Abstract
Insects and ectoparasites are causes for major concern throughout the world due to their economic and welfare impacts on livestock agriculture. Current control measures involve chemicals such as acaricides which pose challenges like chemical resistance and longer withholding periods. To enable more sustainable agriculture practices, it is important to develop technologies that combine targeted effectiveness with minimal environmental footprint. RNA interference (RNAi) is a eukaryotic process in which transcript expression is reduced in a sequence-specific manner. This makes it a perfect tool for developing efficient and effective biological control against pests and pathogens. Double-stranded RNA (dsRNA) is the key trigger molecule for inducing RNAi; this concept is widely studied for development of RNA-based biopesticides as an alternative to chemical controls in crop protection for targeting pests and pathogens with accuracy and specificity. In this review, we discuss key advances made using RNAi technology and how they can be applied to improve health in livestock industries. This includes research focused on different delivery mechanisms of dsRNA, important developments in regulatory frameworks, and risk identification, that will enable the future adoption of RNAi technologies to improve animal health.
1. Introduction
Pests and pathogens have threatened primary production since farming began. According to the Food and Agriculture Organization, parasites affect 80% of the world’s livestock population [1,2,3]. In livestock production systems, chemical pesticides such as insecticides are commonly utilised to control and combat pests like insects, because of their low prices, high availability, fast mode of action, and perceived reliability. However, the extreme dependence on the use of these compounds has resulted in ubiquitous, low-level exposure, which is potentially harmful to both human health and ecosystems [4]. Besides risks to human health, widespread and repeated insecticide application can also result in environmental concentration build-up in soils [5]. Furthermore, direct application of these chemical pesticides have other important limitations like, poor specificity, short duration of protection, poor water-solubility, difficulties with site-specific uptake by the targeted pest, and inducing chemical-resistant pathogen strains [4,6]. Some novel applications have been investigated to increase the efficiency of insecticides, such as site-specific spraying and human–robot collaborative sprayer. However, these methods require significant investment and expensive maintenance cost, making them less practical in many agricultural applications [7,8]. Thereby developing safe, environmentally friendly, effective, and sustainable pest management strategies has become a much-needed requirement to improve the productivity and sustainability of global agriculture.
RNA interference (RNAi) has emerged as a promising candidate for the development of biological based control strategies, offering excellent target species specificity and sustainability management of pests and pathogens affecting livestock agriculture. Unlike conventional pesticides used to control pests, RNAi based control uses the target pests’ own molecular mechanisms to initiate silencing of essential genes. Ideally, the RNAi approach drives mRNA degradation to block targeted protein production and inhibits pest growth, depending on the developmental stage of the pest and gene of interest targeted. The RNAi mechanism is a conserved, nucleic acid metabolism, which can be initiated by exogenously applied or endogenously expressed double-stranded RNAs (dsRNA) in many species [9,10].
RNAi technology has shown great potential in crop protection for controlling hemipteran pests, including aphids, whiteflies, planthoppers and psyllids [10]. The first commercial application of RNAi was done using SmartStax-PRO, developed by Monsanto and Dow AgroSciences to protect maize against corn rootworms. Transgenic maize MON874411 expressed dsRNA targeting DvSnf7 in Western corn rootworm (WCR), which was used on-field for controlling WCR, and other corn rootworms, lepidopteran pests and weeds [11,12]. In 2011, the Monsanto company published a patent for using polynucleotide molecules for gene regulation in plants [13]. However, fewer studies have focused on using this innovative technology for controlling pests affecting animals in agriculture. Recently, there have been an increasing number of reports on the application of RNAi to improve the control of livestock pest, that have proven to be recalcitrant to conventional control strategies. For example, limited research on nematode parasites in pigs, such as Trichinella spiralis and Ascaris suum, and ticks in cattle have been reported to have potential to be controlled efficiently with RNAi technology [14,15,16,17].
In this review, the potential advantages that RNAi technology offers are detailed, along with the scope and significance of using RNAi to control pests impacting the health of livestock. Additionally, key attributes of RNAi target identification, effector molecule design and different delivery methods used for the delivery of dsRNA are reviewed. In addition, the challenges associated with the use of this technology in field are also discussed.
2. RNA-Interference (RNAi)
RNAi is an endogenous, post-transcriptional gene regulation mechanism which has been identified in almost all eukaryotes; from plants, fungi, algae, protozoans, invertebrates, to vertebrates [18,19]. It was first reported by Napoli and Jorgensen in 1990 when they observed endogenous gene co-suppression while studying pigmented petunias [20,21]. In animals, RNAi was first documented in the nematode Caenorhabditis elegans [22,23]. While the intracellular components of RNAi are similar across species, it was initially known as quelling in fungi, and post transcriptional gene silencing in plants [24]. It is a highly conserved mechanism, which is highly sequence specific and selective in its activity [25].
2.1. RNAi Mechanism
While a comprehensive review on RNAi mechanism discussing all the different facets of this technology is outside the scope of this review, the basic mechanism of RNAi when initiated via delivered exogenously applied dsRNA are well described and are illustrated in Figure 1.
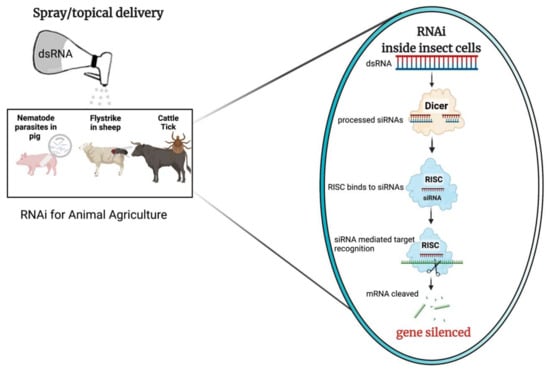
Figure 1.
Schematic example of RNA interference (RNAi) delivery via spray/topical application for animal agriculture; this image was made using BioRender. After application over the animal fur/surface, the dsRNA would enter the cells of the insect pest or pathogen. dsRNA is cleaved by dicer-2 into pre-siRNA duplexes, that are further processed into small-interfering RNA (siRNAs, 21–24 nt) effector molecules. The RNA-induced silencing complex (RISC) binds to the siRNAs and guides sequence-dependant degradation or translational inhibition of homologous mRNAs, which results in RNAi-mediated gene silencing.
RNAi-mediated post-transcriptional gene silencing is triggered by the processing of a dsRNA precursor into short single stranded RNA effector molecules [18]. There are three RNAi pathways, depending on the RNA class of effector involved, small-interfering RNA (siRNA), piwi-interacting RNA (piRNA), and microRNA (miRNA) [18,25,26,27]. Of these, siRNA is considered to be the ‘classical’ pathway, with dsRNA being the trigger molecule for gene silencing in insects and other species [27]. In the siRNA pathway, once taken up by cells dsRNA is cleaved by an endonuclease, Dicer, into pre-siRNA duplexes [24]. The pre-siRNA is a 21–23 nucleotide (nt) long duplex with 2 nt overhangs at each of the 3′ termini. The pre-siRNA duplex is bound by the RNA-induced silencing complex (RISC), with one strand, the guide strand, being retained within the complex, while the complementary or passenger strand of the pre-siRNA duplex is degraded [4,28]. The guide siRNA strand with the Argonaut proteins (Ago) within the activated RISC, identify matching mRNA in a sequence dependent manner, resulting in suppression of translation or mRNA degradation [29]. This results in loss of protein function which may lead to lethality or stunted growth of the target organism [4,27].
