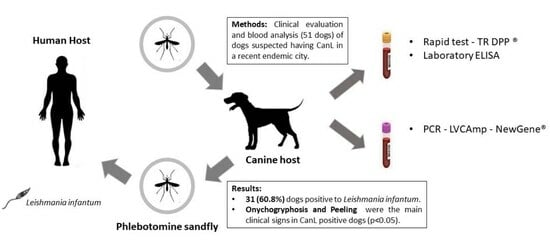
Canine Leishmaniasis in Southern Brazil: Diagnosis and Clinical Features in Domestic Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
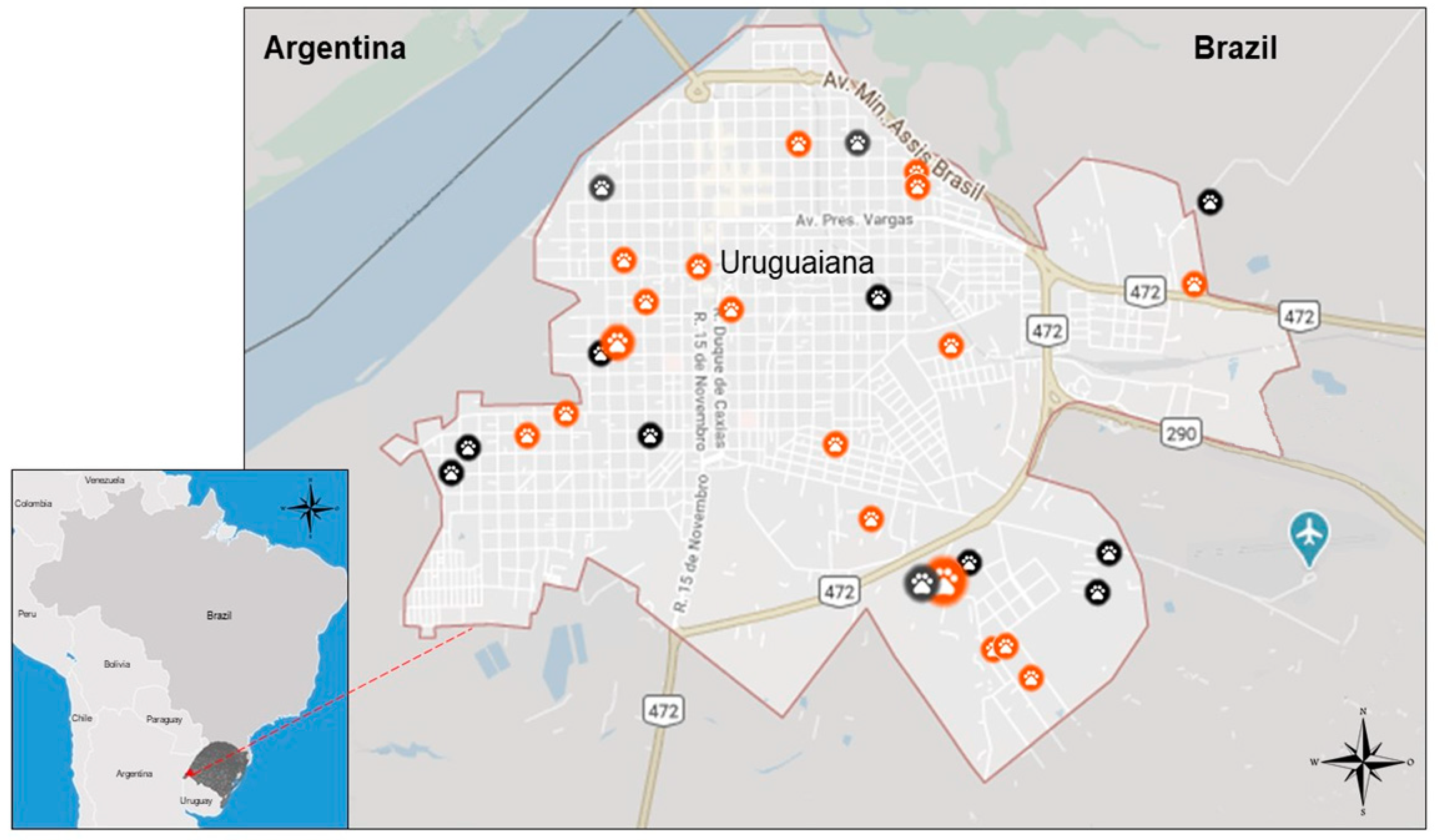
2.1. Study Area
2.2. Animal Sampling
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Overall CanL Prevalence and Epidemiological Data
3.2. Clinical Signs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dantas-Torres, F. Canine vector-borne diseases in Brazil. Parasites Vectors 2008, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Travi, B.L.; Cordeiro-da-Silva, A.; Dantas-Torres, F.; Miró, G. Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLoS Negl. Trop. Dis. 2018, 12, e0006082. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Filho, J.D.; Scholte, R.G.C.; Amaral, A.L.G.; Shimabukuro, P.H.F.; Carvalho, O.S.; Caldeira, R.L. Occurrence and probability maps of Lutzomyia longipalpis and Lutzomyia cruzi (Diptera: Psychodidae: Phlebotominae) in Brazil. J. Med. Entomol. 2017, 54, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.S.C.; Esmeraldino, A.T.; Ávila, V.P.F.; Witz, M.I.; Fischer, C.D.B.; Tartarotti, A.L. Leishmaniose visceral canina autóctone no município de São Borja, Rio Grande do Sul, Brasil: Relato de caso. Veterinária Em Foco 2009, 7, 52–61. [Google Scholar]
- Acardi, S.A.; Liotta, D.J.; Santini, M.S.; Romagosa, C.M.; Salomón, O.D. Detection of Leishmania infantum in naturally infected Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae) and Canis familiaris in Misiones, Argentina: The first report of a PCR-RFLP and sequencing-based confirmation assay. Mem. Inst. Oswaldo Cruz. 2010, 105, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Acosta, L.; Díaz, R.; Torres, P.; Silva, G.; Ramos, M.; Fattore, G.; Bornay-Llinares, F.J. Identification of Leishmania infantum in Puerto Iguazú, Misiones, Argentina. Rev. Inst. Med. Trop. São Paulo 2015, 57, 175–176. [Google Scholar] [CrossRef]
