Anatomy of Cerebral Arteries with Clinical Aspects in Patients with Ischemic Stroke
Abstract
1. Introduction
2. Description of Vascular Anatomy
2.1. Extracranial Circulation
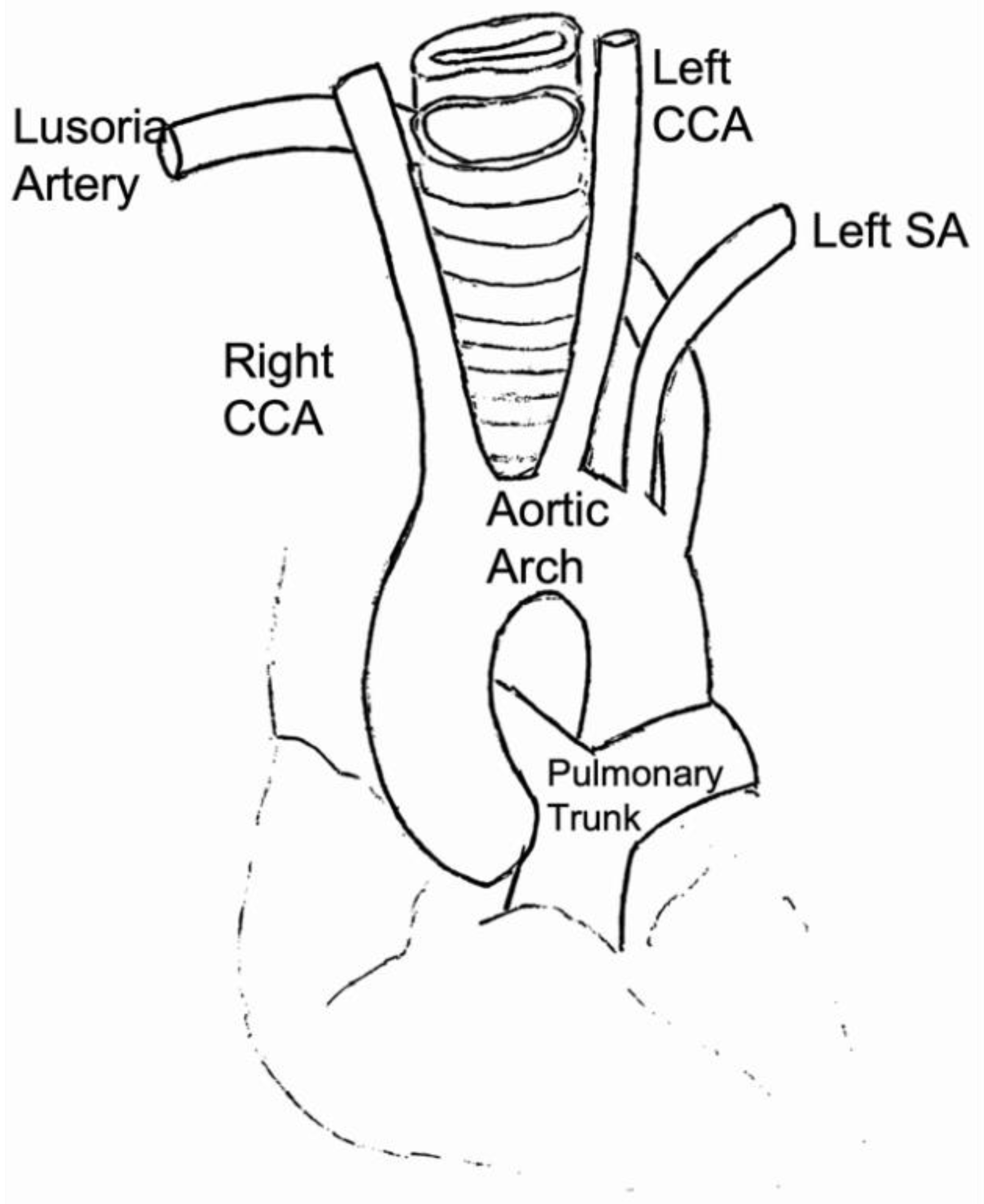
2.1.1. Arch of the Aorta
2.1.2. Supra-Aortic Vessels (SAT)
2.1.3. Common Carotid Artery (CCA)
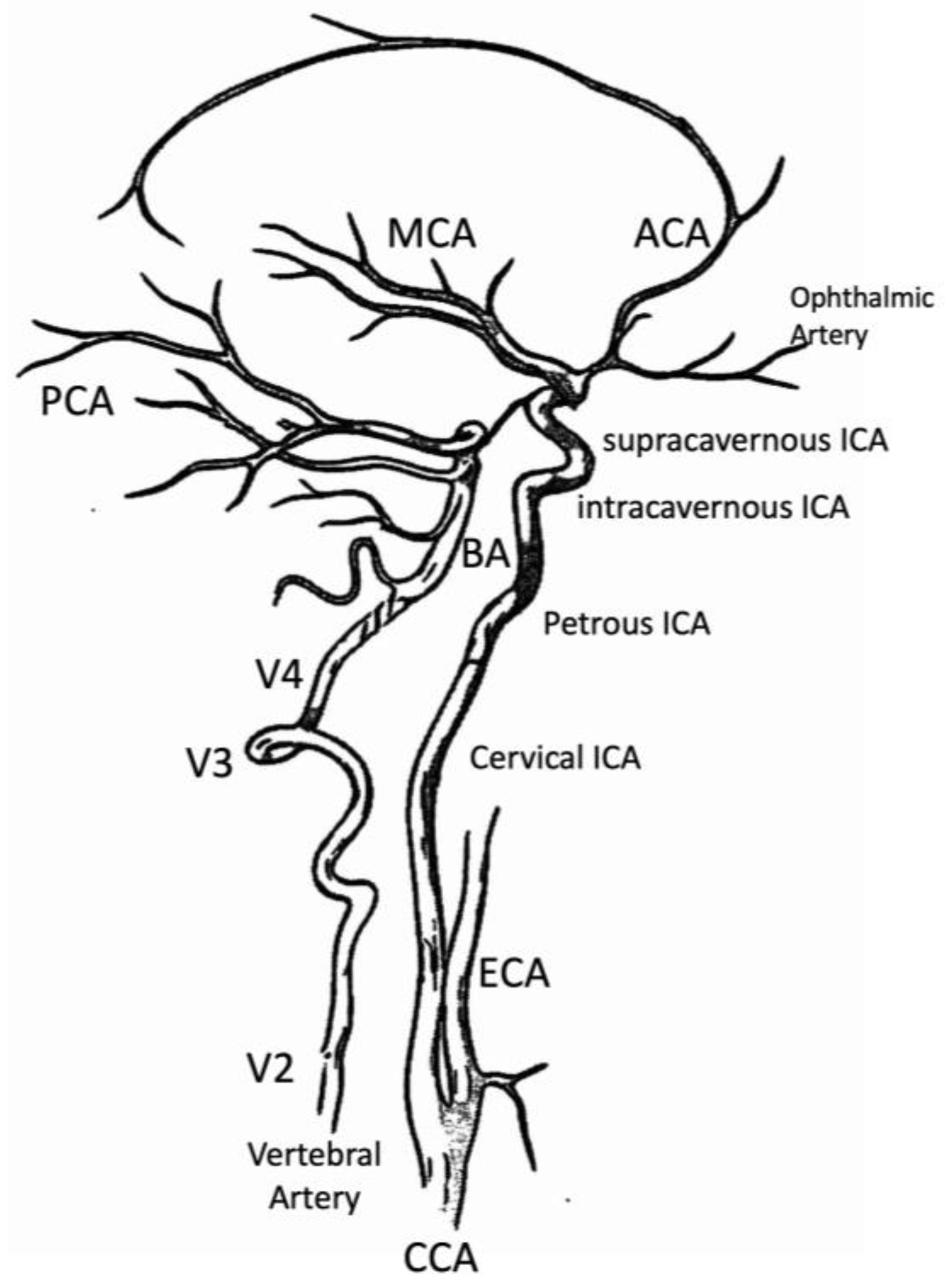
2.1.4. Internal Carotid Artery (ICA)—Extracranial Tract
2.1.5. Vertebral Artery (VA)
- V1 segment, the segment of the vertebral artery before its entry into the transverse canal (C6);
- V2 segment, part of the vertebral artery that runs in the transverse canal of C6 up to C2;
- V3 segment, which bypasses the lateral masses of C1 before redirecting medially to penetrate the atlanto-occipital membrane and through the occipital hole, makes its entry into the cranial cavity;
- V4 segment of intracranial localization, just before the meeting of the two vertebral arteries. It is noted that this V4 segment is of subarachnoid collocation.
