The Chaperone Hsp90, a Key Player in Salivary Gland Tumorigenesis
Abstract
:1. Introduction
2. Salivary Glands Cancer: Epidemiology and Pathogenesis
3. Salivary Gland Cancer Treatment and Patient Management
- (1)
- Surgery: Surgery is the primary treatment for most salivary gland cancers. The extent of surgery depends on the tumor size, location, and whether it has spread to nearby lymph nodes or other tissues. The goal of surgery is to remove the tumor while preserving as much healthy tissue and salivary gland function as possible.
- (2)
- Radiation therapy: Radiation therapy may be used as the primary treatment for small tumors, in combination with surgery, or after surgery to destroy any remaining cancer cells. External beam radiation delivers focused radiation to the tumor site while sparing surrounding healthy tissues.
- (3)
- Chemotherapy: Chemotherapy is not commonly used for all types of salivary gland cancer but may be considered for certain aggressive or advanced cases. It involves the use of drugs to kill cancer cells or stop their growth.
- (4)
- Targeted therapy: Targeted therapy is a newer approach that focuses on specific molecular targets within cancer cells. It is used in some salivary gland cancers that have specific genetic mutations or alterations.
- (5)
- Immunotherapy: Immunotherapy is being explored in clinical trials for some salivary gland cancers. It involves boosting the patient’s immune system to recognize and attack cancer cells more effectively.
- (6)
- Adjuvant and neoadjuvant therapies: Adjuvant therapy refers to additional treatment after the primary treatment (surgery or radiation) to reduce the risk of cancer recurrence. Neoadjuvant therapy is given before the main treatment to shrink the tumor and improve the chances of successful surgery or radiation.
- (7)
- Palliative care: For advanced or metastatic cases where a cure may not be possible, palliative care focuses on improving the patient’s quality of life. It aims to manage symptoms, alleviate pain, and provide emotional and psychological support to both the patient and their family.
- (8)
