Simple Summary
Lyme disease affects about half a million Americans every year, and cases in Canada are rising. However, the number of studies focusing on the epidemiology of Lyme disease in western North America has been relatively low compared to that of eastern North America. Here, we aim to summarize the current state of knowledge regarding Lyme disease epidemiology in western North America, which includes current surveillance efforts tracking Lyme disease cases, modelling studies clarifying the geographic distributions of vectors for Borrelia burgdorferi, and the dynamics required to maintain and transmit B. burgdorferi in the natural environment. In providing a comprehensive picture of the state of Lyme disease in western North America, this review may be particularly helpful for future studies in the region.
Abstract
Lyme disease is the most common vector-borne disease in the United States and Canada. The causative agent of Lyme disease in North America is the spirochete Borrelia burgdorferi. In western North America, the primary vector of Borrelia burgdorferi is the western black-legged tick, Ixodes pacificus. Surveillance and modelling efforts indicate that I. pacificus is primarily found in coastal California, Oregon, Washington and the southern coastal regions of British Columbia However, infection rates with B. burgdorferi among I. pacificus ticks remain low, ranging from 0.6% to 9.9%. Lyme disease case numbers in western North America are also relatively low compared to eastern North America. Enzootic maintenance of B. burgdorferi by hosts in natural environments and climatic factors may influence Lyme disease risk. The borreliacidal western fence lizard, Sceloporus occidentalis, may contribute to the low infection rates observed in I. pacificus ticks, while the migratory nature of avian hosts can allow for long-distance tick dispersal. Moderately warm and moist environments and protection from sunlight define the suitable habitats of I. pacificus ticks. In this review, we discuss the ecology and epidemiology of Lyme disease in relation to I. pacificus, as well as the need for more studies in western North America.
1. Introduction
Lyme disease is the most common vector-borne disease in the United States, Canada, and Europe [1,2,3]. Every year, nearly half a million Americans are treated and diagnosed with Lyme disease [3,4]. Reported cases in Canada increased from 144 in 2009 to 2634 in 2019 [5]. Lyme disease in humans is caused by bites of ticks infected with bacteria from the Borrelia burgdorferi sensu lato (s. l.) complex and is characterized by fever, headaches, fatigue, and a localized erythema migrans rash [6]. Despite being rarely lethal [7], Lyme disease can manifest into a disseminated illness that can lead to long-term complications associated with autoimmune responses, such as nerve and joint pain, facial palsy, and heart palpitations, if left untreated [6].
The spirochete B. burgdorferi Johnson et al. 1984 sensu stricto (s. s.), a member of the B. burgdorferi s. l. complex is the primary etiologic agent of Lyme disease in North America [8]. Certain tick species function as vectors that can transmit these spirochetes to humans. In the eastern and mid-western United States and mid-southern and southeastern Canada, Ixodes scapularis Say is the primary vector of B. burgdorferi s.s. [9,10]. In western United States and southwestern Canada, I. pacificus Cooley and Kohls is the primary vector [9,10]. Ixodes pacificus has undergone several reclassifications, initially classified as I. californicus in 1908, revised to I. ricinus var californicus in 1911 [11,12,13], then determined as a distinct species in 1943 [11,12,13].
Ticks require blood meals in the larval and nymphal stages to molt to the next stage and in the adult stage to develop eggs. Each of the feeding life stages generally prefers a different set of vertebrate hosts (Figure 1). The life cycle of I. pacificus ticks, at least in California, takes three years (Figure 1), while the life cycle of I. scapularis usually takes 2 years in the United States [14] and 3–4 years in Canada [15].
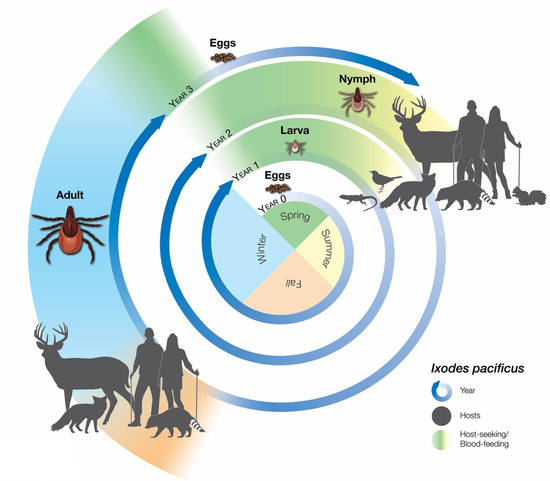
Figure 1.
Life cycle of I. pacificus and the preferred hosts for each developmental life stage. Figure was retrieved from the United States Centers for Disease Control and Prevention [16].
Reported Lyme disease cases have steadily increased in the United States and Canada, but a majority of these cases are concentrated in the Northeast, mid-Atlantic, andupper Midwest of the United States [4,17,18] and Ontario, Quebec, and Nova Scotia in Canada [18]. In contrast, the western provinces of British Columbia, Alberta, and Saskatchewan have reported low numbers of Lyme disease and a higher proportion of travel-related cases acquired outside of the provinces [18].
The main goals of this review are to provide a brief overview of the ecology of I. pacificus and associated transmission dynamics of B. burgdorferi in western North America, to describe the environmental factors that influence the spread and distribution of I. pacificus, and to summarize previous efforts to predict the future distribution of I. pacificus and B. burgdorferi under different climate scenarios. Unless otherwise specified, subsequent mentions of B. burgdorferi refer to B. burgdorferi s.l.
2. Surveillance Efforts to Track Risk of Exposure to Ixodes pacificus
Tick surveillance is essential for assessing Lyme disease risk since ticks are the principal vectors for transmission. Tick surveillance methods can be either active, passive, or a combination of both. Active tick surveillance generally refers to the systematic collection of ticks either by use of carbon dioxide tick traps, direct removal from hosts captured from rodent traps, dragging a large, white cloth behind the investigator, or flagging, which involves waving a large, white cloth over vegetation ahead of the investigator [19]. Locations for active surveillance of ticks with relevance to Lyme disease are typically chosen based on the likelihood of human-tick contact, suitable habitats for ticks, and pre-existing surveillance data. Passive tick surveillance involves the submission of ticks from humans and vertebrate hosts (pets, livestock) by veterinary or medical professionals or members of the public to researchers or government agencies for tick identification or pathogen detection [19]. While useful in estimating the geographic distribution of tick vectors for B. burgdorferi, tick presence data does not indicate the importance of any given tick species in the transmission of Lyme disease. In addition to tick presence data, tick abundance and efficiency in acquiring and transmitting B. burgdorferi should also be considered.
Active tick surveillance done by public health agencies from California [20], Oregon [21], Washington [22], and British Columbia [23,24] indicates that I. pacificus is the most important Ixodes species for the transmission of Lyme disease in the western United States and Canada. From these active surveillance efforts, the current geographic range of I. pacificus is located along most of California, western Oregon, and Washington [21,22,25,26,27], and the southern coastal areas of British Columbia [26,28,29]. Ixodes pacificus adults comprise a majority of ticks obtained from many widescale surveillance studies using passive surveillance for Lyme disease vectors, but I. pacificus immatures are commonly reported from active surveillance efforts by ecological studies confined to smaller study areas [30,31,32,33,34]. This may be in part due to the focus on rodent hosts in such ecological studies.
Passive tick surveillance indicates that I. pacificus ticks account for a majority of human-biting tick submissions in western North America [24,33,34,35]. Most I. pacificus submissions originate from western California, Oregon, Washington, and the southern coastal regions of British Columbia [36,37]. The majority of I. pacificus ticks submitted were found on humans [33,34,36], although there are many cases where ticks are found on domesticated animals [23,33]. Adults are more frequently submitted to passive surveillance programs than immatures, despite peak adult host-seeking activity being greater in the winter or early spring, a season when humans are less likely to participate in outdoor activities [33,34]. The smaller sizes of I. pacificus immatures compared to adults make it less likely for humans to detect and subsequently report them [38]. Additionally, ascending vegetation to seek hosts is commonly done by I. pacificus adults but not nymphs [39]. Humans participating in outdoor recreational activities are likely to contact vegetation and, in turn, expose themselves to questing ticks.
The seasonal abundance of I. pacificus varies in western North America. Based on active [27,40,41,42,43,44,45,46,47,48,49,50,51] and passive [22,33,34,52] surveillance efforts in Northern California, Oregon, and Washington, I. pacificus nymphs are most abundant during the spring and early summer, while I. pacificus adults are most abundant during winter to early spring. In Southern California, both adult and nymphal I. pacificus have a shorter period of abundance compared to those in Northern California [50]. In contrast, seasonal abundance data from British Columbia indicate a slightly extended period of abundance, with nymphal and adult I. pacificus being abundant from spring to late summer and winter to late spring, respectively [26,28]. More work is needed to determine why periods of abundance for I. pacificus vary within western North America.
3. Infection Prevalence of Ixodes pacificus with Borrelia burgdorferi
Despite being the primary vector of B. burgdorferi in western North America, relatively lower proportions of I. pacificus ticks are infected with B. burgdorferi compared to I. scapularis in eastern North America. A common measure of Lyme disease risk is tick infection prevalence, which is the proportion of infected ticks among those collected in a study. Both active and passive surveillance have observed low infection prevalence in I. pacificus adults, ranging from 0.6 to 7.3% [21,22,33,52,53,54,55,56,57,58]. Nymphal infection prevalence is similarly low, although slightly higher than in adults, ranging from 1.4 to 9.9% [31,33,40,53,55,56,58,59,60,61]. These rates are considerably lower when compared with the infection prevalence for I. scapularis nymphs and adults, which can be as high as 23% [62] and 47% [63], respectively.