2.2. Intracranial Circulation
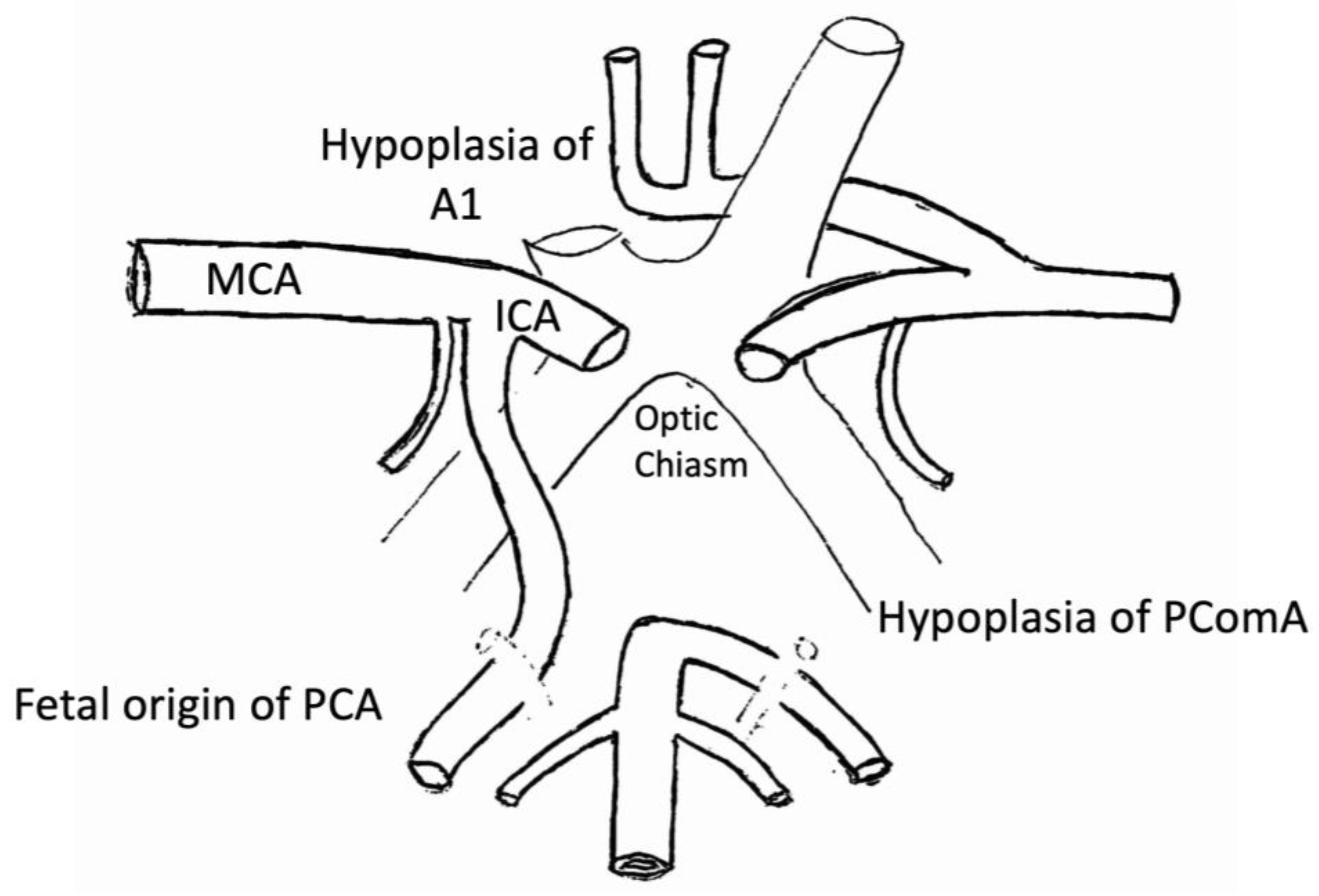
2.2.1. Willis Polygon
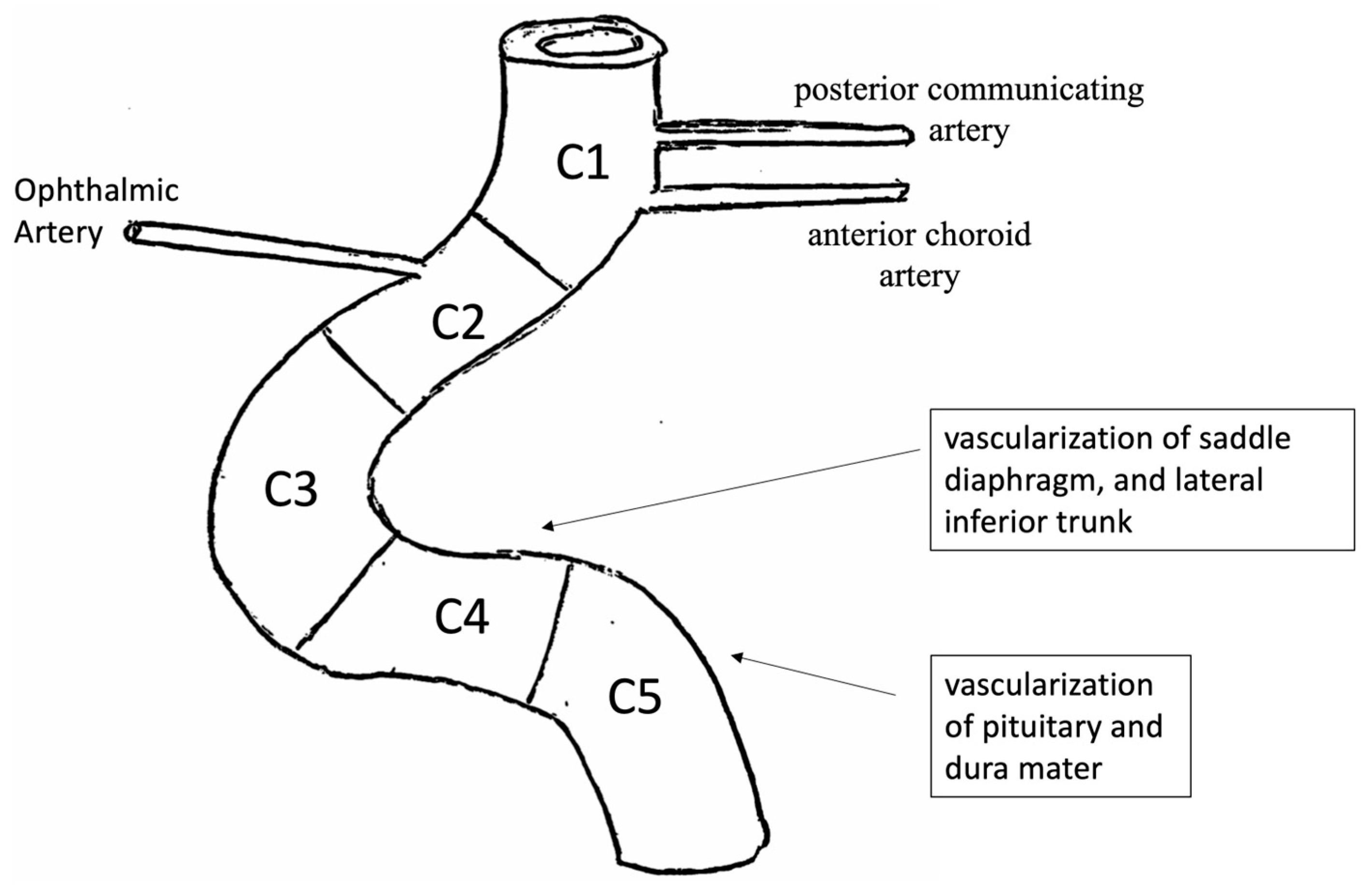
2.2.2. Internal Carotid Artery (ICA)—Intracranial Tract
- C5 segment gives rise to the meningo-pituitary trunk, from which emerges above all some branches that participate in the vascularization of the pituitary and adjacent dura mater;
- C4 segment has a landscape orientation and is directed forward. Its main collaterals are the capsular arteries, which vascularize the saddle diaphragm and the lateral inferior trunk. The lateral inferior trunk is divided into three branches: upper, anterior, and posterior. They participate, respectively, in the vascularization of the roof of the cavernous sinus and oculomotor nerves;
- C3 segment is curved and has a front convexity;
- C2 segment, above cavernous and infra-clinoid, is short and has a posterior, horizontal, or slightly ascending orientation. It gives rise to the superior pituitary arteries, which vascularize the anterior loggia of the pituitary gland. From the anterior part of the upper portion of the C2 segment originates the ophthalmic artery. The ophthalmic artery is of subarachnoid localization, more rarely intradural. It has an anterior orientation to reach the optic canal, where it runs against the lateral inferior face of the optic nerve. From this segment, the intradural arteries do not possess an elastic lamina and are represented by a small tunica adventitia and poor elastic fibers in the tunica media;
- Finally, segment C1 is above cavernous and supraclinoid.