- Patient management also includes regular follow-up visits with the medical team to monitor treatment response, assess for any recurrence, and manage potential side effects. Supportive care, including speech and swallowing therapy, nutrition counseling, and psychological support, is vital for patients dealing with the effects of treatment on salivary gland function and overall well-being.
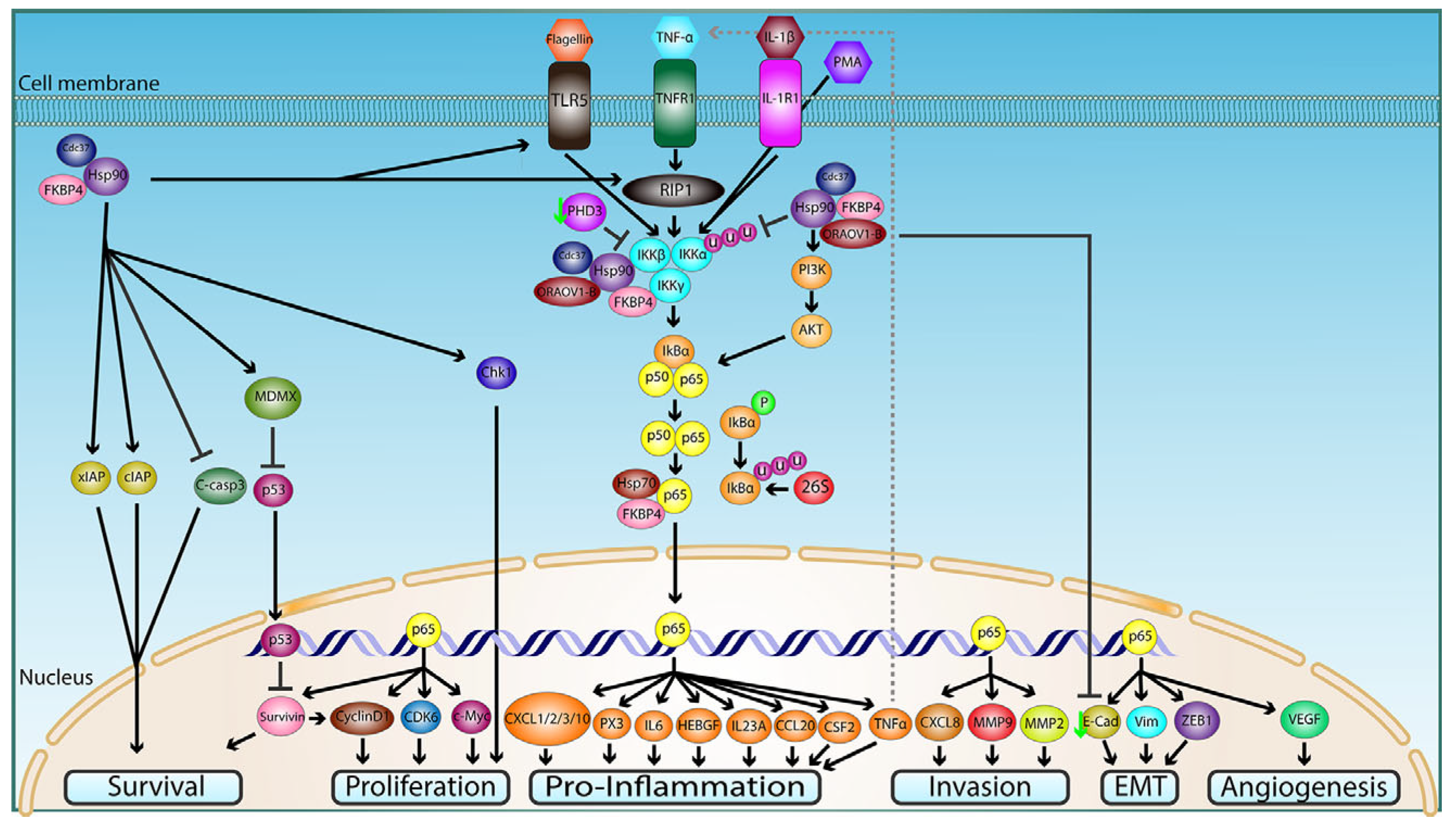
4. Hsp90 in Cancer
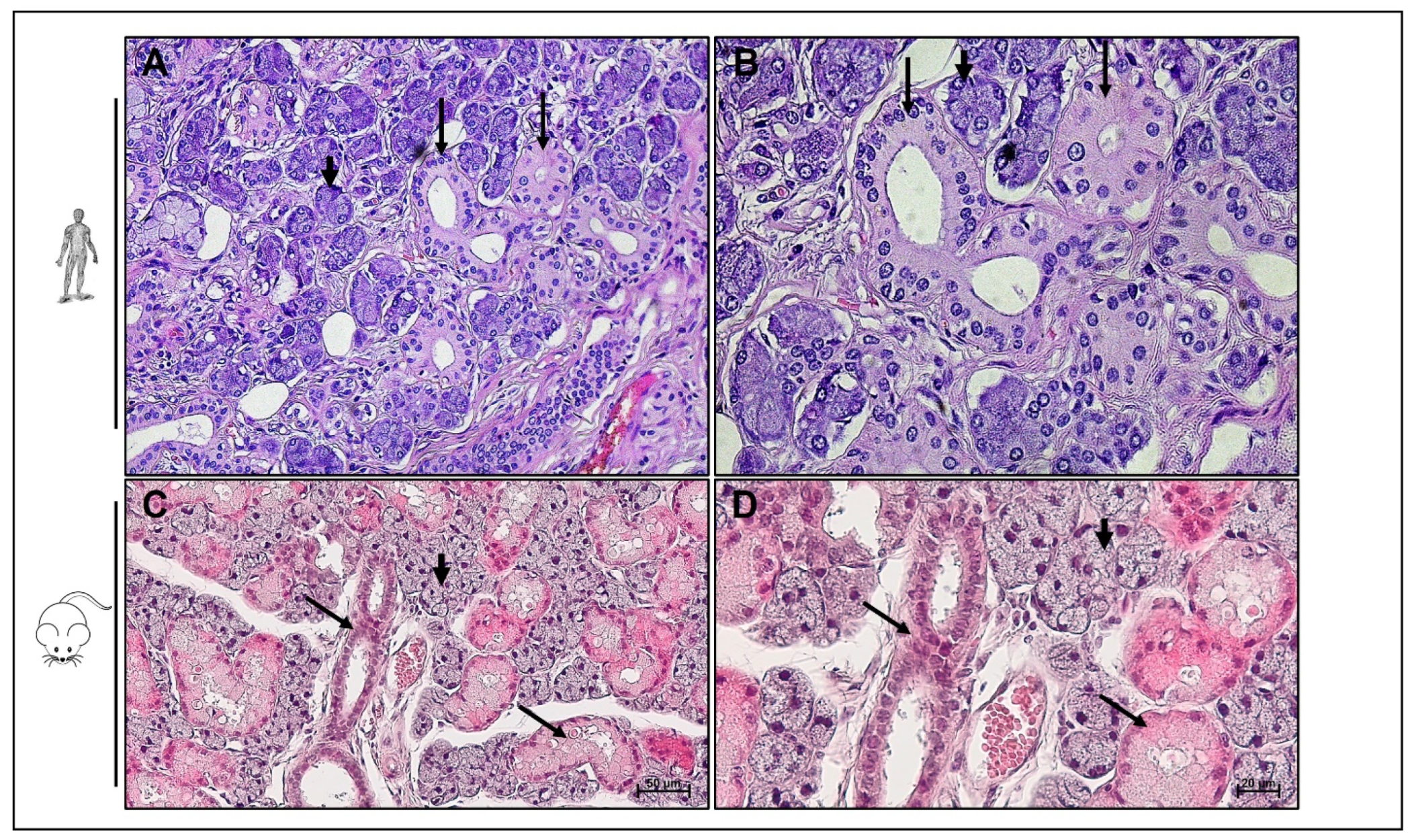
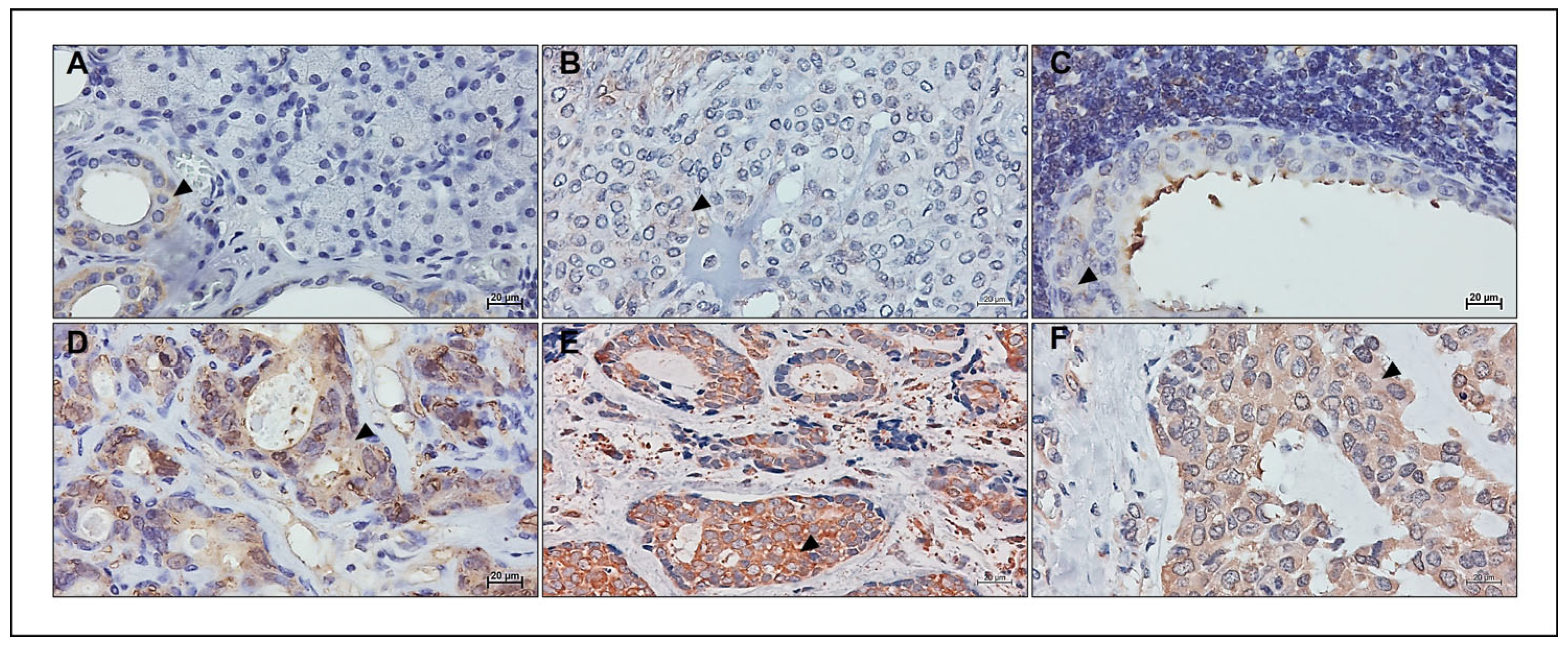
5. Hsp90 in Salivary Gland Tumors
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holmberg, K.V.; Hoffman, M.P. Anatomy, biogenesis and regeneration of salivary glands. Monogr. Oral Sci. 2014, 24, 1–13. [Google Scholar] [PubMed]
- Kessler, A.T.; Bhatt, A.A. Review of the Major and Minor Salivary Glands, Part 1: Anatomy, Infectious, and Inflammatory Processes. J. Clin. Imaging Sci. 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, F.C.; Bui, D.T. Anatomy, Function, and Evaluation of the Salivary Glands. In Salivary Gland Disorders; Myers, E.N., Ferris, R.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–16. [Google Scholar]
- Gatta, G.; Guzzo, M.; Locati, L.D.; McGurk, M.; Prott, F.J. Major and minor salivary gland tumours. Crit. Rev. Oncol. Hematol. 2020, 152, 102959. [Google Scholar] [CrossRef] [PubMed]
- Radha, R.; John Blesswin, A.; Selva Mary, G. A Simple Innovative Approach DNA-Based Saliva Security System for User Authentication. Indian J. Sci. Technol. 2016, 9, 1–6. [Google Scholar] [CrossRef]
- Zolotukhin, S. Metabolic hormones in saliva: Origins and functions. Oral Dis. 2013, 19, 219–229. [Google Scholar] [CrossRef]
- Gröschl, M. The Physiological Role of Hormones in Saliva. BioEssays 2009, 31, 843–852. [Google Scholar] [CrossRef]
- Basset, C.A.; Conway de Macario, E.; Leone, L.G.; Macario, A.J.L.; Leone, A. The chaperone system in cancer therapies: Hsp90. J. Mol. Histol. 2023, 54, 105–118. [Google Scholar] [CrossRef]
- Lin, H.H.; Limesand, K.H.; Ann, D.K. Current State of Knowledge on Salivary Gland Cancers. Crit. Rev. Oncog. 2018, 23, 139–151. [Google Scholar] [CrossRef]
