Green Synthesis and Characterization of Iron Oxide Nanoparticles Using Egeria densa Plant Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
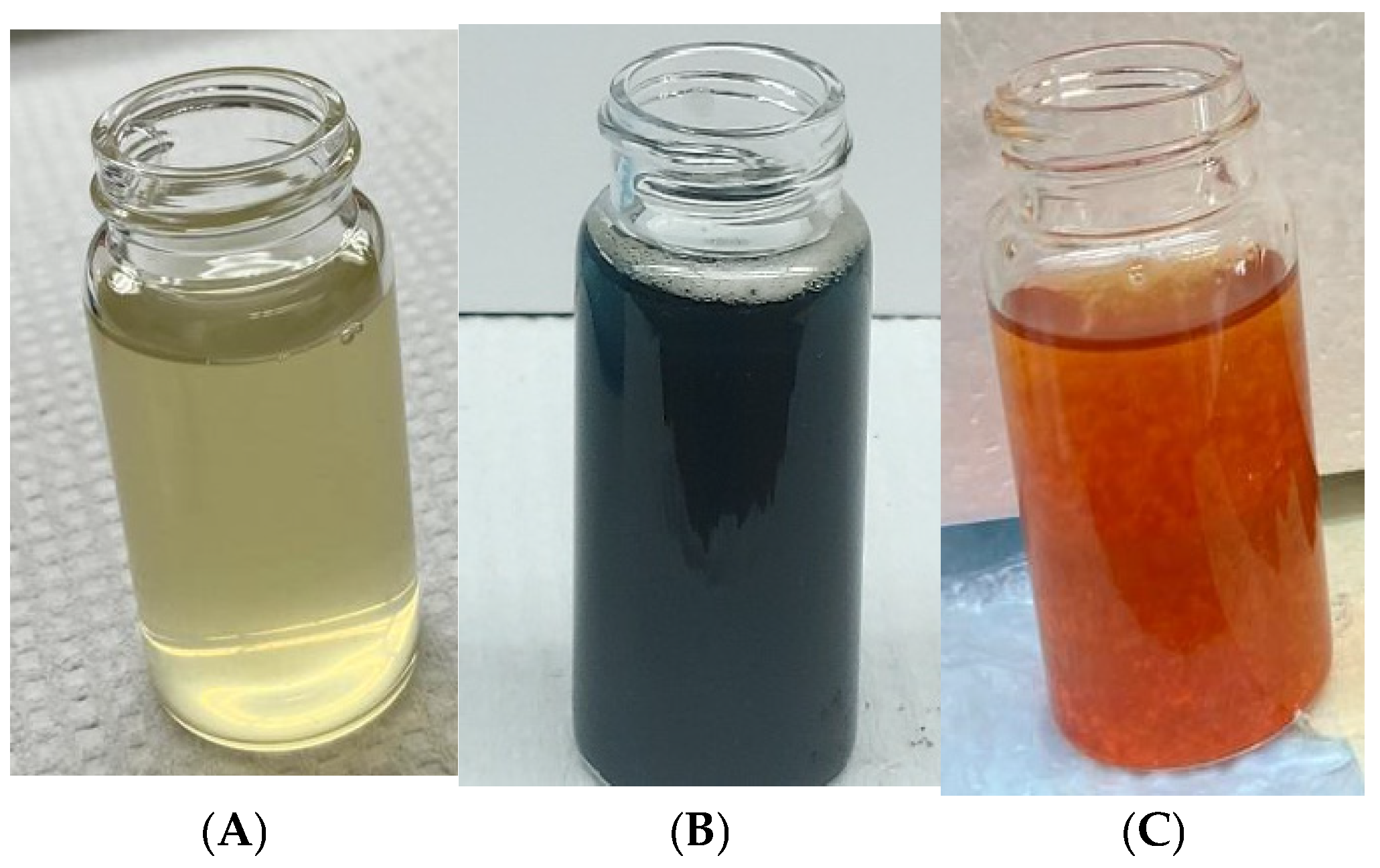
2.2. Preparation of Plant Extracts
- (i).
- Synthesis of iron oxide (FeO) nanoparticles from Fe2+ salts.
- (ii).
- Synthesis of iron oxide (Fe2O3) nanoparticles from Fe3+ salts.
2.3. Characterization of FeNPs
- (a)
- UV–Vis Spectra Analysis
- (b)
- X-Ray Diffraction (XRD) Analysis
- (c)
- Fourier Transform Infrared Spectroscopy
- (d)
- TEM and EDX Analyses
3. Results and Discussion
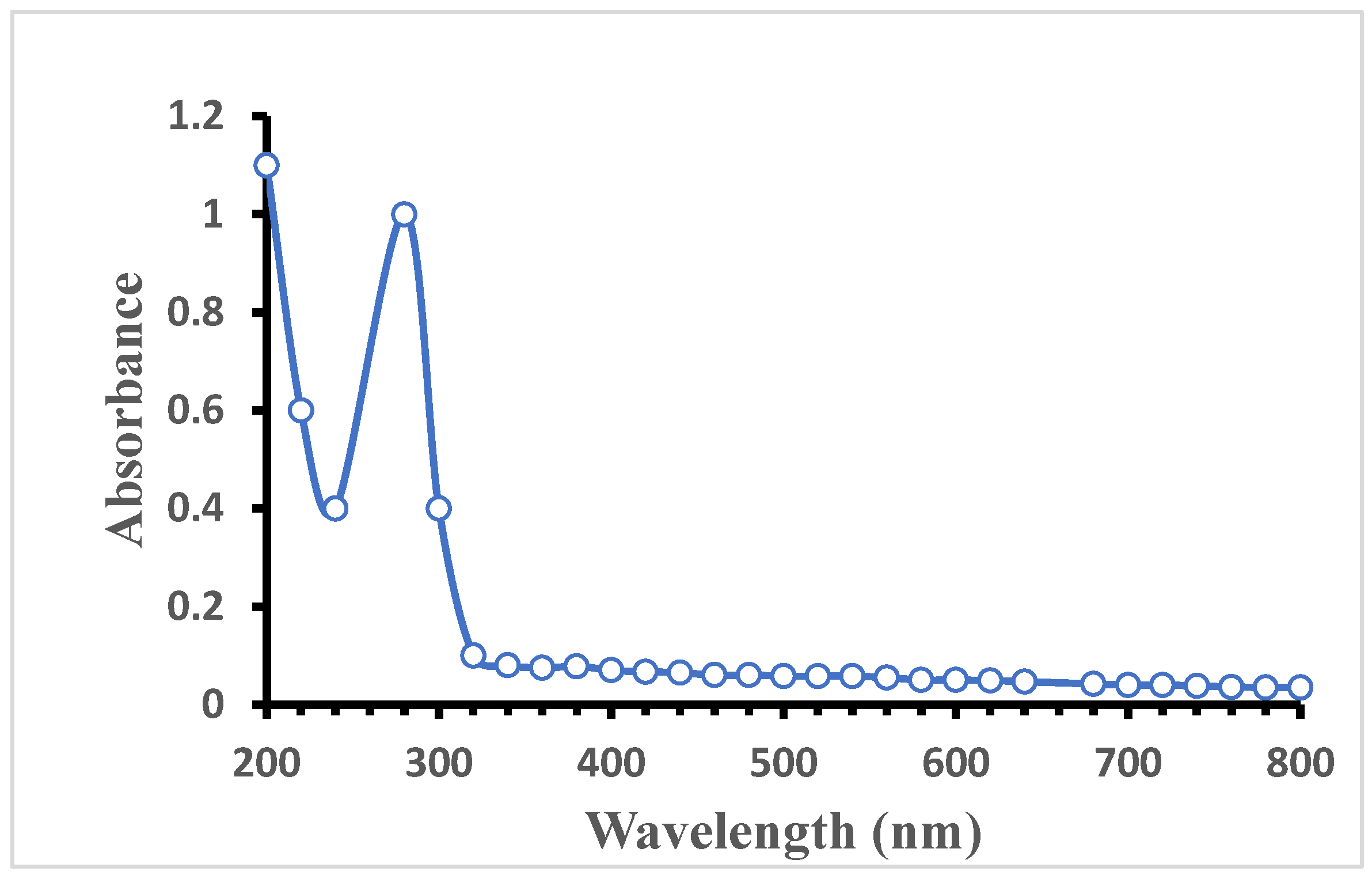
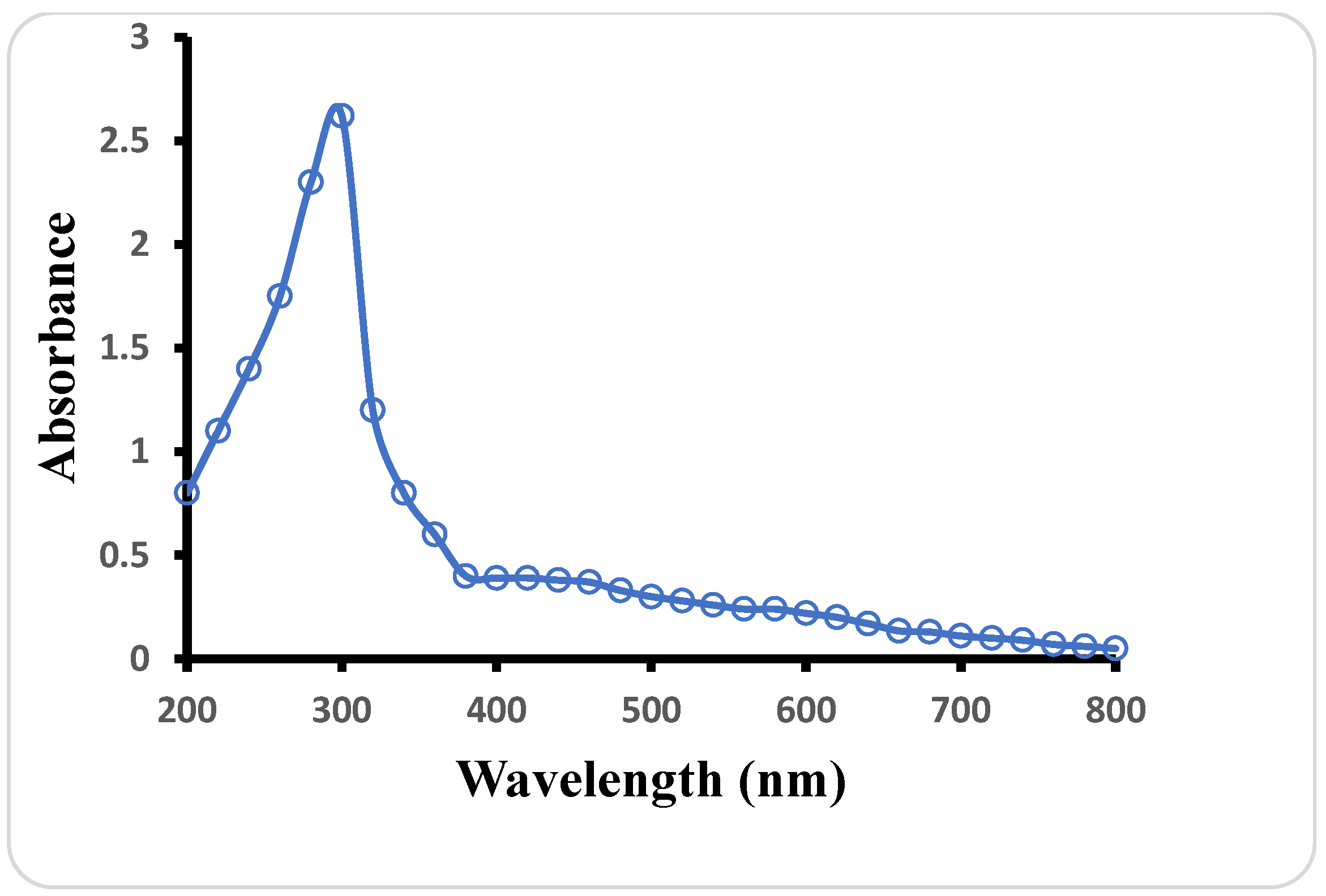
3.1. UV–Vis Spectroscopy
3.2. X-Ray Diffraction (XRD)
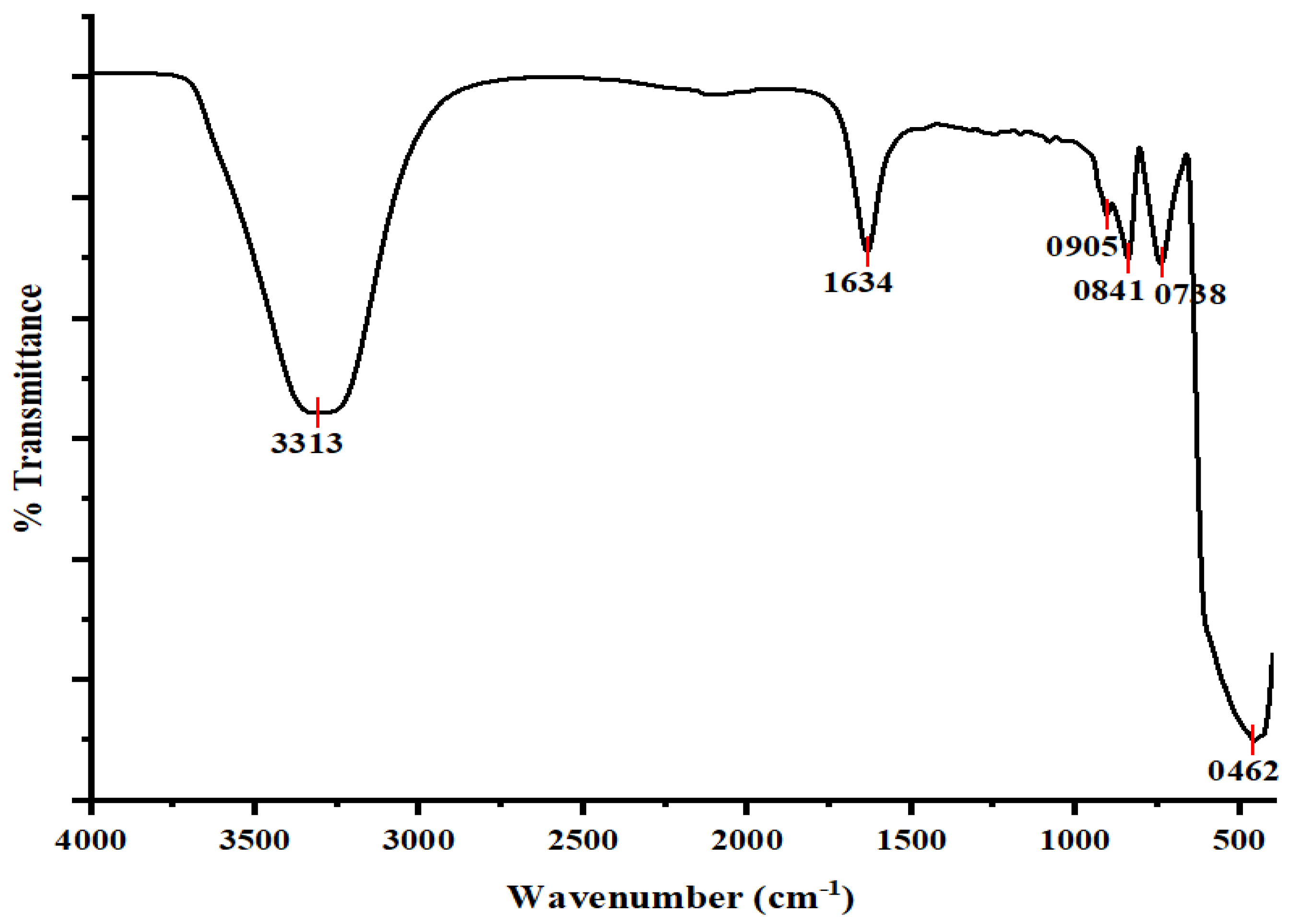
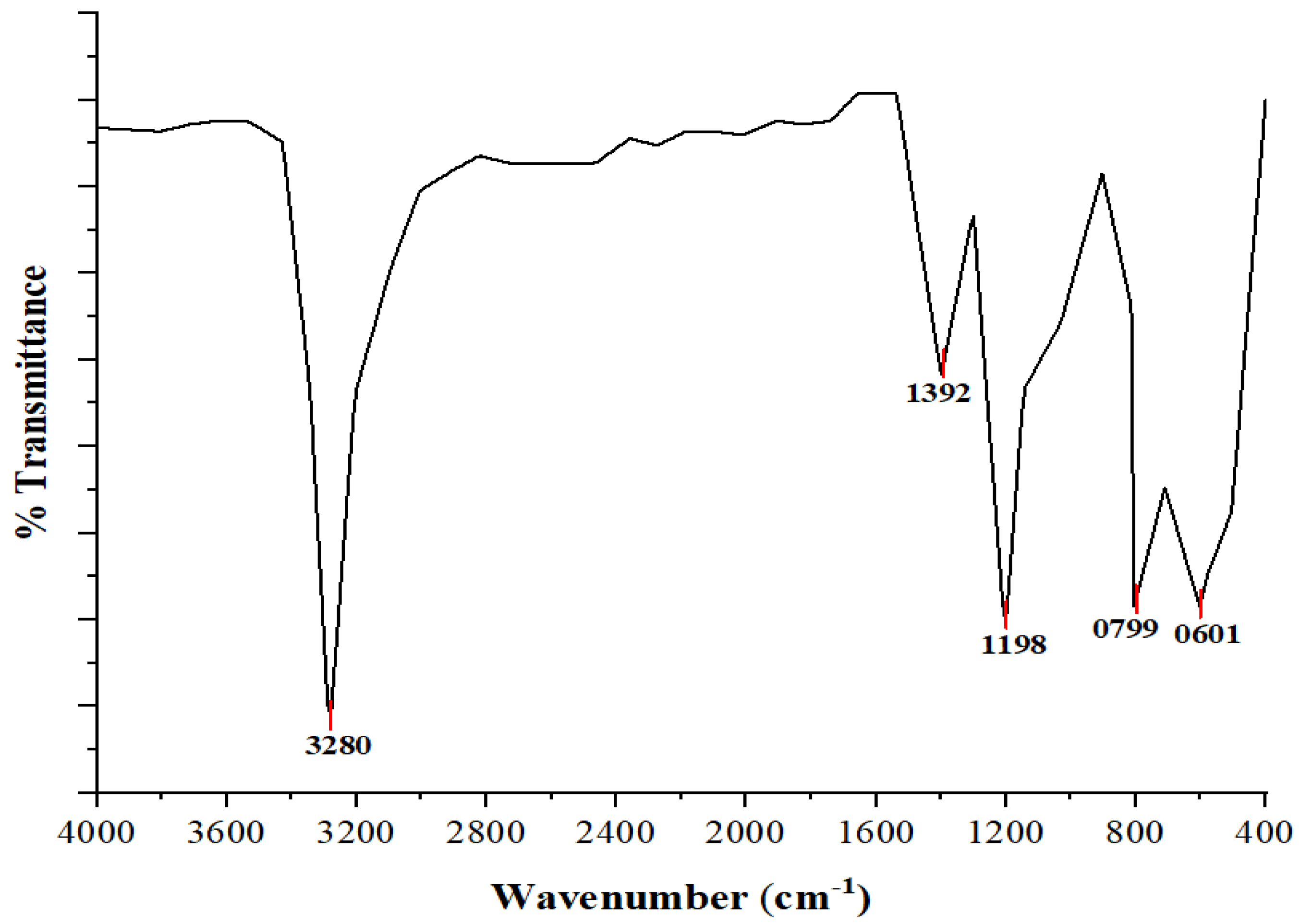
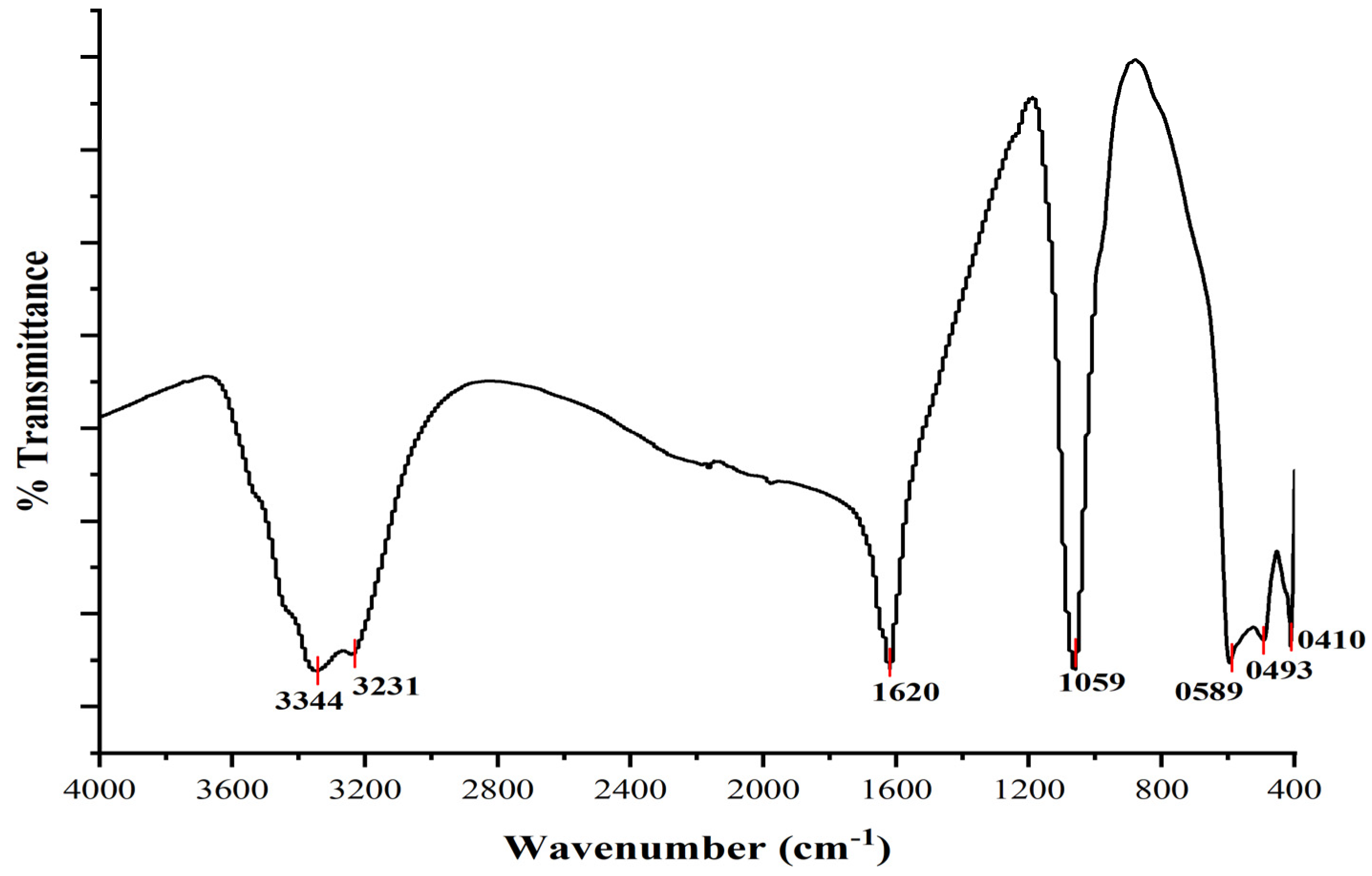
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
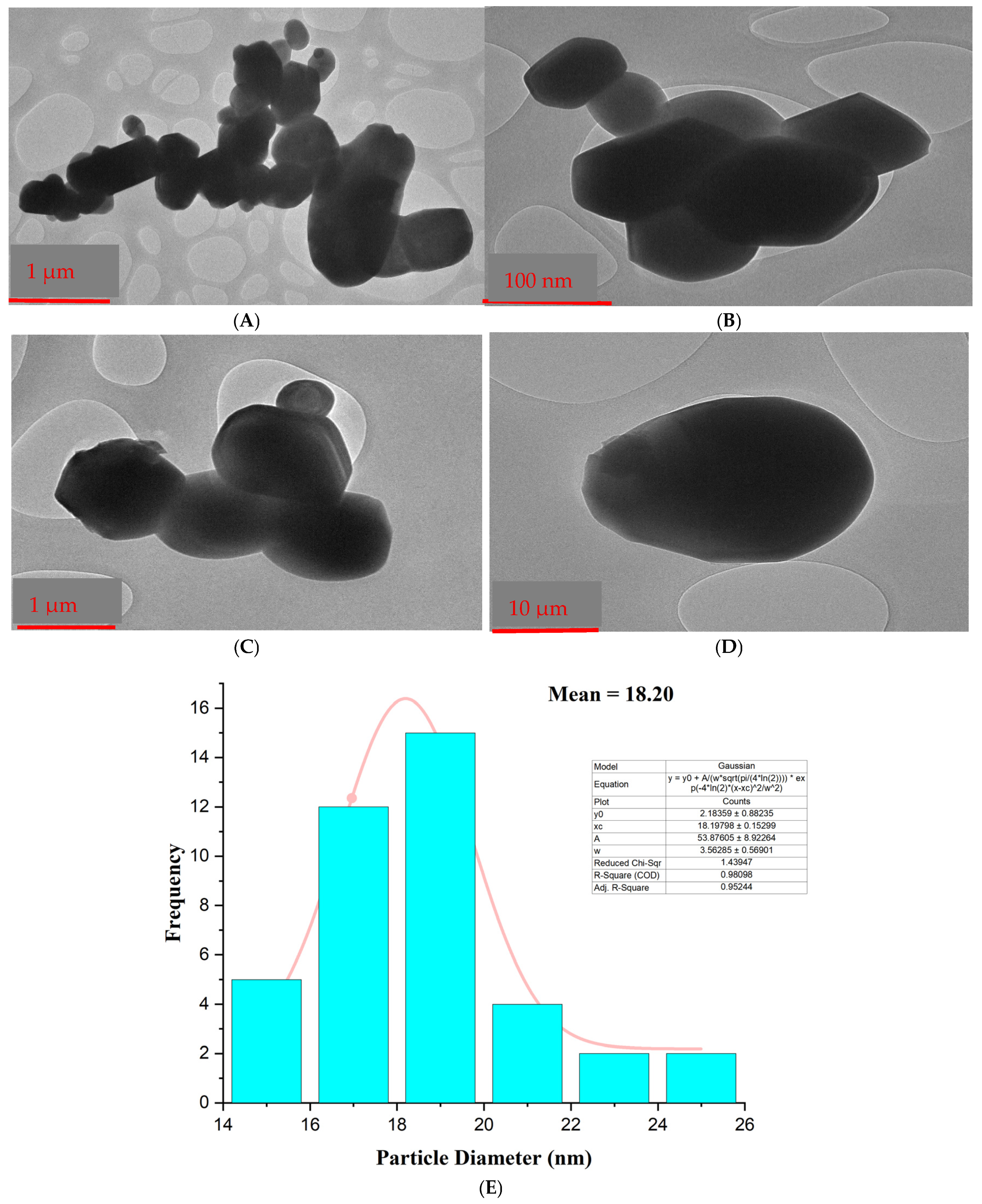
3.4. Transmission Electron Microscopy (TEM)
3.5. EDX
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasan, S.A. Review on Nanoparticles: Their Synthesis and Types. Res. J. Recent. Sci. 2015, 4, 9–11. [Google Scholar]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Ealia, S.A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 1–15. [Google Scholar]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochim. Acta-Part A Mol. Bio. Spe. 2014, 117, 801–804. [Google Scholar] [CrossRef]
