Abstract
Lactiplantibacillus plantarum is omnipresent in vegetable fermentations. Its large metabolic capacity and its ability to adapt to the fermenting microenvironment enable this species, in many cases, to dominate the microecosystem and drive the fermentation. In addition, its metabolic capacity enables it to produce bioactive compounds of great interest for human health. These attributes have directed research for many decades. The widespread application of next-generation sequencing approaches has enabled the genotypic verification of the phenotypically assessed attributes and supplemented them with novel insights, justifying the characterization of a multifunctional tool that has been awarded to this species. However, there are still issues that need to be properly addressed in order to improve our understanding of the microecosystem functionality and to enhance our knowledge regarding the capacities of this species. The aim of the present article is to collect and critically discuss the available information on Lp. plantarum subsistence in vegetable fermentations.
1. Introduction
Lactiplantibacillus plantarum is a lactic acid bacterium that is frequently encountered in fermented products of plant and animal origin. Indeed, its occurrence and, in many cases, dominance in fermented fruits such as olives [1] and cucumbers [2], fermented vegetables such as sauerkraut [3], leek [4], and radish roots [5], fermented sausages [6,7,8,9,10,11,12,13], fermented milks [14,15,16,17], cheeses [18,19,20], and sourdoughs [21,22,23,24,25] has been reported. This omnipresence has been attributed to its large metabolic capacity and the ability to tolerate acidic conditions [26]. These traits have attracted scientific attention towards the elucidation of the potential of this species. The phenotypic assessments revealed the strain-dependent breadth of its physiological attributes, which include persistence in environments with diverse physicochemical characteristics, the decomposition and catabolism of a wide range of macromolecules, and the production of metabolites, many of which are of particular interest for human nutrition and well-being. Thus, Lp. plantarum strains have been effectively employed for a wide range of applications, which extend from the improvement of the silage quality [27,28] and conversion of unpalatable forestry by-products into safe and nutritious feed [29], to the utilization of food industry by-products for the production of technologically important ingredients such as antifungal compounds [30,31] and biosurfactants [32], as well as functional ingredients and products [33,34,35]. However, the main field of application for this species is food fermentation. Several Lp. plantarum strains have the capacity to produce bioactive compounds such as γ-aminobutyric acid (GABA) [36], vitamins, such as riboflavin [37] and folate [38], bioactive peptides [39] and enzymes that increase the nutritional value of fermented food, such as phytase [40], and degrade biogenic amines [41], making them an attractive option for utilization as starter or adjunct cultures. In addition, the lack of virulence factors and toxins [42,43], the capacity to interfere with the virulence mechanisms of an extended range of foodborne pathogens [44], along with the capacity to tolerate and colonize the human gastrointestinal tract also make them an interesting option for the development of probiotic cultures [45]. These attributes were verified by genomic studies and attributed to the extended number of accessory genes.
The use of vegetables as a substrate for lactic acid fermentation is of particular interest due to their nutritional quality and, particularly, the occurrence of bioactive phytochemicals such as dietary fiber, minerals, vitamins, phytoestrogens, polyunsaturated fatty acids sulfur compounds, and terpene derivatives [46]. On the other hand, vegetables may also contain a variety of antinutritional factors, such as phytates, tannins, and oxalates. In both cases, the biotransformations that take place during fermentation may enhance quantitatively and enrich qualitatively the amount of bioactive compounds and reduce the amount of antinutritional compounds [47,48]. Fermented vegetables are also an attractive, yet underexplored, option for the delivery of probiotic microorganisms.
Lactiplantibacillus plantarum is an integral member of the microbiota of spontaneous vegetable fermentations; either forming the dominant population or merely as a part of the microecosystem. The benefits that arise from its presence are multiple; except from effectively driving the fermentation to a low pH value and high acidity level, which, combined with the wide range of antimicrobial compounds that it produces, increases the shelf-life and ensures the safety of the product, it may also enhance the nutritional value of the final product through a variety of metabolic activities. Therefore, its persistence and activities within the particular niche have attracted significant scientific attention. The aim of this article is to collect, analyze, and critically discuss the available information regarding the Lp. plantarum activities that are related to vegetable fermentation in order to systematize current knowledge and highlight research gaps.
2. Lactiplantibacillus plantarum Taxonomic Position and Identification
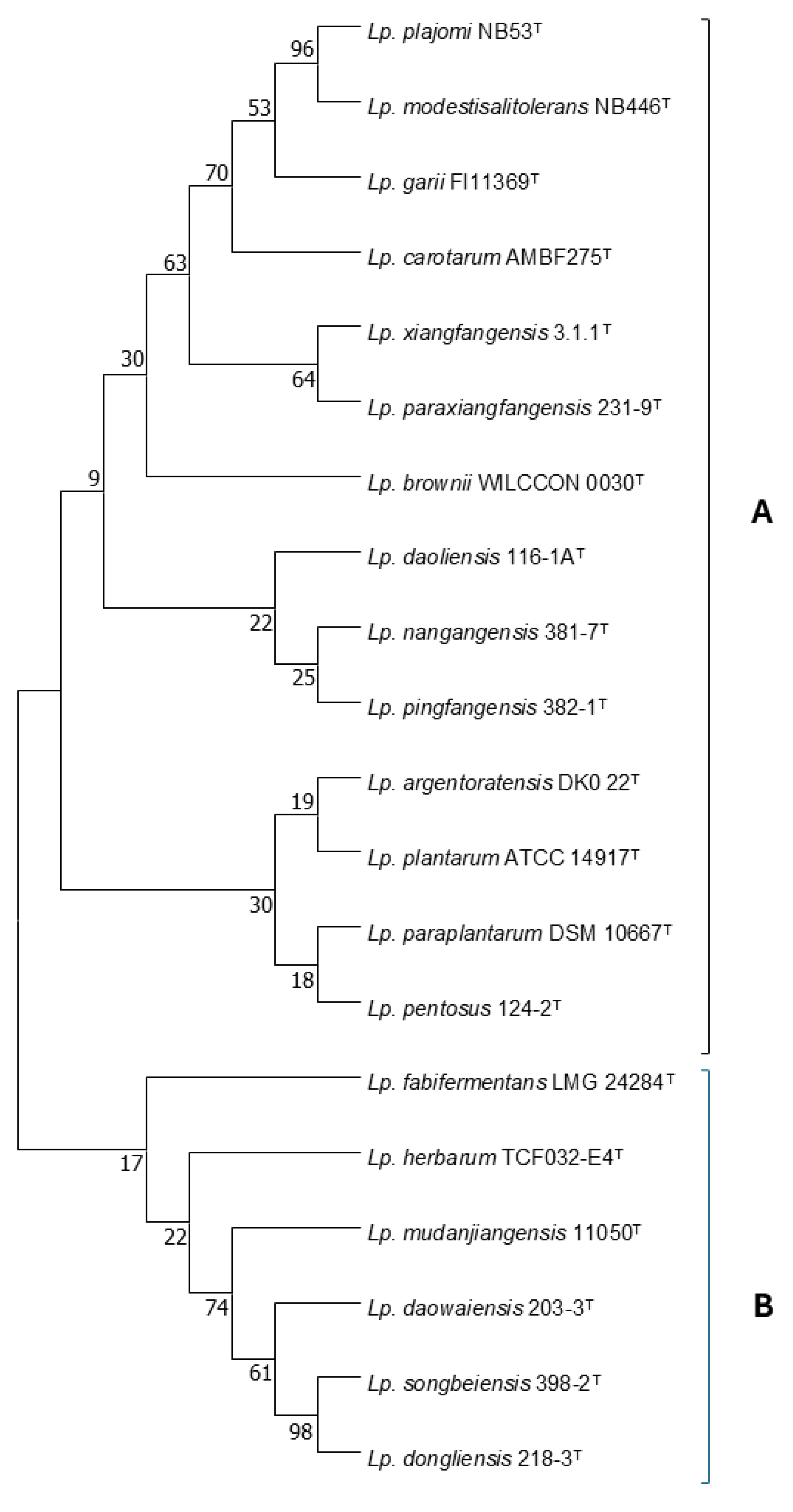
The Lactiplantibacillus genus was established in 2019, and includes species able to ferment a wide range of carbohydrates and which are associated with food fermentations, insect habitats, or the vertebrate intestine [49]. The initial description included 17 species and two subspecies, namely, Lp. plantarum subsp. plantarum and Lp. plantarum subsp. argentoratensis; however, the latter was recently elevated to the species level [50]. The description of two new species, namely, Lp. brownii [51] and Lp. paraxiangfangensis [52], raised the total number of the species belonging to this genus to 20. The species, along with their evolutionary history, based on their 16S rRNA gene sequences, as inferred by using the Maximum Likelihood method and Hasegawa–Kishino–Yano model, are presented in Figure 1.
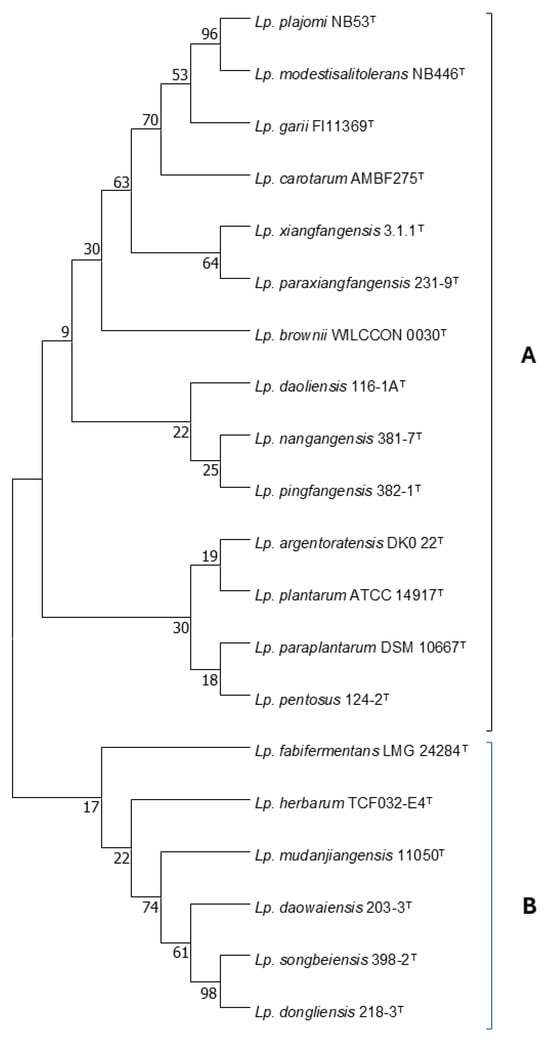
Figure 1.
Evolutionary history of the Lactiplantibacillus spp. based on their 16S rRNA gene sequences, as inferred by using the Maximum Likelihood method and Hasegawa–Kishino–Yano model. Bootstrap values at the node were calculated from 1000 samplings. Evolutionary analyses were conducted in MEGA X, version 6 [53].
According to this model, the Lactiplantibacillus species is divided into two lineages, which are designated in Figure 1 as A and B. The majority of the species that have been assigned to this genus are classified into lineage A. A total of 274 variable sites are available for the differentiation between the Lactiplantibacillus species. This number seems adequate for effective differentiation at the species level, considering the 97% resemblance threshold that has been established as a necessary prerequisite for strains to belong to the same species [54]. These variable sites are not evenly distributed across the sequence of the gene, but most of them are located within the first quarter of the sequence. Certain groups within the genus consist of closely related species, which cannot be differentiated by 16S rRNA gene sequencing. The first group that was recognized as such was the Lp. plantarum group, which originally consisted of Lp. plantarum, Lp. paraplantarum, and Lp. pentosus, and now also includes Lp. argentoratensis. The three former species can be effectively differentiated on the basis of the recA gene sequence [55]. Notably, sequence analysis revealed that, in the 16S rRNA gene sequence, there are only 6 variable sites available for the differentiation between Lp. plantarum, Lp. paraplantarum, and Lp. pentosus, which increased to 11 with the addition of Lp. argentoratensis. Furthermore, the differentiation between Lp. pingfangensis, Lp. nangangensis, and Lp. daoliensis, as well as between Lp. xiangfangesis and Lp. paraxiangfangensis, on the basis of 16S rRNA gene sequencing seems to be at least problematic due to the small number of the available variable sites, which are only four and one, respectively. Until a genotypic protocol for their differentiation is available, this has to be based on their phenotypic properties. In Figure 2, a decision tree based mainly on sugar fermentation patterns of the Lactiplantibacillus species is offered. The phenotypic properties of Lp. paraxiangfangensis were not available at the time of this manuscript preparation, and therefore this species is absent from the decision tree. The ability to produce acid from 26 carbohydrates is necessary for the effective phenotypic differentiation between the species belonging to this genus. However, the decision tree of Figure 2 utilizes only 18 carbohydrates and the ability to grow at 37 °C; the remaining tests can be performed for reasons of confirmation of the identity of an isolate, or for the description of a new species. This decision tree allows the efficient differentiation between the aforementioned closely related species. Regarding the Lp. plantarum group, Lp. argentoratensis is differentiated by its ability to produce acid from D-arabitol and melibiose but not D-mannose. The rest of the species of this group are not able to produce acid from D-arabitol. Lactiplantibacillus plantarum produces acid from methyl-α-D-mannopyranoside and L-arabinose. On the contrary, Lp. paraplantarum and Lp. pentosus are not able to produce acid from methyl-α-D-mannopyranoside. The latter species can be differentiated on the basis of acid production by D-xylose; Lp. pentosus is positive while Lp. paraplantarum is negative. Lactiplantibacillus nangangensis can be differentiated from Lp. pingfangensis and Lp. daoliensis by its inability to produce acid from methyl α-D glucopyranoside. Differentiation between Lp. pingfangesis and Lp. daoliensis can be achieved by examining their ability to grow at 37 °C; the former is capable while the latter is not.