- Skálová, A.; Hyrcza, M.D.; Leivo, I. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Salivary Glands. Head Neck Pathol. 2022, 16, 40–53. [Google Scholar] [CrossRef]
- Guzzo, M.; Locati, L.D.; Prott, F.J.; Gatta, G.; McGurk, M.; Licitra, L. Major and minor salivary gland tumors. Crit. Rev. Oncol. Hematol. 2010, 74, 134–148. [Google Scholar] [CrossRef]
- Del Signore, A.G.; Megwalu, U.C. The Rising incidence of major salivary gland cancer in the United States. Ear Nose Throat J. 2017, 96, E13–E16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, Y.; Li, M.; Yan, H.; Sun, M.; Fan, T. Management of salivary gland carcinomas—A review. Oncotarget 2017, 8, 3946–3956. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.L.; Ismaila, N.; Beadle, B.; Caudell, J.J.; Chau, N.; Deschler, D.; Glastonbury, C.; Kaufman, M.; Lamarre, E.; Lau, H.Y.; et al. Management of Salivary Gland Malignancy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1909–1941. [Google Scholar] [CrossRef]
- Sood, S.; McGurk, M.; Vaz, F. Management of Salivary Gland Tumours: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S142–S149. [Google Scholar] [CrossRef]
- Macario, A.J.L.; Conway de Macario, E. Chaperonins in Cancer: Expression, function, and migration in extracellular vesicles. Semin. Cancer Biol. 2022, 86, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Macario, A.J.L.; Conway de Macario, E. Chaperone Proteins and Chaperonopathies. In Stress: Physiology, Biochemistry, and Pathology; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 3, pp. 135–152. [Google Scholar] [CrossRef]
- Johnson, J.L. Mutations in Hsp90 Cochaperones Result in a Wide Variety of Human Disorders. Front. Mol. Biosci. 2021, 8, 787260. [Google Scholar] [CrossRef] [PubMed]
- Macario, A.J.L.; Conway de Macario, E. Sick chaperones, cellular stress, and disease. N. Engl. J. Med. 2005, 353, 1489–1501. [Google Scholar] [CrossRef]
- Basset, C.A.; Rappa, F.; Barone, R.; Florena, A.M.; Porcasi, R.; Conway de Macario, E.; Macario, A.J.L.; Leone, A. The Chaperone System in Salivary Glands: Hsp90 Prospects for Differential Diagnosis and Treatment of Malignant Tumors. Int. J. Mol. Sci. 2022, 23, 9317. [Google Scholar] [CrossRef]