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef]
- Devi, H.S.; Boda, M.A.; Shah, M.A.; Parveen, S.; Wani, A.H. Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process. Synth. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- Tuček, J.; Tuček, P.; Čuda, J.; Filip, J.; Pechoušek, J.; Machala, L.; Zbořil, R. Iron (III) oxide polymorphs and their manifestations in In-field 57Fe Mössbauer spectra. AIP Conf. Proc. 2012, 1489, 56–74. [Google Scholar] [CrossRef]
- Griffin, S.; Masood, M.I.; Nasim, M.J.; Sarfraz, M.; Ebokaiwe, A.P.; Schäfer, K.; Keck, C.M.; Jacob, C. Natural Nanoparticles: A Particular Matter Inspired by Nature. Antioxidants 2018, 7, 3. [Google Scholar] [CrossRef]
- Roy, A.; Sharma, A.; Yadav, S.; Jule, L.T.; Krishnaraj, R. Nanomaterials for Remediation of Environmental Pollutants. In Bioinorganic Chemistry and Applications; Hindawi Limited: London, UK, 2021. [Google Scholar] [CrossRef]
- Nagime, P.V.; Chandak, V.S. A comprehensive review of nanomaterials synthesis: Physical, chemical, and biological approaches and emerging challenges. Bio. Agr. Bio. 2024, 62, 103458. [Google Scholar] [CrossRef]
