Cellulose Nanocrystals (CNC) Liquid Crystalline State in Suspension: An Overview
Abstract
1. Introduction
2. CNC LCs
2.1. Origin of Chirality of CNCs
2.2. Rheology of CNC LCs
2.3. Phase Separation
2.4. Mechanical Properties
2.5. Orientation
2.6. Pitch Length
2.7. Confinement
3. Applications
3.1. Responsive Materials
3.2. Energy Storage Applications
3.3. Optical and Optoelectronic Applications
3.4. Advanced Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parton, T.G.; van de Kerkhof, G.T.; Narkevicius, A.; Haataja, J.S.; Parker, R.M.; Frka-Petesic, B.; Vignolini, S. Chiral self-assembly of cellulose nanocrystals is driven by crystallite bundles. arXiv 2021, arXiv:2107.04772. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Gahrooee, T.R.; Moud, A.A.; Danesh, M.; Hatzikiriakos, S.G. Rheological Characterization of CNC-CTAB Network below and above Critical Micelle Concentration (CMC). Carbohydr. Polym. 2021, 257, 117552. [Google Scholar] [CrossRef]
- Moud, A.A.; Arjmand, M.; Yan, N.; Nezhad, A.S.; Hejazi, S.H. Colloidal behavior of cellulose nanocrystals in presence of sodium chloride. ChemistrySelect 2018, 3, 4969–4978. [Google Scholar] [CrossRef]
- Moud, A.A.; Arjmand, M.; Liu, J.; Yang, Y.; Sanati-Nezhad, A.; Hejazi, S.H. Cellulose nanocrystal structure in the presence of salts. Cellulose 2019, 26, 9387–9401. [Google Scholar] [CrossRef]
- Domingues, R.M.; Gomes, M.E.; Reis, R.L. The potential of cellulose nanocrystals in tissue engineering strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.; Lee, Y.H.; Chen, W.; Lee, S.Y. Nanocellulose for energy storage systems: Beyond the limits of synthetic materials. Adv. Mater. 2019, 31, 1804826. [Google Scholar] [CrossRef] [PubMed]
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H. Suspensions and hydrogels of cellulose nanocrystals (CNCs): Characterization using microscopy and rheology. Cellulose 2022, 29, 3621–3653. [Google Scholar] [CrossRef]
- Abbasi Moud, A.; Javadi, A.; Nazockdast, H.; Fathi, A.; Altstaedt, V. Effect of dispersion and selective localization of carbon nanotubes on rheology and electrical conductivity of polyamide 6 (PA 6), Polypropylene (PP), and PA 6/PP nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 368–378. [Google Scholar] [CrossRef]
- Arjmand, M.; Moud, A.A.; Li, Y.; Sundararaj, U. Outstanding electromagnetic interference shielding of silver nanowires: Comparison with carbon nanotubes. RSC Adv. 2015, 5, 56590–56598. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the toxicity of carbon nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H.; Sundararaj, U. Viscoelastic properties of poly (vinyl alcohol) hydrogels with cellulose nanocrystals fabricated through sodium chloride addition: Rheological evidence of double network formation. Colloids Surf. Physicochem. Eng. Asp. 2021, 609, 125577. [Google Scholar] [CrossRef]
- Shin, H.; Kim, S.; Kim, J.; Kong, S.; Lee, Y.; Lee, J.C. Preparation of 3-pentadecylphenol-modified cellulose nanocrystal and its application as a filler to polypropylene nanocomposites having improved antibacterial and mechanical properties. J. Appl. Polym. Sci. 2022, 139, 51848. [Google Scholar] [CrossRef]
- Sarfat, M.S.; Setyaningsih, D.; Fahma, F.; Indrasti, N.S. Characterization of mono-diacylglycerols, cellulose nanocrystals, polypropylene, and supporting materials as raw materials for synthesis of antistatic bionanocomposites. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: NIT Raipur, India, 2022; p. 12009. [Google Scholar]
- Rajeev, A.; Natale, G. Anisotropy and Nanomechanics of Cellulose Nanocrystals/Polyethylene Glycol Composite Films. Biomacromolecules 2022, 23, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Cui, L.; Tu, C.; Yan, C.; Guo, Y. Toughen and strengthen alginate fiber by incorporation of polyethylene glycol grafted cellulose nanocrystals. Cellulose 2022, 29, 5021–5035. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Ureña-Benavides, E.E.; Ao, G.; Davis, V.A.; Kitchens, C.L. Rheology and phase behavior of lyotropic cellulose nanocrystal suspensions. Macromolecules 2011, 44, 8990–8998. [Google Scholar] [CrossRef]
- Solomon, E.B.; Niemira, B.A.; Sapers, G.M.; Annous, B.A. Biofilm formation, cellulose production, and curli biosynthesis by Salmonella originating from produce, animal, and clinical sources. J. Food Prot. 2005, 68, 906–912. [Google Scholar] [CrossRef]
- Dong, X.M.; Kimura, T.; Revol, J.-F.; Gray, D.G. Effects of ionic strength on the isotropic− chiral nematic phase transition of suspensions of cellulose crystallites. Langmuir 1996, 12, 2076–2082. [Google Scholar] [CrossRef]
- Hirai, A.; Inui, O.; Horii, F.; Tsuji, M. Phase separation behavior in aqueous suspensions of bacterial cellulose nanocrystals prepared by sulfuric acid treatment. Langmuir 2009, 25, 497–502. [Google Scholar] [CrossRef]
- Onogi, S.; Asada, T. Rheology and rheo-optics of polymer liquid crystals. In Rheology; Springer: Berlin/Heidelberg, Germany, 1980; pp. 127–147. [Google Scholar]
- Bercea, M.; Navard, P. Shear dynamics of aqueous suspensions of cellulose whiskers. Macromolecules 2000, 33, 6011–6016. [Google Scholar] [CrossRef]
- Shafiei-Sabet, S.; Hamad, W.Y.; Hatzikiriakos, S.G. Rheology of nanocrystalline cellulose aqueous suspensions. Langmuir 2012, 28, 17124–17133. [Google Scholar] [CrossRef]
- González-Labrada, E.; Gray, D.G. Viscosity measurements of dilute aqueous suspensions of cellulose nanocrystals using a rolling ball viscometer. Cellulose 2012, 19, 1557–1565. [Google Scholar] [CrossRef]
- Xu, H.; Wu, P.; Zhu, C.; Elbaz, A.; Gu, Z.Z. Photonic crystal for gas sensing. J. Mater. Chem. C 2013, 1, 6087–6098. [Google Scholar] [CrossRef]
- Prum, R.O.; Torres, R.H. Structural colouration of mammalian skin: Convergent evolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 2004, 207, 2157–2172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Chodavarapu, V.P.; Kirk, A.G.; Andrews, M.P.; Carluer, M.; Picard, G. Origin of iridescence in chiral nematic phase nanocrystalline cellulose for encryption and enhanced color. In Emerging Liquid Crystal Technologies VI.; SPIE: San Francisco, CA, USA, 2011; pp. 169–177. [Google Scholar]
- Moud, A.A. Chiral Liquid Crystalline Properties of Cellulose Nanocrystals: Fundamentals and Applications. ACS Omega 2022, 7, 30673. [Google Scholar] [CrossRef]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding nanocellulose chirality and structure–properties relationship at the single fibril level. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Revol, J.-F.; Godbout, L.; Dong, X.-M.; Gray, D.G.; Chanzy, H.; Maret, G. Chiral nematic suspensions of cellulose crystallites; phase separation and magnetic field orientation. Liq. Cryst. 1994, 16, 127–134. [Google Scholar] [CrossRef]
- Casado, U.; Mucci, V.L.; Aranguren, M.I. Cellulose nanocrystals suspensions: Liquid crystal anisotropy, rheology and films iridescence. Carbohydr. Polym. 2021, 261, 117848. [Google Scholar] [CrossRef]
- Xu, Y.; Atrens, A.; Stokes, J.R. A review of nanocrystalline cellulose suspensions: Rheology, liquid crystal ordering and colloidal phase behaviour. Adv. Colloid Interface Sci. 2020, 275, 102076. [Google Scholar] [CrossRef]
- Kádár, R.; Spirk, S.; Nypelö, T. Cellulose Nanocrystal Liquid Crystal Phases: Progress and Challenges in Characterization Using Rheology Coupled to Optics, Scattering, and Spectroscopy. ACS Nano 2021, 15, 7931–7945. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Lin, T.; Duan, M.; Du, H.; Yin, X. Cellulose nanocrystal-based liquid crystal structures and the unique optical characteristics of cellulose nanocrystal films. BioResources 2021, 16, 2116. [Google Scholar] [CrossRef]
