Abstract
The current healthcare environment is at risk due to the facilitated transmission and empowerment of the ESKAPE pathogens, comprising of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. These pathogens have posed significant challenges to global public health and the threat has only amplified over time. These multidrug-resistant bacteria have become adept at escaping the effects of conventional antibiotics utilized, leading to severe healthcare-associated infections and compromising immunocompromised patient outcomes to a greater extent. The impact of ESKAPE pathogens is evident in the rapidly rising rates of treatment failures, increased mortality, and elevated healthcare costs. To combat this looming crisis, diverse strategies have been adopted, ranging from the development of novel antimicrobial agents and combination therapies to the implementation of stringent infection control measures. Additionally, there has been a growing emphasis on promoting antimicrobial stewardship programs to optimize the use of existing antibiotics and reduce the selective pressure driving the evolution of resistance. While progress has been made to some extent, the rapid adaptability of these pathogens and the enhancement of antimicrobial resistance mechanisms proves to be a major hurdle yet to be crossed by healthcare professionals. In this viewpoint, the impending threat heralded by the proliferation of ESKAPE pathogens, and the need for a concerted global effort via international collaborations for the assurance of effective and sustainable solutions, are explored. To curb the possibility of outbreaks in the future and to safeguard public health, better preparation via global awareness and defense mechanisms should be given paramount importance.
1. Introduction
The evolution of modern medicine has ushered in novel methods of treatment within the healthcare sphere, yet it brings an additional concern of microbial adaptability and immunity. Infections stemming from pathogens represent a formidable threat to patient well-being in hospital settings, typically recognized as centers for healing. These facilities, inadvertently, can transform into breeding grounds for various pathogens due to the close proximity of ailing individuals, invasive medical procedures, and the widespread utilization of medical devices [1]. The risk of healthcare-associated infections (HAIs) escalates, particularly in the presence of immunocompromised patients, prolonged hospital stays, and the extensive, often unnecessary, administration of antibiotics [2]. This excessive use of antibiotics stands as a prominent factor contributing to the alarming surge in antimicrobial resistance (AMR). The overuse and misuse of different antibiotics in healthcare settings, where alternative methods could be adopted, significantly foster the development of multidrug-resistant (MDR) strains, amplifying the complexity of treating infections. The development of MDR strains is a complex and concerning phenomenon that emerges when microorganisms, predominantly bacteria, evolve various mechanisms to withstand the specific effects of the antibiotics they are exposed to which can render administered antibiotics highly weakened. Survival and proliferation are favored in strains that have adopted novel genetic mutations or mechanisms that confer heightened resistance to the drugs [3]. Over time, the surviving resistant strains become more prevalent, leading to the proliferation of MDR populations. Horizontal gene transfer, another crucial factor in the development of drug resistance, allows bacteria to share genetic material, including resistance genes, with one another [4]. This facilitates the rapid spread of traits that confer resistance within bacterial communities. Additionally, the widespread use of antibiotics in healthcare and community settings contributes to the environmental dissemination of resistant strains. Figure 1 highlights the various mechanisms involved in the development of antibiotic resistance in bacteria.
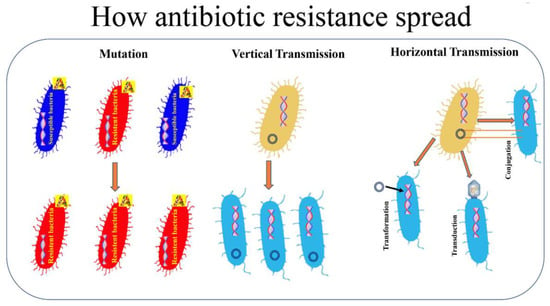
Figure 1.
How antibiotic resistance spread. Bacterial resistance towards antibiotics can be natural, or acquired by vertical or horizontal transmission. A: antibiotic. [5]. Reproduced under the terms of the CC-BY license from Ref. [5] [Pathogens], Copyright 2021, The Authors, published by MDPI.
MDR strains utilize various resistance mechanisms that pose challenges to the efficacy of existing antibiotic treatments. Beta-lactamases and aminoglycoside-modifying enzymes are prevalent mechanisms that confer resistance to beta-lactam antibiotics and aminoglycosides, respectively [5,6]. Beta-lactamases, such as extended-spectrum beta-lactamases (ESBLs) and carbapenemases, hydrolyze beta-lactam rings, rendering antibiotics ineffective [7]. Aminoglycoside-modifying enzymes modify the structure of aminoglycoside antibiotics, reducing their binding affinity to bacterial ribosomes [8]. Target site mutations involve alterations in bacterial cellular structures, such as penicillin-binding proteins or ribosomal subunits, minimizing antibiotic binding and consequently inhibiting their action [9]. Efflux pumps actively expel antibiotics from bacterial cells, limiting intracellular drug concentrations. These pumps function as molecular machines that recognize and pump out a broad spectrum of antimicrobial agents, including antibiotics, from the bacterial cytoplasm [10]. These mechanisms collectively contribute to the resilience of multidrug-resistant bacteria, underscoring the importance of developing novel therapeutic strategies to combat the growing threat of AMR.
The ESKAPE pathogens, consisting of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and various Enterobacter species, are particularly noteworthy due to their adeptness at ‘escaping’ the effects of antimicrobial treatments and the efficient utilization of the commonly observed resistance mechanisms [11]. These pathogens have acquired resistance mechanisms against multiple classes of antibiotics, making them formidable adversaries in the battle against infectious diseases. E. faecium, for instance, has developed resistance to vancomycin, a last-resort antibiotic [12]. In 2018, the European Antimicrobial Resistance Surveillance Network (EARS-Net) noted a significant increase in the mean proportion of vancomycin-resistant E. faecium (VRE) in invasive isolates within the European Union and European Economic Area (EU/EEA), rising from 10.4% in 2014 to 17.3% [13]. S. aureus, including methicillin-resistant strains (MRSA), poses a persistent challenge in healthcare settings [14]. The recent landmark publication of Cassini et al. estimated nearly 150,000 MRSA infections annually in the EU and EEA countries, leading to over 7000 attributable deaths [15]. K. pneumoniae is notorious for its resistance to carbapenems, crucial antibiotics used for severe infections [16]. MDR strains of K. pneumoniae cause over 90,000 infections and 7000 deaths annually, and contribute to 25% of total disability-adjusted life years lost to bacterial infections in Europe alone [15,16].
A. baumannii exhibits resilience against a wide range of antibiotics, earning its designation as a “superbug” [17]. Over the last two decades, global mortality rates for patients with carbapenem-resistant (CR), MDR or extensively drug-resistant (XDR) A. baumannii infections have varied from 24% to 83%, with individuals having multiple comorbidities facing an elevated risk of mortality, particularly from CR A. baumannii infection [18]. The pooled prevalence of MDR P. aeruginosa causing ventilator-associated pneumonia (VAP) was 33%, with varying rates across different regions, ranging from 19.7% in the USA to 87.5% in Iran, and a European prevalence of 29.9% [19]. In the case of Enterobacter spp., according to predictive statistical models (Antimicrobial Resistance, 2022) [20], bacterial resistance in 2019 led to an estimated 4.95 million deaths, with 1.27 million directly attributed to resistance exhibited by Enterobacteriaceae bloodstream infections, alongside significant economic burdens per case of resistant infections [21]. The impact of these ESKAPE pathogens is profound, as AMR undermines the efficacy of conventional antibiotics. This translates to prolonged illnesses for patients, heightened mortality rates, and increased healthcare costs. Infections caused by these pathogens become increasingly difficult to treat, with limited options for effective antibiotics. The dire consequences of AMR extend beyond individual patient outcomes, affecting public health on a global scale.
The practical consequences of AMR demonstrated by ESKAPE pathogens in clinical settings are deeply concerning and demand urgent attention. These pathogens can severely limit treatment options, and increase the risk of treatment failure and patient mortality. Moreover, the rise of AMR variants among these pathogens complicates infection control measures. Addressing this critical issue requires a multifaceted approach involving enhanced surveillance, judicious antibiotic use, the development of novel antimicrobial agents, and the promotion of infection prevention and control strategies to safeguard public health and ensure effective patient care. In this article, the rise and impact of ESKAPE pathogens, the methods currently utilized for eradication, the challenges posed and future perspectives are explored.
