] [CrossRef]
- Schwartz, M.A. Integrins and Extracellular Matrix in Mechanotransduction. Cold Spring Harb. Perspect. Biol. 2010, 2, a005066. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every Step of the Way: Integrins in Cancer Progression and Metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting Integrin Pathways: Mechanisms and Advances in Therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Park, E.J.; Myint, P.K.; Ito, A.; Appiah, M.G.; Darkwah, S.; Kawamoto, E.; Shimaoka, M. Integrin-Ligand Interactions in Inflammation, Cancer, and Metabolic Disease: Insights into the Multifaceted Roles of an Emerging Ligand Irisin. Front. Cell Dev. Biol. 2020, 8, 588066. [Google Scholar] [CrossRef]
- Saraon, P.; Pathmanathan, S.; Snider, J.; Lyakisheva, A.; Wong, V.; Stagljar, I. Receptor Tyrosine Kinases and Cancer: Oncogenic Mechanisms and Therapeutic Approaches. Oncogene 2021, 40, 4079–4093. [Google Scholar] [CrossRef] [PubMed]
- Garmy-Susini, B.; Varner, J.A. Roles of Integrins in Tumor Angiogenesis and Lymphangiogenesis. Lymphat. Res. Biol. 2008, 6, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Farahani, E.; Patra, H.K.; Jangamreddy, J.R.; Rashedi, I.; Kawalec, M.; Rao Pariti, R.K.; Batakis, P.; Wiechec, E. Cell Adhesion Molecules and Their Relation to (Cancer) Cell Stemness. Carcinogenesis 2014, 35, 747–759. [Google Scholar] [CrossRef]
- Schneider, J.G.; Amend, S.R.; Weilbaecher, K.N. Integrins and Bone Metastasis: Integrating Tumor Cell and Stromal Cell Interactions. Bone 2011, 48, 54–65. [Google Scholar] [CrossRef]
- Wagner, C.E.; Wheeler, K.M.; Ribbeck, K. Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annu. Rev. Cell Dev. Biol. 2018, 34, 189–215. [Google Scholar] [CrossRef] [PubMed]
- Wi, D.-H.; Cha, J.-H.; Jung, Y.-S. Mucin in Cancer: A Stealth Cloak for Cancer Cells. BMB Rep. 2021, 54, 344–355. [Google Scholar] [CrossRef]
- Carraway, K.L.; Fregien, N. Mucin Structure and Function: Insights from Molecular Biology. Trends Glycosci. Glycotechnol. 1995, 7, 31–44. [Google Scholar] [CrossRef][Green Version]
- Zaretsky, Z.; Wreschner, H. (Eds.) General Properties and Functions of Mucus and Mucins. In Series Title: Mucins—Potential Regulators of Cell Functions Volume Title: Gel-Forming and Soluble Mucins; Bentham Science Publishers: Sharjah, United Arab Emirates, 2013; pp. 3–10. [Google Scholar]
- Behera, S.K.; Praharaj, A.B.; Dehury, B.; Negi, S. Exploring the Role and Diversity of Mucins in Health and Disease with Special Insight into Non-Communicable Diseases. Glycoconj. J. 2015, 32, 575–613. [Google Scholar] [CrossRef]
- Rajabi, H.; Kufe, D. MUC1-C Oncoprotein Integrates a Program of EMT, Epigenetic Reprogramming and Immune Evasion in Human Carcinomas. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2017, 1868, 117–122. [Google Scholar] [CrossRef]
- Khodabakhsh, F.; Merikhian, P.; Eisavand, M.R.; Farahmand, L. Crosstalk between MUC1 and VEGF in Angiogenesis and Metastasis: A Review Highlighting Roles of the MUC1 with an Emphasis on Metastatic and Angiogenic Signaling. Cancer Cell Int. 2021, 21, 200. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.-S.; Yung, K.K.-L. MUC1: Structure, Function, and Clinic Application in Epithelial Cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef]
- Kufe, D.W. Mucins in Cancer: Function, Prognosis and Therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef]
- Basakran, N.S. CD44 as a Potential Diagnostic Tumor Marker. Saudi Med. J. 2015, 36, 273–279. [Google Scholar] [CrossRef]
- Iczkowski, K.A. Cell Adhesion Molecule CD44: Its Functional Roles in Prostate Cancer. Am. J. Transl. Res. 2010, 3, 1–7. [Google Scholar]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Kimata, K.; Itano, N. Key Roles of Hyaluronan and Its CD44 Receptor in the Stemness and Survival of Cancer Stem Cells. Front. Oncol. 2015, 5, 180. [Google Scholar] [CrossRef]
- Govindaraju, P.; Todd, L.; Shetye, S.; Monslow, J.; Puré, E. CD44-Dependent Inflammation, Fibrogenesis, and Collagenolysis Regulates Extracellular Matrix Remodeling and Tensile Strength during Cutaneous Wound Healing. Matrix Biol. 2019, 75–76, 314–330. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Perschl, A.; Lesley, J.; English, N.; Trowbridge, I.; Hyman, R. Role of CD44 Cytoplasmic Domain in Hyaluronan Binding. Eur. J. Immunol. 1995, 25, 495–501. [Google Scholar] [CrossRef]
- Gupta, A.; Zhou, C.; Chellaiah, M. Osteopontin and MMP9: Associations with VEGF Expression/Secretion and Angiogenesis in PC3 Prostate Cancer Cells. Cancers 2013, 5, 617–638. [Google Scholar] [CrossRef]
- Louderbough, J.M.V.; Schroeder, J.A. Understanding the Dual Nature of CD44 in Breast Cancer Progression. Mol. Cancer Res. 2011, 9, 1573–1586. [Google Scholar] [CrossRef]
- Weber, G.F.; Ashkar, S.; Glimcher, M.J.; Cantor, H. Receptor-Ligand Interaction Between CD44 and Osteopontin (Eta-1). Science 1996, 271, 509–512. [Google Scholar] [CrossRef]
- Desai, B.; Ma, T.; Zhu, J.; Chellaiah, M.A. Characterization of the Expression of Variant and Standard CD44 in Prostate Cancer Cells: Identification of the Possible Molecular Mechanism of CD44/MMP9 Complex Formation on the Cell Surface. J. Cell Biochem. 2009, 108, 272–284. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, C.; Gao, F. The State of CD44 Activation in Cancer Progression and Therapeutic Targeting. FEBS J. 2022, 289, 7970–7986. [Google Scholar] [CrossRef]
- Abd Elhakeem, A.A.E.; Essa, A.A.; Soliman, R.K.; Hamdan, A.R.K. Novel Evaluation of the Expression Patterns CD44 and MMP9 Proteins in Intracranial Meningiomas and Their Relationship to the Overall Survival. Egypt. J. Neurosurg. 2022, 37, 33. [Google Scholar] [CrossRef]