- Lagerwall, J.P.; Schütz, C.; Salajkova, M.; Noh, J.; Hyun Park, J.; Scalia, G.; Bergström, L. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, e80. [Google Scholar] [CrossRef]
- Parton, T.G.; Parker, R.M.; van de Kerkhof, G.T.; Narkevicius, A.; Haataja, J.S.; Frka-Petesic, B.; Vignolini, S. Chiral self-assembly of cellulose nanocrystals is driven by crystallite bundles. Nat. Commun. 2022, 13, 1–9. [Google Scholar] [CrossRef]
- Ritcey, A.M.; Gray, D.G. Cholesteric order in gels and films of regenerated cellulose. Biopolym. Orig. Res. Biomol. 1988, 27, 1363–1374. [Google Scholar] [CrossRef]
- Tran, A.; Boott, C.E.; MacLachlan, M.J. Understanding the Self-Assembly of Cellulose Nanocrystals—Toward Chiral Photonic Materials. Adv. Mater. 2020, 32, 1905876. [Google Scholar] [CrossRef] [PubMed]
- de Souza Lima, M.M.; Borsali, R. Rodlike cellulose microcrystals: Structure, properties, and applications. Macromol. Rapid Commun. 2004, 25, 771–787. [Google Scholar] [CrossRef]
- Chiappini, M.; Dussi, S.; Frka-Petesic, B.; Vignolini, S.; Dijkstra, M. Modeling the cholesteric pitch of apolar cellulose nanocrystal suspensions using a chiral hard-bundle model. J. Chem. Phys. 2022, 156, 014904. [Google Scholar] [CrossRef]
- Bu, L.; Himmel, M.E.; Crowley, M.F. The molecular origins of twist in cellulose I-beta. Carbohydr. Polym. 2015, 125, 146–152. [Google Scholar] [CrossRef]
- Honorato-Rios, C.; Lagerwall, J.P. Interrogating helical nanorod self-assembly with fractionated cellulose nanocrystal suspensions. Commun. Mater. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Tanaka, R.; Li, S.; Kashiwagi, Y.; Inoue, T. A self-build apparatus for oscillatory flow birefringence measurements in a Co-cylindrical geometry. Nihon Reoroji Gakkaishi 2018, 46, 221–226. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Pignon, F. Current progress in rheology of cellulose nanofibril suspensions. Biomacromolecules 2016, 17, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kumar, S. Carbon nanotubes as liquid crystals. Small 2008, 4, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Friedel, G. Les états mésomorphes de la matière. Ann. De Phys. 1922, 9, 273–474. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Chodavarapu, V.P.; Kirk, A.G.; Andrews, M.P. Nanocrystalline cellulose for covert optical encryption. J. Nanophotonics 2012, 6, 063516. [Google Scholar] [CrossRef]
- Conley, K.; Godbout, L.; Whitehead, M.T.; van de Ven, T.G. Origin of the twist of cellulosic materials. Carbohydr. Polym. 2016, 135, 285–299. [Google Scholar] [CrossRef]
- Matthews, J.F.; Skopec, C.E.; Mason, P.E.; Zuccato, P.; Torget, R.W.; Sugiyama, J.; Himmel, M.E.; Brady, J.W. Computer simulation studies of microcrystalline cellulose Iβ. Carbohydr. Res. 2006, 341, 138–152. [Google Scholar] [CrossRef]
- Hadden, J.A.; French, A.D.; Woods, R.J. Unraveling cellulose microfibrils: A twisted tale. Biopolymers 2013, 99, 746–756. [Google Scholar] [CrossRef]
- Paajanen, A.; Ceccherini, S.; Maloney, T.; Ketoja, J.A. Chirality and bound water in the hierarchical cellulose structure. Cellulose 2019, 26, 5877–5892. [Google Scholar] [CrossRef]
- Zhao, Z.; Shklyaev, O.E.; Nili, A.; Mohamed, M.N.A.; Kubicki, J.D.; Crespi, V.H.; Zhong, L. Cellulose microfibril twist, mechanics, and implication for cellulose biosynthesis. J. Phys. Chem. A 2013, 117, 2580–2589. [Google Scholar] [CrossRef]
- Mehandzhiyski, A.Y.; Rolland, N.; Garg, M.; Wohlert, J.; Linares, M.; Zozoulenko, I. A novel supra coarse-grained model for cellulose. Cellulose 2020, 27, 4221–4234. [Google Scholar] [CrossRef]
- Conley, K.; Whitehead, M.; van de Ven, T.G. Probing the structural chirality of crystalline cellulose with induced circular dichroism. Cellulose 2017, 24, 479–486. [Google Scholar] [CrossRef]
- Nystrom, G.; Arcari, M.; Adamcik, J.; Usov, I.; Mezzenga, R. Nanocellulose fragmentation mechanisms and inversion of chirality from the single particle to the cholesteric phase. ACS Nano 2018, 12, 5141–5148. [Google Scholar] [CrossRef]
- Livolant, F.; Leforestier, A. Condensed phases of DNA: Structures and phase transitions. Prog. Polym. Sci. 1996, 21, 1115–1164. [Google Scholar] [CrossRef]
- Tombolato, F.; Ferrarini, A.; Grelet, E. Chiral nematic phase of suspensions of rodlike viruses: Left-handed phase helicity from a right-handed molecular helix. Phys. Rev. Lett. 2006, 96, 258302. [Google Scholar] [CrossRef]
- Nyström, G.; Arcari, M.; Mezzenga, R. Confinement-induced liquid crystalline transitions in amyloid fibril cholesteric tactoids. Nat. Nanotechnol. 2018, 13, 330–336. [Google Scholar] [CrossRef]
- De Michele, C.; Zanchetta, G.; Bellini, T.; Frezza, E.; Ferrarini, A. Hierarchical propagation of chirality through reversible polymerization: The cholesteric phase of DNA oligomers. ACS Macro Lett. 2016, 5, 208–212. [Google Scholar] [CrossRef]
- Dogic, Z.; Fraden, S. Cholesteric phase in virus suspensions. Langmuir 2000, 16, 7820–7824. [Google Scholar] [CrossRef]
- Hanley, S.J.; Revol, J.-F.; Godbout, L.; Gray, D.G. Atomic force microscopy and transmission electron microscopy of cellulose from Micrasterias denticulata; evidence for a chiral helical microfibril twist. Cellulose 1997, 4, 209–220. [Google Scholar] [CrossRef]
- Majoinen, J.; Haataja, J.S.; Appelhans, D.; Lederer, A.; Olszewska, A.; Seitsonen, J.; Aseyev, V.; Kontturi, E.; Rosilo, H.; Österberg, M. Supracolloidal multivalent interactions and wrapping of dendronized glycopolymers on native cellulose nanocrystals. J. Am. Chem. Soc. 2014, 136, 866–869. [Google Scholar] [CrossRef]
- Ogawa, Y. Electron microdiffraction reveals the nanoscale twist geometry of cellulose nanocrystals. Nanoscale 2019, 11, 21767–21774. [Google Scholar] [CrossRef] [PubMed]
- Paavilainen, S.; Róg, T.; Vattulainen, I. Analysis of twisting of cellulose nanofibrils in atomistic molecular dynamics simulations. J. Phys. Chem. B 2011, 115, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Dumitrică, T. Intrinsic twist in Iβ cellulose microfibrils by tight-binding objective boundary calculations. Carbohydr. Polym. 2020, 230, 115624. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, M.; Windle, A. Origin of chiral interactions in cellulose supra-molecular microfibrils. Carbohydr. Polym. 2014, 106, 128–131. [Google Scholar] [CrossRef]
- Moud, A.A. Advanced cellulose nanocrystals (CNC) and cellulose nanofibrils (CNF) aerogels: Bottom-up assembly perspective for production of adsorbents. Int. J. Biol. Macromol. 2022, 222, 1–29. [Google Scholar] [CrossRef]
- Rolland, N.; Mehandzhiyski, A.Y.; Garg, M.; Linares, M.; Zozoulenko, I.V. New patchy particle model with anisotropic patches for molecular dynamics simulations: Application to a coarse-grained model of cellulose Nanocrystal. J. Chem. Theory Comput. 2020, 16, 3699–3711. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Delepierre, G.; Eyley, S.; Thielemans, W.; Weder, C.; Cranston, E.D.; Zoppe, J.O. Patience is a virtue: Self-assembly and physico-chemical properties of cellulose nanocrystal allomorphs. Nanoscale 2020, 12, 17480–17493. [Google Scholar] [CrossRef]
- Uhlig, M.; Fall, A.; Wellert, S.; Lehmann, M.; Prévost, S.; Wagberg, L.; von Klitzing, R.; Nystrom, G. Two-dimensional aggregation and semidilute ordering in cellulose nanocrystals. Langmuir 2016, 32, 442–450. [Google Scholar] [CrossRef]
- Orts, W.J.; Godbout, L.; Marchessault, R.H.; Revol, J.-F. Enhanced ordering of liquid crystalline suspensions of cellulose microfibrils: A small angle neutron scattering study. Macromolecules 1998, 31, 5717–5725. [Google Scholar] [CrossRef]
- Terech, P.; Chazeau, L.; Cavaille, J. A small-angle scattering study of cellulose whiskers in aqueous suspensions. Macromolecules 1999, 32, 1872–1875. [Google Scholar] [CrossRef]