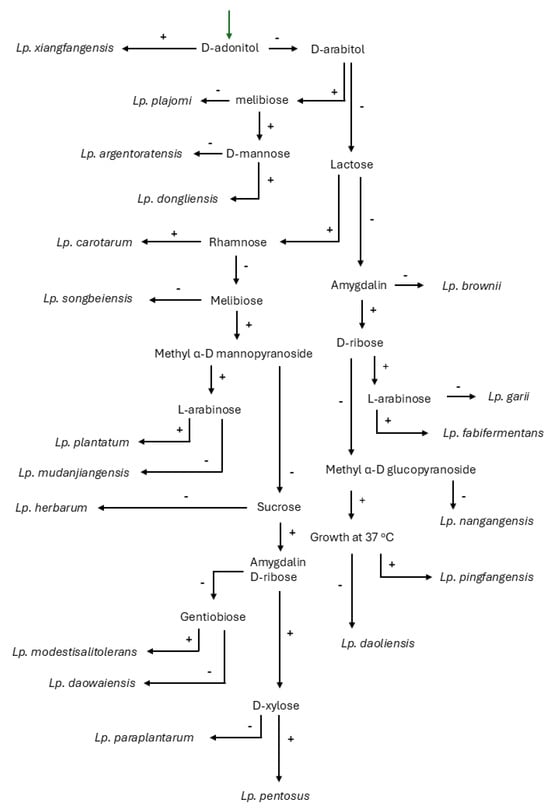
Figure 2.
Decision tree for the effective differentiation of Lactiplantibacillus spp. using phenotypic traits.
Finally, the differentiation between Lp. plantarum, Lp. paraplantarum, Lp. pentosus, and possibly Lp. argentoratensis, through species-specific peaks obtained by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, has been recently reported by Kim et al. [56]. The authors recommended the utilization of the proposed peaks in order to overcome the limitations of commercial databases that are used when MALDI-TOF is employed for identification purposes.
3. Occurrence of Lp. plantarum in Vegetable Fermentation
The microbiota of fermented vegetables has been under intensive study over the last decades. The approach that is selected for this assessment affects the amount of microecosystem-related information that is obtained. More specifically, the application of culture-dependent techniques, provided that colony isolation is performed on the basis of a statistically approved scheme, such as the one described by Harrigan and McCance [57], when combined with suitable genotypic profiling techniques, allows the estimation of the relative abundances at the species and subspecies levels [5,58,59]. The advantage of this approach is that a collection of isolates is generated, and these are available for further assessment. The addition of a culture-independent approach provides insights into the unculturable proportion of the microbiota.
The culture-independent approach that was most frequently employed was polymerase chain reaction–denaturing gradient gel electrophoresis (PCR-DGGE). This technique was very useful for the monitoring of the dynamics of the microbial species throughout fermentation and also for the delineation of the composition of the microecosystem. However, it suffered from significant limitations, resulting from the utilization of the 16S rRNA gene for prokaryotic population assessment. Such limitations included the inability to differentiate between closely related species, the formation of heteroduplexes, and the display of multiple bands for a single species [60,61]. In addition, the application of this technique could only lead to the qualitative assessment of the microecosystem composition and not for the estimation of the relative abundances of the members [62,63,64]. However, the application of a culture-independent approach allowed the recovery of additional members of the microbial community, the presence of which has not been previously reported. This was attributed partly to the detection of the genetic material of microbial species present in the raw materials, which had limited, if any, participation in the fermentation process, and partly to the utilization of DNA and not RNA as a nucleic acid target. It is well known that DNA may persist in the environment after cell lysis and, concomitantly, interfere with the analysis; therefore, RNA seemed to be a better indicator of microbial viability. This was verified by Dolci et al. [65] regarding the microecosystem of Fontina PDO (protected designation of origin) cheese. On the contrary, Iacumin et al. [66] and Syrokou et al. [24] reported that both nucleic acids provided similar results while assessing the composition of the sourdough microcommunity.
The introduction of next-generation sequencing (NGS) technology revealed in more detail the uncultured proportion of the microbiota and also allowed the assessment of their relative abundances [67,68]. The initial concerns regarding the application of this technology referred to the variability of the results according to the platform and the bioinformatic pipeline employed. However, the technological and conceptual advances in both fields, along with the experience that was gained through their widespread application, mitigated these issues, providing a reliable tool to microecosystems study.
The occurrence of Lp. plantarum in the microcommunity of a large number of spontaneously fermented vegetable-based products has been documented (Table 1). In general, the following two types of studies can be distinguished: those that assess the microcommunity of the final product and those that follow the dynamics of the microbial populations throughout fermentation. In the first case, dominance of Lp. plantarum has been reported in many products, such as Awa-bancha [69], Chongqing radish paocai [68], fermented potherbs [70], Mesu [71], Soibum [71], Chongzhou fermented vegetables [72], Dayi fermented vegetables [72], Pujiang fermented vegetables [72], Qionglai fermented vegetables [72], Dua Cu Cai Muoi [73], Miang [74], Khalpi [75], Inziangsang [75], and Sinki [76]. On the other hand, in many products, such as Gundruk [75], Chengdu fermented vegetables [72], Xinjin fermented vegetables [72], Dua Cai Be Muoi [73], fermented Chinese cabbage [70,77], fermented Pakchoi [70], Dua-Cai [64], Mang-Chua [64], Laphet [78], fermented Brassica napobrassica [79], and Zhejiang Xue-Cai [80], Lp. plantarum has been identified as merely a member of the microbial community.

Table 1.
Bacterial microcommunity of fermented vegetables in which Lp. plantarum presence has been reported.
The studies that follow the dynamics of the different lactic acid bacteria (LAB) species throughout fermentation offer unique perspectives and facilitate our understanding of the spontaneous fermentation process. In many cases, such as in Brovada [81], mixed vegetable fermentation [87], leek fermentation [4], kimchi fermented at 8 °C [89] or 10 °C [88], Suan-Cai [92], Takanazuke [90], winter salad [93], Koso [97], Laotan Suan-Cai [99], and non-salted Suan-Cai [67], the participation of Lp. plantarum in the developing microcommunity throughout fermentation has been reported. The dominance of Lp. plantarum is usually reported towards the end of fermentation and not throughout the process. Indeed, the large metabolic capacity that characterizes this species along with the ability to tolerate acidic conditions enables Lp. plantarum to dominate the final stages of fermentation. On the other hand, species that are characterized by a relative sensitivity to acidity, an ability to tolerate salt, and a possibly shorter generation time, such as Leuconostoc mesenteroides, usually dominate the early stages of fermentation. Indeed, during the spontaneous fermentation of sauerkraut [82,83], cauliflower [86], and kimchi fermented at 15 °C or 25 °C [89], the dominance of Ln. mesenteroides during the first days of fermentation, and the substitution by Lp. plantarum after acidity development, has been reported. Similarly, Ln. citreum and Pediococcus pentosaceus have been reported to dominate the early stages of Suguki and radish roots fermentation, respectively [5,85], and a more complex microcommunity has been reported to dominate the early stages of paocai fermentation at 15, 25, or 35 °C [95]. In all cases, the dominance of Lp. plantarum during the later stages of fermentation was evident.
The biotic and abiotic parameters that justify the aforementioned data have been extensively assessed. The capacity of Lp. plantarum to utilize a wide range of carbon sources, along with the ability to tolerate acidic conditions are usually regarded as the factors that play decisive role in its dominance during spontaneous vegetable fermentation, provided that this is performed at temperatures above 10 °C. However, whole-genome sequencing has revealed a number of additional factors, which are presented and discussed in the following paragraph.
4. Metabolic Activities Related to Vegetable Fermentation–Genomic Insights
The ability to utilize the available nutrients within a particular niche, along with the capacity to adapt to the acidic conditions that develop with time, are of paramount importance for effective population development and, ultimately, dominance during fermentation. Regarding vegetables, water is the main constituent, ranging between 91 and 95% of the mass, while digestible carbohydrates are the second most abundant, accounting for 50–80% of the dry weight, with glucose, fructose, and sucrose being the major ones. Non-digestible carbohydrates are also a significant constituent, with cellulose, glycans, lignin, and pectins being the main ones. Proteins and fats constitute only a minor percentage, which accounts for the majority of the cases in less than 1%. In all cases, the nutrient composition is affected by the type of vegetable, cultivar, as well as the agronomic and storage conditions [100,101,102,103,104,105]. Lactiplantibacillus plantarum ferments all three carbohydrates; in fact, there is a wide range of LAB that are able to ferment them; therefore, this cannot be perceived as a competitive advantage from an ecological perspective. On the other hand, the ability of more than 90% of the strains assigned to this species to ferment all carbohydrates used for taxonomic purposes, with the exception of arabinose and xylose, which are fermented by 11–89% of the strains [106], can be regarded as a competitive advantage, since not many species are characterized by such a large metabolic capacity. Therefore, the hydrolysis of non-digestible carbohydrates by endogenous or microbial enzymes may provide additional fermentable carbohydrates. Indeed, the capacity of Lp. plantarum strains to produce hydrolytic enzymes such as glycosidases, esterases, and phytases has been adequately exhibited [107]. Regarding the proteolytic and lipolytic capacity of strains isolated from vegetable fermentations, they have not been adequately studied due to their limited significance to the quality of the product, at least compared to the strains isolated from dairy or sourdough fermentation. Taking into consideration the results obtained from the latter fermentations, their production seems to be a strain-dependent characteristic, and several strains have been reported to possess such traits [107,108,109,110,111]. Regarding the capacity of Lp. plantarum to adapt to the acidic conditions of fermenting material, this has been adequately exhibited.
The metabolic capacity of Lp. plantarum isolates has been traditionally assessed through phenotypic approaches. The most common approach included the incorporation of the substance the hydrolysis of which was assessed, followed by the qualitative or quantitative estimation of its decomposition, the latter mostly by spectrophotometric approaches. The development of the NGS-based techniques, accompanied by suitable bioinformatic analysis, allowed the detection of the genes encoding the respective enzymes. Indeed, the application of NGS-based approaches allowed the whole-genome sequencing of a large number of strains. It was revealed that the size of the genome of Lp. plantarum strains may range from 2.94 to 3.90 Mb, with an average of 3.32 Mb, one of the largest genomes among LAB [112,113,114]. The GC% content may range within 44.1–45.2, with an average of 44.5 [42,112,113,114].
Plasmids may also be present, with the average non-chromosomal genome size calculated at 119 kb [114]. McLeod et al. [115] estimated that only about 25% of the strains are plasmid free. A comparative genomic analysis of 105 plasmid-harboring Lp. plantarum strains revealed the existence of a total of 395 plasmids, with a size ranging from 0.83 to 208.3 kb [116]. Interestingly, the absence of conserved coding sequences (CDSs) across these plasmids was evident, which may contribute to the large diversity of strain-specific phenotypes of this species that has been reported [116]. The majority of the plasmid genes (57.6%) were not detected in the chromosomal DNA; the most abundant encoded protein families included exopolysaccharide biosynthesis, biofilm formation, stress tolerance, and the carbohydrate metabolism, suggesting their contribution to persistence in demanding microhabitats [116,117]. Plasmids have also been reported to accommodate technologically important functions, such as bacteriophage resistance, plantaricin production, and lactose utilization [118].
The number of genes in each chromosome may range from 2763 to 3650 [112,114]. Interestingly, Carpi et al. [114] analyzed the genome of 127 strains and reported the occurrence of a total of 16,911 genes, of which 1436 were detected in 125–127 strains, and therefore characterized as core genes, 414 genes were detected in 120–125 strains, and characterized as soft core genes, 1,858 genes were detected in 19–120 strains, and characterized as shell genes, and 13,203 genes were detected in 1–19 strains, and characterized as cloud genes. Similarly, Li et al. [113] performed a comparative genomic analysis of 455 Lp. plantarum isolates and raised the number of the genes comprising the pan-genome of this species to 57,132. The term ‘pan-genome’ is used to describe the whole set of genes that have been detected in all strains within a particular clade or species. In the case of Lp. plantarum, the pan-genome has been characterized as ‘open’ [114] due to the large number of cloud genes, which is expected to increase further in the future, taking into consideration the abundance of Lp. plantarum in spontaneous fermentations and the widespread application of NGS-based approaches. Li et al. [113] reported that the numbers of genes of core100, core99, and core95 were 661, 1457, and 1692, respectively. Regarding their functional categories, the majority of the genes were assigned to ‘translation, ribosomal structure and biogenesis’, ‘amino acid transport and metabolism’, ‘transcription’, ‘carbohydrate transport and metabolism’, ‘replication, recombination and repair’, ‘nucleotide transport and metabolism’, and ‘inorganic ion transport and metabolism’ [113]. The Lp. plantarum genome may also contain bacteriophages as well as CRISPR-Cas (clustered regularly interspaced short palindromic repeats–CRISPR-associated protein) elements. In the first case, Carpi et al. [114] estimated the size of bacteriophages to be between 35 and 300 kb, which corresponds to 1-8% of the total genome size. Regarding the CRISPR-Cas elements, although their presence is relatively common in bacteria [119], they seem to be rather uncommon in Lp. plantarum [120]. The variability among the strains has also been adequately exhibited and refers to the type of the system as well as the existence and type of cas genes [114,121,122].