- Niraimathee, V.A.; Subha, V.; Ernest Ravindran, R.S.; Renganathan, S. Green synthesis of iron oxide nanoparticles from Mimosa pudica root extract. Int. J. Env. Sus. Dev. 2016, 15, 227–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, H.; Han, A.; Yang, S.; Xu, S.; Wang, X.; Guo, Z.; Chen, N.; Li, A.; Chen, X.; et al. High-performance anodes of Si@B-C/rGO nanoparticles for liquid and all-solid-state lithium-ion batteries. J. Alloys Compd. 2024, 983, 173919. [Google Scholar] [CrossRef]
- Sathya, V.; Jagatheesan, R.; Jeevanantham, V.; Gopi, D.; Muralidharan, V.P.; Rathinam, R. Selective oxidation of benzyl alcohol to benzaldehyde using sustainable catalysts: An overview. J. Indian Chem. Soc. 2024, 101, 101456. [Google Scholar] [CrossRef]
- Liu, J.F.; Jang, B.; Issadore, D.; Tsourkas, A. Use of magnetic fields and nanoparticles to trigger drug release and improve tumor targeting. Int. Rev. Nanomed. Nanobiotechnology 2019, 11, e1571. [Google Scholar] [CrossRef]
- Kirdat, P.N.; Dandge, P.B.; Hagwane, R.M.; Nikam, A.S.; Mahadik, S.P.; Jirange, S.T. Synthesis and characterization of ginger (Z. officinale) extract mediated iron oxide nanoparticles and its antibacterial activity. Mater. Proc. 2021, 43, 2826–2831. [Google Scholar] [CrossRef]
