Review of Emerging and Re-Emerging Zoonotic Pathogens of Dogs in Nigeria: Missing Link in One Health Approach
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Selection and Pathogen Identification
2.2. Search Strategy
2.3. Scope of the Review
2.4. Quality Assessment
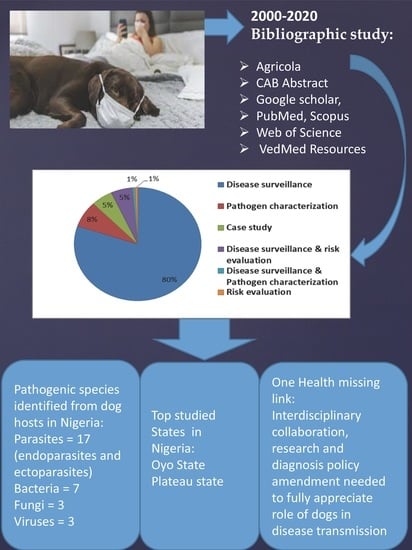
3. Results
3.1. Summary
3.2. Location of Studies
3.3. Study Type
3.4. Bias
4. Discussion
4.1. Overview
4.2. Gastro-Intestinal Parasites
4.3. Ectoparasites
4.4. Haemoparasites and Sprirochetes
4.5. Bacteria
4.5.1. Brucellosis
4.5.2. Campylobacter spp.
4.5.3. Enterobacteriaceae
4.5.4. Leptospirosis
4.5.5. Staphylococcus Infection
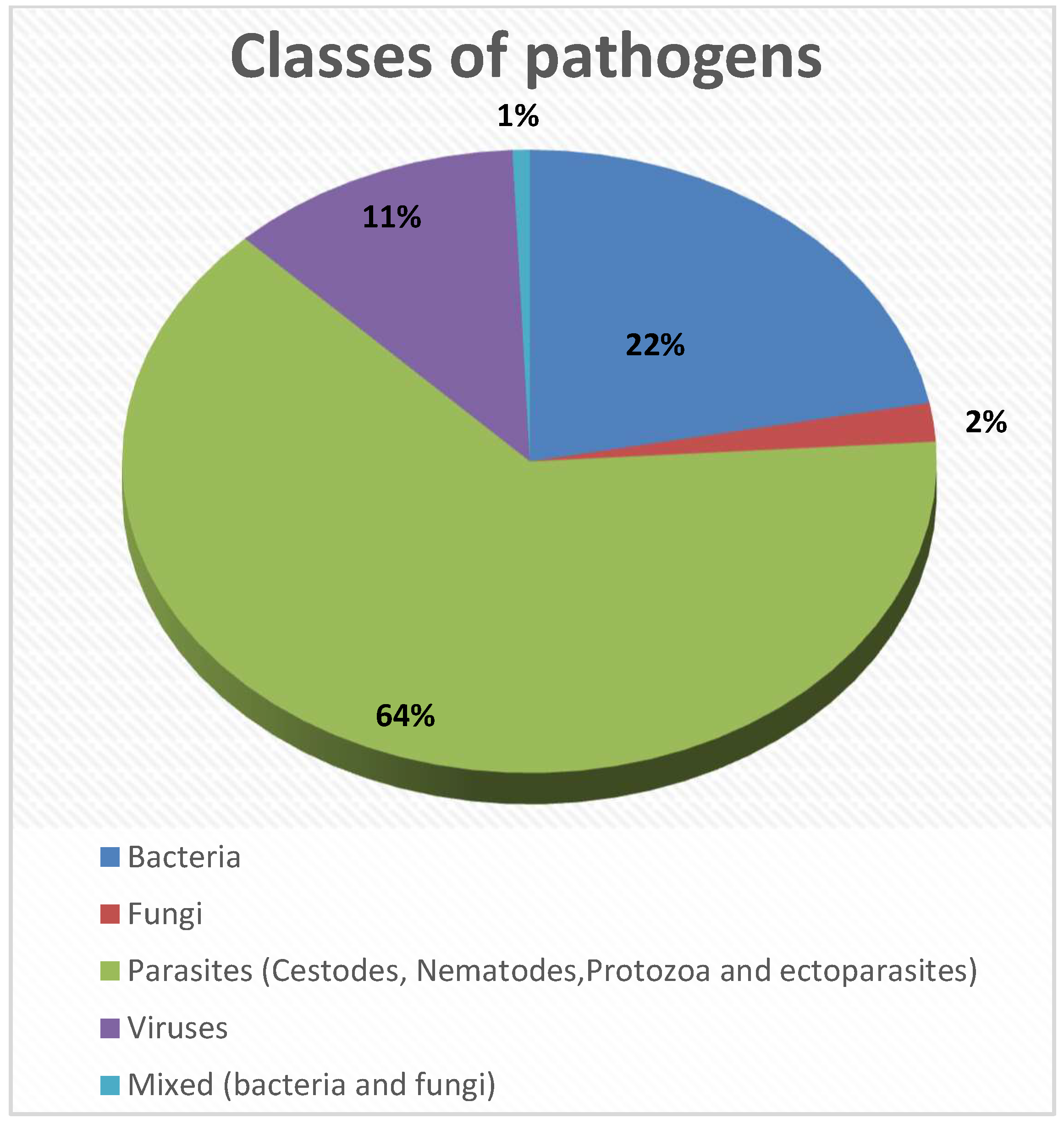
4.6. Fungi
4.7. Viruses
4.7.1. Influenza Viruses
4.7.2. Rabies
4.8. Missing Link in One Health Approach for Zoonotic Disease Diagnosis in Nigeria
4.9. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloom, D.E.; Black, S.; Rappuoli, R. Emerging infectious diseases: A proactive approach. Proc. Natl. Acad. Sci. USA 2017, 114, 4055–4059. [Google Scholar] [CrossRef] [PubMed]
- Mackey, T.K.; Liang, B.A.; Cuomo, R.; Hafen, R.; Brouwer, K.C.; Lee, D.E. Emerging and Reemerging Neglected Tropical Diseases: A Review of Key Characteristics, Risk Factors, and the Policy and Innovation Environment. Clin. Microbiol. Rev. 2014, 27, 949–979. [Google Scholar] [CrossRef]
- Brown, C. Emerging zoonoses and pathogens of public health significance—an overview. Rev. Sci. Et Tech.-Off. Int. Des Epizoot. 2004, 23, 435–442. [Google Scholar] [CrossRef]
- Vorou, R.; Papavassiliou, V.; Tsiodras, S. Emerging zoonoses and vector-borne infections affecting humans in Europe. Epidemiol. Infect. 2007, 135, 1231–1247. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, J.K.; Daszak, P. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization, Coronavirus Disease 2019 (COVID-19): Situation Report, 59. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019?adgroupsurvey={adgroupsurvey}&gclid=EAIaIQobChMIobTOpszL_gIV75JmAh169AbHEAAYASAAEgL8hPD_BwE (accessed on 15 March 2020).
- Siembieda, J.; Kock, R.; McCracken, T.; Newman, S. The role of wildlife in transboundary animal diseases. Anim. Health Res. Rev. 2011, 12, 95–111. [Google Scholar] [CrossRef]
- Gebreyes, W.A.; Dupouy-Camet, J.; Newport, M.J.; Oliveira, C.J.; Schlesinger, L.S.; Saif, Y.M.; Kariuki, S.; Saif, L.J.; Saville, W.; Wittum, T.; et al. The global one health paradigm: Challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Negl. Trop. Dis. 2014, 8, e3257. [Google Scholar] [CrossRef]
- Yeni, F.; Yavaş, S.; Alpas, H.; Soyer, Y. Most Common Foodborne Pathogens and Mycotoxins on Fresh Produce: A Review of Recent Outbreaks. Crit. Rev. Food Sci. Nutr. 2016, 56, 1532–1544. [Google Scholar] [CrossRef]
- Day, M.J. One health: The importance of companion animal vector-borne diseases. Parasites Vectors 2011, 4, 49. [Google Scholar] [CrossRef]
- Cantas, L.; Suer, K. The important bacterial zoonoses in “one health” concept. Front. Public Health 2014, 2, 144. [Google Scholar] [CrossRef]
- Goni, M.D.; Muhammad, I.J.; Bitrus, A.A.; Jajere, S.M.; Shah, M.K.; Aliyu, A.; Goje, M. Public health significance of companion animals in emergence and re-emergence of bacterial zoonoses. J. Adv. Vet. Anim. Res. 2018, 5, 101–109. [Google Scholar] [CrossRef]
- Statista. Number of Dogs and Cats Kept as Pets Worldwide in 2018: Ema Bedford. 2020. Available online: https://www.statista.com/statistics/1044386/dog-and-cat-pet-population-worldwide/ (accessed on 12 March 2020).