- Basset, C.A.; Rappa, F.; Lentini, V.L.; Barone, R.; Pitruzzella, A.; Unti, E.; Cappello, F.; Conway de Macario, E.; Macario, A.J.L.; Leone, A. Hsp27 and Hsp60 in human submandibular salivary gland: Quantitative patterns in healthy and cancerous tissues with potential implications for differential diagnosis and carcinogenesis. Acta Histochem. 2021, 123, 151771. [Google Scholar] [CrossRef]
- Basset, C.A.; Cappello, F.; Rappa, F.; Lentini, V.L.; Jurjus, A.R.; Conway de Macario, E.; Macario, A.J.L.; Leone, A. Molecular Chaperones in Tumors of Salivary Glands. J. Mol. Histol. 2020, 51, 109–115. [Google Scholar] [CrossRef]
- Basset, C.A.; Cappello, F.; Rappa, F.; Jurjus, A.R.; Conway de Macario, E.; Macario, A.J.L.; Leone, A. Chaperonin Hsp60 and Cancer Therapies. In Heat Shock Proteins in Human Disease; Asea, A.A.A., Kaur, P., Eds.; Springer: Cham, Switzerland, 2020; Volume 21, pp. 31–52. [Google Scholar]
- Fouani, M.; Basset, C.A.; Mangano, G.D.; Leone, L.G.; Lawand, N.B.; Leone, A.; Barone, R. Heat Shock Proteins Alterations in Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 2806. [Google Scholar] [CrossRef] [PubMed]
- Macario, A.J.L.; Conway de Macario, E. The chaperone system in autoimmunity, inflammation, and virus-induced diseases: Role of chaperonins. In Stress: Immunology and Inflammation; Fink, G., Ed.; Elsevier/Academic Press: San Diego, CA, USA, 2023; Volume 5, pp. 119–128. [Google Scholar]
- Caruso Bavisotto, C.; Cipolla, C.; Graceffa, G.; Barone, R.; Bucchieri, F.; Bulone, D.; Cabibi, D.; Campanella, C.; Marino Gammazza, A.; Pitruzzella, A.; et al. Immunomorphological Pattern of Molecular Chaperones in Normal and Pathological Thyroid Tissues and Circulating Exosomes: Potential Use in Clinics. Int. J. Mol. Sci. 2019, 20, 4496. [Google Scholar] [CrossRef]
- Rappa, F.; Sciume, C.; Lo Bello, M.; Caruso Bavisotto, C.; Marino Gammazza, A.; Barone, R.; Campanella, C.; David, S.; Carini, F.; Zarcone, F.; et al. Comparative analysis of Hsp10 and Hsp90 expression in healthy mucosa and adenocarcinoma of the large bowel. Anticancer Res. 2014, 34, 4153–4160. [Google Scholar] [PubMed]