2. Treatment and Control Measures Currently Implemented with Limitations
Hospitals and healthcare units implement a range of treatment and control measures to address the contamination and proliferation of MDR strains, recognizing the critical importance of infection prevention and control. For instance, an audit of community-acquired pneumonia (CAP) antimicrobial compliance utilizing an intervention bundle in the Sligo University Hospital (SUH) was collected in Ireland. For the study, patients from August to September 2018 were compared to a post-intervention prospective cohort from May to June 2019. The intervention included implementing a mobile audience response system, promoting an antimicrobial app, creating a physical card with local guidelines, and integrating CURB-65 into the unscheduled admission proforma. The local guidelines aligned with British Thoracic Society CAP guidelines. The results showed that overall compliance with local CAP guidelines significantly improved from 21.6% to 62.5%. Although there was no difference in initial intravenous antibiotic duration, the post-intervention group had a significantly shorter total antibiotic duration. No variations in length of stay or mortality were observed between the groups. Documentation of CURB-65 and streptococcal urinary antigen testing improved, suggesting that a simple, low-cost quality improvement bundle can enhance appropriate antimicrobial prescription and reduce the total antibiotic duration for CAP patients [22]. These measures are essential to safeguard patient safety and limit the spread of resistant strains. However, factors such as resource constraints, such as a lack of financial investments in personnel training, data management systems, and educational resources, and a small number of specialized personnel with training in infectious diseases, microbiology, pharmacy, and other relevant fields, has proven to be detrimental to the success of these endeavours. These limitations can lead to a shift in treatment determination and a lack of guideline adherence. These measures and their limitations involve the following.
2.1. Antibiotic Stewardship Programs
Hospitals establish antibiotic stewardship programs (ASP) to promote the judicious use of antibiotics. They are crucial initiatives designed to optimize the antibiotic usage in healthcare settings. These programs aim to enhance patient outcomes while minimizing the development of antibiotic resistance and reducing unnecessary healthcare costs. Through the implementation of evidence-based practices, such as guidelines for appropriate antibiotic prescribing, education for healthcare professionals and patients, and robust monitoring systems, these programs seek to ensure that antibiotics are used judiciously and only when necessary. By promoting responsible antibiotic use, these stewardship programs play a pivotal role in preserving the effectiveness of antibiotics, safeguarding public health, and mitigating the growing global threat of antibiotic resistance. An ASP was implemented in urgent care clinics within an integrated academic health system, targeting non-antibiotic-appropriate acute respiratory tract infections (ARIs) while maintaining patient satisfaction. This initiative, launched in fiscal year 2020, involved developing measures, providing feedback, and educating clinicians and patients. Over three fiscal years, the antibiotic prescribing rate decreased significantly for visits subject to stewardship measures, dropping from 34% in FY19 to 12% in FY21. Similarly, antibiotic prescribing decreased for diagnosis-shifting visits from 63% to 35%, and overall antibiotic prescribing decreased from 30% to 10%. Despite challenges posed by the COVID-19 pandemic, patient satisfaction at ARI visits increased from 83% in FY19 to 89% in FY20 and FY21, with no significant correlation found between antibiotic prescribing rates and patient satisfaction. These findings underscore the effectiveness of the ASP in reducing unnecessary antibiotic prescriptions while enhancing patient satisfaction in urgent care settings [23].
Educating and screening patients before prescribing antibiotics helps prevent unnecessary antibiotic use in healthcare settings. The Centers for Disease Control and Prevention (CDCP) offer a useful screening checklist, considering factors such as clinical criteria for bacterial infection, patient allergies, and alternative treatments. Monitoring prescribing practices through programs like Plan–Do–Study Act (PDSA) can reduce antibiotic-resistant infections, while updating hospital policies on antimicrobial stewardship and limiting high-risk antibiotic use, are crucial steps. Hand hygiene practices and medication tracing ensure infection prevention and proper medication administration. Computerized alerts and probes aid in monitoring medication use, and the WHO’s Digital Accelerator Kits facilitate the digitalization of guidance. The education of healthcare staff is essential for optimal clinical practice, emphasizing infection control and antibiotic stewardship. Ensuring the right drugs are used at the right time and promoting a culture of prevention through healthy behaviors and infection control measures are integral to effective ASPs. Organizational changes are necessary to implement these strategies effectively [24].
Additionally, outbreak reports indicate that patients can be infected by organisms acquired from the hospital environment, offering insights into specific surfaces and areas within healthcare settings. However, the contribution of the hospital environment to HAIs remains debated, with many infections linked to patients’ endogenous flora or direct transmission via healthcare providers’ hands. Tracing transmission etiologies is challenging outside of intensive epidemiological investigations conducted during reported outbreaks. Despite evidence of environmental transmission, the role of a clean environment in HAI prevention is controversial, with surface cleaning viewed as complementary rather than a substitute for other infection control practices like hand hygiene. While the literature on environmental cleaning mainly originates from resource-rich countries, resource-limited settings face additional challenges, highlighting disparities in hospital environmental conditions worldwide. The WHOs minimum standards emphasize clean water, waste management, and visible dust and soil control as essential temporary measures for healthcare centers with limited resources, underscoring the need for global efforts to address disparities in hospital environmental hygiene standards. By implementing these mechanisms, healthcare facilities can enhance the effectiveness of antimicrobial stewardship practices, ultimately improving patient outcomes and mitigating the development of AMR [25]. Figure 2 highlights the various components of ASPs [26].
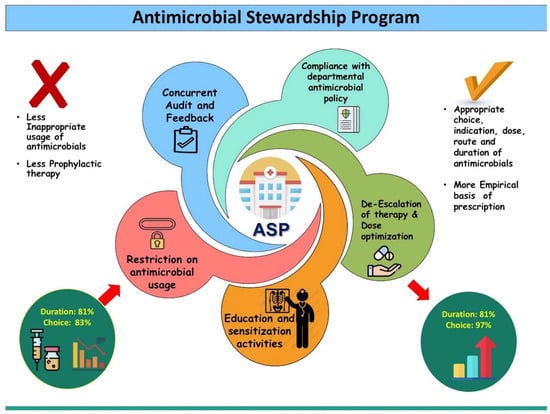
Figure 2.
Main intervention strategies adhered to during the antimicrobial stewardship programme (APS) intervention among patients admitted to a trauma centre of a tertiary care hospital in Chandigarh, India, 2017–2018. [26]. Reprinted from Ref. [26], Copyright (2019), with permission from Elsevier.
2.1.1. Infection Prevention and Control Practices
Hand hygiene: Proper hand hygiene is fundamental in preventing the spread of multidrug-resistant bacteria. Healthcare workers are trained to adhere to strict hand hygiene protocols, including the use of hand sanitizers and thorough handwashing techniques. A systematic review of data from 1980 to 2013 reported that improvements in hand hygiene compliance are associated with a reduction in HAIs in general and multidrug-resistant organisms, such as healthcare-associated MRSA infections in particular [27].
However, despite rigorous training, achieving consistent compliance with hand hygiene protocols can be challenging. Healthcare workers may face time constraints, and busy work environments may contribute to lapses in adherence. Additionally, hand hygiene compliance may vary among different healthcare professionals. Over the last twenty years, research has revealed various factors linked to poor adherence to hand hygiene practices. Challenges like understaffing and overcrowding pose significant barriers to achieving optimal compliance levels, particularly exacerbated during outbreaks of infectious diseases. A study highlights that during the intervention phase, pre-pandemic follow-up, and follow-up during COVID-19, hand hygiene compliance (HHC) rates were 58%, 46%, and 34%, respectively. Comparisons showed a significant decrease in HHC during the pre-pandemic follow-up (46% vs. 58%, p < 0.0001) and the follow-up period during COVID-19 (34% vs. 58%, p < 0.0001) compared to the intervention phase. Despite the pandemic, HHC among healthcare workers significantly declined over time after management discontinued data presentation meetings. This study underscores the tendency of healthcare workers to revert to previous hand hygiene practices once improvement initiatives cease [28]. Additionally, a high workload is identified as another hindrance, particularly in healthcare environments with a substantial number of hand hygiene opportunities per hour of patient care, putting healthcare workers at a heightened risk of lower compliance. Research findings also indicate that doctors exhibit lower compliance compared to nurses in adhering to recommended hygiene practices. The act of wearing gloves is identified as a potential factor contributing to non-compliance, as it is sometimes mistakenly viewed as a replacement for proper hand hygiene. Improper use of gloves may create a false sense of protection and contribute to the transmission of pathogens. Despite clear discouragement of glove reuse in healthcare settings and the absence of a standardized reprocessing method, there is a concerning rise in glove reuse, particularly in resource-limited environments and during health crises [29].