- Cherhal, F.; Cousin, F.; Capron, I. Influence of charge density and ionic strength on the aggregation process of cellulose nanocrystals in aqueous suspension, as revealed by small-angle neutron scattering. Langmuir 2015, 31, 5596–5602. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.; George, K.; Rezvan, G.; Taheri-Qazvini, N.; Zhang, R.; Sadati, M. Capillary Flow Characterizations of Chiral Nematic Cellulose Nanocrystal Suspensions. Langmuir 2022, 38, 2192–2204. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Jarvis, M. Cellulose stacks up. Nature 2003, 426, 611–612. [Google Scholar] [CrossRef]
- Li, Q.; Renneckar, S. Supramolecular structure characterization of molecularly thin cellulose I nanoparticles. Biomacromolecules 2011, 12, 650–659. [Google Scholar] [CrossRef]
- Schutz, C.; Agthe, M.; Fall, A.B.; Gordeyeva, K.; Guccini, V.; Salajková, M.; Plivelic, T.S.; Lagerwall, J.P.; Salazar-Alvarez, G.; Bergstrom, L. Rod packing in chiral nematic cellulose nanocrystal dispersions studied by small-angle X-ray scattering and laser diffraction. Langmuir 2015, 31, 6507–6513. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Q.; Chang, C.; Zhang, L. Phase transition identification of cellulose nanocrystal suspensions derived from various raw materials. J. Appl. Polym. Sci. 2018, 135, 45702. [Google Scholar] [CrossRef]
- Xu, Y.; Atrens, A.D.; Stokes, J.R. Liquid crystal hydroglass formed via phase separation of nanocellulose colloidal rods. Soft Matter 2019, 15, 1716–1720. [Google Scholar] [CrossRef]
- Abitbol, T.; Kam, D.; Levi-Kalisman, Y.; Gray, D.G.; Shoseyov, O. Surface charge influence on the phase separation and viscosity of cellulose nanocrystals. Langmuir 2018, 34, 3925–3933. [Google Scholar] [CrossRef]
- Gray, D.G. Order and gelation of cellulose nanocrystal suspensions: An overview of some issues. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2018, 376, 20170038. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F.; Shen, A.Q. Shear rheology of graphene oxide dispersions. Curr. Opin. Chem. Eng. 2017, 16, 23–30. [Google Scholar] [CrossRef]
- Wissbrun, K.F. Rheology of rod-like polymers in the liquid crystalline state. J. Rheol. 1981, 25, 619–662. [Google Scholar] [CrossRef]
- Denn, M.M. Extrusion instabilities and wall slip. Annu. Rev. Fluid Mech. 2001, 33, 265–287. [Google Scholar] [CrossRef]
- Hobbie, E.K.; Obrzut, J.; Kharchenko, S.; Grulke, E. Charge transport in melt-dispersed carbon nanotubes. J. Chem. Phys. 2006, 125, 044712. [Google Scholar] [CrossRef]
- Pasquali, M. Swell properties and swift processing. Nat. Mater. 2004, 3, 509–510. [Google Scholar] [CrossRef]
- Li, J.; Revol, J.; Marchessault, R. Rheological properties of aqueous suspensions of chitin crystallites. J. Colloid Interface Sci. 1996, 183, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Donald, A.M.; Windle, A.H.; Hanna, S. Liquid Crystalline Polymers; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Conio, G.; Bianchi, E.; Ciferri, A.; Krigbaum, W. Mesophase formation by semirigid polymers: Poly (n-hexyl isocyanate) in dichloromethane and toluene. Macromolecules 1984, 17, 856–861. [Google Scholar] [CrossRef]
- Zhang, S.; Kinloch, I.A.; Windle, A.H. Mesogenicity drives fractionation in lyotropic aqueous suspensions of multiwall carbon nanotubes. Nano Lett. 2006, 6, 568–572. [Google Scholar] [CrossRef]
- Honorato-Rios, C.; Lehr, C.; Schütz, C.; Sanctuary, R.; Osipov, M.A.; Baller, J.; Lagerwall, J.P. Fractionation of cellulose nanocrystals: Enhancing liquid crystal ordering without promoting gelation. NPG Asia Mater. 2018, 10, 455–465. [Google Scholar] [CrossRef]
- Fry, D.; Langhorst, B.; Kim, H.; Grulke, E.; Wang, H.; Hobbie, E.K. Anisotropy of sheared carbon-nanotube suspensions. Phys. Rev. Lett. 2005, 95, 038304. [Google Scholar] [CrossRef] [PubMed]
- Kwolek, S.; Morgan, P.; Schaefgen, J.; Gulrich, L. Synthesis, anisotropic solutions, and fibers of poly (1, 4-benzamide). Macromolecules 1977, 10, 1390–1396. [Google Scholar] [CrossRef]
- Bair, T.; Morgan, P.; Killian, F. Poly (1, 4-phenyleneterephthalamides). Polymerization and novel liquid-crystalline solutions. Macromolecules 1977, 10, 1396–1400. [Google Scholar] [CrossRef]
- Edgar, C.D.; Gray, D.G. Induced circular dichroism of chiral nematic cellulose films. Cellulose 2001, 8, 5–12. [Google Scholar] [CrossRef]
- Guidetti, G.; Atifi, S.; Vignolini, S.; Hamad, W.Y. Flexible photonic cellulose nanocrystal films. Adv. Mater. 2016, 28, 10042–10047. [Google Scholar] [CrossRef]
- Giese, M.; Khan, M.K.; Hamad, W.Y.; MacLachlan, M.J. Imprinting of photonic patterns with thermosetting amino-formaldehyde-cellulose composites. ACS Macro Lett. 2013, 2, 818–821. [Google Scholar] [CrossRef]
- Yao, K.; Meng, Q.; Bulone, V.; Zhou, Q. Flexible and responsive chiral nematic cellulose nanocrystal/poly (ethylene glycol) composite films with uniform and tunable structural color. Adv. Mater. 2017, 29, 1701323. [Google Scholar] [CrossRef]
- Schlesinger, M.; Giese, M.; Blusch, L.K.; Hamad, W.Y.; MacLachlan, M.J. Chiral nematic cellulose–gold nanoparticle composites from mesoporous photonic cellulose. Chem. Commun. 2015, 51, 530–533. [Google Scholar] [CrossRef]
- Querejeta-Fernández, A.; Chauve, G.; Methot, M.; Bouchard, J.; Kumacheva, E. Chiral plasmonic films formed by gold nanorods and cellulose nanocrystals. J. Am. Chem. Soc. 2014, 136, 4788–4793. [Google Scholar] [CrossRef]
- Yuan, H.; Pan, H.; Meng, X.; Zhu, C.; Liu, S.; Chen, Z.; Ma, J.; Zhu, S. Assembly of MnO/CNC/rGO fibers from colloidal liquid crystal for flexible supercapacitors via a continuous one-process method. Nanotechnology 2019, 30, 465702. [Google Scholar] [CrossRef]
- An, Y.; Yang, Y.; Jia, Y.; Han, W.; Cheng, Y. Mechanical properties of biomimetic ceramic with Bouligand architecture. J. Am. Ceram. Soc. 2022, 105, 2385–2391. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Ai, J.; Ji, T.; Nagai, M.; Duan, Y.; Che, S.; Han, L. Chiral hierarchical structure of bone minerals. Nano Res. 2022, 15, 1295–1302. [Google Scholar] [CrossRef]
- Natarajan, B.; Gilman, J.W. Bioinspired Bouligand cellulose nanocrystal composites: A review of mechanical properties. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170050. [Google Scholar] [CrossRef] [PubMed]
- Behera, R.P.; Le Ferrand, H. Impact-resistant materials inspired by the mantis shrimp’s dactyl club. Matter 2021, 4, 2831–2849. [Google Scholar] [CrossRef]
- Guarín-Zapata, N.; Gomez, J.; Yaraghi, N.; Kisailus, D.; Zavattieri, P.D. Shear wave filtering in naturally-occurring Bouligand structures. Acta Biomater. 2015, 23, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bardet, R.; Belgacem, N.; Bras, J. Flexibility and color monitoring of cellulose nanocrystal iridescent solid films using anionic or neutral polymers. ACS Appl. Mater. Interface 2015, 7, 4010–4018. [Google Scholar] [CrossRef]
- Lukach, A.; Therien-Aubin, H.; Querejeta-Fernández, A.; Pitch, N.; Chauve, G.; Méthot, M.; Bouchard, J.; Kumacheva, E. Coassembly of gold nanoparticles and cellulose nanocrystals in composite films. Langmuir 2015, 31, 5033–5041. [Google Scholar] [CrossRef]
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive photonic hydrogels based on nanocrystalline cellulose. Angew. Chem. Int. Ed. 2013, 52, 8912–8916. [Google Scholar] [CrossRef]
- Kose, O.; Tran, A.; Lewis, L.; Hamad, W.Y.; MacLachlan, M.J. Unwinding a spiral of cellulose nanocrystals for stimuli-responsive stretchable optics. Nat. Commun. 2019, 10, 510. [Google Scholar] [CrossRef]
- Oguzlu, H.; Danumah, C.; Boluk, Y. Colloidal behavior of aqueous cellulose nanocrystal suspensions. Curr. Opin. Colloid Interface Sci. 2017, 29, 46–56. [Google Scholar] [CrossRef]