This open pan-genome was also verified by Heo et al. [123], and may justify the dominance of this species during the fermentation of several substrates of plant and animal origin. This open pan-genome regime was early understood, and several studies assessed the effect of ecological niche in shaping the genome of the Lp. plantarum strains. Molenaar et al. [124] attempted to address this question by genotyping 20 Lp. plantarum strains of various sources using microarrays consisting of small genomic fragments of the Lp. plantarum strain WCFS1. A region located between 2.70 and 3.29 Mb of the chromosome of strain WCFS1 was characterized by high genotypic variation. More specifically, the subregions 2.10–2.85 Mb and 3.10–3.29 Mb, which accounted for approximately 10% of the total chromosome, were characterized by high degree of plasticity and included 293 genes, the majority of which belonged to the categories ‘energy metabolism, sugars’ and ‘transport and binding proteins; PTS (phosphotransferase) systems’, indicating a lifestyle-associated adaptation. In addition, it was proposed that the closer to the origin of replication the integration of these genes takes place, the more likely a competitive advantage would be gained within a specific ecological niche. Early studies assessing comparatively the genome of Lp. plantarum strains isolated from different niches reported that the functional diversity that was recorded could not be correlated with the isolation habitat [125,126]. The first indication of an adaptation to the inhabiting niche, possibly followed by genomic evolution, was reported by Cen et al. [127]. In that study, a total of 140 genomes of isolates from human, vegetable, dairy, and Drosophila melanogaster samples were analyzed and the clustering of the majority of the D. melanogaster isolates within one clade, due to the similarities in their core genome, was reported. This habitat-specific genetic evolution was verified by subsequent studies. Indeed, Mao et al. [112] reported that the Lp. plantarum strains isolated from kimchi and fermented sauce could be distinguished on the basis of their phylogenetic analysis and average nucleotide identity. On the contrary, the fecal isolates did not form a unique cluster and were distributed throughout the phylogenetic tree, possibly reflecting their original food source. Similarly, Pan et al. [128] and Li et al. [113] also suggested a niche-specific genetic evolution. In the first case, this evolution was further specified to their fermentation abilities and the interactions with commensal microorganisms. In the study by Li et al. [113], the phylogenetic analysis of the concatenated core100, core99, and core95 genomes of 455 isolates revealed the existence of two clades, namely, A and B. A total of 102 dairy isolates, representing the 83.6% of the dairy isolates included in the study, were clustered in clade B, while a total of 34 meat product-originating isolates, representing the 92.31% of the meat product-originating isolates included in the study, were clustered in clade A. On the contrary, plant-originating isolates, which amounted to 173 isolates, were evenly distributed across the phylogenetic tree. Therefore, on the basis of the core100, core99, and core95 genomes, an association between genome and isolation niche was developed, especially in the case of dairy and meat product isolates. In the case of dairy isolates, this niche-oriented evolution was further supported by the enrichment in genes related to carbohydrate and/or amino acid transport and metabolism, and replication, recombination, and repair, which may enhance growth in a nutrient-rich substrate such as milk. On the contrary, the phylogenetic analysis of core or accessory genes of isolates from pickles, kimchi, kefir, fecal, and Japanese post-fermented tea failed to present a habitat-dependent clustering [129]. Finally, the relation between the proteosurfaceome, i.e., the proteins present on the bacterial surface, with the isolation habitat, was reported by Mazzeo et al. [130].
Cui et al. [131] assessed the carbohydrate metabolic capacity of Lp. plantarum through the analysis of the genome of 165 strains. The strain-dependent character of carbohydrate utilization was highlighted, since the 165 strains could be separated into 89 types on the basis of their carbohydrate metabolism-related genes, highlighting, in genomic terms, the large metabolic capacity of the species. In addition, the existence of more two-component systems in Lp. plantarum than in the other LAB may indicate a larger adaptation capacity, since these systems are implicated in the regulation of diverse physiological processes, including acid tolerance, bacteriocin biosynthesis, and proteolytic activities. The capacity of Lp. plantarum to adapt to acidic conditions is another key attribute that facilitates its participation and, in some cases, dominance in spontaneously fermented vegetables. This capacity can also be assessed genomically through the presence of genes belonging to the F0F1-ATPase system, namely, atpABCDEFGH, which is a key regulator of intracellular pH, the presence of the asp genes that are related to alkaline pH homeostasis and seem to improve acid tolerance [132], as well as genes encoding for sodium–proton antiporters that also seem to play a significant role in acid stress response [133]. Indeed, Jung and Lee [134] assessed the transcriptomic response of the Lp. plantarum strain wikim18 and reported that the genes associated with transport-related functions were differentially expressed, indicating the paramount importance of this function in withstanding the changing environment of fermentation.
A large number of attributes have lately been placed under the umbrella of probiotic-related properties. These include properties associated with the safety of the strain under study, as well as functions enabling the bacterial cells to withstand the harsh conditions of the human gastrointestinal tract, to colonize it, and produce metabolites that provide a benefit for the health of the consumer. An indication of the aforementioned traits can be obtained through the occurrence of associated genes. The presence of genes encoding for virulence factors, toxins, amino acid decarboxylases, and/or deiminases, as well as antibiotic resistance-associated genes, are critical safety-related attributes. In general, Lp. plantarum lacks genes encoding for virulence factors or toxins [42,43]. Issues, such as bacteremia, endocarditis, and peritonitis, that have been reported principally in immunocompromised patients are mostly associated with Lacticaseibacillus casei, La. paracasei, and La. rhamnosus [135]. On the contrary, several studies have indicated that Lp. plantarum strains can interfere with the virulence mechanisms of Listeria monocytogenes, Salmonella, E. coli, Staphylococcus aureus, Helicobacter pylori, Pseudomonas aeruginosa, Streptococcus mutans, and Str. pyogenes, and reduce their pathogenic potential [44]. The production of biogenic amines is a property that is often reported for Lp. plantarum strains [136]. On the other hand, several Lp. plantarum strains have been reported to degrade biogenic amines, thus inhibiting their accumulation [137,138,139]. Regarding antibiotic resistance, Lp. plantarum is inherently resistant to vancomycin [30,140,141], and thus the associated genes are part of its core genome. The occurrence of additional genes in the accessory genome, which confer resistance to additional antibiotics, is frequently reported [114,141,142,143].
The second set of probiotic-related attributes refers to the ability to withstand and colonize the human gastrointestinal tract (GIT). Carpi et al. [114] assigned this ability to 75 probiotic marker genes (PMGs), which were associated with stress resistance (arcB, atpA, atpB, atpC, atpD, etpE, atpF, atpG, atpH, clpB, clpE, clpP_1, clpP_2, copA, dltA, dltD, dnaJ, dnaK, eno, eno2, gadB, gap, gbuB, gla_2, glpF_1, glpF_2, gpmB, groL, groS, grpE, guaA, htrA, ldh_1, luxS, msrB, opuCB, osmV, pgi, pgk, plsC, pyk, recA, relA, tpiA, uvrA, yjbM, and ywaC), bile resistance (arcB, argS, bshA, cbh, copA, dnaJ, dnaK, dps, eno, eno2, glf, glnA, groS, grpE, htrA, luxS, nagB, oppA_1, oppA_2, oppA_3, oppA_4, pdhD_2, pepO, pgk, ponA, pyrG, rplDEF, rpsC, rpsE, and srtA), adhesion ability (exoA, lspA, pycA, and srtA), and gut persistence (celB, treA, and xylA). The assessment of the genome of 127 strains revealed that 70% of these PMGs were detected in more than 95% of the strains. However, important ones, such as bshA, which is responsible for bile tolerance, xylA, which is involved in gut persistence, and srtA, which is associated with the adhesion ability, were detected in a rather limited number of strains.
This pool of determinants can be enlarged by several additions. These may refer to the capacity of the strains to withstand and colonize the human GIT, but mostly to potential probiotic functions, which comprises the third set of probiotic-related attributes. The first category can be enriched by fbpA and gtfA, which encode for fibronectin-binding protein and glucosyltransferase, respectively [42], msa, abpT, and lamA, which encode for a cell-surface anchor protein, a cell-surface persistence protein and a mucus-binding protein, respectively [43], as well as lp_3160, which encodes for a multi-drug resistance transporter and have important role in bile tolerance [43]. The utilization of prebiotics, such as galactooligosaccharides (GOS), fructooligosaccharides (FOS), and inulin, is another important trait. The utilization of GOS with a degree of polymerization of 3–4 has been linked to the presence of a lac operon, consisting of three genes, namely, lacA, lacS, and lacR, encoding a β-galactosidase, a permease, and a divergently oriented regulator [144]. Interestingly, the utilization of FOS or inulin as carbon sources affected the transcription of genes encoding for two-component systems in a similar way [131].
Regarding the potential probiotic functions, the pool of PMGs can be enlarged by the genes encoding the biosynthesis of bioactive compounds such as GABA, riboflavin, cobalamin, folate and biotin, bacteriocins, and exopolysaccharides. GABA biosynthesis may take place either through the transamination of L-glutamine and α-ketoglutarate to L-glutamate, which is catalyzed by the glutamate synthase, an enzyme that consists of two subunits encoded by gltB and gltC, and the subsequent decarboxylation by two homologous glutamate decarboxylases, encoded by gadA and gadB. Then, GABA is transferred extracellularly through the L-glutamate/GABA antiporter, encoded by gadC. The L-glutamate transported intracellularly can be decarboxylated by GadA and/or GadB [145]. The biosynthesis of riboflavin may take place either through guanosine triphosphate or ribulose-5-phosphate through the common intermediate, the immediate riboflavin precursor 6,7-dimethyl-8-ribityllumazine. In the first case, the genes ribBA, ribD, yitU, and ribH are necessary, while, in the second, only ribBA and ribH are necessary. Then, riboflavin is produced by dismutation catalyzed by riboflavin synthase, encoded by ribE. Flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), the biologically active forms of riboflavin, are produced by the bifunctional flavokinase/FAD synthetase, which is encoded by ribF. The genes ribG, ribB, ribA, and ribE are physically gathered in the rib operon, the transcription of which is regulated by the FMN riboswitch, as well as the promoters ribP1 and ribP2 [146,147]. Cobalamin biosynthesis can be separated into three sections. The first section involves the production of precorrin 2 by aminolevulinic acid and necessitates the occurrence of the genes hemB, hemC, hemD, and cobA. Section two is separated into two pathways, an anaerobic and an aerobic one. Both lead to the production of cob(II)yrinic acid a,c-diamine, but the nomenclature uses different prefixes in order to separate them; the prefix cbi is used or the anaerobic and the prefix cob is used for the aerobic one. Thus, the anaerobic pathway relies on the coordinated transcription of cbiK, cbiL, cbiH, cbiF, cbiG, cbiD, cbiJ, cbiE, cbiT, cbiC, and cbiA, while the aerobic pathway relies on cobI, cobG, cobJ, cobM, cobF, cobK, cobL, cobH, cobB, and cobNST. The third section is common, but the differences in nomenclature persist; therefore, for the anaerobic pathway the genes involved are cobA, cbiP, cbiB, cobU, and cobS, while, for the aerobic pathway, the genes involved are cobO, cobQ, cobC, cobD, cobP, and cobV [146]. Folate biosynthesis may take place by guanosine 5-phosphate or chorismite through the common intermediate product 7,8 dihydropteroate. In the first case, the genes folE, folB, folK, and folP are implicated, while, in the latter, pabAB, pabC, and folP are implicated. Then, the production of folate necessitates the occurrence of folC and folA. The genes folE, folK, folB, and folC are physically clustered together, but not always in the same order [146]. The biotin biosynthetic pathway can be divided into the following two stages: during the first one, the pimelate moiety is generated, while, during the second one, the biotin rings are assembled [148]. The first stage can be carried out by different pathways in the bacteria; in E. coli, it is carried out by the genes bioC and bioH, along with several genes of the fatty acid biosynthetic pathway (fabB, fabF, fabG, fabZ, and fabI). On the contrary, the second stage is highly conserved and requires the genes bioA, bioD, and bioB. The occurrence of all or some of the aforementioned genes participating in the production of bioactive compounds is frequently encountered in genomic assessments of Lp. plantarum [42,115,119,149,150]. Of course, phenotypic verification of the production capacity indicated by the genomic studies should always be performed. The analysis of 56 Lp. plantarum genomes by Choi et al. [151] revealed the occurrence of 6 plantaricin-producing operons, namely, plNC8βαc, plNC-IF, plnJKLR, plnMNOP, plnABCD, and plnEFI, along with gene plnQ. In addition, the genes encoding gassericin A, lacticin Z, pediocin, pentocin KCA1, plantaricin 125 beta, plantaricin 423, plantaricin C19, and salivaricin P were also found in plasmids. Their phylogenetic analysis of the genomes revealed the existence of three lineages, A, B, and C. Only the plnEFI operon was observed in all three lineages. In lineage A, several plantaricin-encoding genes were observed, including plnA, plnQ, plnE/F, plnJ/K, and plnN. Regarding lineage B, several pln genes seemed to have lost their activity due to frameshifting, truncation, or disruption by mobile elements. The large variability in the organization of the pln locus between Lp. plantarum strains has been adequately exhibited. This locus may contain any of the aforementioned operons and genes, either functional or truncated [42,43,122,149,152,153,154]. The EPS production may confer technological benefits to the product and functions associated with the adhesion capacity of the strains [155], including health benefits, such as an antitumor capacity [156], and immunomodulatory and anti-inflammatory activities [157]. In Lp. plantarum, four different EPS biosynthetic gene clusters, namely, cps1A-I, cps2A-J, cps3A-J, and cps4A-J, have been described [158]. There is variability among the strains regarding the type of cluster present, which seems justified by their location within the genome, as they are located in genomic recombination hotspots [119]. In addition, a comparative genomic analysis of 54 Lp. plantarum strains revealed that there is significant variation in the size, composition, sequence, and order of the genes across the strains [125], which was also verified by the study of Zhao et al. [159]. Moreover, no correlation could be established between the number of the orthologous groups and the isolation niche of the strains [125].
