Histological Characterization of Ocular and Adnexal Tissues in Dogs (Canis familiaris) and Wolves (Canis lupus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Sample Dissection and Processing
2.3. General Histological Stains
2.4. Lectin Histochemical Labelling
2.5. Imaging and Digital Processing
3. Results
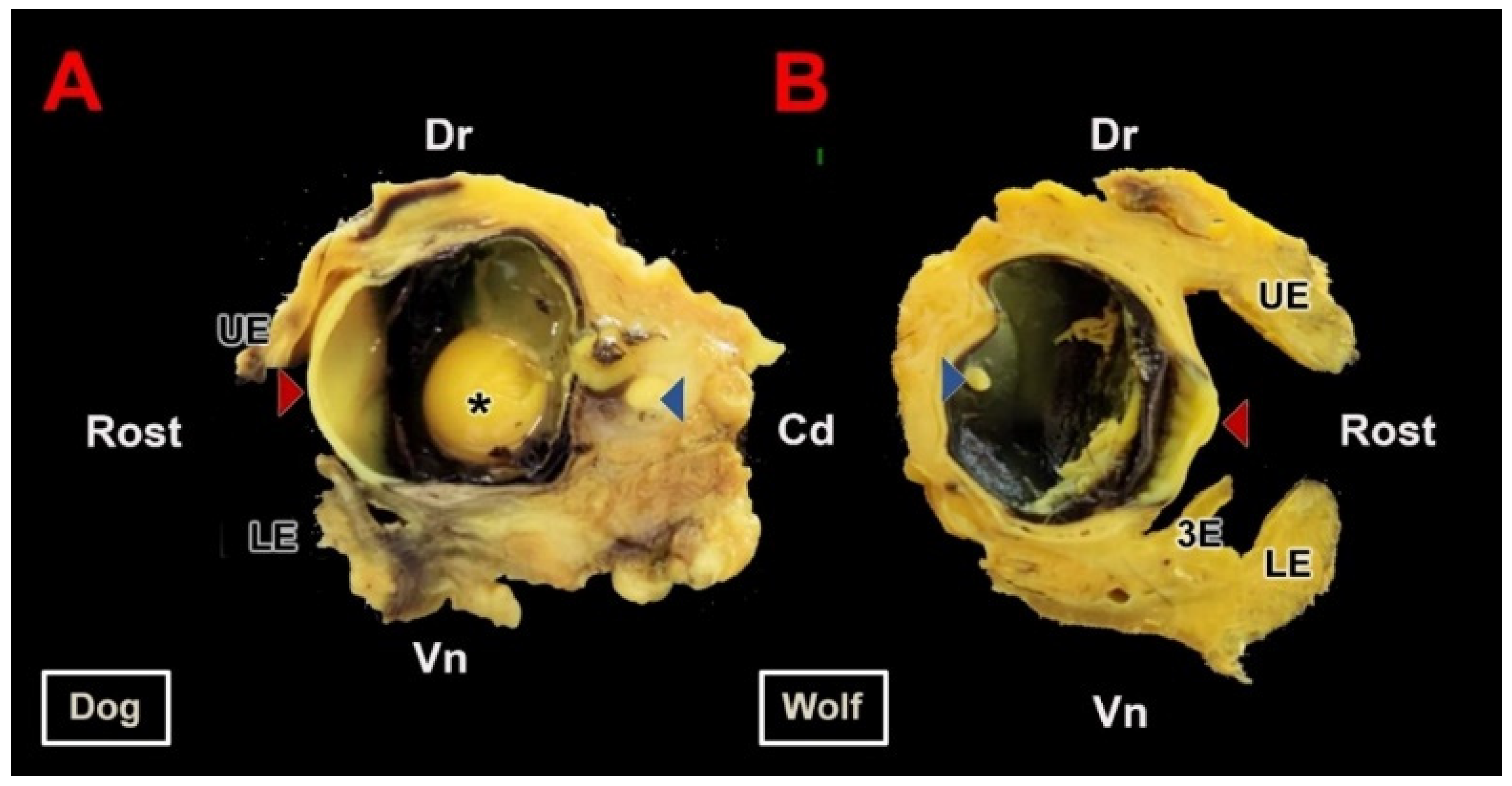
3.1. Macroscopic Study
3.2. Microscopic Study
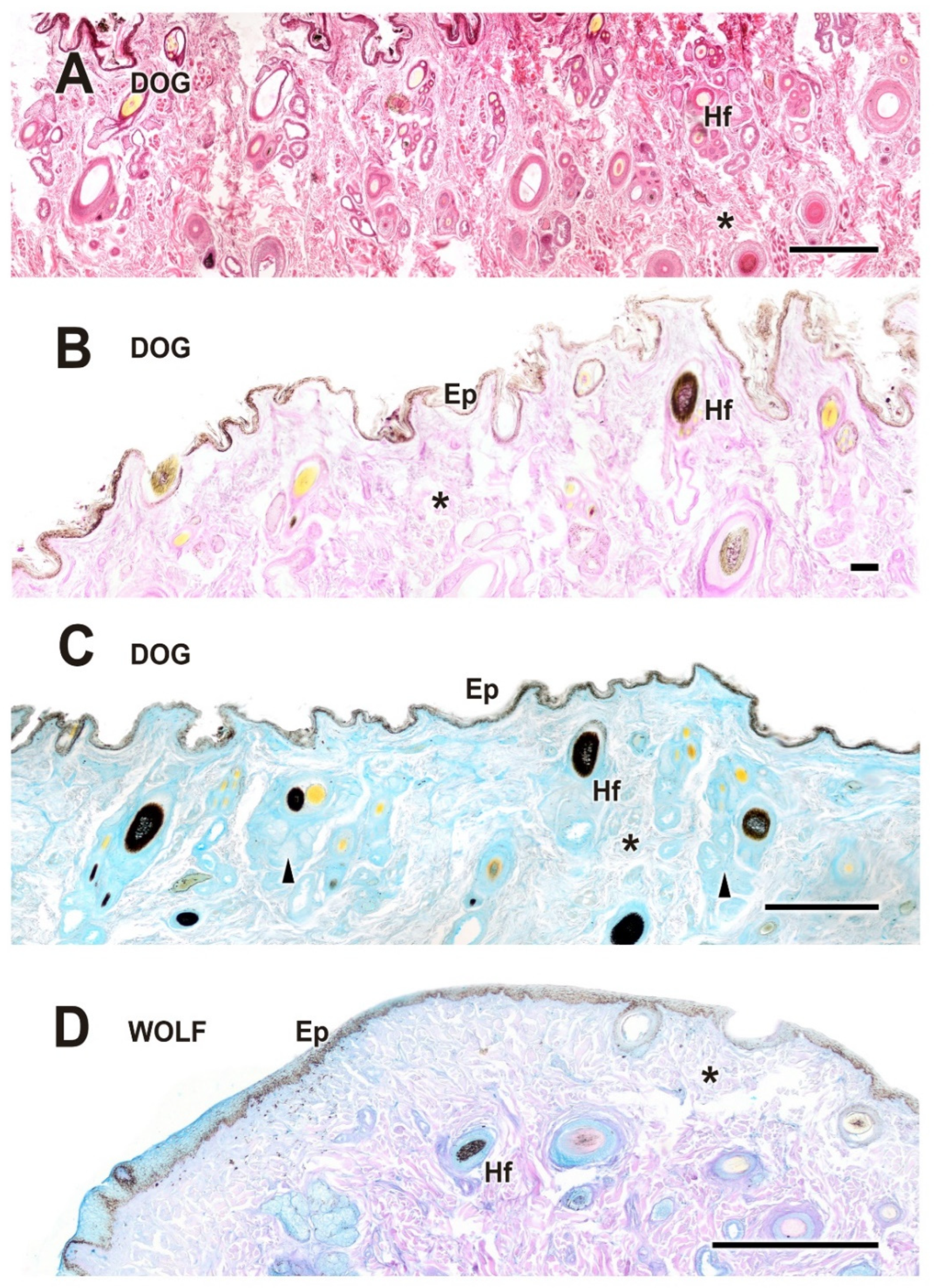
3.2.1. Eyelid Skin Lining
3.2.2. Upper Eyelid and Lower Eyelid
3.2.3. Third Eyelid
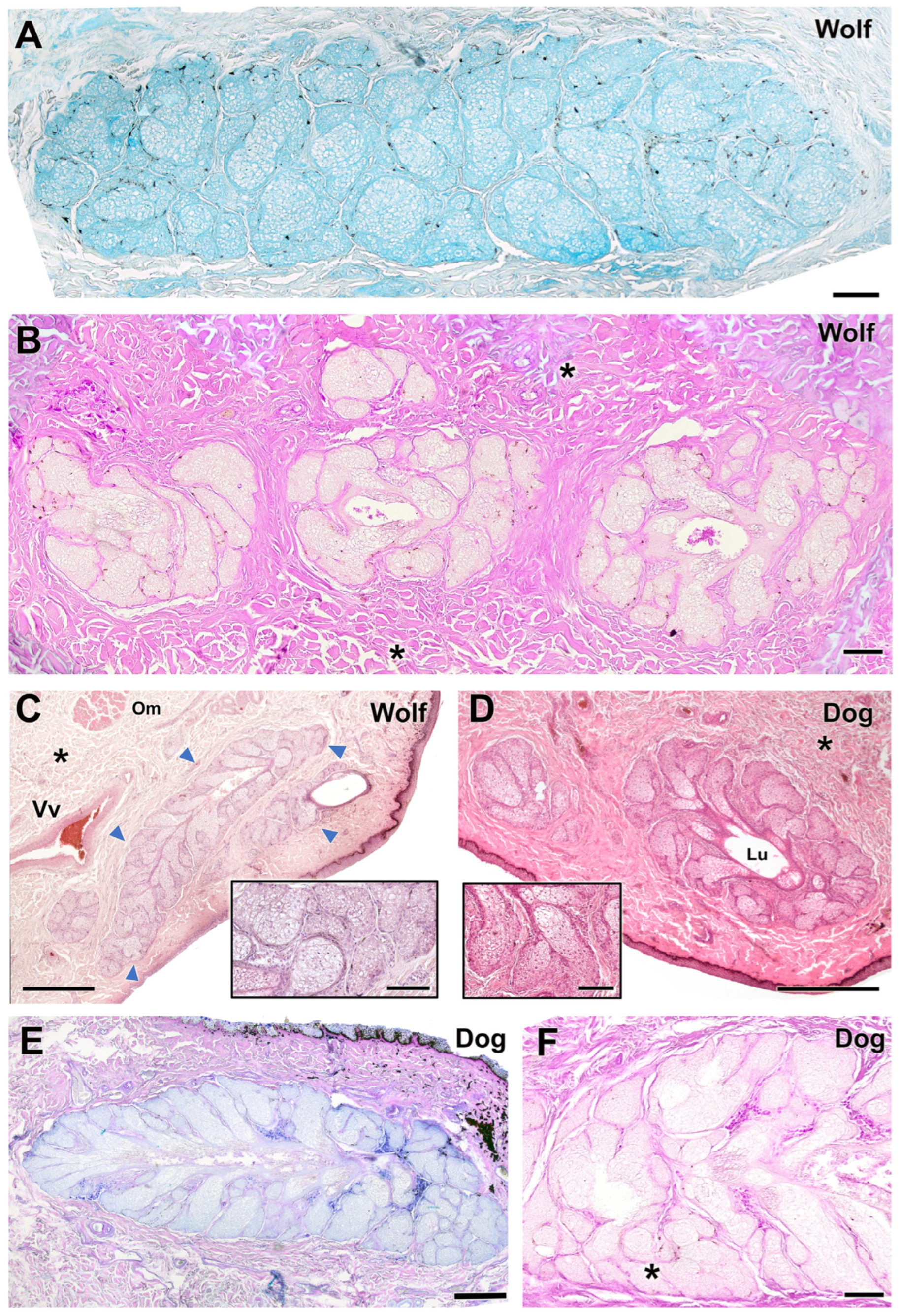
3.2.4. Tarsal Glands
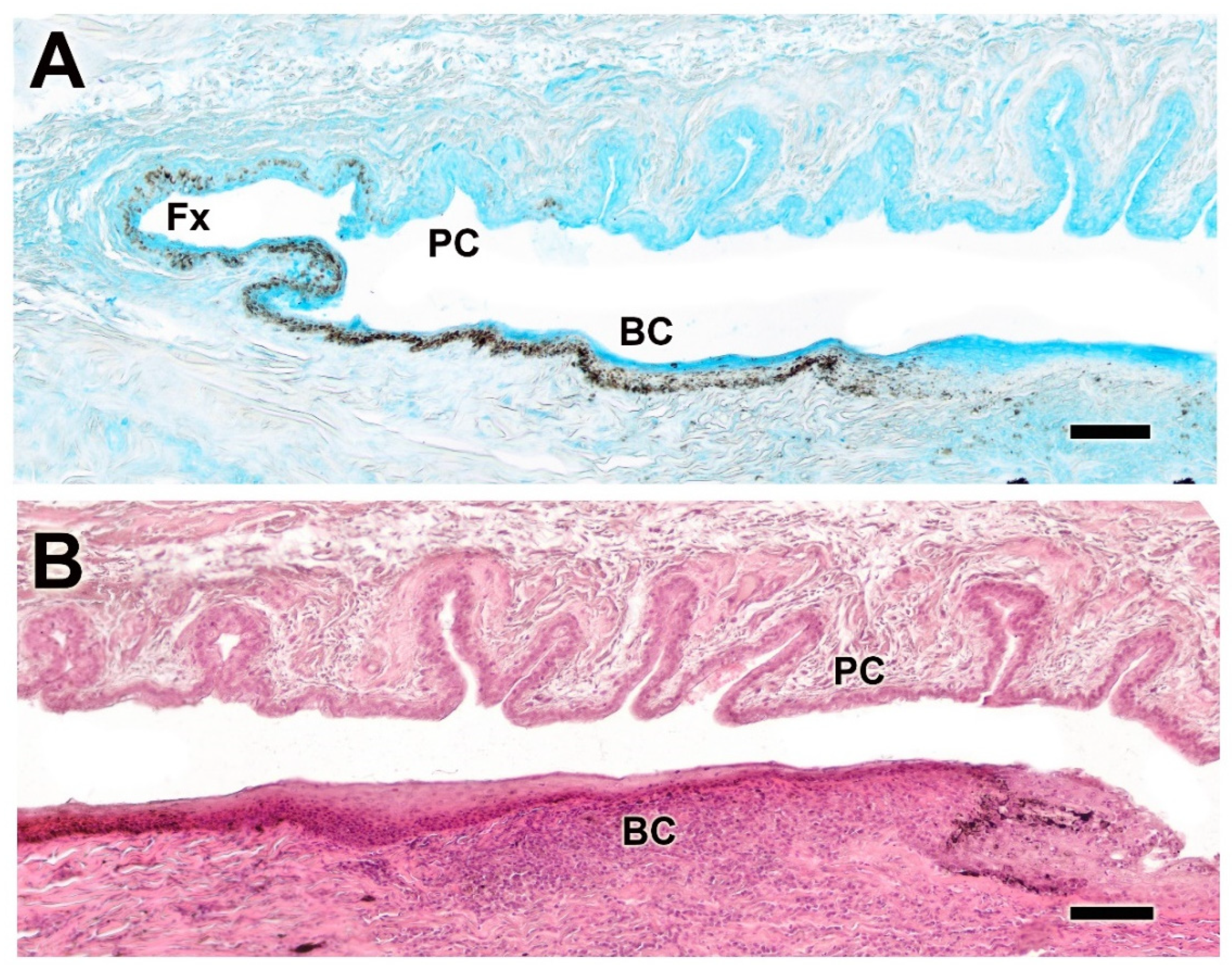
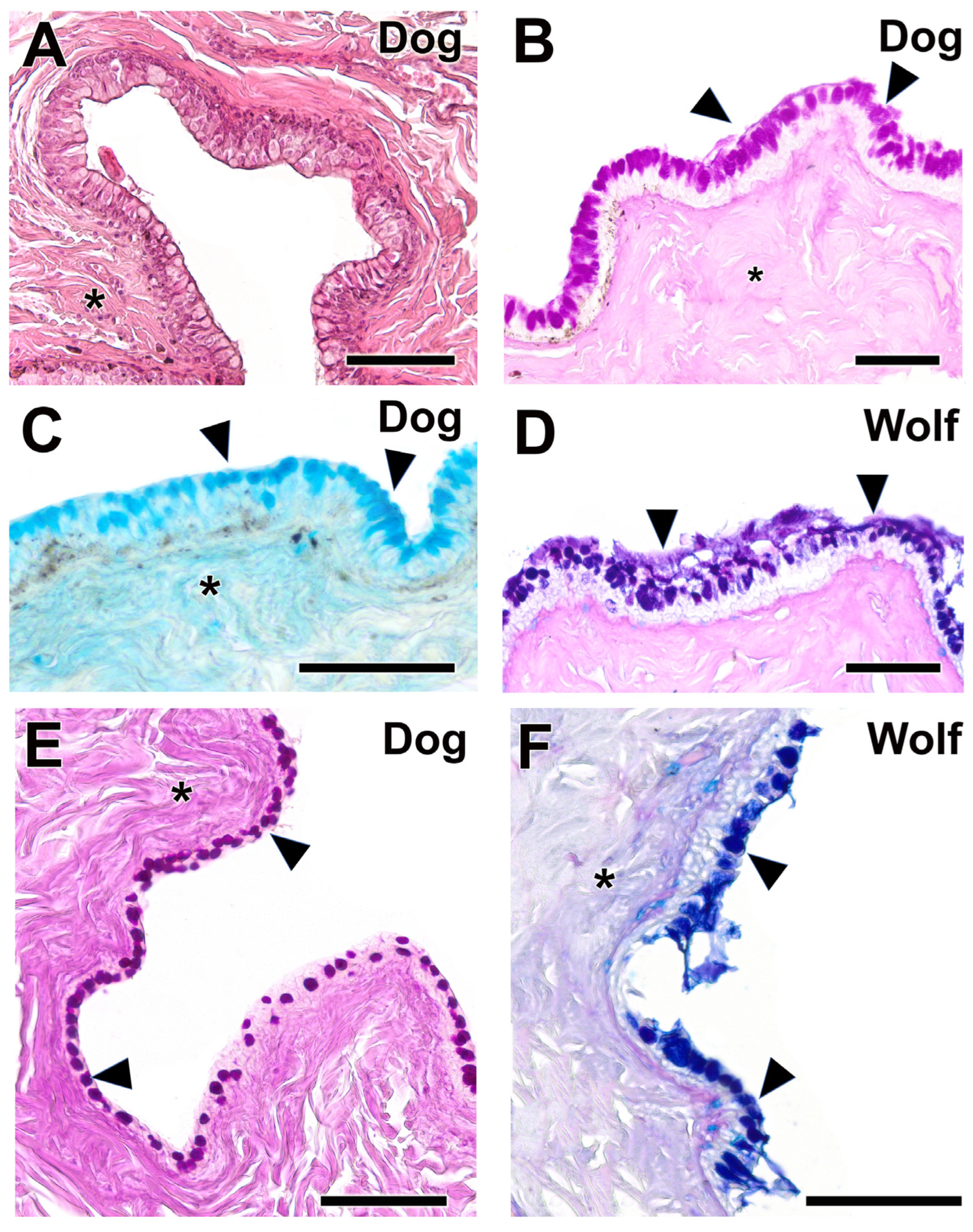
3.2.5. Palpebral Conjunctiva, Bulbar Conjunctiva, and Fornix
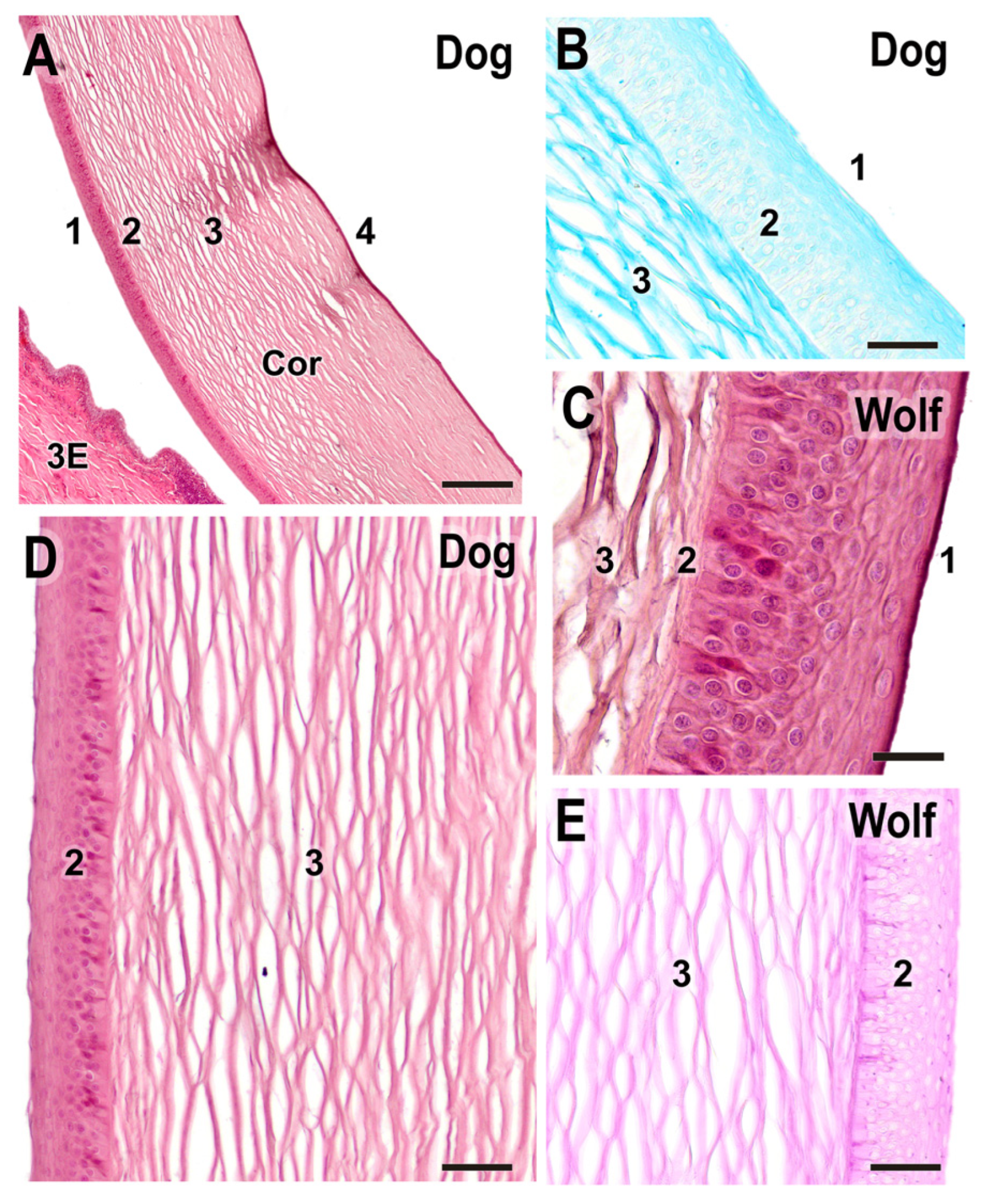
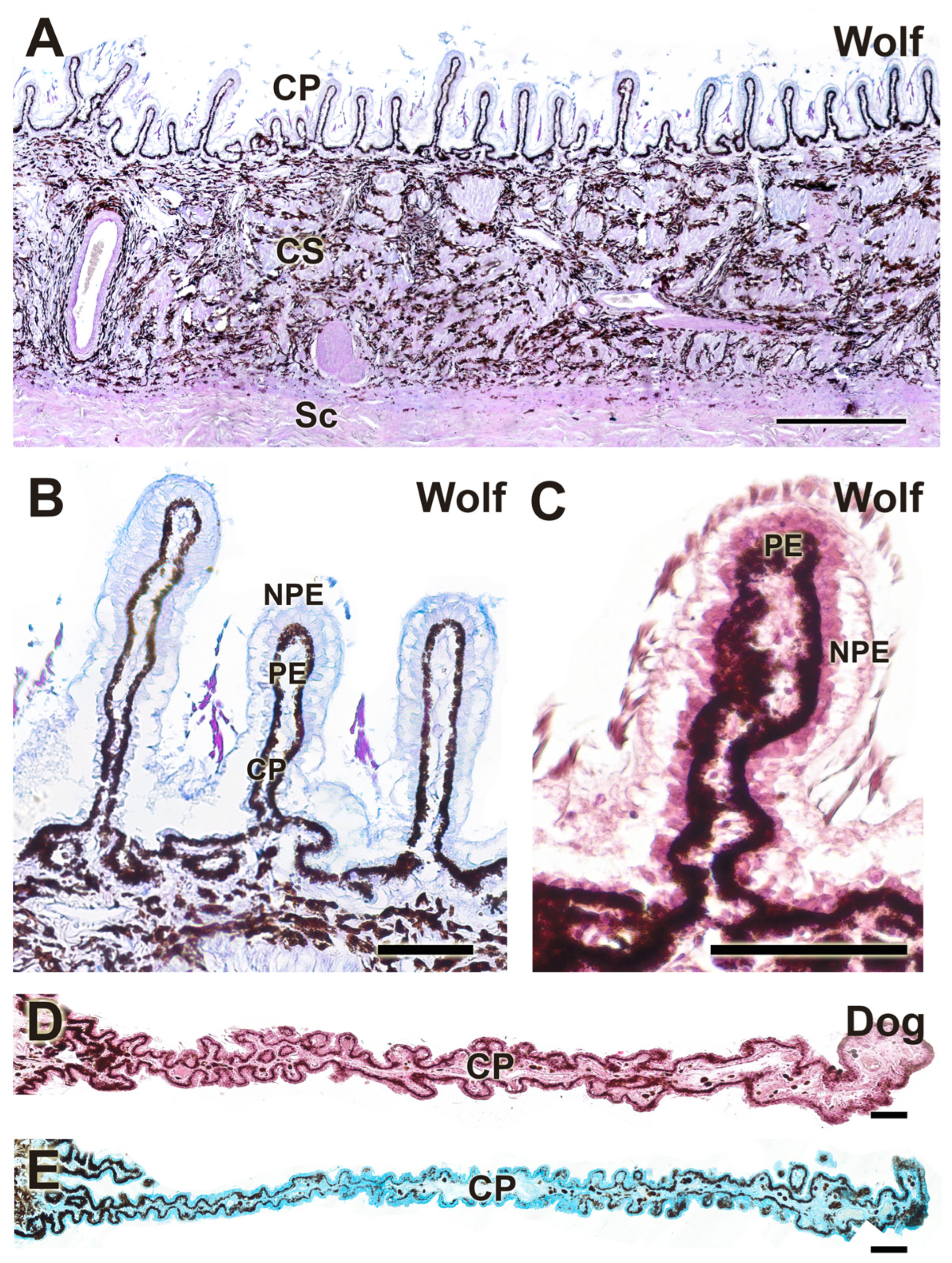
3.2.6. Cornea
3.2.7. Ciliary Body
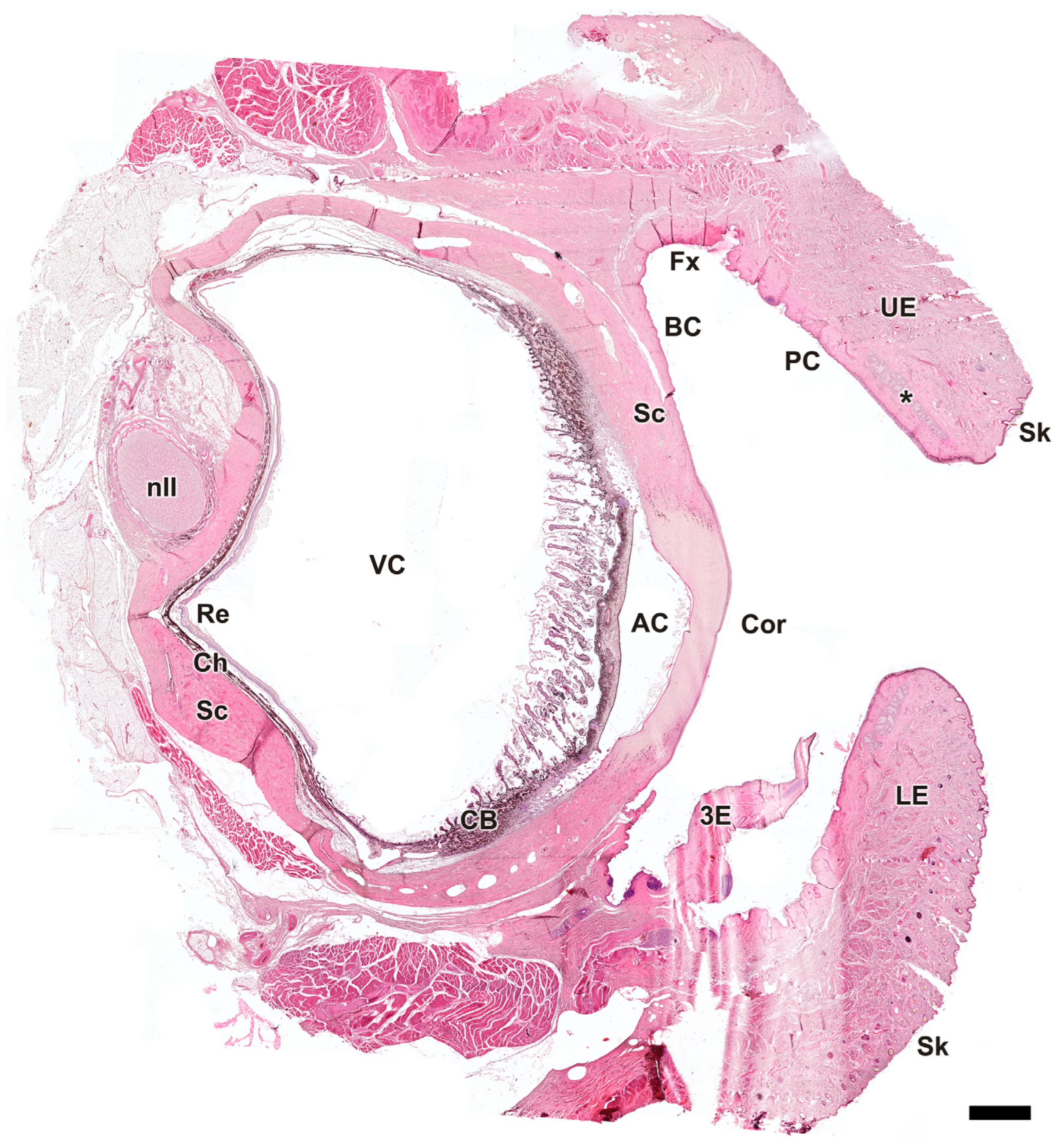
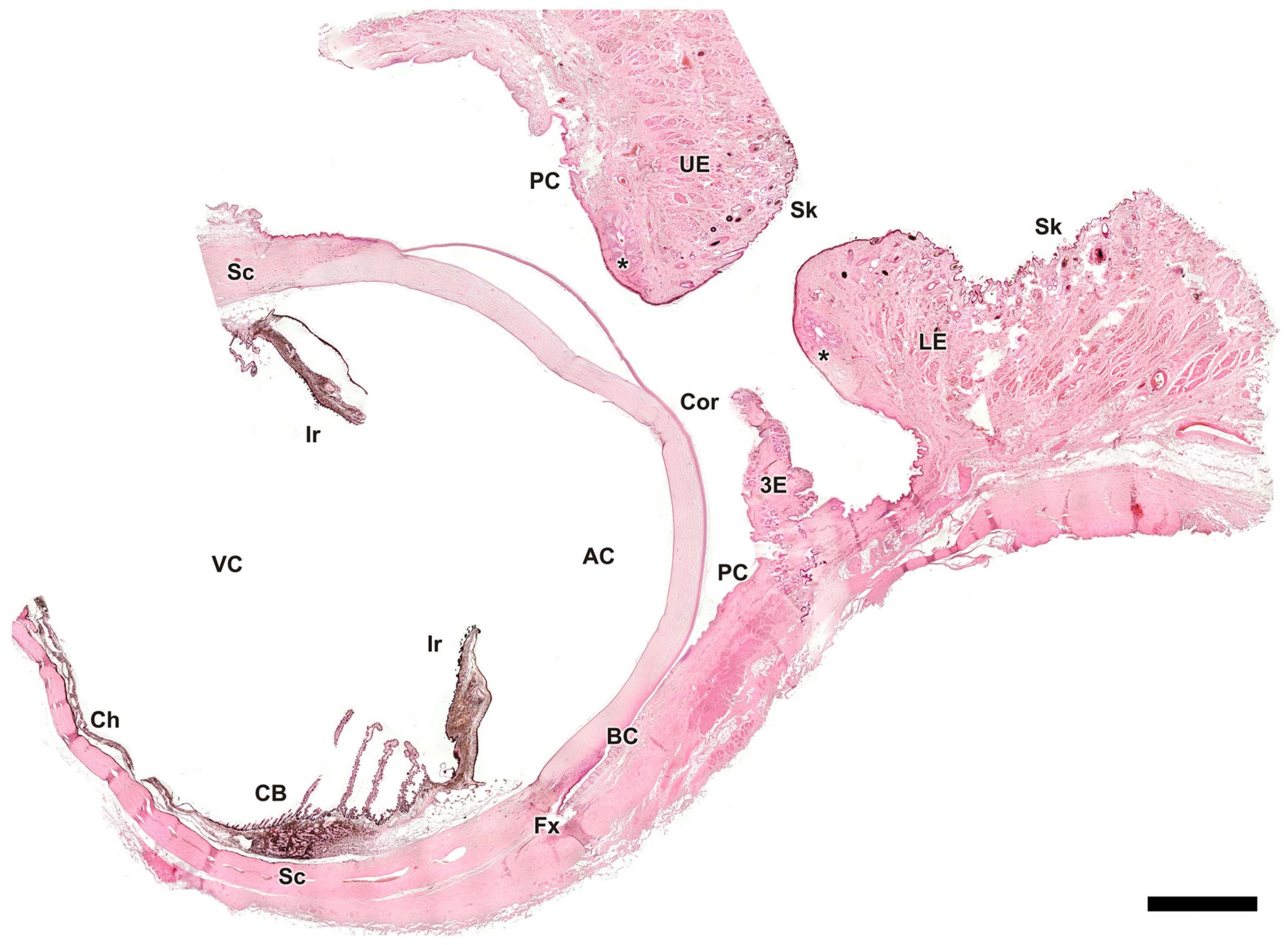
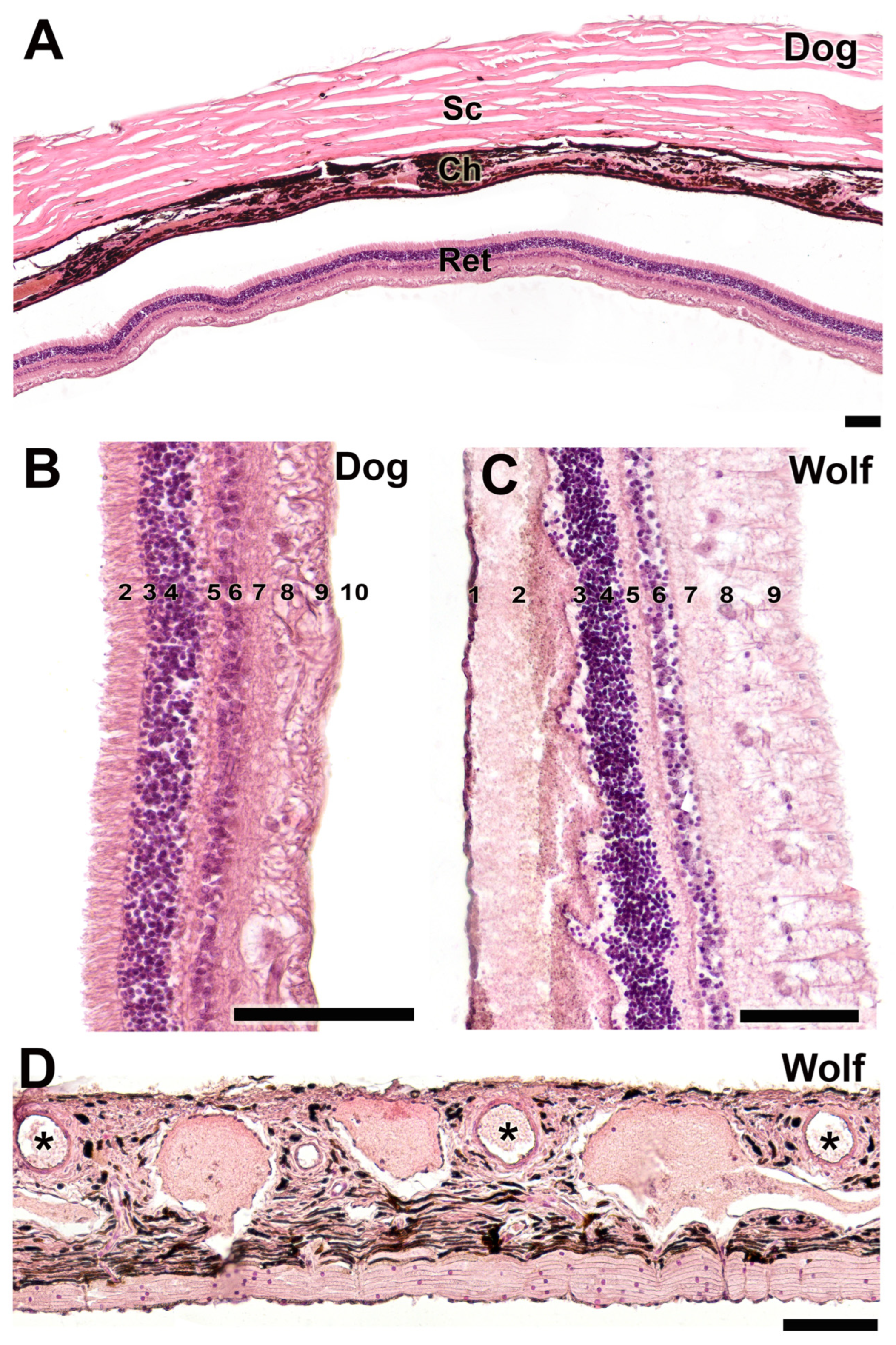
3.2.8. Tunics of the Eye
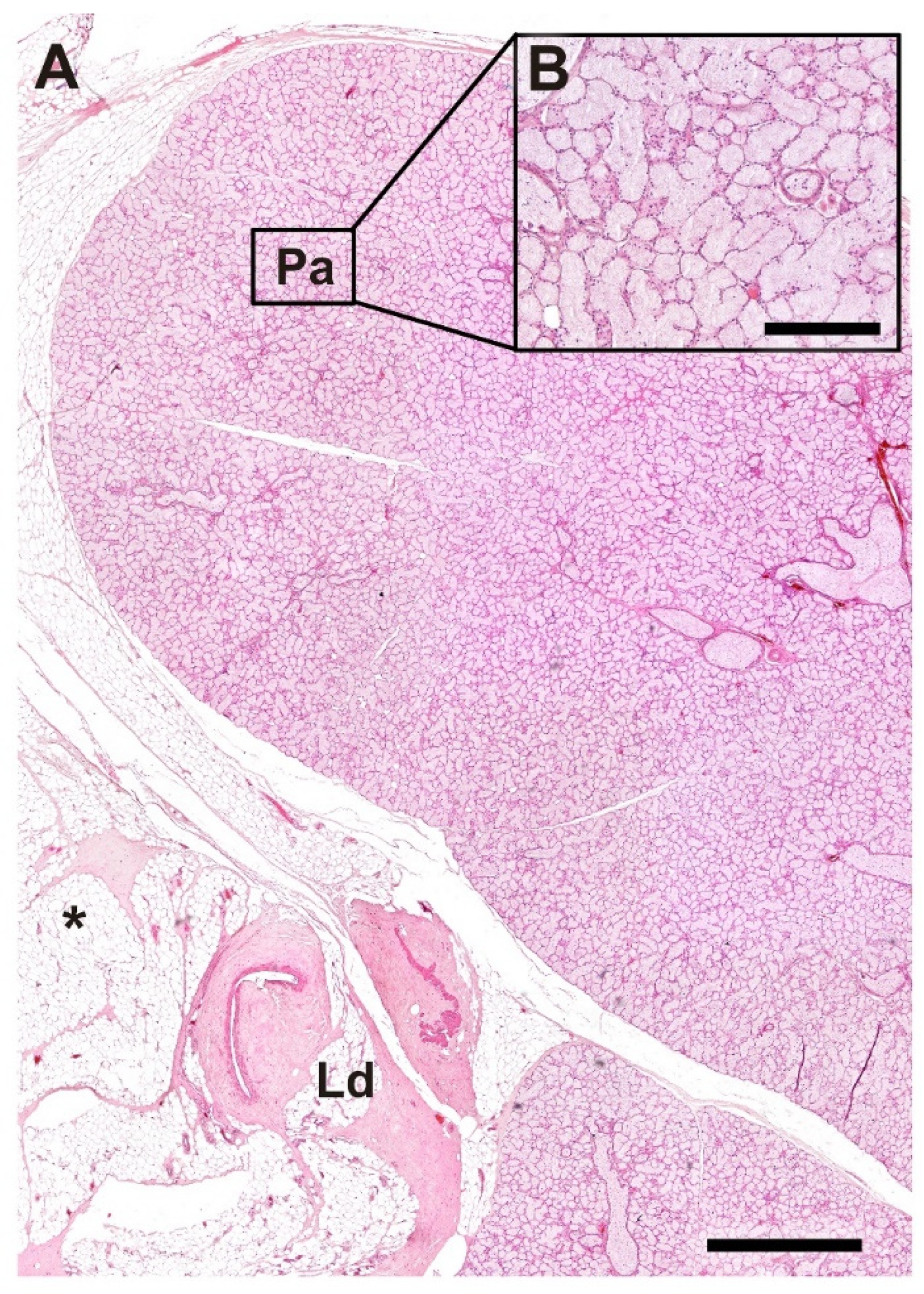
3.2.9. Lacrimal Gland
3.3. Lectin Histochemical Study
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansari, M.W.; Nadeem, A. Anatomy of the Eyelids. In Atlas of Ocular Anatomy; Ansari, M.W., Nadeem, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 53–63. ISBN 978-3-319-42781-2. [Google Scholar]
- Fine, B.S.; Yanoff, M. Ocular Histology: A Text and Atlas, 2nd ed.; Medical Dept., Harper & Row: Hagerstown, MD, USA, 1979. [Google Scholar]
- Crespo-Moral, M.; García-Posadas, L.; López-García, A.; Diebold, Y. Histological and Immunohistochemical Characterization of the Porcine Ocular Surface. PLoS ONE 2020, 15, e0227732. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I.K.; Argüeso, P. Role of Mucins in the Function of the Corneal and Conjunctival Epithelia. Int. Rev. Cytol. 