- Luga, I.; Enemuneme, O.; Apaa, T. Dog population and ecology in Ahmadu Bello University (ABU) main campus and Bomo village, Kaduna state, Nigeria. Sokoto J. Vet. Sci. 2018, 16, 54–59. [Google Scholar] [CrossRef]
- Chidumayo, N.N. Epidemiology of canine gastrointestinal helminths in sub-Saharan Africa. Parasites Vectors 2018, 11, 100. [Google Scholar] [CrossRef]
- Simoons, F.J. Eat Not this Flesh: Food Avoidances from Prehistory to the Present; University of Wisconsin Press: Madison, WI, USA, 1994. [Google Scholar]
- Garba, A.; Dzikwi, A.A.; Okewole, P.A.; Chitunya, W.B.; Tirmidhi, A.B.; Kazeem, H.M.; Umoh, J.U. Evaluation of dog slaughter and consumption practices related to the control of rabies in Nigeria. J. Exp. Biol. Agric. Sci. 2013, 1, 125–130. [Google Scholar]
- Ajoke, E.; Solomon, A.; Ikhide, E. The role of dog trading and slaughter for meat in rabies epidemiology with special reference to Nigeria—A review. J. Exp. Biol. Agric. Sci. 2014, 2, 130–136. [Google Scholar]
- Eze, U.U. Occurrence of Rabies Virus among Apparently Healthy Dogs and Bats Slaughtered for Meat in Some Selected Local Governments of Enugu State. Ph.D. Thesis, University of Nigeria, Nsukka, Enugu State, Nigeria, 2017. [Google Scholar]
- Arnold, K.E.; Williams, N.J.; Bennett, M. ‘Disperse abroad in the land’: The role of wildlife in the dissemination of antimicrobial resistance. Biol. Lett. 2016, 12, 20160137. [Google Scholar] [CrossRef] [PubMed]
- Young, J.K.; Olson, K.A.; Reading, R.P.; Amgalanbaatar, S.; Berger, J. Is wildlife going to the dogs? Impacts of feral and free-roaming dogs on wildlife populations. BioScience 2011, 61, 125–132. [Google Scholar] [CrossRef]
- Hughes, J.; Macdonald, D.W. A review of the interactions between free-roaming domestic dogs and wildlife. Biol. Conserv. 2013, 157, 341–351. [Google Scholar] [CrossRef]
- Orusa, T.; Orusa, R.; Viani, A.; Carella, E.; Borgogno Mondino, E. Geomatics and EO data to support wildlife diseases assessment at landscape level: A pilot experience to map infectious keratoconjunctivitis in chamois and phenological trends in Aosta Valley (NW Italy). Remote Sens. 2020, 12, 3542. [Google Scholar] [CrossRef]
- Carella, E.; Orusa, T.; Viani, A.; Meloni, D.; Borgogno-Mondino, E.; Orusa, R. An integrated, tentative remote-sensing approach based on NDVI entropy to model canine distemper virus in wildlife and to prompt science-based management policies. Animals 2022, 12, 1049. [Google Scholar] [CrossRef]
- Vengust, M.; Anderson, M.; Rousseau, J.; Weese, J. Methicillin-resistant staphylococcal colonization in clinically normal dogs and horses in the community. Lett. Appl. Microbiol. 2006, 43, 602–606. [Google Scholar] [CrossRef]
- Ghasemzadeh, I.; Namazi, S. Review of bacterial and viral zoonotic infections transmitted by dogs. J. Med. Life 2015, 8, 1–5. [Google Scholar]
- Day, M.J.; Breitschwerdt, E.; Cleaveland, S.; Karkare, U.; Khanna, C.; Kirpensteijn, J.; Kuiken, T.; Lappin, M.R.; McQuiston, J.; Mumford, E.; et al. Surveillance of zoonotic infectious disease transmitted by small companion animals. Emerg. Infect. Dis. 2012, 18, e1. [Google Scholar] [CrossRef]
- Woldemeskel, M. Zoonosis due to Bruella suis with special reference to infection in dogs (Carnivores): A brief review. Open J. Vet. Med. 2013, 3, 34279. [Google Scholar] [CrossRef]
- Santos, H.A.; Thomé, S.M.; Baldani, C.D.; Silva, C.B.; Peixoto, M.P.; Pires, M.S.; Vitari, G.L.V.; Costa, R.L.; Santos, T.M.; Angelo, I.C.; et al. Molecular epidemiology of the emerging zoonosis agent Anaplasma phagocytophilum (Foggie, 1949) in dogs and ixodid ticks in Brazil. Parasites Vectors 2013, 6, 348. [Google Scholar] [CrossRef] [PubMed]
- Kolo, A.O.; Sibeko-Matjila, K.P.; Maina, A.N.; Richards, A.L.; Knobel, D.L.; Matjila, P.T. Molecular detection of zoonotic rickettsiae and Anaplasma spp. in domestic dogs and their ectoparasites in Bushbuckridge, South Africa. Vector-Borne Zoonotic Dis. 2016, 16, 245–252. [Google Scholar] [CrossRef]
- Ng-Nguyen, D.; Hii, S.-F.; Hoang, M.-T.T.; Nguyen, V.-A.T.; Rees, R.; Stenos, J.; Traub, R.J. Domestic dogs are mammalian reservoirs for the emerging zoonosis flea-borne spotted fever, caused by Rickettsia felis. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Chomel, B.B. Emerging and re-emerging zoonoses of dogs and cats. Animals 2014, 4, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Odeh, L.E.; Umoh, J.U.; Dzikwi, A.A. Assessment of risk of possible exposure to rabies among processors and consumers of dog meat in Zaria and Kafanchan, Kaduna state, Nigeria. Glob. J. Health Sci. 2014, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Jajere, S.M.; Onyilokwu, S.A.; Adamu, N.B.; Atsanda, N.N.; Saidu, A.S.; Adamu, S.G.; Mustapha, F.B. Prevalence of Salmonella infection in dogs in Maiduguri, Northeastern Nigeria. Int. J. Microbiol. 2014, 2014, 392548. [Google Scholar] [CrossRef]
- Ojo, O.E.; Bello, A.O.; Amosun, E.A.; Ajadi, R.A. Multidrug resistant verocytotoxin-producing Escherichia coli O157:H7 in the faeces of diarrhoeic and non-diarrhoeic dogs in Abeokuta, Nigeria. Vet. Arh. 2014, 84, 63–73. [Google Scholar]
- Kamani, J.; Mani, A.U.; Kumshe, H.A.; Dogo, G.I.; Yidawi, J.P.; Pauline, D.K.; Nnabuife, H.E.; Peter, J.; Egwu, G.O. Serosurvey for Toxoplasma gondii in dogs in Maiduguri, Borno State, Nigeria. J. Infect. Dev. Ctries. 2010, 4, 015–018. [Google Scholar] [CrossRef][Green Version]
- Coker, A.; Isokpehi, R.; Thomas, B.; Fagbenro-Beyioku, A.; Omilabu, S. Zoonotic infections in Nigeria: Overview from a medical perspective. Acta Trop. 2000, 76, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Lindahl, J.; Roesel, K.; Traore, S.G.; Yobouet, B.A.; Ndour, A.P.N.; Carron, M.; Grace, D. Where literature is scarce: Observations and lessons learnt from four systematic reviews of zoonoses in African countries. Anim. Health Res. Rev. 2016, 17, 28–38. [Google Scholar] [CrossRef]
- Sasaki, M.; Omobowale, O.; Tozuka, M.; Ohta, K.; Matsuu, A.; Nottidge, H.O.; Hirata, H.; Ikadai, H.; Oyamada, T. Molecular survey of Babesia canis in dogs in Nigeria. J. Vet. Med. Sci. 2007, 69, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Mshelbwala, P.P.; Lohr, F. Clostridium difficile shedding by healthy dogs in Nigeria and Malawi. Zoonoses Public Health 2019, 66, 618–621. [Google Scholar] [CrossRef]
- Elom, M.; Obeji, N.; Nworie, A.; Usanga, V. Open Access Ectoparasitic infestations of cats and dogs in Izzi Local Government Area of Ebonyi State, Nigeria: Brief communication for ‘One Health’approach to control of potential zoonoses. Afr. J. Clin. Exp. Microbiol. 2020, 21, 72–77. [Google Scholar] [CrossRef]
- Deplazes, P.; van Knapen, F.; Schweiger, A.; Overgaauw, P.A. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet. Parasitol. 2011, 182, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Duscher, G.G.; Leschnik, M.; Fuehrer, H.-P.; Joachim, A. Wildlife reservoirs for vector-borne canine, feline and zoonotic infections in Austria. Int. J. Parasitol. Parasites Wildl. 2015, 4, 88–96. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Mavingui, P.; Boëtsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The one health concept: 10 years old and a long road ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Animal Population [Internet]. OIE WAHIS. 2018. Available online: https://www.oie.int/wahis_2/public/wahid.php/Countryinformation/Animalpopulation (accessed on 4 February 2020).
- Ayinmode, A.B.; Obebe, O.O.; Olayemi, E. Prevalence of potentially zoonotic gastrointestinal parasites in canine faeces in Ibadan, Nigeria. Ghana Med. J. 2016, 50, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Ishola, O.; Awosanya, E.; Adeniyi, I. Management and socio-economic determinants of profitability in dog breeding business in Oyo state, Nigeria. Sokoto J. Vet. Sci. 2016, 14, 32–39. [Google Scholar] [CrossRef]
- Abiola, O.; Babatunde, O.; Adebiyi, A. Sociodemograhic characteristics of dog breeders in some selected states in southwestern Nigeria. Niger. Vet. J. 2018, 39, 194–198. [Google Scholar] [CrossRef]
- Chanding, A.Y.; Umar, Y.A.; Tenshak, T.J.; Ibrahim, S. Prevalence Study of Gastrointestinal Helminth in Domestics Dogs (Canis Familiaris) Slaughtered in Selected Abattoirs in Plateau State, Nigeria. Open Sci. J. 2018, 3, 1–13. [Google Scholar] [CrossRef][Green Version]
- Antoninis, M. Tackling the largest global education challenge? Secular and religious education in northern Nigeria. World Dev. 2014, 59, 82–92. [Google Scholar] [CrossRef]
- Joshua, S.; Olanrewaju, F.O. The Impact of Terrorism on Education: The North-Eastern Nigerian Experience. J. Int. Politics Dev. 2016, 14, 59–74. [Google Scholar]
- Statistics NBo. Demographic Statistics Bulletin; National Bureau of Statistics: Abuja, Nigeria, 2020. [Google Scholar]
- Coker, R.; Rushton, J.; Mounier-Jack, S.; Karimuribo, E.; Lutumba, P.; Kambarage, D.; Pfeiffer, D.U.; Stärk, K.; Rweyemamu, M. Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect. Dis. 2011, 11, 326–331. [Google Scholar] [CrossRef]
- Etikan, I.; Musa, S.A.; Alkassim, R.S. Comparison of convenience sampling and purposive sampling. Am. J. Theor. Appl. Stat. 2016, 5, 1–4. [Google Scholar] [CrossRef]
- Macpherson, C.N. Human behaviour and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005, 35, 1319–1331. [Google Scholar] [CrossRef]
- Robertson, L.J.; Torgerson, P.R.; van der Giessen, J. Foodborne Parasitic Diseases in Europe: Social Cost-Benefit Analyses of Interventions. Trends Parasitol. 2018, 34, 919–923. [Google Scholar] [CrossRef]
- Said DES. Detection of parasites in commonly consumed raw vegetables. Alex. J. Med. 2012, 48, 345–352. [Google Scholar]
- WHO. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/199350/?sequence=1 (accessed on 15 March 2020).
