Nanobiotechnology in Bone Tissue Engineering Applications: Recent Advances and Future Perspectives
Abstract
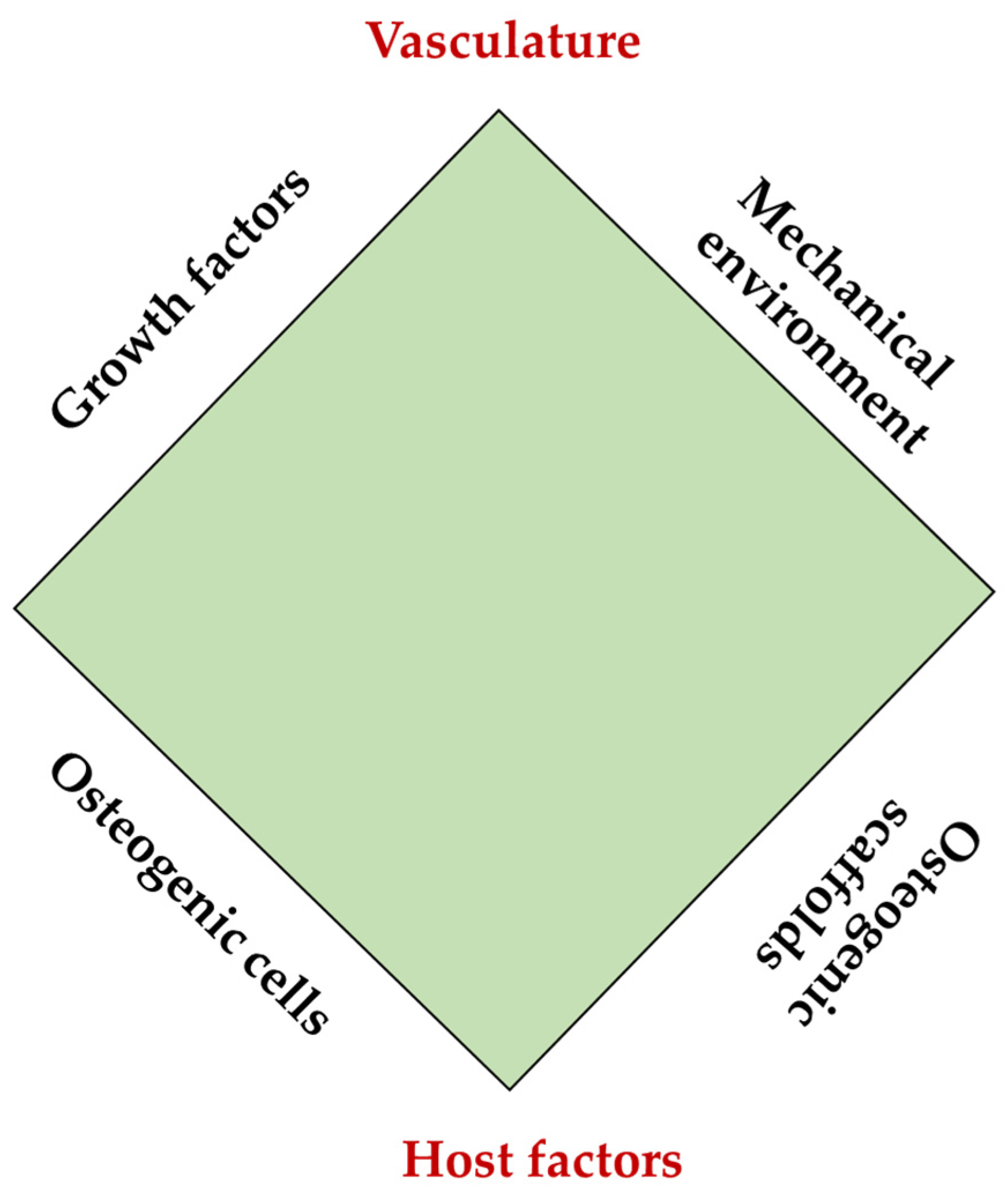
:1. Introduction
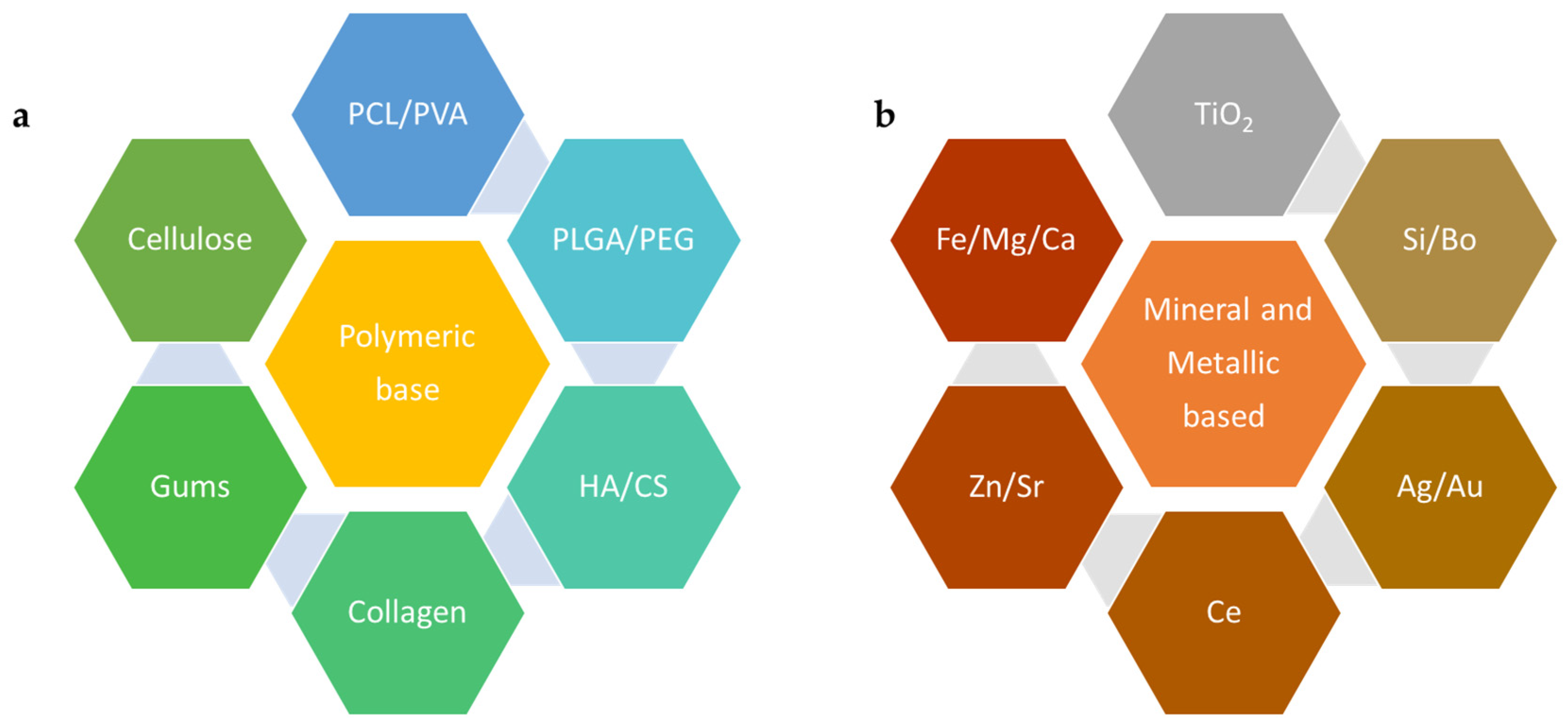
2. Nanoscale Materials for Scaffold Structure and Function
2.1. Polymeric Materials Used at the Nanoscale for BTE
2.1.1. Synthetic Polymers
2.1.2. Natural Polymers
2.2. Mineral-Based and Metallic Nanoscale Materials Used for BTE
2.2.1. Mineral Nanoparticles
Calcium Phosphate
Silicon Dioxide (SiO2)
Zinc Oxide (ZnO)
2.2.2. Metallic NPs
Silver (Ag)
Gold (Au)
Titanium Dioxide (TiO2)
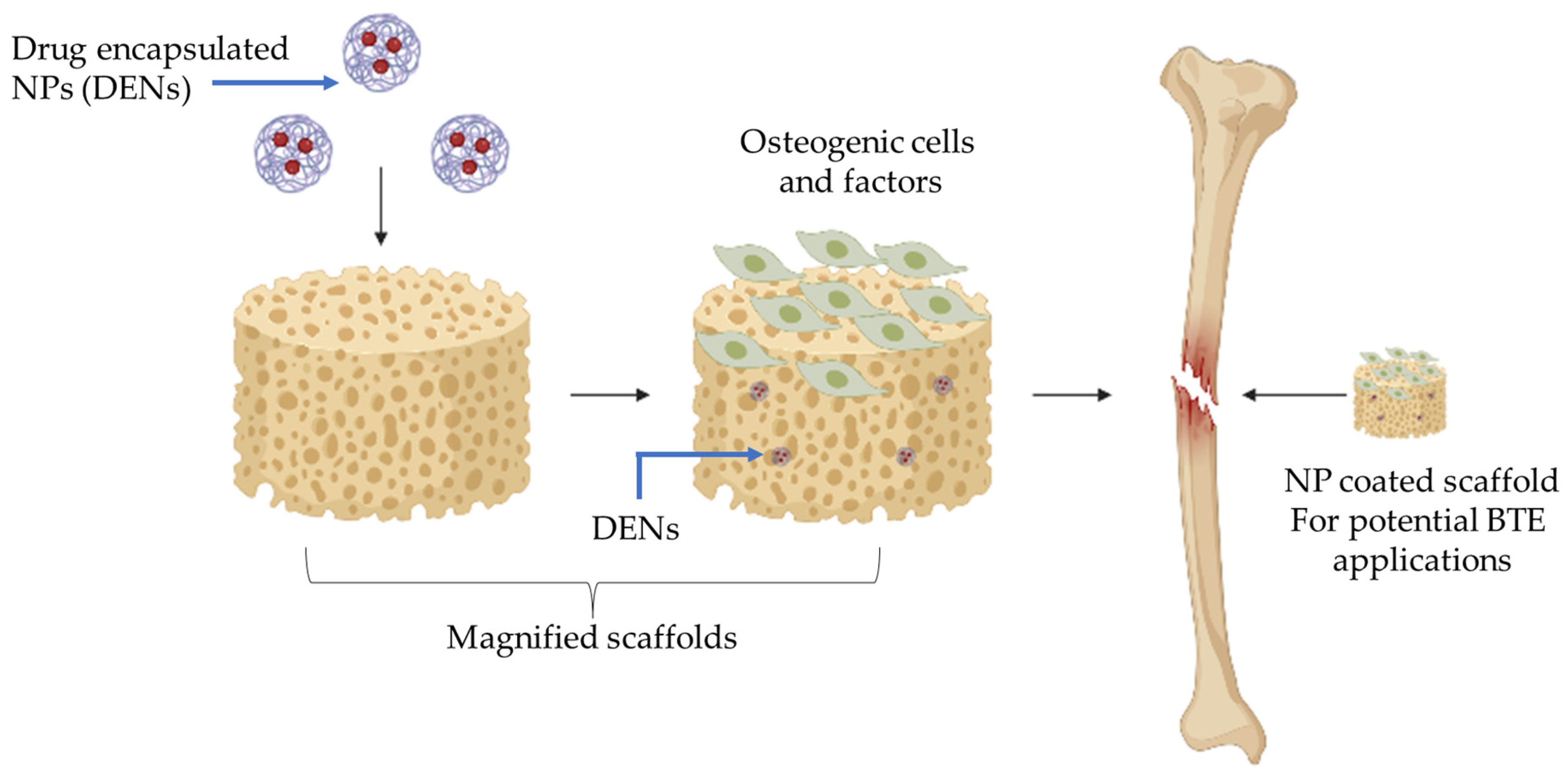
3. Nanomedicine and Drug Delivery in Scaffolds for BTE
3.1. Pharmaceutical Agents in Scaffolds
3.2. Protein Functionalization for Scaffold Surfaces
3.2.1. Plant Proteins
3.2.2. Animal Proteins
| Protein | Source | Scaffold Form | Synthesis Method | Cell/Animal Model | Study Type | Reference |
|---|---|---|---|---|---|---|
| Zein | Plant | HAP/zein nanofibers | Solvothermal | Mouse MSCs | In vitro | [137] |
| Zein/Ca phosphate nanofibrous mats | Electrospinning | Adipose-derived stem cells | In vitro | [132] | ||
| Zein porous scaffold | Salt-leaching | Rabbit MSCs in nude mice | In vivo | [138] | ||
| Zein/chitosan/nanohydroxyapatite porous scaffold | Freeze-drying | MG-63 | In vitro | [139] | ||
| Soy | Plant | Soya protein isolate/polyethylene oxide nanofiber membrane | Crosslinking | Rat MSCs | In vivo | [140] |
| Soya protein isolate/β-tricalcium phosphate/graphene oxide | Freeze-drying | Rat MSCs | In vivo | [141] | ||
| Soy 3D scaffold | Crosslinking/freeze-drying | hMSCs | In vitro | [142] | ||
| Soy 3D scaffold | Electrospinning | Adipose-derived stem cells | In vitro | [143] | ||
| Collagen | Animal | Collagen hydrogel scaffold | Encapsulation | hMSCs | In vitro | [144] |
| Collagen/chitosan/hyaluronic acid hydrogel | Crosslinking | MG-63 | In vitro | [145] | ||
| Collagen/alginate/nanosilica hydrogel | Crosslinking | MG-63 | In vitro | [146] | ||
| Collagen /hydroxyapatite | Biomimetic mineralization | Rabbit rib | In vivo | [147] | ||
| Silk | Animal | Silk fibroin | Lyophilization | Male rabbit | In vivo | [148] |
| polycaprolactone/aloe vera/silk fibroin–hydroxyapatite nanofibrous scaffolds | Electrospinning | Adipose-derived stem cells | In vitro | [149] | ||
| Collagen/dECM/silk fibroin (SF) | 3D printing | Pre-osteoblast MC3T3-E1 cells | In vitro | [150] | ||
| Chitosan-silk sericin 24/hydroxyapatite | Biomimetic mineralization | MG-63 | In vitro | [151] |
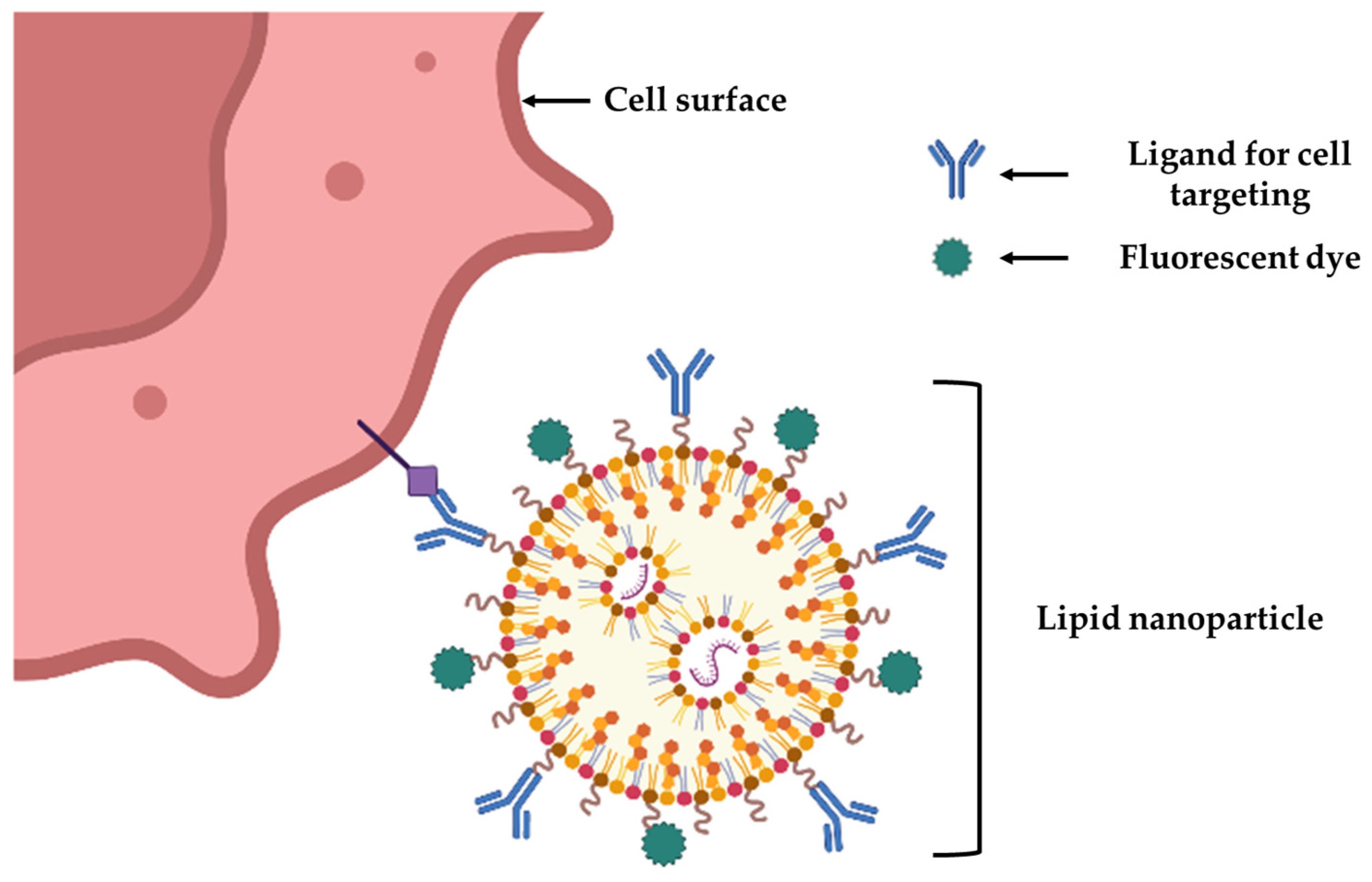
4. Nanotechnology for Cell Targeting and Labeling for BTE
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, T.J.; Goldstein, S.R. Bone health 2022: An update. Climacteric J. Int. Menopause Soc. 2022, 25, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.A.; Aitken, S.A.; Simpson, A.H.R.W. The risk of non-union per fracture: Current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017, 88, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef]
- Steinmetz, S.; Wernly, D.; Moerenhout, K.; Trampuz, A.; Borens, O. Infection after fracture fixation. EFORT Open Rev. 2019, 4, 468–475. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.; Epari, D.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Braddock, M.; Houston, P.; Campbell, C.; Ashcroft, P. Born again bone: Tissue engineering for bone repair. Physiology 2001, 16, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.M.; Giannoudis, P.V. Enhancement of fracture healing with the diamond concept: The role of the biological chamber. Injury 2011, 42, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Andrzejowski, P.; Giannoudis, P.V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef]
- Bartold, M.; Gronthos, S.; Haynes, D.; Ivanovski, S. Mesenchymal stem cells and biologic factors leading to bone formation. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 12–32. [Google Scholar] [CrossRef]
- Arthur, A.; Gronthos, S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int. J. Mol. Sci. 2020, 21, 9759. [Google Scholar] [CrossRef]
- Ganguly, P.; El-Jawhari, J.J.; Giannoudis, P.V.; Burska, A.N.; Ponchel, F.; Jones, E.A. Age-related Changes in Bone Marrow Mesenchymal Stromal Cells: A Potential Impact on Osteoporosis and Osteoarthritis Development. Cell Transplant. 2017, 26, 1520–1529. [Google Scholar] [CrossRef]
- Ganguly, P.; El-Jawhari, J.J.; Burska, A.N.; Ponchel, F.; Giannoudis, P.V.; Jones, E.A. The Analysis of In Vivo Aging in Human Bone Marrow Mesenchymal Stromal Cells Using Colony-Forming Unit-Fibroblast Assay and the CD45 low CD271 + Phenotype. Stem Cells Int. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Iijima, K.; Otsuka, H. Cell Scaffolds for Bone Tissue Engineering. Bioengineering 2020, 7, 119. [Google Scholar] [CrossRef]