2003, 231, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H. Significance of Mucin on the Ocular Surface. Cornea 2002, 21, S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Knop, E.; Knop, N. The Role of Eye-associated Lymphoid Tissue in Corneal Immune Protection. J. Anat. 2005, 206, 271–285. [Google Scholar] [CrossRef]
- García-Posadas, L.; Contreras-Ruiz, L.; Soriano-Romaní, L.; Dartt, D.A.; Diebold, Y. Conjunctival Goblet Cell Function: Effect of Contact Lens Wear and Cytokines. Eye Contact Lens Sci. Clin. Pract. 2016, 42, 83–90. [Google Scholar] [CrossRef]
- Sandøe, P.; Palmer, C.; Corr, S.; Serpell, J. History of Companion Animals and the Companion Animal Sector; John Wiley and Sons: Hoboken, NJ, USA, 2015; pp. 8–23. [Google Scholar]
- Smythe, R.H. The Eye of the Dog. In Vision in the Animal World; Palgrave Macmillan: London, UK, 1975; pp. 60–74. ISBN 978-1-349-02535-0. [Google Scholar]
- Pedraza Aguirre, G.; Beltrán Bareño, A.A. Queratoconjuntivitis Seca Y Cataratas: Algunas Afecciones Oftálmicas Comunes en Caninos. 2019. Available online: https://repository.ucc.edu.co/entities/publication/9631476c-7533-4016-9ed7-5d6c18ba6713 (accessed on 15 April 2025).
- Cabral, V.P.; Laus, J.L.; Dagli, M.L.Z.; Pereira, G.T.; Talieri, I.C.; Monteiro, E.R.; Mamede, F.V. Canine Lacrimal and Third Eyelid Superficial Glands’ Macroscopic and Morphometric Characteristics. Cienc. Rural 2005, 35, 391–397. [Google Scholar] [CrossRef]
- Lantyer-Araujo, N.L.; Silva, D.N.; Estrela-Lima, A.; Muramoto, C.; Libório, F.d.A.; da Silva, É.A.; Oriá, A.P. Anatomical, Histological and Computed Tomography Comparisons of the Eye and Adnexa of Crab-Eating Fox (Cerdocyon thous) to Domestic Dogs. PLoS ONE 2019, 14, e0224245. [Google Scholar] [CrossRef]