Studies of infection prevalence for B. burgdorferi in I. pacificus ticks have been conducted mainly on the west coast of North America, but interior western states and provinces have observed exceedingly low rates of infection. In Alberta, there are no established populations of I. pacificus, and numbers of I. pacificus ticks collected from the field and public submissions are concurrently low, with none reporting to carry B. burgdorferi [64,65]. In Utah, I. pacificus was the most common Ixodes tick found during a state-wide field survey from 2011 to 2013, but all were adults, and none carried B. burgdorferi [66]. Hence, the low infection prevalence of B. burgdorferi among I. pacificus ticks suggest an overall low risk of Lyme disease infection for humans and pets in western North America.
While the overall infection prevalence of B. burgdorferi is quite low for I. pacificus, infection prevalence can vary greatly between sites sampled in a study, and that conclusions taken from one site may not necessarily apply to other sites at different spatial scales. In Washington State, for example, Clallam County has seen elevated rates of B. burgdorferi infection among I. pacificus ticks compared to other counties, likely due to the rarity of the only incompetent host for B. burgdorferi, the northern alligator lizard, Elgaria coerulea (Weigmann), in the sites sampled within the county [22]. The lower infection prevalence of ticks in other counties may be due to the presence of the northern alligator lizard, along with other zooprophylactic lizards present in Washington State, such as the southern alligator lizard, E. multicarinata (Blainville), and the western fence lizard, Sceloporus occidentalis Baird and Girard [22].
Additionally, any interpretations and conclusions regarding tick infection prevalence should not rely on tick infection prevalence alone. For instance, Alberta and Saskatchewan have a higher tick infection prevalence than British Columbia [67], but this does not necessarily mean that Lyme disease risk is higher in those provinces. Historically, Alberta and Saskatchewan have received considerably fewer Ixodes spp. Ticks and most Ixodes ticks submitted are I. scapularis ticks that might have originated outside the provinces [67]. Therefore, a complete picture of Lyme disease risk should also consider endemic tick species composition, tick abundance, tick host availability, and where the ticks submitted to passive surveillance programs originated.
4. Surveillance Efforts Tracking Lyme Disease Cases
The number of reported human Lyme disease cases in western states and provinces is considerably lower than in eastern North America [68,69], which is consistent with the low infection prevalence observed in western North America. Lyme disease case numbers and seropositivity for canines in the west are also lower than in the east [70,71]. The incidence rate, which measures the number of people afflicted with Lyme disease per 100,000 of the population, ranged from 0.1 to 0.9 for California, Oregon, and Washington in 2020, a stark contrast from rates in the northeastern United States, which reached as high as 83.5 in the same year [68]. A similar pattern between the west and east is observed in Canada, where incidence rates ranged from 0.1 to 0.3 for provinces such as British Columbia, Alberta, and Saskatchewan, while eastern provinces have observed incidence rates ranging from 3.8 to 85.6 [5]. The low incidence rates observed for the western United States and British Columbia are unsurprising given the low infection prevalence with B. burgdorferi for I. pacificus. In California, peaks of Lyme disease cases in humans typically occur from May to July, indicating that transmission risk comes primarily from nymphal ticks, which are most active from April to June [72]. Lyme disease reports have also illuminated some behavioral factors and characteristics in humans that might account for increased exposure to I. pacificus and, in turn, Lyme disease. Generally, tick exposure and Lyme disease cases are associated with greater time spent outdoors in natural areas and pet ownership [34,52,73].
5. Mammalian and Reptilian Hosts and Their Roles in Lyme Disease Maintenance and Transmission
Lyme disease risk to humans does not only depend on the chances of encountering infected I. pacificus ticks but also on the effectiveness in which the Lyme disease spirochete itself is maintained and transmitted in nature. I. pacificus ticks must obtain the bacterial pathogen from feeding on infected hosts in their local environment. I. pacificus ticks can feed on a wide variety of animals, a characteristic that is paramount to the species’ survival during each developmental life stage. Each life stage prefers different sets of hosts. Immature life stages of I. pacificus heavily prefer the western fence lizard, the southern alligator lizard, and the northern alligator lizard [26,28,29,47,59,74,75,76,77]. I. pacificus larvae and nymphs are also the predominant life stages found in avian hosts [48,76,78]. Small rodents such as the deer mouse, Peromyscus maniculatus (Wagner), the Pinyon mouse, P. truei (Shufeldt), the brush mouse, P. boylii (Baird), and the dusky-footed woodrat, Neotoma fuscipes Baird are also hosts that are heavily preferred by I. pacificus larvae [28,79]. I. pacificus adults seem to prefer larger mammals such as the black-tailed deer, Odocoileus hemionus columbianus (Richardson) [80].
Hosts that harbor heavy loads of B. burgdorferi can serve as a source of infection for feeding I. pacificus ticks and are termed reservoir hosts, while organisms that cannot carry the pathogen are considered incompetent. Furthermore, the importance of a host’s role in the maintenance of B. burgdorferi in the environment is determined by their ability to successfully carry and spread the pathogen to feeding ticks. Table 1 provides a list of a few hosts that may play key roles in the maintenance of B. burgdorferi in western North America based on previously published studies.

Table 1.
Key and potential hosts of I. pacificus and their efficiency as reservoirs for B. burgdorferi.
Table 1 is not an exhaustive list of hosts, but it indicates key components that can explain both the maintenance of the Lyme disease spirochete in local environments and the lower numbers of Lyme disease cases in western North America compared to the east. Host characteristics relevant to the transmission of the Lyme disease spirochete, such as tick infestations, host infection prevalence, and tick infection prevalence, have been laid out in the table above. The effects of such characteristics on the prevalence of Lyme disease may be best explained through two key actors in the enzootic maintenance and transmission of Lyme disease spirochete: the western fence lizard and the western gray squirrel, Sciurus griseus Ord.
Ixodes pacificus larvae and nymphs prefer to feed on the western fence lizard rather than rodents, but this reptile species is an incompetent host for B. burgdorferi s.s. [47,74,75,76,77,96]. Spirochetal infection rates among ticks that have fed on these lizards are extremely low and attempts to isolate the Lyme disease spirochete have been unsuccessful [47,74,97]. Laboratory studies are underway to determine why western fence lizards are incompetent for the Lyme disease spirochete, but it has been suggested that the blood in this lizard species has a borreliacidal factor that destroys spirochetes present in I. pacificus ticks [98]. In habitats where western fence lizards are abundant, B. burgdorferi s.s. loads in I. pacificus immatures may be heavily diminished [60], which could naturally lead to a lower chance of humans acquiring Lyme disease from biting I. pacificus ticks. However, it is still unclear how the western fence lizard’s refractory nature can affect B. burgdorferi infection [60,88]; hence more work needs to be done before concluding that this lizard species decreases B. burgdorferi s.s. loads in nature and, in turn, Lyme disease cases in nearby localities.
Conversely, the western gray squirrel seems to be a primary player in the enzootic maintenance and transmission of B. burgdorferi s.s. A high proportion of this species has already been reported to have long-lasting infections with B. burgdorferi s.s. it is in frequent contact with both I. pacificus larvae and nymphs and naturally acquired larval ticks that have fed on this species have been reported to carry B. burgdorferi s.s. [60,84,85,91,94,95]. In areas where the western fence lizard is rare or absent, the western gray squirrel may amplify B. burgdorferi s.s. in local enzootic cycles and subsequently increase Lyme disease risk.
In addition, these findings indicate that I. pacificus nymphs may play an important role in maintaining the enzootic cycle for B. burgdorferi. While I. pacificus nymphs highly prefer incompetent reptilian hosts, they are still capable of transmitting B. burgdorferi to a diverse array of mammalian and avian hosts (Table 1). In contrast, I. pacificus adults highly prefer deer, which are dead-end hosts for B. burgdorferi.
6. Avian Hosts and Their Role in Lyme Disease Transmission and Tick Dispersal
In North America, avian hosts may play only a small role in the maintenance of B. burgdorferi s.s. in local habitats compared to rodents and lizards, given the highly variable rates of infection among avian hosts [58,76,77] and the consistently low rates of infections among I. pacificus ticks, regardless of life stage [48,58,78,79]. However, the migratory nature of certain avian hosts, such as the Pacific wren, Troglodytes pacificus Baird, spotted towhee, Pipilo maculatus Swainson, Swainson’s thrush, Catharus ustulatus (Nuttall), and fox sparrow, Passerella iliaca (Merrem) [99], could allow for long-distance tick dispersal and, in turn, the spread of B. burgdorferi s.s. to areas where Lyme disease is non-endemic [64]. Low numbers of I. pacificus immatures have already been found at coastal and inland sites of far-western Canada, likely due to migratory birds carrying I. pacificus ticks from the western United States [64]. Moreover, birds may also be able to disperse B. burgdorferi s.s. for long distances either by maintaining spirochetal infections for long periods of time or by reactivation of latent infection caused by migration stresses [58]. While avian hosts may be of little importance to the local maintenance and transmission of B. burgdorferi, they can have the capacity to introduce ticks infected with B. burgdorferi to areas where the Lyme disease pathogen is not yet established.
7. The Effects of Community-Level Dynamics on Lyme Disease Maintenance and Transmission
Being able to carry B. burgdorferi s.s. and efficiently transmit the pathogen to feeding ticks is important to the enzootic maintenance of B. burgdorferi s.s., but it is important to keep in mind that local community composition and ecological interactions can also influence the maintenance of B. burgdorferi and risk levels for Lyme disease in western North America [89]. For instance, while deer mice seem to act as dilution hosts reducing B. burgdorferi s.s. prevalence in a given habitat, it may be important in maintaining B. burgdorferi s.s. in other habitats where competition with more efficient hosts is absent [89]. Furthermore, increased predator diversity can reduce rodent movement and lead to less contact with tick vectors, which then reduces pathogen transmission in the environment and subsequently lowers Lyme disease risk [90].