- Muthukumar, H.; Mohammed, S.N.; Chandrasekaran, N.I.; Sekar, A.D.; Pugazhendhi, A.; Matheswaran, M. Effect of iron doped Zinc oxide nanoparticles coating in the anode on current generation in microbial electrochemical cells. Int. J. Hydrog. Energy 2019, 44, 2407–2416. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 9. [Google Scholar] [CrossRef]
- Soltys, L.; Olkhovyy, O.; Tatarchuk, T.; Naushad, M. Green Synthesis of Metal and Metal Oxide Nanoparticles: Principles of Green Chemistry and Raw Materials. Magnetochemistry 2021, 7, 145. [Google Scholar] [CrossRef]
- Duman, F.; Ocsoy, I.; Kup, F.O. Chamomile flower extract-directed CuO nanoparticle formation for its antioxidant and DNA cleavage properties. Mater. Sci. Eng. C 2016, 60, 333–338. [Google Scholar] [CrossRef]
- Saranya, S.; Eswari, A.; Gayathri, E.; Eswari, S.; Vijayarani, K. Green Synthesis of Metallic Nanoparticles using Aqueous Plant Extract and their Antibacterial Activity. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1834–1845. [Google Scholar] [CrossRef]
- Sankaranarayanana, V.; Velusamyb, S.; Sankaranb, P.; Kandasamy, K. Phytoremediation potential of Elodea canadensis for reduction of chromium–optimization using response surface methodology. Desalination Water Treat. 2023, 293, 192–199. [Google Scholar] [CrossRef]