- Gupta, A.; Cao, W.; Sadashivaiah, K.; Chen, W.; Schneider, A.; Chellaiah, M.A. Promising Noninvasive Cellular Phenotype in Prostate Cancer Cells Knockdown of Matrix Metalloproteinase 9. Sci. World J. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Chen, L.; Fu, C.; Zhang, Q.; He, C.; Zhang, F.; Wei, Q. The Role of CD44 in Pathological Angiogenesis. FASEB J. 2020, 34, 13125–13139. [Google Scholar] [CrossRef]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Gupta, A.; Cao, W.; Chellaiah, M.A. Integrin Avβ3 and CD44 Pathways in Metastatic Prostate Cancer Cells Support Osteoclastogenesis via a Runx2/Smad 5/Receptor Activator of NF-ΚB Ligand Signaling Axis. Mol. Cancer 2012, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.D.; Funder, J.W. Hormone Receptors. N. Engl. J. Med. 1979, 301, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-R.; Kim, T.-H.; Choi, K.-C. Functions and Physiological Roles of Two Types of Estrogen Receptors, ERα and ERβ, Identified by Estrogen Receptor Knockout Mouse. Lab. Anim. Res. 2012, 28, 71. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for Estrogen Receptor Expression in Human Cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.M.; Horwitz, K.B. Progesterone Receptors, Their Isoforms and Progesterone Regulated Transcription. Mol. Cell Endocrinol. 2012, 357, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, H.; Li, S.; Wu, P.; Mao, X. The Role of Progesterone Receptors in Breast Cancer. Drug Des. Dev. Ther. 2022, 16, 305–314. [Google Scholar] [CrossRef]
- Daniel, A.R.; Hagan, C.R.; Lange, C.A. Progesterone Receptor Action: Defining a Role in Breast Cancer. Expert. Rev. Endocrinol. Metab. 2011, 6, 359–369. [Google Scholar] [CrossRef]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate Cancer Progression after Androgen Deprivation Therapy: Mechanisms of Castrate Resistance and Novel Therapeutic Approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Zhu, M.-L.; Kyprianou, N. Androgen Receptor and Growth Factor Signaling Cross-Talk in Prostate Cancer Cells. Endocr. Relat. Cancer 2008, 15, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Khadka, S.; Druffner, S.R.; Duncan, B.C.; Busada, J.T. Glucocorticoid Regulation of Cancer Development and Progression. Front. Endocrinol. 2023, 14, 1161768. [Google Scholar] [CrossRef] [PubMed]
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment. Front. Immunol. 2020, 11, 568931. [Google Scholar] [CrossRef] [PubMed]
- Laba, S.; Mallett, G.; Amarnath, S. The Depths of PD-1 Function within the Tumor Microenvironment beyond CD8+ T Cells. Semin. Cancer Biol. 2022, 86, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Zak, K.M.; Kitel, R.; Przetocka, S.; Golik, P.; Guzik, K.; Musielak, B.; Dömling, A.; Dubin, G.; Holak, T.A. Structure of the Complex of Human Programmed Death 1, PD-1, and Its Ligand PD-L1. Structure 2015, 23, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and Its Ligands Are Important Immune Checkpoints in Cancer. Oncotarget 2017, 8, 2171–2186. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, L.; Li, J.; Li, Y.; Wang, Y.; Xu, Z.-X. Recent Findings in the Regulation of Programmed Death Ligand 1 Expression. Front. Immunol. 2019, 10, 1337. [Google Scholar] [CrossRef] [PubMed]
- Bally, A.P.R.; Austin, J.W.; Boss, J.M. Genetic and Epigenetic Regulation of PD-1 Expression. J. Immunol. 2016, 196, 2431–2437. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the Tumor Microenvironment in PD-L1/PD-1-Mediated Tumor Immune Escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef]
- Kim, J.M.; Chen, D.S. Immune Escape to PD-L1/PD-1 Blockade: Seven Steps to Success (or Failure). Ann. Oncol. 2016, 27, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Pauken, K.E.; Wherry, E.J. Overcoming T Cell Exhaustion in Infection and Cancer. Trends Immunol. 2015, 36, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 Pathway: Basic Biology and Role in Cancer Immunotherapy. J. Cell Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Hasan, U.; Jeyashree, K.; Mani, R.; Chauhan, M.; Basu, S.M.; Giri, J. Biomaterial-Based Platforms for Modulating Immune Components against Cancer and Cancer Stem Cells. Acta Biomater. 2023, 161, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P.; Paas, Y. Nicotinic Acetylcholine Receptors. In Encyclopedia of Neuroscience; Academic Press: Cambridge, MA, USA, 2009; pp. 1129–1133. [Google Scholar] [CrossRef]
- Mor, I.; Soreq, H. Cholinergic Toxicity and the Male Reproductive System. In Reproductive and Developmental Toxicology; Academic Press: Cambridge, MA, USA, 2011; pp. 863–870. [Google Scholar] [CrossRef]
- Westfall, T.C. Cholinergic Neurotransmission in the Autonomic and Somatic Motor Nervous System. In Encyclopedia of Neuroscience; Academic Press: Cambridge, MA, USA, 2009; pp. 827–834. [Google Scholar] [CrossRef]
- Licitra, L.; Störkel, S.; Kerr, K.M.; Van Cutsem, E.; Pirker, R.; Hirsch, F.R.; Vermorken, J.B.; Von Heydebreck, A.; Esser, R.; Celik, I.; et al. Predictive Value of Epidermal Growth Factor Receptor Expression for First-Line Chemotherapy plus Cetuximab in Patients with Head and Neck and Colorectal Cancer: Analysis of Data from the EXTREME and CRYSTAL Studies. Eur. J. Cancer 2013, 49, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Ciardiello, D.; Martini, G.; Troiani, T.; Cardone, C.; Vitiello, P.P.; Normanno, N.; Rachiglio, A.M.; Maiello, E.; Latiano, T.; et al. Implementing Anti-Epidermal Growth Factor Receptor (EGFR) Therapy in Metastatic Colorectal Cancer: Challenges and Future Perspectives. Ann. Oncol. 2020, 31, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Lemos-González, Y.; Rodríguez-Berrocal, F.J.; Cordero, O.J.; Gómez, C.; Páez De La Cadena, M. Alteration of the Serum Levels of the Epidermal Growth Factor Receptor and Its Ligands in Patients with Non-Small Cell Lung Cancer and Head and Neck Carcinoma. Br. J. Cancer 2007, 96, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Cagle, P.T.; Zhai, Q.J.; Murphy, L.; Low, P.S. Folate Receptor in Adenocarcinoma and Squamous Cell Carcinoma of the Lung: Potential Target for Folate-Linked Therapeutic Agents. Arch. Pathol. Lab. Med. 2013, 137, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.B.; Marth, C.; Coleman, R.L. Role of the Folate Receptor in Ovarian Cancer Treatment: Evidence, Mechanism, and Clinical Implications. Cancer Metastasis Rev. 2015, 34, 41–52. [Google Scholar] [CrossRef]