Greater transmission of B. burgdorferi at a community level can also increase the chances of avian hosts acquiring infected I. pacificus ticks [100]. Rodent species richness, local mammal infection prevalence, and tick infection prevalence in a given site can be significant contributors to avian tick burden and infection prevalence [100].
Changes in the host composition of a given environment can also affect transmission dynamics. For instance, the western gray squirrel is a key player in the enzootic maintenance of B. burgdorferi s.s., yet its displacement by eastern gray squirrels, S. carolinensis Gmelin, and eastern fox squirrels, S. niger L., could dampen its contributions to overall Lyme disease risk in western North America [95,101]. Eastern gray squirrels and eastern fox squirrels have exhibited lower infection rates with B. burgdorferi s.s. [95]. Redwood chipmunks, Neotamias ochrogenys (Merriam), may also have some role in the enzootic maintenance of B. burgdorferi s.s. given that this species has been found with greater infestations of nymphal and larval I. pacificus compared to squirrels [102]. Interestingly, other chipmunk species in parts of the eastern United States and Europe contribute to B. burgdorferi s.l. transmission after introduction to the natural environment, hence future studies determining the effectiveness of redwood chipmunks as reservoir hosts for B. burgdorferi s.s. may be of some importance [103,104].
Thus, hosts contributing greatly to the maintenance of B. burgdorferi s.s. should be given great importance when determining possible areas of elevated Lyme disease risk, but the overall ecology and host composition of a given environment should also be considered when evaluating how efficiently B. burgdorferi s.s. is being maintained and transmitted in a given environment.
8. Additional Tick Vectors of the Lyme Disease Spirochete in Western North America
In addition to I. pacificus, other Ixodid ticks in western North America can also function as vectors for B. burgdorferi s.s. Ixodes angustus Neumann and I. spinipalpis Hadwen and Nuttall are competent vectors for B. burgdorferi s.s. [105,106], although these Ixodes species are more associated with other members of the B. burgdorferi s.l. complex than B. burgdorferi s.s. [86,107,108,109]. Ixodes angustus and I. spinipalpis may still participate in the enzootic transmission of B. burgdorferi in some capacity. I. spinipalpis, especially, is more successful in attaching and feeding to completion on certain rodents than I. pacificus [109] and has reported high infection rates of B. burgdorferi s.s. [110]. In the Pacific Northwest, I. angustus and I. spinipalpis ticks feeding on mammalian and avian hosts have been found in comparable numbers and infection rates to I. pacificus [107,111]. However, more laboratory studies are needed to determine the true efficiency of I. spinipalpis and I. angustus as vectors for B. burgdorferi s.s. In addition, both I. spinipalpis and I. angustus are occasional human biters; hence these tick species are of less importance regarding Lyme disease cases in humans [30].
Ixodes auritulus Neumann could also maintain and transmit B. burgdorferi s.s. in nature. Despite only feeding on avian hosts, I. auritulus may be relevant to the long-distance dispersal of the Lyme disease spirochete to nonendemic areas of Lyme disease, given the migratory nature of avian hosts and reports of B. burgdorferi s.s. infections among members of this species [64,99]. Migratory birds tend to be more infested with I. auritulus than I. pacificus, and based on the high infection prevalence observed among attached I. auritulus ticks, these migratory birds may have the capacity to act as reservoirs for B. burgdorferi s.s. [64,99]. Regardless of whether other Ixodes ticks play a larger role in the epidemiology of Lyme disease than I. pacificus, diverse tick communities may be favourable for the overall maintenance and circulation of the Lyme disease spirochete in local habitats. In southern California, infection of B. burgdorferi s.s. was predicted by diversity, specifically by I. spinipalpis and I. peromysci Augustson [112]. If I. pacificus populations are not frequently infected with B. burgdorferi, then the pathogen may be maintained in the landscape by other competent Ixodes species [112]. More robust maintenance systems might allow I. pacificus ticks to be more frequently infected with B. burgdorferi and, given their status as a bridging vector, increase the risk of Lyme disease to nearby human populations.
9. Environmental and Climatic Factors Affecting Ixodes pacificus and Borrelia burgdorferi
For B. burgdorferi to be successfully transmitted from the natural environment to humans, both a robust system of maintenance by hosts in the natural environment and efficient bridging vectors that have the tendency to bite humans are required. Besides the availability of hosts for I.pacificus, tick abundance, tick biting behaviors, and the presence of alternative tick vectors, the enzootic maintenance and transmission of B. burgdorferi can also be affected by climatic factors such as humidity and temperature. Most published studies in western North America have been done in California and focus on the effects of such climatic factors on the primary tick vector, I. pacificus, to gauge how the maintenance and transmission of B. burgdorferi will be affected by changes in the weather and climate.
In general, environments that are moderately warm and moist support greater numbers of I. pacificus ticks in the west [40,51,113]. Specifically, moderately warm temperatures can activate host-seeking activities and accelerate the development of all three life stages of I. pacificus, leading to an increase in overall numbers for the tick [51,114]. Greater humidity has also been shown to prolong the survival of larval and nymphal I. pacificus [50,115]. The greater numbers of I. pacificus ticks in moderately warm and moist environments typically coincide with a higher infection prevalence for B. burgdorferi [112,113,116]; hence Lyme disease risk may be greater in such areas.
A good example illustrating the effects of humidity and temperature on I. pacificus ticks and B. burgdorferi is the stark contrast between northern and southern California in terms of Lyme disease cases, tick abundance, and infection prevalence among ticks. Infection prevalence among ticks is greater in the environments of northern and coastal California than in the hotter and drier regions of southern California [53,54,117]. This corresponds with the lower Lyme disease cases observed in southern California than in northern California [43]. The relatively unfavourable environment in southern California may cause a truncation in the host-seeking period of I. pacificus ticks [50], which, in turn, could make it less likely for I. pacificus ticks to be infected with B. burgdorferi and subsequently cause Lyme disease infections in humans.
The effects of temperature and humidity on I. pacificus and B. burgdorferi transmission can also be seen in the different habitat types present in western North America. Overall tick burdens are generally higher in forested areas, such as dense oak woodlands and woodland-grass than in chaparral habitats, likely due to the former habitats having more surface ground cover and canopy cover, protecting desiccation-sensitive I. pacificus ticks from extremely high temperatures and lower humidity that are especially evident in warmer months [40,47,78]. Furthermore, B. burgdorferi infections among hosts are more frequently observed within woodland and woodland-grass habitats than chaparral or grassland habitats [85,108,116,118,119]. The former habitats may protect ticks from environmental extremes, encouraging host-seeking behaviors and perhaps a more effective system of maintaining B. burgdorferi in the environment.
Within woodland types, it is interesting to note the infection prevalence of B. burgdorferi among I. pacificus nymphs are slightly greater in hardwood-dominated woodlands, such as oak woodlands, that tend to be hotter and drier than conifer-dominated woodlands, such as redwood and pine woodlands [61,85,112,120,121]. This trend is seemingly the opposite of what has been observed in studies looking at broader spatial scales, further stressing the importance of the effect that specific sites can have on tick abundance and infection prevalence.
Coastal habitats may also provide a beneficial environment for host-seeking I. pacificus and B. burgdorferi, given the higher humidity and more stable temperatures in such habitats [86]. With these environmental conditions, coastal habitats tend to have greater tick burdens on hosts, a greater diversity of Ixodes ticks, and a greater prevalence of B. burgdorferi infection among ticks than hotter and drier areas [86], which could perhaps lead to greater Lyme disease risk. Coastal counties in California [122] and Oregon [21] have already reported the majority of locally acquired Lyme disease cases in their respective states. In British Columbia, the risk of Lyme disease is greatest in coastal areas as well [123].
Environmental conditions at a local scale can also have an indirect impact on Lyme disease risk via effects on existing tick and host populations. The presence of leaf litter and tree cover provides protection from sunlight and facilitates a cool and moist environment that is advantageous both to I. pacificus ticks and certain hosts [42,46,48,84,113,124]. For instance, bird species that frequently use leaf litter as substrates are more heavily infested by I. pacificus than bird species that rarely use or do not have access to such substrate [48,84]. Lizards carry heavier tick loads in woodlands than in open grassland and when leaf litter is present [75]. Moss on trees provides a similar effect as it reduces surface temperature and increases humidity on trees [118]. Models also indicate that nymphal densities are greater in areas with soils that have slow infiltration rates, which hold more moisture and provide favourable microclimatic conditions for nymphs [121].
Picnic areas and trails are areas where humans can encounter I. pacificus ticks during recreational activities. Wooden tables, benches, tree trunks, and logs in picnic areas are common sites for questing I. pacificus nymphs [31,40,46,118]. Questing nymphs more frequently occur in trunks and logs than leaf litter [31,40,118]. Resting against wooden materials also poses a greater risk of exposure to I. pacificus ticks than contacting leaf litter [31,46]. This apparent preference may be due to western fence lizards and western gray squirrels, both primary hosts of I. pacificus, displaying key behavioral activities on wooden materials, and these hosts may be depositing attractant chemicals on logs and trunks [40,118]. Also, I. pacificus adults occur more frequently on trails with adjacent vegetation than on sun-exposed trails and open, grassy hillsides [42,49,124]. However, the chances of encountering host-seeking I. pacificus ticks along trails are still low as visitors most often walk along the centre of such trails away from the vegetation present along trail borders [124]. While picnic areas and certain trails provide a low-to-moderate risk of acquiring host-seeking I. pacificus ticks, there is an even lower risk of acquiring Lyme disease when considering the low infection prevalence of B. burgdorferi among I. pacificus ticks [31,46,124,125].