- Barone, R.; Caruso Bavisotto, C.; Rappa, F.; Gargano, M.L.; Macaluso, F.; Paladino, L.; Vitale, A.M.; Alfano, S.; Campanella, C.; Gorska, M.; et al. JNK pathway and heat shock response mediate the survival of C26 colon carcinoma bearing mice fed with the mushroom Pleurotus eryngii Var. eryngii without affecting tumor growth or cachexia. Food Funct. 2021, 12, 3083–3095. [Google Scholar] [CrossRef]
- Gorska-Ponikowska, M.; Kuban-Jankowska, A.; Marino Gammazza, A.; Daca, A.; Wierzbicka, J.M.; Zmijewski, M.A.; Luu, H.H.; Wozniak, M.; Cappello, F. The Major Heat Shock Proteins, Hsp70 and Hsp90, in 2-Methoxyestradiol-Mediated Osteosarcoma Cell Death Model. Int. J. Mol. Sci. 2020, 21, 616. [Google Scholar] [CrossRef] [PubMed]
- Kamm, A.; Przychodzeń, P.; Kuban-Jankowska, A.; Marino Gammazza, A.; Cappello, F.; Daca, A.; Żmijewski, M.A.; Woźniak, M.; Górska-Ponikowska, M. 2-Methoxyestradiol and Its Combination with a Natural Compound, Ferulic Acid, Induces Melanoma Cell Death via Downregulation of Hsp60 and Hsp90. J. Oncol. 2019, 2019, 9293416. [Google Scholar] [CrossRef]
- Ansa-Addo, E.A.; Thaxton, J.; Hong, F.; Wu, B.X.; Zhang, Y.; Fugle, C.W.; Metelli, A.; Riesenberg, B.; Williams, K.; Gewirth, D.T.; et al. Clients and Oncogenic Roles of Molecular Chaperone gp96/grp94. Curr. Top. Med. Chem. 2016, 16, 2765–2778. [Google Scholar] [CrossRef]
- Chiu, C.C.; Lin, C.Y.; Lee, L.Y.; Chen, Y.J.; Lu, Y.C.; Wang, H.M.; Liao, C.T.; Chang, J.T.C.; Cheng, A.J. Molecular chaperones as a common set of proteins that regulate the invasion phenotype of head and neck cancer. Clin. Cancer Res. 2011, 17, 4629–4641. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Ma, C.; Sun, S.; Zhang, J.; Sun, Y. Expression of heat-shock protein gp96 in gallbladder cancer and its prognostic clinical significance. Int. J. Clin. Exp. Pathol. 2015, 8, 1946–1953. [Google Scholar]
- Feng, J.; Xie, G.; Zhan, Y.; Lu, J.; Xu, L.; Fan, S.; Wang, W. Elevated HSP90 associates with expression of HIF-1α and p-AKT and is predictive of poor prognosis in nasopharyngeal carcinoma. Histopathology 2019, 75, 202–212. [Google Scholar] [CrossRef]