- Płoszyńska, A.; Ruckemann-Dziurdzińska, K.; Jóźwik, A.; Mikosik, A.; Lisowska, K.; Balcerska, A.; Witkowski, J.M. Cytometric Evaluation of Transferrin Receptor 1 (CD71) in Childhood Acute Lymphoblastic Leukemia. Folia Histochem. Cytobiol. 2012, 50, 304–311. [Google Scholar] [CrossRef][Green Version]
- Habashy, H.O.; Powe, D.G.; Staka, C.M.; Rakha, E.A.; Ball, G.; Green, A.R.; Aleskandarany, M.; Paish, E.C.; Douglas MacMillan, R.; Nicholson, R.I.; et al. Transferrin Receptor (CD71) Is a Marker of Poor Prognosis in Breast Cancer and Can Predict Response to Tamoxifen. Breast Cancer Res. Treat. 2010, 119, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Arias-Mejias, S.M.; Warda, K.Y.; Quattrocchi, E.; Alonso-Quinones, H.; Sominidi-Damodaran, S.; Meves, A. The Role of Integrins in Melanoma: A Review. Int. J. Dermatol. 2020, 59, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Suyin, P.C.; Dickinson, J.L.; Holloway, A.F.; Suyin, P.C.; Dickinson, J.L.; Holloway, A.F. Integrins in Prostate Cancer Invasion and Metastasis. In Advances in Prostate Cancer; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef][Green Version]
- Yousefi, H.; Vatanmakanian, M.; Mahdiannasser, M.; Mashouri, L.; Alahari, N.V.; Monjezi, M.R.; Ilbeigi, S.; Alahari, S.K. Understanding the Role of Integrins in Breast Cancer Invasion, Metastasis, Angiogenesis, and Drug Resistance. Oncogene 2021, 40, 1043–1063. [Google Scholar] [CrossRef] [PubMed]
- Raina, D.; Ahmad, R.; Joshi, M.D.; Yin, L.; Wu, Z.; Kawano, T.; Vasir, B.; Avigan, D.; Kharbanda, S.; Kufe, D. Direct Targeting of the Mucin 1 Oncoprotein Blocks Survival and Tumorigenicity of Human Breast Carcinoma Cells. Cancer Res. 2009, 69, 5133–5141. [Google Scholar] [CrossRef]
- Hinoda, Y.; Ikematsu, Y.; Horinochi, M.; Sato, S.; Yamamoto, K.; Nakano, T.; Fukui, M.; Suehiro, Y.; Hamanaka, Y.; Nishikawa, Y.; et al. Increased Expression of MUC1 in Advanced Pancreatic Cancer. J. Gastroenterol. 2003, 38, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Q.; Wang, Q.; Wang, Y.; Chen, J. Prognostic Significance of CD24 and CD44 in Breast Cancer: A Meta-Analysis. Int. J. Biol. Markers 2017, 32, e75–e82. [Google Scholar] [CrossRef]
- Li, W.; Qian, L.; Lin, J.; Huang, G.; Hao, N.; Wei, X.; Wang, W.; Liang, J.; Li, W.; Qian, L.; et al. CD44 Regulates Prostate Cancer Proliferation, Invasion and Migration via PDK1 and PFKFB4. Oncotarget 2017, 8, 65143–65151. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, C.; Chang, K.; Karnad, A.; Jagirdar, J.; Kumar, A.P.; Freeman, J.W. CD44 Expression Level and Isoform Contributes to Pancreatic Cancer Cell Plasticity, Invasiveness, and Response to Therapy. Clin. Cancer Res. 2016, 22, 5592–5604. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Santer, F.R. Androgen Receptor Signaling in Prostate Cancer. Cancer Metastasis Rev. 2014, 33, 413–427. [Google Scholar] [CrossRef]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.G.; Cronin, K.A. US Incidence of Breast Cancer Subtypes Defined by Joint Hormone Receptor and HER2 Status. JNCI J. Natl. Cancer Inst. 2014, 106, dju055. [Google Scholar] [CrossRef]
- Gravelle, P.; Burroni, B.; Péricart, S.; Rossi, C.; Bezombes, C.; Tosolini, M.; Damotte, D.; Brousset, P.; Fournié, J.-J.; Laurent, C.; et al. Mechanisms of PD-1/PD-L1 Expression and Prognostic Relevance in Non-Hodgkin Lymphoma: A Summary of Immunohistochemical Studies. Oncotarget 2017, 8, 44960–44975. [Google Scholar] [CrossRef] [PubMed]
- D’Incecco, A.; Andreozzi, M.; Ludovini, V.; Rossi, E.; Capodanno, A.; Landi, L.; Tibaldi, C.; Minuti, G.; Salvini, J.; Coppi, E.; et al. PD-1 and PD-L1 Expression in Molecularly Selected Non-Small-Cell Lung Cancer Patients. Br. J. Cancer 2014, 112, 95–102. [Google Scholar] [CrossRef]
- Kleffel, S.; Posch, C.; Barthel, S.R.; Mueller, H.; Schlapbach, C.; Guenova, E.; Elco, C.P.; Lee, N.; Juneja, V.R.; Zhan, Q.; et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell 2015, 162, 1242–1256. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Benival, D. Encapsulation of Anabolic Peptide in Lipid Nano Vesicles for Osteoporosis. Curr. Protein Pept. Sci. 2022, 23, 495–503. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Kumar, H.; Kommineni, N.; Benival, D. Anabolic Peptide-Enriched Stealth Nanoliposomes for Effective Anti-Osteoporotic Therapy. Pharmaceutics 2022, 14, 2417. [Google Scholar] [CrossRef] [PubMed]
- Salave, S.; Jain, S.; Shah, R.; Benival, D. Quantification of Anti-Osteoporotic Anabolic Peptide in Stealth Lipid Nanovesicles Through Validated RP-HPLC Method. J. AOAC Int. 2022, 106, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Salave, S.; Shinde, S.D.; Rana, D.; Sahu, B.; Kumar, H.; Patel, R.; Benival, D.; Kommineni, N. Peptide Engraftment on PEGylated Nanoliposomes for Bone Specific Delivery of PTH (1–34) in Osteoporosis. Pharmaceutics 2023, 15, 608. [Google Scholar] [CrossRef]
- Karunakaran, B.; Gupta, R.; Patel, P.; Salave, S.; Sharma, A.; Desai, D.; Benival, D.; Kommineni, N. Emerging Trends in Lipid-Based Vaccine Delivery: A Special Focus on Developmental Strategies, Fabrication Methods, and Applications. Vaccines 2023, 11, 661. [Google Scholar] [CrossRef]