10. Modelling the Geographic Distributions of Ixodes pacificus and Borrelia burgdorferi
Remote sensing, geographic information systems (GIS), and modelling approaches can be useful in delineating the interactions between wildlife, livestock, and humans in the context of disease transmission [126,127,128,129,130]. A common way to apply knowledge of competent tick vectors and reservoir hosts for B. burgdorferi to Lyme disease surveillance efforts is to develop species distribution models that determine the geographical distribution of the tick species and pathogen of interest. Species distribution models can evaluate the suitability of different environments for ticks and the pathogens they carry and aid in the creation of habitat suitability maps [131], which can be especially useful in informing public health surveillance programs and educating the public about high- and low-risk areas of exposure.
Known locations of I. pacificus ticks and I. pacificus ticks infected with B. burgdorferi, respectively, are needed to develop species distribution models that accurately predict suitable habitats of I. pacificus and B. burgdorferi [36,121,122,123,132,133,134,135,136]. Species distribution models also need to incorporate biotic and abiotic variables to accurately predict areas of suitable habitat since environmental factors can affect tick survival and, in turn, the survival of ticks carrying pathogens [120,121,123,133,134,135,136,137,138].
Model building can begin once all the necessary inputs have been collected. Notably, most modelling attempts in western North America focus only on the geographic distribution of I. pacificus. A variety of machine-learning techniques, programs, and algorithms have been used to map the geographic range of I. pacificus (Table 2). Given the different modelling methods available, it is important to be aware of the different biases and assumptions that each method has.
Results from modelling studies have illuminated the geographic ranges for I. pacificus and B. burgdorferi across western North America. In British Columbia, I. pacificus ticks were projected to have suitable habitats along the south, central, and north coast and in valley systems in the interior of the province [123], consistent with areas of known Lyme disease risk in the province [139]. In the western United States, the predicted habitat for I. pacificus is quite wide, concentrating on the coastal and northwest areas of California, western Oregon, and western Washington [136]. In California, particularly, suitable habitat for I. pacificus ticks focuses on areas with moderate amounts of cold-season precipitation and cold-season temperatures greater than 0C [134,140]. These areas include coastal regions in northwest California and along the Sierra Nevada foothills, which are also the same regions where Lyme disease cases are observed [122,134]. In Alaska, the current suitable habitat for I. pacificus ticks is in the southeast region of the state, in valleys around Anchorage, lowlands on the Kenai Peninsula, and the islands north of Kodiak [138].


Table 2.
Species distribution modelling methods used to map the geographic distributions of I. pacificus and B. burgdorferi in western North America. Adapted from Pearson 2010 [141].
Table 2.
Species distribution modelling methods used to map the geographic distributions of I. pacificus and B. burgdorferi in western North America. Adapted from Pearson 2010 [141].
| Species Distribution Modelling Method | References for Method | Species Dataset Required | Sample Studies |
|---|---|---|---|
| Maximum Entropy (MaxEnt) | Phillips, S.J., R.P. Anderson, and R.E. Schapire (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190, pp. 231–259. | Presence | [111,112,122,131,133,134,137] |
| Boosted Regression Trees (BRTs) | Elith, J., C. Graham, and the NCEAS species distribution modeling group. (2006). Novel methods improve prediction of species’ distributions from occurrence data. Ecography, 29(2), pp. 129–151. Elith, J. and Leathwick, J.R. (2007). Predicting species’ distributions from museum and herbarium records using multiresponse models fitted with multivariate adaptive regression splines. Diversity and Distributions, 13, pp. 165–175. Leathwick, J.R., Elith, J., and Hastie, T. (2006). Comparative performance of generalized additive models and multivariate adaptive regression splines for statistical modelling of species distributions. Ecological Modelling, 199(2), pp. 188–196. Lehman, A., Overton, J.M., and Leathwick, J.R. (2002). GRASP: generalized regression analysis and spatial prediction. Ecological Modelling, 157, pp. 189–207. | Presence or Presence/Pseudo-absence | [111,112,133,134,137] |
| Multivariate Adaptive Regression Splines (MARS) | Presence/Absence or Pseudo-absence | [111,112,133,134,137] | |
| Generalized Linear Models (GLMs) | Presence/Absence or Pseudo-absence | [111,112,133,134,137] | |
| Genetic Algorithm for Rule-Set Prediction (GARP) | Stockwell, D.R.B., and D.P. Peters. 1999. The GARP modelling system: Problems and solutions to automated spatial prediction. International Journal of Geographical Information Systems, 13, pp. 143–158. | Presence | [109] |
| Random Forest (RF) | Breiman, L. (2001). Random forests. Machine Learning, 45, pp. 5–32. | Presence/Absence or Pseudo-absence | [111,112,133,134,137] |
Only a few published studies from British Columbia have attempted to map the suitable habitats of I. pacificus ticks carrying B. burgdorferi, with said habitats concentrating along the Vancouver Island coast, southwest coast of the mainland, and in certain valley systems in interior British Columbia [123].
Climate projections and forecasted land use and ecoregions are typically needed to account for future environmental conditions [133] to project future geographic ranges of I. pacificus and B. burgdorferi. Normally, Global Circulation Models (GCMs) and Representative Concentration Pathways (RCPs) are used to determine future suitable habitats. GCMs are numerical models that represent the physical processes in the atmosphere, ocean, cryosphere, and land surface, and they are used to simulate the response of the global climate system to increasing greenhouse gas concentrations [142]. RCPs represent different projections for greenhouse gas concentrations extending up to 2100 [143]. Multiple GCMs and RCPs are used when considering the uncertainty in future bioclimatic predictions and variation in different climate change projections [133,138,140].
Future predictions have mostly focused on the suitable habitat of I. pacificus and are mostly made for California, where forecasts have been conflicting [136,140]. This discrepancy is likely due to a combination of factors, including the use of ensemble modelling versus a single modelling approach, differing tick presence points, data resolution, and geographical ranges used for the study area [133,140]. Variable predictions can be seen between different climate change scenarios as well. In general, more severe RCP scenarios project greater geographical distribution of I. pacificus compared to milder RCP scenarios [133,136,140]. The risk of human exposure to I. pacificus is expected to substantially increase regardless of any future scenario [133,138,140] due to the proximity and overlap of suitable habitats with developed and public land, respectively. So far, no study regarding future predictions for I. pacificus distribution has been conducted for Canada. There have also been no published studies on the future distribution of I. pacificus ticks and Lyme disease in western North America.
While the habitat suitability maps developed in recent years have been helpful in determining the current and future geographic distribution of I. pacificus, it is important to remember that most of these findings are based on tick surveillance data that does not discriminate between I. pacificus life stages. In addition, given the low rates of infection with B. burgdorferi, habitat suitability maps for I. pacificus should not be considered Lyme disease risk maps. The development of more I. pacificus distribution maps beyond California and B. burgdorferi distribution maps in western states and provinces is needed to provide a clearer picture of Lyme disease risk in western North America.
11. Conclusions
Despite the relatively low infection prevalence of B. burgdorferi among I. pacificus ticks, extensive work has been conducted to understand the ecology and epidemiology of Lyme disease in western North America. However, additional research on Lyme disease ecology in the region, particularly in areas where Lyme disease is an emerging risk, is needed to supplement the current literature that is focused primarily on California. It is quite possible that the differing abundances of available mammalian and avian hosts, alternative vector species, and the diverse environments present in western North America provide the opportunity for different tick-host-pathogen dynamics across the region. A more comprehensive view of I. pacificus ecology and Lyme disease epidemiology in the west requires intense research in the region that uses active surveillance methods, the creation of long-term tick collections, and increased collaboration between experts from various fields from public health and ecology to geography and climate science.
With issues regarding climate change and increasing CO2 emissions, there is also more work to be done in determining current and future suitable habitats for both I. pacificus and B. burgdorferi. Much of the published research on this front has, again, focused only on California. Hence there is a strong need to develop habitat suitability maps along western North America. More intensive active surveillance efforts, along with the promotion of online platforms for tick reports, such as e-Tick in Canada [36] and TickReport in the United States [144], can provide a better picture of the geographic distribution of I. pacificus and aid in modelling efforts.
While habitat suitability maps for I. pacificus can be of great importance for public health education and surveillance, they should not be used as proxies for Lyme disease risk, as the infection prevalence of B. burgdorferi is still extremely low in I. pacificus compared to I. scapularis. Still, determining the geographic range of I. pacificus can be useful in deciding where public education initiatives and proper protective measures should be employed to mitigate Lyme disease risk. Furthermore, the creation of more habitat suitability maps for I. pacificus and risk maps for B. burgdorferi will be incredibly helpful in gauging Lyme disease risk at larger spatial scales and will contribute to current literature on the ecology and epidemiology of Lyme disease in western North America.
Author Contributions
Conceptualization, C.D., T.J.L., I.C. and S.C.C.; funding acquisition, S.C.C.; investigation, C.D.; project administration and supervision, S.C.C.; writing—original draft, C.D.; writing—reviewing & editing, C.D., T.J.L., I.C. and S.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Alberta Innovates and the Public Health Agency of Canada (Arrangement #1920-HQ-00069).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital signs: Trends in reported vectorborne disease cases—United States and Territories, 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef]
- Lindsay, L.R. Present state of common vector-borne diseases in Canada. Can. Commun. Dis. Rep. 2016, 42, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.R.; Strle, F.; Wormser, G.P. Comparison of Lyme disease in the United States and Europe. Emerg. Infect. Dis. 2021, 27, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Kugeler, K.J.; Schwartz, A.M.; Delorey, M.J.; Mead, P.S.; Hinckley, A.F. Estimating the frequency of Lyme disease diagnoses, United States, 2010–2018. Emerg. Infect. Dis. 2021, 27, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, S.; Koffi, J.; Nelder, M.; Russell, C.; Graham-Derham, S.; Lachance, L.; Adhikari, B.; Badcock, J.; Baidoobonso, S.; Billard, B.; et al. Surveillance for Lyme disease in Canada, 2009–2019. Can. Commun. Dis. Rep. 2022, 48, 219–227. [Google Scholar] [CrossRef]
- Signs and Symptoms of Lyme Disease. Available online: https://www.cdc.gov/lyme/signs_symptoms/index.html (accessed on 25 October 2022).