Isolation protocols: Isolation protocols utilized in healthcare settings currently include the allocation of private rooms (single occupancy) where infected or colonized patients are often placed in private rooms to minimize direct contact with other patients in the setting. This is particularly crucial when dealing with airborne or highly contagious pathogens. Another method includes the active cohorting of patients afflicted with similar strains, which can prevent the spread of the specific bacteria in question. This grouping strategy helps healthcare providers implement targeted infection control measures. However, placing patients in isolation may negatively impact their psychological well-being and overall experience. It can lead to feelings of isolation, increased anxiety, and a perceived lack of personalized care. Balancing infection control needs with patient-centered care is crucial. Most importantly, overcrowded hospitals often struggle to accommodate the influx of patients, leading to compromised infection prevention measures and increased risk of HAIs. The shortage of beds not only limits the ability to isolate infected individuals effectively but also places strain on healthcare staff to manage patient care efficiently. In crowded environments, the potential for cross-contamination escalates, as close proximity facilitates the spread of pathogens [30].
Personal protective equipment (PPE): Healthcare workers may use PPE, such as gloves and gowns, when caring for patients with multidrug-resistant infections to minimize the risk of contamination, but the consistent use of PPE can be resource-intensive, requiring a constant supply of gloves, gowns, masks, and other protective gear. Some healthcare settings may face challenges in maintaining an adequate and sustainable supply, especially during periods of high demand [26]. For instance, in a study conducted during the COVID-19 pandemic, the appropriate selection and rational use of PPE were shown to be essential to prevent supply shortages and reduce the risk of infection among healthcare workers. While PPE serves as a crucial protective measure, the study emphasized that it should be considered the last line of defense, complemented by effective administrative and engineering controls like the early identification of suspected cases and source control. Given the evolving understanding of transmission risks, PPE recommendations may change accordingly, underscoring the importance of healthcare facilities and healthcare workers staying informed and prepared for future updates [31].
2.1.2. Environmental Cleaning and Disinfection
The rigorous cleaning and disinfection of hospital environments are crucial to eliminate MDR bacteria on surfaces. Enhanced cleaning protocols are often implemented in areas where these bacteria may be prevalent. Environmental cleaning and disinfection in hospitals involve systematic processes to eliminate pathogens from surfaces and healthcare settings, preventing the spread of infections and ensuring a safe environment for patients and healthcare workers. This includes regular cleaning with detergents followed by targeted disinfection of high-touch surfaces using appropriate antimicrobial agents like chlorhexidine, povidone–iodine, hexachlorophene, octenidine dihydrochloride and silver sulfadiazine [25].
Antiseptic resistance and tolerance pose significant challenges in the realm of infection control, particularly in the context of MDR ESKAPE pathogens. While antibiotics primarily target bacterial cells, antiseptics and disinfectants are designed to eliminate microorganisms on surfaces and in the environment. However, some pathogens have developed mechanisms to resist or tolerate the effects of these chemical agents. In addition to the findings by Cai et al., it was discovered that exposure to ultraviolet C (UVC) radiation induced a significant portion of Aeromonas, Pseudomonas, and S. aureus into the viable but nonculturable (VBNC) state. This transition was quantified through assays utilizing 5-cyano-2,3-ditolyl tetrazolium chloride flow cytometry (CTC-FCM) and D2O-labeled Raman spectroscopy. The VBNC state is characterized by bacterial cells that remain metabolically active but are unable to proliferate on standard culture media, posing challenges for traditional detection and eradication methods. This revelation underscores the importance of understanding bacterial responses to environmental stressors, particularly in the context of clinically relevant pathogens such as those belonging to the ESKAPE group [32]. These pathogens, notorious for their ability to evade antimicrobial treatments, may exploit mechanisms such as entering the VBNC state to persist in healthcare environments, potentially leading to recurrent infections and complicating infection control measures.
Additionally, wastewater systems, particularly those exposed to hospital or livestock wastewater, have been identified as major reservoirs of epidemic pathogens associated with high-risk clones. These include E. faecium strains belonging to the high-risk Enterococcal clonal complex (HiRECC) [33], A. baumannii clones carrying OXA-23 (belonging to international clone 2, IC2) and OXA-72 (belonging to international clone 1, IC1) [34], K. pneumoniae strains of sequence types (ST) 11 and 258 [35] and P. aeruginosa strains of ST235, ST111, and ST175. These findings underscore the potential role of wastewater systems as significant contributors to the dissemination of antibiotic-resistant pathogens. Therefore, comprehensive strategies that encompass both conventional and innovative approaches are essential for effectively managing ESKAPE pathogens and minimizing their impact on public health [36].
2.1.3. Surveillance Programs with Patient/Staff Education
Hospitals may implement active surveillance programs to identify patients carrying MDR bacteria upon admission. This allows for early detection and appropriate infection control measures [37]. The continuous monitoring of the prevalence of these resistant strains is essential to track their dynamics over time, identify emerging resistance patterns, and inform timely adjustments to control measures. Examples of surveillance protocols include routine screening of patients for MDR organisms, analyzing antimicrobial susceptibility patterns, and molecular typing to trace the spread of specific strains [38]. Surveillance data not only aid in understanding the prevalence and distribution of MDR strains but also helps identify potential risk factors and sources of transmission within healthcare facilities. For instance, if surveillance reveals an uptick in a particular MDR strain in a specific hospital unit, targeted interventions, such as increased screening, enhanced environmental cleaning, and reinforced staff training, can be implemented to contain the spread [39]. Collaborative efforts between researchers, clinicians, and public health agencies enhance the ability to stay ahead of evolving resistance patterns. In this way, surveillance becomes an integral part of a proactive approach to combatting MDR bacteria, influencing hospital protocols by providing real-time insights that guide decision-making, resource allocation, and the optimization of infection control practices. [36,40].
Surveillance programs, while valuable, may have limitations in detecting all carriers of MDR bacteria. False negatives can occur, leading to potential underestimation of the actual prevalence. For instance, in a study the limitations of national surveillance systems addressing antimicrobial resistance in Europe encompass three primary areas: structural challenges, issues with laboratory-based surveillance, and inadequate coordination with animal and food surveillance programs. These systems often suffer from a lack of coordination, standardization, and harmonization, which impedes effective data collection, sharing, and collaboration. This comprehensive study analyzing 24 national and 14 regional surveillance systems across 19 European countries unveiled significant discrepancies in reporting practices and adherence to international guidelines. Despite the potential benefits of point-prevalence surveys and laboratory-based surveillance, the delayed publication of data diminishes their practicality for timely decision-making and resource allocation. Furthermore, only a minority of surveillance systems offer real-time access to resistance data, underscoring the pressing need for enhanced data accessibility and timeliness [40]. This emphasizes the need for a combination of surveillance methods for improved accuracy. Ongoing research and surveillance efforts also require substantial resources, including funding, skilled personnel, and advanced laboratory facilities. Some healthcare settings, particularly those with limited resources, may struggle to maintain comprehensive surveillance programs consistently.
Education is a key component in the fight against MDR bacteria. Hospitals conduct educational programs for both healthcare staff and patients to raise awareness about the importance of infection prevention, proper antibiotic use, and the potential consequences of MDR infections [41]. The impact of educational programs on long-term behavior change may be challenging to sustain. Continuous reinforcement and adaptation of educational strategies are necessary to keep healthcare professionals and patients consistently aware of the importance of infection prevention and prudent antibiotic use [42]. It empowers patients to understand the appropriate use of antibiotics, reducing antibiotic prescriptions and informed patients are more likely to adhere to treatment plans, minimizing the development of resistant strains. Additionally, educating healthcare staff ensures consistent adherence to antimicrobial stewardship protocols, leading to better patient outcomes and preserving the effectiveness of antibiotics for future generations [43]. Table 1 summarizes the measures implemented and the obstacles and flaws involved.