2.2. RNAi Targets
Target gene selection depends on the class of RNA effector molecule to be used. While both siRNA and piRNA are generated from long, complementary dsRNAs, miRNA is endogenous in nature, usually processed from stem-loops, and siRNA are exogenous and are directly generated from cleaved dsRNAs [30]. The first step in developing an RNAi product is to identify potential target genes and design dsRNA targeting them. In 2010, a research study suggested that there are five factors which play a key role in influencing the efficiency of RNAi as biocontrol, namely, the concentration, nucleotide sequence, length of the dsRNA, effective period of the dsRNA induced silencing, and life stage of the target [31].
The perfect RNAi target gene should be transcribed into an mRNA with a high turn overrate, that is translated into a protein with a short half-life, and is transcribed at all stages of life cycle, with the loss of function leading to mortality or severe impairment in the pest or pathogen of interest. In addition, the target transcript should be poorly conserved across species to maintain maximum specificity of mRNA for minimal environmental effects on non-target species [27]. This is a challenging criterion to meet, as those genes that are essential to cell viability tend to be more conserved between species.
It has reported that the efficiency of dsRNA uptake is length dependent, requiring an optimal length and dosage to induce RNAi successfully [32]. Studies have shown that dsRNA as short as 30 and 60-bp can induce 30% and 70% gene knockdown in the red flour beetle (Tribolium castaneum), and the optimum length 200–520 bps for most insects [31,33]. This ideal dosage changes according to the organism’s susceptibility to RNAi and their potential for systemic RNAi, formulation for delivery, gene expression abundance, life and development stage for gene expression [27]. Unfortunately, genes that often would lead to mortality if targeted by RNAi are highly conserved across nature, due to their evident importance for homeostasis [27].
Various selection methods can be used to develop efficient RNAi targets to minimize side effects and enhance expected silencing. Since RNAi relies on high gene specificity to the selected target species based on sequence divergence, dsRNA needs to be screened and designed to be specific to a target gene or to target genes on a broad spectrum, closer to related species [34]. To increase knockdown or have higher than the expected RNAi result, multiple targets can be selected for a single transcript to ensure variation within the target species is accounted for. A common application that can be used to evaluate off-target genes is the Basic Local Alignment Search Tool (BLAST), and it has been used to find contiguous matching sections of 17 nt or more in the genomes of interesting organisms [34]. BLAST is a common approach for finding regions of identity, and for identifying the functionality of siRNA. Thereafter, a wide range of specialised programs can be used to design the sequences. These include siRNA-Finder (si-Fi), siDirect, dsCheck, and RNAi Designer by ThermoFisher [35].
Amongst insect researchers it is widely accepted that several dsRNA should be screened as a combination as each gene is highly unique to the target gene and the insect species [33]. Whyard et al. demonstrated species specific insecticides could be achieved by targeting the variable 3′-UTR regions of the y-Tubulin transcript, allowing for a specific species knockdown between four closely related Drosophila species [36]. The results of this study clearly demonstrate that with sufficient sequence information and thorough interrogation of this available data, the development of highly specific RNAi based insecticides is highly feasible.
While the application of RNAi animal agriculture is still in its infancy, there are more dosage trials in plant agriculture, such as efficiency of dosage needed per acre regarding insect pests. Despite the fact that sufficient field experimentation is lacking, it is estimated that near 2–10 g of dsRNA is needed per acre of plants [30]. As this depends on the organisms response efficiency and target gene, this number is open to high levels of variability [30].
2.3. dsRNA Uptake Mechanism
The use of RNAi to protect plants and animals by suppressing essential gene function in pest species has been well documented in model invertebrates such as Caenorhabditis elegans, and Drosophila melanogaster [37]. While most of the reported experiments involved injecting dsRNA into the target organism, this is not a viable delivery approach for in-field applications in animal production, thus creating the need for autonomous dsRNA uptake [31,37]. Studies have reported two dsRNA uptake mechanisms, the transmembrane channel-mediated uptake and an alternate endocytosis-mediated uptake [37].
Transmembrane channel-mediated uptake mechanism has been best studied in C. elegans, more specifically with worms carrying mutant sid genes that lack systemic RNAi capacity [31]. This pathway involves two proteins, SID-1 and SID-2, with changes being made to the protein molecule affecting other cells and their activity. SID-1 is a hypothesized transmembrane protein which passively transports dsRNA into the cells [31,38]. The role played by SID-2 has been hypothesized to help facilitate RNAi by coordinating with SID-1 either by modifying the molecule to activate it or binding to dsRNA for delivery or by inducing endocytosis [31]. It has been noted that SID-1 is considered to be a ‘cell surface conduit’ for dsRNA uptake [32]. Meanwhile, endocytosis-mediated mechanism was first studied in D. melanogaster, since it had no sid gene orthologues and the mechanism was later confirmed in C. elegans [31]. This is a cell-autonomous pathway wherein dsRNA is transported intracellularly via vesicles [31,38]. Drosophila S2 cells were able to take up dsRNA and initiate an RNAi response by receptor-mediated endocytosis uptake which is an active process involving vacuolar H+ (V-H) ATPase [31,32]. This pathway is active, specific and is conserved in other animal cells [32].
Some organisms can take up exogenous dsRNA and trigger an RNAi response in the entire animal systemically, like C. elegans, while others cannot transmit this RNAi response in another cell [32]. In Drosophila, it has been confirmed that transposon inversion can activate RNAi, and the homologous gene silencing caused by it is similar to co-inhibition in plants [39]. In a C. elegans assay, it was found that the quelling defective protein, QDE-1, a necessary protein for RNAi process, is homologous to RNA-dependent RNA polymerase (RDRP), supporting RNAi process potential involved in RNA replication and regulation [40]. Similarly, dsRNA injection into phloem of the plant can spread throughout the whole plant to initiate systemic RNAi [41]. Interestingly, RNAi can also be induced by immersing nematodes in dsRNA-containing liquids or by feeding nematodes with E. coli bacteria engineered to express dsRNA [42]. The pathway for entry of dsRNA into the target cell determines RNAi efficiency and provides a starting point for the development of dsRNA delivery systems [32].
2.4. dsRNA Delivery
RNAi efficacy after cellular uptake had been a concern since the immune system and gut enzymes, mainly intestinal nucleases, digest dsRNA. Thereby affecting dsRNA activity as a pest control [43,44]. This identified the need for a carrier system to protect the dsRNA from degradation and thus, the delivery system for dsRNA can play an important role in the success of RNAi activity. There are several delivery systems, such as microinjection, transgenic crops expressing dsRNA, and oral feeding [45].