- Mowat, F.M.; Peichl, L. Ophthalmology of Canidae: Foxes, Wolves, and Relatives. In Wild and Exotic Animal Ophthalmology; Montiani-Ferreira, F., Moore, B.A., Ben-Shlomo, G., Eds.; Springer International Publishing: Cham, Switherland, 2022; pp. 181–214. ISBN 978-3-030-81272-0. [Google Scholar]
- Harwell, G.M.; Angell, J.A.; Merideth, R.E.; Carley, C. Chronic Superficial Keratitis in a Mexican Wolf. J. Am. Vet. Med. Assoc. 1985, 187, 1268. [Google Scholar] [CrossRef]
- Acton, A.E.; Beale, A.B.; Gilger, B.C.; Stoskopf, M.K. Sustained Release Cyclosporine Therapy for Bilateral Keratoconjunctivitis Sicca in a Red Wolf (Canis rufus). J. Zoo Wildl. Med. 2006, 37, 562–564. [Google Scholar] [CrossRef]
- Watsky, M.A.; Jablonski, M.M.; Edelhauser, H.F. Comparison of Conjunctival and Corneal Surface Areas in Rabbit and Human. Curr. Eye Res. 1988, 7, 483–486. [Google Scholar] [CrossRef]
- Loiseau, A.; Raîche-Marcoux, G.; Maranda, C.; Bertrand, N.; Boisselier, E. Animal Models in Eye Research: Focus on Corneal Pathologies. Int. J. Mol. Sci. 2023, 24, 16661. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Chen, J.; Wu, W.; Wei, W.; Yu, Y.; Feng, Y. Comparison of the Rabbit and Human Corneal Endothelial Proteomes Regarding Proliferative Capacity. Exp. Eye Res. 2021, 209, 108629. [Google Scholar] [CrossRef]
- Sebbag, L.; Mochel, J.P. An Eye on the Dog as the Scientist’s Best Friend for Translational Research in Ophthalmology: Focus on the Ocular Surface. Med. Res. Rev. 2020, 40, 2566–2604. [Google Scholar] [CrossRef]
- Schrader, S.; Mircheff, A.K.; Geerling, G. Animal Models of Dry Eye. In Developments in Ophthalmology; Geerling, G., Brewitt, H., Eds.; KARGER: Basel, Switherland, 2008; Volume 41, pp. 298–312. ISBN 978-3-8055-8376-3. [Google Scholar]