- Kugeler, K.J.; Griffith, K.S.; Gould, L.H.; Kochanek, K.; Delorey, M.J.; Biggerstaff, B.J.; Mead, P.S. A review of death certificates listing lyme disease as a cause of death in the United States. Clin. Infect. Dis. 2011, 52, 364–367. [Google Scholar] [CrossRef]
- Johnson, R.C.; Schmid, G.P.; Hyde, F.W.; Steigerwalt, A.G.; Brenner, D.J. Borrelia Burgdorferi sp. nov.: Etiologic agent of Lyme disease. Int. J. Syst. Bacteriol. 1984, 34, 496–497. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef]
- Ogden, N.H.; Lindsay, L.R.; Morshed, M.; Sockett, P.N.; Artsob, H. The emergence of Lyme disease in Canada. Cmaj 2009, 180, 1221–1224. [Google Scholar] [CrossRef]
- Cooley, R.A.; Kohls, G.M. Ixodes californicus Banks, 1904, Ixodes Pacificus n. sp., and Ixodes conepati n. sp. (Acarina: Ixodidae). Pan-Pac. Entomol. 1943, 19, 139–147. [Google Scholar]
- Cooley, R.A.; Kohls, G.M. The genus Ixodes in North America; United States Government Printing Office: Washington, DC, USA, 1945; pp. 21–28. [Google Scholar]
- Lindquist, E.E.; Galloway, T.D.; Artsob, H.; Lindsay, L.R.; Drebot, M.; Wood, H.; Robbins, R.G. A Handbook to the Ticks of Canada (Ixodida: Ixodidae, Argasidae); Biological Survey of Canada: Sackville, NB, Canada, 2016. [Google Scholar]
- Eisen, R.J.; Eisen, L.; Ogden, N.H.; Beard, C.B. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J. Med. Entomol. 2016, 53, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, L.R.; Barker, I.K.; Surgeoner, G.A.; Mcewen, S.A.; Gillespie, T.J.; Addison, E.M. Survival and development of the different life stages of Ixodes scapularis (Acari: Ixodidae) held within four habitats on Long Point, Ontario, Canada. J. Med. Entomol. 1998, 35, 189–199. [Google Scholar] [CrossRef] [PubMed]
- How Ticks Spread Disease. Available online: https://www.cdc.gov/ticks/life_cycle_and_hosts.html#print (accessed on 25 October 2022).
- Schwartz, A.M.; Hinckley, A.F.; Mead, P.S.; Hook, S.A.; Kugeler, K.J. Surveillance for Lyme disease—United States, 2008–2015. MMWR Surveill. Summ. 2017, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, S.; Ogden, N.; Lindsay, L.; Burns, S.; Fleming, S.; Badcock, J.; Hanan, S.; Gaulin, C.; Leblanc, M.; Russell, C.; et al. Surveillance for Lyme disease in Canada: 2009–2015. Can. Commun. Dis. Rep. 2017, 43, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Lyons, L.A.; Brand, M.E.; Gronemeyer, P.; Mateus-Pinilla, N.; Ruiz, M.O.; Stone, C.M.; Tuten, H.C.; Smith, R.L. Comparing contributions of passive and active tick collection methods to determine establishment of ticks of public health concern within Illinois. J. Med. Entomol. 2021, 58, 1849–1864. [Google Scholar] [CrossRef] [PubMed]
- Vector-Borne Disease Section Annual Report 2020. Available online: https://westnile.ca.gov/pdfs/VBDSAnnualReport20.pdf (accessed on 25 October 2022).
- Doggett, J.S.; Kohlhepp, S.; Gresbrink, R.; Metz, P.; Gleaves, C.; Gilbert, D. Lyme disease in Oregon. J. Clin. Microbiol. 2008, 46, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, E.A.; Oltean, H.N.; Kangiser, D.; Marsden-Haug, N.; Rich, S.M.; Xu, G.; Lee, M.-K.; Morshed, M.G.; Graham, C.B.; Eisen, R.J. Ecology and epidemiology of tickborne pathogens, Washington, USA, 2011–2016. Emerg. Infect. Dis. 2020, 26, 648–832. [Google Scholar] [CrossRef]
- Wilson, C.; Gasmi, S.; Bourgeois, A.-C.; Badcock, J.; Chahil, N.; Kulkarni, M.; Lee, M.-K.; Lindsay, R.; Leighton, P.; Morshed, M.; et al. Surveillance for Ixodes scapularis and Ixodes pacificus ticks and their associated pathogens in Canada, 2019. Can. Commun. Dis. Rep. 2022, 48, 208–218. [Google Scholar] [CrossRef]
- Guillot, C.; Badcock, J.; Clow, K.; Cram, J.; Dergousoff, S.; Dibernardo, A.; Evason, M.; Fraser, E.; Galanis, E.; Gasmi, S.; et al. Sentinel surveillance of Lyme disease risk in Canada, 2019: Results from the first year of the Canadian Lyme Sentinel Network (CaLSeN). Can. Commun. Dis. Rep. 2020, 46, 354–361. [Google Scholar] [CrossRef]
- Lyme Disease in California. Available online: https://storymaps.arcgis.com/stories/f64d0c19a3ab42cf90e8ce38397e96e0 (accessed on 25 October 2022).
- Arthur, D.R.; Snow, K.R. Ixodes pacificus Cooley and Kohls, 1943: Its life-history and occurrence. Parasitology 1968, 58, 893–906. [Google Scholar] [CrossRef]
- Easton, E.R.; Keirans, J.E.; Gresbrink, R.A.; Clifford, C.M. The distribution in Oregon of Ixodes pacificus, Dermacentor andersoni, and Dermacentor occidentalis with a note on Dermacentor variabilis (Acarina: Ixodidae). J. Med. Entomol. 1977, 13, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Arnason, C.S. Biology of the Western Black-Legged Tick, Ixodes pacificus (Cooley and Kohls, 1943): A Potential Vector of Lyme Disease in South Coastal British Columbia. Master’s Thesis, Simon Fraser University, Burnaby, BC, Canada, 1992. [Google Scholar]
- Gregson, J.D. A preliminary report of the lizard-tick relationship on the coast of British Columbia. Proc. Entomol. Soc. Br. Columb. 1934, 31, 17–21. [Google Scholar]
- Eisen, L.; Eisen, R.J.; Lane, R.S. Geographical distribution patterns and habitat suitability models for presence of host-seeking Ixodid ticks in dense woodlands of Mendocino County, California. J. Med. Entomol. 2006, 43, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Steinlein, D.B.; Mun, J. Human behaviors elevating exposure to Ixodes pacificus (Acari: Ixodidae) nymphs and their associated bacterial zoonotic agents in a hardwood forest. J. Med. Entomol. 2004, 41, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Swei, A.; Meentemeyer, R.; Briggs, C.J. Influence of abiotic and environmental factors on the density and infection prevalence of Ixodes pacificus (Acari: Ixodidae) with Borrelia burgdorferi. J. Med. Entomol. 2011, 48, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Pearson, P.; Dykstra, E.; Andrews, E.S.; Rich, S.M. Human-biting Ixodes ticks and pathogen prevalence from California, Oregon, and Washington. Vector Borne Zoonotic Dis. 2019, 19, 106–114. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Porter, W.T.; Loh, S.M.; Nieto, N.C. Time of year and outdoor recreation affect human exposure to ticks in California, United States. Ticks Tick Borne Dis. 2019, 10, 1113–1117. [Google Scholar] [CrossRef]
- Bouchard, C.; Dibernardo, A.; Koffi, J.; Wood, H.; Leighton, P.; Lindsay, L. Increased risk of tick-borne diseases with climate and environmental Changes. Can. Commun. Dis. Rep. 2019, 45, 83–89. [Google Scholar] [CrossRef]
- eTick. Available online: https://www.etick.ca/en (accessed on 25 October 2022).
- iNaturalist. Available online: https://www.inaturalist.org/ (accessed on 25 October 2022).
- Transmission of Lyme Disease. Available online: https://www.cdc.gov/lyme/transmission/index.html (accessed on 25 October 2022).
- Surveillance for Ixodes pacificus and Pathogens Found in This Tick Species in the United States. Available online: https://www.cdc.gov/ticks/resources/TickSurveillance_Ipacificus-P.pdf (accessed on 25 October 2022).