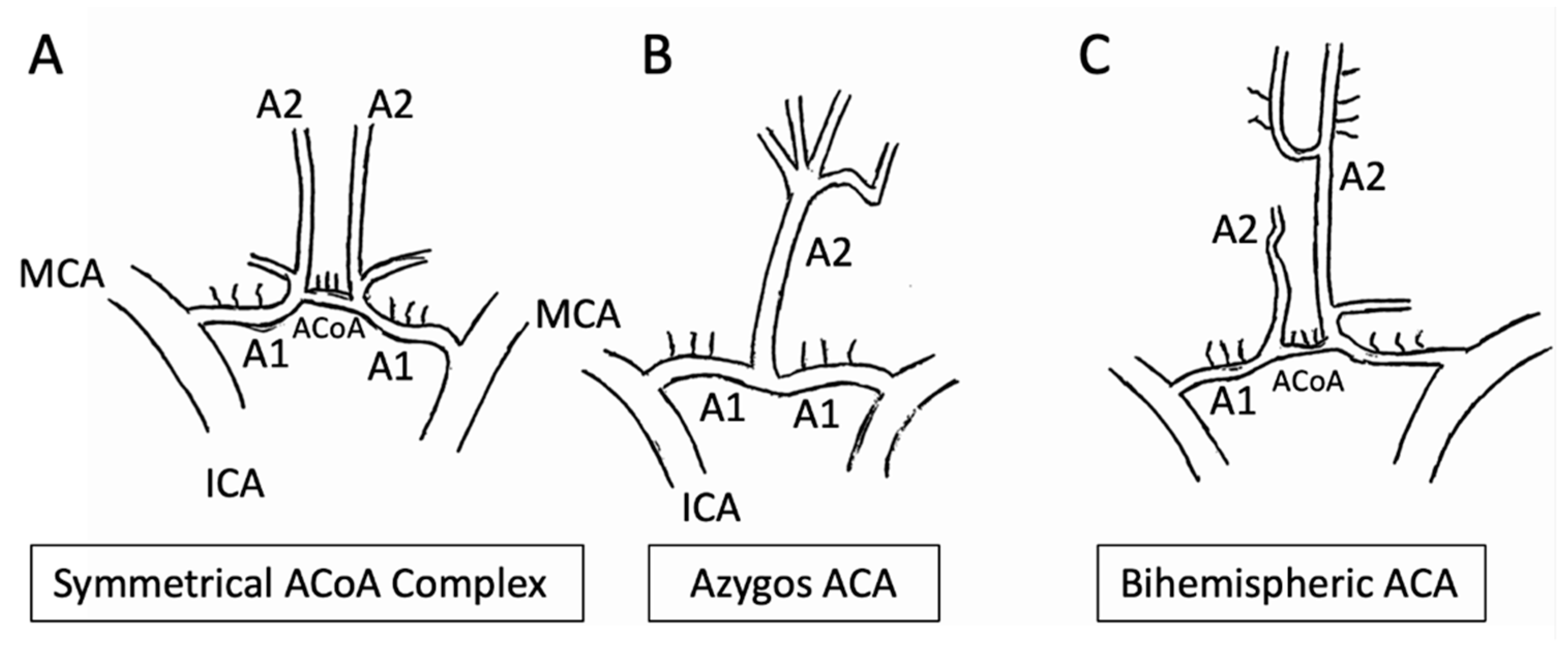
2.2.3. Anterior Cerebral Artery (ACA)
- The A1 segment, known as ‘pre-communicating’;
- The A2 segment, which has an ascending course up to the corpus callosum;
- The A3 segment, which surrounds the knee of the corpus callosum;
- A4 and A5, which continue their course around the corpus callosum.
2.2.4. Middle Cerebral Artery (MCA)
- The frontal orbital artery, which vascularizes the lower face of the frontal lobe;
- The anterior frontal artery, which vascularizes the external faces of the F2 and F3 convolutions and the frontal operculum;
- The ascending frontal artery, which runs in the central and post-central grooves. It ensures the vascularization of the ascending frontal and parietal convolutions;
- The anterior parietal artery;
- The posterior parietal artery;
- The artery of the curved fold or angular artery, which prolongs the axis of the Silvian artery. It ensures the vascularization of the posterior frontal region.