- Mäkeläinen, S.; Gòdia, M.; Hellsand, M.; Viluma, A.; Hahn, D.; Makdoumi, K.; Zeiss, C.J.; Mellersh, C.; Ricketts, S.L.; Narfström, K.; et al. An ABCA4 Loss-of-Function Mutation Causes a Canine Form of Stargardt Disease. PLoS Genet. 2019, 15, e1007873. [Google Scholar] [CrossRef]
- Dufour, V.L.; Aguirre, G.D. Canine Models of Inherited Retinal Diseases: From Neglect to Well-Recognized Translational Value. Mamm. Genome 2025, 36, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Leal, I.; Torres, M.V.; Villamayor, P.R.; Fidalgo, L.E.; López-Beceiro, A.; Sanchez-Quinteiro, P. Can Domestication Shape Canidae Brain Morphology? The Accessory Olfactory Bulb of the Red Fox as a Case in Point. Ann. Anat. 2022, 240, 151881. [Google Scholar] [CrossRef] [PubMed]
- Bera, G.; Das, R.N.; Roy, P.; Ghosh, R.; Islam, N.; Mishra, P.K.; Chatterjee, U. Utility of PAS and β-Catenin Staining in Histological Categorisation and Prediction of Prognosis of Hepatoblastomas. Pediatr. Surg. Int. 2017, 33, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.V.; Ortiz-Leal, I.; Villamayor, P.R.; Ferreiro, A.; Rois, J.L.; Sanchez-Quinteiro, P. The Vomeronasal System of the Newborn Capybara: A Morphological and Immunohistochemical Study. Sci. Rep. 2020, 10, 13304. [Google Scholar] [CrossRef]
- Sanmartín-Vázquez, E.; Ortiz-Leal, I.; Torres, M.V.; Kalak, P.; Kubiak-Nowak, D.; Dzięcioł, M.; Sanchez-Quinteiro, P. Functional Role of the Incisive Duct in Neonatal Dogs. Cells Tissues Organs 2025, 214, 167–184. [Google Scholar] [CrossRef]
- Salazar, I.; Lombardero, M.; Cifuentes, J.M.; Quinteiro, P.S.; Aleman, N. Morphogenesis and Growth of the Soft Tissue and Cartilage of the Vomeronasal Organ in Pigs. J. Anat. 2003, 202, 503–514. [Google Scholar] [CrossRef]