2.4.1. Injection
The first microinjection experiment was reported in 1998 by Kennerdell and Carthew in Drosophila melanogaster where they showed successful downregulating of frizzled (fz) and frizzled 2 (Dfz2) genes [46,47]. dsRNA corresponding to Wnt ortholog wingless (wg) gene was injected into drosophila embryos, resulting in wg-like phenotype at the site of injection with the RNAi effect being localized. Apart from D. melanogaster, dsRNA was injected in T. castaneum, which showed RNAi response in larval, and pupal tissues, and phenotypical defects in adults [48]. This was considered to be a robust system since the previous experiments only reported changes seen in embryos and embryonic RNAi does not persist in the later stages of the insect life cycle [47,48]. Overall, there appears to be a wide range of experimental success rates for different species, with larvae, having injections performed on or between segments, while it was convenient to inject adults under the wings [49]. Although there are many successful cases of injection dsRNA delivery triggering RNAi, it cannot be denied that different microinjection protocols can significantly affect the experimental success rate. There is also the potential to injure the cuticle at the injection site which has necessitated the shift to oral administration of dsRNA [50]. It has been speculated to be a combination of several elements, including the insect’s life stage, injected concentration, and injected dsRNA quantity [49].
2.4.2. Oral Feeding
Ingestion in pests include oral feeding and transgenic plant intake [47]. In 2009, Whyard et al., successfully utilized a liposome carrier for dsRNA and triggered RNAi in four insect pest species; D. melanogaster, T. castaneum, A. pisum, and M. sexta [36]. The first demonstration of dsRNA ingestion in rootworms was been studied and documented, with Dvsnf7 dsRNA being fed to western corn rootworm (WCR—Diabrotica virgifera virgifera). At 24 h post feeding of larvae resulting in them showing high absorbance to 60 bp dsRNA and the rootworm began to die after showing developmental retardation [49]. Similar results had been found in Sri Lanka weevil, a highly polyphagous pest [51]. When Pros2 and Snf7 dsRNAs were orally administered to the target, large decline in gene transcript levels were seen along with significant mortality rates (78.6 and 92.7% respectively) [51]. Collectively, these studies indicate the potential of oral delivery as a more practical approach.
2.4.3. Spraying
Considering that among these delivery systems, microinjection has proven to be not feasible for in-field application as it can develop ecological risks, and feeding on plants tends to show low efficacy due to degradation of dsRNA by enzymatic activity within the digestive system of the target pest, alternate delivery systems are required [52]. One such method is the spray-induced gene silencing (SIGS) delivery system which involves spraying of dsRNA that has been loaded onto plant or animal surfaces [52,53]. Dalakouras et al., reported that applying high-pressure spraying of GFP gene to plant can result in locally silencing of GFP gene [54]. In green fluorescent protein (GFP)-expressing transgenic Nicotiana benthamiana plants, merely spraying and injecting siRNA failed to induce silencing, while high-pressure spraying of multiple sizes of siRNAs (21, 22, and 24 nt) induced localised GFP silencing [54]. These studies supported the idea that a specifically designed application system to different host or targeted gene could be required in some situations.
2.4.4. Nanoparticle Delivery System
The first evidence for using nanoparticle carriers for dsRNA was published [55]. In their study, naked dsRNAs were shown to be ineffective with direct feeding and showed more effective results (95.4% RNAi efficiency and 80.5% reduction in target population) with a nanocarrier-based delivery method [55,56]. The nanoparticle carrier used in the study showed negligible toxicity to non-target cells and aphids [56]. Additionally, nanoparticles have enabled penetration and uptake of dsRNA into the cells using transdermal dsRNA fluorescent nanocarrier delivery system [30,57]. A scientific publication reported significant knockdown (from 86.86 to 58.87%) using this delivery method for Aphis glycines, a soybean pest [57]. The use of nanocarriers is being researched for overcoming such limitation across different delivery systems, especially since it reduced dependence on viral-vectors and is safer and more convenient alternative for mass production [44,57].
One of the most commonly used nanomaterials is chitosan, which is a biodegradable polymer made by deacetylation of chitin [24]. Chitosan was used as a nanocarrier to deliver dsRNA in Anopheles gambiae larvae and was found to protect dsRNA and help stabilize it. This was fed to the mosquito larvae via agarose gel coated food which triggered RNAi and led to increased mortality [58]. Recent studies on nanocarriers which have shown promising results are layered double hydroxide (LDH) clay nanosheets. It was demonstrated by Mitter et al., in 2017, where non-toxic LDH were adsorbed with dsRNA (dsRNA-LDH complexes) to form BioClay™ which can be sprayed onto plant/leaf surfaces and induce an RNAi reaction in the target pest [59]. The dsRNA was protected from by the LDH nanosheets effectively against nucleases, and showed strong adhesion even after rinsing [59]. The LDH nanosheets enhanced the stability of dsRNA under environmental conditions for a longer period, making BioClay™ suitable for spraying on-field [59,60]. Apart from nanocarriers, cationic lipids are another medium for transferring dsRNA into target cells [61]. Lipid-based transfection reagents can also increase RNAi efficiency, these vesicles are referred to as liposomes. Electrostatic interactions between positively charged cationic lipids and negatively charged dsRNA can produce liposomes [62].
More research was done using similar technology, but with a controlled dsRNA release. One such approach is by making dsRNA release pH dependent, with the clay nanocarrier releasing dsRNA either under acidic or basic conditions, based on the target site pH level and the environment conditions to maintain stability [63]. This was investigated in our lab, where the stability of dsRNA was tested after loading on two types of biodegradable clay particles: Clay 1 (releases dsRNA under acidic conditions) and Clay 2 (releases dsRNA under alkaline conditions) on cattle hide. Cattle skin was treated with Cy3 labelled dsRNA alone and Cy3 labelled dsRNA loaded on Clay1 or Clay2. The treated skin samples were imaged using confocal microscopy for the presence of fluorescence signal. We found that the dsRNA loaded on clay particles was stable unlike naked Cy3-dsRNA which degraded and was not visible after washing. This study showed that the clay particles increased inherent stability of the dsRNA molecules, offering promise to provide a sustainable solution for animal health [63]. A study in 2020 compared various inorganic nanomaterials as potential carriers for dsRNA in C. elegans, of which only dsRNA adsorbed to a synthetic cationic nanocarrier, Mg-Al LDH showed effective knockdown with gene expression at the lowest concentration levels being brought down to 66.8% when compared to the control [64].
3. Application of RNAi for Animal Health
Over the past ten years, there has been a significant increase in published papers regarding application of RNAi to control in parasites affecting livestock, the data of which has been figuratively described in Figure 2A. This section highlights those RNAi studies which have been focused on the control of pests that affect pigs, sheep, and cattle pest.