- The anterior temporal artery, which vascularizes the temporal pole;
- The middle temporal artery, which has an oblique course at the bottom and backward in the middle part of the temporal lobe. It ensures the vascularization of the middle and posterior parts of T1eT2;
- The posterior temporal artery or temporo-occipital, which has a course roughly parallel to the previous one. It vascularizes the outer face of the occipital lobe and the back of the outer face of the temporal lobe.
2.2.5. Anterior Choroid Artery
2.2.6. Anterior Communicating Artery (ACoA)
2.2.7. Posterior Communicating Artery (PCoA)
2.2.8. Intracranial Vertebral Artery (VA)
2.2.9. Posteroinferior Cerebellar Artery (PICA)
2.2.10. Basilar Artery
2.2.11. Posterior Cerebral Artery (PCA)
- The P1 or precommunicating segment;
- The P2 segment, which bypasses the cerebral peduncle through III c.n;
- The P3 segment, which runs along the underside of the temporal lobe;
- The P4 segment that arrive at the calcarine fissure.
- The inferior dorsomedial arteries, which vascularize the lower portion of the thalamus and originate from the posterior communicating artery;
- The posteromedian choroid artery, which originates from the P2 segment of the ACP. It runs along the upper margin of the posterior communicating artery, then on the upper edge of the thalamus, emitting branches that vascularize the anterior nuclei of the thalamus. Finally, it penetrates the choroid canvas of the third ventricle, which it sprays;
- The posterolateral choroid artery. Ensures the vascularization of the choroid plexuses. It crosses the inner face of the pulvinar and penetrates the choroid fissure of the lateral ventricle;
- The posterior perichallose artery, which runs on the posterior face of the splenium of the corpus callosum.
- P1 syndrome: midbrain, subthalamic, and thalamic signs, which are due to disease of the proximal P1 segment of the PCA or its penetrating branches (thalamogeniculate, Percheron, and posterior choroidal arteries);
- P2 syndrome: cortical temporal and occipital lobe signs due to occlusion of the segment distal to the junction of the PCA with the posterior communicating artery.
2.2.12. Anteroinferior Cerebellar Artery (or Middle Cerebellar Artery, AICA)
2.2.13. Anterosuperior Cerebellar Artery (or Superior Cerebellar Artery, SCA)
3. Discussion
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.A.; Sterzi, R.; Toso, V.; Consoli, D.; Guidetti, D.; Provinciali, L.; Leone, M.; Beghi, E. The neurologist in the emergency department. An Italian nationwide epidemiological survey. Neurol. Sci. 2008, 29, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sañudo, J.; Vázquez, R.; Puerta, J. Meaning and clinical interest of the anatomical variations in the 21st century. Eur. J. Anat. 2003, 7, 1–3. [Google Scholar]
- Harrigan, M.R.; Deveikis, J.P. Essential Neurovascular Anatomy. In Handbook of Cerebrovascular Disease and Neurointerventional Technique Series: Contemporary Medical Imaging; Harrigan, M.R., Deveikis, J.P., Eds.; Humana Press: New York, NY, USA, 2009; pp. 1–87. [Google Scholar]
- Anastasi, G.; Gaudio, E.; Tacchetti, C. Anatomia Umana—Atlante; Edi.Ermes: Milano, Italy, 2013; Volume 1, ISBN 9788870513486. [Google Scholar]
- Hauser, S. Harrison’s Neurology in Clinical Medicine; Mcgraw-Hill Education: New York, NY, USA, 2016. [Google Scholar]
- Caplan, L.R. Diagnosis and the Clinical Encounter. In Caplan’s Stroke: A Clinical Approach, 4th ed.; Saunders: Philadelphia, PA, USA, 2009; p. 64. [Google Scholar]
- Perry, J.M.; McCabe, K.K. Recognition and initial management of acute ischemic stroke. Emerg. Med. Clin. N. Am. 2012, 30, 637–657. [Google Scholar] [CrossRef]
- Caplan, L.R. Posterior Circulation Disease: Clinical Findings, Diagnosis, and Management; Blackwell Science: Boston, MA, USA, 1996. [Google Scholar]
- Savitz, S.I.; Caplan, L.R. Vertebrobasilar disease. N. Engl. J. Med. 2005, 352, 2618. [Google Scholar] [CrossRef]
- Popieluszko, P.; Henry, B.M.; Sanna, B.; Hsieh, W.C.; Saganiak, K.; Pękala, P.A.; Walocha, J.A.; Tomaszewski, K.A. A systematic review and meta-analysis of variations in branching patterns of the adult aortic arch. J. Vasc. Surg. 2018, 68, 298–306.e10. [Google Scholar] [CrossRef]