- Plendl, J.; Sinowatz, F. Glycobiology of the Olfactory System. Cells Tissues Organs 1998, 161, 234–253. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.V.; Basil-Rose, M.R. Lectins as Ligands for Directing Nanostructured Systems. CDD 2018, 15, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Leal, I.; Torres, M.V.; López-Callejo, L.N.; Fidalgo, L.E.; López-Beceiro, A.; Sanchez-Quinteiro, P. Comparative Neuroanatomical Study of the Main Olfactory Bulb in Domestic and Wild Canids: Dog, Wolf and Red Fox. Animals 2022, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Leal, I.; Torres, M.V.; Barreiro-Vázquez, J.; López-Beceiro, A.; Fidalgo, L.; Shin, T.; Sanchez-Quinteiro, P. The Vomeronasal System of the Wolf (Canis lupus signatus): The Singularities of a Wild Canid. J. Anat. 2024, 245, joa.14024. [Google Scholar] [CrossRef]
- Ortiz-Leal, I.; Torres, M.V.; Vargas-Barroso, V.; Fidalgo, L.E.; López-Beceiro, A.M.; Larriva-Sahd, J.A.; Sánchez-Quinteiro, P. The Olfactory Limbus of the Red Fox (Vulpes vulpes). New Insights Regarding a Noncanonical Olfactory Bulb Pathway. Front. Neuroanat. 2023, 16, 1097467. [Google Scholar] [CrossRef]
- Strom, A.R.; Cortés, D.E.; Rasmussen, C.A.; Thomasy, S.M.; McIntyre, K.; Lee, S.; Kass, P.H.; Mannis, M.J.; Murphy, C.J. In Vivo Evaluation of the Cornea and Conjunctiva of the Normal Laboratory Beagle Using Time- and Fourier-domain Optical Coherence Tomography and Ultrasound Pachymetry. Vet. Ophthalmol. 2016, 19, 50–56. [Google Scholar] [CrossRef]
- Wild and Exotic Animal Ophthalmology: Volume 2: Mammals; Montiani-Ferreira, F., Moore, B.A., Ben-Shlomo, G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-81272-0. [Google Scholar]
- IUCN Canis Lupus; Boitani, L.; Phillips, M.; Jhala, Y. The IUCN Red List of Threatened Species 2023: E.T3746A247624660 2018. Available online: https://www.iucnredlist.org/species/23062/193903628 (accessed on 15 April 2025).