5. Lactiplantibacillus plantarum as a Starter Culture in Vegetable Fermentation
Lactiplantibacillus plantarum has been extensively used as a starter culture, especially in the case of fermented vegetables. In Table 2, representative studies assessing the capacity of various Lp. plantarum strains to serve as a starter culture, drive the lactic acid fermentation of vegetables, and improve the quality of the final product are presented. The brine utilized ranged from 0.5% to 10% NaCl, with 1-4% being the most frequently encountered. In general, the lower the NaCl content, the more complex the developing microecosystem, and, in the majority of the cases, the more complex the organoleptic profile of the final product. The addition of fermentable carbohydrates in the beginning of the fermentation, which has been reported in some cases, may enhance the development of the starter culture along with non-starter microorganisms, depending on the microbiological quality of the raw materials. Increased enterobacterial or yeasts initial populations could create an antagonistic environment to the starter culture and could lead to the production of off-flavors or even divert fermentation.
The fate of the starter culture during fermentation was assessed in only a couple of studies [160,161]. In both cases, the dominance of the starter culture was verified through a suitable approach, namely, the (GTG)5-PCR fingerprinting of the isolates. In general, the acceleration of the fermentation by the addition of starter culture was observed compared to the spontaneously fermented counterparts used as the control. Fermentations were performed at temperatures ranging from 10 to 37 °C for a duration of 2.5 to 30 days, highlighting the capacity of the strains to drive vegetable fermentation at a wide range of temperatures. In general, the lower the temperature the slower the fermentation. The final pH and total titrable acidity (TTA) values ranged, in the majority of the cases, between 3.0 and 3.5, and between 0.065% and 0.62% lactic acid, respectively. Notable exceptions were the pH value of 4, reported by Hang et al. [162], regarding fermented Stachys sieboldii Miq., and the TTA value of 31.78 g/L, reported by Zhao et al. [163], regarding Dongbei Suan-Cai. A very interesting result that was reported by many studies was the reduction in the nitrite concentration during fermentation. Vegetables contain nitrates and nitrites, the concentration of which may range depending on the type of vegetable, harvest season, and agricultural practices [164]. These are most likely extracted through the action of the brine, and the nitrates are converted to nitrites through the action of nitrate reductases, which may be produced by several bacteria, including enterobacteria [165]. Thus, when acidity fails to develop quickly, the enterobacteria population is not inhibited and nitrite is produced, resulting in a peak reported by many studies [166,167,168]. Then, as LAB dominate fermentation and the pH value is reduced below 4.5, a decrease in the nitrite concentration is observed. The latter can be attributed to the inhibition of enterobacteria growth due to the developing acidity, the chemical decomposition of nitrites that occurs at pH values below 4.5 [169], the action of nitrite reductases of the LAB, which takes place at pH values above 4.5 [169], and the highly reactive nature of nitrites themselves [170].

Table 2.
Representative studies assessing the capacity of Lp. plantarum strains as starter culture in vegetable fermentation.
Table 2.
Representative studies assessing the capacity of Lp. plantarum strains as starter culture in vegetable fermentation.
| Product | Strain(s) | Remarks Regarding Fermentation | References |
|---|---|---|---|
| Fermented cauliflower and fermented vegetable mixture consisting of carrot, cauliflower, and green tomato | Lp. plantarum IMDO 788 | Fermentation took place in brine consisting of 3.5% NaCl and 2.0% sucrose at 25 °C for three days, and then at 16 °C for 5 weeks. The inoculum level was 6 log CFU/g. The final pH values were 3.35 and 3.23 for fermented cauliflower and mixed vegetables, respectively. The starter culture prevailed during the fermentation. The addition of the starter culture accelerated the fermentation. | [162] |
| Fermented caper berries | Lp. plantarum Lb9 | Fermentation took place in 10% brine (final concentration) at ambient temperature for six days. The inoculum level was 7 log CFU/g. The final pH value was 3.51. The starter culture dominated during fermentation. The addition of the starter culture accelerated the fermentation, resulted in a more homogeneous microecosystem composition, and a similar sensorial quality compared to spontaneous caper berries fermentation. | [171] |
| Fermented Raphanus sativus roots | Lp. plantarum LQC 740 | Fermentation took place in 5% brine at 20 and 30 °C. The inoculum level was 7.5 log CFU/mL. The fermentation was completed after 14 and 10 days, reaching final pH values of 3.40 and 3.37, and final TTA values of 0.62% and 0.41% lactic acid, at 20 and 30 °C, respectively. The starter culture prevailed during the fermentation. The plantaricin activity (in AU/mL) was reduced compared to the respective in MRS broth. However, this reduction was not accompanied by the downregulation of the 5 pln genes that were detected in the genome of this strain. | [161] |
| Chinese northeast sauerkraut | Ln. mesenteroides HBUAS 51041, Lp. plantarum ORC 2 | Fermentation took place in 0.5, 1.5, 2.5, and 3.5% brine at 18–20 °C for 30 days, with an inoculum level of 6 log CFU/g, with the LAB combined in a mixed culture at a 1:1 ratio. The final pH was approximately 3.2 in all cases except for the 0.5% brine, in which it was approximately 3.3. The acidity ranged between approximately 0.065 and 0.075% lactic acid in all cases, with the exception of the 0.5% brine, in which it was approximately 0.085%. Fermentation in 0.5% brine accelerated the maturation and improved the sensory quality of the product. A reduction in the nitrite content was reported. | [172] |
| Chinese northeast sauerkraut | Ln. mesenteroides, Lp. plantarum, La. paracasei, W. cibaria | Fermentation took place in 1% brine at 18–20 °C for 30 days, with an inoculum level of 6 log CFU/g, with the LAB used as monocultures. The final pH and TTA values ranged between 3.4–3.6 and 0.0930–0.1160% lactic acid, with the Lp. plantarum strain achieving the lowest pH and the highest TTA values. The sensorial quality of the final product depended upon the starter used, as the greatest abundance of esters was reported for the product made by Lp. plantarum, the greatest lactone content was reported for the product made by La. paracasei, while the Ln. mesenteroides and W. cibaria strains presented increased the acid and ketone contents. | [173] |
| Fermented cucumbers | Lp. plantarum NPL 1258, P. pentosaceus NPL 1264 | Fermentation took place in 4% brine at ambient temperature for 3 weeks, with an inoculum level of 7 log CFU/mL, with the LAB used as monocultures or combined as mixed cultures. The final pH value ranged between 3.0 and 3.5, with the mixed culture resulting in the lowest pH value. The highest TTA value of approximately 2.0 g lactic acid/100 mL was achieved by the P. pentosaceus monoculture. The application of the starter cultures accelerated the fermentation and reduced the population of pathogenic microorganisms. | [174] |
| Fermented African nightshade (Solanum scabrum) leaves | Lp. plantarum BFE 5092, Lm. fermentum BFE 6620 | Fermentation took place in 3% NaCl and 3.0% sugar brine at ambient temperature (approximately 25 °C) for 144 h, with an inoculum level of 6–7 log CFU/mL, with the LAB used as mixed cultures in a 1:1 ratio. The pH value decreased to 3.6 within 24 h. The TTA increased to 3–4 g lactic acid/L. The starter cultures used seemed to dominate throughout the fermentation. | [175] |
| Suan-Cai | Lb. plantarum, Lb. brevis, Ln. mesenteroides | Fermentation took place at 15 °C for 30 days, with an inoculum level of 7 log CFU/mL, using each LAB as monoculture. The final pH and TTA values ranged between 3.0–3.5 and 3.0–3.7 g lactic acid/L, with the Lp. plantarum strain achieving the lowest pH value but the lowest TTA. Lp. plantarum was reported as the dominant species after all of the fermentations. The concentration of volatile compound was higher in the products made by the starter cultures compared to the spontaneously fermented one. | [176] |
| Fermented Stachys sieboldii Miq. | Lp. plantarum ZJ316 | Fermentation took place in 0.96% NaCl and 0.46% sugar brine at 37 °C for 7 days, with an inoculum level of 2.4 108 CFU/mL. The final pH value was approximately 4.0. A reduction in the nitrite concentration was reported. | [162] |
| Paocai | Ln. mesenteroides CPTCC 1R3, W. cibaria CPTCC 1R15, Lv. brevis CPTCC 3R8, Lp. plantarum CPTCC 5R10 | Fermentation took place in 4.5% NaCl at 20 °C for 5 days, with an inoculum level of 6 log CFU/mL, either as monocultures or as a mixed culture at a ratio of 1:1:1:1. The final pH and TTA values ranged from approximately 3.0 to 3.7 and 0.25 to 0.5 g lactic acid/100 g, with the Lp. plantarum strain achieving the lowest pH and the highest TTA values. It was reported that paocai made by the mixed culture presented more advantages than the one made by the single cultures, and Ln. mesenteroides and Lp. plantarum were designated as the core microorganisms related to the flavor formation of paocai. | [177] |
| Fermented mustard leaves | Lp. plantarum ZJ316 | Fermentation took place in 8% brine at room temperature for 29 days with an inoculum level of 1.5 106 CFU/mL. The final pH value was below 4.0. A reduction in the nitrite concentration was reported. The supernatant of the fermented mustard leaves exhibited significant antibacterial activity against Staphylococcus aureus D48, Escherichia coli DH5α, and Listeria monocytogenes LM1 | [166] |
| Pickled Suan-Cai | Lp. plantarum strains 8, 11, 32, and 45 | Fermentation took place with a mustard/salt ratio of 8:1 (w/w) at ambient temperature (10 ± 2 °C–18 ± 2 °C) for 2 months, with a 0.5% (v/v) inoculum level, using each strain as a monoculture. The final TTA values ranged between 0.28 and 0.41 g lactic acid/100 g. The volatile compounds detected after fermentation differed by 11.42–32.35%. | [178] |
| Pickled radish roots | Lp. plantarum MC14, Ln. mesenteroides GDMCC 1.774 | Fermentation took place in 3% (m/v) brine at 28 °C for 60 h, with an inoculum level of 2.0 106 CFU/mL, using the strains as a mixed starter at a 1:1 ratio. The final pH and TTA values were 3.01 and above 0.3 g lactic acid/100 g, respectively. The starter culture enhanced both texture and flavor of the final product. | [167] |
| Dongbei Suan-Cai | Lp. plantarum DP189, and Ln. mesenteroides subsp. mesenteroides UA107 | Fermentation took place with the addition of 1% salt (on the fresh weight of cabbage) at 15 °C for 30 days, with an inoculum level of 5.0 106 CFU/g, using the strains as a mixed starter at a 1:1 ratio. The final pH value was 3.79 and the final TTA 31.78 g lactic acid/L. Compared to spontaneously fermented Dongbei Suan-Cai, the utilization of the starter culture resulted in more rapid acid production, the better utilization of soluble protein and reducing sugar, increased levels of umami amino acids, and an increased amount of volatile flavor substances by 12.43%. | [163] |
| Radish paocai | Lp. plantarum, La. rhamnosus, P. acidilactici, Lv. brevis, Le. buchneri, W. paramesenteroides | Fermentation took place in 4% (m/v) brine at 25 °C for 7 days, with an inoculum level of 107 CFU/mL, using each LAB as a monoculture. The lowest pH (3.56 and 3.54) and the highest TTA (8.00 and 8.92 mg lactic acid/mL) values were obtained by Lp. plantarum and La. rhamnosus, respectively. The OPLS-DA analysis, based on 31 quality indicators and fermentation performance ranking by the TOPSIS method, indicated that the highest scores were achieved by the Lp. plantarum and La. rhamnosus strains. | [178] |
| Sichuan radish paocai | Lp. plantarum LB6, Lp. pentosus LB3, W. cibaria W51 | Fermentation took place in 3% brine (after equilibration) at 25 °C for 7 days, with an inoculum level of 106 CFU/mL, either with each LAB as a monoculture or combined at a 1:1:1 ratio. In all cases, the final pH value was approximately 3.25 and the TTA increased to 3.59–3.89 g lactic acid/L. By using these starter cultures, acceleration of the fermentation was achieved, along with a reduction in the nitrite content and the abundance of opportunistic pathogens. | [168] |
OPLS-DA: orthogonal partial least squares discriminant analysis; TOPSIS: technique for order preference by similarity to ideal solution; TTA: total titrable acidity; La.: Lacticaseibacillus; Le.: Lentilactobacillus; Lm.: Limosilactobacillus; Ln.: Leuconostoc; Lp.: Lactiplantibacillus; Lv.: Levilactobacillus; P.: Pediococcus; W.: Weisella.