- Sallent, I.; Capella-Monsonís, H.; Procter, P.; Bozo, I.Y.; Deev, R.V.; Zubov, D.; Vasyliev, R.; Perale, G.; Pertici, G.; Baker, J.; et al. The Few Who Made It: Commercially and Clinically Successful Innovative Bone Grafts. Front. Bioeng. Biotechnol. 2020, 8, 952. [Google Scholar] [CrossRef]
- Chen, J.; Hendriks, M.; Chatzis, A.; Ramasamy, S.K.; Kusumbe, A.P. Bone Vasculature and Bone Marrow Vascular Niches in Health and Disease. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2020, 35, 2103–2120. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Reagan, M.R.; Rosen, C.J. Navigating the bone marrow niche: Translational insights and cancer-driven dysfunction. Nat. Rev. Rheumatol. 2016, 12, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Rausch, M.; Iqbal, N.; Pathak, S.; Owston, H.E.; Ganguly, P. Organoid Models and Next-Generation Sequencing for Bone Marrow and Related Disorders. Organoids 2023, 2, 123–139. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1253–1263. [Google Scholar] [CrossRef]
- Saiz, E.; Zimmermann, E.A.; Lee, J.S.; Wegst, U.G.; Tomsia, A.P. Perspectives on the role of nanotechnology in bone tissue engineering. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013, 29, 103–115. [Google Scholar] [CrossRef]
- Simchi, A.; Mazaheri, M.; Eslahi, N.; Ordikhani, F.; Tamjid, E. Nanomedicine applications in orthopedic medicine: State of the art. Int. J. Nanomed. 2023, 10, 6039–6054. [Google Scholar] [CrossRef]
- Griffin, M.F.; Kalaskar, D.M.; Seifalian, A.; Butler, P.E. An update on the Application of Nanotechnology in Bone Tissue Engineering. Open Orthop. J. 2016, 10, 836–848. [Google Scholar] [CrossRef]
- Schofer, M.D.; Roessler, P.P.; Schaefer, J.; Theisen, C.; Schlimme, S.; Heverhagen, J.T.; Voelker, M.; Dersch, R.; Agarwal, S.; Fuchs-Winkelmann, S.; et al. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects. PLoS ONE 2011, 6, e25462. [Google Scholar] [CrossRef]
- Prabha, R.D.; Kraft, D.C.E.; Harkness, L.; Melsen, B.; Varma, H.; Nair, P.D.; Kjems, J.; Kassem, M. Bioactive nano-fibrous scaffold for vascularized craniofacial bone regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1537–e1748. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, Y.; Selvaratnam, B.; Koodali, R.T.; Sun, H. Mesoporous silicate nanoparticles/3D nanofibrous scaffold-mediated dual-drug delivery for bone tissue engineering. J. Control. Release Off. J. Control. Release Soc. 2018, 279, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Mouriño, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, P.; Gandhimathi, C.; Venugopal, J.R.; Becker, D.L.; Ramakrishna, S.; Srinivasan, D.K. Controlled release of drugs in electrosprayed nanoparticles for bone tissue engineering. Adv. Drug Deliv. Rev. 2015, 94, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Hajiali, H.; Ouyang, L.; Llopis-Hernandez, V.; Dobre, O.; Rose, F.R.A.J. Review of emerging nanotechnology in bone regeneration: Progress, challenges, and perspectives. Nanoscale 2021, 13, 10266–10280. [Google Scholar] [CrossRef]
- Wen, J.; Cai, D.; Gao, W.; He, R.; Li, Y.; Zhou, Y.; Klein, T.; Xiao, L.; Xiao, Y. Osteoimmunomodulatory Nanoparticles for Bone Regeneration. Nanomaterials 2023, 13, 692. [Google Scholar] [CrossRef]
- Scott, T.G.; Blackburn, G.; Ashley, M.; Bayer, I.S.; Ghosh, A.; Biris, A.S.; Biswas, A. Advances in bionanomaterials for bone tissue engineering. J. Nanosci. Nanotechnol. 2013, 13, 1–22. [Google Scholar] [CrossRef]
- Qiao, K.; Xu, L.; Tang, J.; Wang, Q.; Lim, K.S.; Hooper, G.; Woodfield, T.B.F.; Liu, G.; Tian, K.; Zhang, W.; et al. The advances in nanomedicine for bone and cartilage repair. J. Nanobiotechnol. 2022, 20, 141. [Google Scholar] [CrossRef]
- Babuska, V.; Kasi, P.B.; Chocholata, P.; Wiesnerova, L.; Dvorakova, J.; Vrzakova, R.; Nekleionova, A.; Landsmann, L.; Kulda, V. Nanomaterials in Bone Regeneration. Appl. Sci. 2022, 12, 6793. [Google Scholar] [CrossRef]
- Vieira, S.; Vial, S.; Reis, R.L.; Oliveira, J.M. Nanoparticles for bone tissue engineering. Biotechnol. Prog. 2017, 33, 590–611. [Google Scholar] [CrossRef]