- Sobiecka, E.; Mroczkowska, M.; Olejnik, T.P. The Influence of Chlorpyrifos on the Nonenzymatic Antioxidants Content in Macrophytes Leaves. Antioxidants 2022, 11, 684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vishwanath, R.; Negi, B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. Curr. Res. Green Sustain. Chem. 2021, 4, 100205. [Google Scholar] [CrossRef]
- Moreno-Samaniego, M.; Villagomez-Herrera, J.; Dalgo-Flores, V.; Sanchez-Moreno, H. Green synthesis of copper oxide nanoparticles using Eucalyptus globulus extract with effective bactericidal activity for river water treatment. Results Eng. 2024, 24, 103441. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.B.; Salimi, M.N.; Letchumanan, I.; Subramaniam, S. Green synthesized strontium oxide nanoparticles by Elodea canadensis extract and their antibacterial activity. J. Nanostructure Chem. 2021, 12, 365–373. [Google Scholar] [CrossRef]
- Kaminskiene, Z.; Prosyevasa, I.; Stonkute, J.; Guobiene, A. Evaluation of optical properties of ag, cu, and co nanoparticles synthesized in organic medium. Acta Phys. Pol. A 2013, 123, 111–114. [Google Scholar] [CrossRef]
- Dhananjayan, B.; Badmapriya, D.; Asharani, I.V. Dye Degradation Studies Catalysed by Green Synthesized Iron Oxide Nanoparticles. 2016. Available online: https://www.researchgate.net/publication/306208710 (accessed on 27 October 2024).