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Fèvre, E.M.; Sripa, B.; et al. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.M.; Fischer-Walker, C.L.; Lanata, C.F.; Devleesschauwer, B.; Hall, A.J.; Kirk, M.D.; Duarte, A.S.; Black, R.E.; Angulo, F.J. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS ONE 2015, 10, e0142927. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Alvarado, M.; Basáñez, M.-G.; Bolliger, I.; Bourne, R.; Boussinesq, M.; Brooker, S.J.; Brown, A.S.; Buckle, G.; Budke, C.M.; et al. The global burden of disease study 2010: Interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014, 8, e2865. [Google Scholar] [CrossRef]
- Epe, C.; Rehkter, G.; Schnieder, T.; Lorentzen, L.; Kreienbrock, L. Giardia in symptomatic dogs and cats in Europe—Results of a European study. Vet. Parasitol. 2010, 173, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Guardone, L.; Magi, M.; Prati, M.; Macchioni, F. Cardiorespiratory and gastrointestinal parasites of dogs in north-west Italy. Helminthologia 2016, 53, 318–325. [Google Scholar] [CrossRef]
- Woodhall, D.M.; Eberhard, M.L.; Parise, M.E. Neglected parasitic infections in the United States: Toxocariasis. Am. J. Trop. Med. Hyg. 2014, 90, 810–813. [Google Scholar] [CrossRef]
- Zewdu, E.; Semahegn, Y.; Mekibib, B. Prevalence of helminth parasites of dogs and owners awareness about zoonotic parasites in Ambo town, central Ethiopia. Ethiop. Vet. J. 2010, 14, 17–30. [Google Scholar] [CrossRef]
- Mukaratirwa, S.; Singh, V.P. Prevalence of gastrointestinal parasites of stray dogs impounded by the Society for the Prevention of Cruelty to Animals (SPCA), Durban and Coast, South Africa. J. S. Afr. Vet. Assoc. 2010, 81, 123–125. [Google Scholar] [CrossRef]
- Sowemimo, O.A.; Asaolu, S.O. Epidemiology of intestinal helminth parasites of dogs in Ibadan, Nigeria. J. Helminthol. 2008, 82, 89–93. [Google Scholar] [CrossRef]
- Magaji, A.; Mohammed, M.; Saulawa, M.; Salihu, M. Survey of zoonotic gastrointestinal parasites of dogs (Canis familiaris) slaughtered at Zuru area, Kebbi state, Nigeria. Sci. J. Vet. Adv. 2012, 1, 132–136. [Google Scholar]
- Felix, P.M.; Mde, I.T.P.; Uwondo, A.E.; Esonu, D.O. Prevalence of Zoonotic Gastrointestinal Helminth Parasites (ZGIHP) of Dogs Presented to the Small Animal Clinic of the Veterinary Teaching Hospital, University of Agriculture, Makurdi, Benue State (October 2016–January 2017). 2019. Available online: http://www.scirj.org/papers-0819/scirj-P0819686.pdf (accessed on 12 March 2020).
- Ogbaje, C.I.; Ofukwu, R.A.; Ajogi, I.A. Zoonotic gastrointestinal parasite burden of local dogs in Zaria, Northern Nigeria: Implications for human health. Int. J. One Health 2015, 1, 32–36. [Google Scholar] [CrossRef]
- Onyenwe, I.; Ikpegbu, E. Prevalence of Gastroıntestınal Helmınth Parasıtes (GIHP) of dogs presented at the Unıversıty of Nıgerıa Veterınary Teachıng Hospıtal (UNVTH) between 1994–2002. Niger. Vet. J. 2004, 25, 21–25. [Google Scholar] [CrossRef]
- Mbaya, A.; Aliyu, M.; Nwosu, C.; Ibrahim, U.; Shallanguwa, J. A ten-year retrospective study of the prevalence of parasitic infections of dogs at the University of Maiduguri Veterinary Teaching Hospital, Nigeria. Niger. Vet. J. 2008, 29, 31–36. [Google Scholar] [CrossRef]
- Onyeabor, A. Prevalence of Gastrointestinal Helminths of Dogs: A Retrospective Study. J. Vet. Adv. 2014, 4, 746–751. [Google Scholar] [CrossRef][Green Version]
- Craft, M.E.; Vial, F.; Miguel, E.; Cleaveland, S.; Ferdinands, A.; Packer, C. Interactions between domestic and wild carnivores around the greater Serengeti ecosystem. Anim. Conserv. 2017, 20, 193–204. [Google Scholar] [CrossRef]
- Butler, J.R.A.; du Toit, J.T.; Bingham, J. Free-ranging domestic dogs (Canis familiaris) as predators and prey in rural Zimbabwe: Threats of competition and disease to large wild carnivores. Biol. Conserv. 2004, 115, 369–378. [Google Scholar] [CrossRef]
- Curi, N.; Paschoal, A.; Massara, R.; Santos, H.; Guimarães, M.; Passamani, M.; Chiarello, A.G. Risk factors for gastrointestinal parasite infections of dogs living around protected areas of the Atlantic Forest: Implications for human and wildlife health. Braz. J. Biol. 2017, 77, 388–395. [Google Scholar] [CrossRef]
- Prociv, P.; Croese, J. Human eosinophilic enteritis caused by dog hookworm Ancylostoma caninum. Lancet 1990, 335, 1299–1302. [Google Scholar] [CrossRef]
- Rubinsky-Elefant, G.; Hirata, C.; Yamamoto, J.; Ferreira, M. Human toxocariasis: Diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann. Trop. Med. Parasitol. 2010, 104, 3–23. [Google Scholar] [CrossRef]
- Torgerson, P.; Budke, C. Echinococcosis–an international public health challenge. Res. Vet. Sci. 2003, 74, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, L.; Wang, J.; Luo, Y. Knowledge domain and emerging trends on echinococcosis research: A scientometric analysis. Int. J. Environ. Res. Public Health 2019, 16, 842. [Google Scholar] [CrossRef] [PubMed]
- Ohiolei, J.A.; Yan, H.-B.; Li, L.; Zhu, G.-Q.; Muku, R.J.; Wu, Y.-T.; Jia, W.Z. Review of cystic echinococcosis in Nigeria: A story of neglect. Acta Parasitol. 2019, 65, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ojo, G.; Adekeye, T.; Awobode, H. Prevalence of single and mixed parasitic infections of dogs in Egbeda communities, Ibadan, Oyo State, Nigeria. Sokoto J. Vet. Sci. 2019, 17, 25–34. [Google Scholar] [CrossRef]
- Okewole, E. The prevalence, pathogenesis and control of canine and human toxocariosis in Ibadan, Nigeria. Sokoto J. Vet. Sci. 2016, 14, 34–42. [Google Scholar] [CrossRef]
- Heukelbach, J.; Feldmeier, H. Ectoparasites—The underestimated realm. Lancet 2004, 363, 889–891. [Google Scholar] [CrossRef]
- Colella, V.; Nguyen, V.L.; Tan, D.Y.; Lu, N.; Fang, F.; Zhijuan, Y.; Wang, J.; Liu, X.; Chen, X.; Dong, J.; et al. Zoonotic Vectorborne Pathogens and Ectoparasites of Dogs and Cats in Eastern and Southeast Asia. Emerg. Infect. Dis. 2020, 26, 1221. [Google Scholar] [CrossRef]
- Ojemudia, T.; Olabode, A.; Okeke, O.; Chukwu, C.; Duru, B.; Adeyanju, O.; Ashi, R.I.; Nedosa, A.U.; Dogo, G.I.A. Emergence of Zoonotic Myiasis in Vom and Bukuru Metropolis, Jos South LGA, Plateau State, Nigeria. 2011. Available online: https://dspace.unijos.edu.ng/jspui/handle/123456789/682 (accessed on 11 March 2020).
- Odeniran, P.O.; Ademola, I.O. A meta-analysis of the prevalence of African animal trypanosomiasis in Nigeria from 1960 to 2017. Parasites Vectors 2018, 11, 280. [Google Scholar] [CrossRef]
- Phillips, I.; Casewell, M.; Cox, T.; De Groot, B.; Friis, C.; Jones, R.; Nightingale, C.; Preston, R.; Waddell, J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2004, 53, 28–52. [Google Scholar] [CrossRef]
- Marcotty, T.; Matthys, F.; Godfroid, J.; Rigouts, L.; Ameni, G.; Gey van Pittius, N.; Kazwala, R.; Muma, J.; van Helden, P.; Walravens, K.; et al. Zoonotic tuberculosis and brucellosis in Africa: Neglected zoonoses or minor public-health issues? The outcomes of a multi-disciplinary workshop. Ann. Trop. Med. Parasitol. 2009, 103, 401–411. [Google Scholar] [CrossRef]
- Cadmus, S.; Ijagbone, I.; Oputa, H.; Adesokan, H.; Stack, J. Serological survey of brucellosis in livestock animals and workers in Ibadan, Nigeria. Afr. J. Biomed. Res. 2006, 9, 163–168. [Google Scholar] [CrossRef]
- Ducrotoy, M.J.; Bertu, W.J.; Ocholi, R.A.; Gusi, A.M.; Bryssinckx, W.; Welburn, S.; Moriyón, I. Brucellosis as an emerging threat in developing economies: Lessons from Nigeria. PLoS Negl. Trop. Dis. 2014, 8, e3008. [Google Scholar] [CrossRef]
- Karshima, S.N.; Bobbo, A.A. Isolation and PCR Characterisation of Thermophilic Campylobacter Species in Dogs Presented to Selected Veterinary Clinics in Jos, Nigeria. Alex. J. Vet. Sci. 2016, 50, 70–77. [Google Scholar] [CrossRef]
- Chaban, B.; Ngeleka, M.; Hill, J.E. Detection and quantification of 14 Campylobacter species in pet dogs reveals an increase in species richness in feces of diarrheic animals. BMC Microbiol. 2010, 10, 73. [Google Scholar] [CrossRef]
- Karama, M.; Cenci-Goga, B.T.; Prosperi, A.; Etter, E.; El-Ashram, S.; McCrindle, C.; Ombui, J.N.; Kalake, A. Prevalence and risk factors associated with Campylobacter spp. occurrence in healthy dogs visiting four rural community veterinary clinics in South Africa. Onderstepoort J. Vet. Res. 2019, 86, 1–6. [Google Scholar] [CrossRef]
- Salihu, M.D.; Magaji, A.A.; Abdulkadir, J.U.; Kolawale, A. Survey of thermophilic Campylobacter species in cats and dogs in north-western Nigeria. Vet Ital 2010, 46, 425–430. [Google Scholar]
- Hlashwayo, D.F.; Sigaúque, B.; Bila, C.G. Epidemiology and antimicrobial resistance of Campylobacter spp. in animals in Sub-Saharan Africa: A systematic review. Heliyon 2020, 6, e03537. [Google Scholar] [CrossRef]