- Guillén-Carvajal, K.; Valdez-Salas, B.; Beltrán-Partida, E.; Salomón-Carlos, J.; Cheng, N. Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration. Polymers 2023, 15, 2762. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Al-Arjan, W.S.; Binkadem, M.S.; Mehboob, H.; Haider, A.; Raza, M.A.; Razak, S.I.A.; Hasan, A.; Amin, R. Development of Biopolymeric Hybrid Scaffold-Based on AAc/GO/nHAp/TiO2 Nanocomposite for Bone Tissue Engineering: In-Vitro Analysis. Nanomaterials 2021, 11, 1319. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.J.R.; Silva, J.C.; Soares, P.I.P.; Borges, J.P. Polyvinylpyrrolidone Nanofibers Incorporating Mesoporous Bioactive Glass for Bone Tissue Engineering. Biomimetics 2023, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Chahal, S.; Kumar, A.; Hussian, F.S.J. Development of biomimetic electrospun polymeric biomaterials for bone tissue engineering. A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 1308–1355. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Sathiyasree, M.; Rahim, S.B.A.; Renitta, R.E.; Kasipandian, K.; Shree, S.K.; Rajalakshmi, D.; Shobana, N.; Dhiva, S.; Abirami, S.; et al. Scaffold Using Chitosan, Agarose, Cellulose, Dextran and Protein for Tissue Engineering—A Review. Polymers 2023, 15, 1525. [Google Scholar] [CrossRef]
- Sagadevan, S.; Schirhagl, R.; Rahman, Z.; Bin Ismail, M.F.; Lett, J.A.; Fatimah, I.; Kaus, N.H.M.; Oh, W.-C. Recent advancements in polymer matrix nanocomposites for bone tissue engineering applications. J. Drug Deliv. Sci. Technol. 2023, 82, 104313. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Dohle, E.; Lim, J.; Weigl, P.; Teoh, S.H.; Sader, R.; Ghanaati, S. Biologization of Pcl-Mesh Using Platelet Rich Fibrin (Prf) Enhances Its Regenerative Potential In Vitro. Int. J. Mol. Sci. 2021, 22, 2159. [Google Scholar] [CrossRef]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef]
- Hassan, M.; Sulaiman, M.; Yuvaraju, P.D.; Galiwango, E.; Rehman, I.u.; Al-Marzouqi, A.H.; Khaleel, A.; Mohsin, S. Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration. J. Funct. Biomater. 2022, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, W.-M.; Fei, Z.-Q.; Chen, J.-L.; Xiong, J.-Y.; Zhang, J.-F.; Duan, L.; Huang, J.; Liu, Z.; Wang, D.; et al. The study on biocompatibility of porous nHA/PLGA composite scaffolds for tissue engineering with rabbit chondrocytes in vitro. BioMed Res. Int. 2013, 2013, 412745. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zou, Q.; Boerman, O.C.; Nijhuis, A.W.; Jansen, J.A.; Li, Y.; Leeuwenburgh, S.C. Combined delivery of BMP-2 and bFGF from nanostructured colloidal gelatin gels and its effect on bone regeneration in vivo. J. Control. Release Off. J. Control. Release Soc. 2013, 166, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Alqahtani, M.S.; Alhifzi, R. Recent Developments in Polymer Nanocomposites for Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 3312. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.I.; Llano, C.H.V.; Tenorio, D.L.; Saavedra, M.; Zapata, P.; Navia-Porras, D.P.; Delgado-Ospina, J.; Chaur, M.N.; Hernández, J.H.M.; Grande-Tovar, C.D. Biocompatibility Assessment of Polylactic Acid (PLA) and Nanobioglass (n-BG) Nanocomposites for Biomedical Applications. Molecules 2022, 27, 3640. [Google Scholar] [CrossRef]
- Suaza, M.L.M.; Rivera, J.C.L.; Padilla, M.C.R.; Acevedo, M.E.M.; Orozco, C.P.O.; Triviño, D.G.Z. Poly(vinyl alcohol)/Silk Fibroin/Ag-NPs Composite Nanofibers as a Substrate for MG-63 Cells’ Growth. Polymers 2023, 15, 1838. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Jeng, U.-S.; Hsu, S.-H. Biodegradable Water-Based Polyurethane Shape Memory Elastomers for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 1397–1406. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.S.; Deepthi, S.; Chennazhi, K.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 399, 1644–1667. [Google Scholar] [CrossRef]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Scher, J.; Muniglia, L. Enzymatic synthesis of chitosan derivatives and their potential applications. J. Mol. Catal. B Enzym. 2015, 112, 25–39. [Google Scholar] [CrossRef]
- Yildizbakan, L.; Iqbal, N.; Ganguly, P.; Kumi-Barimah, E.; Do, T.; Jones, E.; Giannoudis, P.V.; Jha, A. Fabrication and Characterisation of the Cytotoxic and Antibacterial Properties of Chitosan-Cerium Oxide Porous Scaffolds. Antibiotics 2023, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Braxton, T.M.; Anastasiou, A.; Raif, E.M.; Chung, C.K.Y.; Kumar, S.; Giannoudis, P.V.; Jha, A. Dicalcium Phosphate Dihydrate Mineral Loaded Freeze-Dried Scaffolds for Potential Synthetic Bone Applications. Materials 2022, 15, 6245. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Zairy, E.M.R.E. Chitosan as a Biomaterial—Structure, Properties, and Electrospun Nanofibers. In Concepts, Compounds and the Alternatives of Antibacterials; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Lee, J.S.; Baek, S.D.; Venkatesan, J.; Bhatnagar, I.; Chang, H.K.; Kim, H.T.; Kim, S.-K. In vivo study of chitosan-natural nano hydroxyapatite scaffolds for bone tissue regeneration. Int. J. Biol. Macromol. 2014, 67, 360–366. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Mehdi Jorfi, E.J.F. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2014, 132, 41719. [Google Scholar] [CrossRef]