- Rêgo, F.D.; Souza, G.D.; Miranda, J.B.; Peixoto, L.V.; Andrade-Filho, J.D. Potential Vectors of Leishmania Parasites in a Recent Focus of Visceral Leishmaniasis in Neighborhoods of Porto Alegre, State of Rio Grande do Sul, Brazil. J. Med. Entomol. 2020, 57, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.O.; Carvalho, D.; Frizzo, C.; Lopes, K.; Tessari, G.B.; Catecati, T.; Dhom-Lemos, L.C.; Pasquali, A.K.S.; Quaresma, P.F.; Stoco, P.H. First case of canine visceral leishmaniasis in the midwestern of Santa Catarina State, Brazil. Braz. J. Biol. 2021, 82, e241162. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, V.C.; Pruzinova, K.; Sadlova, J.; Volfova, V.; Myskova, J.; Filho, S.P.; Volf, P. Lutzomyia migonei is a permissive vector competent for Leishmania infantum. Parasites Vectors 2016, 9, 159. [Google Scholar] [CrossRef]
- Galvis-Ovallos, F.; Ueta, A.E.; Marques, G.O.; Sarmento, A.M.C.; Araujo, G.; Sandoval, C.; Dhom-Lemos, L.C.; Pasquali, A.K.S.; Quaresma, P.F.; Stoco, P.H. Detection of Pintomyia fischeri (Diptera: Psychodidae) with Leishmania infantum (Trypanosomatida: Trypanosomatidae) Promastigotes in a Focus of Visceral Leishmaniasis in Brazil. J. Med. Entomol. 2021, 58, 830–836. [Google Scholar] [CrossRef]
- Carvalho Junior, C.G.; Teixeira Neto, R.G.; Lopes, V.V.; Belo, V.S.; Alves, N.R.; de Paula, T.B.; Ribeiro, R.I.M.A.; Silva, E.S. Parasitism and inflammation in ear skin and in genital tissues of symptomatic and asymptomatic male dogs with visceral leishmaniasis. Parasitol. Res. 2017, 116, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.V.; Michalsky, É.M.; Pereira, N.C.L.; Paula, A.J.V.; Souza, A.G.M.; Pinheiro, L.C.; Lima, A.C.V.M.d.R.; de Avelar, D.M.; França-Silva, J.C.; Lanzetta, V.A.S.; et al. Canine visceral leishmaniasis in area with recent Leishmania transmission: Prevalence, diagnosis, and molecular identification of the infecting species. