Leishmania Infection during Chemotherapy in a Dog Diagnosed with Multicentric Large B-Cell Lymphoma—A Diagnostic Challenge
Abstract
:Simple Summary
Abstract
1. Introduction
2. Case Report
2.1. Case History
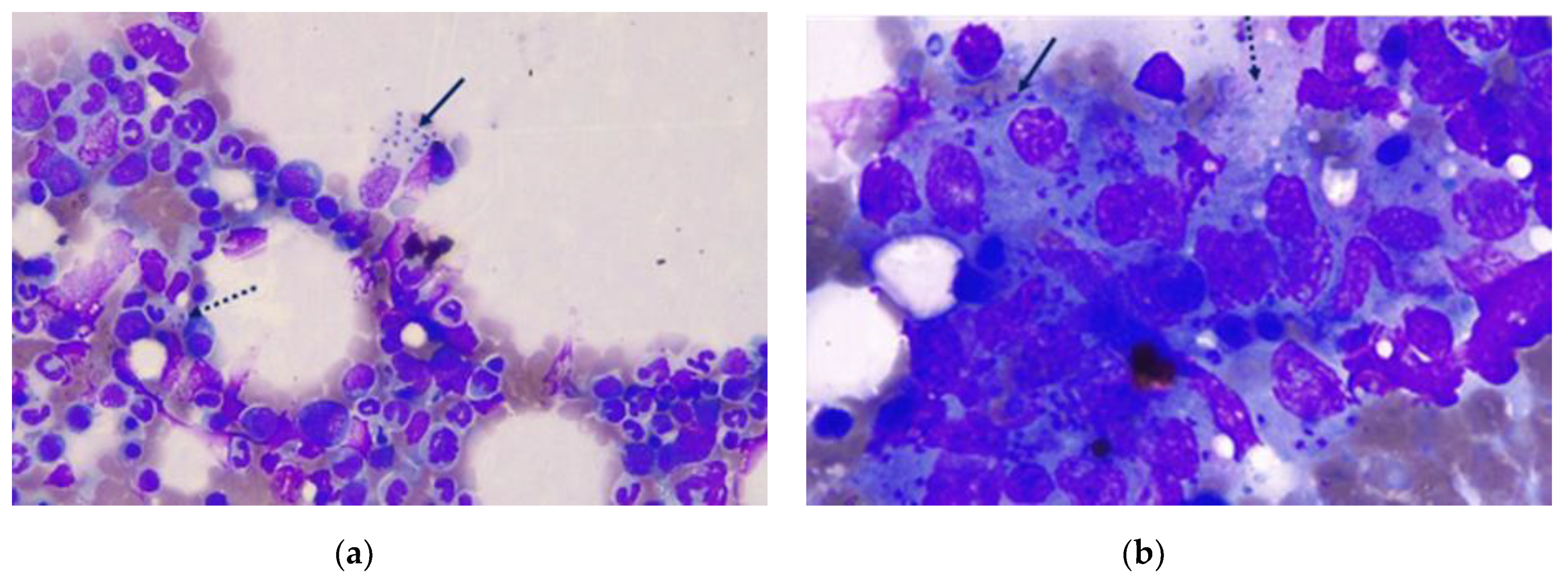
2.2. Clinical and Laboratory Findings and Treatment
2.3. Clinical Follow-Up and Treatment
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martineau, M.M.C. Multicentric canine lymphoma in a 12-year-old keeshond: Chemotherapy options. Can. Vet. J. 2002, 43, 709–711. [Google Scholar]
- Peixoto, T.C.; Freitas, J.L.; Farias, S.S.; Vieira-Filho, C.H.; Laranjeira, D.F.; Mascarenhas, M.B.; Nogueira, V.A.; Barrouin-Melo, S.M. Linfoma primário cardíaco associado à leishmaniose visceral em cão—Relato de caso. Rev. Bras. Med. Vet. 2016, 38 (Suppl. 1), 47–54. [Google Scholar]
- Valli, V.E.; Kass, P.H.; San Myint, M.; Scott, F. Canine lymphomas: Association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 2013, 50, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; Pinkerton, M.; Young, K.M. Section A: Canine Lymphoma and Lymphocytic Leukemias. Lymphoma. In Hemato-poietic Tumors. Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Vail, D., Thamm, D.H., Liptak, J.M., Eds.; Elsevier: St. Louis, MI, USA, 2019; pp. 688–772. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7161413/ (accessed on 13 November 2021).