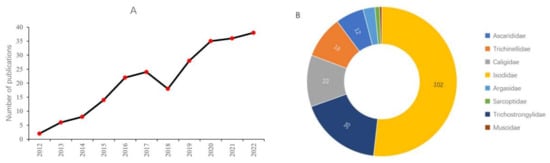
Figure 2.
Recent publications of RNAi based pest management associated with livestock. (A) Number of publications in the past 10 years based on a query-based PubMed search. (B) Recent quantitative distribution of RNAi studies in major livestock pest families. Based on PubMed search: More than 203 publications have been identified with queries “RNAi, gene silencing, specific species name, pest management, and specific livestock animals” by PubMed search.
RNAi technology has been described as a novel method for determining gene function and has provided an appealing approach to pest and disease management [65]. There has been abundant transcriptomic/genomic resources available for Ascaris suum, making it an experimental model among nematode parasites [66]. Apart from studies focused on nematode parasite management in pigs, there were studies done with regard to tick control in cattle. Ticks are ectoparasites of both animals and humans and are a significant disease vector affecting cattle husbandry globally [67,68].
With regard to human health, large-scale production of mRNA has been achieved for commercial production with the development of mRNA vaccines for COVID in the recent years [69]. Lipid nanoparticles (LNPs) which are composed of ionisable lipids, helper phospholipids, cholesterol, and PEGylated lipids are used to deliver mRNA vaccines developed by biopharmaceutical companies such as Moderna against COVID-19 and Zika viruses and BioNTech against COVID-19 spike proteins [69]. Over the past few years, research in pest management has further extended towards other livestock pest species. Table 1 enumerates some of the major experiments conducted in the past decade on the application of RNAi based pest control in the agricultural sector.

Table 1.
A summary of the RNAi studies focused on the management of pest associated with livestock production.
Table 1.
A summary of the RNAi studies focused on the management of pest associated with livestock production.
| RNAi Target | Parasites | Delivery | Livestock | Efficiency | Ref |
|---|---|---|---|---|---|
| As-eft-1, As-eft-2, As-gmpr, As-tnc-1, As-rab-3, As-hb-1, As-unc-29, As-unc-38 | Ascaris suum | Soaking | Pig | Different targets in adult A. suum were tested for gene knockdown. All targets were significantly silenced in different region of adult A. suum. Furthermore, all genes indicate they are susceptible to RNAi in adults A. suum, especially neuronal genes. | [15] |
| As-enol-1 | Ascaris suum | Soaking | Pig | Enolase gene expression was significantly silenced, and the mortality rate increased by 20.11% (p < 0.01) after soaking expressed sequence tag (EST) representing As-enol-1 dsRNA derived from A. suum for 72 h. | [65] |
| EST-06G09 | Ascaris suum | Soaking | Pig | The gene of EST 06G09 was silenced after 72 h soaking. The survival rate achieved highest peak at 48 h (20.37%, p < 0.01). The gene 06G09 has function in larval development. | [70] |
| TsSPIs | Trichinella spiralis | Soaking | Pig | TsSPI mRNA and protein expression levels in larvae decreased by 68.7% and 68.4%, respectively (p < 0.05). RNAi-mediated suppression of TsSPI expression in T. spiralis drastically decreased larval infectivity and survival inside the host. | [71] |
| LsalMS | Lepeophtheirus salmonis | Soaking | Salmon Lice | Myosuppressin (LsalMS) knockdown resulted in reduction in the amount of muscle, with skeletal and visceral muscles both showing anomalies in the lice. Additionally, LsalMS down-regulation also had an impact on feeding, spermatophore deposition, and moulting. | [72] |
| Ubiquitin-63E | Rhipicephalus microplus; | Injection | Cattle | All ticks treated with dsRNA did not have offspring. Ubiquitin-63E knockdown was confirmed by microarray and qRT-PCR. | [73] |
| HBP | Amblyomma mericanum | Injection | Cattle | dsRNA specific for histamine binding protein (HBP) transcript was injected into the haemolymph. Less expression of HBP mRNA was shown by molecular evidence, resulting in lower histamine binding ability. | [74] |
| Subolesin | Ornithodoros spp. | Injection | Cattle | By injecting dsRNA to silence the subolesin gene (Silencing 70–78%), oviposition was prevented (88.6% and 95.4%). | [75] |
| Fhteg1, Fhteg5, Fhteg8, | Fasciola hepatica | Soaking | Cattle | 92.9% transcript reduction of Fhteg1, 74.1% transcript reduction of Fhteg5. But no observable effect on phenotype. | [76] |
| SsGST-mu1 | Sarcoptes scabiei | Soaking | Sheep | Compared to controls, knockdown caused a 35% decrease in transcription of the target gene. | [77] |
| Hyaluronidase | Haemonchus contortus | Oral feeding | Sheep | A significant drop in worm burden and significant decrease in hyaluronidase activity in siRNA treated group. | [78] |
The data from Table 1 demonstrates that RNAi has the potential to be successfully applied for parasite management and improve animal husbandry practices. RNAi technology has been described as a novel method for determining gene function and has provided an appealing disease management approach in parasitic nematodes. Out of the examples illustrated, the most promising results were obtained for nematode management, highlighting its potential for improving pig health. More than half of the studies focusing on tick control were on Ixodidae and Argasidae families, covering the following species, Amblyomma mericanum, Ixodes scapularis, Haemaphysalis longicornis, Rhipicephalus sanguineus, Dermacentor marginatus, and Boophilus microlpolu.
The field application of RNAi product for pest control In animal is still in an early stage. One specific research example is the application of RNAi against Haemonchus contortus commonly known as barber’s pole worm in sheep. Researchers successfully used siRNA treatment for 8000 barber’s pole worms [78]. They used a dosage of ~1 mg/10,000 larval to observe a RNAi effect. For this study, the quantity of siRNA needed for RNAi effect was ~1 mg per animal [78]. In animal agriculture, each animal needs to be treated individually against pests and the exact quantity of RNA precursors required RNAi silencing can be very subjective depending on various factors like: (1) Size, animal types, animal density and living conditions of the farm. (2) Delivery system (nanoparticle/directly) and methods (injection/feeding). (3) Knockdown of genes with different function. (4) Species, stages of the pest. (5) Infection abilities associated with different pest and animal also need to be considered.
DsRNA has been delivered to the various tick species using different techniques, including microinjection, soaking, and feeding. As a major parasite of cattle, research studies focusing on RNAi strategy for tick species have achieved considerable gene knockdown or mortality increase [67,68]. Many parasites that affect livestock remain significant research subjects for flystrike in sheep, anaplasmosis in cattle, nematode infection in pigs and ectoparasites of salmon. While large-scale production of dsRNA has been expensive in the past, there has been a significant drop in production costs. This is due to the development of mass production systems, such as bacterial expression vectors for in vivo synthesis [79]. Thus, the use of RNAi as biopesticide has an excellent potential as control strategy for these pests affecting livestock industries.