On the basis of the aims of each study, a wide range of interesting results were also reported, including the organoleptic evaluation of the final product [167,171,172,173,177,178], the transcription of the pln genes encoding for plantaricins [161], the concentration of the volatile compounds [163,176,178], the antimicrobial capacity of the brine [166,168], and the enhancement of the nutritional value of the final product, through the utilization of probiotic strains [174].
6. Future Perspectives
Lactiplantibacillus plantarum is indeed a multifunctional tool of vegetable fermentations, as it can direct the fermentation and increase the nutritional value of the final product through the production of bioactive compounds or its function as a probiotic. However, there are still aspects of its subsistence in vegetable fermentation that require further assessment. Apart from elucidating the physiological responses of this species triggered by biotic and abiotic stimuli during fermentation, the following two issues are of particular interest from both microbiological and technological perspectives. The first one refers to its inability to dominate vegetable fermentations as a whole, despite its ubiquitous nature in the specific microenvironment and despite the advantages that its genetic background provides. This issue should be addressed in a case-by-case manner in order to be adequately explained. The second issue refers to the under-exploitation of its large metabolic capacity. Indeed, the production of fermented vegetables nutritionally enhanced through the utilization of suitable Lp. plantarum strains is lacking, despite the fact that several strains have been reported that are capable of producing bioactive compounds, such as GABA or vitamins.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozès, N. Lactic acid bacteria from fermented table olives. Food Microbiol. 2012, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díaz, M.; Hayes, J.; Medina, E.; Anekella, K.; Daughtry, K.; Dieck, S.; Levi, M.; Price, R.; Butz, N.; Lu, Z.; et al. Reassessment of the succession of lactic acid bacteria in commercial cucumber fermentations and physiological and genomic features associated with their dominance. Food Microbiol. 2017, 63, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Beganovic, J.; Kos, B.; Lebos Pavunc, A.; Uroic, K.; Jokic, M.; Suskovi, J. Traditionally produced sauerkraut as source of autochthonous functional starter cultures. Microbiol. Res. 2014, 169, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Wouters, D.; Bernaert, N.; Conjaerts, W.; Van Droogenbroeck, B.; De Loose, M.; De Vuyst, L. Species diversity, community dynamics, and metabolite kinetics of spontaneous leek fermentations. Food Microbiol. 2013, 33, 185–196. [Google Scholar] [CrossRef]
- Pardali, E.; Paramithiotis, S.; Papadelli, M.; Mataragas, M.; Drosinos, E.H. Lactic acid bacteria population dynamics during spontaneous fermentation of radish (Raphanus sativus L.) roots in brine. World J. Microbiol. Biotechnol. 2017, 33, 110. [Google Scholar] [CrossRef] [PubMed]
- Aquilanti, L.; Santarelli, S.; Silvestri, G.; Osimani, A.; Petruzzelli, A.; Clementi, F. The microbial ecology of a typical Italian salami during its natural fermentation. Int. J. Food Microbiol. 2007, 120, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.; Cocconcelli, P.S.; Vignolo, G. Monitoring the bacterial population dynamics during fermentation of artisanal Argentinean sausages. Int. J. Food Microbiol. 2005, 103, 131–142. [Google Scholar] [CrossRef]
- Drosinos, E.H.; Paramithiotis, S.; Kolovos, G.; Tsikouras, I.; Metaxopoulos, I. Phenotypic and technological diversity of lactic acid bacteria and staphylococci isolated from traditionally fermented sausages in southern Greece. Food Microbiol. 2007, 24, 260–270. [Google Scholar] [CrossRef]
- Rebecchi, A.; Crivori, S.; Sarra, P.G.; Cocconcelli, P.S. Physiological and molecular techniques for the study of bacterial community development in sausage fermentation. J. Appl. Microbiol. 1998, 84, 1043–1049. [Google Scholar] [CrossRef]
- Pisacane, V.; Callegari, M.L.; Puglisi, E.; Dallolio, G.; Rebecchi, A. Microbial analyses of traditional Italian salami reveal microorganisms transfer from the natural casing to the meat matrix. Int. J. Food Microbiol. 2015, 207, 57–65. [Google Scholar] [CrossRef]
- Garcia Fontan, M.C.; Lorenzo, J.M.; Martinez, S.; Franco, I.; Carballo, J. Microbiological characteristics of Botillo, a Spanish traditional pork sausage. LWT-Food Sci. Technol. 2007, 40, 610–622. [Google Scholar] [CrossRef]
- Garcia Fontan, M.C.; Lorenzo, J.M.; Parada, A.; Franco, I.; Carballo, J. Microbiological characteristics of ‘androlla’, a Spanish traditional pork sausage. Food Microbiol. 2007, 24, 52–58. [Google Scholar] [CrossRef]
- Prado, N.; Sampayo, M.; González, P.; Lombó, F.; Díaz, J. Physicochemical, sensory and microbiological characterisation of Asturian Chorizo, a traditional fermented sausage manufactured in northern Spain. Meat Sci. 2019, 156, 118–124. [Google Scholar] [CrossRef]
- Mbawala, A.; Mahbou, P.Y.; Mouafo, H.T.; Tatsadjieu, L.N. Antibacterial activity of some lactic acid bacteria isolated from a local fermented milk product (pendidam) in ngaoundere, Cameroon. J. Anim. Plant Sci. 2013, 23, 157–166. [Google Scholar]
- Ohenhen, R.E.; Imarenezor, E.P.K.; Kihuha, A.N. Microbiome of madila—A southern African fermented milk product. Int. J. Basic Appl. Sci. 2013, 2, 170–175. [Google Scholar] [CrossRef]
- Wullschleger, S.; Lacroix, C.; Bonfoh, B.; Sissoko-Thiam, A.; Hugenschmidt, S.; Romanens, E.; Baumgartner, S.; Traore, I.; Yaffee, M.; Jans, C.; et al. Analysis of lactic acid bacteria communities and their seasonal variations in a spontaneously fermented dairy product (Malian fene) by applying a cultivation/genotype based binary model. Int. Dairy J. 2013, 29, 28–35. [Google Scholar] [CrossRef]
- Mo, L.; Jin, H.; Pan, L.; Hou, Q.; Li, C.; Darima, I.; Zhang, H.; Yu, J. Biodiversity of lactic acid bacteria isolated from fermented milk products in Xinjiang, China. Food Biotechnol. 2019, 33, 174–192. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Zheng, X.; Ge, Z.; Lin, K.; Zhang, D.; Chen, Y.; Wang, B.; Shi, X. Investigation of the lactic acid bacteria in Kazak cheese and their contributions to cheese fermentation. Front. Microbiol. 2020, 11, 228. [Google Scholar] [CrossRef]
- Bozoudi, D.; Torriani, S.; Zdragas, A.; Litopoulou-Tzanetaki, E. Assessment of microbial diversity of the dominant microbiota in fresh and mature PDO Feta cheese made at three mountainous areas of Greece. LWT-Food Sci. Technol. 2016, 72, 525–533. [Google Scholar] [CrossRef]
- Tsigkrimani, M.; Bakogianni, M.; Paramithiotis, S.; Bosnea, L.; Pappa, E.; Drosinos, E.H.; Skandamis, P.Ν.; Mataragas, M. Microbial ecology of artisanal Feta and Kefalograviera cheeses. Part I: Bacterial community and its functional characteristics with focus on lactic acid bacteria as determined by culture-dependent methods and phenotype microarrays. Microorganisms 2022, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Nionelli, L.; Curri, N.; Curiel, J.A.; Di Cagno, R.; Pontonio, E.; Cavoski, I.; Gobbetti, M.; Rizzello, C.G. Exploitation of Albanian wheat cultivars: Characterisation of the flours and lactic acid bacteria microbiota, and selection of starters for sourdough fermentation. Food Microbiol. 2014, 44, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, S.; Tsiasiotou, S.; Drosinos, E.H. Comparative study of spontaneously fermented sourdoughs originating from two regions of Greece: Peloponnesus and Thessaly. Eur. Food Res. Technol. 2010, 231, 883–890. [Google Scholar] [CrossRef]
- Boreczek, J.; Litwinek, D.; Żylińska-Urban, J.; Izak, D.; Buksa, K.; Gawor, J.; Gromadka, R.; Bardowski, J.K.; Kowalczyk, M. Bacterial community dynamics in spontaneous sourdoughs made from wheat, spelt, and rye wholemeal flour. MicrobiologyOpen 2020, 9, e1009. [Google Scholar] [CrossRef] [PubMed]
- Syrokou, M.K.; Themeli, C.; Paramithiotis, S.; Mataragas, M.; Bosnea, L.; Argyri, A.; Chorianopoulos, N.G.; Skandamis, P.N.; Drosinos, E.H. Microbial ecology of Greek wheat sourdoughs identified by culture-dependent and culture-independent approach. Foods 2020, 9, 1603. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef]
- Paramithiotis, S. Microorganisms associated with food fermentation. In Bioactive Compounds in Fermented Foods: Health Aspects; Rai, A.K., Anu Appaiah, K.A., Eds.; CRC Press: Cleveland, OH, USA, 2021; pp. 3–47. [Google Scholar]
- Du, G.; Zhang, G.; Shi, J.; Zhang, J.; Ma, Z.; Liu, X.; Yuan, C.; Li, X.; Zhang, B. Keystone taxa Lactiplantibacillus and Lacticaseibacillus directly improve the ensiling performance and microflora profile in co-ensiling cabbage byproduct and rice straw. Microorganisms 2021, 9, 1099. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela Saldinger, S. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biotechnol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Tišma, M.; He, B.; Zhai, X.; Yuan, C.; Su, Z.; Shi, J.; Zhang, B. Valorization of the Caragana waste via two-stage bioaugmentation: Optimizing nutrition composition, palatability, and microbial contaminant control. J. Bioresour. Bioprod. 2024, 9, 518–533. [Google Scholar]
- Iosca, G.; Turetta, M.; De Vero, L.; Bang-Berthelsen, C.H.; Gullo, M.; Pulvirenti, A. Valorization of wheat bread waste and cheese whey through cultivation of lactic acid bacteria for bio-preservation of bakery products. LWT-Food Sci. Technol. 2023, 176, 114524. [Google Scholar] [CrossRef]
- Dopazo, V.; Navarré, A.; Calpe, J.; Riolo, M.; Moreno, A.; Meca, G.; Luz, C. Revalorization of beer brewing waste as an antifungal ingredient for bread biopreservation. Food Biosci. 2024, 58, 103588. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Papadaki, A.; Lappa, I.; Papastergiou, S.; Kleisiari, D.; Kopsahelis, N. Biosurfactant production from Lactobacilli: An insight on the interpretation of prevailing assessment methods. Appl. Biochem. Biotechnol. 2022, 194, 882–900. [Google Scholar] [CrossRef]
- Deba-Rementeria, S.; Paz, A.; Estrada, O.; Vázquez-Araújo, L. Consumer perception and physicochemical characterization of a new product made from lactic acid fermented orange peels. Int. J. Gastron. Food Sci. 2023, 31, 100647. [Google Scholar] [CrossRef]
- Chiarini, E.; Alessandria, V.; Buzzanca, D.; Giordano, M.; Seif Zadeh, N.; Mancuso, F.; Zeppa, G. Valorization of fruit by-products through lactic acid fermentation for innovative beverage formulation: Microbiological and physiochemical effects. Foods 2024, 13, 3715. [Google Scholar] [CrossRef]
- Montemurro, M.; Casertano, M.; Vilas-Franquesa, A.; Rizzello, C.G.; Fogliano, V. Exploitation of spent coffee ground (SCG) as a source of functional compounds and growth substrate for probiotic lactic acid bacteria. LWT-Food Sci. Technol. 2024, 198, 115974. [Google Scholar] [CrossRef]
- Moore, J.F.; Johanningsmeier, S.D.; Pérez-Díaz, I.M. Enhancement of γ-aminobutyric acid in fermented cucumbers. J. Food Sci. 2024, 89, 9678–9691. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Amrutha, R.; Ahire, J.J.; Taneja, N.K. Techno-functional assessment of riboflavin-enriched yogurt-based fermented milk prepared by supplementing riboflavin-producing probiotic strains of Lactiplantibacillus plantarum. Probiotics Antimicrob. Proteins 2024, 16, 152–162. [Google Scholar] [CrossRef]
- Ashagrie, H.; Baye, K.; Guibert, B.; Rochette, I.; Tisseyre, P.; Humblot, C. The use of propionic and lactic acid bacteria to produce cobalamin and folate in injera, an Ethiopian cereal-based fermented food. Int. J. Food Microbiol. 2025, 426, 110909. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, G.; Chen, L.; Dai, C.; Yao, C.; Zhang, M.; Dong, X. ACE inhibitory and antioxidant peptides from alcalase-assisted Lactiplantibacillus plantarum L60 and Lacticaseibacillus rhamnosus LR22 fermentation of goat milk: Optimization and identification. J. Food Process. Preserv. 2022, 46, e16514. [Google Scholar] [CrossRef]