- Maddalena, F.; Simeon, V.; Vita, G.; Bochicchio, A.; Possidente, L.; Sisinni, L.; Lettini, G.; Condelli, V.; Matassa, D.S.; Li Bergolis, V.; et al. TRAP1 protein signature predicts outcome in human metastatic colorectal carcinoma. Oncotarget 2017, 8, 21229–21240. [Google Scholar] [CrossRef] [PubMed]
- Pick, E.; Kluger, Y.; Giltnane, J.M.; Moeder, C.; Camp, R.L.; Rimm, D.L.; Kluger, H.M. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007, 67, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Zagouri, F.; Sergentanis, T.; Nonni, A.; Papadimitriou, C.; Pazaiti, A.; Michalopoulos, N.V.; Safioleas, P.; Lazaris, A.; Theodoropoulos, G.; Patsouris, E.; et al. Decreased Hsp90 expression in infiltrative lobular carcinoma: An immunohistochemical study. BMC Cancer 2010, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gu, X.; Chen, L.; Wang, Y.; Cao, B.; Qun, E. Comparison of the expression of 5 heat shock proteins in benign and malignant salivary gland tumor tissues. Oncol. Lett. 2013, 5, 1363–1369. [Google Scholar] [CrossRef]
- Shigeishi, H.; Sugiyama, M.; Tahara, H.; Ono, S.; Bhawal, U.K.; Okura, M.; Kogo, M.; Shinohara, M.; Shindoh, M.; Shintani, S.; et al. Increased telomerase activity and hTERT expression in human salivary gland carcinomas. Oncol. Lett. 2011, 2, 845–850. [Google Scholar] [CrossRef]
- Stepanova, L.; Finegold, M.; DeMayo, F.; Schmidt, E.V.; Harper, J.W. The oncoprotein kinase chaperone CDC37 functions as an oncogene in mice and collaborates with Both c-Myc and cyclin D1 in transformation of multiple tissues. Mol. Cell Biol. 2000, 20, 4462–4473. [Google Scholar] [CrossRef]
- Sanchez, J.; Carter, T.R.; Cohen, M.S.; Blagg, B.S.J. Old and New Approaches to Target the Hsp90 Chaperone. Curr. Cancer Drug Targets 2019, 20, 253–270. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, L.; Jiang, J.; Zheng, Z.; Shang, J.; Wang, C.; Chen, W.; Bao, Q.; Xu, X.; et al. Small-molecule inhibitor targeting the Hsp90-Cdc37 protein-protein interaction in colorectal cancer. Sci. Adv. 2019, 5, eaax2277. [Google Scholar] [CrossRef]