- Cerrada, I.; Leiva, M.; Vilao, R.; Peña, T.; Ríos, J. Follicular Conjunctivitis in Dogs: A Retrospective Study (2007–2022). Vet. Ophthalmol. 2024, 27, 310–317. [Google Scholar] [CrossRef]
- Ollivier, F.J. Bacterial Corneal Diseases in Dogs and Cats. Clin. Tech. Small Anim. Pract. 2003, 18, 193–198. [Google Scholar] [CrossRef]
- Startup, F.G. Corneal Ulceration in the Dog. J. Small Anim. Pract. 1984, 25, 737–752. [Google Scholar] [CrossRef]
- Peña, M.T.; Leiva, M. Canine Conjunctivitis and Blepharitis. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 233–249. [Google Scholar] [CrossRef]
- El-naseery, N.; El-behery, E.; El-Ghazali, H.; El-Hady, E. The Structural Characterization of the Lacrimal Gland in the Adult Dog (Canis familiaris). Benha Vet. Med. J. 2016, 31, 106–116. [Google Scholar] [CrossRef]
- Zwingenberger, A.L.; Park, S.A.; Murphy, C.J. Computed Tomographic Imaging Characteristics of the Normal Canine Lacrimal Glands. BMC Vet. Res. 2014, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Tighe, A. Immunohistochemical Evaluation of Lymphocyte Populations in the Nictitans Glands of Normal Dogs and Dogs with Keratoconjunctivitis Sicca. Open Vet. J. 2018, 8, 47. [Google Scholar] [CrossRef]
- Araújo, R.L.S.; Corrêa, J.R.; Galera, P.D. Ultrastructural Morphology of Goblet Cells of the Conjunctiva of Dogs. Vet. Ophthalmol. 2019, 22, 891–897. [Google Scholar] [CrossRef]
- Moore, C.P.; Wilsman, N.J.; Nordheim, E.V.; Majors, L.J.; Collier, L.L. Density and Distribution of Canine Conjunctival Goblet Cells. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1925–1932. [Google Scholar]
- Umeda, Y.; Nakamura, S.; Fujiki, K.; Toshida, H.; Saito, A.; Murakami, A. Distribution of Goblet Cells and MUC5AC mRNA in the Canine Nictitating Membrane. Exp. Eye Res. 2010, 91, 721–726. [Google Scholar] [CrossRef]
- Murphy, C.; Samuelson, D.A.; Pollock, R.V.H. The Eye. In Miller’s Anatomy of the Dog; Elsevier: Amsterdam, The Netherlands, 2012; pp. 746–785. Available online: https://www.researchgate.net/publication/280090396_C_J_Murphy_DA_Samuelson_RVH_Pollock_Ch_21_The_Eye_Miller's_Anatomy_of_the_Dog_2012_746-785 (accessed on 15 April 2025).
- Paszta, W.; Klećkowska-Nawrot, J.E.; Goździewska-Harłajczuk, K. Anatomical and Morphometric Evaluation of the Orbit, Eye Tunics, Eyelids and Orbital Glands of the Captive Females of the South African Painted Dog (Lycaon pictus pictus Temminck, 1820) (Caniformia: Canidae). PLoS ONE 2021, 16, e0249368. [Google Scholar] [CrossRef]
- Hartstone-Rose, A.; Werdelin, L.; De Ruiter, D.J.; Berger, L.R.; Churchill, S.E. The Plio-Pleistocene Ancestor of Wild Dogs, Lycaon sekowei n. Sp. J. Paleontol. 2010, 84, 299–308. [Google Scholar] [CrossRef]
- Gasser, K.; Fuchs-Baumgartinger, A.; Tichy, A.; Nell, B. Investigations on the Conjunctival Goblet Cells and on the Characteristics of Glands Associated with the Eye in the Guinea Pig. Vet. Ophthalmol. 2011, 14, 26–40. [Google Scholar] [CrossRef]
- Peichl, L. Catecholaminergic Amacrine Cells in the Dog and Wolf Retina. Vis. Neurosci. 1991, 7, 575–587. [Google Scholar] [CrossRef]
- Peichl, L. Morphological Types of Ganglion Cells in the Dog and Wolf Retina. J. Comp. Neurol. 1992, 324, 590–602. [Google Scholar] [CrossRef]