- Gupta, R.; Salave, S.; Rana, D.; Karunakaran, B.; Butreddy, A.; Benival, D.; Kommineni, N. Versatility of Liposomes for Antisense Oligonucleotide Delivery: A Special Focus on Various Therapeutic Areas. Pharmaceutics 2023, 15, 1435. [Google Scholar] [CrossRef]
- Rana, D.; Salave, S.; Jain, S.; Shah, R.; Benival, D. Systematic Development and Optimization of Teriparatide-Loaded Nanoliposomes Employing Quality by Design Approach for Osteoporosis. J. Pharm. Innov. 2023, 18, 548–562. [Google Scholar] [CrossRef]
- Eloy, J.O.; Petrilli, R.; Chesca, D.L.; Saggioro, F.P.; Lee, R.J.; Marchetti, J.M. Anti-HER2 Immunoliposomes for Co-Delivery of Paclitaxel and Rapamycin for Breast Cancer Therapy. Eur. J. Pharm. Biopharm. 2017, 115, 159–167. [Google Scholar] [CrossRef]
- Moase, E.H.; Qi, W.; Ishida, T.; Gabos, Z.; Longenecker, B.M.; Zimmermann, G.L.; Ding, L.; Krantz, M.; Allen, T.M. Anti-MUC-1 Immunoliposomal Doxorubicin in the Treatment of Murine Models of Metastatic Breast Cancer. Biochim. Biophys. Acta Biomembr. 2001, 1510, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, S.; Li, Y.; Li, X.; Yang, G.; Li, M.; Xie, Y.; Su, W.; Wu, J.; Jia, L.; et al. Cationic Liposomes Co-Deliver Chemotherapeutics and SiRNA for the Treatment of Breast Cancer. Eur. J. Med. Chem. 2022, 233, 114198. [Google Scholar] [CrossRef]
- Rana, D.; Salave, S.; Patel, R.; Khunt, D.; Misra, M.; Prajapati, B.; Patel, G.; Patel, J. Solid Lipid Nanoparticles in Tuberculosis. In Tubercular Drug Delivery Systems: Advances in Treatment of Infectious Diseases; Springer International Publishing: Cham, Switzerland, 2023; pp. 99–121. [Google Scholar] [CrossRef]
- Kanojia, N.; Sharma, N.; Gupta, N.; Singh, S. Applications of Nanostructured Lipid Carriers: Recent Advancements and Patent Review. Biointerface Res. Appl. Chem. 2022, 12, 638–652. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Duong, V.A.; Nguyen, T.T.L.; Maeng, H.J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Tran, T.H.; Choi, J.Y.; Ramasamy, T.; Truong, D.H.; Nguyen, C.N.; Choi, H.G.; Yong, C.S.; Kim, J.O. Hyaluronic Acid-Coated Solid Lipid Nanoparticles for Targeted Delivery of Vorinostat to CD44 Overexpressing Cancer Cells. Carbohydr. Polym. 2014, 114, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.R.; Carrião, M.S.; Pacheco, M.T.; Branquinho, L.C.; de Souza, A.L.R.; Bakuzis, A.F.; Lima, E.M. Triggered Release of Paclitaxel from Magnetic Solid Lipid Nanoparticles by Magnetic Hyperthermia. Mater. Sci. Eng. C 2018, 92, 547–553. [Google Scholar] [CrossRef]
- Murugesan, M.P.; Venkata Ratnam, M.; Mengitsu, Y.; Kandasamy, K. Evaluation of Anti-Cancer Activity of Phytosomes Formulated from Aloe Vera Extract. Mater. Today Proc. 2021, 42, 631–636. [Google Scholar] [CrossRef]
- Talaat, S.M.; Elnaggar, Y.S.R.; El-Ganainy, S.O.; Gowayed, M.A.; Allam, M.; Abdallah, O.Y. Self-Assembled Fisetin-Phospholipid Complex: Fisetin-Integrated Phytosomes for Effective Delivery to Breast Cancer. Eur. J. Pharm. Biopharm. 2023, 189, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.H.; Alcantara, K.P.; Bulatao, B.P.I.; Sorasitthiyanukarn, F.N.; Muangnoi, C.; Nalinratana, N.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-Coated Nanostructured Lipid Carriers for Transdermal Delivery of Tetrahydrocurcumin for Breast Cancer Therapy. Carbohydr. Polym. 2022, 288, 119401. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, L.; Mahoutforoush, A.; Dorreyatim, S.S.; Soltanfam, T.; Paiva-Santos, A.C.; Peixoto, D.; Veiga, F.; Hamishehkar, H.; Zeinali, M.; Abbaspour-Ravasjani, S. Co-Delivery of Erlotinib and Resveratrol via Nanostructured Lipid Carriers: A Synergistically Promising Approach for Cell Proliferation Prevention and ROS-Mediated Apoptosis Activation. Int. J. Pharm. 2022, 624, 122027. [Google Scholar] [CrossRef]
- Shehata, E.M.M.; Gowayed, M.A.; El-Ganainy, S.O.; Sheta, E.; Elnaggar, Y.S.R.; Abdallah, O.Y. Pectin Coated Nanostructured Lipid Carriers for Targeted Piperine Delivery to Hepatocellular Carcinoma. Int. J. Pharm. 2022, 619, 121712. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, T.H.; Kim, B.D.; Kim, H.K.; Lyu, M.J.; Jung, H.M.; Goo, Y.T.; Kang, M.J.; Lee, S.; Choi, Y.W. Co-Administration of Tariquidar Using Functionalized Nanostructured Lipid Carriers Overcomes Resistance to Docetaxel in Multidrug Resistant MCF7/ADR Cells. J. Drug Deliv. Sci. Technol. 2022, 71, 103323. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing Dendrimers for Biological Applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Kaneshiro, T.L.; Lu, Z.R. Targeted Intracellular Codelivery of Chemotherapeutics and Nucleic Acid with a Well-Defined Dendrimer-Based Nanoglobular Carrier. Biomaterials 2009, 30, 5660–5666. [Google Scholar] [CrossRef]
- Liu, C.; Gao, H.; Zhao, Z.; Rostami, I.; Wang, C.; Zhu, L.; Yang, Y. Improved Tumor Targeting and Penetration by a Dual-Functional Poly(Amidoamine) Dendrimer for the Therapy of Triple-Negative Breast Cancer. J. Mater. Chem. B 2019, 7, 3724–3736. [Google Scholar] [CrossRef]
- Zamani, S.; Shafeie-Ardestani, M.; Bitarafan-Rajabi, A.; Khalaj, A.; Sabzevari, O. Synthesis, Radiolabelling, and Biological Assessment of Folic Acid-Conjugated G-3 99m Tc-Dendrimer as the Breast Cancer Molecular Imaging Agent. IET Nanobiotechnol. 2020, 14, 628–634. [Google Scholar] [CrossRef]
- Nwe, K.; Bryant, L.H.; Brechbiel, M.W. Poly(Amidoamine) Dendrimer Based MRI Contrast Agents Exhibiting Enhanced Relaxivities Derived via Metal Preligation Techniques. Bioconjug Chem. 2010, 21, 1014–1017. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block Copolymer Micelles for Drug Delivery: Design, Characterization and Biological Significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, P.; Luo, Q.; Li, X.; Zhu, W. Supramolecular Polymeric Prodrug Micelles for Efficient Anticancer Drug Delivery. Polym. Chem. 2022, 13, 2964–2970. [Google Scholar] [CrossRef]