- Dimmick, S.J.; Faulder, K.C. Normal variants of the cerebral circulation at multidetector CT angiography. Radiographics 2009, 29, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Okahara, M.; Kiyosue, H.; Mori, H.; Tanoue, S.; Sainou, M.; Nagatomi, H. Anatomic variations of the cerebral arteries and their embryology: A pictorial review. Eur. Radiol. 2002, 12, 2548–2561. [Google Scholar] [CrossRef] [PubMed]
- Caldemeyer, K.S.; Carrico, J.B.; Mathews, V.P. The radiology and embryology of anomalous arteries of the head and neck. Am. J. Roentgenol. 1998, 170, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Valvano, A.; Bosso, G.; Apuzzi, V.; Mercurio, V.; Di Simone, V.; Panicara, V.; De Luca, M.; Tomas, C.; Cammarota, F.; Cittadini, A.; et al. Long-term follow-up in high-risk hypertensive patients with carotid dolicoarteriopathies. Int. Angiol. 2020, 39, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Petty, G.W.; Brown, R.D., Jr.; Whisnant, J.P.; Sicks, J.D.; O’Fallon, W.M.; Wiebers, D.O. Ischemic stroke subtypes: A population-based study of incidence and risk factors. Stroke 1999, 30, 2513. [Google Scholar] [CrossRef]
- Ekici, F.; Tekbas, G.; Onder, H.; Gumus, H.; Cetincakmak, M.G.; Palanci, Y.; Bakir, S.; Bilici, A. Course anomalies of extracranial internal carotid artery and their relationship with pharyngeal wall: An evaluation with multislice CT. Surg. Radiol. Anat. 2012, 34, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Shaban, A.; Albright, K.C.; Boehme, A.K.; Martin-Schild, S. Circle of Willis variants: Fetal PCA. Stroke Res. Treat. 2013, 2013, 105937. [Google Scholar] [CrossRef]
- Hakim, A.; Gralla, J.; Rozeik, C.; Mordasini, P.; Leidolt, L.; Piechowiak, E.; Ozdoba, C.; El-Koussy, M. Anomalies and Normal Variants of the Cerebral Arterial Supply: A Comprehensive Pictorial Review with a Proposed Workflow for Classification and Significance. J. Neuroimaging 2018, 28, 14–35. [Google Scholar] [CrossRef]
- Horowitz, M.; Bansal, S.; Dastur, K. Aortic Arch Origin of the Left External Carotid Artery and Type II Proatlantal Fetal Anastomosis. Am. J. Neuroradiol. Mar. 2003, 24, 323–325. [Google Scholar]
- Menshawi, K.; Mohr, J.P.; Gutierrez, J. A functional perspective on the embryology and anatomy of the cerebral blood supply. J. Stroke 2015, 17, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Thierfelder, K.M.; Baumann, A.B.; Sommer, W.H. Vertebral artery hypoplasia: Frequency and effect on cerebellar blood flow characteristics. Stroke 2014, 45, 1363–1368. [Google Scholar] [CrossRef]
- Saade, C.; Bourne, R.; Wilkinson, M.; Brennan, P.C. MDCT angiography of the major congenital anomalies of the extracranial arteries: Pictorial review. J. Med. Imaging Radiat. Oncol. 2013, 57, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kovač, J.D.; Stanković, A.; Stanković, D.; Kovač, B.; Šaranović, D. Intracranial arterial variations: A comprehensive evaluation using CT angiography. Med. Sci. Monit. 2014, 20, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Compter, A.; Labeyrie, M.A.; Uyttenboogaart, M.; Metso, T.M.; Majersik, J.J.; Goeggel-Simonetti, B.; Engelter, S.T.; Pezzini, A.; Bijlenga, P.; et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015, 14, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Hudák, I.; Lenzsér, G.; Lunenkova, V.; Dóczi, T. Cerebral arterial fenestrations: A common phenomenon in unexplained subarachnoid haemorrhage. Acta Neurochir. 2013, 155, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hoksbergen, A.W.J.; Fulesdi, B.; Legemate, D.A.; Csiba, L. Collateral Configuration of the Circle of Willis. Transcranial Color-Coded Duplex Ultra- sonography and Comparison with Postmortem Anatomy. Stroke 2000, 31, 1346–1351. [Google Scholar] [CrossRef]
- Iqbal, S. A comprehensive study of the anatomical variations of the circle of willis in adult human brains. J. Clin. Diagn. Res. 2013, 7, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, R.; Ciszek, B. Internal Carotid Artery Classification Systems. Pol. J. Aviat. Med. Bioeng. Psychol. 2018, 24, 27–35. [Google Scholar] [CrossRef]
- Chandra, A.; Li, W.A.; Stone, C.R.; Geng, X.; Ding, Y. The cerebral circulation and cerebrovascular disease I: Anatomy. Brain Circ. 2017, 3, 45–56. [Google Scholar] [PubMed]
- Shapiro, M. Anterior Cerebral Artery. Available online: http://neuroangio.org/anatomy-and-variants/anterior-cerebral-artery/ (accessed on 17 February 2017).