- Zandvliet, M. Canine lymphoma: A review. Vet. Q. 2000, 36, 76–104. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.L.; Jansen, A.M. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int. J. Parasitol. Parasites Wildl. 2014, 3, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemayehu, B.; Alemayehu, M. Leishmaniasis: A Review on Parasite, Vector and Reservoir. Host. Health Sci. J. 2017, 11, 519. [Google Scholar] [CrossRef]
- Naucke, T.J.; Menn, B.; Massberg, D.; Lorentz, S. Sandflies and leishmaniasis in Germany. Parasitol. Res. 2008, 103, 65–68. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Solano-Gallego, L.; Fondati, A.; Lubas, G.; Gradoni, L.; Castagnaro, M.; Crotti, A.; Maroli, M.; Oliva, G.; Roura, X.; et al. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasites Vectors 2011, 4, 86. [Google Scholar] [CrossRef] [Green Version]
- Tánczos, B.; Balogh, N.; Király, L.; Biksi, I.; Szeredi, L.; Gyurkovsky, M.; Scalone, A.; Fiorentino, E.; Gramiccia, M.; Farkas, R. First record of autochthonous canine Leishmaniasis in Hungary. Vector Borne Zoonotic Dis. 2012, 12, 588–594. [Google Scholar] [CrossRef] [Green Version]
- Morosetti, G.; Toson, M.; Trevisiol, K.; Idrizi, I.; Natale, A.; Lucchese, L.; Michelutti, A.; Ceschi, P.; Lorenzi, G.; Piffer, C.; et al. Canine leishmaniosis in the Italian northeastern Alps: A survey to assess serological prevalence in dogs and distribution of phlebotomine sand flies in the Autonomous Province of Bolzano-South Tyrol, Italy. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100432. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Lazo, A.; Jasensky, A.K.; Jolly-Frahija, I.T.; Kehl, A.; Müller, E.; Mesa-Sánchez, I. Clonality testing in the lymph nodes from dogs with lymphadenomegaly due to Leishmania infantum infection. PLoS ONE 2019, 14, e0226336. [Google Scholar] [CrossRef]
- Ferro, S.; Palmieri, C.; Cavicchioli, L.; De Zan, G.; Aresu, L.; Benali, S.L. Leishmania amastigotes in neoplastic cells of 3 nonhistiocytic canine tumors. Vet. Pathol. 2013, 50, 749–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foglia Manzillo, V.; Restucci, B.; Pagano, A.; Gradoni, L.; Oliva, G. Pathological changes in the bone marrow of dogs with leishmaniosis. Vet. Rec. 2006, 158, 690–694. [Google Scholar] [CrossRef]
- Loiola, M.T.A.; Silva, M.C.; Albuquerque, B.F.; Vago, P.B. Desenvolvimento de linfoma em cão com leishmaniose visceral. Ci. Anim. 2019, 29, 128–136. [Google Scholar]
- Sözmen, M.; Tasca, S.; Carli, E.; De Lorenzi, D.; Furlanello, T.; Caldin, M. Use of fine needle aspirates and flow cytometry for the diagnosis, classification, and immunophenotyping of canine lymphomas. J. Vet. Diagn. Investig. 2005, 17, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Roldan, J.A.; Benelli, G.; Bezerra-Santos, M.A.; Nguyen, V.-L.; Conte, G.; Latta, R.; Furlanello, T.; Otranto, D. Seropositivity to canine tick-borne pathogens in a population of sick dogs in Italy. Parasites Vectors 2021, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009–2019: Changing distribution patterns. Parasites Vectors 2020, 13, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavazza, A.; Lubas, G.; Fridman, A.; Peruzzi, D.; Impellizeri, J.A.; Luberto, L.; Marra, E.; Roscilli, G.; Ciliberto, G.; Aurisicchio, L. Safety and efficacy of a genetic vaccine targeting telomerase plus chemotherapy for the therapy of canine B-cell lymphoma. Hum. Gene Ther. 2013, 24, 728–738. [Google Scholar] [CrossRef]