- Barve, A.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Enzyme-Responsive Polymeric Micelles of Cabazitaxel for Prostate Cancer Targeted Therapy. Acta Biomater. 2020, 113, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Rafael, D.; Vilar-Hernández, M.; Montero, S.; Martínez-Trucharte, F.; Seras-Franzoso, J.; Díaz-Riascos, Z.V.; Boullosa, A.; García-Aranda, N.; Cámara-Sánchez, P.; et al. Polymeric Micelles Targeted against CD44v6 Receptor Increase Niclosamide Efficacy against Colorectal Cancer Stem Cells and Reduce Circulating Tumor Cells in Vivo. J. Control. Release 2021, 331, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, J.; Pan, H.; Sang, Y.; Wang, D.; Wang, Z.; Ai, J.; Lin, B.; Chen, L. PH-Redox Responsive Polymer-Doxorubicin Prodrug Micelles Studied by Molecular Dynamics, Dissipative Particle Dynamics Simulations and Experiments. J. Drug Deliv. Sci. Technol. 2022, 69, 103136. [Google Scholar] [CrossRef]
- Liang, T.J.; Zhou, Z.M.; Cao, Y.Q.; Ma, M.Z.; Wang, X.J.; Jing, K. Gemcitabine-Based Polymer-Drug Conjugate for Enhanced Anticancer Effect in Colon Cancer. Int. J. Pharm. 2016, 513, 564–571. [Google Scholar] [CrossRef]
- Rychahou, P.; Bae, Y.; Reichel, D.; Zaytseva, Y.Y.; Lee, E.Y.; Napier, D.; Weiss, H.L.; Roller, N.; Frohman, H.; Le, A.T.; et al. Colorectal Cancer Lung Metastasis Treatment with Polymer–Drug Nanoparticles. J. Control. Release 2018, 275, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Li, M.; Wang, Z.; Zheng, X.; Lao, Y.H.; Chang, Z.; Zhang, F.; Lu, M.; Yue, J.; Hu, H.; et al. Bioinspired Diselenide-Bridged Mesoporous Silica Nanoparticles for Dual-Responsive Protein Delivery. Adv. Mater. 2018, 30, 1801198. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhang, X.; Huang, J.; Huang, Y.; Huang, Z.; Pan, X.; Quan, G.; Liu, H.; Wang, L.; Wu, C. Improved Gene Transfer with Functionalized Hollow Mesoporous Silica Nanoparticles of Reduced Cytotoxicity. Materials 2017, 10, 731. [Google Scholar] [CrossRef]
- Rahmani, S.; Budimir, J.; Sejalon, M.; Daurat, M.; Aggad, D.; Vivès, E.; Raehm, L.; Garcia, M.; Lichon, L.; Gary-Bobo, M.; et al. Large Pore Mesoporous Silica and Organosilica Nanoparticles for Pepstatin A Delivery in Breast Cancer Cells. Molecules 2019, 24, 332. [Google Scholar] [CrossRef]
- Cha, B.G.; Jeong, J.H.; Kim, J. Extra-Large Pore Mesoporous Silica Nanoparticles Enabling Co-Delivery of High Amounts of Protein Antigen and Toll-like Receptor 9 Agonist for Enhanced Cancer Vaccine Efficacy. ACS Cent. Sci. 2018, 4, 484–492. [Google Scholar] [CrossRef]
- Rana, K.; Kumar Pandey, S.; Chauhan, S.; Preet, S. Anticancer Therapeutic Potential of 5-Fluorouracil and Nisin Co-Loaded Chitosan Coated Silver Nanoparticles against Murine Skin Cancer. Int. J. Pharm. 2022, 620, 121744. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.R.; Venkatesan, J.; Prabhu, A. Anticancer Activity of Silver Nanoparticles from the Aqueous Extract of Dictyota Ciliolata on Non-Small Cell Lung Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 74, 103525. [Google Scholar] [CrossRef]
- Pourshohod, A.; Zeinali, M.; Ghaffari, M.A.; Kheirollah, A.; Jamalan, M. Improvement of Specific Aiming of X-Ray Radiotherapy on HER2-Overexpressing Cancerous Cell Lines by Targeted Delivery of Silver Nanoparticle. J. Drug Deliv. Sci. Technol. 2022, 76, 103746. [Google Scholar] [CrossRef]
- Mao, W.; Kim, H.S.; Son, Y.J.; Kim, S.R.; Yoo, H.S. Doxorubicin Encapsulated Clicked Gold Nanoparticle Clusters Exhibiting Tumor-Specific Disassembly for Enhanced Tumor Localization and Computerized Tomographic Imaging. J. Control. Release 2018, 269, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Jia, H.R.; Duan, Q.Y.; Liu, X.; Yang, J.; Liu, Y.; Wu, F.G. Photosensitizer-Doped and Plasma Membrane-Responsive Liposomes for Nuclear Drug Delivery and Multidrug Resistance Reversal. ACS Appl. Mater. Interfaces 2020, 12, 36882–36894. [Google Scholar] [CrossRef] [PubMed]
- Maity, R.; Chatterjee, M.; Banerjee, A.; Das, A.; Mishra, R.; Mazumder, S.; Chanda, N. Gold Nanoparticle-Assisted Enhancement in the Anti-Cancer Properties of Theaflavin against Human Ovarian Cancer Cells. Mater. Sci. Eng. C 2019, 104, 109909. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gao, Q.; Lv, C.; Liu, L. Improved Photothermal Therapy of Brain Cancer Cells and Photogeneration of Reactive Oxygen Species by Biotin Conjugated Gold Photoactive Nanoparticles. J. Photochem. Photobiol. B 2021, 215, 112102. [Google Scholar] [CrossRef]
- Swami, R.; Shahiwala, A. Impact of Physiochemical Properties on Pharmacokinetics of Protein Therapeutics. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 231–239. [Google Scholar] [CrossRef]
- Kaur, N.; Popli, P.; Tiwary, N.; Swami, R. Small Molecules as Cancer Targeting Ligands: Shifting the Paradigm. J. Control. Release 2023, 355, 417–433. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Canevari, S.; Thigpen, T. Targeting the Folate Receptor: Diagnostic and Therapeutic Approaches to Personalize Cancer Treatments. Ann. Oncol. 2015, 26, 2034–2043. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ritter, C.A.; von Woedtke, T.; Bekeschus, S.; Wende, K. Package Delivered: Folate Receptor-Mediated Transporters in Cancer Therapy and Diagnosis. Chem. Sci. 2024, 15, 1966–2006. [Google Scholar] [CrossRef]
- Bellotti, E.; Cascone, M.G.; Barbani, N.; Rossin, D.; Rastaldo, R.; Giachino, C.; Cristallini, C. Targeting Cancer Cells Overexpressing Folate Receptors with New Terpolymer-Based Nanocapsules: Toward a Novel Targeted DNA Delivery System for Cancer Therapy. Biomedicines 2021, 9, 1275. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Huang, J.; Xu, Y.; Zhang, X.; Teng, Y.; Huang, C.; Wu, Y.; Zhang, X.; Zhang, H.; Sun, W.; et al. Co-Delivery of Cisplatin and Paclitaxel by Folic Acid Conjugated Amphiphilic PEG-PLGA Copolymer Nanoparticles for the Treatment of Non-Small Lung Cancer. Oncotarget 2015, 6, 42150–42168. [Google Scholar] [CrossRef]