- Hacker, G.M.; Jackson, B.T.; Niemela, M.; Andrews, E.S.; Danforth, M.E.; Pakingan, M.J.; Novak, M.G. A comparison of questing substrates and environmental factors that influence nymphal Ixodes pacificus (Acari: Ixodidae) abundance and seasonality in the Sierra Nevada Foothills of California. J. Med. Entomol. 2021, 58, 1880–1890. [Google Scholar] [CrossRef]
- Clover, J.R.; Lane, R.S. Evidence implicating nymphal Ixodes pacificus (Acari: Ixodidae) in the epidemiology of Lyme disease in California. Am. J. Trop. Med. Hyg. 1995, 53, 237–240. [Google Scholar] [CrossRef]
- Kramer, V.L.; Beesley, C. Temporal and spatial distribution of Ixodes pacificus and Dermacentor occidentalis (Acari: Ixodidae) and prevalence of Borrelia burgdorferi in Contra Costa County, California. J. Med. Entomol. 1993, 30, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Fedorova, N.; Kleinjan, J.E.; Maxwell, M. Eco-epidemiological factors contributing to the low risk of human exposure to Ixodid tick-borne borreliae in Southern California, USA. Ticks Tick Borne Dis. 2013, 4, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Mun, J.; Peribáñez, M.A.; Fedorova, N. Differences in prevalence of Borrelia burgdorferi and Anaplasma spp. infection among host-seeking Dermacentor occidentalis, Ixodes pacificus, and Ornithodoros coriaceus ticks in Northwestern California. Ticks Tick Borne Dis. 2010, 1, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Kucera, T.F.; Barrett, R.H.; Mun, J.; Wu, C.; Smith, V.S. Wild turkey (Meleagris gallopavo) as a host of Ixodid ticks, lice, and Lyme disease spirochetes (Borrelia burgdorferi sensu lato) in California state parks. J. Wildl. Dis. 2006, 42, 759–771. [Google Scholar] [CrossRef]
- Padgett, K.A.; Bonilla, D.L. Novel exposure sites for nymphal Ixodes pacificus within picnic areas. Ticks Tick Borne Dis. 2011, 2, 191–195. [Google Scholar] [CrossRef]
- Lane, R.S.; Loye, J.E. Lyme disease in California: Interrelationship of Ixodes pacificus (Acari: Ixodidae), the western fence lizard (Sceloporus occidentalis), and Borrelia burgdorferi. J. Med. Entomol. 1989, 26, 272–278. [Google Scholar] [CrossRef]
- Dingler, R.J.; Wright, S.A.; Donohue, A.M.; Macedo, P.A.; Foley, J.E. Surveillance for Ixodes pacificus and the tick-borne pathogens Anaplasma phagocytophilum and Borrelia burgdorferi in birds from California’s Inner Coast Range. Ticks Tick Borne Dis. 2014, 5, 436–445. [Google Scholar] [CrossRef]
- Billeter, S.A.; Yoshimizu, M.H.; Hu, R. Species composition and temporal distribution of adult Ixodid ticks and prevalence of Borrelia burgdorferi sensu lato and Rickettsia species in Orange County, California. J. Vector Ecol. 2017, 42, 189–192. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Briggs, C.J. Truncated seasonal activity patterns of the western blacklegged tick (Ixodes pacificus) in Central and Southern California. Ticks Tick Borne Dis. 2016, 7, 234–242. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J.; Lane, R.S. Seasonal activity patterns of Ixodes pacificus nymphs in relation to climatic conditions. Med. Vet. Entomol. 2002, 16, 235–244. [Google Scholar] [CrossRef]
- Lane, R.S.; Manweiler, S.A.; Stubbs, H.A.; Lennette, E.T.; Madigan, J.E.; Lavoie, P.E. Risk factors for Lyme disease in a small rural community in Northern California. Am. J. Epidemiol. 1992, 136, 1358–1368. [Google Scholar] [CrossRef]
- Rose, I.; Yoshimizu, M.H.; Bonilla, D.L.; Fedorova, N.; Lane, R.S.; Padgett, K.A. Phylogeography of Borrelia spirochetes in Ixodes pacificus and Ixodes spinipalpis ticks highlights differential acarological risk of tick-borne disease transmission in Northern versus Southern California. PLoS ONE 2019, 14, e0214726. [Google Scholar] [CrossRef] [PubMed]
- Padgett, K.; Bonilla, D.; Kjemtrup, A.; Vilcins, I.-M.; Yoshimizu, M.H.; Hui, L.; Sola, M.; Quintana, M.; Kramer, V. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus. PLoS ONE 2014, 9, e110853. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, N.; Kleinjan, J.E.; James, D.; Hui, L.T.; Peeters, H.; Lane, R.S. Remarkable diversity of tick or mammalian-associated borreliae in the metropolitan San Francisco Bay Area, California. Ticks Tick Borne Dis. 2014, 5, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Salkeld, D.J.; Lagana, D.M.; Wachara, J.; Porter, W.T.; Nieto, N.C. Examining prevalence and diversity of tick-borne pathogens in questing Ixodes pacificus ticks in California. Appl. Environ. Microbiol. 2021, 87, e00319-21. [Google Scholar] [CrossRef] [PubMed]
- Schwan, T.G.; Schrumpf, M.E.; Karstens, R.H.; Clover, J.R.; Wong, J.; Daugherty, M.; Struthers, M.; Rosa, P.A. Distribution and molecular analysis of Lyme disease spirochetes, Borrelia burgdorferi, isolated from ticks throughout California. J. Clin. Microbiol. 1993, 31, 3096–3108. [Google Scholar] [CrossRef]
- Wright, S.A.; Thompson, M.A.; Miller, M.J.; Knerl, K.M.; Elms, S.L.; Karpowicz, J.C.; Young, J.F.; Kramer, V.L. Ecology of Borrelia burgdorferi in ticks (Acari: Ixodidae), rodents, and birds in the Sierra Nevada Foothills, Placer County, California. J. Med. Entomol. 2000, 37, 909–918. [Google Scholar] [CrossRef]
- Wright, S.A.; Lane, R.S.; Clover, J.R. Infestation of the southern alligator lizard (Squamata: Anguidae) by Ixodes pacificus (Acari: Ixodidae) and its susceptibility to Borrelia burgdorferi. J. Med. Entomol. 1998, 35, 1044–1049. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Lane, R.S. Community ecology and disease risk: Lizards, squirrels, and the Lyme disease spirochete in California, USA. Ecology 2010, 91, 293–298. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Girard, Y.A.; Fedorova, N.; Mun, J.; Slikas, B.; Leonhard, S.; Kitron, U.; Lane, R.S. A spatially-explicit model of acarological risk of exposure to Borrelia burgdorferi-infected Ixodes pacificus nymphs in Northwestern California based on woodland type, temperature, and water vapor. Ticks Tick Borne Dis. 2010, 1, 35–43. [Google Scholar] [CrossRef]
- Feldman, K.A.; Connally, N.P.; Hojgaard, A.; Jones, E.H.; White, J.L.; Hinckley, A.F. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J. Vector Ecol. 2015, 40, 198–201. [Google Scholar] [CrossRef]
- Hutchinson, M.L.; Strohecker, M.D.; Simmons, T.W.; Kyle, A.D.; Helwig, M.W. Prevalence rates of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in host-seeking Ixodes scapularis (Acari: Ixodidae) from Pennsylvania. J. Med. Entomol. 2015, 52, 693–698. [Google Scholar] [CrossRef]
- Morshed, M.G.; Scott, J.D.; Fernando, K.; Beati, L.; Mazerolle, D.F.; Geddes, G.; Durden, L.A. Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J. Parasitol. 2005, 91, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Kanji, J.N.; Isaac, A.; Gregson, D.; Mierzejewski, M.; Shpeley, D.; Tomlin, P.; Groeschel, M.; Lindsay, L.R.; Lachance, L.; Kowalewska-Grochowska, K. Epidemiology of ticks submitted from human hosts in Alberta, Canada (2000–2019). Emerg. Microbes Infect. 2022, 11, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.S.; Ramirez, R.A.; Anderson, J.L.; Bernhardt, S.A. Distribution and habitat of Ixodes pacificus (Acari: Ixodidae) and prevalence of Borrelia burgdorferi in Utah. J. Med. Entomol. 2015, 52, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- TCC-3W. Tick Species Submitted in Western Canada, 2022. Available online: http://www.bccdc.ca/Documents/T3WProjectInfographic-FINAL-220615.pdf (accessed on 21 October 2022).
- Surveillance Data. Available online: https://www.cdc.gov/lyme/datasurveillance/surveillance-data.html (accessed on 25 October 2022).
- Lyme Disease Surveillance Report 2019. Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/diseases-conditions/lyme-disease-surveillance-report-2019/LD-REPORT2019-ENG-Final.pdf (accessed on 25 October 2022).
- Watson, S.C.; Liu, Y.; Lund, R.B.; Gettings, J.R.; Nordone, S.K.; McMahan, C.S.; Yabsley, M.J. A Bayesian spatio-temporal model for forecasting the prevalence of antibodies to Borrelia burgdorferi, causative agent of Lyme disease, in domestic dogs within the contiguous United States. PLoS ONE 2017, 12, e0174428. [Google Scholar] [CrossRef]
- Parasite Prevalence Maps. Available online: https://www.petsandparasites.org/parasite-prevalence-maps#/ (accessed on 25 October 2022).