- Saion, E.; Gharibshahi, E.; Naghavi, K. Size-Controlled and Optical Properties of Monodispersed Silver Nanoparticles Synthesized by the Radiolytic Reduction Method. Int. J. Mol. Sci. 2013, 14, 7880–7896. [Google Scholar] [CrossRef]
- Ahmmad, B.; Leonard, K.; Shariful Islam, M.; Kurawaki, J.; Muruganandham, M.; Ohkubo, T.; Kuroda, Y. Green synthesis of mesoporous hematite (α-Fe2O3) nanoparticles and their photocatalytic activity. Adv. Powder Technol. 2013, 24, 160–167. [Google Scholar] [CrossRef]
- Paredes-García, V.; Toledo, N.; Denardin, J.; Venegas-Yazigi, D.; Cruz, C.; Spodine, E.; Luo, Z. One Pot Solvothermal Synthesis of Organic Acid Coated Magnetic Iron Oxide Nanoparticles. Int. J. Chil. Chem. Soc. 2013, 58, 2011–2015. [Google Scholar] [CrossRef]
- InstaNANO. FTIR Functional Group Database Table with Search. Available online: https://instanano.com/all/characterization/ftir/ftir-functional-group-search/ (accessed on 1 December 2024).
- Katata-Seru, L.; Moremedi, T.; Aremu, O.S.; Bahadur, I. Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: Removal of nitrate from water and antibacterial activity against Escherichia coli. J. Mol. Liq. 2018, 256, 296–304. [Google Scholar] [CrossRef]
- Kulkarni, N.J.; Kulkarni, N.; Dauthal, P.; Mukhopadhyay, M. Green Synthesis of Iron Complex Nanoparticles Using Delonix regia Leaf. In Proceedings of the 68th Annual Conference of Indian Institute of Chemical Engineers, Assam, India, 27–30 December 2015; Available online: https://www.researchgate.net/publication/290426479 (accessed on 8 February 2025).