- Boluk, Y.; Zhao, L.; Incani, V. Dispersions of nanocrystalline cellulose in aqueous polymer solutions: Structure formation of colloidal rods. Langmuir 2012, 28, 6114–6123. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Jansson, R.; Widhe, M.; Benselfelt, T.; Hakansson, K.M.; Lundell, F.; Hedhammar, M.; Söderberg, L.D. Ultrastrong and bioactive nanostructured bio-based composites. ACS Nano 2017, 11, 5148–5159. [Google Scholar] [CrossRef] [PubMed]
- Phan-Xuan, T.; Thuresson, A.; Skepö, M.; Labrador, A.; Bordes, R.; Matic, A. Aggregation behavior of aqueous cellulose nanocrystals: The effect of inorganic salts. Cellulose 2016, 23, 3653–3663. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Tanaka, R.; Saito, T.; Isogai, A. Dispersion stability and aggregation behavior of TEMPO-oxidized cellulose nanofibrils in water as a function of salt addition. Cellulose 2014, 21, 1553–1559. [Google Scholar] [CrossRef]
- Bast, L.K.; Klockars, K.W.; Greca, L.G.; Rojas, O.J.; Tardy, B.L.; Bruns, N. Infiltration of proteins in cholesteric cellulose structures. Biomacromolecules 2021, 22, 2067–2080. [Google Scholar] [CrossRef]
- Tripathi, A.; Tardy, B.L.; Khan, S.A.; Liebner, F.; Rojas, O.J. Expanding the upper limits of robustness of cellulose nanocrystal aerogels: Outstanding mechanical performance and associated pore compression response of chiral-nematic architectures. J. Mater. Chem. A 2019, 7, 15309–15319. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Grosset, L.; Seppälä, J. Liquid crystalline thermosets based on anisotropic phases of cellulose nanocrystals. Cellulose 2013, 20, 2569–2582. [Google Scholar] [CrossRef]
- Abbasi Moud, A. Gel Development Using Cellulose Nanocrystals. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, June 2020. [Google Scholar]
- Gupta, S.; Goswami, S.; Sinha, A. A combined effect of freeze--thaw cycles and polymer concentration on the structure and mechanical properties of transparent PVA gels. Biomed. Mater. 2012, 7, 015006. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Sun, F.; Xiao, R.; Guo, Y.; Song, J. Liquid crystal microphase separation of cellulose nanocrystals in wet-spun PVA composite fibers. RSC Adv. 2014, 4, 30784–30789. [Google Scholar] [CrossRef]
- Mu, X.; Gray, D.G. Droplets of cellulose nanocrystal suspensions on drying give iridescent 3-D “coffee-stain” rings. Cellulose 2015, 22, 1103–1107. [Google Scholar] [CrossRef]
- Wang, P.-X.; Hamad, W.Y.; MacLachlan, M.J. Structure and transformation of tactoids in cellulose nanocrystal suspensions. Nat. Commun. 2016, 7, 11515. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Noh, J.; Schütz, C.; Salazar-Alvarez, G.; Scalia, G.; Bergström, L.; Lagerwall, J.P. Macroscopic control of helix orientation in films dried from cholesteric liquid-crystalline cellulose nanocrystal suspensions. ChemPhysChem 2014, 15, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Cherpak, V.; Korolovych, V.; Geryak, R.; Turiv, T.; Nepal, D.; Kelly, J.; Bunning, T.; Lavrentovich, O.; Heller, W.; Tsukruk, V. Robust chiral organization of cellulose nanocrystals in capillary confinement. Nano Lett. 2018, 18, 6770–6777. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.G.; Mu, X. Chiral nematic structure of cellulose nanocrystal suspensions and films; polarized light and atomic force microscopy. Materials 2015, 8, 7873–7888. [Google Scholar] [CrossRef]
- Honorato-Rios, C.; Kuhnhold, A.; Bruckner, J.R.; Dannert, R.; Schilling, T.; Lagerwall, J.P. Equilibrium liquid crystal phase diagrams and detection of kinetic arrest in cellulose nanocrystal suspensions. Front. Mater. 2016, 21. [Google Scholar] [CrossRef]
- de Heer, W.A.; Bacsa, W.; Chatelain, A.; Gerfin, T.; Humphrey-Baker, R.; Forro, L.; Ugarte, D. Aligned carbon nanotube films: Production and optical and electronic properties. Science 1995, 268, 845–847. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, P.; Nan, F.; Zhou, L.; Zhang, J. Tuning the iridescence of chiral nematic cellulose nanocrystal films with a vacuum-assisted self-assembly technique. Biomacromolecules 2014, 15, 4343–4350. [Google Scholar] [CrossRef]
- Bardet, R.; Roussel, F.; Coindeau, S.; Belgacem, N.; Bras, J. Engineered pigments based on iridescent cellulose nanocrystal films. Carbohydr. Polym. 2015, 122, 367–375. [Google Scholar] [CrossRef]
- Jiang, F.; Esker, A.R.; Roman, M. Acid-catalyzed and solvolytic desulfation of H2SO4-hydrolyzed cellulose nanocrystals. Langmuir 2010, 26, 17919–17925. [Google Scholar] [CrossRef]
- Kloser, E.; Gray, D.G. Surface grafting of cellulose nanocrystals with poly (ethylene oxide) in aqueous media. Langmuir 2010, 26, 13450–13456. [Google Scholar] [CrossRef]
- Beck, S.; Bouchard, J. Auto-catalyzed acidic desulfation of cellulose nanocrystals. Nord. Pulp Pap. Res. J. 2014, 29, 6–14. [Google Scholar] [CrossRef]
- Nan, F.; Nagarajan, S.; Chen, Y.; Liu, P.; Duan, Y.; Men, Y.; Zhang, J. Enhanced toughness and thermal stability of cellulose nanocrystal iridescent films by alkali treatment. ACS Sustain. Chem. Eng. 2017, 5, 8951–8958. [Google Scholar] [CrossRef]
- Jia, W.; Liu, Y. Two characteristic cellulose nanocrystals (CNCs) obtained from oxalic acid and sulfuric acid processing. Cellulose 2019, 26, 8351–8365. [Google Scholar] [CrossRef]
- Orts, W.; Revol, J.-F.; Godbout, L.; Marchessault, R. SANS study of chirality and order in liquid crystalline cellulose suspensions. MRS Online Proc. Libr. (OPL) 1994, 376, 317–322. [Google Scholar] [CrossRef]
- Timbrell, V. Alignment of carbon and other man-made fibers by magnetic fields. J. Appl. Phys. 1972, 43, 4839–4840. [Google Scholar] [CrossRef]
- Schmitt, Y.; Paulick, C.; Royer, F.; Gasser, J. Magnetic field induced orientational order of conductive fibers in non-conductive liquids. J. Non-Cryst. Solids 1996, 205, 135–138. [Google Scholar] [CrossRef]
- Fujiwara, M.; Oki, E.; Hamada, M.; Tanimoto, Y.; Mukouda, I.; Shimomura, Y. Magnetic orientation and magnetic properties of a single carbon nanotube. J. Phys. Chem. A 2001, 105, 4383–4386. [Google Scholar] [CrossRef]
- Kimura, T.; Yamato, M.; Koshimizu, W.; Koike, M.; Kawai, T. Magnetic orientation of polymer fibers in suspension. Langmuir 2000, 16, 858–861. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Cudmore, B.; Dunlop, M.J.; Acharya, B.; Bissessur, R.; Ahmed, M.; Whelan, W.M. Effect of magnetic field alignment of cellulose nanocrystals in starch nanocomposites: Physicochemical and mechanical properties. Carbohydr. Polym. 2020, 247, 116688. [Google Scholar] [CrossRef]
- De France, K.J.; Yager, K.G.; Hoare, T.; Cranston, E.D. Cooperative ordering and kinetics of cellulose nanocrystal alignment in a magnetic field. Langmuir 2016, 32, 7564–7571. [Google Scholar] [CrossRef]
- Kimura, F.; Kimura, T.; Tamura, M.; Hirai, A.; Ikuno, M.; Horii, F. Magnetic alignment of the chiral nematic phase of a cellulose microfibril suspension. Langmuir 2005, 21, 2034–2037. [Google Scholar] [CrossRef] [PubMed]
- Vignolini, S. Cellulose Nanocrystal Holograms. In Proceedings of the APS March Meeting 2021, Virtual, 15–19 March 2021; APS March Meeting Abstracts. American Physical Society (APS): College Park, MD, USA, 2021. B09.004. [Google Scholar]
- Dhar, P.; Kumar, A.; Katiyar, V. Magnetic cellulose nanocrystal based anisotropic polylactic acid nanocomposite films: Influence on electrical, magnetic, thermal, and mechanical properties. ACS Appl. Mater. Interfaces 2016, 8, 18393–18409. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Zussman, E. Electro-responsive liquid crystalline nanocelluloses with reversible switching. J. Phys. Chem. Lett. 2020, 11, 6697–6703. [Google Scholar] [CrossRef]