- Chuang, Y.M.; Liu, C.Y.; Pan, P.J.; Lin, C.-P. Anterior cerebral artery A1 segment hypoplasia may contribute to A1 hypoplasia syndrome. Eur. Neurol. 2007, 57, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Uchino, A.; Nomiyama, K.; Takase, Y.; Kudo, S. Anterior cerebral artery variations detected by MR angiography. Neuroradiology 2006, 48, 647–652. [Google Scholar] [CrossRef]
- Komiyama, M.; Nakajima, H.; Nishikawa, M.; Yasui, T. Middle cerebral artery variations: Duplicated and accessory arteries. Am. J. Neuroradiol. 1998, 19, 45–49. [Google Scholar]
- Takahashi, S.; Hoshino, F.; Uemura, K.; Takahashi, A.; Sakamoto, K. Accessory middle cerebral artery: Is it a variant form of the recurrent artery of Heubner? Am. J. Neuroradiol. 1989, 10, 563–568. [Google Scholar]
- Shapiro, M. Anterior Choroidal Artery. Available online: http://neuroangio.org/anatomy-and-variants/anterior-choroidal-artery/ (accessed on 2 February 2017).
- De Gast, A.N.; van Rooij, W.J.; Sluzewski, M. Fenestrations of the Anterior Communicating Artery: Incidence on 3D Angiography and Relationship to Aneurysms. Am. J. Neuroradiol. 2008, 29, 296–298. [Google Scholar] [CrossRef]
- Barbato, F.; Allocca, R.; Serra, C.; Bosso, G.; Numis, F.G. Spontaneous eye movements in myxedematous coma. Intern. Emerg. Med. 2022, 17, 2063–2064. [Google Scholar] [CrossRef] [PubMed]
- Percheron, G. Arteries of the human thalamus. Rev. Neurol. 1976, 132, 297–307. [Google Scholar] [PubMed]
- Amarenco, P.; Hauw, J.J. Anatomy of the cerebellar arteries. Rev. Neurol. 1989, 145, 267. [Google Scholar] [PubMed]
- Coupland, A.P.; Thapar, A.; Qureshi, M.I.; Jenkins, H.; Davies, A.H. The definition of stroke. J. R. Soc. Med. 2017, 110, 9–12. [Google Scholar] [CrossRef]
- Kluytmans, M.; van der Grond, J.; van Everdingen, K.J.; Klijn, C.J.M.; Kappelle, L.J.; Viergever, M.A. Cerebral hemodynamics in relation to patterns of collateral flow. Stroke 1999, 30, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbato, F.; Allocca, R.; Bosso, G.; Numis, F.G. Anatomy of Cerebral Arteries with Clinical Aspects in Patients with Ischemic Stroke. Anatomia 2022, 1, 152-169. https://doi.org/10.3390/anatomia1020016
Barbato F, Allocca R, Bosso G, Numis FG. Anatomy of Cerebral Arteries with Clinical Aspects in Patients with Ischemic Stroke. Anatomia. 2022; 1(2):152-169. https://doi.org/10.3390/anatomia1020016
Chicago/Turabian StyleBarbato, Francesco, Roberto Allocca, Giorgio Bosso, and Fabio Giuliano Numis. 2022. "Anatomy of Cerebral Arteries with Clinical Aspects in Patients with Ischemic Stroke" Anatomia 1, no. 2: 152-169. https://doi.org/10.3390/anatomia1020016
APA StyleBarbato, F., Allocca, R., Bosso, G., & Numis, F. G. (2022). Anatomy of Cerebral Arteries with Clinical Aspects in Patients with Ischemic Stroke. Anatomia, 1(2), 152-169. https://doi.org/10.3390/anatomia1020016