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200141. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, V.E.; Pinheiro, L.C.; Almeida, M.C.; de Menezes, F.C.; Morais, M.H.; Reis, I.A.; Carneiro, M. Relative risk of visceral leishmaniasis in Brazil: A spatial analysis in urban area. PLoS Negl. Trop. Dis. 2013, 7, e2540. [Google Scholar] [CrossRef] [PubMed]
- Belo, V.S.; Werneck, G.L.; Barbosa, D.S.; Simões, T.C.; Nascimento, B.W.; da Silva, E.S.; Struchiner, C.J. Factors associated with visceral leishmaniasis in the americas: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2013, 7, e2182. [Google Scholar] [CrossRef]
- Alvar, J.; Cañavate, C.; Molina, R.; Moreno, J.; Nieto, J. Canine leishmaniasis. Adv. Parasitol. 2004, 57, 1–88. [Google Scholar] [PubMed]
- Pennisi, M.G.; Cardoso, L.; Baneth, G.; Bourdeau, P.; Koutinas, A.; Miró, G.; Oliva, G.; Solano-Gallego, L. LeishVet update and recommendations on feline leishmaniosis. Parasites Vectors 2015, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Brasil—Ministério da Saúde (MS). Manual de Vigilância e Controle da Leishmaniose Visceral; Brasil—Ministério da Saúde (MS): Uruguaiana, Brazil, 2014; 122p, (accessed on 27 December 2023).
- Sevá, A.D.P.; Brandão, A.P.D.; Godoy, S.N.; Soares, R.M.; Langoni, H.; Rodrigues, B.C.; Zanotto, P.F.d.C.; Jimenez-Villegas, T.; Hiramoto, R. Investigation of canine visceral leishmaniasis in a non-endemic area in Brazil and the comparison of serological and molecular diagnostic tests. Rev. Soc. Bras. Med. Trop. 2021, 54, e01822021. [Google Scholar] [CrossRef] [PubMed]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Uruguaiana. 2023. Available online: https://www.ibge.gov.br/cidades-e-estados/rs/uruguaiana.html (accessed on 8 April 2023).