- Brenner, D.J.; Farmer, J., III. Enterobacteriaceae. Bergey’s Man. Syst. Archaea Bact. 2015, 1–24. [Google Scholar]
- Rubin, J.E.; Pitout, J.D. Extended-spectrum β-lactamase, carbapenemase and AmpC producing Enterobacteriaceae in companion animals. Vet. Microbiol. 2014, 170, 10–18. [Google Scholar] [CrossRef]
- Carattoli, A. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 2227–2238. [Google Scholar] [CrossRef]
- Paterson, D.L. Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Control. 2006, 34, S20–S28. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.; Moura, R.A.; Ramires, P.; de Pestana Castro, A.; Lincopan, N. Current status of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae in animals. Microb. Pathog. Strateg. Combat. Sci. Technol. Educ. Badajoz Formatex Res. Cent. 2013, 1600–1607. [Google Scholar]
- Hetsa, B.; Ateba, T.; Moroane, T.; Nyirenda, M.; Gopane, R.; Ateba, C. Detection of antibiotic resistant Enterobacteriaceae from dogs in North West University (South Africa) animal health hospital. Afr. J. Microbiol. Res. 2013, 7, 5004–5010. [Google Scholar] [CrossRef][Green Version]
- Köck, R.; Daniels-Haardt, I.; Becker, K.; Mellmann, A.; Friedrich, A.W.; Mevius, D.; Schwarz, S.; Jurke, A. Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: A systematic review. Clin. Microbiol. Infect. 2018, 24, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.; de la Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hartskeerl, R.A.; Collares-Pereira, M.; Ellis, W.A. Emergence, control and re-emerging leptospirosis: Dynamics of infection in the changing world. Clin. Microbiol. Infect. 2011, 17, 494–501. [Google Scholar] [CrossRef]
- Levett, P.N. Leptospirosis: A forgotten zoonosis? Clin. Appl. Immunol. Rev. 2004, 4, 435–448. [Google Scholar] [CrossRef]
- Lau, C.; Smythe, L.; Weinstein, P. Leptospirosis: An emerging disease in travellers. Travel Med. Infect. Dis. 2010, 8, 33–39. [Google Scholar] [CrossRef]
- Schuller, S.; Francey, T.; Hartmann, K.; Hugonnard, M.; Kohn, B.; Nally, J.; Sykes, J. European consensus statement on leptospirosis in dogs and cats. J. Small Anim. Pract. 2015, 56, 159–179. [Google Scholar] [CrossRef]
- de Vries, S.G.; Visser, B.J.; Nagel, I.M.; Goris, M.G.A.; Hartskeerl, R.A.; Grobusch, M.P. Leptospirosis in Sub-Saharan Africa: A systematic review. Int. J. Infect. Dis. 2014, 28, 47–64. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Duquette, R.; Nuttall, T. Methicillin-resistant Staphylococcus aureus in dogs and cats: An emerging problem? J. Small Anim. Pract. 2004, 45, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Van Duijkeren, E.; Wolfhagen, M.J.; Box, A.T.; Heck, M.E.; Wannet, W.J.; Fluit, A.C. Human-to-dog transmission of methicillin-resistant Staphylococcus aureus. Emerg. Infect. Dis. 2004, 10, 2235. [Google Scholar] [CrossRef]
- Morris, D.O.; Mauldin, E.A.; O’Shea, K.; Shofer, F.S.; Rankin, S.C. Clinical, microbiological, and molecular characterization of methicillin-resistant Staphylococcus aureus infections of cats. Am. J. Vet. Res. 2006, 67, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. Occurrence of methicillin-resistant Staphylococcus aureus strains from cattle and chicken, and analyses of their mecA, mecR1 and mecI genes. Vet. Microbiol. 2006, 114, 155–159. [Google Scholar] [CrossRef]
- Wendlandt, S.; Kadlec, K.; Feßler, A.T.; Monecke, S.; Ehricht, R.; van de Giessen, A.W.; Hengeveld, P.D.; Huijsdens, X.; Schwarz, S.; van Duijkeren, E. Resistance phenotypes and genotypes of methicillin-resistant Staphylococcus aureus isolates from broiler chickens at slaughter and abattoir workers. J. Antimicrob. Chemother. 2013, 68, 2458–2463. [Google Scholar] [CrossRef]
- Chah, K.F.; Gómez-Sanz, E.; Nwanta, J.A.; Asadu, B.; Agbo, I.C.; Lozano, C.; Zarazaga, M.; Torres, C. Methicillin-resistant coagulase-negative staphylococci from healthy dogs in Nsukka, Nigeria. Braz. J. Microbiol. 2014, 45, 215–220. [Google Scholar] [CrossRef]
- van Duijkeren, E.; Moleman, M.; van Oldruitenborgh-Oosterbaan, M.S.; Multem, J.; Troelstra, A.; Fluit, A.; van Wamel, W.J.; Houwers, D.J.; de Neeling, A.J.; Wagenaar, J.A. Methicillin-resistant Staphylococcus aureus in horses and horse personnel: An investigation of several outbreaks. Vet. Microbiol. 2010, 141, 96–102. [Google Scholar] [CrossRef]
- Baptiste, K.E.; Williams, K.; Willams, N.J.; Wattret, A.; Clegg, P.D.; Dawson, S.; Corkill, J.E.; O’Neill, T.; Hart, C.A. Methicillin-resistant staphylococci in companion animals. Emerg. Infect. Dis. 2005, 11, 1942. [Google Scholar] [CrossRef]
- Adebiyi, A.I.; Oluwayelu, D.O. Zoonotic fungal diseases and animal ownership in Nigeria. Alex. J. Med. 2018, 54, 397–402. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Bosco, S.D.M.; De Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. 2018, 56 (suppl. 1), S165–S187. [Google Scholar] [CrossRef] [PubMed]
- Murmu, S.; Debnath, C.; Pramanik, A.; Mitra, T.; Jana, S.; Dey, S.; Banerjee, S.; Batabyal, K. Detection and characterization of zoonotic dermatophytes from dogs and cats in and around Kolkata. Vet. World 2015, 8, 1078. [Google Scholar] [CrossRef]
- Watt, P.; Robins, G.; Galloway, A.; O’boyle, D. Disseminated opportunistic fungal disease in dogs: 10 cases (1982–1990). J. Am. Vet. Med. Assoc. 1995, 207, 67–70. [Google Scholar]
- Oyedele, O.; Oluwayelu, D.; Cadmus, S.; Odemuyiwa, S.; Adu, F. Protective levels of canine distemper virus antibody in an urban dog population using plaque reduction neutralization test. Onderstepoort J. Vet. Res. 2004, 71, 227–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Pesavento, P.A.; Shan, T.; Leutenegger, C.M.; Wang, C.; Delwart, E. Viruses in diarrhoeic dogs include novel kobuviruses and sapoviruses. J. Gen. Virol. 2011, 92, 2534. [Google Scholar] [CrossRef] [PubMed]
- Babalola, E.; Ijaopo, O.; Okonko, I. Evaluation of Immunity and Seropositivity of IgG Antibodies to Canine Parvoviruses in Vaccinated and Unvaccinated Dogs in Abeokuta, Nigeria. J. Immunoass. Immunochem. 2016, 37, 16–28. [Google Scholar] [CrossRef]
- Vakuru, C.T.; Manu, S.A.; Ahmed, G.I.; Junaidu, K.; Newman, S.; Nyager, J.; Iwar, V.N.; Mshelbwala, G.M.; Joannis, T.; Maina, J.A.; et al. Situation-based survey of avian influenza viruses in possible “bridge” species of wild and domestic birds in Nigeria. Influenza Res. Treat. 2012, 2012, 567–601. [Google Scholar]
- Parrish, C.R.; Voorhees, I.E.H. H3N8 and H3N2 canine influenza viruses: Understanding these new viruses in dogs. Vet. Clin. Small Anim. Pract. 2019, 49, 643–649. [Google Scholar] [CrossRef]
- Song, D.; Moon, H.; Jung, K.; Yeom, M.; Kim, H.; Han, S.; An, D.; Oh, J.; Kim, J.; Park, B.; et al. Association between nasal shedding and fever that influenza A (H3N2) induces in dogs. Virol. J. 2011, 8, 1–4. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, Y.-N.; Zhou, H.-B.; Wei, Z.-Z.; Wang, G.-J.; Yu, Q.-X.; Xiao, X.; Yang, W.J.; Huang, W.J. Emergence of human-like H3N2 influenza viruses in pet dogs in Guangxi, China. Virol. J. 2015, 12, 10. [Google Scholar] [CrossRef]
- Gupta, R.; Dhawan, S.; Gupta, B. World Rabies Research Output: A Scientometric Assessment of Publication Output during 2006–2015. J. Sci. Res. 2016, 5, 220–229. [Google Scholar] [CrossRef]
- Mshelbwala, P.; Ogunkoya, A.; Maikai, B. Detection of rabies antigen in the saliva and brains of apparently healthy dogs slaughtered for human consumption and its public health implications in Abia State, Nigeria. Int. Sch. Res. Not. 2013, e468043. [Google Scholar] [CrossRef]
- Cleaveland, S.; Kaare, M.; Knobel, D.; Laurenson, M.K. Canine vaccination—Providing broader benefits for disease control. Vet. Microbiol. 2006, 117, 43–50. [Google Scholar] [CrossRef]
- Lerner, H.; Berg, C. The concept of health in One Health and some practical implications for research and education: What is One Health? Infect. Ecol. Epidemiol. 2015, 5, 25300. [Google Scholar] [CrossRef]
- Aragrande, M.; Canali, M.; Roccaro, M.; Ferraro, E.; Bonoli, A.; Savini, F.; Piva, S.; Gallina, L.; Peli, A.; Sambri, V.; et al. One Health Evaluation: A Case Study at the University of Bologna. Front. Public Health 2021, 9, e661490. [Google Scholar] [CrossRef]
- Torrey, E.F.; Yolken, R.H. Beasts of the Earth: Animals, Humans, and Disease; Rutgers University Press: New Brunswick, NJ, USA, 2005. [Google Scholar]
- Ehizibolo, D.; Ehizibolo, P.; Ehizibolo, E.; Sugun, M.; Idachaba, S. The control of neglected zoonotic diseases in Nigeria through animal intervention. Afr. J. Biomed. Res. 2011, 14, 81–88. [Google Scholar]
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef]
- Levy, S. Reduced Antibiotic Use in Livestock: How Denmark Tackled Resistance. NLM-Export. 2014. Available online: https://ehp.niehs.nih.gov/doi/full/10.1289/ehp.122-A160 (accessed on 8 March 2020).