- Sharif, F.; Arjmand, M.; Moud, A.A.; Sundararaj, U.; Roberts, E.P. Segregated hybrid poly (methyl methacrylate)/graphene/magnetite nanocomposites for electromagnetic interference shielding. ACS Appl. Mater. Interface 2017, 9, 14171–14179. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi Meddeb, A.; Chae, I.; Han, A.; Kim, S.H.; Ounaies, Z. Magnetic field effects on cellulose nanocrystal ordering in a non-aqueous solvent. Cellulose 2020, 27, 7901–7910. [Google Scholar] [CrossRef]
- Moud, A.A. Fluorescence Recovery after Photobleaching in Colloidal Science: Introduction and Application. ACS Biomater. Sci. Eng. 2022, 8, 1028–1048. [Google Scholar] [CrossRef]
- Fraden, S.; Hurd, A.; Meyer, R.; Cahoon, M.; Caspar, D. Magnetic-field-induced alignment and instabilities in ordered colloids of tobacco mosaic virus. J. Phys. Colloq. 1985, 46, C3–C85. [Google Scholar] [CrossRef]
- Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Vignolini, S. Controlling the photonic properties of cholesteric cellulose nanocrystal films with magnets. Adv. Mater. 2017, 29, 1701469. [Google Scholar] [CrossRef]
- Habibi, Y.; Heim, T.; Douillard, R. AC electric field-assisted assembly and alignment of cellulose nanocrystals. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1430–1436. [Google Scholar] [CrossRef]
- Frka-Petesic, B.; Sugiyama, J.; Kimura, S.; Chanzy, H.; Maret, G. Negative diamagnetic anisotropy and birefringence of cellulose nanocrystals. Macromolecules 2015, 48, 8844–8857. [Google Scholar] [CrossRef]
- Frka-Petesic, B.; Radavidson, H.; Jean, B.; Heux, L. Dynamically controlled iridescence of cholesteric cellulose nanocrystal suspensions using electric fields. Adv. Mater. 2017, 29, 1606208. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Han, T.H.; Lee, S.H.; Kim, J.Y.; Ahn, C.W.; Yun, J.M.; Kim, S.O. Graphene oxide liquid crystals. Angew. Chem. 2011, 123, 3099–3103. [Google Scholar] [CrossRef]
- Hull, R.; Hills, G.; Markham, R. Studies on Alfalfa mosaic virus: II. The structure of the virus components. Virology 1969, 37, 416–428. [Google Scholar] [CrossRef]
- He, Q.-Q.; Lan, Y.; Quan, Y.-Y.; Li, C.-Y.; Liu, Y.-P.; Wang, X.-J.; Jia, Y.-G.; Tian, M.; Yao, D.-S. The influence of the structure of terminal groups and cores on the properties of schiff base star-shaped liquid crystals. Liq. Cryst. 2021, 48, 1309–1320. [Google Scholar] [CrossRef]
- Kvien, I.; Oksman, K. Orientation of cellulose nanowhiskers in polyvinyl alcohol. Appl. Phys. A 2007, 87, 641–643. [Google Scholar] [CrossRef]
- Ebeling, T.; Paillet, M.; Borsali, R.; Diat, O.; Dufresne, A.; Cavaille, J.; Chanzy, H. Shear-induced orientation phenomena in suspensions of cellulose microcrystals, revealed by small angle X-ray scattering. Langmuir 1999, 15, 6123–6126. [Google Scholar] [CrossRef]
- Hoeger, I.; Rojas, O.J.; Efimenko, K.; Velev, O.D.; Kelley, S.S. Ultrathin film coatings of aligned cellulose nanocrystals from a convective-shear assembly system and their surface mechanical properties. Soft Matter 2011, 7, 1957–1967. [Google Scholar] [CrossRef]
- Abbasi Moud, A. Cellulose through the lens of microfluidics: A review. Appl. Biosci. 2022, 1, 1–37. [Google Scholar] [CrossRef]
- Yoshiharu, N.; Shigenori, K.; Masahisa, W.; Takeshi, O. Cellulose microcrystal film of high uniaxial orientation. Macromolecules 1997, 30, 6395–6397. [Google Scholar] [CrossRef]
- Diaz, J.A.; Wu, X.; Martini, A.; Youngblood, J.P.; Moon, R.J. Thermal expansion of self-organized and shear-oriented cellulose nanocrystal films. Biomacromolecules 2013, 14, 2900–2908. [Google Scholar] [CrossRef]
- Ličen, M.; Majaron, B.; Noh, J.; Schütz, C.; Bergström, L.; Lagerwall, J.; Drevenšek-Olenik, I. Correlation between structural properties and iridescent colors of cellulose nanocrystalline films. Cellulose 2016, 23, 3601–3609. [Google Scholar] [CrossRef]
- Brostow, W. Mechanical and Thermophysical Properties of Polymer Liquid Crystals; Springer Science & Business Media: London, UK, 2013; Volume 3. [Google Scholar]
- Esmaeili, M.; George, K.; Taheri-Qazvini, N.; Sadati, M. 3D Printing of Chiral Liquid Crystal Elastomers Using Cellulose Nanocrystals. In 2021 AIChE Annual Meeting; AIChE: Boston, MA, USA, 2021. [Google Scholar]
- Folgar, F.; Tucker III, C.L. Orientation behavior of fibers in concentrated suspensions. J. Reinf. Plast. Compos. 1984, 3, 98–119. [Google Scholar] [CrossRef]
- Jeffery, G.B. The motion of ellipsoidal particles immersed in a viscous fluid. Proc. R. Soc. London. Ser. A Contain. Pap. A Math. Phys. Character 1922, 102, 161–179. [Google Scholar]
- Werbowyj, R.S.; Gray, D.G. Optical properties of hydroxypropyl cellulose liquid crystals. I. Cholesteric pitch and polymer concentration. Macromolecules 1984, 17, 1512–1520. [Google Scholar] [CrossRef]
- Dumanli, A.G.; Kamita, G.; Landman, J.; van der Kooij, H.; Glover, B.J.; Baumberg, J.J.; Steiner, U.; Vignolini, S. Controlled, bio-inspired self-assembly of cellulose-based chiral reflectors. Adv. Opt. Mater. 2014, 2, 646–650. [Google Scholar] [CrossRef]
- Dumanli, A.G.M.; Van Der Kooij, H.M.; Kamita, G.; Reisner, E.; Baumberg, J.J.; Steiner, U.; Vignolini, S. Digital color in cellulose nanocrystal films. ACS Appl. Mater. Interface 2014, 6, 12302–12306. [Google Scholar] [CrossRef]
- De Vries, H. Rotatory power and other optical properties of certain liquid crystals. Acta Crystallogr. 1951, 4, 219–226. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Sierra, E.; Eguiraun, H.; Lizundia, E. Iridescent cellulose nanocrystal films: The link between structural colour and Bragg’s law. Eur. J. Phys. 2018, 39, 045803. [Google Scholar] [CrossRef]
- Lagerwall, S.T. On some important chapters in the history of liquid crystals. Liq. Cryst. 2013, 40, 1698–1729. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Zhai, S.; Sugiyama, J.; Pan, M.; Shi, J.; Lu, H. Dual response of photonic films with chiral nematic cellulose nanocrystals: Humidity and formaldehyde. ACS Appl. Mater. Interface 2020, 12, 17833–17844. [Google Scholar] [CrossRef]
- Qin, J.; Wang, Z.; Hu, J.; Yuan, Y.; Liu, P.; Cheng, L.; Kong, Z.; Liu, K.; Yan, S.; Zhang, J. Distinct liquid crystal self-assembly behavior of cellulose nanocrystals functionalized with ionic liquids. Colloids Surf. Physicochem. Eng. Asp. 2022, 632, 127790. [Google Scholar] [CrossRef]
- Lin, M.; Raghuwanshi, V.S.; Browne, C.; Simon, G.P.; Garnier, G. Modulating transparency and colour of cellulose nanocrystal composite films by varying polymer molecular weight. J. Colloid Interface Sci. 2021, 584, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Li, X.; Zhang, L.; Li, X.; Liu, P.; Jiang, Z.; Yu, Z.-Z. Rapidly responsive and flexible chiral nematic cellulose nanocrystal composites as multifunctional rewritable photonic papers with eco-friendly inks. ACS Appl. Mater. Interface 2018, 10, 5918–5925. [Google Scholar] [CrossRef] [PubMed]
- Klockars, K.W.; Tardy, B.L.; Borghei, M.; Tripathi, A.; Greca, L.G.; Rojas, O.J. Effect of anisotropy of cellulose nanocrystal suspensions on stratification, domain structure formation, and structural colors. Biomacromolecules 2018, 19, 2931–2943. [Google Scholar] [CrossRef]
- Jin, S.-A.; Facchine, E.G.; Khan, S.A.; Rojas, O.J.; Spontak, R.J. Mesophase characteristics of cellulose nanocrystal films prepared from electrolyte suspensions. J. Colloid Interface Sci. 2021, 599, 207–218. [Google Scholar] [CrossRef]
- Cheng, Z.; Ye, H.; Cheng, F.; Li, H.; Ma, Y.; Zhang, Q.; Natan, A.; Mukhopadhyay, A.; Jiao, Y.; Li, Y. Tuning chiral nematic pitch of bioresourced photonic films via coupling organic acid hydrolysis. Adv. Mater. Interface 2019, 6, 1802010. [Google Scholar] [CrossRef]
- Dong, X.; Gray, D. Effect of counterions on ordered phase formation in suspensions of charged rodlike cellulose crystallites. Langmuir 1997, 13, 2404–2409. [Google Scholar] [CrossRef]