- Trotta, M.; Nicetto, M.; Fogliazza, A.; Montarsi, F.; Caldin, M.; Furlanello, T.; Solano-Gallego, L. Detection of Leishmania infantum, Babesia canis, and rickettsiae in ticks removed from dogs living in Italy. Ticks Tick Borne Dis. 2012, 3, 293–296. [Google Scholar] [CrossRef]
- Trotta, M.; Fogliazza, A.; Furlanello, T.; Solano-Gallego, L. A molecular and serological study of exposure to tick-borne pathogens in sick dogs from Italy. Clin. Microbiol. Infect. 2009, 15 (Suppl. 2), 62–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solano-Gallego, L.; Caprì, A.; Pennisi, M.G.; Caldin, M.; Furlanello, T.; Trotta, M. Acute febrile illness is associated with Rickettsia spp infection in dogs. Parasites Vectors 2015, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Childress, M.O.; Ramos-Vara, J.A.; Ruple, A. Retrospective analysis of factors affecting clinical outcome following CHOP-based chemotherapy in dogs with primary nodal diffuse large B-cell lymphoma. Vet. Comp. Oncol. 2017, 16, E159–E168. [Google Scholar] [CrossRef] [PubMed]
- Schwing, A.; Pomares, C.; Majoor, A.; Boyer, L.; Marty, P.; Michel, G. Leishmania infection: Misdiagnosis as cancer and tumor-promoting potential. Acta Trop. 2019, 197, 104855. [Google Scholar] [CrossRef] [PubMed]
- Kopterides, P.; Mourtzoukou, E.G.; Skopelitis, E.; Tsavaris, N.; Falagas, M.E. Aspects of the association between leishmaniasis and malignant disorders. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Albanese, F.; Poli, A.; Millanta, F.; Abramo, F. Primary cutaneous extragenital canine transmissible venereal tumour with Leishmania-laden neoplastic cells: A further suggestion of histiocytic origin? Vet. Dermatol. 2002, 13, 243–246. [Google Scholar] [CrossRef]
- Catone, G.; Marino, G.; Poglayen, G.; Gramiccia, M.; Ludovisi, A.; Zanghì, A. Canine transmissible venereal tumour parasitized by Leishmania infantum. Vet. Res. Commun. 2003, 27, 549–553. [Google Scholar] [CrossRef]
- Marino, G.; Gaglio, G.; Zanghì, A. Clinicopathological study of canine transmissible venereal tumour in leishmaniotic dogs. J. Small Anim. Pract. 2012, 53, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Kegler, K.; Habierski, A.; Hahn, K.; Amarilla, S.P.; Seehusen, F.; Baumgärtner, W. Vaginal canine transmissible venereal tumour associated with intra-tumoural Leishmania spp. amastigotes in an asymptomatic female dog. J. Comp. Pathol. 2013, 149, 156–161. [Google Scholar] [CrossRef]
- Carreira, V.S.; Ferrari, H.F.; Langohr, I.M.; Mackenzie, C.; Montezzo, L.C.; Taira, E.; Floeter-Winter, L.M.; Luvizotto, M.C.R. Leishmania sp. amastigotes identification in canine transmissible venereal tumor. Case Rep. Vet. Med. 2014, 2014, 603852. [Google Scholar] [CrossRef] [Green Version]
- Di Mattia, D.; De Bellis, F. Leishmania spp. in perianal adenoma in a dog: A case report. Top. Companion Anim. Med. 2019, 34, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, M.T.; Birettoni, F.; Miglio, A.; Lalli, P.; Porciello, F.; Mangili Pecci, V. Monoclonal gammopathy associated with multiple myeloma and visceral leishmaniasis in the dog: A comparison of two cases. Vet. Res. Commun. 2010, 34 (Suppl. 1), 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezerra, J.A.B.; Rodrigues, R.T.G.A.; Lima, I.O.; Vale, A.M.; Filgueira, K.D. Acute myeloid leukemia in a dog chronically infected with Leishmania spp. and other infectious agents. Acta Sci. Vet. 2018, 46 (Suppl. 1), 260. [Google Scholar] [CrossRef] [Green Version]