4. Regulatory Framework
The excitement surrounding the development of RNAi for different agricultural applications has been building for years, with the full breadth of these potential benefits across many areas being thrown into sharp focus. Specifically, RNAi has the potential to replace conventional chemical pesticides with the ability to target genes selectively, and therefore only induce mortality in the target organism. As an emerging agricultural technology, RNAi has already shown its appealing capabilities in multiple areas. RNAi for developing sustainable agriculture has growing number of publications and patents (according to a search in the World Intellectual Property Organization database using the terms ‘RNAi’ and ‘agriculture’, ~1100 patent applications have been lodged), yet very few agricultural applications have made it to market. Despite the potential of RNAi there are still many challenges to be addressed to enable its application in the field.
One important limitation in the development of regulatory framework for RNAi include difference in the efficacy of each dsRNA under environmental conditions. RNAi-based products can be applied via some of the delivery methods described above. The exogenous spray application of RNAi as SIGS to improve plant health has the potential to become a fast-growing market. Despite existing framework available for regulatory assessment and approval of genetically modified plants, appropriate safety evaluations, and authorization procedures for SIGS-based products are less clear [80]. The degradation profile of each target dsRNA entering the environment needs to be extensively studied to assess the risk of unanticipated persistence [81]. The three environmental and highly researched microcosms of interest in this context are soil, surface water and sediment. Within soil foliarly applied dsRNA degradation rate for DT50 (Time to 50% degradation) and DT90 (Time to 90% degradation) was <30 h and <35 h, respectively, [81]. Interesting enough, insect bioassays demonstrate that the loss of functional capacity of dsRNA is unlikely to be caused by soil texture, clay content, pH, or other differences. Instead, it is commonly believed that microbial degradation is the primary driver of dsRNA degradation [81].Fischer et al., demonstrated that dsRNA remained stable in sterile water for three days. While this information lays a good foundation of dsRNA degradation in the ecosystem suggesting low risk of persistence [82]. As RNAi is a rapidly evolving field with most research focused on model organisms it is still not fully understood, making the design and implementation of regulations to be in constant flux.
Safe consumption of dsRNAs by humans and other vertebrates holds a long history; as dsRNA and its processed products are natural components of food and feed [83,84]. Oral uptake of dsRNA or siRNA by humans or farm animals has been reported to have negligible impact due to degradation and multiple barriers in the gastrointestinal tract of mammals [85,86]. The regulatory status of RNAi technology is different for each country. Topical dsRNA will be the likely treatment for animal agriculture, hence topical dsRNA regulatory approval is discussed below for selected jurisdictions. European Union (EU) regulations have been initially set on chemicals as active substances, no specific guidance documents defining the data requirements for the authorization of dsRNA-based application for plant protection are outlined. However, “the Commission may…adopt or amend technical and other guidance documents such as explanatory notes, guidance documents on the content of the application concerning microorganisms, pheromones, and biological products, for the implementation of this regulation. The Commission may ask the Authority to prepare or to contribute to such guidance documents” [87].
The Environmental Protection Authority (EPA) in New Zealand regulates RNAi under the Hazardous Substances and New Organisms (HSNO) Act. The NZ EPA and Decision-Making Committee decided that due to topical dsRNA lacking the ability to alter the genetic code, it is out of the scope of HSNO, and instead may be classed as a hazardous substance. However, EPA databases of hazardous chemical databases does not list dsRNA, making the regulations on dsRNA almost non-existent [88]. In the United States, pesticide goods must be registered with the US Environmental Protection Agency (EPA) before they can be manufactured, transported, or sold [89]. In 2017, the EPA approved the first RNAi pesticide [90]. SmartStax Pro is a GM maize seed that will use both transgenic insecticidal proteins and RNAi to combat western and northern corn rootworm [11,90]. The EPA’s standards in Title 40 of the Code of Federal Regulations (CFR) Part 158 lay out the data requirements for assessing pesticide products [91]. These data criteria aid in the evaluation of pesticide active components as well as pesticide products [91]. In addition to traditional chemical pesticide data requirements, 40 CFR Part 158 defines data requirements for biochemical and microbiological insecticides, which are often less toxic intrinsically than conventional chemical pesticides [91].
In Australia, as of the 8 October 2019, the Gene Technology Regulations 2001 legislation was amended, to include a provision that explained that gene technology does not include approaches involving the introduction of RNA into an organism, if the RNA cannot be translated into a polypeptide, an infectious agent cannot be produced, or the organisms’ genomic sequence is not altered as a result. If these conditions are met, the organism treated with dsRNA is not classified as a GMO under the Gene Technology Act of 2000 and is thus not regulated by the OTGR [34]. However, topically applied dsRNA is still subject to regulatory oversight by the Australian Pesticides and Veterinary Medicines Authority (APVMA) under the category of agricultural chemical products. To be approved by the APVMA, appropriate evidence must be presented for human health and safety, worker health and safety, environmental fate and toxicity, target efficacy and crop safety, foreign trade implications, and chemistry and manufacturing processes [34].
RNAi for controlling pests (O: Diptera, Hemiptera, Lepidoptera, Coleoptera), which affect plants/crops are proving to be efficient, hence promoting the transition of the novel and innovative RNAi to the market [92,93]. This existing data on RNAi research available to regulators to identify, assess, manage, and mitigate risks can help expand on traditional laws, biosafety regulations, market-based or economic regulatory schemes to create a specific niche assessment for non-GMO RNAi-products globally. The legal regulatory framework surrounding RNAi is looking more promising than ever due to the completed assessment to date and the regulatory framework already in places such as Australia regarding SIGS-products. While these outcomes have been focused on the plant/crop sector, they will have a profound impact on our knowledge of gene silencing and inform the way we apply this technology to improve the productivity of livestock.
5. Conclusions
RNAi-based biological controls can offer high target specificity, non-GM, environmentally friendly strategy against pests and pathogens. As described above, the RNA-mediated management for animal agriculture is still in its infancy and substantial research focus will be required for it to reach its full potential. Additionally, RNAi applications also need to be explored and adopted from the regulatory and community acceptance aspects of this technology. The breadth and success reported for RNAi studies conducted to control pests affecting plants, suggest dsRNA has a promising future in addressing current deficiencies in the control of livestock pests. If development is conducted diligently and thoroughly, encompassing bioinformatic identification, in silico best-design of RNAi effectors, and laboratory- and field-based RNAi toxicity studies, RNAi-based biopesticides have the potential to revolutionize pest management in a safe, specific, and effective manner. For successful development of RNAi strategies to improve animal health this issue must be addressed by understanding the movement of the dsRNA within the pest of interest to improve the penetration and persistence in the environment.