- Bourbour, M.; Khayam, N.; Noorbazargan, H.; Tavakkoli Yaraki, M.; Asghari Lalami, Z.; Akbarzadeh, I.; Eshrati Yeganeh, F.; Dolatabadi, A.; Mirzaei Rad, F.; Tan, Y.N. Evaluation of Anti-Cancer and Anti-Metastatic Effects of Folate-PEGylated Niosomes for Co-Delivery of Letrozole and Ascorbic Acid on Breast Cancer Cells. Mol. Syst. Des. Eng. 2022, 7, 1102–1118. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Lv, P.; Zhang, P. Transferrin-Conjugated Doxorubicin-Loaded Lipid-Coated Nanoparticles for the Targeting and Therapy of Lung Cancer. Oncol. Lett. 2015, 9, 1065–1072. Available online: https://www.spandidos-publications.com/10.3892/ol.2014.2840 (accessed on 25 November 2023). [CrossRef]
- Jiang, X.; Xin, H.; Ren, Q.; Gu, J.; Zhu, L.; Du, F.; Feng, C.; Xie, Y.; Sha, X.; Fang, X. Nanoparticles of 2-Deoxy-d-Glucose Functionalized Poly(Ethylene Glycol)-Co-Poly(Trimethylene Carbonate) for Dual-Targeted Drug Delivery in Glioma Treatment. Biomaterials 2014, 35, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Huennekens, F.M. The Methotrexate Story: A Paradigm for Development of Cancer Chemotherapeutic Agents. Adv. Enzym. Regul. 1994, 34, 397–419. [Google Scholar] [CrossRef]
- Thomas, T.P.; Huang, B.; Choi, S.K.; Silpe, J.E.; Kotlyar, A.; Desai, A.M.; Zong, H.; Gam, J.; Joice, M.; Baker, J.R. Polyvalent Dendrimer-Methotrexate as a Folate Receptor-Targeted Cancer Therapeutic. Mol. Pharm. 2012, 9, 2669–2676. [Google Scholar] [CrossRef]
- Wong, P.T.; Choi, S.K. Mechanisms and Implications of Dual-Acting Methotrexate in Folate-Targeted Nanotherapeutic Delivery. Int. J. Mol. Sci. 2015, 16, 1772–1790. [Google Scholar] [CrossRef]
- Thomas, T.P.; Choi, S.K.; Li, M.H.; Kotlyar, A.; Baker, J.R. Design of Riboflavin-Presenting PAMAM Dendrimers as a New Nanoplatform for Cancer-Targeted Delivery. Bioorg Med. Chem. Lett. 2010, 20, 5191–5194. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Wang, Y.; Liu, H.; Yin, Z. A Biotin-Modified and H2O2-Activatable Theranostic Nanoplatform for Enhanced Photothermal and Chemical Combination Cancer Therapy. Eur. J. Pharm. Biopharm. 2022, 177, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Swami, R.; Jeengar, M.K.; Khan, W.; Sistla, R. p-Aminophenyl-α-d-Mannopyranoside Engineered Lipidic Nanoparticles for Effective Delivery of Docetaxel to Brain. Chem. Phys. Lipids 2015, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gautam, L.; Sharma, R.; Shrivastava, P.; Vyas, S.; Vyas, S.P. Development and Characterization of Biocompatible Mannose Functionalized Mesospheres: An Effective Chemotherapeutic Approach for Lung Cancer Targeting. AAPS PharmSciTech 2020, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Bae, P.K.; Chung, B.H. Self-Assembled Levan Nanoparticles for Targeted Breast Cancer Imaging. Chem. Commun. 2014, 51, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.; Mahajan, K.; Vavia, P. In Vivo Anticancer Efficacy and Toxicity Studies of a Novel Polymer Conjugate N-Acetyl Glucosamine (NAG)–PEG–Doxorubicin for Targeted Cancer Therapy. AAPS PharmSciTech 2017, 18, 3021–3033. [Google Scholar] [CrossRef]
- Tian, B.; Ding, Y.; Han, J.; Zhang, J.; Han, Y.; Han, J. N-Acetyl-D-Glucosamine Decorated Polymeric Nanoparticles for Targeted Delivery of Doxorubicin: Synthesis, Characterization and in Vitro Evaluation. Colloids Surf. B Biointerfaces 2015, 130, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Swami, R.; Singh, I.; Jeengar, M.K.; Naidu, V.G.M.; Khan, W.; Sistla, R. Adenosine Conjugated Lipidic Nanoparticles for Enhanced Tumor Targeting. Int. J. Pharm. 2015, 486, 287–296. [Google Scholar] [CrossRef]
- Mathur, R.; Chauhan, R.P.; Singh, G.; Singh, S.; Varshney, R.; Kaul, A.; Jain, S.; Mishra, A.K. Tryptophan Conjugated Magnetic Nanoparticles for Targeting Tumors Overexpressing Indoleamine 2,3 Dioxygenase (IDO) and L-Type Amino Acid Transporter. J. Mater. Sci. Mater. Med. 2020, 31, 87. [Google Scholar] [CrossRef]
- Bayat, P.; Pakravan, P.; Salouti, M.; Dolatabadi, J.E.N. Lysine Decorated Solid Lipid Nanoparticles of Epirubicin for Cancer Targeting and Therapy. Adv. Pharm. Bull. 2021, 11, 96–103. [Google Scholar] [CrossRef]
- Li, L.; Di, X.; Wu, M.; Sun, Z.; Zhong, L.; Wang, Y.; Fu, Q.; Kan, Q.; Sun, J.; He, Z. Targeting Tumor Highly-Expressed LAT1 Transporter with Amino Acid-Modified Nanoparticles: Toward a Novel Active Targeting Strategy in Breast Cancer Therapy. Nanomedicine 2017, 13, 987–998. [Google Scholar] [CrossRef]
- Li, K.; Zang, X.; Meng, X.; Li, Y.; Xie, Y.; Chen, X. Targeted Delivery of Quercetin by Biotinylated Mixed Micelles for Non-Small Cell Lung Cancer Treatment. Drug Deliv. 2022, 29, 970–985. [Google Scholar] [CrossRef]
- Hao, Z.F.; Cui, Y.X.; Li, M.H.; Du, D.; Liu, M.F.; Tao, H.Q.; Li, S.; Cao, F.Y.; Chen, Y.L.; Lei, X.H.; et al. Liposomes Modified with P-Aminophenyl-α-d-Mannopyranoside: A Carrier for Targeting Cerebral Functional Regions in Mice. Eur. J. Pharm. Biopharm. 2013, 84, 505–516. [Google Scholar] [CrossRef]
- Wang, T.; Li, M.; Wei, R.; Wang, X.; Lin, Z.; Chen, J.; Wu, X. Small Molecule-Drug Conjugates Emerge as a New Promising Approach for Cancer Treatment. Mol. Pharm. 2024, 21, 1038–1055. [Google Scholar] [CrossRef] [PubMed]