- Sandez Penidez, S.H.; De Moreno De Le Blanc, A.; Gerez, C.L.; Rollán, G.C. Quinoa snack elaborated with Lactiplantibacillus plantarum CRL 1964 sourdough increases the mineral bioavailability in mice. J. Sci. Food Agric. 2025, 105, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernández, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic amines degradation by Lactobacillus plantarum: Toward a potential application in wine. Front. Microbiol. 2012, 3, 122. [Google Scholar] [CrossRef]
- Karaseva, O.; Ozhegov, G.; Khusnutdinova, D.; Siniagina, M.; Anisimova, E.; Akhatova, F.; Fakhrullin, R.; Yarullina, D. Whole genome sequencing of the novel probiotic strain Lactiplantibacillus plantarum FCa3L. Microorganisms 2023, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Isaac, S.L.; Malek, A.Z.A.; Hazif, N.S.; Roslan, F.S.; Hashim, A.M.; Song, A.A.-L.; Rahim, R.A.; Ismah, W.A.K.W.N. Genome mining of Lactiplantibacillus plantarum PA21: Insights into its antimicrobial potential. BMC Genom. 2024, 25, 571. [Google Scholar] [CrossRef] [PubMed]
- Colautti, A.; Orecchia, E.; Comi, G.; Iacumin, L. Lactobacilli, a weapon to counteract pathogens through the inhibition of their virulence factors. J. Bacteriol. 2022, 204, e00272-22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Niu, H.; Qu, Q.; Guo, D.; Wan, X.; Yang, Q.; Mo, Z.; Tan, S.; Xiang, Q.; Tian, X.; et al. Advancements in Lactiplantibacillus plantarum: Probiotic characteristics, gene editing technologies and applications. Crit. Rev. Food Sci. Nutr. 2025, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Domínguez, R.; Munekata, P.E.S.; Nieto, G.; Bangar, S.P.; Dhama, K.; Lorenzo, J.M. Bioactive compounds from leaf vegetables as preservatives. Foods 2023, 12, 637. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Das, G.; Shin, H.-S.; Patra, J.K. Fate of bioactive compounds during lactic acid fermentation of fruits and vegetables. Foods 2022, 11, 733. [Google Scholar] [CrossRef]
- Naseem, A.; Akhtar, S.; Ismail, T.; Qamar, M.; Sattar, D.-E.-S.; Saeed, W.; Esatbeyoglu, T.; Bartkiene, E.; Rocha, J.M. Effect of growth stages and lactic acid fermentation on anti-nutrients and nutritional attributes of spinach (Spinacia oleracea). Microorganisms 2023, 11, 2343. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Li, T.T.; Liu, D.D.; Fu, M.L.; Gu, C.T. Proposal of Lactobacillus kosoi Chiou et al. 2018 as a later heterotypic synonym of Lactobacillus micheneri McFrederick et al. 2018, elevation of Lactobacillus plantarum subsp. argentoratensis to the species level as Lactobacillus argentoratensis sp. nov., and Lactobacillus zhaodongensis sp. nov., isolated from traditional Chinese pickle and the intestinal tract of a honey bee (Apis mellifera). Int. J. Syst. Evol. Microbiol. 2020, 70, 3123–3133. [Google Scholar] [PubMed]
- Heng, Y.C.; Silvaraju, S.; Lee, J.K.Y.; Kittelmann, S. Lactiplantibacillus brownii sp. nov., a novel psychrotolerant species isolated from sauerkraut. Int. J. Syst. Evol. Microbiol. 2023, 73, 006194. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Gu, C.T. Lactiplantibacillus paraxiangfangensis sp. nov., isolated from traditional Chinese pickle. Int. J. Syst. Evol. Microbiol. 2024, 74, 006278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef] [PubMed]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Environ. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.-M.; Kim, H.-B.; Kim, H.-Y. Novel specific peaks for differentiating the Lactobacillus plantarum group using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Microbiol. Meth. 2020, 178, 106064. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, W.F.; McCance, M.E. Laboratory Methods in Food and Dairy Microbiology; Academic Press: London, UK, 1976; pp. 47–49. [Google Scholar]
- Paramithiotis, S.; Doulgeraki, A.I.; Karahasani, A.; Drosinos, E.H. Microbial population dynamics during spontaneous fermentation of Asparagus officinalis L. young sprouts. Eur. Food Res. Technol. 2014, 239, 297–304. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Kouretas, K.; Drosinos, E.H. Effect of ripening stage on the development of the microbial community during spontaneous fermentation of green tomatoes. J. Sci. Food Agric. 2014, 94, 1600–1606. [Google Scholar] [CrossRef]
- Garofalo, C.; Bancalari, E.; Milanovic, V.; Cardinali, F.; Osimani, A.; Sardaro, M.L.S.; Bottari, B.; Bernini, V.; Aquilanti, L.; Clementi, F. Study of the bacterial diversity of foods: PCR-DGGE versus LHPCR. Int. J. Food Microbiol. 2017, 242, 24–36. [Google Scholar] [CrossRef]
- Scheirlinck, I.; Van der Meulen, R.; Van Schoor, A.; Vancanneyt, M.; De Vuyst, L.; Vandamme, P.; Huys, G. Taxonomic structure and stability of the bacterial community in Belgian sourdough ecosystems as assessed by culture and population fingerprinting. Appl. Environ. Microbiol. 2008, 74, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zhang, L.; Han, X.; Du, M.; Zhang, Y.; Li, J.; Sun, K.; Hou, Y. Isolation and applied potential of lactic acid bacteria from Chinese traditional fermented food in specific ecological localities. Food Sci. Biotechnol. 2011, 20, 1685–1690. [Google Scholar] [CrossRef]
- Cheng, L.; Luo, J.; Li, P.; Yu, H.; Huang, J.; Luo, L. Microbial diversity and flavor formation in onion fermentation. Food Funct. 2014, 5, 2338. [Google Scholar] [CrossRef] [PubMed]
- Phan, Y.T.N.; Tang, M.T.; Tran, T.T.M.; Nguyen, V.H.; Nguyen, T.H.; Tsuruta, T.; Nishino, N. Diversity of lactic acid bacteria in vegetable-based and meat-based fermented foods produced in the central region of Vietnam. AIMS Microbiol. 2017, 3, 61–70. [Google Scholar] [CrossRef]
- Dolci, P.; Zenato, S.; Pramotton, R.; Barmaz, A.; Alessandria, V.; Rantsiou, K.; Cocolin, L. Cheese surface microbiota complexity: RT-PCR-DGGE, a tool for a detailed picture? Int. J. Food Microbiol. 2013, 162, 8–12. [Google Scholar] [CrossRef]
- Iacumin, L.; Cecchini, F.; Manzano, M.; Osualdini, M.; Boscolo, D.; Orlic, S.; Comi, G. Description of the microflora of sourdoughs by culture dependent and culture-independent methods. Food Microbiol. 2009, 26, 128–135. [Google Scholar] [CrossRef]
- Lai, H.; Yan, L.; Wang, Y.; Mei, Y.; Huang, Y.; Zeng, X.; Ge, L.; Zhao, J.; Zhu, Y.; Huang, Q.; et al. Effects of substrates and suppliers of ingredients on microbial community and metabolites of traditional non-salt Suancai. Microbiome Res. Rep. 2024, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, Y.; Li, T.; Yang, Y.; Zeng, F.; Wang, H.; Suo, H.; Song, J.; Zhang, Y. Microbial composition and correlation between microbiota and quality-related physiochemical characteristics in chongqing radish paocai. Food Chem. 2022, 369, 130897. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, H.; Ohno, T.; Iwahashi, H.; Horie, M. Diversity of lactic acid bacteria involved in the fermentation of awa-bancha. Microbes Environ. 2021, 36, ME21029. [Google Scholar] [CrossRef] [PubMed]
- Jun, Z.; Shuaishuai, W.; Lihua, Z.; Qilong, M.; Xi, L.; Mengyang, N.; Tong, Z.; Hongli, Z. Culture-dependent and -independent analysis of bacterial community structure in Jiangshui, a traditional Chinese fermented vegetable food. LWT-Food Sci. Technol. 2018, 96, 244–250. [Google Scholar] [CrossRef]
- Tamang, B.; Tamang, J.P.; Schillinger, U.; Franz, C.M.A.P.; Gores, M.; Holzapfel, W.H. Phenotypic and genotypic identification of lactic acid bacteria isolated from ethnic fermented bamboo tender shoots of North East India. Int. J. Food Microbiol. 2008, 121, 35–40. [Google Scholar] [CrossRef]
- Yu, J.; Gao, W.; Qing, M.; Sun, Z.; Wang, W.; Liu, W.; Pan, L.; Sun, T.; Wang, H.; Bai, N.; et al. Identification and characterization of lactic acid bacteria isolated from traditional pickles in Sichuan, China. J. Gen. Appl. Microbiol. 2012, 58, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.L.; Van Hoorde, K.; Cnockaert, M.; De Brandt, E.; Aerts, M.; Thanh, L.B.; Vandamme, P. A description of the lactic acid bacteria microbiota associated with the production of traditional fermented vegetables in Vietnam. Int. J. Food Microbiol. 2013, 163, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chaikaew, S.; Baipong, S.; Sone, T.; Kanpiengjai, A.; Chui-chai, N.; Asano, K.; Khanongnuch, C. Diversity of lactic acid bacteria from Miang, a traditional fermented tea leaf in northern Thailand and their tannin-tolerant ability in tea extract. J. Microbiol. 2017, 55, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Tamang, B.; Schillinger, U.; Franz, C.M.A.P.; Gores, M.; Holzapfel, W.H. Identification of predominant lactic acid bacteria isolated from traditionally fermented vegetable products of the Eastern Himalayas. Int. J. Food Microbiol. 2005, 105, 347–356. [Google Scholar] [CrossRef]
- Tamang, J.P.; Sarkar, P.K. Sinki: A traditional radish tap root lactic acid fermented root product. J. Gen. Appl. Microbiol. 1993, 39, 395–408. [Google Scholar] [CrossRef]
- Hong, Y.; Yang, H.-S.; Li, J.; Han, S.-K.; Chang, H.-C.; Kim, H.-Y. Identification of lactic acid bacteria in salted Chinese cabbage by SDS-PAGE and PCR-DGGE. J. Sci. Food Agric. 2014, 94, 296–300. [Google Scholar] [CrossRef]
- Bo, B.; Kim, S.-A.; Han, N.S. Bacterial and fungal diversity in Laphet, traditional fermented tea leaves in Myanmar, analyzed by culturing, DNA amplicon-based sequencing, and PCR-DGGE methods. Int. J. Food Microbiol. 2020, 320, 108508. [Google Scholar] [CrossRef]
- Liang, T.; Xie, X.; Wu, L.; Li, L.; Li, H.; Xi, Y.; Feng, Y.; Xue, L.; Chen, M.; Chen, X.; et al. Microbial communities and physiochemical properties of four distinctive traditionally fermented vegetables from north China and their influence on quality and safety. Foods 2022, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, C.; Wu, W.; Lv, X.; Xin, X.; Liu, D.; Hu, H.; Guo, S. Correlation of the bacterial communities with umami components, and chemical characteristics in Zhejiang xuecai and fermented brine. Food Res. Int. 2021, 140, 109986. [Google Scholar] [CrossRef] [PubMed]
- Maifreni, M.; Marino, M.; Conte, L. Lactic acid fermentation of Brassica rapa: Chemical and microbial evaluation of a typical Italian product (brovada). Eur. Food Res. Technol. 2004, 218, 469–473. [Google Scholar] [CrossRef]
- Plengvidhya, V.; Breidt, F., Jr.; Lu, Z.; Fleming, H.P. DNA fingerprinting of lactic acid bacteria in sauerkraut fermentations. Appl. Environ. Microbiol. 2007, 73, 7697–7702. [Google Scholar] [CrossRef]
- Gaudioso, G.; Weil, T.; Marzorati, G.; Solovyev, P.; Bontempo, L.; Franciosi, E.; Bertoldi, L.; Pedrolli, C.; Tuohy, K.M.; Fava, F. Microbial and metabolic characterization of organic artisanal sauerkraut fermentation and study of gut health-promoting properties of sauerkraut brine. Front. Microbiol. 2022, 13, 929738. [Google Scholar] [CrossRef]
- Endo, A.; Mizuno, H.; Okada, S. Monitoring the bacterial community during fermentation of sunki, an unsalted, fermented vegetable traditional to the Kiso area of Japan. Lett. Appl. Microbiol. 2008, 47, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, H.; Kawarai, T.; Furukawa, S.; Miyao, S.; Yamasaki, M. Microfloral and chemical changes of salted pickles (suguki) during its manufacturing process. Jpn. J. Food Microbiol. 2009, 26, 98–106. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Hondrodimou, O.L.; Drosinos, E.H. Development of the microbial community during spontaneous cauliflower fermentation. Food Res. Int. 2010, 43, 1098–1103. [Google Scholar] [CrossRef]
- Wouters, D.; Grosu-Tudor, S.; Zamfir, M.; De Vuyst, L. Bacterial community dynamics, lactic acid bacteria species diversity and metabolite kinetics of traditional Romanian vegetable fermentations. J. Sci. Food Agric. 2013, 93, 749–760. [Google Scholar] [CrossRef]