Table 1.
Current ASPs with advantages and limitations of implementation.
3. Effects of ASP on ESKAPE Pathogens
Recognizing the formidable threat posed by ESKAPE pathogens, ASPs play a pivotal role in promoting responsible antibiotic use, curbing resistance, and ensuring effective infection control measures in healthcare environments. The cases highlight the effectiveness and limitations of these programs in the context of ESKAPE pathogens in a clinical environment. Some of the cases are extensively tabulated in Table 2.

Table 2.
A comprehensive summary of the various studies involving ASP implementation and their role in reducing antibiotic consumption in patients affected by ESKAPE pathogens.
4. Future Measures for Successful Mitigation
While the measures currently implemented can be fine-tuned for the mitigation of MDR bacteria, a few more measures can be implemented for their effective eradication. Addressing the prevalence of ESKAPE pathogens necessitates a comprehensive approach, including stringent hygiene practices, judicious antibiotic use, and ongoing research to develop novel antimicrobial agents.
Addressing the challenge of multidrug-resistant bacteria requires a forward-looking approach that involves innovative measures and a comprehensive strategy. Below are potential measures that could be implemented in the future to aid in the successful mitigation of multidrug-resistant bacteria.
4.1. Incentives for Novel Antibiotic Development
Dedicating resources to research and development is crucial for the discovery and creation of novel antibiotics that boast distinctive mechanisms of action. This investment fosters innovation in the pharmaceutical industry, driving the pursuit of antibiotics less susceptible to resistance. By incentivizing the development of antibiotics with a lower likelihood of resistance, we can fortify our arsenal against evolving microbial threats [57]. Miethke et al. recommend the establishment of an international coalition of experienced advocates for AMR. The Global AMR R&D Hub is proposed as a central hub for coordinating such efforts, supported by various consortia, including the authors’ initiative, the International Research Alliance for Antibiotic Discovery and Development (IRAADD). This alliance aligns with the mission outlined in the current roadmap and has received backing from the JPIAMR Virtual Research Institute [58]. By offering funding, governments and organizations encourage pharmaceutical companies to allocate resources to antibiotic research, which is often financially challenging due to the high costs and lengthy timelines involved [59]. Making the research and development costs more manageable, tax credits can encourage sustained efforts in this critical area [60]. This approach addresses concerns about the financial viability of antibiotic development by ensuring a return on investment for pharmaceutical companies [61]. For instance, initiatives like public–private partnerships can leverage the strengths of both sectors, pooling resources and expertise to address the challenges associated with antibiotic development. These collaborations can lead to joint research efforts, shared infrastructure, and accelerated development timelines [62].
The development of novel antibiotics for ESKAPE pathogens remains challenging due to the rapid emergence of multidrug resistance and difficulties in identifying new drug targets. This poses a significant threat to global health as effective treatment options dwindle. Consequently, researchers are exploring alternative strategies, such as drug repurposing, to combat antibiotic resistance and extend the usefulness of existing antibiotics. Drug repurposing offers a cost-effective approach to combat antibiotic resistance while extending the lifespan of existing antibiotics. For instance, research discovered BMS-833923 (a phosphatidylglycerol-selective adjuvant to colistin) as a potent compound that directly kills Gram-positive bacteria and enhances colistin’s effectiveness against Gram-negative strains. BMS showed no evidence of inducing antibiotic resistance and effectively treated drug-resistant bacteria in tests by disrupting membranes. This study suggests BMS as a promising antibacterial candidate against Gram-positive pathogens like MRSA and VRE, and as an adjunct to bolster colistin’s activity against various Gram-negative pathogens, including multidrug-resistant strains and ESKAPE pathogens [63]. Additionally, encouraging pharmaceutical companies to explore neglected or underexplored classes of antibiotics can broaden the spectrum of available treatments. The pursuit of antibiotics with decreased resistance potential aligns with global health goals and addresses the urgent need for sustainable solutions to combat bacterial infections. For instance, a comprehensive review discusses the current utilization of quinolones for these pathogens, their subsequent deterioration and the need for various quinolone hybrids with different heterocyclic moieties, exploring their potential as antibacterial agents. The article highlights the effectiveness of quinolone–thiazole/thiadiazole hybrids, which exhibit antibacterial effects by inhibiting bacterial cell wall and folic acid synthesis. Quinolone–oxadiazole/oxazolidinone hybrids are emphasized for their unique mechanism of action in protein synthesis. The study also explores quinolone hybrids with 1,2,3-/1,2,4-triazole/tetrazole, imidazole, pyrimidine/pyridine/quinazolinone, sulfonamide/sulfonyl hydrazide/sulfenamide, kanglemycin/macrolide, isatin/quinoline, and miscellaneous compounds. Notable findings include promising antibacterial activity of ciprofloxacin/norfloxacin-1,2,3-triazole hybrids and ciprofloxacin–isatin hybrids against a range of pathogens. Additionally, hybrids like 2-quinolone–thiazole–coumarin demonstrated profound activity against specific antibiotic-resistant strains [64].
4.2. Interdisciplinary Research
Promoting collaborative and interdisciplinary research is essential for devising comprehensive strategies to combat multidrug-resistant bacteria. This approach involves fostering collaboration among experts from diverse fields such as microbiology, epidemiology, pharmacology, and social sciences. By pooling the expertise of researchers from these distinct disciplines, a more holistic understanding of the multifaceted challenges posed by multidrug-resistant bacteria can be achieved [65]. For example, microbiologists can contribute by delving into the genetic and molecular aspects of antibiotic resistance mechanisms, while epidemiologists can provide insights into the spread and dynamics of resistant strains within populations. Pharmacologists play a crucial role in studying the pharmacokinetics and pharmacodynamics of antimicrobial agents, helping to optimize dosages and treatment regimens. Social scientists contribute valuable perspectives on behavioral factors, public awareness, and healthcare infrastructure, influencing the success of interventions [66].
This interdisciplinary collaboration not only broadens the scope of research but also enhances the potential for innovative solutions. Understanding the socio-cultural factors influencing antibiotic misuse can guide the development of targeted public awareness campaigns, promoting responsible antibiotic use. Moreover, this collaborative approach fosters a more robust and adaptable response to the evolving challenges posed by MDR bacteria, ensuring that strategies are not only effective but also sustainable in diverse settings. Overall, encouraging interdisciplinary research is a key element in the concerted effort to address the complex issue of antibiotic resistance.
4.3. Alternative Therapies and Treatment Modalities
In advocation for the exploration of innovative therapeutic approaches beyond traditional antibiotics, recognizing the need for diversified strategies in combating bacterial infections is experiencing a boom. Examples of these alternative approaches include phage therapy, which utilizes bacteriophages to specifically target and eliminate bacteria, offering a promising avenue for treatment. Additionally, monoclonal antibodies, designed to target specific bacterial components, and immunotherapies, harnessing the body’s immune system to combat infections, are suggested as potential alternatives or complementary options to conventional antibiotic treatments [67].
Phage therapy, in particular, involves the use of bacteriophages, which are viruses that infect and kill bacteria. These viruses can be tailored to target specific bacterial strains, providing a highly targeted and precise method for combating infections [68]. Monoclonal antibodies, on the other hand, are laboratory-created molecules designed to bind to specific targets on bacteria, enhancing the immune system’s ability to recognize and eliminate them. Immunotherapies aim to bolster the body’s natural defenses against bacterial infections, potentially offering a more sustainable and adaptive approach to treatment [69].
By diversifying the arsenal of therapeutic options to include these innovative approaches, the medical community aims to address the challenges posed by antibiotic resistance while improving treatment efficacy and minimizing adverse effects. This holistic exploration of alternative strategies represents a crucial step towards a more comprehensive and sustainable approach to managing bacterial infections in the face of increasing antibiotic resistance.