- Dal Corso, A.; Neri, D. Linker Stability Influences the Anti-Tumor Activity of Acetazolamide-Drug Conjugates for the Therapy of Renal Cell Carcinoma. J. Control. Release 2017, 246, 39–45. [Google Scholar] [CrossRef]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The Joint IAEA, EANM, and SNMMI Practical Guidance on Peptide Receptor Radionuclide Therapy (PRRNT) in Neuroendocrine Tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Wang, C.; Ma, Y.; Feng, S.; Liu, K.; Zhou, N. Gonadotropin-Releasing Hormone Receptor-Targeted Paclitaxel–Degarelix Conjugate: Synthesis and in Vitro Evaluation. J. Pept. Sci. 2015, 21, 569–576. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Benival, D. Peptide Functionalised Nanocarriers for Bone Specific Delivery of PTH (1-34) in Osteoporosis. Curr. Nanomed. 2021, 11, 142–148. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Benival, D. Dual Targeting Anti-Osteoporotic Therapy Through Potential Nanotherapeutic Approaches. Pharm. Nanotechnol. 2022, 10, 384–392. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Prayag, K.; Shah, S.; Rawat, G.; Sharma, N.; Jindal, A.B.; Patel, R.; Benival, D. Recent Advances in Teriparatide Delivery By-Virtue-of Novel Drug Delivery Approaches for the Management of Osteoporosis. Crit. Rev. Trade Ther. Drug Carr. Syst. 2023, 40, 93–113. [Google Scholar] [CrossRef]
- Jiang, Z.; Guan, J.; Qian, J.; Zhan, C. Peptide Ligand-Mediated Targeted Drug Delivery of Nanomedicines. Biomater. Sci. 2019, 7, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.B.; Gao, Y.J.; Wang, L.; Wang, H. Self-Assembled Peptide-Based Nanomaterials for Biomedical Imaging and Therapy. Adv. Mater. 2018, 30, 1703444. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Jumeaux, C.; Gupta, B.; Stevens, M.M. Peptide and Protein Nanoparticle Conjugates: Versatile Platforms for Biomedical Applications. Chem. Soc. Rev. 2018, 47, 3574–3620. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, D.; Geng, L.; Qu, S.; Scarfone, C.; Giorgio, T.; Donnelly, E.; Gao, X.; Clanton, J. Integrin-Mediated Targeting of Drug Delivery to Irradiated Tumor Blood Vessels. Cancer Cell 2003, 3, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, I.; Wang, X.; Thakur, A.; Iyaswamy, A.; Thakur, S.; Chen, X.; Kumar, G.; Li, M.; Yang, Z. Peptide-Conjugated Nano Delivery Systems for Therapy and Diagnosis of Cancer. Pharmaceutics 2021, 13, 1433. [Google Scholar] [CrossRef] [PubMed]
- Bibby, D.C.; Talmadge, J.E.; Dalal, M.K.; Kurz, S.G.; Chytil, K.M.; Barry, S.E.; Shand, D.G.; Steiert, M. Pharmacokinetics and Biodistribution of RGD-Targeted Doxorubicin-Loaded Nanoparticles in Tumor-Bearing Mice. Int. J. Pharm. 2005, 293, 281–290. [Google Scholar] [CrossRef]
- Garg, A.; Tisdale, A.W.; Haidari, E.; Kokkoli, E. Targeting Colon Cancer Cells Using PEGylated Liposomes Modified with a Fibronectin-Mimetic Peptide. Int. J. Pharm. 2009, 366, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Garbuzenko, O.B.; Kirkpatrick, P.; Pandya, I.; Savla, R.; Pozharov, V.P.; He, H.; Minko, T. Surface-Engineered Targeted PPI Dendrimer for Efficient Intracellular and Intratumoral SiRNA Delivery. J. Control. Release 2009, 140, 284–293. [Google Scholar] [CrossRef]
- Zhan, C.; Yan, Z.; Xie, C.; Lu, W. Loop 2 of Ophiophagus Hannah Toxin b Binds with Neuronal Nicotinic Acetylcholine Receptors and Enhances Intracranial Drug Delivery. Mol. Pharm. 2010, 7, 1940–1947. [Google Scholar] [CrossRef]
- Wei, X.; Gao, J.; Zhan, C.; Xie, C.; Chai, Z.; Ran, D.; Ying, M.; Zheng, P.; Lu, W. Liposome-Based Glioma Targeted Drug Delivery Enabled by Stable Peptide Ligands. J. Control. Release 2015, 218, 13–21. [Google Scholar] [CrossRef]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent Advances in SELEX Technology and Aptamer Applications in Biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Malczyk, A.H.; Greten, H.J.; Efferth, T. Aptamers as a Novel Tool for Diagnostics and Therapy. Investig. New Drugs 2015, 33, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In Vitro Selection of RNA Molecules That Bind Specific Ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, J.; Ali, M.M.; Mahmood, M.A.I.; Labanieh, L.; Lu, M.; Iqbal, S.M.; Zhang, Q.; Zhao, W.; Wan, Y. Nucleic Acid Aptamers in Cancer Research, Diagnosis and Therapy. Chem. Soc. Rev. 2015, 44, 1240–1256. [Google Scholar] [CrossRef]
- Ulrich, H.; Trujillo, C.; Nery, A.; Alves, J.; Majumder, P.; Resende, R.; Martins, A. DNA and RNA Aptamers: From Tools for Basic Research Towards Therapeutic Applications. Comb. Chem. High. Throughput Screen. 2006, 9, 619–632. [Google Scholar] [CrossRef]
- Stadler, A.; Chi, C.; Van Der Lelie, D.; Gang, O. DNA-Incorporating Nanomaterials in Biotechnological Applications. Nanomedicine 2010, 5, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yigit, M.V.; Mazumdar, D.; Lu, Y. Molecular Diagnostic and Drug Delivery Agents Based on Aptamer-Nanomaterial Conjugates. Adv. Drug Deliv. Rev. 2010, 62, 592–605. [Google Scholar] [CrossRef]
- Wang, H.; Yang, R.; Yang, L.; Tan, W. Nucleic Acid Conjugated Nanomaterials for Enhanced Molecular Recognition. ACS Nano 2009, 3, 2451–2460. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Cheng, J.; Teply, B.A.; Sherifi, I.; Jon, S.; Kantoff, P.W.; Richie, J.P.; Langer, R. Targeted Nanoparticle-Aptamer Bioconjugates for Cancer Chemotherapy in Vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 6315–6320. [Google Scholar] [CrossRef]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhza, O.C.; Lippard, S.J. Targeted Delivery of Cisplatin to Prostate Cancer Cells by Aptamer Functionalized Pt(IV) Prodrug-PLGA—PEG Nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 17356–17361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Luo, Z.; Liu, J.; Ding, X.; Li, J.; Cai, K. Cytochrome c End-Capped Mesoporous Silica Nanoparticles as Redox-Responsive Drug Delivery Vehicles for Liver Tumor-Targeted Triplex Therapy in Vitro and in Vivo. J. Control. Release 2014, 192, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, F.; Zhang, H.; Lu, Y.; Lian, S.; Lin, H.; Gao, Y.; Jia, L. EpCAM Aptamer-Functionalized Mesoporous Silica Nanoparticles for Efficient Colon Cancer Cell-Targeted Drug Delivery. Eur. J. Pharm. Sci. 2016, 83, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.M.; Gherghel, D.; Popa, M. “In Vitro” Behaviour of Aptamer-Functionalized Polymeric Nanocapsules Loaded with 5-Fluorouracil for Targeted Therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Lavaee, P.; Jalalian, S.H.; Robati, R.Y.; Abnous, K. Double Targeting and Aptamer-Assisted Controlled Release Delivery of Epirubicin to Cancer Cells by Aptamers-Based Dendrimer in Vitro and in Vivo. Eur. J. Pharm. Biopharm. 