- Salkeld, D.J.; Castro, M.B.; Bonilla, D.; Kjemtrup, A.; Kramer, V.L.; Lane, R.S.; Padgett, K.A. Seasonal activity patterns of the western black-legged tick, Ixodes pacificus, in relation to onset of human Lyme disease in Northwestern California. Ticks Tick Borne Dis. 2014, 5, 790–796. [Google Scholar] [CrossRef]
- Lane, R.S.; Voie, P.E.L. Lyme borreliosis in California acarological, clinical, and epidemiological Studies. Ann. N. Y. Acad. Sci. 1988, 539, 192–203. [Google Scholar] [CrossRef]
- Manweiler, S.A.; Lane, R.S.; Tempelis, C.H. The western fence lizard Sceloporus occidentalis: Evidence of field exposure to Borrelia burgdorferi in relation to infestation by Ixodes pacificus (Acari: Ixodidae). Am. J. Trop. Med. Hyg. 1992, 47, 328–336. [Google Scholar] [CrossRef]
- Tälleklint-Eisen, L.; Eisen, R.J. Abundance of Ticks (Acari: Ixodidae) Infesting the western fence lizard, Sceloporus occidentalis, in relation to environmental factors. Exp. Appl. Acarol. 1999, 23, 731–740. [Google Scholar] [CrossRef]
- Wright, S.A.; Lemenager, D.A.; Tucker, J.R.; Armijos, M.V.; Yamamoto, S.A. An avian contribution to the presence of Ixodes pacificus (Acari: Ixodidae) and Borrelia burgdorferi on the Sutter Buttes of California. J. Med. Entomol. 2006, 43, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Slowik, T.J.; Lane, R.S. Birds and their ticks in Northwestern California: Minimal contribution to Borrelia burgdorferi enzootiology. J. Parasitol. 2001, 87, 755–761. [Google Scholar] [CrossRef]
- Newman, E.A.; Eisen, L.; Eisen, R.J.; Fedorova, N.; Hasty, J.M.; Vaughn, C.; Lane, R.S. Borrelia burgdorferi sensu lato spirochetes in wild birds in Northwestern California: Associations with ecological factors, bird behavior and tick infestation. PLoS ONE 2015, 10, e0118146. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.A.; Tucker, J.R.; Donohue, A.M.; Castro, M.B.; Kelley, K.L.; Novak, M.G.; Macedo, P.A. Avian hosts of Ixodes pacificus (Acari: Ixodidae) and the detection of Borrelia burgdorferi in larvae feeding on the Oregon junco. J. Med. Entomol. 2011, 48, 852–859. [Google Scholar] [CrossRef]
- Lane, R.S.; Burgdorfer, W. Potential role of native and exotic deer and their associated ticks (Acari: Ixodidae) in the ecology of Lyme disease in California, USA. Zentralbl. Bakteriol. Mikrobiol. Hyg. A. 1986, 263, 55–64. [Google Scholar] [CrossRef]
- Morshed, M.G.; Lee, M.-K.; Man, S.; Fernando, K.; Wong, Q.; Hojgaard, A.; Tang, P.; Mak, S.; Henry, B.; Patrick, D.M. Surveillance for Borrelia burgdorferi in Ixodes ticks and small rodents in British Columbia. Vector Borne Zoonotic Dis. 2015, 15, 701–705. [Google Scholar] [CrossRef]
- Peavey, C.A.; Lane, R.S. Transmission of Borrelia burgdorferi by Ixodes pacificus nymphs and reservoir competence of deer mice (Peromyscus maniculatus) infected by tick-bite. J. Parasitol. 1995, 81, 175–178. [Google Scholar] [CrossRef]
- Lane, R.S.; Loye, J.E. Lyme disease in California: Interrelationship of Ixodid ticks (Acari), rodents, and Borrelia burgdorferi. J. Med. Entomol. 1991, 28, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Eisen, R.J.; Lane, R.S. The roles of birds, lizards, and rodents as hosts for the western black-legged tick Ixodes pacificus. J. Vector Ecol. 2004, 29, 295–308. [Google Scholar] [PubMed]
- Eisen, L.; Eisen, R.J.; Mun, J.; Salkeld, D.J.; Lane, R.S. Transmission cycles of Borrelia burgdorferi and B. bissettii in relation to habitat type in Northwestern California. J. Vector Ecol. 2009, 34, 81–91. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Weinstein, S.B.; O’Connor, K.E.; Swei, A. Circulation of tick-borne spirochetes in tick and small mammal communities in Santa Barbara County, California, USA. J. Med. Entomol. 2020, 57, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.N.; Lane, R.S. Reservoir competence of four chaparral-dwelling rodents for Borrelia burgdorferi in California. Am. J. Trop. Med. Hyg. 1996, 54, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Swei, A.; Ostfeld, R.S.; Lane, R.S.; Briggs, C.J. Impact of the experimental removal of lizards on Lyme disease risk. Proc. R. Soc. B Biol. Sci. 2011, 278, 2970–2978. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Hyon, D.W.; McDaniels, A.; O’Connor, K.E.; Swei, A.; Briggs, C.J. Risk of vector tick exposure initially increases, then declines through time in response to wildfire in California. Ecosphere 2018, 9, e02227. [Google Scholar] [CrossRef]
- Salomon, J.; Lawrence, A.; Crews, A.; Sambado, S.; Swei, A. Host infection and community composition predict vector burden. Oecologia 2021, 196, 305–316. [Google Scholar] [CrossRef]
- Brown, R.N.; Lane, R.S. Lyme disease in California: A novel enzootic transmission cycle of Borrelia burgdorferi. Science 1992, 256, 1439–1442. [Google Scholar] [CrossRef]
- Foley, J.E.; Nieto, N.C. The ecology of tick-transmitted infections in the redwood chipmunk (Tamias ochrogenys). Ticks Tick Borne Dis. 2011, 2, 88–93. [Google Scholar] [CrossRef]
- Lane, R.S.; Mun, J.; Eisen, R.J.; Eisen, L. Western gray squirrel (Rodentia: Sciuridae): A primary reservoir host of Borrelia burgdorferi in Californian oak woodlands? J. Med. Entomol. 2005, 42, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Leonhard, S.; Jensen, K.; Salkeld, D.J.; Lane, R.S. Distribution of the Lyme disease spirochete Borrelia burgdorferi in naturally and experimentally infected western gray squirrels (Sciurus griseus). Vector Borne Zoonotic Dis. 2010, 10, 441–447. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Leonhard, S.; Girard, Y.A.; Hahn, N.; Mun, J.; Padgett, K.A.; Lane, R.S. Identifying the reservoir hosts of the Lyme disease spirochete Borrelia burgdorferi in California: The role of the western gray squirrel (Sciurus griseus). Am. J. Trop. Med. Hyg. 2008, 79, 535–540. [Google Scholar] [CrossRef]
- Lane, R.S.; Mun, J.; Eisen, L.; Eisen, R.J. Refractoriness of the western fence lizard (Sceloporus occidentalis) to the Lyme disease group spirochete Borrelia bissettii. J. Parasitol. 2006, 92, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S. Susceptibility of the western fence lizard (Sceloporus occidentalis) to the Lyme borreliosis spirochete (Borrelia burgdorferi). Am. J. Trop. Med. Hyg. 1990, 42, 75–82. [Google Scholar] [CrossRef]
- Lane, R.S.; Quistad, G.B. Borreliacidal factor in the blood of the western fence lizard (Sceloporus occidentalis). J. Parasitol. 1998, 84, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Durden, L.A.; Anderson, J.F. Infection prevalence of Borrelia Burgdorferi in ticks collected from songbirds in Far-Western Canada. Open J. Anim. Sci. 2015, 5, 232. [Google Scholar] [CrossRef]
- Lilly, M.; Amaya-Mejia, W.; Pavan, L.; Peng, C.; Crews, A.; Tran, N.; Sehgal, R.; Swei, A. Local community composition drives avian Borrelia burgdorferi infection and tick infestation. Vet. Sci. 2022, 9, 55. [Google Scholar] [CrossRef]
- Linders, M.J.; Stinson, D.W. Western Gray Squirrel Recovery Plan; Washington Department of Fish and Wildlife: Olympia, WA, USA, 2007; p. 140. [Google Scholar]
- Nieto, N.C.; Foley, J.E. Evaluation of squirrels (Rodentia: Sciuridae) as ecologically significant hosts for Anaplasma phagocytophilum in California. J. Med. Ent. 2008, 45, 763–769. [Google Scholar] [CrossRef]
- Marsot, M.; Chapuis, J.-L.; Gasqui, P.; Dozières, A.; Masséglia, S.; Pisanu, B.; Ferquel, E.; Vourc’h, G. Introduced siberian chipmunks (Tamias sibiricus barberi) contribute more to Lyme borreliosis risk than native reservoir rodents. PLoS ONE 2013, 8, e55377. [Google Scholar] [CrossRef] [PubMed]
- Slajchert, T.; Kitron, U.D.; Jones, C.J.; Mannelli, A. Role of the eastern chipmunk (Tamias striatus) in the epizootiology of Lyme borreliosis in Northwestern Illinois, USA. J. Wildl. Dis. 1997, 33, 40–46. [Google Scholar] [CrossRef]
- Peavey, C.A.; Lane, R.S.; Damrow, T. Vector competence of Ixodes angustus (Acari: Ixodidae) for Borrelia burgdorferi sensu stricto. Exp. Appl. Acarol. 2000, 24, 77–84. [Google Scholar] [CrossRef]
- Marcum, L. LYME SCI: Lyme-Carrying Ticks in West Differ from Their Eastern Cousins. Lyme Disease. 2022. Available online: https://www.lymedisease.org/ixodes-pacificus-review/# (accessed on 21 October 2022).