- Eze, U.U.; Ngoepe, E.C.; Anene, B.M.; Ezeokonkwo, R.C.; Nwosuh, C.I.; Sabeta, C.T. Molecular Detection of Rabies Lyssaviruses from Dogs in Southeastern Nigeria: Evidence of Transboundary Transmission of Rabies in West Africa. Viruses 2020, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Ndumari, W.; Se-ember, A.; Oluwatosin, A.; Terzugwe, T. Seroprevalence of Brucellosis in Nigerian Breed of Dog in North Bank Area of Makurdi, Benue State Nigeria. Int. J. Intern. Med. Geriatr. 2020, 2, 80–86. [Google Scholar]
- Abulude, O.A. Prevalence of Intestinal Helminth Infections of Stray Dogs of Public Health Significance in Lagos Metropolis, Nigeria. Int. Ann. Sci. 2020, 9, 24–32. [Google Scholar] [CrossRef]
- Kamani, J.; González-Miguel, J.; Mshelbwala, F.; Shekaro, A.; Apanaskevich, D. Ticks (Acari: Ixodidae) infesting dogs in Nigeria: Epidemiological and public health implications. Exp. Appl. Acarol. 2019, 78, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Ezema, K.U.; Malgwi, S.A.; Zango, M.K.; Kyari, F.; Tukur, S.M.; Mohammed, A.; Kayeri, B.K. Gastrointestinal parasites of dogs (Canis familiaris) in Maiduguri, Borno State, Northeastern Nigeria: Risk factors and zoonotic implications for human health. Vet. World 2019, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Umeakuana, P.U.; Gibson, W.; Ezeokonkwo, R.C.; Anene, B.M. Identification of Trypanosoma brucei gambiense in naturally infected dogs in Nigeria. Parasites Vectors 2019, 12, 420. [Google Scholar] [CrossRef]
- Moro, K.K.; Abah, A.E. Epizootiology of zoonotic parasites of dogs in Abua area of Rivers State, Nigeria. Vet. Anim. Sci. 2019, 7, 100045. [Google Scholar] [CrossRef]
- Pam, D.; Pam, V.; Felix, L.; Adejoh, V.; Ombugadu, A.; Terhemen, S. AHC-22 Prevalence of Parasites among Dogs Undergoing Treatment at Polo Veterinary Clinic Jos, North Central Nigeria. Available online: https://www.researchgate.net/profile/Pam-Dung/publication/325805514_AHC-22_Prevalence_of_Parasites_among_Dogs_Undergoing_Treatment_at_Polo_Veterinary_Clinic_Jos_North_Central_Nigeria/links/5b250d25a6fdcc697468ca88/AHC-22-Prevalence-of-Parasites-among-Dogs-Undergoing-Treatment-at-Polo-Veterinary-Clinic-Jos-North-Central-Nigeria.pdf (accessed on 11 March 2020).
- Tirmidhi, A.; Kazeem, H.; Jibril, A.; Jahun, B.; Orakpoghenor, O. Detection of rabies virus antigen in brain tissue of dogs slaughtered for human consumption in Taraba State, Nigeria. Sokoto J. Vet. Sci. 2019, 17, 9–13. [Google Scholar] [CrossRef]
- Kamani, J.; Rojas, A.; Msheliza, E.; Shand, M.; Harrus, S.; Baneth, G. Molecular detection of filarioid worms in dogs in Nigeria, West Africa. Vet. Arh. 2019, 89, 821–830. [Google Scholar] [CrossRef]
- Amapu, T.; Latu, M.; Dapiya, H.S.H.; Pam, K.; Job, M.; Dawen, D.; Brengshak, S.B.; Ajang, Y.; Hero, G.U.; Dingmun, P.J.; et al. Occurrence of Gastrointestinal Parasitic Associated with Exotic Dogs in Commercial Breeding Mills in Jos Metropolis-Nigeria. 2019. Available online: https://irepos.unijos.edu.ng/jspui/handle/123456789/2781 (accessed on 11 March 2020).
- Eze, U.U.; Ezeh, I.O.; Nzeakor, T.A.; Attama, S.C.; Ezenduka, E.V.; Onah, D.N. Prevalence and risk factors associated with Cryptosporidium spp. infection in local breed of dogs in Enugu State, Nigeria. Vet. World 2019, 12, 729. [Google Scholar] [CrossRef]
- Peter, O.M.; Ikejiofor, O.K.; Sabo, J.A.A.W.; Ibrahim, M.A.A.; Terzungwe, T.M.; Samuel, O.O.; Yola, Y.D.; Okonkwo, R.A.; Malgwi, R.I. Prevalence of Campylobacter Species in Dogs in Bassa, Plateau State, Nigeria. Ann. Microbiol. Infect. Dis. 2019, 2, 21–25. [Google Scholar]
- Opeyemi, O.A.; Babamale, O.A.; Shittu, O.; Mohammad, M.U.; Ugbomoiko, U.S. Seasonal distribution and common management practices of ectoparasites of domestic dogs in Ilorin, Nigeria. Anim. Res. Int. 2019, 16, 3265–3272. [Google Scholar]
- Daodu, O.; Adebiyi, A.; Oluwayelu, D. Serological and molecular surveillance for influenza A virus in dogs and their human contacts in Oyo State, Nigeria. Trop. Biomed. 2019, 36, 1054–1060. [Google Scholar]
- Nwufoh, O.C.; Sadiq, A.N.; Emikpe, B.O. The seroprevalence of Sarcoptes scabiei var. canis and its associated risk factors in dogs in Ibadan, Southwest Nigeria. J. Immunoass. Immunochem. 2019, 40, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Ezema, K.U.; Bukar, Y.M.; Konto, M.; Malgwi, S.A. Serological and Parasitological Survey of Canine Dirofilaria immitis Infection in Maiduguri, Borno state, Northeastern Nigeria. Alex. J. Vet. Sci. 2019, 62, 11–15. [Google Scholar] [CrossRef]
- Ayinmode, A.; Oliveira, B.; Obebe, O.; Dada-Adgebola, H.; Ayede, A.; Widmer, G. Genotypic Characterization of Cryptosporidium Species in Humans and Peri-Domestic Animals in Ekiti and Oyo States, Nigeria. J. Parasitol. 2018, 104, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, B.O.; Christy, A.L.; Samuel, U.U. Prevalence of ectoparasite infestations in owned dogs in Kwara State, Nigeria. Parasite Epidemiol. Control. 2019, 4, e00079. [Google Scholar] [CrossRef]
- Okpara, E.O.; Ojo, O.E.; Awoyomi, O.J.; Dipeolu, M.A.; Oyekunle, M.A.; Schwarz, S. Antimicrobial usage and presence of extended-spectrum β-lactamase-producing Enterobacteriaceae in animal-rearing households of selected rural and peri-urban communities. Vet. Microbiol. 2018, 218, 31–39. [Google Scholar] [CrossRef]
- Happi, A.N.; Toepp, A.J.; Ugwu, C.; Petersen, C.A.; Sykes, J.E. Detection and identification of blood-borne infections in dogs in Nigeria using light microscopy and the polymerase chain reaction. Vet. Parasitol. Reg. Stud. Rep. 2018, 11, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ogbu, K.; Olaolu, O.; Ochai, S. Detection of Antibodies to Non-Vaccinal Leptospira Serovars in Dogs in Jos North and Jos South Local Government Area. Ann. Microbiol. Infect. Dis. 2018, 1, 17–22. [Google Scholar]
- Audu, Y.; Maikai, B.; Okolocha, E. Survey for Brucella antibodies in dogs in Billiri Local government area of Gombe state, Nigeria. Sci. Res. J. (SCIRJ) 2018, 6, 43–51. [Google Scholar] [CrossRef]
- David, O.-F.S.; Goria, K.P.; Abraham, D.G.A. Haemoparasite fauna of domestic animals in plateau state, north central Nigeria. Bayero J. Pure Appl. Sci. 2018, 11, 156–161. [Google Scholar] [CrossRef]
- Daniel, L.; Adamma, A.; Ibukunoluwa, M. Microbial association with suspected cutaneous leishmaniasis (cl) lesions on dogs in Jos-South Plateau State North-central, Nigeria. Int. J. Sci. Appl. Res. 2018, 3, 79–87. [Google Scholar]
- Kia, G.S.; Huang, Y.; Zhou, M.; Zhou, Z.; Gnanadurai, C.; Leysona, C.; Umoh, J.U.; Kazeem, H.M.; Ehizibolo, D.O.; Kwaga, J.K.P.; et al. Molecular characterization of a rabies virus isolated from trade dogs in Plateau State, Nigeria. Sokoto J. Vet. Sci. 2018, 16, 54–62. [Google Scholar] [CrossRef]
- Ayinmode, A.; Obebe, O.; Falohun, O. Molecular detection of Cryptosporidium species in street-sampled dog faeces in Ibadan, Nigeria. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Ehimiyein, A.; Maishanu, D.; Ehimiyein, I. Prevalence of gastrointestinal and haemo-parasites in hunting dogs in Zaria, Nigeria. Sokoto J. Vet. Sci. 2018, 16, 55–60. [Google Scholar] [CrossRef]
- Akande, F.; Adebowale, A.; Idowu, O.; Sofela, O. Prevalence of ticks on indigenous breed of hunting dogs in Ogun State, Nigeria. Sokoto J. Vet. Sci. 2018, 16, 66–71. [Google Scholar] [CrossRef]
- Akwuobu, C.A.; Agbo, J.O.; Ofukwu, R.A.-P. Salmonella infection in clinically healthy dogs in Makurdi, Benue State, North-central Nigeria: A potential source of infection to humans. J. Adv. Vet. Anim. Res. 2018, 5, 405–409. [Google Scholar] [CrossRef]
- Daramola, O.O.; Takeet, M.I.; Oyewusi, I.K.; Oyekunle, M.A.; Talabi, A.O. Detection and molecular characterisation of Ehrlichia canis in naturally infected dogs in South West Nigeria. Acta Vet. Hung. 2018, 66, 85–95. [Google Scholar] [CrossRef]
- Abalaka, S.; Ubah, S.; Umeakuana, P.; Idoko, I.; Sani, N.; Obeta, S.; Hikosaka, K.; Inaoka, D.K.; Kita, K.; Watanabe, Y.I.; et al. Pathological and molecular diagnosis of canine babesiosis in Nigeria: A case report. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 150. [Google Scholar] [CrossRef]
- Kamani, J.; Chung, P.-J.; Lee, C.-C.; Chung, Y.-T. In search of the vector(s) of Babesia rossi in Nigeria: Molecular detection of B. rossi DNA in Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) ticks collected from dogs, circumstantial evidence worth exploring. Exp. Appl. Acarol. 2018, 76, 243–248. [Google Scholar] [CrossRef]
- Maurice, N.A.; Luka, P.D.; Maurice, M.N.; Ngbede, E.O.; Zhakom, P.N.; Mshelbwala, P.P.; Tekki, I.S.; Udoh, U.H.; Inyang, U.A.; Ekanem, N.J.; et al. Rabies in a set of eight-week old puppies in Nigeria: The need for review of current dog antirabies vaccination schedule. Afr. J. Infect. Dis. 2018, 12, 72–77. [Google Scholar] [CrossRef]