- Blair, L.J.; Genest, O.; Mollapour, M. The multiple facets of the Hsp90 machine. Nat. Struct. Mol. Biol. 2019, 26, 92–95. [Google Scholar] [CrossRef]
- Honma, Y.; Kurokawa, Y.; Sawaki, A.; Naito, Y.; Iwagami, S.; Baba, H.; Komatsu, Y.; Nishida, T.; Doi, T. Randomized, double-blind, placebo (PL)-controlled, phase III trial of pimitespib (TAS-116), an oral inhibitor of heat shock protein 90 (HSP90), in patients (Pts) with advanced gastrointestinal stromal tumor (GIST) refractory to imatinib (IM), sunitinib (SU) and regorafenib (REG). J. Clin. Oncol. 2021, 39, 11524. [Google Scholar] [CrossRef]
| Water 99.5% |
| Solid constituents 0.5% |
| Organic solid constituents 0.3%: mucin, serum albumin, serum globulin, amino acids, amylase, lysozyme, IgA, IgG, glucose, citrate, lactate, ammonia, urea, uric acid, creatinine, cholesterol, cyclic nucleotides: cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) |
| Inorganic solid constituents 0.2%: NaCl; KCl; NaHCO3; Na2; HPO4; CaCO3; KSCN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basset, C.A.; Hajj Hussein, I.; Jurjus, A.R.; Cappello, F.; Conway de Macario, E.; Macario, A.J.L.; Leone, A. The Chaperone Hsp90, a Key Player in Salivary Gland Tumorigenesis. Appl. Biosci. 2023, 2, 607-616. https://doi.org/10.3390/applbiosci2040038
Basset CA, Hajj Hussein I, Jurjus AR, Cappello F, Conway de Macario E, Macario AJL, Leone A. The Chaperone Hsp90, a Key Player in Salivary Gland Tumorigenesis. Applied Biosciences. 2023; 2(4):607-616. https://doi.org/10.3390/applbiosci2040038
Chicago/Turabian StyleBasset, Charbel A., Inaya Hajj Hussein, Abdo R. Jurjus, Francesco Cappello, Everly Conway de Macario, Alberto J. L. Macario, and Angelo Leone. 2023. "The Chaperone Hsp90, a Key Player in Salivary Gland Tumorigenesis" Applied Biosciences 2, no. 4: 607-616. https://doi.org/10.3390/applbiosci2040038
APA StyleBasset, C. A., Hajj Hussein, I., Jurjus, A. R., Cappello, F., Conway de Macario, E., Macario, A. J. L., & Leone, A. (2023). The Chaperone Hsp90, a Key Player in Salivary Gland Tumorigenesis. Applied Biosciences, 2(4), 607-616. https://doi.org/10.3390/applbiosci2040038