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Anderson, J.F.; Bierman, B.C.; Durden, L.A. Extensive distribution of the Lyme disease bacterium, Borrelia burgdorferi sensu lato, in multiple tick species parasitizing avian and mammalian hosts across Canada. Healthcare 2018, 6, 131. [Google Scholar] [CrossRef]
- Brown, R.N.; Peot, M.A.; Lane, R.S. Sylvatic Maintenance of Borrelia burgdorferi (Spirochaetales) in Northern California: Untangling the web of transmission. J. Med. Entomol. 2006, 43, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Dolan, M.C.; Piesman, J.; Lane, R.S. Vector competence of Ixodes pacificus and I. spinipalpis (Acari: Ixodidae), and reservoir competence of the dusky-footed woodrat (Neotoma fuscipes) and the deer mouse (Peromyscus maniculatus), for Borrelia bissettii. J. Med. Entomol. 2003, 40, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Burkot, T.R.; Clover, J.R.; Happ, C.M.; DeBess, E.; Maupin, G.O. Isolation of Borrelia burgdorferi from Neotoma fuscipes, Peromyscus maniculatus, Peromyscus boylii, and Ixodes pacificus in Oregon. Am. J. Trop. Med. Hyg. 1999, 60, 453–457. [Google Scholar] [CrossRef]
- Scott, J.D.; Anderson, J.F.; Durden, L.A. Widespread dispersal of Borrelia burgdorferi–infected ticks collected from songbirds across Canada. J. Parasitol. 2012, 98, 49–59. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Hyon, D.W.; Brewington, J.B.; O’Connor, K.E.; Swei, A.; Briggs, C.J. Lyme disease risk in Southern California: Abiotic and environmental drivers of Ixodes pacificus (Acari: Ixodidae) density and infection prevalence with Borrelia burgdorferi. Parasites Vectors 2017, 10, 7. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J.; Chang, C.-C.; Mun, J.; Lane, R.S. Acarologic risk of exposure to Borrelia burgdorferi spirochaetes: Long-term evaluations in North-Western California, with implications for Lyme borreliosis risk-assessment models. Med. Vet. Entomol. 2004, 18, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Padgett, K.A.; Lane, R.S. Life cycle of Ixodes pacificus (Acari: Ixodidae): Timing of developmental processes under field and laboratory conditions. J. Med. Entomol. 2001, 38, 684–693. [Google Scholar] [CrossRef]
- Nieto, N.C.; Holmes, E.A.; Foley, J.E. Survival rates of immature Ixodes pacificus (Acari: Ixodidae) ticks estimated using field-placed enclosures. J. Vector Ecol. 2010, 35, 43–49. [Google Scholar] [CrossRef]
- Foley, J.E.; Queen, E.V.; Sacks, B.; Foley, P. GIS-facilitated spatial epidemiology of tick-borne diseases in coyotes (Canis latrans) in Northern and Coastal California. Comp. Immunol. Microbiol. Infect. Dis. 2005, 28, 197–212. [Google Scholar] [CrossRef]
- Fleshman, A.C.; Graham, C.B.; Maes, S.E.; Foster, E.; Eisen, R.J. Reported county-level distribution of Lyme disease spirochetes, Borrelia burgdorferi sensu stricto and Borrelia mayonii (Spirochaetales: Spirochaetaceae), in host-seeking Ixodes scapularis and Ixodes pacificus ticks (Acari: Ixodidae) in the contiguous United States. J. Med. Entomol. 2021, 58, 1219–1233. [Google Scholar]
- Lane, R.S.; Mun, J.; Peribáñez, M.A.; Stubbs, H.A. Host-seeking behavior of Ixodes pacificus (Acari: Ixodidae) nymphs in relation to environmental parameters in dense-woodland and woodland-grass habitats. J. Vector Ecol. 2007, 32, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Salkeld, D.J.; Nieto, N.C.; Carbajales-Dale, P.; Carbajales-Dale, M.; Cinkovich, S.S.; Lambin, E.F. Disease risk & landscape attributes of tick-borne Borrelia pathogens in the San Francisco Bay Area, California. PLoS ONE 2015, 10, e0134812. [Google Scholar]
- Eisen, R.J.; Clark, R.J.; Monaghan, A.J.; Eisen, L.; Delorey, M.J.; Beard, C.B. Host-seeking phenology of Ixodes pacificus (Acari: Ixodidae) nymphs in Northwestern California in relation to calendar week, woodland type, and weather conditions. J. Med. Entomol. 2016, 54, 125–131. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Lane, R.S. Predicting density of Ixodes pacificus nymphs in dense woodlands in Mendocino County, California, based on geographic information systems and remote sensing versus field-derived data. Am. J. Trop. Med. Hyg. 2006, 74, 632–640. [Google Scholar] [CrossRef]
- Eisen, R.J.; Lane, R.S.; Fritz, C.L.; Eisen, L. Spatial patterns of Lyme disease risk in California based on disease incidence data and modeling of vector-tick exposure. Am. J. Trop. Med. Hyg. 2006, 75, 669–676. [Google Scholar] [CrossRef]
- Mak, S.; Morshed, M.; Henry, B. Ecological niche modeling of Lyme disease in British Columbia, Canada. J. Med. Entomol. 2010, 47, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peavey, C.A.; Lane, R.S. Density and spatial distribution of Ixodes pacificus (Acari: Ixodidae) in two recreational areas in North Coastal California. Am. J. Trop. Med. Hyg. 2000, 62, 415–422. [Google Scholar] [CrossRef]
- Lane, R.S. Risk of human exposure to vector ticks (Acari: Ixodidae) in a heavily used recreational area in Northern California. Am. J. Trop. Med. Hyg. 1996, 55, 165–173. [Google Scholar] [CrossRef]
- Carella, E.; Orusa, T.; Viani, A.; Meloni, D.; Borgogno-Mondino, E.; Orusa, R. An integrated, tentative remote-sensing approach based on NDVI entropy to model canine distemper virus in wildlife and to prompt science-based management policies. Animals 2022, 12, 1049. [Google Scholar] [CrossRef]
- Orusa, T.; Orusa, R.; Viani, A.; Carella, E.; Borgogno Mondino, E. Geomatics and EO data to support wildlife diseases assessment at landscape level: A pilot experience to map infectious keratoconjunctivitis in chamois and phenological trends in Aosta Valley (NW Italy). Remote Sens. 2020, 12, 3542. [Google Scholar] [CrossRef]
- Suresh, K.P.; Bylaiah, S.; Patil, S.; Kumar, M.; Indrabalan, U.B.; Panduranga, B.A.; Srinivas, P.T.; Shivamallu, C.; Kollur, S.P.; Cull, C.A.; et al. A new methodology to comprehend the effect of El Niño and La Niña oscillation in early warning of anthrax epidemic among livestock. Zoonotic Dis. 2022, 2, 267–290. [Google Scholar] [CrossRef]
- De Marinis, P.; De Petris, S.; Sarvia, F.; Manfron, G.; Momo, E.J.; Orusa, T.; Corvino, G.; Sali, G.; Borgogno, E.M. Supporting pro-poor reforms of agricultural systems in Eastern DRC (Africa) with remotely sensed data: A possible contribution of spatial entropy to interpret land management practices. Land 2021, 10, 1368. [Google Scholar] [CrossRef]
- Orusa, T.; Borgogno Mondino, E. Exploring short-term climate change effects on rangelands and broad-leaved forests by free satellite data in Aosta Valley (Northwest Italy). Climate 2021, 9, 47. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- MacDonald, A.J.; O’Neill, C.; Yoshimizu, M.H.; Padgett, K.A.; Larsen, A.E. Tracking seasonal activity of the western blacklegged tick across California. J. Appl. Ecol. 2019, 56, 2562–2573. [Google Scholar] [CrossRef]
- MacDonald, A.J.; McComb, S.; O’Neill, C.; Padgett, K.A.; Larsen, A.E. Projected climate and land use change alter western blacklegged tick phenology, seasonal host-seeking suitability and human encounter risk in California. Glob. Chang. Biol. 2020, 26, 5459–5474. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Feirer, S.; Padgett, K.A.; Hahn, M.B.; Monaghan, A.J.; Kramer, V.L.; Lane, R.S.; Kelly, M. Modeling climate suitability of the western blacklegged tick in California. J. Med. Entomol. 2018, 55, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.B.; Jarnevich, C.S.; Monaghan, A.J.; Eisen, R.J. Modeling the geographic distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. J. Med. Entomol. 2016, 53, 1176–1191. [Google Scholar] [CrossRef]
- Porter, W.T.; Barrand, Z.A.; Wachara, J.; DaVall, K.; Mihaljevic, J.R.; Pearson, T.; Salkeld, D.J.; Nieto, N.C. Predicting the current and future distribution of the western black-legged tick, Ixodes pacificus, across the Western US using citizen science collections. PLoS ONE 2021, 16, e0244754. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Lane, R.S. Remote sensing (normalized difference vegetation index) classification of risk versus minimal risk habitats for human exposure to Ixodes pacificus (Acari: Ixodidae) nymphs in Mendocino County, California. J. Med. Entomol. 2005, 42, 75–81. [Google Scholar] [CrossRef]
- Witmer, F.D.W.; Nawrocki, T.W.; Hahn, M. Modeling geographic uncertainty in current and future habitat for potential populations of Ixodes pacificus (Acari: Ixodidae) in Alaska. J. Med. Entomol. 2022, 59, 976–986. [Google Scholar] [CrossRef] [PubMed]
- BC Centres for Disease Control. Lyme Disease Risk Areas in British Columbia. Available online: http://www.bccdc.ca/resource-gallery/Documents/Statistics%20and%20Research/Statistics%20and%20Reports/Epid/Vector-bourne/Lyme_Disease_Risk_Areas_Map_BC.pdf (accessed on 21 October 2022).
- Hahn, M.B.; Feirer, S.; Monaghan, A.J.; Lane, R.S.; Eisen, R.J.; Padgett, K.A.; Kelly, M. Modeling future climate suitability for the western blacklegged tick, Ixodes pacificus, in California with an emphasis on land access and ownership. Ticks Tick Borne Dis. 2021, 12, 101789. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Species’ distribution modeling for conservation educators and practitioners. Lessons Conserv. 2010, 3, 54–89. [Google Scholar]
- What is a GCM? Available online: https://www.ipcc-data.org/guidelines/pages/gcm_guide.html (accessed on 25 October 2022).
- IPCC DDC Glossary. Available online: https://www.ipcc-data.org/guidelines/pages/glossary/glossary_r.html (accessed on 25 October 2022).
- TickReport. Available online: https://www.tickreport.com/ (accessed on 25 October 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).