- Takeet, M.I.; Oyewusi, A.J.; Abakpa, S.A.; Daramola, O.O.; Peters, S.O. Genetic diversity among Babesia rossi detected in naturally infected dogs in Abeokuta, Nigeria, based on 18S rRNA gene sequences. Acta Parasitol. 2017, 62, 192–198. [Google Scholar] [CrossRef]
- Atting, I.A.; Etim, N.J.; Ebere, N. Comparative Study of Ectoparasites of Exotic and Locally Bred Dogs in IkotEkpene Local Government Area, Niger-Delta Region of Nigeria. Int. J. Sci. Res. 2017, 6, ART20171226. [Google Scholar]
- Omonijo, A.; Sowemimo, O. Prevalence of ectoparasites of dogs and cats in Ijero and Moba LGAs, Ekiti State, Nigeria. Niger. J. Parasitol. 2017, 38, 278–283. [Google Scholar] [CrossRef]
- Anyanwu, M.U.; Ezekwelu, M.O.; Okorafor, O.N. Prevalence and Antibiogram of Generic Extended-Spectrum β-Lactam-Resistant Enterobacteria in Healthy Dogs. Not. Sci. Biol. 2017, 9, 22–33. [Google Scholar] [CrossRef]
- Dogo, A.G.; Karaye, G.P.; Patrobas, M.; Galadima, M.; Gosomji, I. Prevalence of gastrointestinal parasites and their impact in domestic animals in Vom, Nigeria. Saudi J. Med. Nd Pharm. Sci. 2017, 3, 211–216. [Google Scholar]
- Daodu, O.B.; Amosun, E.A.; Oluwayelu, D.O. Antibiotic resistance profiling and microbiota of the upper respiratory tract of apparently healthy dogs in Ibadan, south west Nigeria. Afr. J. Infect. Dis. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Akande, F.A.; Adebowale, A.F.; Takeet, M.I.; Omisile, O.K. Prevalence of Babesia species in hunting dogs in Ogun State South West Nigeria. Alex. J. Vet. Sci. 2017, 54, 1–7. [Google Scholar] [CrossRef]
- Opara, M.; Adewumi, T.; Mohammed, B.; Obeta, S.; Simon, M.; Jegede, O.; Agbede, R. Investigations on the haemoprotozoan parasites of Nigerian local breed of dogs in Gwagwalada Federal Capital Territory (FCT) Nigeria. Res. J. Parasitol. 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Umeakuana, P.; Mohammed, B.; Anene, B. Canine trypanosomosis in the University of Nigeria Veterinary Teaching Hospital(UNVTH), Enugu State, Nigeria, sub-Saharan Africa. J. Vet. Adv. 2016, 6, 1350–1356. [Google Scholar]
- Aquino, L.; Kamani, J.; Haruna, A.; Paludo, G.; Hicks, C.; Helps, C.; Tasker, S. Analysis of risk factors and prevalence of haemoplasma infection in dogs. Vet. Parasitol. 2016, 221, 111–117. [Google Scholar] [CrossRef]
- Olabanji, G.M.; Maikai, B.V.; Otolorin, G.R. Prevalence and risk factors associated with faecal shedding of Cryptosporidium oocysts in dogs in the Federal Capital Territory, Abuja, Nigeria. Vet. Med. Int. 2016, 2016, 4591238. [Google Scholar] [CrossRef]
- Matthew, T.T.; Seer, I.J.; David, O.K. The prevalence of gastrointestinal helminths (GIH) infection of dogs in Makurdi metropolis. IJIR 2016, 2, 1042–1049. [Google Scholar]
- Adediran, O.A.; Kolapo, T.U.; Uwalaka, E.C. Seroprevalence of canine leishmaniasis in Kwara, Oyo and Ogun states of Nigeria. J. Parasit. Dis. 2016, 40, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Ogbaje, C.I.; Danjuma, A. Prevalence and risk factors associated with Dirofilaria immitis infection in dogs in Makurdi, Benue State, Nigeria. J. Adv. Vet. Anim. Res. 2016, 3, 338–344. [Google Scholar] [CrossRef]
- Isaac, C.; Igbinosa, I.; Nmorsi, O. Parasites and pathogens of ticks (Rhipicephalus species Acari: Ixodidae) among dogs in Edo state, Nigeria. Niger. J. Parasitol. 2016, 37, 129–134. [Google Scholar] [CrossRef]
- Mailafia, S.; Olabode, H.; Adah, B.; Waziri, H. Isolation and Characterization of Candida albicans Associated with Canine Conjunctivitis. J. Etiol. Anim. Health 2016, 2, JEAH-2-005. [Google Scholar]
- Mustapha, F.; Balami, S.; Malgwi, S.; Adamu, S.; Wakil, Y. Prevalence of gastrointestinal parasites of hunting dogs in Maiduguri, Borno State, Nigeria. IOSR J. Agric. Vet. Sci. 2016, 9, 39–42. [Google Scholar] [CrossRef]
- Ugochukwu, C.I.I.; Omekam, N.; Ugochukwu, E.I. Incidence of Dirofilaria immitis in dogs presented at University of Nigeria, Nsukka Veterinary Teaching Hospital using wet smear and buffy coat techniques. Asian Pac. J. Trop. Dis. 2016, 6, 627–630. [Google Scholar] [CrossRef]
- Mustapha, M.; Bukar-Kolo, Y.M.; Geidam, Y.A.; Gulani, I.A. Phenotypic and genotypic detection of methicillin-resistant Staphylococcus aureus in hunting dogs in Maiduguri metropolitan, Borno State, Nigeria. Vet. World 2016, 9, 501. [Google Scholar] [CrossRef]
- Ayoola, M.C.; Ogugua, A.J.; Akinseye, V.O.; Joshua, T.O.; Banuso, M.F.; Adedoyin, F.J.; Adesokan, H.K.; Omobowale, T.O.; Abiola, J.O. Sero-epidemiological survey and risk factors associated with brucellosis in dogs in south-western Nigeria. Pan Afr. Med. J. 2016, 23, 29. [Google Scholar]
- Tono, R.R.; Faleke, O.O.; Magaji, A.; Alayande, M.O.; Fajinmi, A.O.; Ibitoye, E.B. Presence of Trypanosome Species and Anemic Status of Dogs in Zuru, Nigeria. Maced. Vet. Rev. 2015, 38, 217–222. [Google Scholar] [CrossRef]
- Nongo, N.; Tion, M.; Apaa, T.; Ogunro, B. A case of canine trypanosomosis with epistaxis in a two-year old alsatian dog. J. Agric. Vet. Sci. 2015, 8, 68–72. [Google Scholar]
- Jegede, O.; Daniels, T.; Obeta, S. A case of dipylidiasis and babesiosis in a 2-year old mongrel (bitch). Vom J. Vet. Sci. 2015, 10, 143–147. [Google Scholar]
- Oluwayelu, D.O.; Adebiyi, A.I.; Ohore, O.G. A survey of rabies virus antibodies in confined, hunting and roaming dogs in Ogun and Oyo States, Southwestern Nigeria. Asian Pac. J. Trop. Dis. 2015, 5, 17–21. [Google Scholar] [CrossRef]
- Iboh, C.; Ajang, R.; Abraham, J. Comparison of gastrointestinal helminthes in dogs and awareness of zoonotic infection among dog owners in calabar, South Eastern Nigeria. Afr. J. Parasitol. Res. 2015, 2, 041–045. [Google Scholar]
- Momoh, H.A.; Ijale, G.O.; Ajogi, I.; Okolocha, E.C. Risk factors and level of awareness of canine brucellosis in Jos, Plateau State, Nigeria. J. Vet. Med. Anim. Health 2015, 7, 39–44. [Google Scholar]
- Ayinmode, A.B.; Ishola, O.O.; Oderinu, T.A. Seroprevalence of Toxoplasma gondii in dogs slaughtered for food in Southwestern Nigeria and assessment of consumer’s knowledge and behavior. Alex. J. Vet. Sci. 2015, 45, 161–165. [Google Scholar] [CrossRef]
- Adamu, M.; Troskie, M.; Oshadu, D.O.; Malatji, D.P.; Penzhorn, B.L.; Matjila, P.T. Occurrence of tick-transmitted pathogens in dogs in Jos, Plateau State, Nigeria. Parasites Vectors 2014, 7, 119. [Google Scholar] [CrossRef]
- Ameh, V.O.; Dzikwi, A.A.; Umoh, J.U. Assessment of knowledge, attitude and practice of dog owners to canine rabies in Wukari Metropolis, Taraba State Nigeria. Glob. J. Health Sci. 2014, 6, 226. [Google Scholar] [CrossRef]
- Momoh, H.; Ijale, G.; Ajogi, I.; Okolocha, E. Seroprevalence of Canine Brucellosis in Jos, Plateau State, Nigeria. Asian J. Epidemiol. 2014, 7, 36. [Google Scholar] [CrossRef][Green Version]
- Oluwasina, O.S.; ThankGod, O.E.; Augustine, O.O.; Gimba, F.I. Linguatula serrata (Porocephalida: Linguatulidae) infection among client-owned dogs in Jalingo, North Eastern Nigeria: Prevalence and public health implications. J. Parasitol. Res. 2014, 2014, 916120. [Google Scholar] [CrossRef]
- Akeredolu, A.; Sowemimo, O. Prevalence, intensity and associated risk factors for Toxocara canis infection in Nigerian dogs. J. Parasitol. Vector Biol. 2014, 6, 111–116. [Google Scholar]
- Adediran, O.A.; Kolapo, T.U.; Uwalaka, E.C. Echinococcus granulosus prevalence in dogs in Southwest Nigeria. J. Parasitol. Res. 2014, 2014, 124358. [Google Scholar] [CrossRef] [PubMed]
- Oluwayelu, D.; Bankole, O.; Ajagbe, O.; Adebiyi, A.; Abiola, J.; Otuh, P.; Omobowale, O.T. Serological survey for emerging canine H3N8 and H3N2 influenza viruses in pet and village dogs in Nigeria. Afr. J. Med. Med. Sci. 2014, 43, 111–115. [Google Scholar] [PubMed]
- Adediran, O.A.; Kolapo, T.U. Canine Echinococcosis in hunting and companion dogs in Oyo State, Nigeria: The public health significance. Acta Parasitol. Glob. 2014, 5, 59–64. [Google Scholar]
- Jegede, O.; Obeta, S.; Faisal, B. Infection of dogs with Babesia canis in Gwagwalada metropolis of Federal Capital Territory, Abuja, Nigeria. Sokoto J. Vet. Sci. 2014, 12, 37–41. [Google Scholar] [CrossRef]
- Nwiyi, P.; Okonkwo, C.; Enwere, S. Isolation of pathogenic bacteria and antibiotic susceptibility testing of dogs with otitis externa in Aba, Abia state, Nigeria. Sky J. Microbiol. Res. 2014, 2, 59–62. [Google Scholar]
- Adedoja, A.; Oshodi, J.A.; Akanbi, A.A.; Babatunde, S. Prevalence of intestinal protozoan parasites in stray and domicile dogs in Ilorin, North Central, Nigeria. Int. J. Biol. Chem. Sci. 2014, 8, 2054–2061. [Google Scholar] [CrossRef]