- Kelly, J.A.; Shopsowitz, K.E.; Ahn, J.M.; Hamad, W.Y.; MacLachlan, M.J. Chiral nematic stained glass: Controlling the optical properties of nanocrystalline cellulose-templated materials. Langmuir 2012, 28, 17256–17262. [Google Scholar] [CrossRef]
- Van Rie, J.; González-Rubio, G.; Kumar, S.; Schütz, C.; Kohlbrecher, J.; Vanroelen, M.; Van Gerven, T.; Deschaume, O.; Bartic, C.; Liz-Marzán, L.M. SANS study of mixed cholesteric cellulose nanocrystal–gold nanorod suspensions. Chem. Commun. 2020, 56, 13001–13004. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, Q.; Meng, X.; Li, Y.; Peng, H.; Whittaker, A.K.; Zhu, S. Ultrasensitive magnetic tuning of optical properties of films of cholesteric cellulose nanocrystals. ACS Nano 2020, 14, 9440–9448. [Google Scholar] [CrossRef]
- Grelet, E.; Fraden, S. What is the origin of chirality in the cholesteric phase of virus suspensions? Phys. Rev. Lett. 2003, 90, 198302. [Google Scholar] [CrossRef] [PubMed]
- Seč, D.; Porenta, T.; Ravnik, M.; Žumer, S. Geometrical frustration of chiral ordering in cholesteric droplets. Soft Matter 2012, 8, 11982–11988. [Google Scholar] [CrossRef]
- Nayani, K.; Chang, R.; Fu, J.; Ellis, P.W.; Fernandez-Nieves, A.; Park, J.O.; Srinivasarao, M. Spontaneous emergence of chirality in achiral lyotropic chromonic liquid crystals confined to cylinders. Nat. Commun. 2015, 6, 8067. [Google Scholar] [CrossRef]
- Kossyrev, P.; Ravnik, M.; Žumer, S. Branching of colloidal chains in capillary-confined nematics. Phys. Rev. Lett. 2006, 96, 048301. [Google Scholar] [CrossRef] [PubMed]
- Busseron, E.; Ruff, Y.; Moulin, E.; Giuseppone, N. Supramolecular self-assemblies as functional nanomaterials. Nanoscale 2013, 5, 7098–7140. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.D. Liquid crystal models of biological materials and processes. Soft Matter 2010, 6, 3402–3429. [Google Scholar] [CrossRef]
- Seago, A.E.; Brady, P.; Vigneron, J.-P.; Schultz, T.D. Gold bugs and beyond: A review of iridescence and structural colour mechanisms in beetles (Coleoptera). J. R. Soc. Interface 2009, 6, S165–S184. [Google Scholar] [CrossRef] [PubMed]
- Agez, G.; Bayon, C.; Mitov, M. Multiwavelength micromirrors in the cuticle of scarab beetle Chrysina gloriosa. Acta Biomater. 2017, 48, 357–367. [Google Scholar] [CrossRef]
- Parker, R.M.; Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Consani, G.; Abell, C.; Vignolini, S. Hierarchical self-assembly of cellulose nanocrystals in a confined geometry. ACS Nano 2016, 10, 8443–8449. [Google Scholar] [CrossRef]
- Parker, R.M.; Guidetti, G.; Williams, C.A.; Zhao, T.; Narkevicius, A.; Vignolini, S.; Frka-Petesic, B. The self-assembly of cellulose nanocrystals: Hierarchical design of visual appearance. Adv. Mater. 2018, 30, 1704477. [Google Scholar] [CrossRef]
- Li, Y.; Jun-Yan Suen, J.; Prince, E.; Larin, E.M.; Klinkova, A.; Thérien-Aubin, H.; Zhu, S.; Yang, B.; Helmy, A.S.; Lavrentovich, O.D. Colloidal cholesteric liquid crystal in spherical confinement. Nat. Commun. 2016, 7, 12520. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Prince, E.; Cho, S.; Salari, A.; Mosaddeghian Golestani, Y.; Lavrentovich, O.D.; Kumacheva, E. Periodic assembly of nanoparticle arrays in disclinations of cholesteric liquid crystals. Proc. Natl. Acad. Sci. USA 2017, 114, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Prince, E.; Wang, Y.; Smalyukh, I.I.; Kumacheva, E. Cylindrical confinement of nanocolloidal cholesteric liquid crystal. J. Phys. Chem. B 2021, 125, 8243–8250. [Google Scholar] [CrossRef]
- Giese, M.; Blusch, L.K.; Khan, M.K.; Hamad, W.Y.; MacLachlan, M.J. Responsive mesoporous photonic cellulose films by supramolecular cotemplating. Angew. Chem. 2014, 126, 9026–9030. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Li, J.; Ye, S.; Wei, J.; Guo, J. A bio-inspired cellulose nanocrystal-based nanocomposite photonic film with hyper-reflection and humidity-responsive actuator properties. J. Mater. Chem. C 2016, 4, 9687–9696. [Google Scholar] [CrossRef]
- Lu, T.; Pan, H.; Ma, J.; Li, Y.; Bokhari, S.W.; Jiang, X.; Zhu, S.; Zhang, D. Cellulose nanocrystals/polyacrylamide composites of high sensitivity and cycling performance to gauge humidity. ACS Appl. Mater. Interface 2017, 9, 18231–18237. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, C.; Lei, S.; Chen, J.; Lei, S.; Li, Z. Ultraviolet light-, temperature-and pH-responsive fluorescent sensors based on cellulose nanocrystals. Polym. Chem. 2018, 9, 3098–3107. [Google Scholar] [CrossRef]
- Feng, K.; Dong, C.; Gao, Y.; Jin, Z. A green and iridescent composite of cellulose nanocrystals with wide solvent resistance and strong mechanical properties. ACS Sustain. Chem. Eng. 2021, 9, 6764–6775. [Google Scholar] [CrossRef]
- He, Y.-D.; Zhang, Z.-L.; Xue, J.; Wang, X.-H.; Song, F.; Wang, X.-L.; Zhu, L.-L.; Wang, Y.-Z. Biomimetic optical cellulose nanocrystal films with controllable iridescent color and environmental stimuli-responsive chromism. ACS Appl. Mater. Interface 2018, 10, 5805–5811. [Google Scholar] [CrossRef]
- Ganesan, K.; Barowski, A.; Ratke, L. Gas permeability of cellulose aerogels with a designed dual pore space system. Molecules 2019, 24, 2688. [Google Scholar] [CrossRef]
- Park, H.-S.; Kang, S.-W.; Tortora, L.; Kumar, S.; Lavrentovich, O.D. Condensation of self-assembled lyotropic chromonic liquid crystal sunset yellow in aqueous solutions crowded with polyethylene glycol and doped with salt. Langmuir 2011, 27, 4164–4175. [Google Scholar] [CrossRef] [PubMed]
- Vasilevskaya, V.; Khokhlov, A.; Matsuzawa, Y.; Yoshikawa, K. Collapse of single DNA molecule in poly (ethylene glycol) solutions. J. Chem. Phys. 1995, 102, 6595–6602. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Ma, C.; Yu, H.; Wu, Y.; Wang, Y.; Chen, Z.; Li, J.; Liu, S. Multifunctional chiral nematic cellulose nanocrystals/glycerol structural colored nanocomposites for intelligent responsive films, photonic inks and iridescent coatings. J. Mater. Chem. C 2018, 6, 5391–5400. [Google Scholar] [CrossRef]
- Sheikhi, A.; Hayashi, J.; Eichenbaum, J.; Gutin, M.; Kuntjoro, N.; Khorsandi, D.; Khademhosseini, A. Recent advances in nanoengineering cellulose for cargo delivery. J. Control Release 2019, 294, 53–76. [Google Scholar] [CrossRef] [PubMed]
- John, W.S.; Fritz, W.; Lu, Z.; Yang, D.-K. Bragg reflection from cholesteric liquid crystals. Phys. Rev. E 1995, 51, 1191. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, M.; Reyes, C.G.; Noh, J.; Sharma, A.; Geng, Y.; Jampani, V.S.R.; Lagerwall, J.P. Liquid crystals in micron-scale droplets, shells and fibers. J. Phys. Condens. Matter 2017, 29, 133003. [Google Scholar] [CrossRef]
- Ji, Q.; Lefort, R.; Busselez, R.; Morineau, D. Structure and dynamics of a Gay–Berne liquid crystal confined in cylindrical nanopores. J. Chem. Phys. 2009, 130, 234501. [Google Scholar] [CrossRef]
- Guo, M.; Li, Y.; Yan, X.; Song, J.; Liu, D.; Li, Q.; Su, F.; Shi, X. Sustainable iridescence of cast and shear coatings of cellulose nanocrystals. Carbohydr. Polym. 2021, 273, 118628. [Google Scholar] [CrossRef]
- Feng, K.; Gao, X.; Gu, Z.; Jin, Z. Improving homogeneity of iridescent cellulose nanocrystal films by surfactant-assisted spreading self-assembly. ACS Sustain. Chem. Eng. 2019, 7, 19062–19071. [Google Scholar] [CrossRef]
- Giese, M.; De Witt, J.C.; Shopsowitz, K.E.; Manning, A.P.; Dong, R.Y.; Michal, C.A.; Hamad, W.Y.; MacLachlan, M.J. Thermal switching of the reflection in chiral nematic mesoporous organosilica films infiltrated with liquid crystals. ACS Appl. Mater. Interface 2013, 5, 6854–6859. [Google Scholar] [CrossRef]
- Yi, J.; Xu, Q.; Zhang, X.; Zhang, H. Temperature-induced chiral nematic phase changes of suspensions of poly (N, N-dimethylaminoethyl methacrylate)-grafted cellulose nanocrystals. Cellulose 2009, 16, 989–997. [Google Scholar] [CrossRef]