- Yeun, H.; Yang, H.-S.; Chang, H.-C.; Kim, H.-Y. Comparison of bacterial community changes in fermenting kimchi at two different temperatures using a denaturing gradient gel electrophoresis analysis. J. Microbiol. Biotechnol. 2013, 23, 76–84. [Google Scholar]
- Lee, H.Y.; Haque, M.A.; Cho, K.M. Changes in physicochemical property and lactic acid bacterial community during kimchi fermentation at different temperatures. J. Appl. Biol. Chem. 2020, 63, 429–437. [Google Scholar] [CrossRef]
- Sakai, M.; Ohta, H.; Niidome, T.; Morimura, S. Changes in microbial community composition during production of takanazuke. Food Sci. Technol. Res. 2014, 20, 693–698. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Zou, H.; Qu, C.; Zhang, L.; Liu, T.; Wu, H.; Li, Y. Dominant microorganisms during the spontaneous fermentation of suan cai, a Chinese fermented vegetable. Food Sci. Technol. Res. 2014, 20, 915–926. [Google Scholar] [CrossRef]
- Wu, R.; Yu, M.; Liu, X.; Meng, L.; Wang, Q.; Xue, Y.; Wu, J.; Yue, X. Changes in flavour and microbial diversity during natural fermentation of suan-cai, a traditional food made in Northeast China. Int. J. Food Microbiol. 2015, 211, 23–31. [Google Scholar] [CrossRef]
- Saeedi, M.; Shahidi, F.; Mortazavi, S.A.; Milani, E.; Yazdi, F.T. Isolation and identification of lactic acid bacteria in winter salad (local pickle) during fermentation using 16S rRNA gene sequence analysis. J. Food Saf. 2015, 35, 287–294. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, A.; Wu, Z.; Liu, C.; Zhang, W. Characterization of microbial community during the fermentation of Chinese homemade paocai, a traditional fermented vegetable food. Food Sci. Technol. Res. 2016, 22, 467–475. [Google Scholar] [CrossRef]
- Wang, D.; Chena, G.; Tang, Y.; Li, H.; Shen, W.; Wang, M.; Liu, S.; Qin, W.; Zhang, Q. Effects of temperature on paocai bacterial succession revealed by culture dependent and culture-independent methods. Int. J. Food Microbiol. 2020, 317, 1084. [Google Scholar] [CrossRef]
- Chen, A.-J.; Luo, W.; Peng, Y.-T.; Niu, K.-L.; Liu, X.-Y.; Shen, G.-H.; Zhang, Z.-Q.; Wan, H.; Luo, Q.-Y.; Li, S.-S. Quality and microbial flora changes of radish paocai during multiple fermentation rounds. Food Control 2019, 106, 106733. [Google Scholar] [CrossRef]
- Chiou, T.-Y.; Suda, W.; Oshima, K.; Hattori, M.; Takahashi, T. Changes in the bacterial community in the fermentation process of kôso, a Japanese sugar-vegetable fermented beverage. Biosci. Biotechnol. Biochem. 2017, 81, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, S.; Syrokou, M.K.; Papadia-Nikolaidou, A.; Papoutsis, G.; Drosinos, E.H. Towards recreation of food commodities based on ancient texts; the case of avyrtake. Appl. Sci. 2022, 12, 1697. [Google Scholar] [CrossRef]
- Xiong, S.; Xu, X.; Zhang, L.; Du, T.; Huang, T.; Huang, J.; Ren, H.; Xiong, T.; Xie, M. Integrated metatranscriptomics and metabolomics reveal microbial succession and flavor formation mechanisms during the spontaneous fermentation of Laotan Suancai. Food Res. Int. 2024, 177, 113865. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.; David, M.; Gomes, M.H. Glucose, fructose and sucrose content in broccoli, white cabbage and Portuguese cabbage grown in early and late seasons. J. Sci. Food Agric. 2001, 81, 1145–1149. [Google Scholar] [CrossRef]
- Vincente, A.R.; Manganaris, G.A.; Ortiz, C.M.; Sozzi, G.O.; Crisosto, C.H. Nutritional quality of fruits and vegetables. In Postharvest Handling, 3rd ed.; Florkowski, W.J., Shewfelt, R.L., Brueckner, B., Prussia, S.E., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 69–122. [Google Scholar]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Muller, U.; Hoglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Lisciani, S.; Gambelli, L.; Durazzo, A.; Marconi, S.; Camilli, E.; Rossetti, C.; Gabrielli, P.; Aguzzi, A.; Temperini, O.; Marletta, L. Carbohydrates components of some Italian local landraces: Garlic (Allium sativum L.). Sustainability 2017, 9, 1922. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Choi, C.S.; Rhee, J.; Jo, J.S.; Shin, Y.K.; Song, J.W.; Lee, J.G. Seasonal variation in agronomic characteristics and sugar content of cabbage genotypes. Chil. J. Agric. Res. 2021, 81, 80–91. [Google Scholar] [CrossRef]
- Yusuf, E.; Tkacz, K.; Turkiewicz, I.P.; Wojdyło, A.; Nowicka, P. Analysis of chemical compounds’ content in different varieties of carrots, including qualification and quantification of sugars, organic acids, minerals, and bioactive compounds by UPLC. Eur. Food Res. Technol. 2021, 247, 3053–3062. [Google Scholar] [CrossRef]
- Hammes, W.P.; Hertel, C.; Genus, I. Lactobacillus Beijerinck 1901, 212AL. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; De Vos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.-H., Whitman, W.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 3, The Firmicutes; pp. 465–510. [Google Scholar]
- Paventi, G.; Di Martino, C.; Crawford, T.W., Jr.; Iorizzo, M. Enzymatic activities of Lactiplantibacillus plantarum: Technological and functional role in food processing and human nutrition. Food Biosci. 2024, 61, 104944. [Google Scholar] [CrossRef]
- Syrokou, M.K.; Tziompra, S.; Psychogiou, E.-E.; Mpisti, S.-D.; Paramithiotis, S.; Bosnea, L.; Mataragas, M.; Skandamis, P.N.; Drosinos, E.H. Technological and safety attributes of lactic acid bacteria and yeasts isolated from spontaneously fermented Greek wheat sourdoughs. Microorganisms 2021, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Tsigkrimani, M.; Panagiotarea, K.; Paramithiotis, S.; Bosnea, L.; Pappa, E.; Drosinos, E.H.; Skandamis, P.Ν.; Mataragas, M. Microbial ecology of sheep milk, artisanal Feta and Kefalograviera cheeses. Part II: Technological, safety and probiotic attributes of lactic acid bacteria isolates. Foods 2022, 11, 459. [Google Scholar] [CrossRef]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Manuel Lorenzo, J.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The impacts of Lactiplantibacillus plantarum on the functional properties of fermented foods: A review of current knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Nadeem, M.; Al-Asmari, F.; Imran, M.; Ambreen, S.; Rahim, M.A.; Oranab, S.; Esatbeyoglu, T.; Bartkiene, E.; Rocha, J.M. Effect of Lactiplantibacillus plantarum on the conversion of linoleic acid of vegetable oil to conjugated linoleic acid, lipolysis, and sensory properties of cheddar cheese. Microorganisms 2023, 11, 2613. [Google Scholar] [CrossRef]
- Mao, B.; Yin, R.; Li, X.; Cui, S.; Zhang, H.; Zhao, J.; Chen, W. Comparative genomic analysis of Lactiplantibacillus plantarum isolated from different niches. Genes 2021, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, S.; Liu, W.; Kwok, L.-Y.; Bilige, M.; Zhang, W. Comparative genomic analysis of 455 Lactiplantibacillus plantarum isolates: Habitat-specific genomes shaped by frequent recombination. Food Microbiol. 2022, 104, 103989. [Google Scholar] [CrossRef]
- Carpi, F.M.; Coman, M.M.; Silvi, S.; Picciolini, M.; Verdenelli, M.C.; Napolioni, V. Comprehensive pan-genome analysis of Lactiplantibacillus plantarum complete genomes. J. Appl. Microbiol. 2022, 132, 592–604. [Google Scholar] [CrossRef]
- McLeod, A.; Fagerlund, A.; Rud, I.; Axelsson, L. Large plasmid complement resolved: Complete genome sequencing of Lactobacillus plantarum MF1298, a candidate probiotic strain associated with unfavorable effect. Microorganisms 2019, 7, 262. [Google Scholar] [CrossRef]
- Davray, D.; Bawane, H.; Kulkarni, R. Non-redundant nature of Lactiplantibacillus plantarum plasmidome revealed by comparative genomic analysis of 105 strains. Food Microbiol. 2023, 109, 104153. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Yang, Y.; Wang, J.; Wang, G.; Ren, F.; Hao, Y. Complete genome sequencing of Lactobacillus plantarum CAUH2 reveals a novel plasmid pCAUH203 associated with oxidative stress tolerance. 3 Biotech 2019, 9, 116. [Google Scholar] [CrossRef]
- Flórez, A.B.; Vázquez, L.; Rodríguez, J.; Mayo, B. Phenotypic and safety assessment of the cheese strain Lactiplantibacillus plantarum LL441, and sequence analysis of its complete genome and plasmidome. Int. J. Mol. Sci. 2023, 24, 605. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Bottacini, F.; van Sinderen, D.; Gahan, C.G.M.; Corsetti, A. comparative genomics of Lactiplantibacillus plantarum: Insights into probiotic markers in strains isolated from the human gastrointestinal tract and fermented foods. Front. Microbiol. 2022, 13, 854266. [Google Scholar] [CrossRef]
- Crawley, A.B.; Henriksen, E.D.; Stout, E.; Brandt, K.; Barrangou, R. Characterizing the activity of abundant, diverse and active CRISPR-Cas systems in lactobacilli. Sci. Rep. 2018, 8, 11544. [Google Scholar] [CrossRef] [PubMed]
- Surve, S.; Shinde, D.B.; Kulkarni, R. Isolation, characterization and comparative genomics of potentially probiotic Lactiplantibacillus plantarum strains from Indian foods. Sci. Rep. 2022, 12, 1940. [Google Scholar] [CrossRef]
- Syrokou, M.K.; Paramithiotis, S.; Drosinos, E.H.; Bosnea, L.; Mataragas, M. Comparative genomics and safety assessment of six Lactiplantibacillus plantarum subsp. argentoratensis strains isolated from spontaneously fermented Greek wheat sourdoughs for potential biotechnological application. Int. J. Mol. Sci. 2022, 23, 2487. [Google Scholar] [CrossRef]
- Heo, S.; Jung, E.J.; Park, M.-K.; Sung, M.-H.; Jeong, D.-W. Evolution and competitive struggles of Lactiplantibacillus plantarum under different oxygen contents. Int. J. Mol. Sci. 2024, 25, 8861. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, D.; Bringel, F.; Schuren, F.H.; de Vos, W.M.; Siezen, R.J.; Kleerebezem, M. Exploring Lactobacillus plantarum genome diversity by using microarrays. J. Bacteriol. 2005, 187, 6119–6127. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.E.; Bayjanov, J.R.; Caffrey, B.E.; Wels, M.; Joncour, P.; Hughes, S.; Gillet, B.; Kleerebezem, M.; van Hijum, S.A.F.T.; Leulier, F. Nomadic lifestyle of Lactobacillus plantarum revealed by comparative genomics of 54 strains isolated from different habitats. Environ. Microbiol. 2016, 18, 4974–4989. [Google Scholar] [CrossRef]
- Choi, S.; Jin, G.-D.; Park, J.; You, I.; Kim, E.B. Pan-Genomics of Lactobacillus plantarum revealed group-specific genomic profiles without habitat association. J. Microbiol. Biotechnol. 2018, 28, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Cen, S.; Yin, R.; Mao, B.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Comparative genomics shows niche-specific variations of Lactobacillus plantarum strains isolated from human, Drosophila melanogaster, vegetable and dairy sources. Food Biosci. 2020, 35, 100581. [Google Scholar] [CrossRef]
- Pan, Q.; Cen, S.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Niche-specific adaptive evolution of Lactobacillus plantarum strains isolated from human feces and paocai. Front. Cell. Infect. Microbiol. 2021, 10, 615876. [Google Scholar] [CrossRef]
- Sato, K.; Ikagawa, Y.; Niwa, R.; Nishioka, H.; Horie, M.; Iwahashi, H. Genome sequencing unveils nomadic traits of Lactiplantibacillus plantarum in Japanese post-fermented tea. Curr. Microbiol. 2024, 81, 52. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, M.F.; Sorrentino, A.; Morandi, S.; Abouloifa, H.; Asehraou, A.; Brasca, M.; Siciliano, R.A. Catalogue of surface proteins of Lactiplantibacillus plantarum strains of dairy and vegetable niches. Int. J. Food Microbiol. 2025, 426, 110922. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, M.; Zheng, Y.; Miao, K.; Qu, X. The carbohydrate metabolism of Lactiplantibacillus plantarum. Int. J. Mol. Sci. 2021, 22, 13452. [Google Scholar] [CrossRef]