- Massia, L.I.; Lamadril, R.D.Q.; Wellicks, J.R.; Bittencourt, R.A.; Bittencourt, D.G.; Marques, G.D.; Celis, E.L.H.; Pellegrini, D.D.C.P. Leishmaniose visceral canina em três bairros de Uruguaiana—RS. Vigil. Sanit. Debate. 2016, 4, 113–119. [Google Scholar] [CrossRef]
- Escobar, T.A.; Dowich, G.; Dos Santos, T.P.; Zuravski, L.; Duarte, C.A.; Lübeck, I.; Manfredini, V. Assessment of Leishmania infantum infection in equine populations in a canine visceral leishmaniosis transmission area. BMC Vet. Res. 2019, 15, 381. [Google Scholar] [CrossRef]
- Pradella, G.D.; Escobar, T.A.; Santos, T.P.D.; Vargas, R.C.; Góss, G.C.; Ferrareze, P.A.G.; Zuravski, L.; Pereira, K.B.; Duarte, C.A. PCR-RLFP characterization of Leishmania spp. in domestic animals from the south-western border of Brazil. Rev. Bras. Parasitol. Vet. 2022, 31, e005222. [Google Scholar] [CrossRef]
- Garay, A.F.G.; Fraenkel, S.; Diaz, J.J.A.R.; Recalde, O.D.S.; Gómez, M.C.V.; Riquelme, J.A.M.; Arze, P.V.; Centurión, G.N.R.; Britos, M.; Rolón, M. Sensitivity comparison for the Leishmania spp. detection in different canine tissues using PCR-HRM. Rev. Soc. Bras. Med. Trop. 2022, 55, e0069-2022. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Gradoni, L.; Roura, X.; Zatelli, A.; Zini, E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet. Clin. Pathol. 2016, 45, 552–578. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Di Filippo, L.; Ordeix, L.; Planellas, M.; Roura, X.; Altet, L.; Montserrat, S. Early reduction of Leishmania infantum-specific antibodies and blood parasitemia during treatment in dogs with moderate or severe disease. Parasites Vectors 2016, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.C.A.; Teixeira-Neto, R.G.; Lopes, V.V.; Pena, H.P.; Paz, G.F.; Custodio, C.H.X.; Belo, V.S.; Júnior, A.A.d.F.; da Silva, E.S. Development of quantitative PCR and digital PCR for the quantification of Leishmania infantum in dogs. Mol. Cell. Biochem. 2023, 478, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Neto, R.G.; Giunchetti, R.C.; Carneiro, C.M.; de Almeida, R.W.V.; Coura-Vital, W.; Quaresma, P.F.; Ker, H.G.; de Melo, L.A.; Gontijo, C.M.F.; Reis, A.B. Relationship of Leishmania-specific IgG levels and IgG avidity with parasite density and clinical signs in canine leishmaniasis. Vet. Parasitol. 2010, 169, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Galán-Relaño, Á.; Maldonado, A.; Gómez-Gascón, L.; Tarradas, C.; Astorga, R.J.; Luque, I.; Huerta, B. Pre-test probability and likelihood ratios for clinical findings in canine leishmaniasis. Transbound Emerg. Dis. 2022, 69, 3540–3547. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.; Neto, M.B.O.; Bezerra, T.L.; Da Silva, W.S.I.; da Paz, W.S.; dos Santos, I.G.; Bezerra-Santos, M.; Lima, V.F.S. Canine leishmaniasis in an endemic region, Northeastern Brazil: A comparative study with four groups of animals. Parasitol. Res. 2021, 120, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.R.D.; Mendonça, V.R.R.D.; Silva, K.M.; Nascimento, L.F.M.D.; Mendes-Sousa, A.F.; Pinho, F.A.D.; Barral-Netto, M.; Barral, A.M.P.; e Cruz, M.D.S.P. Scoring clinical signs can help diagnose canine visceral leishmaniasis in a highly endemic area in Brazil. Mem. Inst. Oswaldo Cruz. 2017, 112, 53–63. [Google Scholar] [CrossRef][Green Version]
- Koutinas, A.F.; Carlotti, D.N.; Koutinas, C.; Papadogiannakis, E.I.; Spanakos, G.K.; Saridomichelakis, M.N. Claw histopathology and parasitic load in natural cases of canine leishmaniosis associated with Leishmania infantum. Vet. Dermatol. 2010, 21, 572–577. [Google Scholar] [CrossRef]
- Koutinas, A.F.; Koutinas, C.K. Pathologic mechanisms underlying the clinical findings in canine leishmaniosis due to Leishmania infantum/chagasi. Vet. Parasitol. 2014, 51, 527–538. [Google Scholar]
- LeishVet—LeishVet Guidelines for the Practical Management of Canine and Feline Leishmaniosis: A Brief for the Practicing Veterinarian. 2023. Available online: https://www.leishvet.org/wp-content/uploads/2023/01/ALIVE-dec22-web-EN.pdf (accessed on 27 December 2023).