Author Contributions
Conceptualization, K.T.M.; methodology, writing, review, and editing—Y.Y. (Yakun Yan), T.J.M., P.S.M., Y.Y. (Yunjia Yang), N.M. and K.T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Advance Queensland Industry Research Fellowship with grant number CTS 25856/19.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Y.Y. (Yunjia Yang), T.M., P.M., Y.Y. (Yakun Yan), N.M. and K.M. would like to thank the support of The University of Queensland and Queensland Alliance of Agriculture and Food Innovation (QAAFI) and School of Chemistry and Biology (SCMB). This project is supported by K.M.’s Advance Queensland Industry Research Fellowship by Queensland Government, Queensland, Australia and YY’s (Yunjia Yang and Yakun Yan) Ph.D. scholarship from The University of Queensland, Brisbane, Queensland, Australia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of Food and Agriculture 2001; Food & Agriculture Organization: Rome, Italy, 2001. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.D.; McRoberts, N.; Nelson, A.D. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Narladkar, B. Projected economic losses due to vector and vector-borne parasitic diseases in livestock of India and its significance in implementing the concept of integrated practices for vector management. Vet. World 2018, 11, 151. [Google Scholar] [CrossRef]
- Worrall, E.; Hamid, A.; Mody, K.; Mitter, N.; Pappu, H. Nanotechnology for Plant Disease Management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.D.; Morrissey, C.A.; Noome, D.A.; Van der Sluijs, J.P. Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. Int. 2014, 22, 119–134. [Google Scholar] [CrossRef]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Sala, S.; Vighi, M. GIS-based procedure for site-specific risk assessment of pesticides for aquatic ecosystems. Ecotoxicol. Environ. Saf. 2008, 69, 1–12. [Google Scholar] [CrossRef]
- Karimzadeh, R.; Hejazi, M.J.; Helali, H.; Iranipour, S.; Mohammadi, S.A. Assessing the impact of site-specific spraying on control of Eurygaster integriceps (Hemiptera: Scutelleridae) damage and natural enemies. Precis. Agric. 2011, 12, 576–593. [Google Scholar] [CrossRef]
- Kim, Y.H.; Issa, M.S.; Cooper, A.M.; Zhu, K.Y. RNA interference: Applications and advances in insect toxicology and insect pest management. Pestic. Biochem. Physiol. 2015, 120, 109–117. [Google Scholar] [CrossRef]
- Jain, R.G.; Robinson, K.E.; Fletcher, S.J.; Mitter, N. RNAi-based functional genomics in Hemiptera. Insects 2020, 11, 557. [Google Scholar] [CrossRef]
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: Efficacy and resistance management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef]
- Rodrigues, T.; Sridharan, K.; Manley, B.; Cunningham, D.; Narva, K. Development of dsRNA as a sustainable bioinsecticide: From laboratory to field. In Crop Protection Products for Sustainable Agriculture; ACS Publications: Washington, DC, USA, 2021; pp. 65–82. [Google Scholar]
- Sammons, R.D.; Ivashuta, S.I.; Liu, H.; Wang, D.; Feng, P.C.; Kouranov, A.Y.; Andersen, S.E. Polynucleotide Molecules for Gene Regulation in Plants. US Patent 2011/0296556 A1, 1 December 2011. [Google Scholar]
- Maule, A.G.; McVeigh, P.; Dalzell, J.J.; Atkinson, L.; Mousley, A.; Marks, N.J. An eye on RNAi in nematode parasites. Trends Parasitol. 2011, 27, 505–513. [Google Scholar] [CrossRef]
- McCoy, C.J.; Warnock, N.D.; Atkinson, L.E.; Atcheson, E.; Martin, R.J.; Robertson, A.P.; Maule, A.G.; Marks, N.J.; Mousley, A. RNA interference in adult Ascaris suum–an opportunity for the development of a functional genomics platform that supports organism-, tissue-and cell-based biology in a nematode parasite. Int. J. Parasitol. 2015, 45, 673–678. [Google Scholar] [CrossRef]
- Yang, D.; Zeng, Y.; Sun, X.; Yue, X.; Hu, C.; Jiang, P.; Liu, R.; Ciu, J.; Wang, Z. Trichinella spiralis: RNAi-mediated silencing of serine protease results in reduction of intrusion, development and fecundity. Trop. Biomed. 2020, 37, 932–946. [Google Scholar] [PubMed]
- Almazán, C.; Lagunes, R.; Villar, M.; Canales, M.; Rosario-Cruz, R.; Jongejan, F.; de la Fuente, J. Identification and characterization of Rhipicephalus (Boophilus) microplus candidate protective antigens for the control of cattle tick infestations. Parasitol. Res. 2010, 106, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Miesen, P.; van Rij, R.P. Antiviral RNAi in insects and mammals: Parallels and differences. Viruses 2019, 11, 448. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Y.; Zhang, H.; Wang, K.; Zhao, C.; Zhu, G.; Reddy Palli, S.; Han, Z. Off-target effects of RNAi correlate with the mismatch rate between dsRNA and non-target mRNA. RNA Biol. 2021, 18, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Sen, G.L.; Blau, H.M. A brief history of RNAi: The silence of the genes. FASEB J. 2006, 20, 1293–1299. [Google Scholar] [CrossRef]
- Guo, S.; Kemphues, K.J. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 1995, 81, 611–620. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. dsRNA uptake in plant pests and pathogens: Insights into RNAi-based insect and fungal control technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef]
- Bramlett, M.; Plaetinck, G.; Maienfisch, P. RNA-based biocontrols—A new paradigm in crop protection. Engineering 2020, 6, 522–527. [Google Scholar] [CrossRef]
- Darlington, M.; Reinders, J.D.; Sethi, A.; Lu, A.L.; Ramaseshadri, P.; Fischer, J.R.; Boeckman, C.J.; Petrick, J.S.; Roper, J.M.; Narva, K.E. RNAi for Western Corn Rootworm Management: Lessons Learned, Challenges, and Future Directions. Insects 2022, 13, 57. [Google Scholar] [CrossRef]
- Vogel, E.; Santos, D.; Mingels, L.; Verdonckt, T.-W.; Broeck, J.V. RNA interference in insects: Protecting beneficials and controlling pests. Front. Physiol. 2019, 9, 1912. [Google Scholar] [CrossRef]
- Nunes, C.C.; Dean, R.A. Host-induced gene silencing: A tool for understanding fungal host interaction and for developing novel disease control strategies. Mol. Plant Pathol. 2012, 13, 519–529. [Google Scholar] [CrossRef]
- You, L.; Zhang, F.; Huang, S.; Merchant, A.; Zhou, X.; Li, Z. Over-expression of rna interference (rnai) core machinery improves susceptibility to rnai in silkworm larvae. Insect Mol. Biol. 2020, 29, 353–362. [Google Scholar] [CrossRef]