- Md Nazrul Islam, F.R. Chapter 6—Production and modification of nanofibrillated cellulose composites and potential applications. In Green Composites for Automotive Applications; Woodhead Publishing Series in Composites Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–141. [Google Scholar] [CrossRef]
- Osorio, D.A.; Lee, B.E.J.; Kwiecien, J.M.; Wang, X.; Shahid, I.; Hurley, A.L.; Cranston, E.D.; Grandfield, K. Cross-linked cellulose nanocrystal aerogels as viable bone tissue scaffolds. Acta Biomater. 2019, 87, 152–165. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Jones, E.; Panagiotopoulou, V.; Jha, A.; Blanchy, M.; Antimisiaris, S.; Anton, M.; Dhuiège, B.; Marotta, M.; Marjanovic, N.; et al. Electrospun and 3D printed polymeric materials for one-stage critical-size long bone defect regeneration inspired by the Masquelet technique: Recent Advances. Injury 2022, 53 (Suppl. S2), s2–s12. [Google Scholar] [CrossRef] [PubMed]
- Di Marzio, N.; Eglin, D.; Serra, T.; Moroni, L. Bio-Fabrication: Convergence of 3D Bioprinting and Nano-Biomaterials in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic Mineralization of Biomaterials Using Simulated Body Fluids for Bone Tissue Engineering and Regenerative Medicine. Tissue Eng. Part A 2017, 23, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Hindy, O.A.; Goker, M.; Yilgor Huri, P. Nanoscale agents within 3D-printed constructs: Intersection of nanotechnology and personalized bone tissue engineering. Emergent Mater. 2022, 5, 195–205. [Google Scholar] [CrossRef]
- Chen, Y.; Kawazoe, N.; Chen, G. Preparation of dexamethasone-loaded biphasic calcium phosphate nanoparticles/collagen porous composite scaffolds for bone tissue engineering. Acta Biomater. 2018, 67, 341–353. [Google Scholar] [CrossRef]
- Sokolova, V.; Kostka, K.; Shalumon, K.T.; Prymak, O.; Chen, J.-P.; Epple, M. Synthesis and characterization of PLGA/HAP scaffolds with DNA-functionalised calcium phosphate nanoparticles for bone tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 102. [Google Scholar] [CrossRef]
- Ha, S.-W.; Viggeswarapu, M.; Habib, M.M.; Beck, G.R. Bioactive effects of silica nanoparticles on bone cells are size, surface, and composition dependent. Acta Biomater. 2018, 82, 184–196. [Google Scholar] [CrossRef]
- Echazú, M.I.A.; Renou, S.J.; Alvarez, G.S.; Desimone, M.F.; Olmedo, D.G. Synthesis and Evaluation of a Chitosan–Silica-Based Bone Substitute for Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 13379. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, F.; Shuai, Y.; Peng, S.; Chen, S.; Deng, Y.; Feng, P. Silicon dioxide nanoparticles decorated on graphene oxide nanosheets and their application in poly(l-lactic acid) scaffold. J. Adv. Res. 2023, 48, 175–190. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Qing, Y.; Li, R.; Tang, X.; Guo, D.; Qin, Y. Enhancing ZnO-NP Antibacterial and Osteogenesis Properties in Orthopedic Applications: A Review. Int. J. Nanomed. 2020, 15, 6247–6262. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Narayanan, V.; Sumathi, S. Zinc and manganese substituted hydroxyapatite/CMC/PVP electrospun composite for bone repair applications. Int. J. Biol. Macromol. 2020, 145, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Kim, H.-K.; Ghim, M.-S.; Hong, M.W.; Kim, Y.Y.; Cho, Y.-S. Evaluation of the Antibacterial Activity and Cell Response for 3D-Printed Polycaprolactone/Nanohydroxyapatite Scaffold with Zinc Oxide Coating. Polymers 2020, 12, 2193. [Google Scholar] [CrossRef]
- Forero, J.C.; Roa, E.; Reyes, J.G.; Acevedo, C.; Osses, N. Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) Scaffold. Materials 2017, 10, 1177. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Baradaran, B.; de la Guardia, M. Nanomaterial-based biosensors for detection of pathogenic virus. Trends Anal. Chem. TRAC 2017, 97, 445–457. [Google Scholar] [CrossRef]
- Chenab, K.K.; Eivazzadeh-Keihan, R.; Maleki, A.; Pashazadeh-Panahi, P.; Hamblin, M.R.; Mokhtarzadeh, A. Biomedical applications of nanoflares: Targeted intracellular fluorescence probes. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 342–358. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lin, S.-H.; Chien, C.-S.; Kung, J.-C.; Shih, C.-J. In Vitro Bioactivity and Antibacterial Effects of a Silver-Containing Mesoporous Bioactive Glass Film on the Surface of Titanium Implants. Int. J. Mol. Sci. 2022, 23, 9291. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Chenab, K.K.; Jafari, A.; Radinekiyan, F.; Hashemi, S.M.; Ahmadpour, F.; Behboudi, A.; Mosafer, J.; Mokhtarzadeh, A.; et al. Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1687–1714. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, V.P.; Sivashanmugam, P.; Kulandaivelu, R.; Sagadevan, S.; Sridevi, B.; Govindasamy, R.; Thiruvengadam, M. Biosynthesised Silver Nanoparticles Loading onto Biphasic Calcium Phosphate for Antibacterial and Bone Tissue Engineering Applications. Antibiotics 2022, 11, 1780. [Google Scholar] [CrossRef] [PubMed]