- Weiss, D.J.; Evanson, O.A.; Sykes, J. A retrospective study of canine pancytopenia. Vet. Clin. Pathol. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Frezoulis, P.S.; Angelidou, E.; Karnezi, D.; Oikonomidis, I.L.; Kritsepi-Konstantinou, M.; Kasabalis, D.; Mylonakis, M.E. Canine pancytopoenia in a Mediterranean region: A retrospective study of 119 cases (2005 to 2013). J. Small Anim. Pract. 2017, 58, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Momo, C.; Jacintho, A.P.; Moreira, P.R.; Munari, D.P.; Machado, G.F.; Vasconcelos, R.O. Morphological changes in the bone marrow of the dogs with visceral leishmaniasis. Vet. Med. Int. 2014, 2014, 150582. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Smith, S. Primary Myelodysplastic Syndromes of Dogs: A Report of 12 Cases. J. Vet. Intern. Med. 2000, 14, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Aird, B. Cytologic evaluation of primary and secondary syndromes in the dog. Vet. Clin. Pathol. 2001, 30, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Paparcone, R.; Fiorentino, E.; Cappiello, S.; Gizzarelli, M.; Gradoni, L.; Oliva, L.; Foglia Manzillo, V. Sternal aspiration of bone marrow in dogs: A practical approach for canine leishmaniasis diagnosis and monitoring. J. Vet. Med. 2013, 2013, 217314. [Google Scholar] [CrossRef] [Green Version]
- Marconato, L.; Stefanello, D.; Valenti, P.; Bonfanti, U.; Comazzi, S.; Roccabianca, P.; Caniatti, M.; Romanelli, G.; Massari, F. Predictors of long-term survival in dogs with high-grade multicentric lymphoma. J. Am. Vet. Med. Assoc. 2011, 238, 480–485. [Google Scholar] [CrossRef]
- Maroli, M.; Mizzon, V.; Siragusa, C.; D’Orazi, A.; Gradoni, L. Evidence for an impact on the incidence of canine leishmaniasis by the mass use of deltamethrin-impregnated dog collars in southern Italy. Med. Vet. Entomol. 2001, 15, 358–363. [Google Scholar] [CrossRef]
- Brianti, E.; Gaglio, G.; Napoli, E.; Falsone, L.; Prudente, C.; Solari Basano, F.; Latrofa, M.S.; Tarallo, V.D.; Dantas-Torres, F.; Capelli, G.; et al. Efficacy of a slow-release imidacloprid (10%)/flumethrin (4.5%) collar for the prevention of canine leishmaniosis. Parasites Vectors 2014, 7, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coura-Vital, W.; de Almeida Leal, G.G.; Araújo Marques, L.; da Costa Pinheiro, A.; Carneiro, M.; Reis, A.B. Effectiveness of deltamethrin-impregnated dog collars on the incidence of canine infection by Leishmania infantum: A large scale intervention study in an endemic area in Brazil. PLoS ONE 2018, 13, e0208613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, E.G.; Sevá, A.P.; Ferreira, F.; Nunes, C.M.; Keid, L.B.; Hiramoto, R.M.; Ferreira, H.L.; Oliveira, T.M.F.S.; Ovallos, F.G.; Galati, E.A.B.; et al. Vaccine effectiveness and use of collar impregnated with insecticide for reducing incidence of Leishmania infection in dogs in an endemic region for visceral leishmaniasis, in Brazil. Epidemiol. Infect. 2018, 146, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtenay, O.; Dilger, E.; Calvo-Bado, L.A.; Kravar-Garde, L.; Carter, V.; Bell, M.J.; Alves, G.B.; Goncalves, R.; Makhdoomi, M.M.; González, M.A.; et al. Sand fly synthetic sex-aggregation pheromone co-located with insecticide reduces the incidence of infection in the canine reservoir of visceral leishmaniasis: A stratified cluster randomised trial. PLoS Negl. Trop. Dis. 2019, 13, e0007767. [Google Scholar] [CrossRef] [PubMed]