2016, 102, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Engelberg, S.; Netzer, E.; Assaraf, Y.G.; Livney, Y.D. Selective Eradication of Human Non-Small Cell Lung Cancer Cells Using Aptamer-Decorated Nanoparticles Harboring a Cytotoxic Drug Cargo. Cell Death Dis. 2019, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, W.; Tan, W.; Lai, Z.; Fang, D.; Jiang, L.; Zuo, C.; Yang, N.; Lai, Y. An Efficient Cell-Targeting Drug Delivery System Based on Aptamer-Modified Mesoporous Silica Nanoparticles. Nanoscale Res. Lett. 2019, 14, 390. [Google Scholar] [CrossRef] [PubMed]
- Darabi, F.; Saidijam, M.; Nouri, F.; Mahjub, R.; Soleimani, M. Anti-CD44 and EGFR Dual-Targeted Solid Lipid Nanoparticles for Delivery of Doxorubicin to Triple-Negative Breast Cancer Cell Line: Preparation, Statistical Optimization, and in Vitro Characterization. Biomed. Res. Int. 2022, 2022, 6253978. [Google Scholar] [CrossRef] [PubMed]
- Ara, M.N.; Matsuda, T.; Hyodo, M.; Sakurai, Y.; Hatakeyama, H.; Ohga, N.; Hida, K.; Harashima, H. An Aptamer Ligand Based Liposomal Nanocarrier System That Targets Tumor Endothelial Cells. Biomaterials 2014, 35, 7110–7120. [Google Scholar] [CrossRef]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein Nanoparticles as Drug Carriers in Clinical Medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.C.; Velho, S.; Amaral, M.H. Lipid Nanoparticles Functionalized with Antibodies for Anticancer Drug Therapy. Pharmaceutics 2023, 15, 216. [Google Scholar] [CrossRef] [PubMed]
- Farahavar, G.; Abolmaali, S.S.; Gholijani, N.; Nejatollahi, F. Antibody-Guided Nanomedicines as Novel Breakthrough Therapeutic, Diagnostic and Theranostic Tools. Biomater. Sci. 2019, 7, 4000–4016. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ding, Y.; Zhang, Y.; Ho, R.J.Y.; Zhao, Y.; Zhang, T.; Guo, C. Antibody-Modified Liposomes for Tumor-Targeting Delivery of Timosaponin AIII. Int. J. Nanomed. 2018, 13, 1927–1944. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, M.M.J.; Johnston, A.P.R.; Such, G.K.; Dam, H.H.; Evans, R.A.; Scott, A.M.; Nice, E.C.; Heath, J.K.; Caruso, F. Targeting of Cancer Cells Using Click-Functionalized Polymer Capsules. J. Am. Chem. Soc. 2010, 132, 15881–15883. [Google Scholar] [CrossRef] [PubMed]
- Diniz, F.; Azevedo, M.; Sousa, F.; Osório, H.; Campos, D.; Sampaio, P.; Gomes, J.; Sarmento, B.; Reis, C.A. Polymeric Nanoparticles Targeting Sialyl-Tn in Gastric Cancer: A Live Tracking under Flow Conditions. Mater. Today Bio 2022, 16, 100417. [Google Scholar] [CrossRef]
- Vivek, R.; Thangam, R.; Nipunbabu, V.; Rejeeth, C.; Sivasubramanian, S.; Gunasekaran, P.; Muthuchelian, K.; Kannan, S. Multifunctional HER2-Antibody Conjugated Polymeric Nanocarrier-Based Drug Delivery System for Multi-Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2014, 6, 6469–6480. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Kim, G.J.; Nie, S.; Shin, D.M. Nanotechnology in Cancer Therapeutics: Bioconjugated Nanoparticles for Drug Delivery. Mol. Cancer Ther. 2006, 5, 1909–1917. [Google Scholar] [CrossRef]
- Jain, N.K.; Tare, M.S.; Mishra, V.; Tripathi, P.K. The Development, Characterization and in Vivo Anti-Ovarian Cancer Activity of Poly(Propylene Imine) (PPI)-Antibody Conjugates Containing Encapsulated Paclitaxel. Nanomedicine 2015, 11, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Anisuzzman, M.; Komalla, V.; Tarkistani, M.A.M.; Kayser, V. Anti-Tumor Activity of Novel Nimotuzumab-Functionalized Gold Nanoparticles as a Potential Immunotherapeutic Agent against Skin and Lung Cancers. J. Funct. Biomater. 2023, 14, 407. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, X.; Taratula, O.; Treado, S.; Urbas, A.; Holbrook, R.D.; Cavicchi, R.E.; Avedisian, C.T.; Mitra, S.; Savla, R.; et al. Anti-HER2 IgY Antibody-Functionalized Single-Walled Carbon Nanotubes for Detection and Selective Destruction of Breast Cancer Cells. BMC Cancer 2009, 9, 351. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Stanczyk, M.; Janaszewska, A.; Sobierajska, E.; Chworos, A.; Klajnert-Maculewicz, B. Multicomponent Conjugates of Anticancer Drugs and Monoclonal Antibody with PAMAM Dendrimers to Increase Efficacy of HER-2 Positive Breast Cancer Therapy. Pharm. Res. 2019, 36, 154. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prajapati, A.; Rangra, S.; Patil, R.; Desai, N.; Jyothi, V.G.S.S.; Salave, S.; Amate, P.; Benival, D.; Kommineni, N. Receptor-Targeted Nanomedicine for Cancer Therapy. Receptors 2024, 3, 323-361. https://doi.org/10.3390/receptors3030016
Prajapati A, Rangra S, Patil R, Desai N, Jyothi VGSS, Salave S, Amate P, Benival D, Kommineni N. Receptor-Targeted Nanomedicine for Cancer Therapy. Receptors. 2024; 3(3):323-361. https://doi.org/10.3390/receptors3030016
Chicago/Turabian StylePrajapati, Arvee, Shagun Rangra, Rashmi Patil, Nimeet Desai, Vaskuri G. S. Sainaga Jyothi, Sagar Salave, Prakash Amate, Derajram Benival, and Nagavendra Kommineni. 2024. "Receptor-Targeted Nanomedicine for Cancer Therapy" Receptors 3, no. 3: 323-361. https://doi.org/10.3390/receptors3030016
APA StylePrajapati, A., Rangra, S., Patil, R., Desai, N., Jyothi, V. G. S. S., Salave, S., Amate, P., Benival, D., & Kommineni, N. (2024). Receptor-Targeted Nanomedicine for Cancer Therapy. Receptors, 3(3), 323-361. https://doi.org/10.3390/receptors3030016








