Valorization of Black Beans (Phaseolus vulgaris L.) for the Extraction of Bioactive Compounds Using Solid-State Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Proximal and Physicochemical Characterization
2.3. Strain Reactivation
2.4. Kinetics of Accumulation of Condensed Tannins
2.5. SSF Conditions
2.6. Condensed Polyphenols Content
2.7. HPLC-MS Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Physicochemical and Proximal Characterization
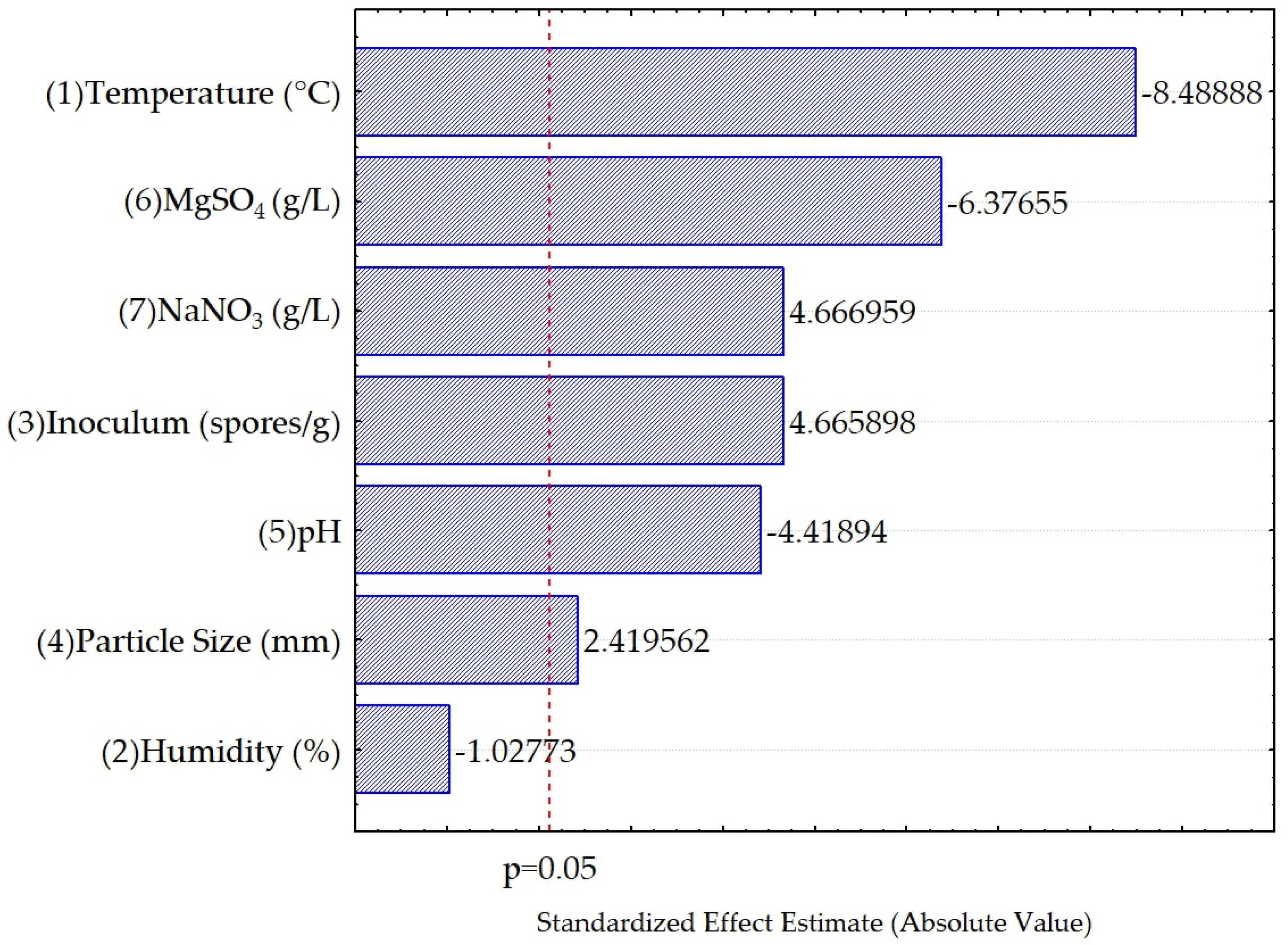
3.2. Evaluation of Fermentation Conditions for the Accumulation of Condensed Polyphenols
3.3. Identification of the Main Phenolics Compounds by HPLC-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SAGARPA (Frijol, Mexicano). Available online: https://www.gob.mx/cms/uploads/attachment/file/256428/B_sico-Frijol.pdf (accessed on 28 February 2025).