- Akturk, A.; Taygun, M.E.; Goller, G. Optimization of the electrospinning process variables for gelatin/silver nanoparticles/bioactive glass nanocomposites for bone tissue engineering—Akturk—2020—Polymer Composites—Wiley Online Library. Polym. Compos. 2020, 41, 2411–2425. [Google Scholar] [CrossRef]
- Vaidhyanathan, B.; Vincent, P.; Vadivel, S.; Karuppiah, P.; Al-Dhabi, N.A.; Sadhasivam, D.R.; Vimalraj, S.; Saravanan, S. Fabrication and Investigation of the Suitability of Chitosan-Silver Composite Scaffolds for Bone Tissue Engineering Applications. Process Biochem. 2021, 100, 178–187. [Google Scholar] [CrossRef]
- Lopes, J.; Ferreira-Gonçalves, T.; Ascensão, L.; Viana, A.S.; Carvalho, L.; Catarino, J.; Faísca, P.; Oliva, A.; Barros, D.P.C.d.; Rodrigues, C.M.P.; et al. Safety of Gold Nanoparticles: From In Vitro to In Vivo Testing Array Checklist. Pharmaceutics 2023, 15, 1120. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shen, Y.; Huang, L.; Lv, G.; Lei, C.; Fan, X.; Lin, F.; Zhang, Y.; Wu, L.; Yang, Y. In vitro cytotoxicity of gold nanorods in A549 cells. Environ. Toxicol. Pharmacol. 2015, 39, 871–878. [Google Scholar] [CrossRef]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Rachamalla, S.S.; Sistla, R. Xanthan gum stabilized gold nanoparticles: Characterization, biocompatibility, stability and cytotoxicity. Carbohydr. Polym. 2014, 110, 1–9. [Google Scholar] [CrossRef]
- Ko, W.-C.; Wang, S.-J.; Hsiao, C.-Y.; Hung, C.-T.; Hsu, Y.-J.; Chang, D.-C.; Hung, C.-F. Pharmacological Role of Functionalized Gold Nanoparticles in Disease Applications. Molecules 2022, 27, 1551. [Google Scholar] [CrossRef]
- Nekounam, H.; Allahyari, Z.; Gholizadeh, S.; Mirzaei, E.; Shokrgozar, M.A.; Faridi-Majidi, R. Simple and robust fabrication and characterization of conductive carbonized nanofibers loaded with gold nanoparticles for bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111226. [Google Scholar] [CrossRef]
- Yu, M.; Lei, B.; Gao, C.; Yan, J.; Ma, P.X. Optimizing surface-engineered ultra-small gold nanoparticles for highly efficient miRNA delivery to enhance osteogenic differentiation of bone mesenchymal stromal cells. Nano Res. 2016, 10, 49–63. [Google Scholar] [CrossRef]
- Hou, Y.; Cai, K.; Li, J.; Chen, X.; Lai, M.; Hu, Y.; Luo, Z.; Ding, X.; Xu, D. Effects of titanium nanoparticles on adhesion, migration, proliferation, and differentiation of mesenchymal stem cells. Int. J. Nanomed. 2013, 8, 3619–3630. [Google Scholar] [CrossRef]
- Kumar, P. Nano-TiO2 Doped Chitosan Scaffold for the Bone Tissue Engineering Applications. Int. J. Biomater. 2018, 2018, 6576157. [Google Scholar] [CrossRef] [PubMed]
- Pattanashetti, N.A.; Hiremath, C.; Naik, S.R.; Heggannavar, G.B.; Kariduraganavar, M.Y. Development of nanofibrous scaffolds by varying the TiO2 content in crosslinked PVA for bone tissue engineering. New J. Chem. 2020, 44, 2111–2121. [Google Scholar] [CrossRef]
- Yang, J.; Jia, C.; Yang, J. Designing Nanoparticle-based Drug Delivery Systems for Precision Medicine. Int. J. Med. Sci. 2021, 18, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Lu, V.; Zhang, J.; Patel, R.; Zhou, A.K.; Thahir, A.; Krkovic, M. Fracture Related Infections and Their Risk Factors for Treatment Failure—A Major Trauma Centre Perspective. Diagnostics 2022, 12, 1289. [Google Scholar] [CrossRef]
- Johnson, C.T.; García, A.J. Scaffold-based anti-infection strategies in bone repair. Ann. Biomed. Eng. 2015, 43, 515–528. [Google Scholar] [CrossRef]
- Xu, C.; Cao, Y.; Lei, C.; Li, Z.; Kumeria, T.; Meka, A.K.; Xu, J.; Liu, J.; Yan, C.; Luo, L.; et al. Polymer–Mesoporous Silica Nanoparticle Core–Shell Nanofibers as a Dual-Drug-Delivery System for Guided Tissue Regeneration. Appl. Nano Mater. 2020, 3, 1457–1467. [Google Scholar] [CrossRef]
- Sreeja, S.; Muraleedharan, C.; Varma, P.H.; Sailaja, G. Surface-transformed osteoinductive polyethylene terephthalate scaffold as a dual system for bone tissue regeneration with localized antibiotic delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110491. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [PubMed]