- Zotti, M.; Dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef]
- Saleh, M.-C.; van Rij, R.P.; Hekele, A.; Gillis, A.; Foley, E.; O’Farrell, P.H.; Andino, R. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat. Cell Biol. 2006, 8, 793–802. [Google Scholar] [CrossRef]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi Efficiency, Systemic Properties, and Novel Delivery Methods for Pest Insect Control: What We Know So Far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A Perspective on RNAi-Based Biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.; Kreszies, T.; Strickert, M.; Schweizer, P.; Kuhlmann, M.; Douchkov, D. siRNA-Finder (si-Fi) Software for RNAi-Target Design and Off-Target Prediction. Front. Plant Sci. 2019, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Whyard, S.; Singh, A.D.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Katoch, R.; Sethi, A.; Thakur, N.; Murdock, L.L. RNAi for insect control: Current perspective and future challenges. Appl. Biochem. Biotechnol. 2013, 171, 847–873. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, E.H.; Hunter, C.P. Transport of dsRNA into cells by the transmembrane protein SID-1. Science 2003, 301, 1545–1547. [Google Scholar] [CrossRef]
- Okamura, K.; Chung, W.-J.; Ruby, J.G.; Guo, H.; Bartel, D.P.; Lai, E.C. The Drosophila hairpin RNA pathway generates endogenous short interfering RNAs. Nature 2008, 453, 803–806. [Google Scholar] [CrossRef]
- Mourrain, P.; Béclin, C.; Elmayan, T.; Feuerbach, F.; Godon, C.; Morel, J.-B.; Jouette, D.; Lacombe, A.-M.; Nikic, S.; Picault, N. Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell 2000, 101, 533–542. [Google Scholar] [CrossRef]
- Price, D.R.; Gatehouse, J.A. RNAi-mediated crop protection against insects. Trends Biotechnol. 2008, 26, 393–400. [Google Scholar] [CrossRef]
- Alper, S.; McBride, S.J.; Lackford, B.; Freedman, J.H.; Schwartz, D.A. Specificity and complexity of the Caenorhabditis elegans innate immune response. Mol. Cell. Biol. 2007, 27, 5544–5553. [Google Scholar]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Wei, H.; Tan, S.; Yan, S.; Li, Z.; Shen, J.; Liu, X. Nanocarrier-mediated transdermal dsRNA-NPF1 delivery system contributes to pest control via inhibiting feeding behavior in Grapholita molesta. J. Pest Sci. 2022, 95, 983–995. [Google Scholar] [CrossRef]
- Yan, S.; Ren, B.Y.; Shen, J. Nanoparticle-mediated double-stranded RNA delivery system: A promising approach for sustainable pest management. Insect Sci. 2021, 28, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Kennerdell, J.R.; Carthew, R.W. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 1998, 95, 1017–1026. [Google Scholar] [CrossRef]
- Yu, N.; Christiaens, O.; Liu, J.; Niu, J.; Cappelle, K.; Caccia, S.; Huvenne, H.; Smagghe, G. Delivery of dsRNA for RNAi in insects: An overview and future directions. Insect Sci. 2013, 20, 4–14. [Google Scholar] [CrossRef]
- Tomoyasu, Y.; Denell, R.E. Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev. Genes Evol. 2004, 214, 575–578. [Google Scholar] [CrossRef]
- Bolognesi, R.; Ramaseshadri, P.; Anderson, J.; Bachman, P.; Clinton, W.; Flannagan, R.; Ilagan, O.; Lawrence, C.; Levine, S.; Moar, W. Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte). PLoS ONE 2012, 7, e47534. [Google Scholar] [CrossRef]
- Narva, K.E.; Siegfried, B.D.; Storer, N.P. Transgenic approaches to western corn rootworm control. In Yellow Biotechnology II; Springer: Heidelberg, Germany, 2013; Volume 163, pp. 135–162. [Google Scholar]
- Pinheiro, D.H.; Taylor, C.E.; Wu, K.; Siegfried, B.D. Delivery of gene-specific dsRNA by microinjection and feeding induces RNAi response in Sri Lanka weevil, Myllocerus undecimpustulatus undatus Marshall. Pest Manag. Sci. 2020, 76, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Ma, Z.Z.; Zhou, H.; Chao, Z.J.; Yan, S.; Shen, J. Nanocarrier-delivered dsRNA suppresses wing development of green peach aphids. Insect Sci. 2022, 29, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jin, H. Spray-Induced Gene Silencing: A Powerful Innovative Strategy for Crop Protection. Trends Microbiol. 2017, 25, 4–6. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M.; McMillan, J.N.; Cardoza, V.; Maegele, I.; Dadami, E.; Runne, M.; Krczal, G.; Wassenegger, M. Induction of silencing in plants by high-pressure spraying of in vitro-synthesized small RNAs. Front. Plant Sci. 2016, 7, 1327. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, Y.; Hu, Y.; Christie, P.; Zhang, J.; Li, X. Maize yield and soil fertility with combined use of compost and inorganic fertilizers on a calcareous soil on the North China Plain. Soil Tillage Res. 2016, 155, 85–94. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Y.; Yan, S.; Zhou, H.; Song, D.; Yin, M.; Shen, J. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest Manag. Sci. 2019, 75, 1993–1999. [Google Scholar] [CrossRef]
- Yan, S.; Qian, J.; Cai, C.; Ma, Z.; Li, J.; Yin, M.; Ren, B.; Shen, J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. J. Pest Sci. 2020, 93, 449–459. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhu, K. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Worrall, E.A.; Bravo-Cazar, A.; Nilon, A.T.; Fletcher, S.J.; Robinson, K.E.; Carr, J.P.; Mitter, N. Exogenous application of RNAi-inducing double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Front. Plant Sci. 2019, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Lechanteur, A.; Sanna, V.; Duchemin, A.; Evrard, B.; Mottet, D.; Piel, G. Cationic liposomes carrying siRNA: Impact of lipid composition on physicochemical properties, cytotoxicity and endosomal escape. Nanomaterials 2018, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Zuhorn, I.S.; Engberts, J.B.; Hoekstra, D. Gene delivery by cationic lipid vectors: Overcoming cellular barriers. Eur. Biophys. J. 2007, 36, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.T.; Zhang, B.; Li, X.; Jain, R.; Li, P.; James, P.; Mahony, T.J.; Xu, Z.; Mitter, N. Topical RNAi for Sustainable Animal Health. Multidiscip. Digit. Publ. Inst. Proc. 2020, 36, 170. [Google Scholar]
- Lichtenberg, S.S.; Laisney, J.; Elhaj Baddar, Z.; Tsyusko, O.V.; Palli, S.R.; Levard, C.; Masion, A.; Unrine, J.M. Comparison of nanomaterials for delivery of double-stranded RNA in Caenorhabditis elegans. J. Agric. Food Chem. 2020, 68, 7926–7934. [Google Scholar] [CrossRef]