- Ren, X.; Chen, Q.; Wang, F.; Guo, H.; Wang, Y.; Gu, F. Dynamic changes in nutritions and antioxidant activities of germinated black bean based on solid-state fermentation of Ganoderma oregonense. Innov. Food Sci. Emerg. Technol. 2025, 100, 103934. [Google Scholar] [CrossRef]
- Escobedo, A.; Avalos-Flores, L.; Mojica, L.; Lugo-Cervantes, E.; Gschaedler, A.; Alcazar, M. Native Mexican black bean purified anthocyanins fractionated by high-performance counter-current chromatography modulate inflammatory pathways. Food Chem. 2024, 458, 140216. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Condensed tannins, a viable solution to meet the need for sustainable and effective multifunctionality in food packaging: Structure, sources, and properties. J. Agric. Food Chem. 2022, 70, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, Y.; Ucar, Y.; Tadesse, E.E.; Rathod, N.; Kulawik, P.; Trif, M.; Esatbeyoglu, T.; Ozogul, F. Tannins for food preservation and human health: A review of current knowledge. Appl. Food Res. 2025, 5, 100738. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef]
- Cano y Postigo, L.O.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; Garcia, A.L.E.; García-Cayuela, T. Solid-state fermentation for enhancing the nutraceutical content of agrifood by-products: Recent advances and its industrial feasibility. Food Biosci. 2021, 41, 100926. [Google Scholar] [CrossRef]
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-state fermentation: Applications and future perspectives for biostimulant and biopesticides production. Microorganisms 2023, 11, 1408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, B.; Li, Q.; Yang, C.; Yu, P.; Yang, X.; Li, T. Dynamic changes on sensory property, nutritional quality and metabolic profiles of green kernel black beans during Eurotium cristatum-based solid-state fermentation. Food Chem. 2024, 455, 139846. [Google Scholar] [CrossRef]
- Rochín-Medina, J.J.; Gutiérrez-Dorado, R.; Sánchez-Magaña, L.M.; Milán-Carrillo, J.; Cuevas-Rodríguez, E.O.; Mora-Rochín, S.; Valdez-Ortiz, A.; Reyes-Moreno, C. Enhancement of nutritional properties, and antioxidant and antihypertensive potential of black common bean seeds by optimizing the solid state bioconversion process. Int. J. Food Sci. Nutr. 2015, 66, 498–504. [Google Scholar] [CrossRef]
- Flores-Medellín, S.A.; Camacho-Ruiz, R.M.; Guízar-González, C.; Rivera-Leon, E.A.; Llamas-Covarrubias, I.M.; Mojica, L. Protein hydrolysates and phenolic compounds from fermented black beans inhibit markers related to obesity and type-2 diabetes. Legume Sci. 2021, 3, e64. [Google Scholar] [CrossRef]
- Espitia-Hernández, P.; Ruelas-Chacón, X.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Flores-Naveda, A.; Sepúlveda-Torre, L. Solid-state fermentation of sorghum by Aspergillus oryzae and Aspergillus niger: Effects on tannin content, phenolic profile, and antioxidant activity. Foods 2022, 11, 3121. [Google Scholar] [CrossRef]
- Mein’da, C.D.; Maimouna, A.; Nguetnkam, J.-P.; Tatchum, L.T.; Megueni, C. Effect of pyroclastic powder on the growth and seeds nutritional values of common bean (Phaseolus vulgaris L.) on ferralitic soils in adamawa (Cameroon, Central Africa). Int. J. Plant Soil Sci. 2022, 34, 211–225. [Google Scholar] [CrossRef]
- Fan, G.; Beta, T. Proximate composition, phenolic profiles and antioxidant capacity of three common bean varieties (Phaseolus vulgaris L.). J. Food Chem. Nanotechnol. 2016, 2, 147–152. [Google Scholar] [CrossRef]