- Nisar, P.; Ali, N.; Rahman, L.; Ali, M.; Shinwari, Z.K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. J. Biol. Inorg. Chem. JBIC A Publ. Soc. Biol. Inorg. Chem. 2019, 24, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Lee, C.-S.; Hsu, G.C.-Y.; Sono, T.; Lee, M.; James, A.W. Development of a Biomaterial Scaffold Integrated with Osteoinductive Oxysterol Liposomes to Enhance Hedgehog Signaling and Bone Repair. Mol. Pharm. 2021, 18, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Liu, L.; Xiang, Y.; Lu, Y.; Deng, L.; Zhang, H.; Santos, H.A.; Cui, W. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials 2020, 232, 119706. [Google Scholar] [CrossRef]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A.; et al. Drug Delivery (Nano)Platforms for Oral and Dental Applications: Tissue Regeneration, Infection Control, and Cancer Management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ou, K.-L.; Mao, C.; Hosseinkhani, H. Importance of dual delivery systems for bone tissue engineering. J. Control. Release Off. J. Control. Release Soc. 2016, 225, 152–169. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Y.; Luo, Y.; Ma, J. cLayer-by-layer coated porous 3D printed hydroxyapatite composite scaffolds for controlled drug delivery. Colloids Surf. B Biointerfaces 2019, 179, 121–127. [Google Scholar] [CrossRef]
- Lee, D.; Wufuer, M.; Kim, I.; Choi, T.H.; Kim, B.J.; Jung, H.G.; Jeon, B.; Lee, G.; Jeon, O.H.; Chang, H.; et al. Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration. Sci. Rep. 2021, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Franco, D.; Petralia, S.; Monforte, F.; Condorelli, G.G.; Squarzoni, S.; Traina, F.; Conoci, S. Dual-Functional Nano-Functionalized Titanium Scaffolds to Inhibit Bacterial Growth and Enhance Osteointegration. Nanomaterials 2021, 11, 2634. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Pei, X.; Zhang, B.; Su, Z.; Gui, X.; Gao, C.; Guo, L.; Fan, H.; Jiang, Q.; Zhao, L.; et al. 3D-printed HAp bone regeneration scaffolds enable nano-scale manipulation of cellular mechanotransduction signals. Chem. Eng. J. 2023, 455, 140699. [Google Scholar] [CrossRef]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; da Silva, C.E.R.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef]
- Soundarya, S.P.; Menon, A.H.; Chandran, S.V.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Biggs, M.J.P.; Richards, R.G.; Dalby, M.J. Nanotopographical modification: A regulator of cellular function through focal adhesions. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 619–633. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem. 2019, 21, 4839–4867. [Google Scholar] [CrossRef]
- Jahangirian, H.; Azizi, S.; Rafiee-Moghaddam, R.; Baratvand, B.; Webster, T.J. Status of Plant Protein-Based Green Scaffolds for Regenerative Medicine Applications. Biomolecules 2019, 9, 40. [Google Scholar] [CrossRef]
- Lian, H.; Liu, X.; Meng, Z. Enhanced mechanical and osteogenic differentiation performance of hydroxyapatite/zein composite for bone tissue engineering. J. Mater. Sci. 2018, 54, 719–729. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Jia, Y.; Han, H.; Sun, D. Preparation and Evaluation of Electrospun Zein/HA Fibers Based on Two Methods of Adding HA Nanoparticles. J. Bionic Eng. 2014, 11, 115–124. [Google Scholar] [CrossRef]
- Silva, S.S.; Oliveira, J.M.; Mano, J.F.; Reis, R.L. Physicochemical Characterization of Novel Chitosan-Soy Protein/TEOS Porous Hybrids for Tissue Engineering Applications. Mater. Sci. Forum 2006, 514–516, 1000–1004. [Google Scholar] [CrossRef]
- Chien, K.B.; Aguado, B.A.; Bryce, P.J.; Shah, R.N. In vivo acute and humoral response to three-dimensional porous soy protein scaffolds. Acta Biomater. 2013, 9, 8983–8990. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.M.; Wang, L.; Huang, J.; Rowe, D.W.; Wei, M. Bone tissue engineering with a collagen-hydroxyapatite scaffold and culture expanded bone marrow stromal cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 243–253. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Zhang, W.; Mao, L.-B.; Zhao, Y.; Yu, S.-H. Biomimetic mineralization of zein/calcium phosphate nanocomposite nanofibrous mats for bone tissue scaffolds. CrystEngComm 2014, 16, 9513–9519. [Google Scholar] [CrossRef]
- Tu, J.; Wang, H.; Li, H.; Dai, K.; Wang, J.; Zhang, X. The in vivo bone formation by mesenchymal stem cells in zein scaffolds. Biomaterials 2009, 30, 4369–4376. [Google Scholar] [CrossRef]
- Shahbazarab, Z.; Teimouri, A.; Chermahini, A.N.; Azadi, M. Fabrication and characterization of nanobiocomposite scaffold of zein/chitosan/nanohydroxyapatite prepared by freeze-drying method for bone tissue engineering. Int. J. Biol. Macromol. 2018, 108, 1017–1027. [Google Scholar] [CrossRef]
- Lee, H.-J.; Abueva, C.D.; Padalhin, A.R.; Lee, B.-T. Soya protein isolate-polyethylene oxide electrospun nanofiber membrane with bone marrow-derived mesenchymal stem cell for enhanced bone regeneration. J. Biomater. Appl. 2020, 34, 1142–1149. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Zheng, B.; He, J.; Liu, J.; Chen, C.; Lee, I.-s.; Wang, X.; Liu, Y. Synergistic Effects on Incorporation of β-Tricalcium Phosphate and Graphene Oxide Nanoparticles to Silk Fibroin/Soy Protein Isolate Scaffolds for Bone Tissue Engineering. Polymers 2020, 12, 69. [Google Scholar] [CrossRef]