- Peichl, L. Topography of Ganglion Cells in the Dog and Wolf Retina. J. Comp. Neurol. 1992, 324, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Caeiro, C.; Guo, K.; Mills, D. Dogs and Humans Respond to Emotionally Competent Stimuli by Producing Different Facial Actions. Sci. Rep. 2017, 7, 15525. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.F.; Felix, M.A.; Rocco, F.A.; Lynch, L.M.; Valdez, D. Adaptations to Sociality in the Mimetic and Auricular Musculature of the African Wild Dog (Lycaon pictus). Anat. Rec. 2024, 307, 3327–3343. [Google Scholar] [CrossRef]
- Ruiz-Rubio, S.; Ortiz-Leal, I.; Torres, M.V.; Elsayed, M.G.A.; Somoano, A.; Sanchez-Quinteiro, P. The Accessory Olfactory Bulb in Arvicola Scherman: A Neuroanatomical Study in a Subterranean Mammal. Animals 2024, 14, 3285. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.V.; Ortiz-Leal, I.; Sanchez-Quinteiro, P. Pheromone Sensing in Mammals: A Review of the Vomeronasal System. Anatomia 2023, 2, 346–413. [Google Scholar] [CrossRef]
- Salazar, I.; Sánchez Quinteiro, P. Differential Development of Binding Sites for Four Lectins in the Vomeronasal System of Juvenile Mouse: From the Sensory Transduction Site to the First Relay Stage. Brain Res. 2003, 979, 15–26. [Google Scholar] [CrossRef]
- Tuori, A.; Virtanen, I.; Uusitalo, H. Lectin Binding in the Anterior Segment of the Bovine Eye. Histochem. J. 1994, 26, 787–798. [Google Scholar] [CrossRef]
- Qaddoumi, M.; Lee, V.H.L. Lectins as Endocytic Ligands: An Assessment of Lectin Binding and Uptake to Rabbit Conjunctival Epithelial Cells. Pharm. Res. 2004, 21, 1160–1166. [Google Scholar] [CrossRef]
- Rittig, M.; Brigel, C.; Lutjen-Drecoll, E. Lectin-Binding Sites in the Anterior Segment of the Human Eye. Graefe’s Arch. Clin. Exp. Ophthalmol. 1990, 228, 528–532. [Google Scholar] [CrossRef]
- Ralph, R.A. Conjunctival Goblet Cell Density in Normal Subjects and in Dry Eye Syndromes. Investig. Ophthalmol. 1975, 14, 299–302. [Google Scholar]

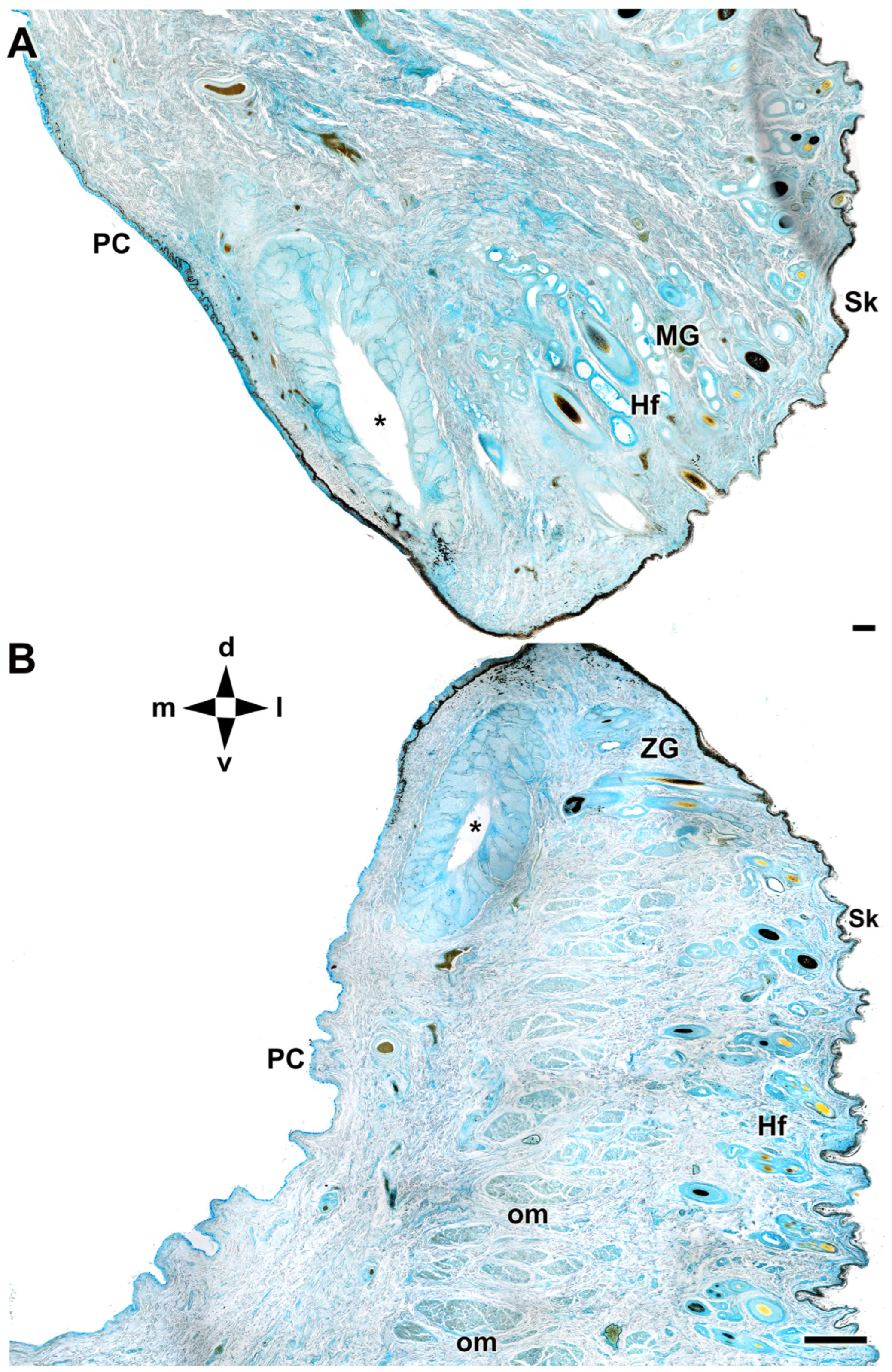

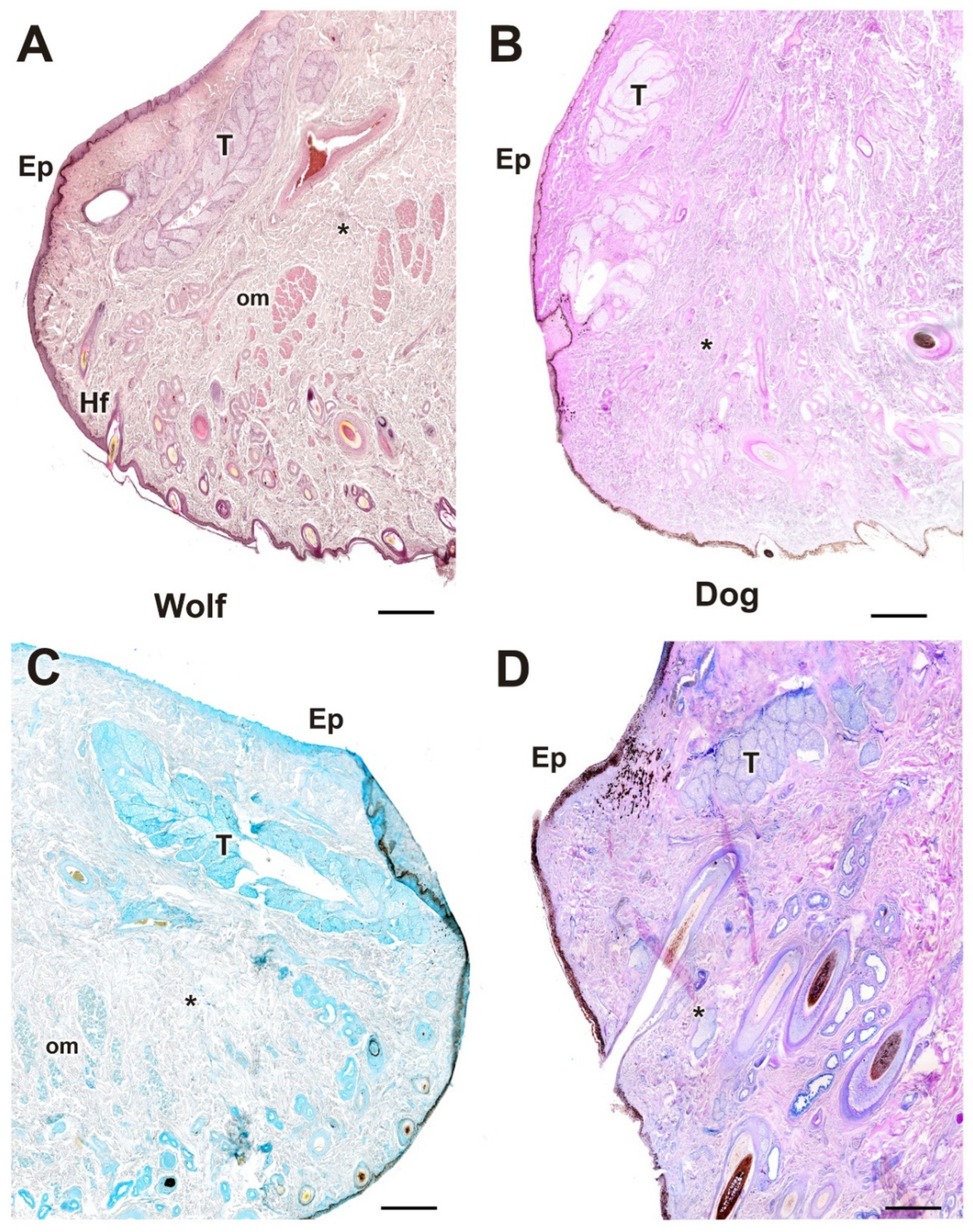
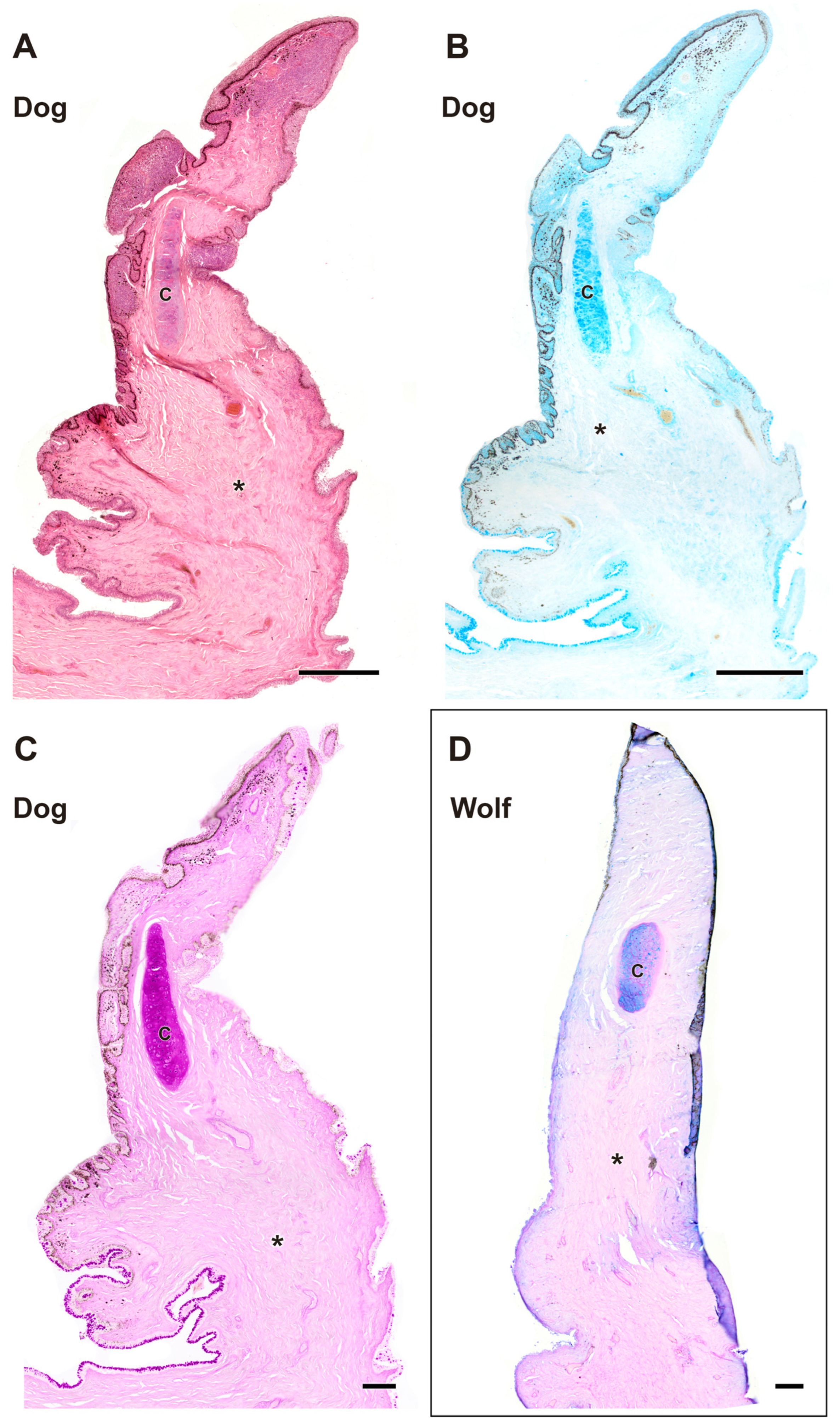
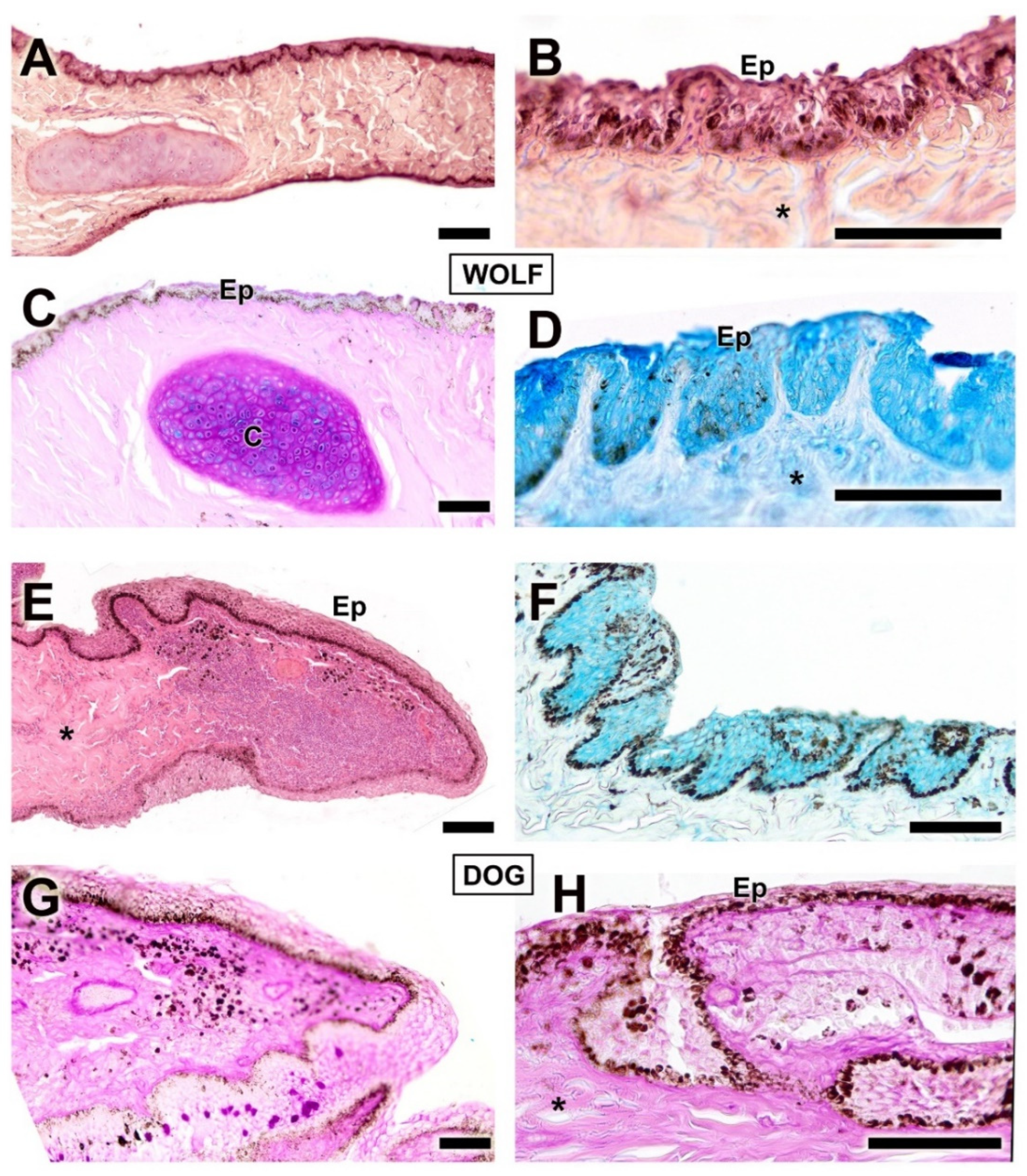


| Structure | Dog | Wolf | Appreciable Difference |
|---|---|---|---|
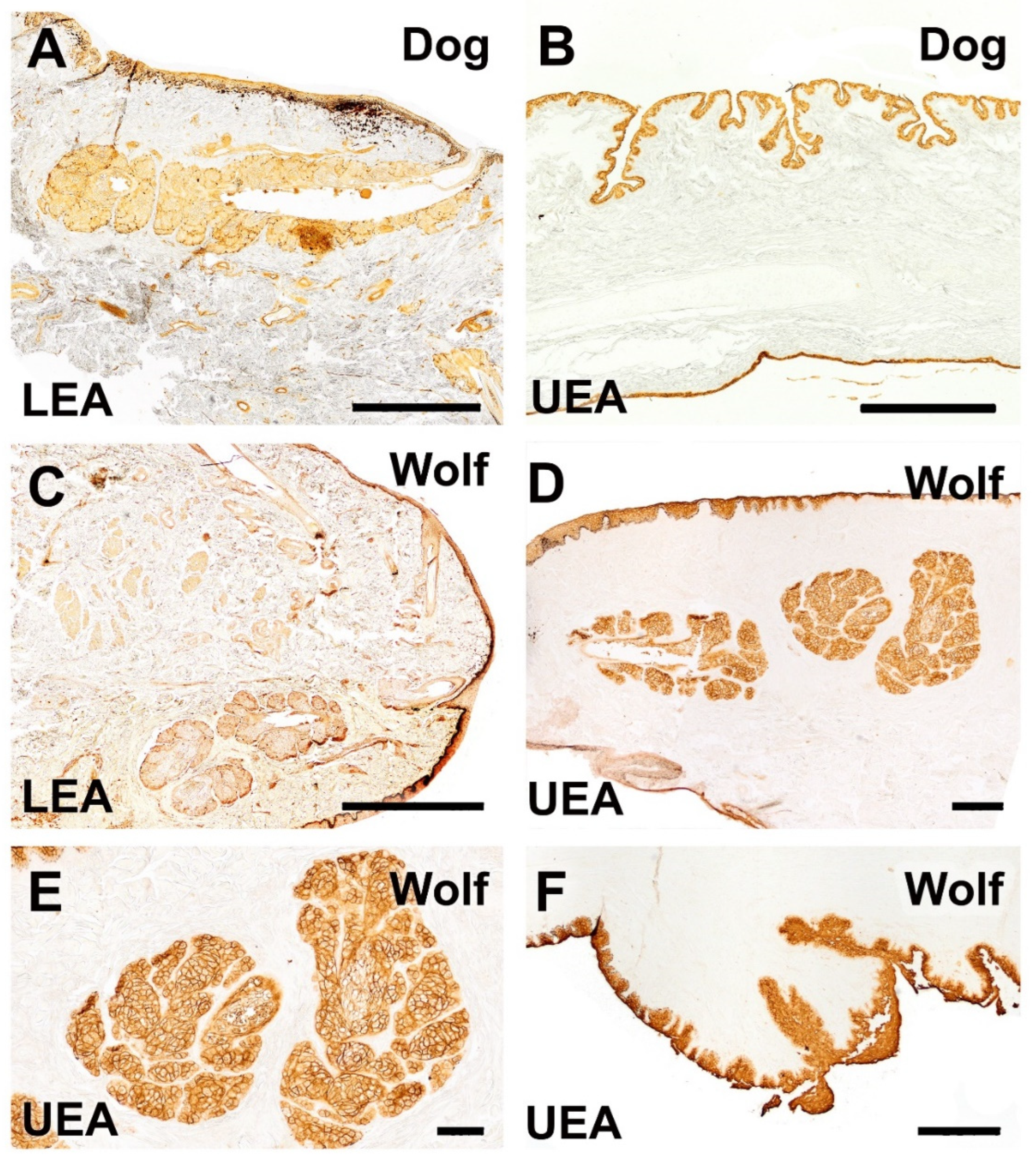
| Eyelid Skin lining | Histology: Non-keratinized stratified squamous epithelium; abundant superficial dermal glands | Histology: Same epithelial type; fewer dermal glands | Greater glandular density in the dog |
| Lectin: Strong UEA lectin labeling in skin, palpebral conjunctiva | Lectin: Similar strong UEA lectin labeling in skin, palpebral conjunctiva | No difference in UEA labeling pattern | |
| Upper and lower eyelids | Histology: Well-developed; Meibomian, Moll, and Zeis glands; dense collagen; more elastic due to richer connective tissue | Histology: Similar structure; dense collagen; less connective tissue, more fibrous | Greater elasticity and connective tissue in the dog |
| Lectin: Strong UEA lectin labeling in upper and lower eyelid mucosal epithelia | Lectin: Similar strong UEA lectin labeling in upper and lower eyelid mucosal epithelia | No difference in UEA labeling pattern | |
| Third eyelid | Histology: Thicker epithelium; stronger PAS and AB staining (more secretory activity) | Histology: Thinner epithelium; weaker PAS and AB staining | More developed epithelium and secretory activity in the dog |