- Duprey, Z.H.; Steurer, F.J.; Rooney, J.A.; Kirchhoff, L.V.; Jackson, J.E.; Rowton, E.D.; Schantz, P.M. Canine visceral leishmaniasis, United States and Canada, 2000–2003. Emerg. Infect. Dis. 2006, 12, 440–446. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.; Charalampos, A.; Tasker, S.; Augusto, M. Leishmaniosis in a dog with no travel history outside of the UK. Vet. Rec. 2019, 184, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, S.E.; Langton, D.A.; Hillman, T.J. Canine leishmaniosis in the United Kingdom: A zoonotic disease waiting for a vector? Vet. Parasitol. 2009, 163, 281–285. [Google Scholar] [CrossRef]
- Silvestrini, P.; Batchelor, D.; Allenspach, K.; Maunder, C.; Seth, M.; Mas, A.; Hill, T.; Serrano, G.; Roura, X.; Planellas, M.; et al. Clinical leishmaniasis in dogs living in the UK. J. Small Anim. Pract. 2016, 57, 453–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameters and Units | Reference Ranges | D0 † | D14 ‡ | D24 | D35 | D62 | D85 | D115 | D128 | D207 | D250 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RBC M/µL | 5.65–8.87 | 4.74 | 4.48 | 4.58 | 5.22 | 5.83 | 6.20 | 6.35 | 5.92 | 7.00 | 6.72 |
| HCT% | 37.3–61.7 | 31.4 | 29.3 | 29.0 | 32.7 | 35.7 | 37.1 | 38.7 | 36.0 | 41.9 | 40.2 |
| HGB g/dL | 13.1–20.5 | 10.5 | 9.6 | 9.5 | 10.7 | 11.6 | 12.2 | 12.9 | 12.2 | 14.1 | 14.1 |
| MCV fL | 61.6–73.5 | 66.7 | 65.4 | 63.3 | 62.6 | 61.2 | 59.8 | 60.9 | 60.8 | 59.9 | 59.8 |
| MCH pg | 21.2–25.9 | 22.2 | 21.4 | 20.7 | 20.5 | 19.9 | 19.7 | 20.3 | 20.6 | 20.1 | 21.0 |
| MCHC g/dL | 32.0–37.9 | 33.4 | 32.8 | 32.8 | 32.7 | 32.5 | 32.9 | 33.3 | 33.9 | 33.7 | 35.1 |
| RDW% | 13.6–21.7 | 19.8 | 19.5 | 20.4 | 22.0 | 22.9 | 24.1 | 24.1 | 23.5 | 23.3 | 24.3 |
| Retics K/µL | 10.0–110.0 | 35.6 | 19.3 | 29.3 | 49.1 | 29.7 | 45.3 | 30.5 | 26.0 | 42.7 | 28.9 |
| Retic-HGB pg | 22.3–29.6 | 19.2 | 21.0 | 19.4 | 21.2 | 19.2 | 20.2 | 23.5 | 23.7 | 22.4 | 25.3 |
| WBC K/µL | 5.05–16.76 | 2.59 | 2.96 | 2.54 | 2.47 | 4.03 | 3.56 | 4.34 | 1.84 | 5.05 | 15.34 |
| NEU seg K/µL | 3.7–11.9 | 1.76 | 2.43 | 1.93 | 1.98 | 2.74 | 2.78 | 3.39 | 0.96 | 4.17 | 10.74 |
| NEU band K/µL | 0.0–0.3 | 0.00 | 0.00 | 0.05 | 0.00 | 0,00 | 0.00 | 0.04 | 0.00 | 0.05 | 0.00 |
| EOS K/µL | 0.1–1.35 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.07 | 0.04 | 0.00 | 0.05 | 0.00 |
| BAS K/µL | 0.0–0.1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| LYM K/µL | 0.7–5.1 | 0.36 | 0.36 | 0.46 | 0.30 | 1.05 | 0.43 | 0.69 | 0.66 | 0.50 | 4.14 |
| MON K/µL | 0.2–1.5 | 0.47 | 0.18 | 0.10 | 0.10 | 0.24 | 0.28 | 0.17 | 0.22 | 0.25 | 0.46 |
| PLT K/µL | 148–484 | 37 | 67 | 72 | 81 | 77 | 43 | 41 | 47 | 116 | 68 |
| MPV fL | 8.7–13.2 | 13.9 | 12.9 | 13.9 | 12.9 | 12.3 | 12.1 | 11.0 | 10.3 | 11.2 | 10.0 |
| PLT estimate | Adequate | Inadequate | Inadequate | Inadequate | Inadequate | Inadequate | Inadequate | Inadequate | Inadequate | Inadequate | Inadequate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Feo, G.; Simčič, P.; Lubas, G.; Papini, R.A. Leishmania Infection during Chemotherapy in a Dog Diagnosed with Multicentric Large B-Cell Lymphoma—A Diagnostic Challenge. Zoonotic Dis. 2021, 1, 25-36. https://doi.org/10.3390/zoonoticdis1010003
De Feo G, Simčič P, Lubas G, Papini RA. Leishmania Infection during Chemotherapy in a Dog Diagnosed with Multicentric Large B-Cell Lymphoma—A Diagnostic Challenge. Zoonotic Diseases. 2021; 1(1):25-36. https://doi.org/10.3390/zoonoticdis1010003
Chicago/Turabian StyleDe Feo, Giulia, Petra Simčič, George Lubas, and Roberto Amerigo Papini. 2021. "Leishmania Infection during Chemotherapy in a Dog Diagnosed with Multicentric Large B-Cell Lymphoma—A Diagnostic Challenge" Zoonotic Diseases 1, no. 1: 25-36. https://doi.org/10.3390/zoonoticdis1010003