- Qasim, S.; Zafar, A.; Saif, M.S.; Ali, Z.; Nazar, M.; Waqas, M.; Haq, A.U.; Tariq, T.; Hassan, S.G.; Iqbal, F.; et al. Green synthesis of iron oxide nanorods using Withania coagulans extract improved photocatalytic degradation and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111784. [Google Scholar] [CrossRef]

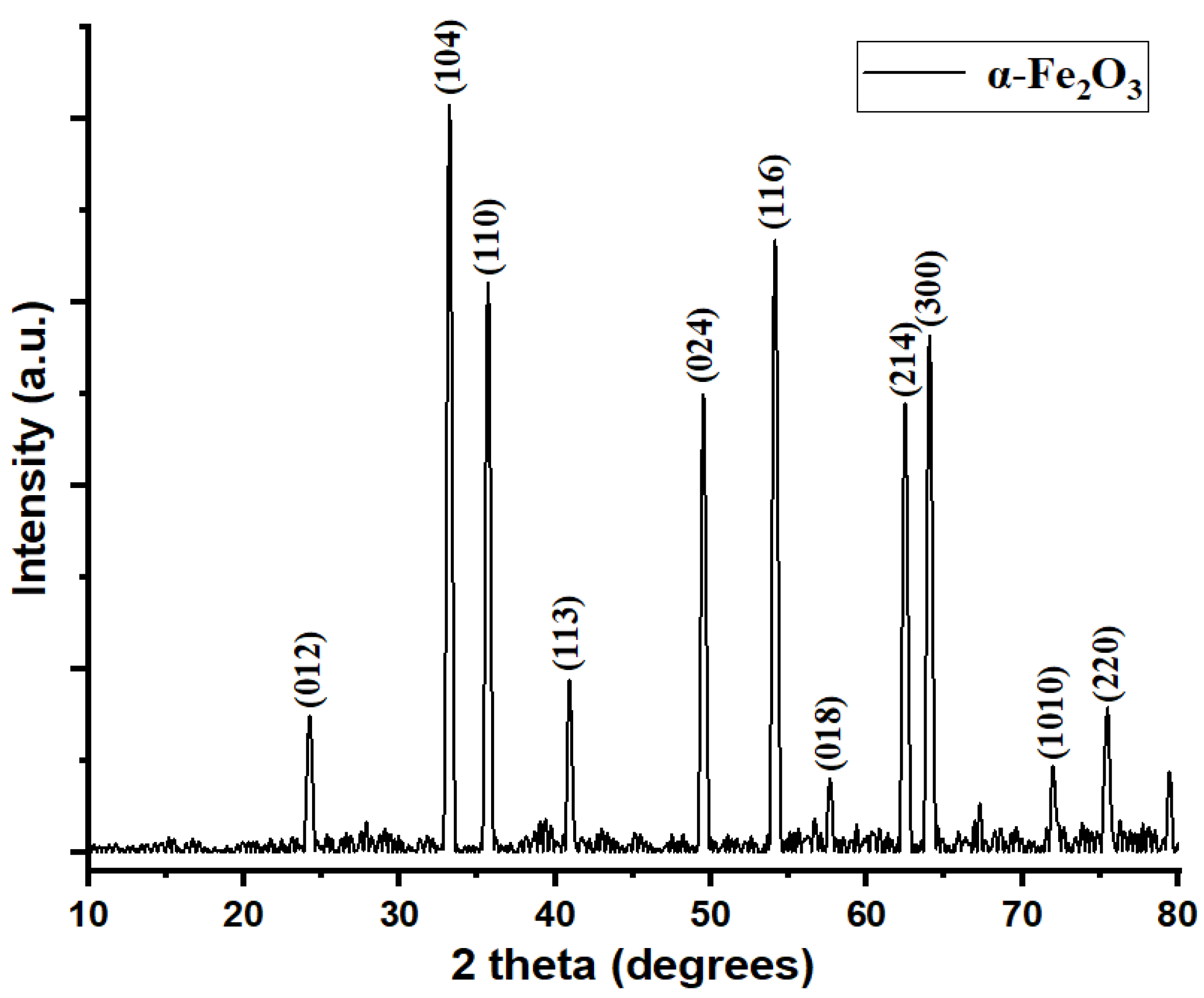
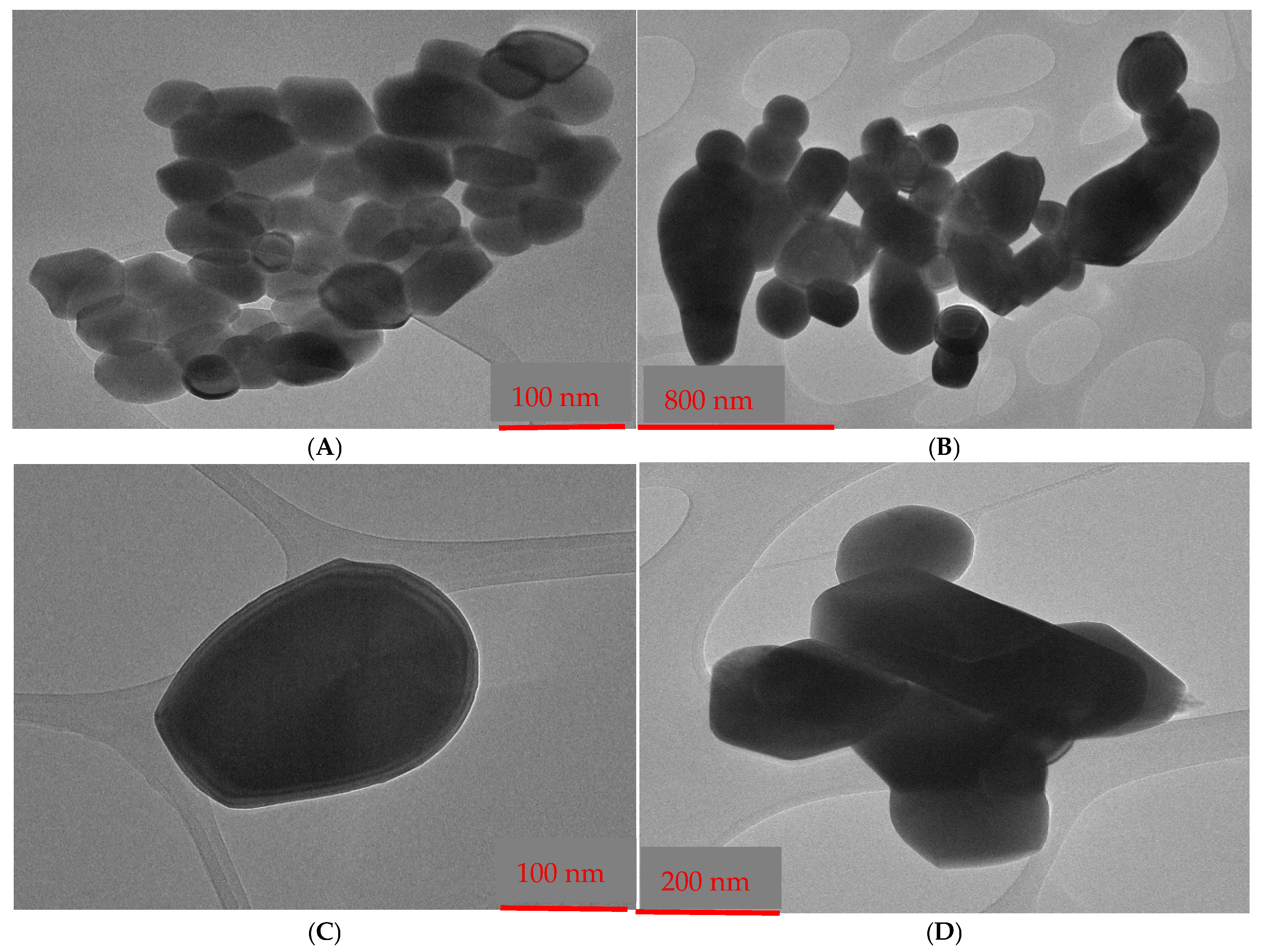
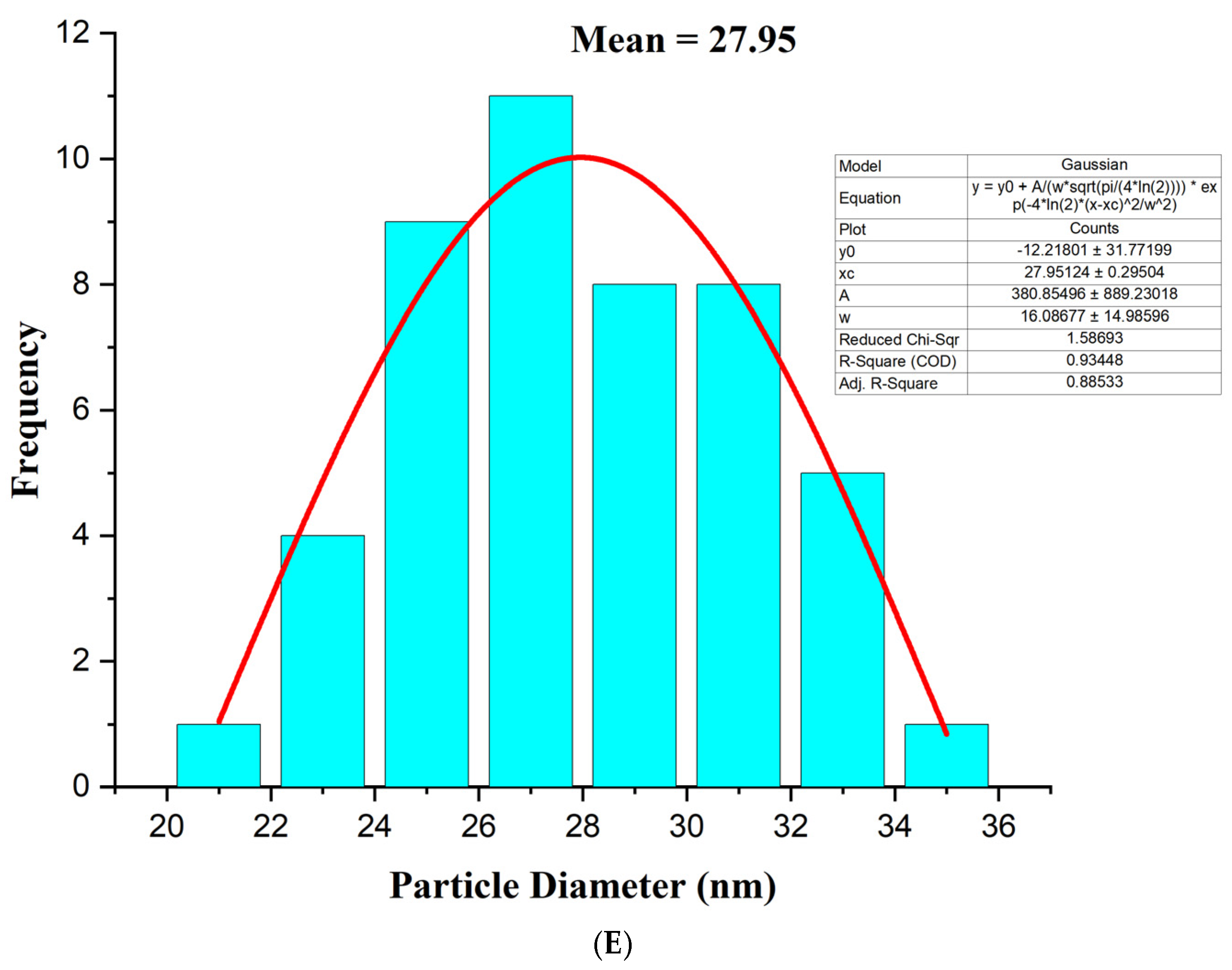
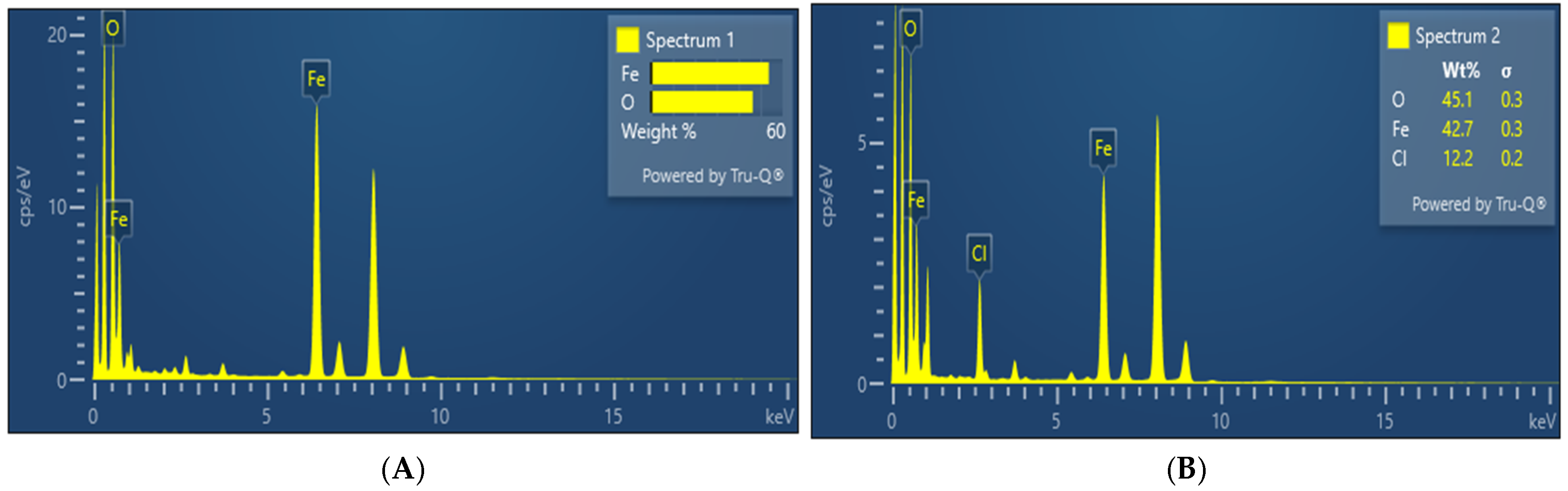
| Sample (2θ) | Reference (2θ) (JCPDS) | Reference (hkl) | Reference Relative Intensity (%) | Sample Relative Intensity (%) | Match Assessment |
|---|---|---|---|---|---|
| 23.7° | 24.18° | 012 | 28.8 | 23.91 | Close shift (≈ −0.48°) |
| 32.7° | 33.15° | 104 | 100 | 100 | Close shift (≈ −0.45°) |
| 35.1° | 35.61° | 110 | 60 | 81.52 | Slight shift (≈ −0.51°) |
| 40.4° | 40.92° | 113 | 18 | 27.17 | Slight shift (≈ −0.52°) |
| 49.05° | 49.53° | 024 | 30 | 61.96 | Close shift (≈ −0.48°) |
| 53.6° | 54.13° | 116 | 55 | 77.17 | Slight shift (≈ −0.53°) |
| 62.0° | 62.53° | 214 | 35 | 56.52 | Slight shift (≈ −0.53°) |
| 63.7° | 64.0° | 300 | 25 | 55.43 | Close shift (≈ −0.3°) |
| 71.65° | 72.06° | 1010 | 10.8 | 21.74 | Close shift (≈ −0.41°) |
| 75.1° | 75.57° | 220 | 7.0 | 16.30 | Close shift (≈ −0.47°) |
| Sample (2θ) | Reference (2θ) (JCPDS) | Reference (hkl) | Reference Relative Intensity (%) | Sample Relative Intensity (%) | Match Assessment |
|---|---|---|---|---|---|
| 23.7° | 24.18° | 012 | 28.8 | 17.44 | Close (shift ≈ −0.48°) |
| 32.7° | 33.15° | 104 | 100 | 100 | Close (shift ≈ −0.45°) |