- Arcolezi, G.; Luders, D.; Sampaio, A.; Simões, M.; Braga, W.; Santos, O.; Palangana, A.; Kimura, N. Computational method to determine the pitch length in cholesteric liquid crystals. J. Mol. Liq. 2020, 298, 111752. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Weissman, I.; Levi, E.; Ein-Eli, Y. On the correlation between surface chemistry and performance of graphite negative electrodes for Li ion batteries. Electrochim. Acta 1999, 45, 67–86. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, C.Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.Q.; Yu, D.; Liu, Y.; Titirici, M.M.; Chueh, Y.L. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Posada, J.O.G.; Rennie, A.J.; Villar, S.P.; Martins, V.L.; Marinaccio, J.; Barnes, A.; Glover, C.F.; Worsley, D.A.; Hall, P.J. Aqueous batteries as grid scale energy storage solutions. Renew. Sustain. Energy Rev. 2017, 68, 1174–1182. [Google Scholar] [CrossRef]
- Hu, B.L.-H.; Wu, F.; Lin, C.; Khlobystov, A.; Li, L. Graphenemodified LiFePO4 cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K.; Hara, Y.; Adachi, Y. Effect of carbon coating on electrochemical performance of treated natural graphite as lithium-ion battery anode material. J. Electrochem. Soc. 2000, 147, 1245. [Google Scholar] [CrossRef]
- Peled, E.; Menachem, C.; Bar-Tow, D.; Melman, A. Improved graphite anode for lithium-ion batteries chemically: Bonded solid electrolyte interface and nanochannel formation. J. Electrochem. Soc. 1996, 143, L4. [Google Scholar] [CrossRef]
- Ko, S.; Lee, J.I.; Yang, H.S.; Park, S.; Jeong, U. Mesoporous CuO particles threaded with CNTs for high-performance lithium-ion battery anodes. Adv. Mater. 2012, 24, 4451–4456. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, Y.; Liu, Y.; Wang, C. Electrochemical performance of porous carbon/tin composite anodes for sodium-ion and lithium-ion batteries. Adv. Energy Mater. 2013, 3, 128–133. [Google Scholar] [CrossRef]
- Zhou, J.; Qin, J.; Zhang, X.; Shi, C.; Liu, E.; Li, J.; Zhao, N.; He, C. 2D space-confined synthesis of few-layer MoS2 anchored on carbon nanosheet for lithium-ion battery anode. ACS Nano 2015, 9, 3837–3848. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Zhang, Y.; Chen, L.; Zhao, Q.; Meng, Y.; Guo, Y.; Xiao, D. Nitrogen-rich porous carbon derived from biomass as a high performance anode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 6534–6541. [Google Scholar] [CrossRef]
- Wang, L.; Schnepp, Z.; Titirici, M.M. Rice husk-derived carbon anodes for lithium ion batteries. J. Mater. Chem. A 2013, 1, 5269–5273. [Google Scholar] [CrossRef]
- Kim, P.J.; Fontecha, H.D.; Kim, K.; Pol, V.G. Toward high-performance lithium–sulfur batteries: Upcycling of LDPE plastic into sulfonated carbon scaffold via microwave-promoted sulfonation. ACS Appl. Mater. Interface 2018, 10, 14827–14834. [Google Scholar] [CrossRef]
- Tang, J.; Etacheri, V.; Pol, V.G. Wild fungus derived carbon fibers and hybrids as anodes for lithium-ion batteries. ACS Sustain. Chem. Eng. 2016, 4, 2624–2631. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-density sodium and lithium ion battery anodes from banana peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef]
- Etacheri, V.; Hong, C.N.; Pol, V.G. Upcycling of packing-peanuts into carbon microsheet anodes for lithium-ion batteries. Environ. Sci. Technol. 2015, 49, 11191–11198. [Google Scholar] [CrossRef]
- Lim, D.G.; Kim, K.; Razdan, M.; Diaz, R.; Osswald, S.; Pol, V.G. Lithium storage in structurally tunable carbon anode derived from sustainable source. Carbon 2017, 121, 134–142. [Google Scholar] [CrossRef]
- Kim, K.; Lim, D.G.; Han, C.W.; Osswald, S.; Ortalan, V.; Youngblood, J.P.; Pol, V.G. Tailored carbon anodes derived from biomass for sodium-ion storage. ACS Sustain. Chem. Eng. 2017, 5, 8720–8728. [Google Scholar] [CrossRef]
- Kim, K.; Kim, P.J.; Youngblood, J.P.; Pol, V.G. Surface Functionalization of Carbon Architecture with Nano-MnO2 for Effective Polysulfide Confinement in Lithium–Sulfur Batteries. ChemSusChem 2018, 11, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.; Lee, J.W.; Song, T.; Paik, U. 3D-interconnected nanoporous RGO-CNT structure for supercapacitors application. Electrochim. Acta 2014, 125, 536–542. [Google Scholar] [CrossRef]
- Shokhen, V.; Zitoun, D. Platinum-group metal grown on vertically aligned MoS2 as electrocatalysts for hydrogen evolution reaction. Electrochim. Acta 2017, 257, 49–55. [Google Scholar] [CrossRef]
- Liu, W.; Lee, S.W.; Lin, D.; Shi, F.; Wang, S.; Sendek, A.D.; Cui, Y. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat. Energy 2017, 2, 17035. [Google Scholar] [CrossRef]
- Walters, C.M.; Matharu, G.K.; Hamad, W.Y.; Lizundia, E.; MacLachlan, M.J. Chiral nematic cellulose nanocrystal/germania and carbon/germania composite aerogels as supercapacitor materials. Chem. Mater. 2021, 33, 5197–5209. [Google Scholar] [CrossRef]
- Lizundia, E.; Nguyen, T.-D.; Vilas, J.L.; Hamad, W.Y.; MacLachlan, M.J. Chiroptical, morphological and conducting properties of chiral nematic mesoporous cellulose/polypyrrole composite films. J. Mater. Chem. A 2017, 5, 19184–19194. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Li, J.; Lizundia, E.; Niederberger, M.; Hamad, W.Y.; MacLachlan, M.J. Black titania with nanoscale helicity. Adv. Funct. Mater. 2019, 29, 1904639. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Chiral nematic mesoporous carbon derived from nanocrystalline cellulose. Angew. Chem. Int. Ed. 2011, 50, 10991–10995. [Google Scholar] [CrossRef]
- Tang, H.; Chen, R.; Huang, Q.; Ge, W.; Zhang, X.; Yang, Y.; Wang, X. Scalable manufacturing of leaf-like MXene/Ag NWs/cellulose composite paper electrode for all-solid-state supercapacitor. EcoMat 2022, e12247. [Google Scholar] [CrossRef]
- Yu, R.; Lan, T.; Jiang, J.; Peng, H.; Liang, R.; Liu, G. Facile fabrication of functional cellulose paper with high-capacity immobilization of Ag nanoparticles for catalytic applications for tannery wastewater. J. Leather Sci. Eng. 2020, 2, 6. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Flexible and iridescent chiral nematic mesoporous organosilica films. J. Am. Chem. Soc. 2012, 134, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Shopsowitz, K.E.; Qi, H.; Hamad, W.Y.; MacLachlan, M.J. Free-standing mesoporous silica films with tunable chiral nematic structures. Nature 2010, 468, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fuentes-Hernandez, C.; Khan, T.M.; Liu, J.-C.; Hsu, J.; Shim, J.W.; Dindar, A.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Recyclable organic solar cells on cellulose nanocrystal substrates. Sci. Rep. 2013, 3, 1536. [Google Scholar] [CrossRef]
- Kim, K.; Kim, P.J.; Chowdhury, R.A.; Kantharaj, R.; Candadai, A.; Marconnet, A.; Pol, V.G.; Youngblood, J.P. Structural orientation effect of cellulose nanocrystals (CNC) films on electrochemical kinetics and stability in lithium-ion batteries. Chem. Eng. J. 2021, 417, 128128. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Xuan, Y.; Zhou, L.; Wang, D.; Li, Z.; Lin, H.; Tretiak, S.; Wang, H.; Wang, L. Multifunctional Cellulose Nanocrystals as a High-Efficient Polysulfide Stopper for Practical Li–S Batteries. ACS Appl. Mater. Interfaces 2020, 12, 17592–17601. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Z.; Xiang, Y.; Liu, D.; Xie, Z.; Qu, D.; Sun, M.; Tang, H.; Li, J. Cellulose-based material in lithium-sulfur batteries: A review. Carbohydr. Polym. 2021, 255, 117469. [Google Scholar] [CrossRef]
- Subramanian, G. Chiral Separation Techniques: A Practical Approach; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Shukla, N.; Gellman, A.J. Chiral metal surfaces for enantioselective processes. Nat. Mater. 2020, 19, 939–945. [Google Scholar] [CrossRef]