4.4. Precision Medicine for Infectious Diseases
Precision medicine, also known as personalized medicine, is an innovative approach to healthcare that tailors medical treatment and interventions to individual characteristics, such as genetic makeup, molecular profiles, and environmental factors. In the context of multidrug-resistant pathogens, precision medicine seeks to develop targeted therapies and interventions that are specifically designed to address the unique characteristics of each patient’s infection [70]. Some of these methods include
- -
- Genetic variability: Precision medicine often involves genomic analysis to identify genetic variations in pathogens that contribute to drug resistance. For example, the identification of specific mutations in the genes responsible for antibiotic targets can inform the development of drugs that overcome these resistance mechanisms. In the case of Mycobacterium tuberculosis, whole-genome sequencing can reveal mutations associated with resistance to anti-tuberculosis drugs, guiding the selection of personalized treatment regimens [71].
- -
- Tailored treatments: Precision medicine aims to develop drugs that specifically target the vulnerabilities of multidrug-resistant pathogens. The use of targeted therapies, such as monoclonal antibodies, that focus on specific components of the bacterial cell wall can be explored for bacteria like MRSA [72].
- -
- Pharmacogenomic studies: They can help understand how an individual’s genetic makeup influences their response to medications, aiding in the selection of the most effective and least toxic drugs. Identifying genetic markers associated with resistance to certain antibiotics can guide clinicians in choosing alternative drugs for treating infections caused by multidrug-resistant bacteria [73,74].
- -
- Host factors: Precision medicine recognizes the importance of host–pathogen interactions and incorporates host factors into treatment strategies. Understanding the host’s immune response and genetic factors can inform the development of immunotherapies or adjunctive therapies that enhance the host’s ability to combat infections caused by multidrug-resistant pathogens [75].
4.5. Technological Innovations
In the ongoing effort to combat antimicrobial resistance, this study emphasizes the importance of integrating advanced technologies into healthcare practices. Notably, the incorporation of cutting-edge tools like artificial intelligence (AI), machine learning (ML), and rapid diagnostic tools has proven instrumental in augmenting the early detection of multidrug-resistant strains [76].
Artificial intelligence plays a pivotal role by analyzing vast datasets to identify patterns and trends that might be indicative of drug resistance. Machine learning algorithms, through continuous learning and adaptation, contribute to refining diagnostic capabilities, making them more accurate and efficient over time. Rapid diagnostic tools, leveraging the latest technological advancements, enable healthcare professionals to swiftly identify specific resistance mechanisms, facilitating targeted and precise treatment decisions [77].
For instance, AI algorithms can analyze patient data, including previous antibiotic exposure and microbial genomic information, to predict the likelihood of drug resistance. Machine learning models can adapt to evolving resistance patterns, providing real-time insights into the emergence of multidrug-resistant strains. Rapid diagnostic tools, equipped with advanced molecular techniques, can quickly identify genetic markers associated with resistance, allowing for tailored interventions [78].
The synergy of these advanced technologies not only enhances the speed and accuracy of detecting multidrug-resistant strains but also enables healthcare providers to adopt a more targeted and effective approach in prescribing antimicrobial treatments. This integration represents a promising step forward in the battle against antimicrobial resistance, emphasizing the need for a comprehensive and technologically advanced approach to safeguard public health.
4.6. Global Surveillance Networks
The establishment and enhancement of global surveillance networks play a pivotal role in combating the spread of multidrug-resistant strains, providing a comprehensive approach to monitor resistance patterns and fostering timely information sharing across healthcare facilities, regions, and countries. One key aspect of these networks involves the systematic collection and analysis of data on antimicrobial resistance. By leveraging advanced technologies and collaborative efforts, countries and healthcare facilities can contribute information on emerging resistance trends, enabling the identification of hotspots and high-risk areas. For instance, a global surveillance network might involve the real-time monitoring of antibiotic usage, genetic sequencing of resistant strains, and sharing of treatment outcomes [79].
International collaborations, such as the Global Antimicrobial Resistance Surveillance System (GLASS), exemplify the effectiveness of such networks. GLASS, coordinated by the WHO, brings together data from various countries, allowing for a comprehensive understanding of global resistance patterns. Through shared information, participating nations can adapt their antimicrobial stewardship programs to align with emerging threats, enhancing their ability to respond effectively. In their report for the European region in 2020, it has been reported that surveillance of nosocomial infections, antimicrobial consumption (AMC), and AMR has been ongoing in EU and EEA countries for nearly two decades, providing valuable trends for policymakers. In 2011, the European Strategic Action Plan highlighted the need for systematic data collection on AMC and AMR in non-EU/EEA countries, leading to the establishment of the AMC and CAESAR networks by the WHO Regional Office for Europe and partner institutions. The CAESAR Network, established in 2012, aids 19 countries in improving laboratory testing and data management for AMR surveillance, with 11 countries currently submitting data. Annual meetings and joint conferences have facilitated collaboration and technical support. Moreover, GLASS includes 25 European Region countries, with plans for further expansion and targeted interventions, such as promoting systematic antibiotic susceptibility testing. Successful pilot projects have already been conducted in Georgia and Armenia, with ongoing initiatives in Tajikistan and Uzbekistan [80].
Moreover, these networks facilitate the exchange of best practices in antimicrobial stewardship and infection control strategies. For instance, a healthcare facility in one region, facing success in reducing antibiotic resistance through a specific intervention, can share its experience with counterparts globally. This knowledge exchange fosters a collaborative learning environment, enabling healthcare providers worldwide to adopt successful strategies and tailor them to their local contexts. Timely information sharing is critical during outbreaks of multidrug-resistant strains, as demonstrated during the COVID-19 pandemic. The rapid dissemination of data enabled a more effective global response. Similarly, a robust global surveillance network for antimicrobial resistance would expedite the identification of emerging threats, allowing for proactive measures to mitigate the spread of resistant strains.
4.7. One Health Approach
Embracing a holistic and comprehensive “One Health” approach is paramount in combating antibiotic resistance, acknowledging the intricate links between human health, animal health, and the environment. This approach advocates for collaborative efforts across various sectors, including human and veterinary medicine, agriculture, and environmental health. By fostering synergy among these domains, strategies can be developed to tackle the broader ecosystem that contributes to the rise of antibiotic resistance [81]. For instance, in veterinary medicine, responsible antibiotic use in livestock is crucial. This involves implementing judicious prescribing practices, monitoring antibiotic consumption, and adopting vaccination strategies to reduce the need for antibiotics. In agriculture, promoting sustainable farming practices, such as organic farming and rotational grazing, can mitigate the overuse of antibiotics in animals raised for food production [82].
Environmental health considerations encompass understanding how antibiotic residues in water sources can contribute to resistance. Efforts to manage pharmaceutical waste, improve wastewater treatment, and reduce environmental contamination play a pivotal role in preventing the spread of antibiotic resistance. By integrating these diverse elements under the One Health umbrella, a more robust defense against antibiotic resistance can be established. The collaborative approach ensures a comprehensive understanding of the complex interplay between human health, animal health, and the environment, leading to more effective strategies to address the multifaceted challenges posed by antibiotic resistance [83].
Most importantly, methods to evaluate global AMR utilizing the One Health approach prove to be enlightening. For example, this study aimed to develop a comprehensive evaluation tool for global AMR using a One Health approach. Incorporating data from 146 countries, the Global One Health Index (GOHI)–AMR scheme was developed, comprising five key indicators and 49 sub-indicators. Results showed that high-income countries (HICs) generally performed better on AMR indicators compared to lower-income countries, with unexpected variations among income groups. GOHI-AMR scores correlated positively with gross domestic product and life expectancy but negatively with natural growth rate and chronic respiratory disease. Significant disparities were observed within and between countries, emphasizing the need for tailored interventions. Notably, collaborations between countries like China and Russia demonstrated promising outcomes. This study underscores the importance of global collaboration and provides targeted recommendations to enhance AMR management in low-ranking countries, positioning GOHI-AMR as a valuable tool for global AMR assessment and intervention planning [84].
Implementing these measures requires a concerted effort from governments, healthcare systems, pharmaceutical industries, researchers, and the public. The complex nature of antibiotic resistance demands a multifaceted and collaborative approach to ensure the successful mitigation of multidrug-resistant bacteria in the future.