- Diaz, R.G.; Salvatierra, K.A.; Silva, G.A.; Deschutter, E.J.; Bornay-Llinares, F.J.; Acosta, L. First molecular characterization of Leishmania infantum species in patients infected with visceral leishmaniasis in Misiones province, Argentina. Biomedica 2019, 39, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Lamattina, D.; Berrozpe, P.E.; Casas, N.; Moya, S.L.; Giuliani, M.G.; Costa, S.A.; Arrabal, J.P.; Martínez, M.F.; Rivero, M.R.; Salas, M.; et al. Twice upon a time: The progression of canine visceral leishmaniasis in an Argentinean city. PLoS ONE 2019, 14, e0219395. [Google Scholar] [CrossRef] [PubMed]
- Rio Grande do Sul—Secretaria da Saúde (MS). Leishmaniose Visceral Humana—Situação Epidemiológica/Dados 2023. Available online: https://www.cevs.rs.gov.br/lvh-situacao-epidemiologica-dados (accessed on 27 December 2023).
| DPP+ | DPP− | |
|---|---|---|
| qPCR+ | 22 (43.1%) | 3 (5.9%) |
| qPCR− | 6 (11.8%) | 20 (39.2%) |
| Variables | Positive (n = 31) | Negative (n = 20) | OR (95% Cl) | p-Value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Age | ||||||
| 0–2 years | 12 | 38.7 | 11 | 55.0 | 0.91 (0.22–3.84) | 0.897 a |
| 3–5 years | 11 | 35.5 | 4 | 20.0 | 2.30 (0.44–11.91) | 0.324 a |
| 6 or more years | 6 | 19.4 | 5 | 25.0 | 1.Ref | |
| Missing | 2 | 6.4 | 0 | 0 | ||
| Sex | ||||||
| Males | 18 | 58.1 | 11 | 55.0 | 1.13 (0.36–3.52) | 0.082 |
| Females | 13 | 41.9 | 9 | 45.0 | 1.Ref | |
| Breed | ||||||
| Mixed bred | 27 | 87.1 | 14 | 70.0 | 2.41 (0.55–10.42) | 0.231 a |
| Purebred | 4 | 12.9 | 5 | 25.0 | 1.Ref | |
| Missing | 0 | 0 | 1 | 5.0 | ||
| Living with more dogs | ||||||
| Yes | 28 | 90.3 | 17 | 85.0 | 1.65 (0.30–9.10) | 0.565 a |
| No | 3 | 9.7 | 3 | 15.0 | 1.Ref | |
| Hair | ||||||
| Short | 28 | 90.3 | 17 | 85.0 | 1.65 (0.30–9.10) | 0.565 a |
| Long | 3 | 9.7 | 3 | 15 | 1.Ref | |
| Variables | Cases (n = 31) | Controls (n = 20) | OR (95% Cl) | p-Value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Apathy | ||||||
| Yes | 6 | 19.4 | 8 | 40 | 0.36 (0.10–1.27) | 0.107 |
| No | 25 | 80.6 | 12 | 60 | 1.Ref | |
| Emaciation | ||||||
| Yes | 17 | 54.8 | 14 | 70 | 0.52 (0.16–1.70) | 0.279 |
| No | 14 | 45.2 | 6 | 30 | 1.Ref | |
| Muscular Atrophy | ||||||
| Yes | 10 | 32.3 | 8 | 40 | 0.72 (0.22–2.30) | 0.572 |
| No | 21 | 67.7 | 12 | 60 | 1.Ref | |
| Opaque hair | ||||||
| Yes | 26 | 83.9 | 14 | 70 | 0.23 (0.58–8.62) | 0.245 a |
| No | 5 | 16.1 | 6 | 30 | 1.Ref | |
| Alopecia | ||||||
| Yes | 22 | 71 | 10 | 50 | 2.44 (0.76–7.88) | 0.131 |
| No | 9 | 29 | 10 | 50 | 1.Ref | |
| Peeling | ||||||
| Yes | 20 | 64.5 | 7 | 35 | 3.37 (1.04–10.95) | 0.039 |