- Gin, D.L.; Lu, X.; Nemade, P.R.; Pecinovsky, C.S.; Xu, Y.; Zhou, M. Recent advances in the design of polymerizable lyotropic liquid-crystal assemblies for heterogeneous catalysis and selective separations. Adv. Funct. Mater. 2006, 16, 865–878. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Lizundia, E.; Niederberger, M.; Hamad, W.Y.; MacLachlan, M.J. Self-assembly route to TiO2 and TiC with a liquid crystalline order. Chem. Mater. 2019, 31, 2174–2181. [Google Scholar] [CrossRef]
- Duan, C.; Cheng, Z.; Wang, B.; Zeng, J.; Xu, J.; Li, J.; Gao, W.; Chen, K. Chiral photonic liquid crystal films derived from cellulose nanocrystals. Small 2021, 17, 2007306. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.N.; Almeida, P.L.; Monge, N.; Aguirre, L.E.; Reis, D.; de Oliveira, C.L.; Neto, A.M.; Pieranski, P.; Godinho, M.H. Mind the microgap in iridescent cellulose nanocrystal films. Adv. Mater. 2017, 29, 1603560. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, Y.; Zhang, B.; Feng, P.; Dang, C.; Li, M.; Zhao, L.; Gao, L. Dual-Mode Circularly Polarized Light Emission and Metal-Enhanced Fluorescence Realized by the Luminophore–Chiral Cellulose Nanocrystal Interfaces. ACS Appl. Mater. Interfaces 2021, 13, 59132–59141. [Google Scholar] [CrossRef]
- Cao, Y.; Hamad, W.Y.; MacLachlan, M.J. Broadband circular polarizing film based on chiral nematic liquid crystals. Adv. Opt. Mater. 2018, 6, 1800412. [Google Scholar] [CrossRef]
- Frka-Petesic, B.; Kamita, G.; Guidetti, G.; Vignolini, S. Angular optical response of cellulose nanocrystal films explained by the distortion of the arrested suspension upon drying. Phys. Rev. Mater. 2019, 3, 045601. [Google Scholar] [CrossRef]
- Li, J.; Bisoyi, H.K.; Tian, J.; Guo, J.; Li, Q. Optically rewritable transparent liquid crystal displays enabled by light-driven chiral fluorescent molecular switches. Adv. Mater. 2019, 31, 1807751. [Google Scholar] [CrossRef] [PubMed]
- Gençer, A.; Schütz, C.; Thielemans, W. Influence of the particle concentration and marangoni flow on the formation of cellulose nanocrystal films. Langmuir 2017, 33, 228–234. [Google Scholar] [CrossRef]
- Thérien-Aubin, H.; Lukach, A.; Pitch, N.; Kumacheva, E. Coassembly of nanorods and nanospheres in suspensions and in stratified films. Angew. Chem. 2015, 127, 5710–5714. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Anderson, V.J.; Lekkerkerker, H.N. Insights into phase transition kinetics from colloid science. Nature 2002, 416, 811–815. [Google Scholar] [CrossRef]
- Asakura, S.; Oosawa, F. On interaction between two bodies immersed in a solution of macromolecules. J. Chem. Phys. 1954, 22, 1255–1256. [Google Scholar] [CrossRef]
- Mondiot, F.; Botet, R.; Snabre, P.; Mondain-Monval, O.; Loudet, J.-C. Colloidal aggregation and dynamics in anisotropic fluids. Proc. Natl. Acad. Sci. USA 2014, 111, 5831–5836. [Google Scholar] [CrossRef] [PubMed]
- van der Schoot, P. Depletion interactions in lyotropic nematics. J. Chem. Phys. 2000, 112, 9132–9138. [Google Scholar] [CrossRef][Green Version]
- Ye, X.; Millan, J.A.; Engel, M.; Chen, J.; Diroll, B.T.; Glotzer, S.C.; Murray, C.B. Shape alloys of nanorods and nanospheres from self-assembly. Nano Lett. 2013, 13, 4980–4988. [Google Scholar] [CrossRef]
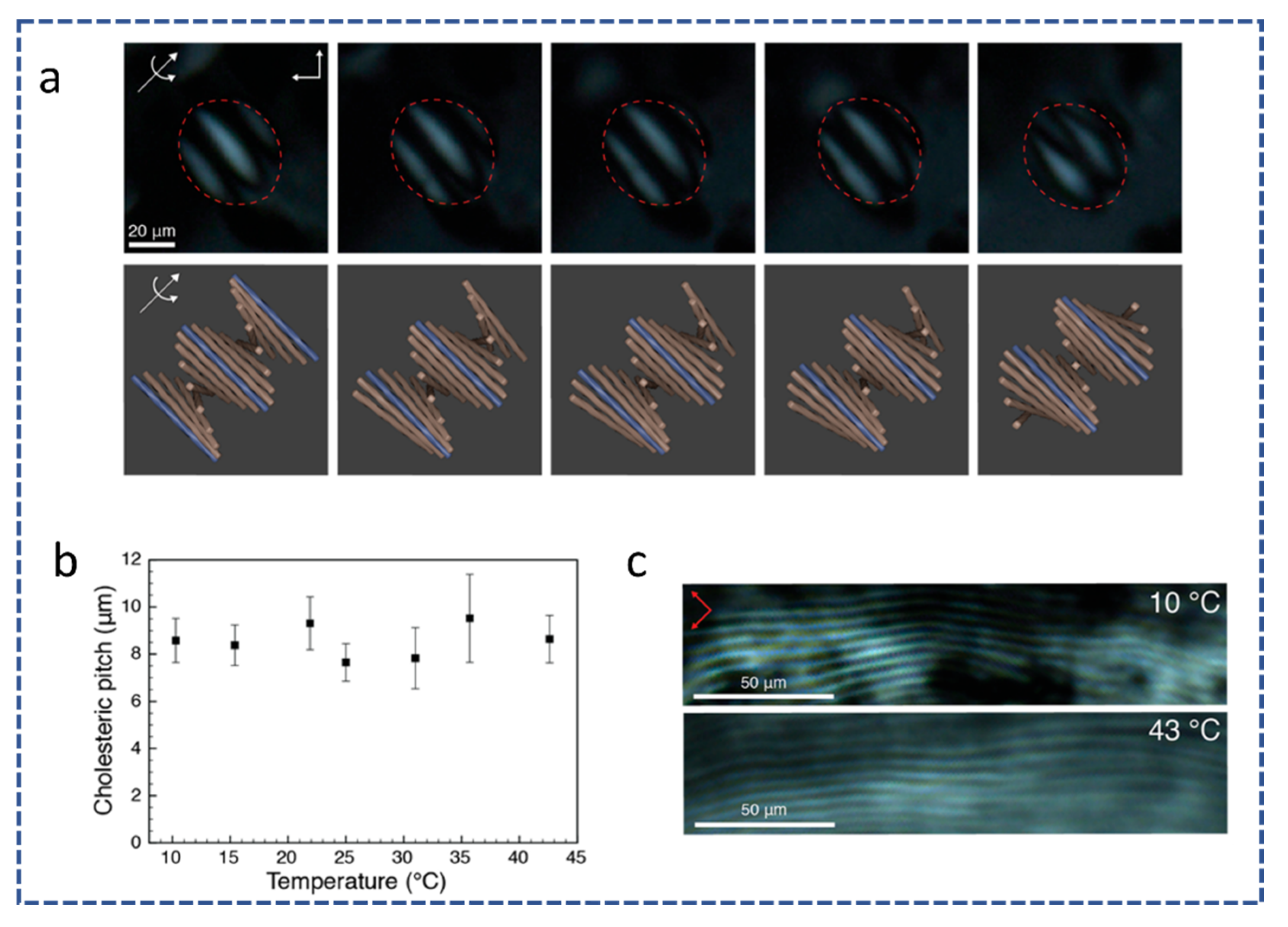

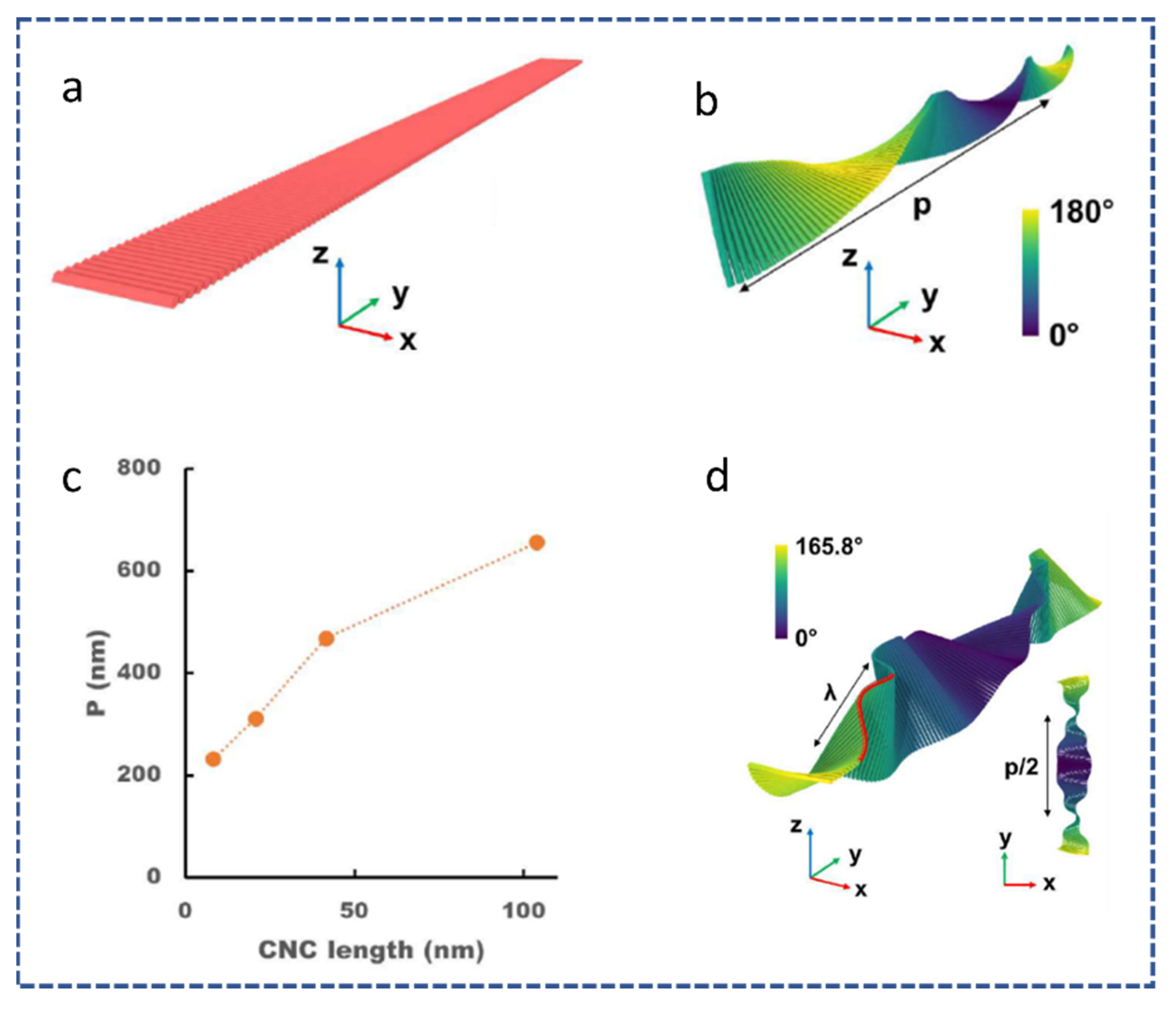
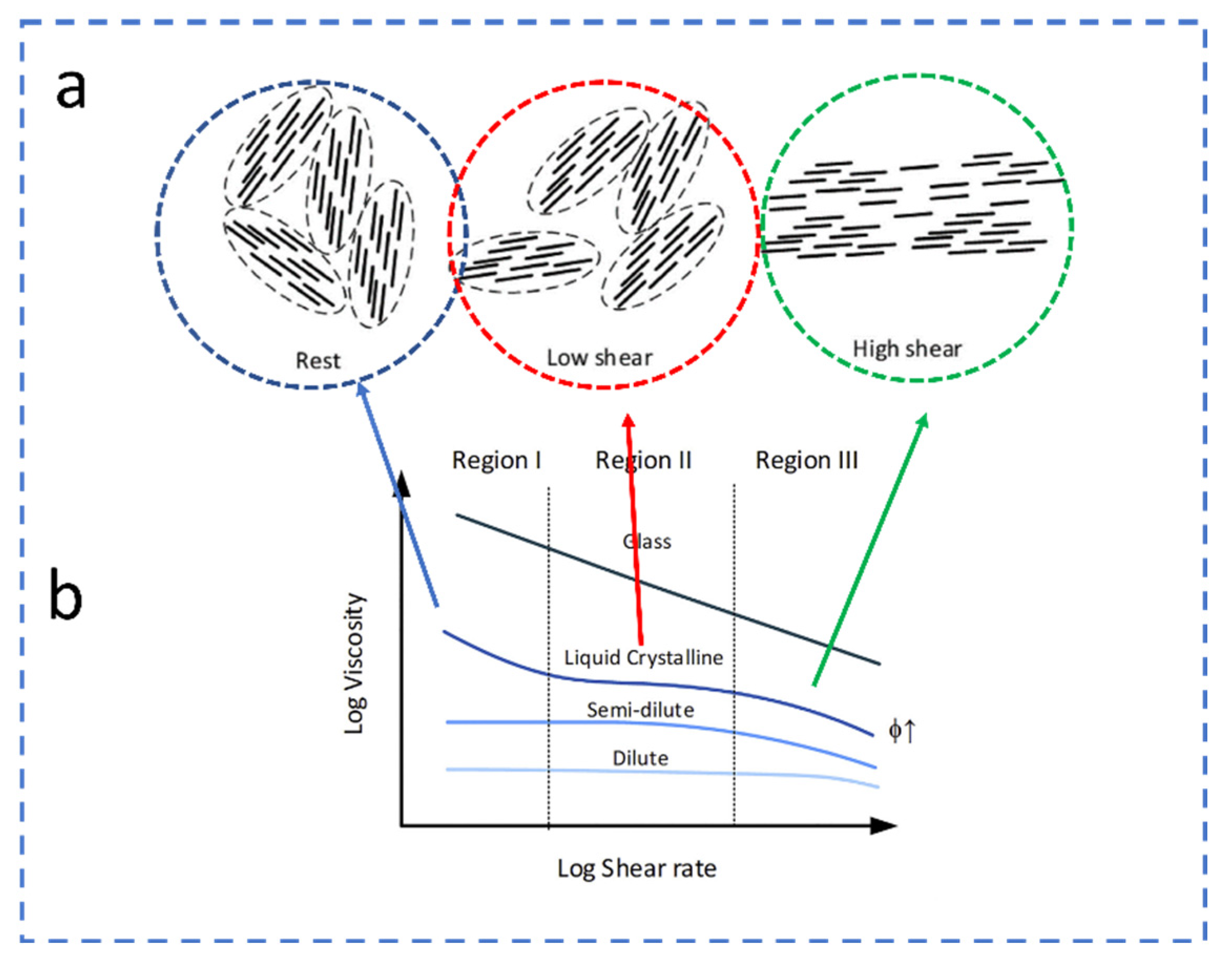
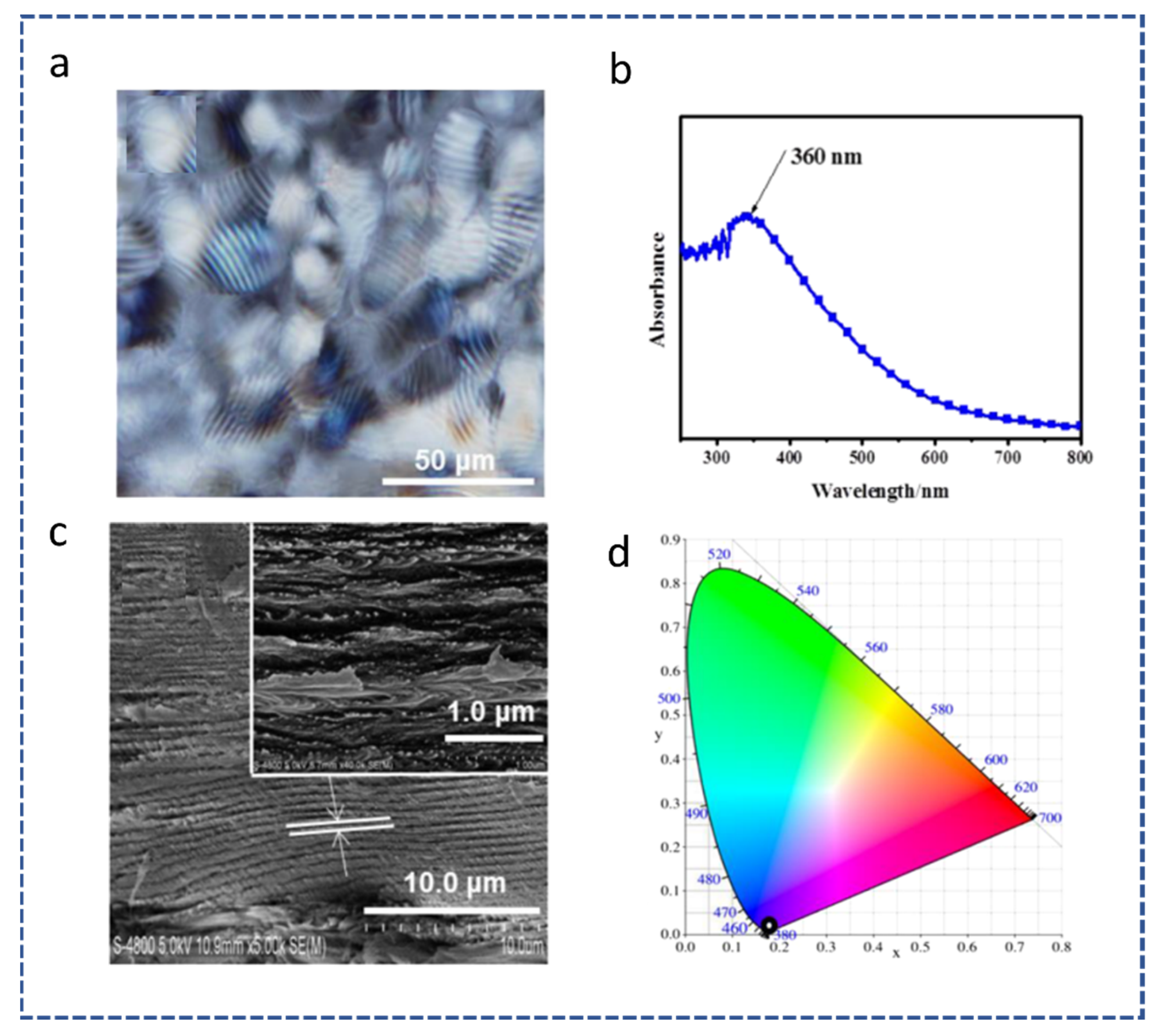
| Reference | Highlight |
| [37] | CNCs are bio-derived colloidal particles that can self-assemble into chiral photonic structures. Their mesophase chirality’s genesis is unknown. CNC crystallite bundle might be the missing piece in the molecular-to-colloidal hierarchical transfer. |
| [38] | Congo red displays induced optical activity when attached to regenerated cellulose in gel films produced by gradual precipitation from LiCl/N,N,dimethylacetamide solution. The colour generation is structural rather than the result of dye interaction with chiral centres on the cellulose chain. |
| [39] | The bio-renewable resource CNCs spontaneously arrange into chiral nematic LCs. They reflect light, giving them an iridescent appearance. Recent breakthroughs in photonic material development employing CNCs are described in this manuscript. |
| [28] | In aqueous suspension, CNCs cause anisotropic order, which results in iridescence from the fluid phase. The effects of hydrolysis duration, wood pulp species, and sonication on LC phase separation are examined. |
| [40] | Cellulose microcrystallites can be made from wood, cotton, or animal sources. When cellulose fibres are acid hydrolysed, they produce stable aqueous suspensions. These whiskers are arbitrarily suspended in the water and create an isotropic phase at very small doses. |
| [41] | CNCs are elongated nanocolloids derived from nature that generate cholesteric phases in water and apolar solvents. They are made up of bundles of crystalline microfibrils that are grouped together. The genesis of chiral interactions between CNCs is unknown. |
| [42] | The major alcohol in cellulose I is in a trans-glycosidic (TG)-linkage orientation, resulting in the production of twist-causing hydrogen bonds. Other crystalline forms of cellulose exhibit microfibril-scale twisting. This study indicates that it is partly attributable to the absence of secondary alcohol in the TG orientation in those forms. |
| [43] | The helical self-assembly of cholesteric LCs is a strong, naturally formed assembly pattern. Attempts to emulate these extraordinary materials frequently result in films having a non-uniform mosaic-like quality. The researcher’s results reconcile contradictory facts and pave the way for biomimetic artificial materials. |
| Reference | I-N (%) | LC (%) | |
|---|---|---|---|
| [18] | 5 | 2.5 | 6.8 |
| [80] | 67 | 2.1 | 4.9 |
| [81] | 28 | 1.2 | 5.1 |
| [81] | 19 | 0.5 | 4.3 |
| [21] | 31 | 0.5 | 2.6 |
| [82] | 14.3 | 2.0 | 5.1 |
| [81] | 22 | 3.0 | 5.8 |
| [83] | 18 | 3.5 | 8.9 |
| [70] | 28 | 3.4 | 7.8 |
| [70] | 30 | 3.1 | 7.4 |
| [20] | 16.5 | 3.1 | 5.1 |
| [70] | 24 | 4.7 | 9.1 |
| [70] | 24 | 3.5 | 8.2 |
| Reference | Highlight |
|---|---|
| [263] | Widening of left circularly polarization reflected band with addition of micelles |
| [264] | Distortion of helix during drying phase; impact of vertical compression |
| [184] | Molecular dynamic simulations proving that increase surface charge increases pitch size |
| [265] | The pitch of the LC in the box varied due to the isomerization of photosensitive molecules when exposed to alternating ultraviolet and blue light, resulting in a shift in the reflection wavelength. |
| [133] | Using grinded CNC iridescent film as pigments |
| [266] | Coffee ring effect leading to non-uniform optical characteristics. |
| [127] | Employing circular shear flow is applied in the drying process to improve CNC films uniformity. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi Moud, A.; Abbasi Moud, A. Cellulose Nanocrystals (CNC) Liquid Crystalline State in Suspension: An Overview. Appl. Biosci. 2022, 1, 244-278. https://doi.org/10.3390/applbiosci1030016
Abbasi Moud A, Abbasi Moud A. Cellulose Nanocrystals (CNC) Liquid Crystalline State in Suspension: An Overview. Applied Biosciences. 2022; 1(3):244-278. https://doi.org/10.3390/applbiosci1030016
Chicago/Turabian StyleAbbasi Moud, Aref, and Aliyeh Abbasi Moud. 2022. "Cellulose Nanocrystals (CNC) Liquid Crystalline State in Suspension: An Overview" Applied Biosciences 1, no. 3: 244-278. https://doi.org/10.3390/applbiosci1030016
APA StyleAbbasi Moud, A., & Abbasi Moud, A. (2022). Cellulose Nanocrystals (CNC) Liquid Crystalline State in Suspension: An Overview. Applied Biosciences, 1(3), 244-278. https://doi.org/10.3390/applbiosci1030016