- Tchumou, M.; Kouangbe, M.A.; Goly, K.C.; Tano, K. Effect of cooking time on the nutritional and anti-nutritional properties of red and black beans of Phaseolus lunatus (L.) consumed in south and east of Côte d’Ivoire. GSC Biol. Pharm. Sci. 2023, 22, 269–281. [Google Scholar] [CrossRef]
- Casas-Rodríguez, A.D.; Ascacio-Valdés, J.A.; Dávila-Medina, M.D.; Medina-Morales, M.A.; Londoño-Hernández, L.; Sepúlveda, L. Evaluation of solid-state fermentation conditions from pineapple peel waste for release of bioactive compounds by Aspergillus niger spp. Appl. Microbiol. 2024, 4, 934–947. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Estrada-Gil, L.E.; Torres-León, C.; Chávez-González, M.L.; Aguilar, C.N.; Ascacio-Valdés, J.A. Enhancing the release of ellagic acid from Mexican rambutan peel using solid-state fermentation. Biomass 2024, 4, 1005–1016. [Google Scholar] [CrossRef]
- Fernández-Valenciano, A.F.; Sánchez-Chávez, E. Study of physicochemical properties and nutritional quality in different varieties of beans consumed in Mexico. Nova Sci. 2017, 9, 133–148. [Google Scholar]
- Neder-Suárez, D.; Quintero-Ramos, A.; Meléndez-Pizarro, C.O.; Zazueta-Morales, J.J.; Paraguay-Delgado, F.; Ruiz-Gutiérrez, M.G. Evaluation of the physicochemical properties of third-generation snacks made from blue corn, black beans, and sweet chard produced by extrusion. LWT 2021, 146, 111414. [Google Scholar] [CrossRef]
- Perucini-Avendaño, M.; Arzate-Vázquez, I.; Perea-Flores, M.J.; Tapia-Maruri, D.; Méndez-Méndez, J.V.; Nicolás-García, M.; Dávila-Ortiz, G. Effect of cooking on structural changes in the common black bean (Phaseolus vulgaris var. Jamapa). Heliyon 2024, 10, e25620. [Google Scholar] [CrossRef]
- De la Rosa-Millán, J.; Heredia-Olea, E.; Pérez-Carrillo, E.; Guajardo-Flores, D.; Serna-Saldívar, S.R.O. Effect of decortication, germination and extrusion on physicochemical and in vitro protein and starch digestion characteristics of black beans (Phaseolus vulgaris L.). LWT 2019, 102, 330–337. [Google Scholar] [CrossRef]
- Sánchez-Quezada, V.; Luzardo-Ocampo, I.; Gaytán-Martínez, M.; Loarca-Piña, G. Physicochemical, nutraceutical, and sensory evaluation of a milk-type plant-based beverage of extruded common bean (Phaseolus vulgaris L.) added with iron. Food Chem. 2024, 453, 139602. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Peraza, M.; Chel-Guerrero, L.; Betancur-Ancona, D. Some physicochemical and functional properties of the rich fibrous fraction of hardened beans (Phaseolus vulgaris L.) and its addition in the formulation of beverages. Int. J. Gastron. Food Sci. 2021, 26, 100440. [Google Scholar] [CrossRef]
- Hernández-Melgar, M.A.; Martínez-Hernández, E.G.; Carranza-Estrada, F.A.; Bonilla-de-Torres, B.L.; Cuadra-Soto, J.A.; Vi-var-de Figueroa, M.E. Proximal bromatological analysis and culinary quality of creole black grain bean (Phaseolus vulgaris L.) cultivated in western El Salvador. Rev. Agrociencia 2020, 3, 46–54. [Google Scholar] [CrossRef]
- Jan, S.; Rather, I.A.; Sofi, P.A.; Wani, M.A.; Sheikh, F.A.; Bhat, M.A.; Mir, R.R. Characterization of common bean (Phaseolus vulgaris L.) germplasm for morphological and seed nutrient traits from Western Himalayas. Legume Sci. 2021, 3, e86. [Google Scholar] [CrossRef]
- Sepúlveda, L.; de la Cruz, R.; Buenrostro, J.J.; Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Prado, A.; Rodríguez-Herrera, R.; Aguilar, C.N. Effect of different polyphenol sources on the efficiency of ellagic acid release by Aspergillus niger. Rev. Argent. Microbiol. 2016, 48, 71–77. [Google Scholar] [CrossRef]