5. Conclusions
The landscape of modern medicine has indeed transformed healthcare, ushering in innovative treatments that, unfortunately, bring forth the escalating challenge of microbial adaptability and immunity. While hospitals have traditionally been sanctuaries for healing, certain factors such as close patient proximity, invasive medical procedures, and the extensive use of medical devices can unintentionally turn them into breeding grounds for pathogens. The surge in HAIs, exacerbated by prolonged hospital stays and unnecessary antibiotic administration, has played a pivotal role in the alarming rise of AMR. The emergence of drug-resistant strains, a complex and concerning phenomenon, is rooted in the selective pressure exerted by the overuse and misuse of antibiotics. This process favors the survival and proliferation of microbial strains with heightened resistance, adding layers of complexity to infection treatment. The notorious ESKAPE pathogens, adept at ‘escaping’ antimicrobial treatments, pose a formidable challenge. Their resistance spans multiple classes of antibiotics, including last-resort options, underscoring the urgent need to address AMR. The repercussions of AMR extend beyond individual patient outcomes, impacting global public health with prolonged illnesses, heightened mortality rates, and increased healthcare costs. In this evolving landscape, a concerted effort is imperative to mitigate the impact of AMR and safeguard the efficacy of current and future antimicrobial treatments for clinical applications.
To confront these challenges, a comprehensive approach is indispensable. Stringent hygiene practices, judicious antibiotic use, regulatory and governmental initiatives, and ongoing research for new antimicrobial agents are crucial components. This multifaceted strategy not only addresses the evolving threats posed by ESKAPE pathogens but also ensures the sustainability of our ability to combat infectious diseases amid increasing antimicrobial resistance. It is crucial to recognize the urgency of this issue and to foster international collaboration, research initiatives, and public awareness to curb the growing impact of ESKAPE pathogens on global health. The continuous evolution and spread of antimicrobial resistance underscore the importance of proactive measures to mitigate the challenges posed by these elusive and resilient microorganisms. International programs including global collaborations, education, and public awareness efforts are pivotal in this ongoing battle to safeguard global health. As we navigate the complexities of MDR bacteria, measures like global awareness and defense mechanisms offer a beacon of hope for a future where effective treatments remain within our grasp for public health and well-being.
Author Contributions
Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, visualization and writing—original draft preparation, B.S. and K.R.; writing—review and editing and supervision, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This article received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Raoofi, S.; Kan, F.P.; Rafiei, S.; Hosseinipalangi, Z.; Mejareh, Z.N.; Khani, S.; Abdollahi, B.; Talab, F.S.; Sanaei, M.; Zarabi, F.; et al. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0274248. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Long, Y.; Greenhalgh, C.; Steeg, S.; Wilkinson, J.; Li, H.; Verma, A.; Spencer, A. A systematic review and meta-analysis of risk factors associated with healthcare-associated infections among hospitalised patients in Chinese general hospitals from 2001 to 2022. J. Hosp. Infect. 2023, 135, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Paritosh, K.; Sanyal, P.; Khan, S.; Singh, Y.; Varshney, U.; Nandicoori, V.K. GWAS and functional studies suggest a role for altered DNA repair in the evolution of drug resistance in Mycobacterium tuberculosis. elife 2023, 12, e75860. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, C.; Grohmann, E. Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Bodendoerfer, E.; Marchesi, M.; Imkamp, F.; Courvalin, P.; Böttger, E.C.; Mancini, S. Co-occurrence of aminoglycoside and β-lactam resistance mechanisms in aminoglycoside- non-susceptible Escherichia coli isolated in the Zurich area, Switzerland. Int. J. Antimicrob. Agents 2020, 56, 106019. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Tiwari, A.; Varadwaj, P. An Extensive Review on β-lactamase Enzymes and their Inhibitors. Curr. Med. Chem. 2023, 30, 783–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Luo, J.; Deng, F.; Huang, Y.; Zhou, H. Antibiotic combination therapy: A strategy to overcome bacterial resistance to aminoglycoside antibiotics. Front. Pharmacol. 2022, 13, 839808. [Google Scholar] [CrossRef]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial resistance to antimicrobial agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Kulshrestha, M.; Tiwari, M.; Tiwari, V. Bacteriophage therapy against ESKAPE bacterial pathogens: Current status, strategies, challenges, and future scope. Microb. Pathog. 2023, 186, 106467. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, R.F.; Leong, K.W.; Cumming, V.; Van Hal, S.J. Vancomycin-resistant Enterococcus faecium and the emergence of new sequence types associated with hospital infection. Res. Microbiol. 2023, 174, 104046. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance Surveillance in Europe 2022–2020 Data; World Health Organization, Re-gional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital signs: Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible staphylococcus aureus bloodstream infections—United States. Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: An approach based on the mechanisms of resistance to car-bapenems. Infection 2020, 48, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Chung, K.T. Alexander Fleming and the discovery of penicillin. Adv. Appl. Microb. 2001, 49, 163–184. [Google Scholar]
- Du, X.; Xu, X.; Yao, J.; Deng, K.; Chen, S.; Shen, Z.; Yang, L.; Feng, G. Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: A systematic review and meta-analysis. Am. J. Infect. Control 2019, 47, 1140–1145. [Google Scholar] [CrossRef]
- Li, Y.; Roberts, J.A.; Walker, M.M.; Aslan, A.T.; Harris, P.N.; Sime, F.B. The global epidemiology of ventilator-associated pneumonia caused by multi-drug resistant Pseudomonas aeruginosa: A systematic review and meta-analysis. Int. J. Infect. Dis. 2023, 139, 78–85. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Wozniak, T.M.; Barnsbee, L.; Lee, X.J.; Pacella, R.E. Using the best available data to estimate the cost of antimicrobial resistance: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 1–12. [Google Scholar] [CrossRef]
- O’kelly, B.; Rueda-Benito, A.; O’regan, M.; Finan, K. An audit of community-acquired pneumonia antimicrobial compliance using an intervention bundle in an Irish hospital. J. Glob. Antimicrob. Resist. 2020, 23, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Ng, T.; Madani, L.S.; Persell, S.D.; Greg, M.; Roemer, P.E.; Oberoi, S.K.; Linder, J.A. Antibiotic stewardship to reduce inappropriate antibiotic prescribing in integrated academic health-system urgent care clinics. Infect. Control. Hosp. Epidemiology 2023, 44, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Bankar, N.J.; Ugemuge, S.; Ambad, R.S.; Hawale, D.V.; Timilsina, D.R. Implementation of antimicrobial stewardship in the healthcare setting. Cureus 2022, 14, e26664. [Google Scholar] [CrossRef] [PubMed]
- Doll, M.; Stevens, M.; Bearman, G. Environmental cleaning and disinfection of patient areas. Int. J. Infect. Dis. 2018, 67, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Shafiq, N.; Tripathy, J.P.; Nagaraja, S.B.; Kathirvel, S.; Chouhan, D.K.; Arora, P.; Singh, T.; Jain, K.; Gautam, V.; et al. Antimicrobial stewardship programme in a trauma centre of a tertiary care hospital in North India: Effects and implementation challenges. J. Glob. Antimicrob. Resist. 2019, 17, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Shekelle, P.G.; Wachter, R.M.; Pronovost, P.J.; Schoelles, K.; McDonald, K.M.; Dy, S.M.; Shojania, K.; Reston, J.; Berger, Z.; Johnsen, B.; et al. Making health care safer II: An updated critical analysis of the evidence for patient safety practices. Evid. Rep. Technol. Assess. Full Rep. 2013, 211, 1–945. Available online: https://pubmed.ncbi.nlm.nih.gov/24423049/ (accessed on 10 April 2024).