- Awoyomi, O.; Ojo, O. Antimicrobial resistance in aerobic bacteria isolated from oral cavities of hunting dogs in rural areas of Ogun State, Nigeria. Sokoto J. Vet. Sci. 2014, 12, 47–51. [Google Scholar] [CrossRef]
- Atuman, Y.; Adawa, Y.; Solomon, A.; Mshelbwala, P.; Ogunkoya, A. Potential Risks for Rabies Spill-Over from Apparently Healthy Dogs to Wildlife in Bauchi State, Nigeria. J. Vet. Adv. 2014, 4, 493–498. [Google Scholar] [CrossRef]
- Kamani, J.; Lee, C.-C.; Haruna, A.M.; Chung, P.-J.; Weka, P.R.; Chung, Y.-T. First detection and molecular characterization of Ehrlichia canis from dogs in Nigeria. Res. Vet. Sci. 2013, 94, 27–32. [Google Scholar] [CrossRef]
- Kamani, J.; Baneth, G.; Mumcuoglu, K.Y.; Waziri, N.E.; Eyal, O.; Guthmann, Y.; Harrus, S. Molecular detection and characterization of tick-borne pathogens in dogs and ticks from Nigeria.(Report). PLoS Negl. Trop. Dis. 2013, 7, e2108. [Google Scholar] [CrossRef] [PubMed]
- Okubanjo, O.; Adeshina, O.; Jatau, I.; Natala, A. Prevalence of Babesia canis and Hepatozoon canis in Zaria, Nigeria. Sokoto J. Vet. Sci. 2013, 11, 15–20. [Google Scholar] [CrossRef]
- Odeniran, P.; Ademola, I. Prevalence of Zoonotic Gastrointestinal Helminth in Dogs and Knowledge of Risk of Infection by Dog Owners in Ibadan, Nigeria. Niger. Vet. J. 2013, 34, 851–858. [Google Scholar]
- Qasim, A.; Obadua, A.; Okewole, P.; Tekki, I.; Omoleye, O. Rabies in a vaccinated 9-month-old German shepherd dog, Akure, 2010: A case report. Case Rep. Vet. Med. 2013, 2013, 280603. [Google Scholar] [CrossRef]
- Isek, T.; Umoh, J.; Dzikwi, A. Detection of Rabies Antigen in the Brain Tissues of Apparetly Healthy Dogs Slaughteres in Ogoja-Cross River State, Nigeria. Niger. Vet. J. 2013, 34, 789–794. [Google Scholar]
- Ojo, O.; Bello, A.; Ogunjobi, O.; Ajadi, R. Antimicrobial resistance of gram-negative aerobic bacteria isolates from the faeces of diarrhoeic and non-diarrhoeic dogs in Abeokuta, Nigeria. Trop. Vet. 2013, 31, 55–64. [Google Scholar]
- Ogo, N.I.; Onovoh, E.; Okubanjo, O.O.; Galindo, R.C.; Lastra, J.-M.P. Molecular identification of Cordylobia anthropophaga Blanchard (Diptera: Calliphoridae) larvae collected from dogs (Canis familiaris) in Jos South, Plateau State, Nigeria. Onderstepoort J. Vet. Res. 2012, 79, 01–04. [Google Scholar] [CrossRef]
- Mahmuda, A.; Magaji, A.A.; Yakubu, Y.; Salihu, M.; Lawal, M.; Mahmud, U.; Nazeef, S.; Daanmaigoro, A. Prevalence of intestinal parasites of dogs slaughtered at Mami market area, Sokoto, Nigeria. Sci. J. Anim. Sci. 2012, 1, 126–130. [Google Scholar]
- Adamu, N.; Adamu, J.; Salisu, L. Prevalence of ecto-, endo-and haemoparasites in slaughtered dogsin Maiduguri, Nigeria. Rev. De Médecine Vétérinaire 2012, 163, 178–182. [Google Scholar]
- Chukwu, C.O.; Ogo, N.I.; Jimoh, A.; Chukwu, D.I. Pathogenic bacteria associated with cutaneous canine myiasis due to Cordylobia anthropophaga. Vet. World 2012, 5, 617–620. [Google Scholar] [CrossRef]
- Ogo, N.I.; de Mera, I.G.F.; Galindo, R.C.; Okubanjo, O.O.; Inuwa, H.M.; Agbede, R.I.; Torina, A.; Alongi, A.; Vicente, J.; Gortázar, C.; et al. Molecular identification of tick-borne pathogens in Nigerian ticks. Vet. Parasitol. 2012, 187, 572–577. [Google Scholar] [CrossRef]
- Ogunleye, A.; Omobowale, T.; Okunlade, A.; Ajuwape, A.; Adetosoye, A. Multi-Drug Resistant Bacteria Isolated From Dogs Presented with Otitis Externa in a Veterinary Teaching Hospital in Nigeria. Trop. Vet. 2012, 30, 74–80. [Google Scholar]
- Cadmus, S.; Adesokan, H.; Ajala, O.; Odetokun, W.; Perrett, L.; Stack, J. Seroprevalence of Brucella abortus and B. canis in household dogs in southwestern Nigeria: A preliminary report. J. S. Afr. Vet. Assoc. 2011, 82, 56–57. [Google Scholar] [CrossRef]
- Oluwayelu, D.; Aiki-Raji, C.; Neba, C.; Ahmadu, O. Prevalence of avian origin H5 and H7 influenza virus antibodies in dogs in Ibadan and Sagamu, Southwestern Nigeria. Afr. J. Biomed. Res. 2011, 14, 23–26. [Google Scholar]
- Ugwoke, E.; Audu, P.; Umoh, J.; Adakole, J. Prevalence of intestinal helminthes of dogs that have been disposed off at non-descript abattoirs in Zaria, Nigeria. Bayero J. Pure Appl. Sci. 2011, 4, 44–47. [Google Scholar]
- Kamani, J.; Weka, P.; Gbise, S. Parasitic cause of anaemia in dogs in Vom, Nigeria. Int. J. Agro Vet. Med. Sci. 2011, 5, 283–289. [Google Scholar]
- Nweze, E.I. Dermatophytoses in domesticated animals. Rev. Do Inst. De Med. Trop. De São Paulo 2011, 53, 94–99. [Google Scholar] [CrossRef]
- Olugasa, B.O.; Aiyedun, J.O.; Emikpe, B.O. Prevalence of antibody against rabies among confined, free-roaming and stray dogs in a transit city of Nigeria. Vet Ital 2011, 47, 453–460. [Google Scholar]
- Okoye, I.C.; Obiezue, N.R.; Okorie, C.E.; Ofoezie, I.E. Epidemiology of intestinal helminth parasites in stray dogs from markets in south-eastern Nigeria. J. Helminthol. 2011, 85, 415–420. [Google Scholar] [CrossRef]
- Ekanem, M.; Mbagwu, H.; Opara, K.; Agbata, Q. Ticks infestation of domestic dogs (Canis familiaris lupus) in Uyo, Akwa Ibom State, Nigeria. World J. Appl. Sci. Technol. 2010, 2, 191–196. [Google Scholar]
- Kamani, J.; Sannusi, A.; Dogo, A.G.; Tanko, J.T.; Egwu, K.O.; Tafarki, A.E.; Ogo, I.N.; Kemza, S.; Onovoh, E.; Shamaki, D.; et al. Babesia canis and Babesia rossi co-infection in an untraveled Nigerian dog. Vet. Parasitol. 2010, 173, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Omudu, E.; Okpe, G.; Adelusi, S. Studies on dog population in Makurdi, Nigeria (II): A survey of ectoparasite infestation and its public health implications. J. Res. For. Wildl. Environ. 2010, 2, 94–106. [Google Scholar]
- Nwoha, R.; Ekwuruike, J. Prevalence of gastrointestinal parasites in dogs from Umuahia City of Abia State. Glob. J. Med. Sci. 2010, 9, 34–42. [Google Scholar]
- Amuta, E.; Houmsou, R.; Ogabiela, M. Tick infestation of dogs in Makurdi metropolis, Benue State-Nigeria. Internet J. Vet. Med. 2010, 7, 1–7. [Google Scholar]
- Dzikwi, A.; Umoh, J.; Kwaga, J.; Ahmed, A. Serological surveillance for non-rabies lyssaviruses among apparently healthy dogs in Zaria, Nigeria. Niger. Vet. J. 2010, 31, 214–218. [Google Scholar] [CrossRef]
- Adejinmi, J.; Osayomi, J. Prevalence of intestinal protozoan parasites of dogs in Ibadan, south western Nigeria. J. Anim. Plant Sci. 2010, 7, 783–785. [Google Scholar]
- Sowemimo, O.A. The prevalence and intensity of gastrointestinal parasites of dogs in Ile-Ife, Nigeria. J. Helminthol. 2009, 83, 27–31. [Google Scholar] [CrossRef]
- Ojo, O.E.; Adetosoye, A.I. Salmonella Typhimurium infection in diarrhoeic and non-diarrhoiec dogs in Ibadan, Nigeria. Vet. Arh. 2009, 79, 371–377. [Google Scholar]
- Umar, Y. Intestinal helminthoses in dogs in Kaduna metropolis, Kaduna state, Nigeria. Iran. J. Parasitol. 2009, 4, 34–39. [Google Scholar]
- Ezeh, I.; Agbo, L.; Emehelu, C.; Nweze, E.; Ezeokonkwo, R.; Onah, D. Berenil-Resistant Trypanosoma brucei brucei infection in a dog in Nsukka area of Enugu state, Nigeria. NVJ 2009, 29, 34–42. [Google Scholar]
- Adeniyi Okewole, E.; Oluremi Ayoola, M. Seroprevalence of leptospiral serovars other than Canicola and Icterohaemorrhagiae in dogs in the Southwestern Nigeria. Vet. Arh. 2009, 79, 87–96. [Google Scholar]
- Agbolade, O.M.; Soetan, E.; Awesu, A.; Ojo, J.A.; Somoye, O.; Raufu, S. Ectoparasites of domestic dogs in some Ijebu communities, Southwest Nigeria. World Appl. Sci. J. 2008, 3, 916–920. [Google Scholar]
- Sowemimo, O.; Asaolu, S. Survey of intestinal helminth parasites of puppies in Ile-Ife, Nigeria. Ife J. Sci. 2008, 10, 67–81. [Google Scholar]
- Ugbomoiko, U.S.; Ariza, L.; Heukelbach, J. Parasites of importance for human health in Nigerian dogs: High prevalence and limited knowledge of pet owners.(Research article)(Report). BMC Vet. Res. 2008, 4, 49. [Google Scholar] [CrossRef]
- Uaboi-Egbenni, P.; Okolie, P.; Adesanya, O.; Omonigbehin, E.; Sobande, A. Epidemiological studies of the incidence of pathogenic Campylobacter spp. amongst animals in Lagos metropolis. Afr. J. Biotechnol. 2008, 7, 2852–2956. [Google Scholar]
- Ofukwu, R.; Akwuobu, C.; Oboegbulem, S. Presence and Isolation Pattern of Zoonotic Bacteria in Oral of Cavities of Dogs in Peri-Urban Areas of Makurdi, Nigeria. J. Appl. Biosci. 2008, 11, 602–606. [Google Scholar]
- Ikpeze, O.; Eneanya, C.; Nwokedi, O. Environmental surveillance of canine babesiosis as an early alert system on emerging human babesiosis. J. Adv. Med. Pharm. Sci. 2007, 1, 19–23. [Google Scholar]
- Omudu, E.A.; Atu, B.O.; Ayashar, J. Epidemiological survey of canine babesiosis in Makurdi, Nigeria. Anim. Res. Int. 2007, 4, 745–749. [Google Scholar] [CrossRef]