| No | 11 | 35.5 | 13 | 65 | 1.Ref | |
| Hyperkeratosis | ||||||
| Yes | 10 | 32.3 | 5 | 25 | 1.43 (0.40–5.04) | 0.579 a |
| No | 21 | 67.7 | 15 | 75 | 1.Ref | |
| Skin Ulcers | ||||||
| Yes | 10 | 32.3 | 10 | 50 | 0.48 (0.15–1.51) | 0.205 |
| No | 21 | 67.7 | 10 | 50 | 1.Ref | |
| Ocular lesions | ||||||
| Yes | 20 | 64.5 | 12 | 60 | 1.21 (0.38–3.86) | 0.745 |
| No | 11 | 35.5 | 8 | 40 | 1.Ref | |
| Onychogryphosis | ||||||
| Yes | 16 | 51.6 | 4 | 20 | 4.27 (1.16–15.69) | 0.024 a |
| No | 15 | 48.4 | 16 | 80 | 1.Ref | |
| Hepatosplenomegaly | ||||||
| Yes | 5 | 16.1 | 2 | 10 | 1.73 (0.30–9.92) | 0.535 a |
| No | 26 | 83.9 | 18 | 90 | 1.Ref | |
| Lymphadenopathy | ||||||
| Yes | 26 | 83.9 | 17 | 85 | 0.92 (0.19–4.35) | 0.914 a |
| No | 5 | 16.1 | 3 | 15 | 1.Ref | |
| Epistaxis | ||||||
| Yes | 0 | 0 | 1 | 5 | 0.20 (0.1–5.32) | 0.209 a |
| No | 31 | 100 | 19 | 95 | 1.Ref | |
| Diarrhea | ||||||
| Yes | 2 | 6.5 | 2 | 10 | 0.62 (0.08–4.80) | 0.645 a |
| No | 29 | 93.5 | 18 | 90 | 1.Ref | |
| Limb paresis | ||||||
| Yes | 0 | 0 | 1 | 5 | 0.20 (0.01–5.32) | 0.209 a |
| No | 31 | 100 | 19 | 95 | 1.Ref | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraga, A.P.d.; da Silveira, V.P.; Freitas Salla, P.d.; Goulart, F.G.d.O.; Streck, A.F.; Pereira, V.R.Z.B.; de Mello, L.S.; Fonseca, A.S.K.; Ikuta, N.; Lunge, V.R. Canine Leishmaniasis in Southern Brazil: Diagnosis and Clinical Features in Domestic Dogs. Zoonotic Dis. 2024, 4, 114-122. https://doi.org/10.3390/zoonoticdis4010011
Fraga APd, da Silveira VP, Freitas Salla Pd, Goulart FGdO, Streck AF, Pereira VRZB, de Mello LS, Fonseca ASK, Ikuta N, Lunge VR. Canine Leishmaniasis in Southern Brazil: Diagnosis and Clinical Features in Domestic Dogs. Zoonotic Diseases. 2024; 4(1):114-122. https://doi.org/10.3390/zoonoticdis4010011
Chicago/Turabian StyleFraga, Aline Padilha de, Vinicius Proença da Silveira, Patrícia de Freitas Salla, Fernanda Gass de Oliveira Goulart, André Felipe Streck, Vagner Reinaldo Zingalli Bueno Pereira, Lauren Santos de Mello, André Salvador Kazantzi Fonseca, Nilo Ikuta, and Vagner Ricardo Lunge. 2024. "Canine Leishmaniasis in Southern Brazil: Diagnosis and Clinical Features in Domestic Dogs" Zoonotic Diseases 4, no. 1: 114-122. https://doi.org/10.3390/zoonoticdis4010011
APA StyleFraga, A. P. d., da Silveira, V. P., Freitas Salla, P. d., Goulart, F. G. d. O., Streck, A. F., Pereira, V. R. Z. B., de Mello, L. S., Fonseca, A. S. K., Ikuta, N., & Lunge, V. R. (2024). Canine Leishmaniasis in Southern Brazil: Diagnosis and Clinical Features in Domestic Dogs. Zoonotic Diseases, 4(1), 114-122. https://doi.org/10.3390/zoonoticdis4010011