- Stangerup, M.; Hansen, M.B.; Hansen, R.; Sode, L.P.; Hesselbo, B.; Kostadinov, K.; Olesen, B.S.; Calum, H. Hand hygiene compliance of healthcare workers before and during the COVID-19 pandemic: A long-term follow-up study. Am. J. Infect. Control. 2021, 49, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Lotfinejad, N.; Peters, A.; Tartari, E.; Fankhauser-Rodriguez, C.; Pires, D.; Pittet, D. Hand hygiene in health care: 20 years of ongoing advances and perspectives. Lancet Infect. Dis. 2021, 21, e209–e221. [Google Scholar] [CrossRef] [PubMed]
- Chernet, A.Z.; Dasta, K.; Belachew, F.; Zewdu, B.; Melese, M.; Ali, M.M. Burden of healthcare-associated infections and associated risk factors at Adama Hospital Medical College, Adama, Oromia, Ethiopia. Drug Heal. Patient Saf. 2020, 12, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H. Personal protective equipment for healthcare workers during the COVID-19 pandemic. Infect. Chemother. 2020, 52, 165–182. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, J.; Li, G.; Wong, P.K.; An, T. Formation mechanisms of viable but nonculturable bacteria through induction by light-based disinfection and their antibiotic resistance gene transfer risk: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3651–3688. [Google Scholar] [CrossRef]
- Sadowy, E.; Luczkiewicz, A. Drug-resistant and hospital-associated Enterococcus faecium from wastewater, riverine estuary and anthropogenically impacted marine catchment basin. BMC Microbiol. 2014, 14, 66. [Google Scholar] [PubMed]
- Higgins, P.G.; Hrenovic, J.; Seifert, H.; Dekic, S. Characterization of Acinetobacter baumannii from water and sludge line of secondary wastewater treatment plant. Water Res. 2018, 140, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Surleac, M.; Barbu, I.C.; Paraschiv, S.; Popa, L.I.; Gheorghe, I.; Marutescu, L.; Popa, M.; Sarbu, I.; Talapan, D.; Nita, M.; et al. Whole genome sequencing snapshot of multi-drug resistant Klebsiella pneumoniae strains from hospitals and receiving wastewater treatment plants in Southern Romania. PLoS ONE 2020, 15, e0228079. [Google Scholar]
- Merrill, K.; Hanson, S.F.; Sumner, S.; Vento, T.; Veillette, J.; Webb, B. Antimicrobial stewardship: Staff nurse knowledge and attitudes. Am. J. Infect. Control 2019, 47, 1219–1224. [Google Scholar] [PubMed]
- Wilken, D.; Baur, X.; Barbinova, L.; Preisser, A.; Meijer, E.; Rooyackers, J.; Heederik, D. What are the benefits of medical screening and surveillance? Eur. Respir. Rev. 2012, 21, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Blake, K.S.; Choi, J.; Dantas, G. Approaches for characterizing and tracking hospital-associated multidrug-resistant bacteria. Cell. Mol. Life Sci. 2021, 78, 2585–2606. [Google Scholar] [CrossRef] [PubMed]
- Schinas, G.; Polyzou, E.; Spernovasilis, N.; Gogos, C.; Dimopoulos, G.; Akinosoglou, K. Preventing Multidrug-Resistant Bacterial Transmission in the Intensive Care Unit with a Comprehensive Approach: A Policymaking Manual. Antibiotics 2023, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Sifakis, F.; Harbarth, S.; Schrijver, R.; van Mourik, M.; Voss, A.; Sharland, M.; Rajendran, N.B.; Rodríguez-Baño, J.; Bielicki, J.; et al. Surveillance for control of antimicrobial resistance. Lancet Infect. Dis. 2018, 18, e99–e106. [Google Scholar] [CrossRef]
- Dyer, C.; Hutt, L.P.; Burky, R.; Joshi, L.T. Biocide resistance and transmission of Clostridium difficile spores spiked onto clinical surfaces from an American health care facility. Appl. Environ. Microbiol. 2019, 85, e01090-19. [Google Scholar] [CrossRef]
- Sargeant, J.M.; O’Connor, A.M.; Winder, C.B. Editorial: Systematic reviews reveal a need for more, better data to inform antimicrobial stewardship practices in animal agriculture. Anim. Heal. Res. Rev. 2019, 20, 103–105. [Google Scholar] [CrossRef]
- Walker, S. Effective antimicrobial resistance communication: The role of information design. Palgrave Commun. 2019, 5, 24. [Google Scholar] [CrossRef]
- Kinnear, C.L.; Patel, T.S.; Young, C.L.; Marshall, V.; Newton, D.W.; Read, A.F.; Woods, R.J. Impact of an antimicrobial stewardship inter-vention on within-and between-patient daptomycin resistance evolution in vancomycin-resistant Enterococcus faecium. Timicrobial Agents Chemother. 2019, 63, 10–128. [Google Scholar]
- O’Riordan, F.; Shiely, F.; Byrne, S.; O’Brien, D.; Ronayne, A.; Fleming, A. Antimicrobial use and antimicrobial resistance in Entero-bacterales and Enterococcus faecium: A time series analysis. J. Hosp. Infect. 2022, 120, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Gandhi, T.; Chenoweth, C.; Lassiter, J.; Dela Pena, J.; Eschenauer, G.; Nagel, J.L. Impact of an antimicrobial stewardship-led intervention for Staphylococcus aureus bacteraemia: A quasi-experimental study. J. Antimicrob. Chemother. 2015, 70, 3390–3396. [Google Scholar]
- Jover-Sáenz, A.; Ramírez-Hidalgo, M.; Bellés, A.B.; Murillo, E.R.; Bosch, M.B.; Miró, A.R.; López, A.M.; Cabanillas, J.C.; Palacín, N.P.; Garrido-Calvo, S.; et al. Effects of a Primary Care Antimicrobial Stewardship Program on Meticillin-Resistant Staphylococcus aureus Strains across a Region of Catalunya (Spain) over 5 Years. Antibiotics 2024, 13, 92. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Salsano, A.; Del Puente, F.; Campanini, F.; Mariscalco, G.; Marchese, A.; Viscoli, C.; Santini, F. Reduced incidence of carbapenem-resistant klebsiella pneumoniae infections in cardiac surgery patients after implementation of an antimicrobial stewardship project. Antibiotics 2019, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hu, S.; Sun, J.; Zhang, L.; Dong, H.; Feng, W.; Lei, J.; Dong, Y. Antibiotic consumption versus the prevalence of carbapenem-resistant Gram-negative bacteria at a tertiary hospital in China from 2011 to 2017. J. Infect. Public Heal. 2019, 12, 195–199. [Google Scholar] [CrossRef]
- Marra, A.R.; de Almeida, S.M.; Correa, L.; Silva, M., Jr.; Martino, M.D.; Silva, C.V.; Cal, R.G.; Edmond, M.B.; dos Santos, O.F. The effect of limiting antimicrobial therapy duration on antimicrobial resistance in the critical care setting. Am. J. Infect. Control 2009, 37, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Yusef, D.; A Hayajneh, W.; Issa, A.B.; Haddad, R.; Al-Azzam, S.; A Lattyak, E.; Lattyak, W.J.; Gould, I.; Conway, B.R.; Bond, S.; et al. Impact of an antimicrobial stewardship programme on reducing broad-spectrum antibiotic use and its effect on carbapenem-resistant Acinetobacter baumannii (CRAb) in hospitals in Jordan. J. Antimicrob. Chemother. 2020, 76, 516–523. [Google Scholar] [CrossRef]
- Rizk, N.A.; Zahreddine, N.; Haddad, N.; Ahmadieh, R.; Hannun, A.; Bou Harb, S.; Haddad, S.F.; Zeenny, R.M.; Kanj, S.S. The Impact of antimicrobial stewardship and infection control interventions on Acinetobacter baumannii resistance rates in the ICU of a ter-tiary care center in Lebanon. Antibiotics 2022, 11, 911. [Google Scholar] [CrossRef] [PubMed]
- Sid Ahmed, M.A.; Abdel Hadi, H.; Abu Jarir, S.; Al Khal, A.L.; Al-Maslamani, M.A.; Jass, J.; Ibrahim, E.B.; Ziglam, H. Impact of an antimi-crobial stewardship programme on antimicrobial utilization and the prevalence of MDR Pseudomonas aeruginosa in an acute care hospital in Qatar. JAC-Antimicrob. Resist. 2020, 2, dlaa050. [Google Scholar] [CrossRef] [PubMed]