- Padan, E.; Bibi, E.; Ito, M.; Krulwich, T.A. Alkaline pH homeostasis in bacteria: New insights. Biochim. Biophys. Acta. 2005, 1717, 67–88. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Boekhorst, J.; van Kranenburg, R.; Molenaar, D.; Kuipers, O.P.; Leer, R.; Tarchini, R.; Peters, S.A.; Sandbrink, H.M.; Fiers, M.W.E.J.; et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl Acad. Sci. USA 2003, 100, 1990–1995. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.-H. Characterization of transcriptional response of Lactobacillus plantarum under acidic conditions provides insight into bacterial adaptation in fermentative environments. Sci. Rep. 2020, 10, 19203. [Google Scholar] [CrossRef] [PubMed]
- Colautti, A.; Arnoldi, M.; Comi, G.; Iacumin, L. Antibiotic resistance and virulence factors in lactobacilli: Something to carefully consider. Food Microbiol. 2022, 103, 103934. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef]
- Li, S.; Du, X.; Feng, L.; Mu, G.; Tuo, Y. The microbial community, biogenic amines content of soybean paste, and the degradation of biogenic amines by Lactobacillus plantarum HM24. Food Sci. Nutr. 2021, 9, 6458–6470. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, T.; Zhai, Y.; Sun, L.; Zhai, M.; Kang, L.; Zhao, X.; Wang, B.; Duan, Y.; Jin, Y. Effect of Lactiplantibacillus plantarum X22-2 on biogenic amine formation and quality of fermented lamb sausage during storage. Fermentation 2023, 9, 883. [Google Scholar] [CrossRef]
- Zhang, S.; Oh, J.-H.; Alexander, L.M.; eOzcam, M.; van Pijkeren, J.-P. d-alanyl-d-alanine ligase as a broad-host-range counterselection marker in vancomycin-resistant lactic acid bacteria. J. Bacteriol. 2018, 200, e00607-17. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Gaur, G.; Modesto, M.; Chinnici, F.; Scarafile, D.; Borruso, L.; Marin, A.C.; Spiezio, C.; Valente, D.; Sandri, C.; et al. Physiological and genomic characterization of Lactiplantibacillus plantarum isolated from Indri indri in Madagascar. J. Appl. Microbiol. 2023, 134, lxad255. [Google Scholar] [CrossRef] [PubMed]
- Sunardi, J.; Purnama, E.T.; Sugata, M.; Victor, H.; Jan, T.T.; Jo, J. A comparative assessment of Lactiplantibacillus plantarum isolated from chicken and humans as candidates for probiotics. Biodiversitas 2023, 24, 5198–5206. [Google Scholar] [CrossRef]
- Pulido-Mateos, E.C.; Lessard-Lord, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Comprehensive analysis of the metabolic and genomic features of tannin-transforming Lactiplantibacillus plantarum Strains. Sci. Rep. 2022, 12, 22406. [Google Scholar] [CrossRef]
- Fuhren, J.; Schwalbe, M.; Peralta-Marzal, L.; Rösch, C.; Schols, H.A.; Kleerebezem, M. Phenotypic and genetic characterization of differential galacto-oligosaccharide utilization in Lactobacillus plantarum. Sci. Rep. 2020, 10, 21657. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, S.; Pateraki, C. Lactic acid bacterial cell factories for the production of gamma aminobutyric acid. In Lactic Acid Bacteria as Cell Factories; Montet, D., Ray, R.C., Azevedo, V.A.D.C., Paramithiotis, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 121–152. [Google Scholar]
- Syrokou, M.K.; Paramithiotis, S.; Drosinos, E.H. Health promoting functional genomic features of lactic acid bacteria. In Lactic Acid Bacteria in Food Biotechnology Innovations and Functional Aspects; Paramithiotis, S., Azevedo, V.A.D.C., Montet, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 221–244. [Google Scholar]
- Paramithiotis, S.; Pateraki, C. Lactic acid bacteria for riboflavin production. In Lactic Acid Bacteria as Cell Factories; Montet, D., Ray, R.C., Azevedo, V.A.D.C., Paramithiotis, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 109–120. [Google Scholar]
- Sirithanakorn, C.; Cronan, J.E. Biotin, a universal and essential cofactor: Synthesis, ligation and regulation. FEMS Microbiol. Rev. 2021, 45, fuab003. [Google Scholar] [CrossRef]
- Tenea, G.N.; Ortega, C. Genome Characterization of Lactiplantibacillus plantarum strain UTNGt2 originated from Theobroma grandiflorum (White Cacao) of Ecuadorian Amazon: Antimicrobial peptides from safety to potential applications. Antibiotics 2021, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Weiman, M.; Guyot, J.-P.; Lajus, A.; Cruveiller, S.; Humblot, C. The genomic and transcriptomic basis of the potential of Lactobacillus plantarum A6 to improve the nutritional quality of a cereal based fermented food. Int. J. Food Microbiol. 2018, 266, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Baek, M.G.; Chung, M.J.; Lim, S.; Yi, H. Distribution of bacteriocin genes in the lineages of Lactiplantibacillus plantarum. Sci. Rep. 2021, 11, 20063. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Halami, P.M.; Tamang, J.P. Genome analysis of Lactobacillus plantarum isolated from some Indian fermented foods for bacteriocin production and probiotic marker genes. Front. Microbiol. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, Z.; Ikram-ul-Haq; Mukhtar, H.; Tayyeb, A.; Manzoor, A. Next-generation sequencing to elucidate adaptive stress response and plantaricin genes among Lactobacillus plantarum strains. Future Microbiol. 2020, 15, 333–348. [Google Scholar] [CrossRef]
- Kim, E.; Chang, H.C.; Kim, H.-Y. Complete genome sequence of Lactobacillus plantarum EM, a putative probiotic strain with the cholesterol-lowering effect and antimicrobial activity. Curr. Microbiol. 2020, 77, 1871–1882. [Google Scholar] [CrossRef]
- Lee, I.C.; Caggianiello, G.; van Swam, I.I.; Taverne, N.; Meijerink, M.; Bron, P.A. Strain-specific features of extracellular polysaccharides and their impact on Lactobacillus plantarum-host interactions. Appl. Environ. Microbiol. 2016, 82, 3959–3970. [Google Scholar] [CrossRef]
- Wang, Y.; Ahmed, Z.; Feng, W.; Li, C.; Song, S. Physicochemical properties of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir. Int. J. Biol. Macromol. 2008, 43, 283–288. [Google Scholar] [CrossRef]
- Bachtarzi, N.; Gomri, M.A.; Meradji, M.; Gil-Cardoso, K.; Ortega, N.; Chomiciute, G.; Del Bas, J.M.; López, Q.; Martínez, V.; Kharroub, K. In vitro assessment of biofunctional properties of Lactiplantibacillus plantarum strain Jb21-11 and the characterization of its exopolysaccharide. Int. Microbiol. 2024, 27, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Remus, D.M.; van Kranenburg, R.; van Swam, I.I.; Taverne, N.; Bongers, R.S.; Wels, M.; Wells, J.M.; Bron, P.A.; Kleerebezem, M. Impact of 4 Lactobacillus plantarum capsular polysaccharide clusters on surface glycan composition and host cell signaling. Microb. Cell Fact. 2012, 11, 149. [Google Scholar] [CrossRef]
- Zhao, X.F.; Liang, Q.; Song, X.M.; Zhang, Y. Whole genome sequence of Lactiplantibacillus plantarum MC5 and comparative analysis of eps gene clusters. Front. Microbiol. 2023, 14, 1146566. [Google Scholar] [CrossRef] [PubMed]
- Wouters, D.; Grosu-Tudor, S.; Zamfir, M.; De Vuyst, L. Applicability of Lactobacillus plantarum IMDO 788 as a starter culture to control vegetable fermentations. J. Sci. Food Agric. 2013, 93, 3352–3361. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Papadelli, M.; Pardali, E.; Mataragas, M.; Drosinos, E.H. Evaluation of plantaricin genes expression during fermentation of Raphanus sativus roots with a plantaricin producing Lactobacillus plantarum starter. Curr. Microbiol. 2019, 76, 909–916. [Google Scholar] [CrossRef]
- Hang, S.; Zeng, L.; Han, J.; Zhang, Z.; Zhou, Q.; Meng, X.; Gu, Q.; Li, P. Lactobacillus plantarum ZJ316 improves the quality of Stachys sieboldii Miq. pickle by inhibiting harmful bacteria growth, degrading nitrite and promoting the gut microbiota health in vitro. Food Funct. 2022, 13, 1551. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Gao, Y.; Yang, G.; Liu, X.; Huang, R.; Liang, W.; Li, S. Assessment of autochthonous lactic acid bacteria as starter culture for improving traditional Chinese Dongbei Suancai fermentation. LWT-Food Sci. Technol. 2023, 178, 114615. [Google Scholar] [CrossRef]
- Luetic, S.; Knezovic, Z.; Jurcic, K.; Majic, Z.; Tripkovic, K.; Sutlovic, D. Leafy vegetable nitrite and nitrate content: Potential health effects. Foods 2023, 12, 1655. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vivián, C.; Cabello, P.; Martínez-Luque, M.; Blasco, R.; Castillo, F. Prokaryotic nitrate reduction: Molecular properties and functional distinction among bacterial nitrate reductases. J. Bacteriol. 1999, 181, 6573–6584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, J.; Zheng, X.; Yan, J.; Chen, X.; Zhou, Q.; Zhao, X.; Gu, Q.; Li, P. Use of Lactiplantibacillus plantarum ZJ316 as a starter culture for nitrite degradation, foodborne pathogens inhibition and microbial community modulation in pickled mustard fermentation. Food Chem. X 2022, 14, 100344. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, Y.-Y.; Yousaf, M.; Liu, D.-M. Improvement of physicochemical properties and flavour of pickled radish through the use of a direct-vat set starter consisting of Lactiplantibacillus plantarum and Leuconostoc mesenteroides. Int. J. Food Sci. Technol. 2023, 58, 6272–6284. [Google Scholar] [CrossRef]
- Xu, B.; Mi, T.; Ma, S.; Yi, X.; Huang, P.; Huang, P.; Wu, C. Insight into the autochthonous lactic acid bacteria as starter culture for improving the quality of Sichuan radish paocai: Changes in microbial diversity and metabolic profiles. Int. J. Food Microbiol. 2024, 425, 110877. [Google Scholar] [CrossRef]
- Yuan, J.; Zeng, X.; Zhang, P.; Leng, L.; Du, Q.; Pan, D. Nitrite reductases of lactic acid bacteria: Regulation of enzyme synthesis and activity, and different applications. Food Biosci. 2024, 59, 103833. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in cured meats, health risk issues, alternatives to nitrites: A review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.M.; del Arbol, J.T.; Benomar, N.; Abriouel, H.; Martínez Canamero, M.; Galvez, A.; Perez Pulido, R. Application of Lactobacillus plantarum Lb9, as starter culture in caper berry fermentation. LWT-Food Sci. Technol. 2015, 60, 788–794. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Jiang, A.; Xiu, Z.; Ji, Y.; Guan, Y.; Sarengaowa; Yang, X. Effect of salt concentration on quality of Chinese northeast sauerkraut fermented by Leuconostoc mesenteroides and Lactobacillus plantarum. Food Biosci. 2019, 30, 100421. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Sarengaowa; Ji, Y.; Guan, Y.; Feng, K. Comparison of northeast sauerkraut fermentation between single lactic acid bacteria strains and traditional fermentation. Food Res. Int. 2020, 137, 109553. [Google Scholar] [CrossRef]
- Ahmed, S.; Ashraf, F.; Tariq, M.; Zaidi, A. Aggrandizement of fermented cucumber through the action of autochthonous probiotic cum starter strains of Lactiplantibacillus plantarum and Pediococcus pentosaceus. Ann. Microbiol. 2021, 71, 33. [Google Scholar] [CrossRef]
- Stoll, D.A.; Wafula, E.N.; Mathara, J.M.; Trierweiler, B.; Kulling, S.E.; Huch, M. Fermentation of African nightshade leaves with lactic acid bacterial starter cultures. Int. J. Food Microbiol. 2021, 342, 109056. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, M.; Zou, S.; Li, Z.; Wu, Y.; Ji, C.; Chen, Y.; Dong, L.; Zhang, S.; Liang, H. Effect of autochthonous lactic acid bacteria-enhanced fermentation on the quality of suancai. Foods 2022, 11, 3310. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Tang, Y.; Ming, J.; Huang, R.; Li, J.; Ye, M.; Fan, Z.; Chi, Y.; Zhang, Q.; et al. Study of bacterial community succession and reconstruction of the core lactic acid bacteria to enhance the flavor of paocai. Int. J. Food Microbiol. 2022, 375, 109702. [Google Scholar] [CrossRef]
- Yang, M.; Lai, H.; Wang, Y.; Mei, Y.; Huang, Y.; Zeng, X.; Ge, L.; Zhao, J.; Zhu, Y.; Huang, Q.; et al. Characterizing the impact of species/strain-specific Lactiplantibacillus plantarum with community assembly and metabolic regulation in pickled Suancai. Food Res. Int. 2023, 174, 113650. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yang, X.; Hu, C.; Wei, B.; Xu, F.; Guo, Q. Fermentation performance evaluation of lactic acid bacteria strains for Sichuan radish paocai production. Foods 2024, 13, 1813. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).