| Lectin: Intense UEA lectin labeling along both lining epithelia; UEA-positive pits with tubular glands | Lectin: Similar intense UEA lectin labeling in the lining epithelia and tubular glands | No difference in UEA lectin pattern | |
| Tarsal glands | Histology: Similar parenchymal structure; larger glandular lumen | Histology: Similar parenchymal structure; more frequent glandular aggregates (up to 3 lobes) | Dog: larger lumens; Wolf: more glandular lobes |
| Lectin: Intense UEA and strong LEA lectin labeling | Lectin: Intense UEA but weak LEA lectin labeling | LEA lectin labeling is markedly weaker in the wolf | |
| Palpebral, bulbar conjunctiva, and fornix | Thick palpebral mucosa; deep tubular invaginations; strong PAS and AB staining; well-developed bulbar epithelium with negative PAS, mild AB staining | Strong AB in fornix and palpebral mucosa; thin bulbar epithelium with slight PAS staining and strong AB. | Dog: thick epithelium, more neutral mucins; Wolf: thin epithelium, more acidic mucins |
| Cornea | Typical layered structure; stroma stains strongly with AB | Same layered structure; stroma stains strongly with PAS | No differences |
| Ciliary body | Similar morphology; pigmented and non-pigmented epithelia; low PAS/AB reactivity | Similar morphology; pigmented and non-pigmented epithelia; low PAS/AB reactivity | No morphological differences |
| Ocular tunics and retina | Typical three tunics; detailed retinal layering observed | Greater density of ganglionar cells in the retina. Same tunic organization | Greater density of ganglionar cells in the retina of the wolf |
| Lacrimal gland | Morphology not detailed in the current data | Well-organized glandular parenchyma; lacrimal duct surrounded by adipose tissue | Detailed description available only for the wolf |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diz López, A.; Torres, M.V.; Martínez Gómez, F.; Fraga Abelleira, S.A.; López-Beceiro, A.; Fidalgo, L.; Sanchez-Quinteiro, P.; Ortiz-Leal, I. Histological Characterization of Ocular and Adnexal Tissues in Dogs (Canis familiaris) and Wolves (Canis lupus). Anatomia 2025, 4, 10. https://doi.org/10.3390/anatomia4030010
Diz López A, Torres MV, Martínez Gómez F, Fraga Abelleira SA, López-Beceiro A, Fidalgo L, Sanchez-Quinteiro P, Ortiz-Leal I. Histological Characterization of Ocular and Adnexal Tissues in Dogs (Canis familiaris) and Wolves (Canis lupus). Anatomia. 2025; 4(3):10. https://doi.org/10.3390/anatomia4030010
Chicago/Turabian StyleDiz López, Abel, Mateo V. Torres, Fabio Martínez Gómez, Silvia Alejandra Fraga Abelleira, Ana López-Beceiro, Luis Fidalgo, Pablo Sanchez-Quinteiro, and Irene Ortiz-Leal. 2025. "Histological Characterization of Ocular and Adnexal Tissues in Dogs (Canis familiaris) and Wolves (Canis lupus)" Anatomia 4, no. 3: 10. https://doi.org/10.3390/anatomia4030010
APA StyleDiz López, A., Torres, M. V., Martínez Gómez, F., Fraga Abelleira, S. A., López-Beceiro, A., Fidalgo, L., Sanchez-Quinteiro, P., & Ortiz-Leal, I. (2025). Histological Characterization of Ocular and Adnexal Tissues in Dogs (Canis familiaris) and Wolves (Canis lupus). Anatomia, 4(3), 10. https://doi.org/10.3390/anatomia4030010