| 35.1° | 35.61° | 110 | 60 | 76.74 | Slight shift (≈−0.51°) |
| 40.4° | 40.92° | 113 | 18 | 23.26 | Slight shift (≈ −0.52°) |
| 49.05° | 49.53° | 024 | 30 | 61.63 | Close (shift ≈ −0.48°) |
| 53.6° | 54.13° | 116 | 55 | 81.40 | Slight shift (≈ −0.53°) |
| 57.1° | 57.5° | 018 | 40 | 9.30 | Close (shift (≈ −0.4°) |
| 62.0° | 62.53° | 214 | 35 | 60.47 | Slight shift (≈ −0.53°) |
| 63.7° | 64.0° | 300 | 25 | 69.77 | Close (shift ≈ −0.3°) |
| 71.65° | 72.06° | 1010 | 10.8 | 11.63 | Close (shift ≈ −0.41°) |
| 75.1° | 75.57° | 220 | 7.0 | 76.74 | Close (shift ≈ −0.47°) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yekeen, M.O.; Ibrahim, M.; Wachira, J.; Pramanik, S. Green Synthesis and Characterization of Iron Oxide Nanoparticles Using Egeria densa Plant Extract. Appl. Biosci. 2025, 4, 27. https://doi.org/10.3390/applbiosci4020027
Yekeen MO, Ibrahim M, Wachira J, Pramanik S. Green Synthesis and Characterization of Iron Oxide Nanoparticles Using Egeria densa Plant Extract. Applied Biosciences. 2025; 4(2):27. https://doi.org/10.3390/applbiosci4020027
Chicago/Turabian StyleYekeen, Maruf Olaide, Mubarak Ibrahim, James Wachira, and Saroj Pramanik. 2025. "Green Synthesis and Characterization of Iron Oxide Nanoparticles Using Egeria densa Plant Extract" Applied Biosciences 4, no. 2: 27. https://doi.org/10.3390/applbiosci4020027
APA StyleYekeen, M. O., Ibrahim, M., Wachira, J., & Pramanik, S. (2025). Green Synthesis and Characterization of Iron Oxide Nanoparticles Using Egeria densa Plant Extract. Applied Biosciences, 4(2), 27. https://doi.org/10.3390/applbiosci4020027