- Chien, K.B.; Shah, R.N. Novel soy protein scaffolds for tissue regeneration: Material characterization and interaction with human mesenchymal stem cells. Acta Biomater. 2012, 8, 694–703. [Google Scholar] [CrossRef]
- Xu, H.; Cai, S.; Sellers, A.; Yang, Y. Intrinsically water-stable electrospun three-dimensional ultrafine fibrous soy protein scaffolds for soft tissue engineering using adipose derived mesenchymal stem cells. RSC Adv. 2014, 4, 15451–15457. [Google Scholar] [CrossRef]
- Nguyen, B.B.; Moriarty, R.A.; Kamalitdinov, T.; Etheridge, J.M.; Fisher, J.P. Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/chitosan/hyaluronic acid—Based injectable hydrogels for tissue engineering applications—Design, physicochemical and biological characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Khatami, N.; Khoshfetrat, A.B.; Khaksar, M.; Zamani, A.R.N.; Rahbarghazi, R. Collagen-alginate-nano-silica microspheres improved the osteogenic potential of human osteoblast-like MG-63 cells. J. Cell. Biochem. 2019, 120, 15069–15082. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-Z.; Wang, D.-W.; Zhang, Y.-J.; Lv, Z.Y.; Sun, X.-D.; Li, K.-Y.; Zhang, B.; Wang, X.-M.; Cui, F.-Z. Comparison of rabbit rib defect regeneration with and without graft. J. Mater. Sci. Mater. Med. 2017, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Guo, J.; Chen, C.; Yao, C.; Chung, S.-M.; Yao, J.; Lee, I.-S.; Kong, X. Silk fibroin membrane used for guided bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 148–154. [Google Scholar] [CrossRef]
- Shanmugavel, S.; Reddy, V.J.; Ramakrishna, S.; Lakshmi, B.; Dev, V.G. Precipitation of hydroxyapatite on electrospun polycaprolactone/aloe vera/silk fibroin nanofibrous scaffolds for bone tissue engineering. J. Biomater. Appl. 2014, 29, 46–58. [Google Scholar] [CrossRef]
- Lee, H.; Yang, G.H.; Kim, M.; Lee, J.; Huh, J.; Kim, G. Fabrication of micro/nanoporous collagen/dECM/silk-fibroin biocomposite scaffolds using a low temperature 3D printing process for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 140–147. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.; Ran, J.; Shen, X.; Tong, H. A novel nanocomposite for bone tissue engineering based on chitosan–silk sericin/hydroxyapatite: Biomimetic synthesis and its cytocompatibility. RCS Adv. 2015, 69, 56410–56422. [Google Scholar] [CrossRef]
- Fragogeorgi, A.E.; Rouchota, M.; Georgiou, M.; Velez, M.; Bouziotis, P.; Loudos, G. In vivo imaging techniques for bone tissue engineering. J. Tissue Eng. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Gil, H.M.; Price, T.W.; Chelani, K.; Bouillard, J.-S.G.; Calaminus, S.D.; Stasiuk, G.J. NIR-quantum dots in biomedical imaging and their future. iScience 2021, 24, 102189. [Google Scholar] [CrossRef] [PubMed]
- Jahed, V.; Vasheghani-Farahani, E.; Bagheri, F.; Zarrabi, A.; Jensen, H.H.; Larsen, K.L. Quantum dots-βcyclodextrin-histidine labeled human adipose stem cells-laden chitosan hydrogel for bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2020, 27, 102217. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Feng, S.; Guo, J.; Hou, J.; Zhu, X.; Chen, L.; Yang, H.; Chen, M.; Li, Y.; Chen, S.; et al. In vivo live imaging of bone using shortwave infrared fluorescent quantum dots. Nanoscale 2020, 12, 22022–22029. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, N.; Justa, P.; Kumar, H.; Deepshikha; Krishna; Pani, B.; Kumar, P. Biomedical Applications of Superparamagnetic Iron Oxide Nanoparticles (SPIONS) as a Theranostic Agent. In Iron Ores and Iron Oxide; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Cai, Z.; Wu, C.; Yang, L.; Wang, D.; Ai, H. Assembly-Controlled Magnetic Nanoparticle Clusters as MRI Contrast Agents. ACS Biomater. Sci. Eng. 2020, 6, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Seifalian, A.M.; Bull, E.; Madani, S.Y.; Sheth, R.; Green, M.; Seifalian, A. Stem cell tracking using iron oxide nanoparticles. Int. J. Nanomed. 2014, 9, 1641–1653. [Google Scholar] [CrossRef]
- Jing, X.-H.; Yang, L.; Duan, X.-J.; Xie, B.; Chen, W.; Li, Z.; Tan, H.-B. In vivo MR imaging tracking of magnetic iron oxide nanoparticle labeled, engineered, autologous bone marrow mesenchymal stem cells following intra-articular injection. Jt. Bone Spine 2008, 75, 432–438. [Google Scholar] [CrossRef]
- Kim, H.; Dae, H.-M.; Park, C.; Kim, E.O.; Kim, D.; Kim, I.-H.; Kim, Y.-H.; Choi, Y. A highly sensitive magnetite nanoparticle as a simple and rapid stem cell labelling agent for MRI tracking. J. Mater. Chem. 2011, 21, 7742–7747. [Google Scholar] [CrossRef]