- Lee, I.-H.; Hung, Y.-H.; Chou, C.-C. Solid-state fermentation with fungi to enhance the antioxidative activity, total phenolic and anthocyanin contents of black bean. Int. J. Food Microbiol. 2008, 121, 150–156. [Google Scholar] [CrossRef]
- Dhull, S.B.; Punia, S.; Kidwai, M.K.; Kaur, M.; Chawla, P.; Purewal, S.S.; Sangwan, M.; Palthania, S. Solid-state fermentation of lentil (Lens culinaris L.) with Aspergillus awamori: Effect on phenolic compounds, mineral content, and their bioavailability. Legume Sci. 2020, 2, e37. [Google Scholar] [CrossRef]
- Liu, W.; Dun, M.; Liu, X.; Zhang, G.; Ling, J. Effects on total phenolic and flavonoid content, antioxidant properties, and angiotensin I-converting enzyme inhibitory activity of beans by solid-state fermentation with Cordyceps militaris. Int. J. Food Prop. 2022, 25, 477–491. [Google Scholar] [CrossRef]
- Perwez, M.; Al Asheh, S. Valorization of agro-industrial waste through solid-state fermentation: Mini review. Biotechnol. Rep. 2025, 45, e00873. [Google Scholar] [CrossRef]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Manan, M.A.; Webb, C. Solid-state fermentation of food industry wastes. In Food Industry Wastes, Assessment and Recuperation of Commodities; Kosseva, M.R., Webb, C., Eds.; Academic Press: London, UK, 2020; pp. 135–161. [Google Scholar]
- Cebrián, M.; Ibarruri, J. Filamentous fungi processing by solid-state fermentation. In Current Developments in Biotechnology and Bioengineering; Taherzadeh, M.J., Ferreira, J.A., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 251–292. [Google Scholar]
- Meini, M.R.; Cabezudo, I.; Galetto, C.S.; Romanini, D. Production of grape pomace extracts with enhanced antioxidant and prebiotic activities through solid-state fermentation by Aspergillus niger and Aspergillus oryzae. Food Biosci. 2021, 42, 101168. [Google Scholar] [CrossRef]
- Akbari, M.; Gómez-Urios, C.; Razavi, S.H.; Khodaiyan, F.; Blesa, J.; Esteve, M.J. Optimization of solid-state fermentation conditions to improve phenolic content in corn bran, followed by extraction of bioactive compounds using natural deep eutectic solvents. Innov. Food Sci. Emerg. Technol. 2024, 93, 103621. [Google Scholar] [CrossRef]
- Hussain, M.K.; Khatoon, S.; Khan, M.F.; Akhtar, M.S.; Ahamad, S.; Saquib, M. Coumarins as versatile therapeutic phytomolecules: A systematic review. Phytomedicine 2024, 134, 155972. [Google Scholar] [CrossRef]
- Naseem, N.; Ahmad, M.F.; Imam, N.; Ahsan, H.; Siddiqui, W.A. The potential of esculin as a therapeutic modality in diabetes mellitus and its complications. Hum. Nutr. Metab. 2023, 33, 200207. [Google Scholar] [CrossRef]
- León-Cortés, D.; Arce-Villalobos, K.; Bogantes-Ledezma, D.; Irías-Mata, A.; Chaves-Barrantes, N.; Vinas, M. Anti-aflatoxin potential of phenolic compounds from common beans (Phaseolus vulgaris L.). Food Chem. 2025, 469, 142597. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yong, H.; Liu, Y.; Bai, R. Recent advances in the preparation, structural characteristics, biological properties and applications of gallic acid grafted polysaccharides. Int. J. Biol. Macromol. 2020, 156, 1539–1555. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Gan, R.-Y.; Ge, Y.-Y.; Zhang, D.; Corke, H. Polyphenols in common beans (Phaseolus vulgaris L.): Chemistry, analysis, and factors affecting composition. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1518–1539. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 16 March 2025).