- Slain, D.; Sarwari, A.R.; Petros, K.O.; McKnight, R.L.; Sager, R.B.; Mullett, C.J.; Wilson, A.; Thomas, J.G.; Moffett, K.; Palmer, H.C.; et al. Impact of a multimodal antimicrobial stewardship program on Pseudomonas aeruginosa susceptibility and antimicrobial use in the intensive care unit setting. Crit. Care Res. Pr. 2011, 2011, 416426. [Google Scholar]
- Strazzulla, A.; Bokobza, S.; Ombandza, E.; Kherallah, K.; Hommel, S.; Draidi, R.; Bonutto, C.; Zamponi, D.B.; Gauzit, R.; Diamantis, S. Impact of an antimicrobial stewardship program on resistance to fluoroquinolones of urinary enterobacteriaceae isolated from nursing home residents: A retrospective cohort study. J. Am. Med. Dir. Assoc. 2020, 21, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Cipko, K.; Cuenca, J.; Wales, E.; Harris, J.; Bond, S.; Newton, P.; Miyakis, S. Implementation of an antimicrobial stewardship programme and reduction in carbapenemase-producing Enterobacterales in an Australian local health district. JAC-Antimicrob. Resist. 2020, 2, dlaa041. [Google Scholar] [CrossRef] [PubMed]
- Udaondo, Z.; Matilla, M.A. Mining for novel antibiotics in the age of antimicrobial resistance. Microb. Biotechnol. 2020, 13, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Morel, C.M.; Lindahl, O.; Harbarth, S.; de Kraker, M.E.; Edwards, S.; Hollis, A. Industry incentives and antibiotic resistance: An intro-duction to the antibiotic susceptibility bonus. J. Antibiot. 2020, 73, 421–428. [Google Scholar] [CrossRef]
- Ehsan, F. Boosting innovation in small- and medium-sized enterprises through tax incentives: Lessons from the UK. Sci. Public Policy 2021, 48, 712–726. [Google Scholar] [CrossRef]
- Dutescu, I.A.; Hillier, S.A. Encouraging the development of new antibiotics: Are financial incentives the right way forward? A systematic review and case study. Infect. Drug Resist. 2021, 14, 415–434. [Google Scholar] [CrossRef]
- Arslan, B.; Vasudeva, G.; Hirsch, E.B. Public–Private and Private–Private Collaboration as Pathways for Socially Beneficial Innovation: Evidence from Antimicrobial Drug-Development Tasks. Acad. Manag. J. 2023. [Google Scholar] [CrossRef]
- Zhang, N.; Shan, W.; Gao, L.; Kou, S.H.; Lu, C.; Yang, H.; Peng, B.; Tam, K.Y.; Lee, L.T.O.; Zheng, J. Repurposing the Hedgehog pathway inhibitor, BMS-833923, as a phosphatidylglycerol-selective membrane-disruptive colistin adjuvant against ESKAPE pathogens. Int. J. Antimicrob. Agents 2023, 62, 106888. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hou, H.; Gao, F. Current scenario of quinolone hybrids with potential antibacterial activity against ESKAPE pathogens. Eur. J. Med. Chem. 2023, 247, 115026. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sheldenkar, A.; Lwin, M.O. A decade of antimicrobial resistance research in social science fields: A scientometric review. Antimicrob. Resist. Infect. Control. 2020, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Gong, Y.; Yan, X.; Wang, L.; Zheng, W.; Ai, H.; Zhao, Y. Recent advances in nanoantibiotics against multidrug-resistant bacteria. Nanoscale Adv. 2023, 5, 6278–6317. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.R.; Sinha, D.K.; Agrawal, R.K.; Singh, V. Alternative Approaches to Mitigate Antimicrobial Drug Resistance. Bareilly: Division of Epidemiology; ICAR-Indian Veterinary Research Institute: Bareilly, India, 2021. [Google Scholar]
- Düzgüneş, N.; Sessevmez, M.; Yildirim, M. Bacteriophage therapy of bacterial infections: The rediscovered frontier. Pharmaceuticals 2021, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ding, M.; Song, Y.; Hu, Y.; Xiu, W.; Yuwen, L.; Xie, Y.; Song, Y.; Shao, J.; Song, X.; et al. Nanotherapeutics with immunoregulatory functions for the treatment of bacterial infection. Biomater. Res. 2023, 27, 73. [Google Scholar] [CrossRef] [PubMed]
- Merker, M.; Tueffers, L.; Vallier, M.; Groth, E.E.; Sonnenkalb, L.; Unterweger, D.; Baines, J.F.; Niemann, S.; Schulenburg, H. Evolutionary approaches to combat antibiotic resistance: Opportunities and challenges for precision medicine. Front. Immunol. 2020, 11, 568485. [Google Scholar] [CrossRef] [PubMed]
- Watney, A.A.; Cosgrove, C.A.; Arnold, A.; Hinds, J.; Stoker, N.G.; Butcher, P.D. Clinical use of whole genome sequencing for Myco-bacterium tuberculosis. BMC Med. 2016, 14, 46. [Google Scholar]
- Speziale, P.; Pietrocola, G. Monoclonal antibodies targeting surface-exposed and secreted proteins from staphylococci. Vaccines 2021, 9, 459. [Google Scholar] [CrossRef]
- Marimani, M.; Ahmad, A.; Duse, A. The Role of Advanced Therapeutic Techniques to Combat Multi-drug Resistance. In Non-traditional Approaches to Combat Antimicrobial Drug Resistance; Springer Nature: Singapore, 2023; pp. 29–55. [Google Scholar] [CrossRef]
- Topp, E.; Larsson, D.G.J.; Miller, D.N.; Eede, C.V.D.; Virta, M.P.J. Antimicrobial resistance and the environment: Assessment of advances, gaps and recommendations for agriculture, aquaculture and pharmaceutical manufacturing. FEMS Microbiol. Ecol. 2017, 94, fix185. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Nijnik, A.; Philpott, D.J. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 2012, 10, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, N.E.; Price, V.; Cunningham-Oakes, E.; Tsang, K.K.; Nunn, J.G.; Midega, J.T.; Anjum, M.F.; Wade, M.J.; Feasey, N.A.; Peacock, S.J.; et al. Innovations in genomic antimicrobial resistance surveillance. Lancet Microbe 2023, 4, e1063–e1070. [Google Scholar] [CrossRef] [PubMed]
- Nayarisseri, A.; Khandelwal, R.; Tanwar, P.; Madhavi, M.; Sharma, D.; Thakur, G.; Speck-Planche, A.; Singh, S.K. Artificial intelligence, big data and machine learning approaches in precision medicine & drug discovery. Curr. Drug Targets 2021, 22, 631–655. [Google Scholar] [PubMed]
- Sakagianni, A.; Koufopoulou, C.; Feretzakis, G.; Kalles, D.; Verykios, V.S.; Myrianthefs, P. Using Machine Learning to Predict Anti-microbial Resistance―A Literature Review. Antibiotics 2023, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, G.S. Antimicrobial resistance surveillance in Europe and beyond. Eurosurveillance 2018, 23, 1800560–1800564. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- White, A.; Hughes, J.M. Critical Importance of a One Health Approach to Antimicrobial Resistance. Ecohealth 2019, 16, 404–409. [Google Scholar] [CrossRef]
- Golding, S.E. Exploring Antimicrobial Stewardship in UK Veterinary Medicine and Livestock Agriculture: A Mixed-Method, one Health Approach. Ph.D. Thesis, University of Surrey, Surrey, UK, 2020. [Google Scholar]
- Zhang, X.; Liu, J.; Han, L.; Xia, S.; Li, S.; Li, O.; Kassegne, K.; Li, M.; Yin, K.; Hu, Q.; et al. Towards a global One Health index: A potential assessment tool for One Health performance. Infectious diseases of poverty. Infect. Dis. Poverty 2022, 11, 12–26. [Google Scholar] [CrossRef]
- Zhou, N.; Cheng, Z.; Zhang, X.; Lv, C.; Guo, C.; Liu, H.; Dong, K.; Zhang, Y.; Liu, C.; Chang, Y.F.; et al. Global antimicrobial resistance: A system-wide comprehensive investigation using the Global One Health Index. Infect. Dis. Poverty 2022, 11, 92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).