- Yuan, D.; Ellis, C.M.; Davis, J.J. Mesoporous Silica Nanoparticles in Bioimaging. Materials 2020, 13, 3795. [Google Scholar] [CrossRef]
- Wu, Y.; Ali, M.R.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold nanoparticles in biological optical imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Bouché, M.; Hsu, J.C.; Dong, Y.C.; Kim, J.; Taing, K.; Cormode, D.P. Recent Advances in Molecular Imaging with Gold Nanoparticles. Bioconjugate Chem. 2020, 31, 303–314. [Google Scholar] [CrossRef]
- Raghavendran, H.R.B.; Puvaneswary, S.; Talebian, S.; Murali, M.R.; Naveen, S.V.; Krishnamurithy, G.; McKean, R.; Kamarul, T. A comparative study on in vitro osteogenic priming potential of electron spun scaffold PLLA/HA/Col, PLLA/HA, and PLLA/Col for tissue engineering application. PLoS ONE 2014, 9, e104389. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; He, M.; Liu, H.; Niu, Y.; Crawford, A.; Coates, P.D.; Chen, D.; Shi, R.; Zhang, L. Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials 2014, 35, 9395–9405. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Castro, N.J.; Lee, S.-J.; Noh, H.; Zhu, W.; Zhang, L.G. Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel. Nanoscale 2017, 9, 5055–5062. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Su, Y.-Y.; Jiang, X.-C.; Gao, J.-Q. Cell membrane-coated nanoparticles: A novel multifunctional biomimetic drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 716–737. [Google Scholar] [CrossRef]
- Fang, R.H.; Kroll, A.V.; Gao, W.W.; Zhang, L.F. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Wang, M.; Liu, R.; Song, H.; Li, N.; Lu, Q.; Zhang, W.; Du, Y.; Yang, W.; et al. Bioinspired nanoplatelets for chemo-photothermal therapy of breast cancer metastasis inhibition. Biomaterials 2019, 206, 1–12. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Lu, Q.; Zhao, J.; Wang, M.; Kan, Q.; Zhang, H.; Wang, Y.; He, Z.; Sun, J. Nanosponges of circulating tumor-derived exosomes for breast cancer metastasis inhibition. Biomaterials 2020, 242, 119932. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Fallica, A.N.; Virzì, N.; Kesharwani, P.; Pittalà, V.; Greish, K. The Promise of Nanotechnology in Personalized Medicine. J. Pers. Med. 2022, 12, 673. [Google Scholar] [CrossRef]
| Trial id | Title | Phase | NBT Used | Target |
|---|---|---|---|---|
| NCT04316091 | A Phase I Clinical Trial of Neoadjuvant Chemotherapy With/Without SPIONs/SMF for Patients with Osteosarcomas | I | SPIONS | Osteosarcoma/bone cancer |
| NCT01323894 | Osteogenic Effects on Human Mesenchymal Stem Cells Enhanced by Wnt Signaling (using HA NPs) | Observational | NP | Osteoblastogenesis of human MSCs |
| NCT05258006 | Assessment of Autogenous Dentin Graft in Treatment of Infra-bony Defect (using demineralized Dentin NPs) | NA | NP | Stage III periodontitis |
| NCT03678883 | 9-ING-41 in Patients with Advanced Cancers | II | NP | Cancers (including bone) |
| NCT04803500 | Simvastatin Around Immediate Implant (using simvastatin gel (1.2 mg/0.1 mL of solid lipid nanoparticles)) | II | Lipid NPs | Alveolar bone regeneration |
| NCT03140657 | The Effects of Nanocurcumin on Treg Cells and Th17 Cells Responses in Ankylosing Spondylitis Patients | II | Nanocurcumin | Intervertebral and sacroiliac joints |
| NCT05906563 | Evaluations of Melatonin and Metformin Loaded Nanoparticles in the Treatment of Periodontal Intra-bony Defects | II | NP | Bone loss in the jaw |
| NCT05101655 | Construction of Microfluidic Exosome Chip for Diagnosis of Lung Metastasis of Osteosarcoma (using NP tracking analysis (NTA)) | Observational | NP | Osteosarcomas, pulmonary metastases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, N.; Pant, T.; Rohra, N.; Goyal, A.; Lawrence, M.; Dey, A.; Ganguly, P. Nanobiotechnology in Bone Tissue Engineering Applications: Recent Advances and Future Perspectives. Appl. Biosci. 2023, 2, 617-638. https://doi.org/10.3390/applbiosci2040039
Iqbal N, Pant T, Rohra N, Goyal A, Lawrence M, Dey A, Ganguly P. Nanobiotechnology in Bone Tissue Engineering Applications: Recent Advances and Future Perspectives. Applied Biosciences. 2023; 2(4):617-638. https://doi.org/10.3390/applbiosci2040039
Chicago/Turabian StyleIqbal, Neelam, Tejal Pant, Nanda Rohra, Abhishek Goyal, Merin Lawrence, Anomitra Dey, and Payal Ganguly. 2023. "Nanobiotechnology in Bone Tissue Engineering Applications: Recent Advances and Future Perspectives" Applied Biosciences 2, no. 4: 617-638. https://doi.org/10.3390/applbiosci2040039
APA StyleIqbal, N., Pant, T., Rohra, N., Goyal, A., Lawrence, M., Dey, A., & Ganguly, P. (2023). Nanobiotechnology in Bone Tissue Engineering Applications: Recent Advances and Future Perspectives. Applied Biosciences, 2(4), 617-638. https://doi.org/10.3390/applbiosci2040039