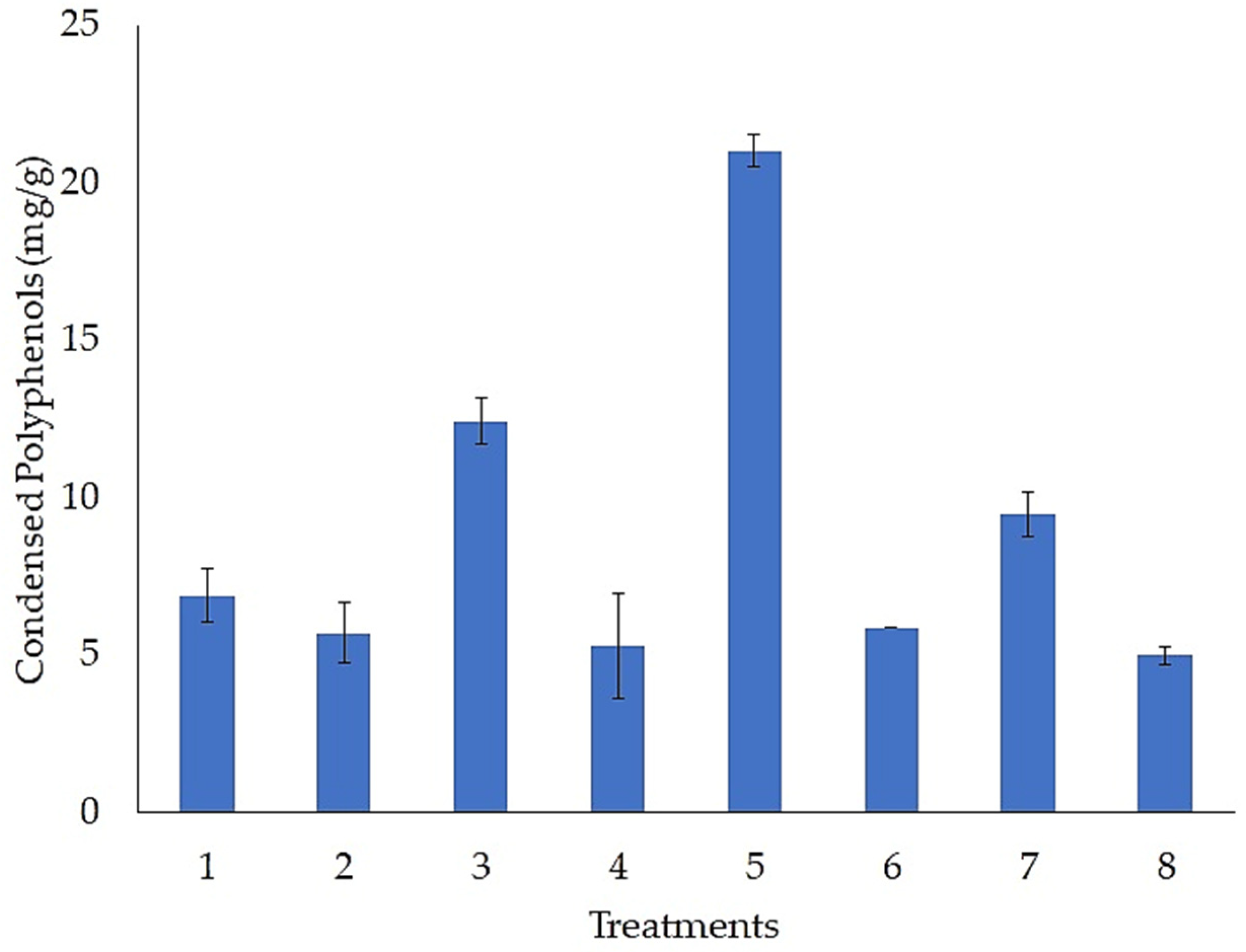
| Treatments | Temperature (°C) | Humidity (%) | Inoculum (Spores/g) | Particle Size (mm) | pH | MgSO4 (g/L) | NaNO3 (g/L) |
|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 |
| 2 | 1 | −1 | −1 | −1 | −1 | 1 | 1 |
| 3 | −1 | 1 | −1 | −1 | 1 | −1 | 1 |
| 4 | 1 | 1 | −1 | 1 | −1 | −1 | −1 |
| 5 | −1 | −1 | 1 | 1 | −1 | −1 | 1 |
| 6 | 1 | −1 | 1 | −1 | 1 | −1 | −1 |
| 7 | −1 | 1 | 1 | −1 | −1 | 1 | −1 |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Factors | High Level (+1) | Low Level (−1) | |||||
| Temperature (°C) | 30 | 25 | |||||
| Humidity (%) | 80 | 60 | |||||
| Inoculum (spores/g) | 1 × 108 | 1 × 106 | |||||
| Particle size (mm) | 1.41 mm | 0.354 mm | |||||
| pH | 6 | 5 | |||||
| MgSO4 (g/L) | 15.6 | 7.8 | |||||
| NaNO3 (g/L) | 3.04 | 1.50 | |||||
| Parameters | Results (%) |
|---|---|
| Protein | 43.13 ± 0.88 |
| Lipids | 8.14 ± 0.38 |
| Fiber | 15.57 ± 0.04 |
| Carbohydrates | 20.84 ± 0.87 |
| Ash | 3.13 ± 0.11 |
| Humidity | 5.2 ± 0.53 |
| Minerals | |
| Fluorine (F) | 2.86 |
| Iron (Fe) | 2.77 |
| Zinc (Zn) | 1.32 |
| Manganese (Mn) | 0.78 |
| Copper (Cu) | 0.44 |
| Strontium (Sr) | 0.41 |
| Molybdenum (Mo) | 0.23 |
| Cobalt (Co) | 0.22 |
| Others 1 | 0.80 |
| Factors | SS | dF | MS | F | p |
| (1) Temperature (°C) | 217.7458 | 1 | 217.7458 | 72.0610 | 0.000000 |
| (2) Humidity (%) | 3.1916 | 1 | 3.1916 | 1.0562 | 0.319364 |
| (3) Inoculum (spores/g) | 65.7839 | 1 | 65.7839 | 21.7706 | 0.000258 |
| (4) Particle Size (mm) | 17.6898 | 1 | 17.6898 | 5.8542 | 0.027814 |
| (5) pH | 59.0046 | 1 | 59.0046 | 19.5270 | 0.000430 |
| (6) MgSO4 (g/L) | 122.8630 | 1 | 122.8630 | 40.6604 | 0.000009 |
| (7) NaNO3 (g/L) | 65.8138 | 1 | 65.8138 | 21.7805 | 0.000258 |
| Error | 48.3469 | 16 | 3.0217 | ||
| Total SS | 600.4393 | 23 |
| Tentative Identification | Structure 1 | Retention Time | Elemental Formula | [M-H]− |