- Sowemimo, O.A. Prevalence and intensity of Toxocara canis (Werner, 1782) in dogs and its potential public health significance in Ile-Ife, Nigeria. J. Helminthol. 2007, 81, 433–438. [Google Scholar] [CrossRef]
- Shittu, O.; Nwagboniwe, C.; George, O. Antibiotic Resistance patterns of Escherichia Coli isolates from human, pet, livestock and poultry living in close contact. ASSET Ser. B 2007, 6, 164–170. [Google Scholar]
- Okewole, E.A. Seroprevalence of antibodies to Toxoplasma gondii in some food and companion animals in the Southwestern Nigeria. Folia Vet. 2007, 51, 113–117. [Google Scholar]
- Omudu, E.; Amuta, E. Parasitology and urban livestock farming in Nigeria: Prevalence of ova in faecal and soil samples and animal ectoparasites in Makurdi. J. S. Afr. Vet. Assoc. 2007, 78, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Awoniyi, T.; Adebayo, I.; Osho, L. Public Health Implication of Man and Livestock Cohabitation: Cordylobia Infestation in SW Nigeria: A Case Study. Int. J. Trop. Med. 2006, 1, 178–181. [Google Scholar]
- Nottidge, H.O.; Omobowale, T.; Oladiran, O. Mokola. Virus antibodies in humans, dogs, cats, cattle, sheep, and goats in Nigeria. Int. J. Appl. Res. Vet. Med. 2007, 5, 105. [Google Scholar]
- Ajuwape, A.T.P.; Oyebanji, M.O.; Adetosoye, A.I. Bacteriological examination of normal upper respiratory tract of puppies with particular reference to staphylococci. Vet. Arh. 2006, 76, 179–184. [Google Scholar]
- Ibidapo, C.A. Prevalence of intestinal helminth parasites of dogs in Lagos, Nigeria. Pak. J. Sci. Ind. Res. 2005, 48, 279. [Google Scholar]
- Osinubi, M.; Ajogi, I.; Ehizibol, O. Brucella abortus agglutinins in dogs in Zaria, Nigeria. Niger. Vet. J. 2004, 25, 35–38. [Google Scholar] [CrossRef]
- Anosike, J.; Nwoke, B.; Ukaga, C.; Madu, N.; Dozie, I. Aspects of intestinal helminth parasites of dogs in World Bank-assisted housing estate, New Oweri, Nigeria. Afr. J. Appl. Zool. Environ. Biol. 2004, 6, 26–30. [Google Scholar]
- Efuntoye, M.O.; Fashanu, S.O. Fungi isolated from skins and pens of healthy animals in Nigeria. Mycopathologia 2002, 153, 21–23. [Google Scholar] [CrossRef]


| Main Search Terms | Synonyms/Related Terms |
|---|---|
| Dog | Canine, puppy, Canis familiaris |
| Nigeria | Nigerian, West Africa |
| Man | Human/humans |
| Pathogen | Parasites, Viruses, Bacteria, microorganisms, gastro-intestinal parasites, |
| * Zoonoses | Zoono *, zoonotic viral disease, zoonotic bacterial disease, zoonotic parasitic disease, zoonotic viral infection, zoonotic bacterial infection, zoonotic parasitic infection, Brucella, Campylobacter, Cryptosporidium, Echinococcus, Giardia, Hookworm, Leptospira, Rabies, Rotavirus, Roundworm, Ringworm, Salmonella, Scabies, Methicillin resistant Staphoccus aureus, Tapeworm, Tuberculosis |
| Considerations | Title and Abstract | Full Text | ||
|---|---|---|---|---|
| Include If | Exclude If | Include If | Exclude If | |
| Study population | Study includes dogs | Species aside from dogs | Study includes dogs | Species aside from dogs |
| Geographical location | Data from Nigeria | Data from countries aside from Nigeria | Data from Nigeria | Data from countries aside from Nigeria |
| Pathogen | Zoonotic pathogens of dogs transmitted via vectors, fomites or aerosol | Potentially zoonotic Commensal | Zoonotic pathogens of dogs transmitted via vectors, fomites or aerosol | Potentially zoonotic Commensal |
| Date | Published between 2000 and March 2020 | Published earlier than 2000 | Published between 2000 and March 2020 | Published earlier than 2000 |
| Article type | Peer-reviewed journal article | Thesis, Review article. Textbooks, Newspaper articles, Conference proceeding | Peer-reviewed journal article, Full text available | Thesis, Review article. Textbooks, Newspaper articles, Conference proceedings, Only abstract was published, |
| Study type | Cross-sectional studies, case studies, retrospective studies | Clinical trial studies, studies without laboratory confirmation, physiological studies | Cross-sectional studies, case studies, retrospective studies | Clinical trial studies, studies without laboratory confirmation, physiological studies, molecular techniques development studies, phylogenetic studies with no epidemiological data |
| Language | Written in English | Not written in English | Full text available in English | Full text not available in English |
| Good Quality | Medium Quality | Poor Quality |
|---|---|---|
| The selection of subjects was unbiased | Biased selection of subjects is acknowledged where unavoidable and fully accounted for | Biased selection of subjects not acknowledged or accounted for |
| Appropriate data analysis | Limitations in data analysis are acknowledged and accounted for | Use of inappropriate data analysis |
| Use of validated and scientifically sound methods | Use of scientifically sound methods, even though may not be the most appropriate | Use of inappropriate methods |
| Detailed and accurately described methods | Comprehensible methods and valid results | Unclear or incomplete methods |
| Reported results are accurate and complete | Accurately reported results | Incomplete or inaccurate results |
| Infectious Agent | Host |
|---|---|
| Parasites: Ancyclostoma spp., Cordylobia anthropophaga, Dipylidium caninum, Dirofilaria immitis, Echinococcus granulosus, Ehrlichia canis, Filarioid worms, Leishmania spp., Linguatula serrata, Rickettsia spp., Scabies, Toxocara canis, Toxoplasma gondii, Trichuris spp., and Trypanosoma spp. | Dog |
| Bacteria: Brucella spp., Campylobacter spp., Clostidium spp., Cryptosporidium spp., Enterobacteria, Salmonella spp., Leptospira spp., and Staphylococcus spp. | |
| Fungi: Candida spp., Microsporum spp., and Trychophyton spp. | |
| Virus: Influenza virus, Mokola virus, and Rabies virus. | |
| Anasplasma spp., Ehrlichia spp. and Rickettsa spp. | Ticks (from dog host) |
| Ancyclostoma spp., Ascaris lumbricoides, Cordylobia anthropophaga, Cryptosporidium spp., and Toxocara canis. | Human (infection with dog strain of pathogen/evidence of transmission from dog) |
| Parasite | Number of Studies Reported |
|---|---|
| Alaria spp. | 1 |
| Ascaris lumbricoides | 2 |
| Baylisascaris procyonis | 1 |
| Coccidia spp. | 2 |
| Diphylidium caninum | 1 |
| Diphylobothrium latum | 4 |
| Entamoeba histolytica | 1 |
| Fasciola spp. | 1 |
| Filaroides osleri | 1 |
| Giardia spp. | 2 |
| Gongylonema pulchrum | 1 |
| Graphidium strigosum | 1 |
| Isospora spp. | 11 |
| Necator americanus | 1 |
| Nanophytus salmincola | 1 |
| Sarcocystis spp. | 2 |
| Spirocerca lupi | 2 |
| Strongyloides spp. | 7 |
| Taenia spp. | 17 |
| Taenidae spp. | 1 |
| Trichostrongylus spp. | 1 |
| Troglotrema salmincolo | 1 |
| Toxoscaris leonina | 4 |
| Uncinaria spp. | 6 |
| Ectoparasites | Species |
|---|---|
| Ticks | Rhipicephalus sanguineus, Rhipicephalus Pulchellus, Rhipicephalus Decoloratus, Rhipicephalus turanicus, Rhipicephalus lunulatus, Rhipicephalus muhsamae, Rhipicephalus senegalensis Haemaphysalis leachii, Haemaphysalis elliptica, Haemaphysalis longicornis, Boophilus spp., Boophilus annulatus, Ixodes scapularis, Ixodes spp., Hyalomma truncatum, Amblyomma variegatum, Amblyomma spp., and Dermacentor andersoni |
| Fleas | Ctenocephalides canis, Ctenocephalides felis, Pulex irritans, and Tunga penetrans |
| Lice | Damalina spp., Heterodoxus spiniger, Linognathus spp. and Trichodectes canis |
| Mites | Demodex canis, Otodectes spp. and Sarcop Sarcoptes scabiei |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gado, D.A.; Ehizibolo, D.O.; Meseko, C.A.; Anderson, N.E.; Lurz, P.W.W. Review of Emerging and Re-Emerging Zoonotic Pathogens of Dogs in Nigeria: Missing Link in One Health Approach. Zoonotic Dis. 2023, 3, 134-161. https://doi.org/10.3390/zoonoticdis3020012
Gado DA, Ehizibolo DO, Meseko CA, Anderson NE, Lurz PWW. Review of Emerging and Re-Emerging Zoonotic Pathogens of Dogs in Nigeria: Missing Link in One Health Approach. Zoonotic Diseases. 2023; 3(2):134-161. https://doi.org/10.3390/zoonoticdis3020012
Chicago/Turabian StyleGado, Dorcas A., David O. Ehizibolo, Clement A. Meseko, Neil E. Anderson, and Peter W. W. Lurz. 2023. "Review of Emerging and Re-Emerging Zoonotic Pathogens of Dogs in Nigeria: Missing Link in One Health Approach" Zoonotic Diseases 3, no. 2: 134-161. https://doi.org/10.3390/zoonoticdis3020012
APA StyleGado, D. A., Ehizibolo, D. O., Meseko, C. A., Anderson, N. E., & Lurz, P. W. W. (2023). Review of Emerging and Re-Emerging Zoonotic Pathogens of Dogs in Nigeria: Missing Link in One Health Approach. Zoonotic Diseases, 3(2), 134-161. https://doi.org/10.3390/zoonoticdis3020012