- Chen, N.; Xu, M.-J.; Nisbet, A.J.; Huang, C.-Q.; Lin, R.-Q.; Yuan, Z.-G.; Song, H.-Q.; Zhu, X.-Q. Ascaris suum: RNAi mediated silencing of enolase gene expression in infective larvae. Exp. Parasitol. 2011, 127, 142–146. [Google Scholar] [CrossRef]
- Jex, A.R.; Liu, S.; Li, B.; Young, N.D.; Hall, R.S.; Li, Y.; Yang, L.; Zeng, N.; Xu, X.; Xiong, Z. Ascaris suum draft genome. Nature 2011, 479, 529–533. [Google Scholar] [CrossRef]
- Peter, R.; Van den Bossche, P.; Penzhorn, B.L.; Sharp, B. Tick, fly, and mosquito control—Lessons from the past, solutions for the future. Vet. Parasitol. 2005, 132, 205–215. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Xu, M.; Chen, N.; Song, H.; Lin, R.; Huang, C.; Yuan, Z.; Zhu, X. RNAi-mediated silencing of a novel Ascaris suum gene expression in infective larvae. Parasitol. Res. 2010, 107, 1499–1503. [Google Scholar] [CrossRef]
- Yi, N.; Yu, P.; Wu, L.; Liu, Z.; Guan, J.; Liu, C.; Liu, M.; Lu, Y. RNAi-mediated silencing of Trichinella spiralis serpin-type serine protease inhibitors results in a reduction in larval infectivity. Vet. Res. 2020, 51, 139. [Google Scholar] [CrossRef]
- Komisarczuk, A.Z.; Kongshaug, H.; Li, M.; Nilsen, F. RNAi mediated myosuppressin deficiency affects muscle development and survival in the salmon louse (Lepeophtheirus salmonis). Sci. Rep. 2019, 9, 6944. [Google Scholar] [CrossRef]
- Lew-Tabor, A.; Kurscheid, S.; Barrero, R.; Gondro, C.; Moolhuijzen, P.; Valle, M.R.; Morgan, J.; Covacin, C.; Bellgard, M. Gene expression evidence for off-target effects caused by RNA interference-mediated gene silencing of Ubiquitin-63E in the cattle tick Rhipicephalus microplus. Int. J. Parasitol. 2011, 41, 1001–1014. [Google Scholar] [CrossRef]
- Aljamali, M.; Bior, A.; Sauer, J.; Essenberg, R. RNA interference in ticks: A study using histamine binding protein dsRNA in the female tick Amblyomma americanum. Insect Mol. Biol. 2003, 12, 299–305. [Google Scholar] [CrossRef]
- Manzano-Román, R.; Díaz-Martín, V.; Oleaga, A.; Siles-Lucas, M.; Pérez-Sánchez, R. Subolesin/akirin orthologs from Ornithodoros spp. soft ticks: Cloning, RNAi gene silencing and protective effect of the recombinant proteins. Vet. Parasitol. 2012, 185, 248–259. [Google Scholar] [CrossRef] [PubMed]
- McCusker, P.; Toet, H.; Rathinasamy, V.; Young, N.; Beddoe, T.; Anderson, G.; Dempster, R.; McVeigh, P.; McCammick, E.; Wells, D. Molecular characterisation and vaccine efficacy of two novel developmentally regulated surface tegument proteins of Fasciola hepatica. Vet. Parasitol. 2020, 286, 109244. [Google Scholar] [CrossRef]
- Fernando, D.D.; Marr, E.J.; Zakrzewski, M.; Reynolds, S.L.; Burgess, S.T.; Fischer, K. Gene silencing by RNA interference in Sarcoptes scabiei: A molecular tool to identify novel therapeutic targets. Parasites Vectors 2017, 10, 289. [Google Scholar] [CrossRef]
- Yang, X.; Khan, S.; Zhao, X.; Zhang, J.; Nisar, A.; Feng, X. Suppression of hyaluronidase reduces invasion and establishment of Haemonchus contortus larvae in sheep. Vet. Res. 2020, 51, 106. [Google Scholar] [CrossRef]
- Cagliari, D.; Dias, N.P.; Galdeano, D.M.; Dos Santos, E.Á.; Smagghe, G.; Zotti, M.J. Management of pest insects and plant diseases by non-transformative RNAi. Front. Plant Sci. 2019, 10, 1319. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.; Dubruel, P.; Smagghe, G. RNAi-Based Biocontrol Products: Market Status, Regulatory Aspects, and Risk Assessment. Front. Insect Sci. 2022, 1, 1–9. [Google Scholar] [CrossRef]
- Bachman, P.; Fischer, J.; Song, Z.; Urbanczyk-Wochniak, E.; Watson, G. Environmental Fate and Dissipation of Applied dsRNA in Soil, Aquatic Systems, and Plants. Front. Plant Sci. 2020, 11, 21. [Google Scholar] [CrossRef]
- Fischer, J.R.; Zapata, F.; Dubelman, S.; Mueller, G.M.; Uffman, J.P.; Jiang, C.; Jensen, P.D.; Levine, S.L. Aquatic fate of a double-stranded RNA in a sediment–water system following an over-water application. Environ. Toxicol. Chem. 2017, 36, 727–734. [Google Scholar] [CrossRef]
- Dávalos, A.; Henriques, R.; Latasa, M.J.; Laparra, M.; Coca, M. Literature review of baseline information on non-coding RNA (ncRNA) to support the risk assessment of ncRNA-based genetically modified plants for food and feed. EFSA Support. Publ. 2019, 16, 1688E. [Google Scholar] [CrossRef]
- OECD. Considerations for the Environmental Risk Assessment of the Application of Sprayed or Externally Applied dsRNA-Based Pesticides; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2020; Volume 104. [Google Scholar]
- Petrick, J.S.; Brower-Toland, B.; Jackson, A.L.; Kier, L.D. Safety assessment of food and feed from biotechnology-derived crops employing RNA-mediated gene regulation to achieve desired traits: A scientific review. Regul. Toxicol. Pharmacol. 2013, 66, 167–176. [Google Scholar] [CrossRef]
- O’Neill, M.J.; Bourre, L.; Melgar, S.; O’Driscoll, C.M. Intestinal delivery of non-viral gene therapeutics: Physiological barriers and preclinical models. Drug Discov. Today 2011, 16, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Commission, E. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union L 2009, 309, 1–50. [Google Scholar]
- Heinemann, J.A. Should dsRNA treatments applied in outdoor environments be regulated? Env. Int. 2019, 132, 104856. [Google Scholar] [CrossRef] [PubMed]
- Leahy, J.; Mendelsohn, M.; Kough, J.; Jones, R.; Berckes, N. Biopesticide oversight and registration at the US Environmental Protection Agency. Biopestic. State Art Future Oppor. 2014, 1172, 3–18. [Google Scholar]
- Shaffer, L. Inner Workings: RNA-based pesticides aim to get around resistance problems. Proc. Natl. Acad. Sci. USA 2020, 117, 32823–32826. [Google Scholar] [CrossRef]
- Dietz-Pfeilstetter, A.; Mendelsohn, M.; Gathmann, A.; Klinkenbuß, D. Considerations and regulatory approaches in the USA and in the EU for dsRNA-based externally applied pesticides for plant protection. Front. Plant Sci. 2021, 12, 974. [Google Scholar] [CrossRef]
- Cooper, A.M.; Silver, K.; Zhang, J.; Park, Y.; Zhu, K.Y. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef]
- Baum, J.; Roberts, J. Progress Towards RNAi-Mediated Insect Pest Management. Adv. Insect Physiology. 2014, 47, 249–295. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).