|---|---|---|---|---|
| Esculin | 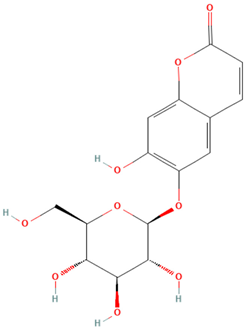 | 5.38 | C15H16O9 | 338.9 |
| Gallic acid 3-O-gallate | 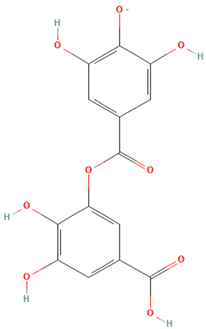 | 17.66 | C14H9O9 | 320.1 |
| 5-5′Dehydrodiferulic acid | 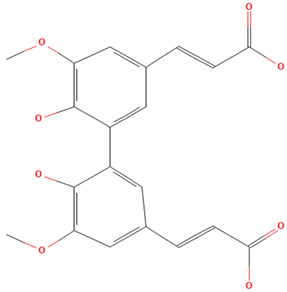 | 21.8 | C20H18O8 | 385.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Martínez, D.W.; Casas-Rodríguez, A.D.; Coronado-Contreras, S.A.; Flores-Gallegos, A.C.; López-Badillo, C.M.; Ascacio-Valdés, J.A.; Flores-Naveda, A.; Sepúlveda, L. Valorization of Black Beans (Phaseolus vulgaris L.) for the Extraction of Bioactive Compounds Using Solid-State Fermentation. Waste 2025, 3, 13. https://doi.org/10.3390/waste3020013
González-Martínez DW, Casas-Rodríguez AD, Coronado-Contreras SA, Flores-Gallegos AC, López-Badillo CM, Ascacio-Valdés JA, Flores-Naveda A, Sepúlveda L. Valorization of Black Beans (Phaseolus vulgaris L.) for the Extraction of Bioactive Compounds Using Solid-State Fermentation. Waste. 2025; 3(2):13. https://doi.org/10.3390/waste3020013
Chicago/Turabian StyleGonzález-Martínez, Dulce W., Alma D. Casas-Rodríguez, Sergio A. Coronado-Contreras, Adriana C. Flores-Gallegos, Claudia M. López-Badillo, Juan A. Ascacio-Valdés, Antonio Flores-Naveda, and Leonardo Sepúlveda. 2025. "Valorization of Black Beans (Phaseolus vulgaris L.) for the Extraction of Bioactive Compounds Using Solid-State Fermentation" Waste 3, no. 2: 13. https://doi.org/10.3390/waste3020013
APA StyleGonzález-Martínez, D. W., Casas-Rodríguez, A. D., Coronado-Contreras, S. A., Flores-Gallegos, A. C., López-Badillo, C. M., Ascacio-Valdés, J. A., Flores-Naveda, A., & Sepúlveda, L. (2025). Valorization of Black Beans (Phaseolus vulgaris L.) for the Extraction of Bioactive Compounds Using Solid-State Fermentation. Waste, 3(2), 13. https://doi.org/10.3390/waste3020013









