Harnessing the Power of Zinc-Solubilizing Bacteria: A Catalyst for a Sustainable Agrosystem
Abstract
1. Introduction
2. Zinc Bioavailability in Soil
3. Effectiveness of Zn Fertilizers in Soil
4. Physiological Functions of Zn in Plants
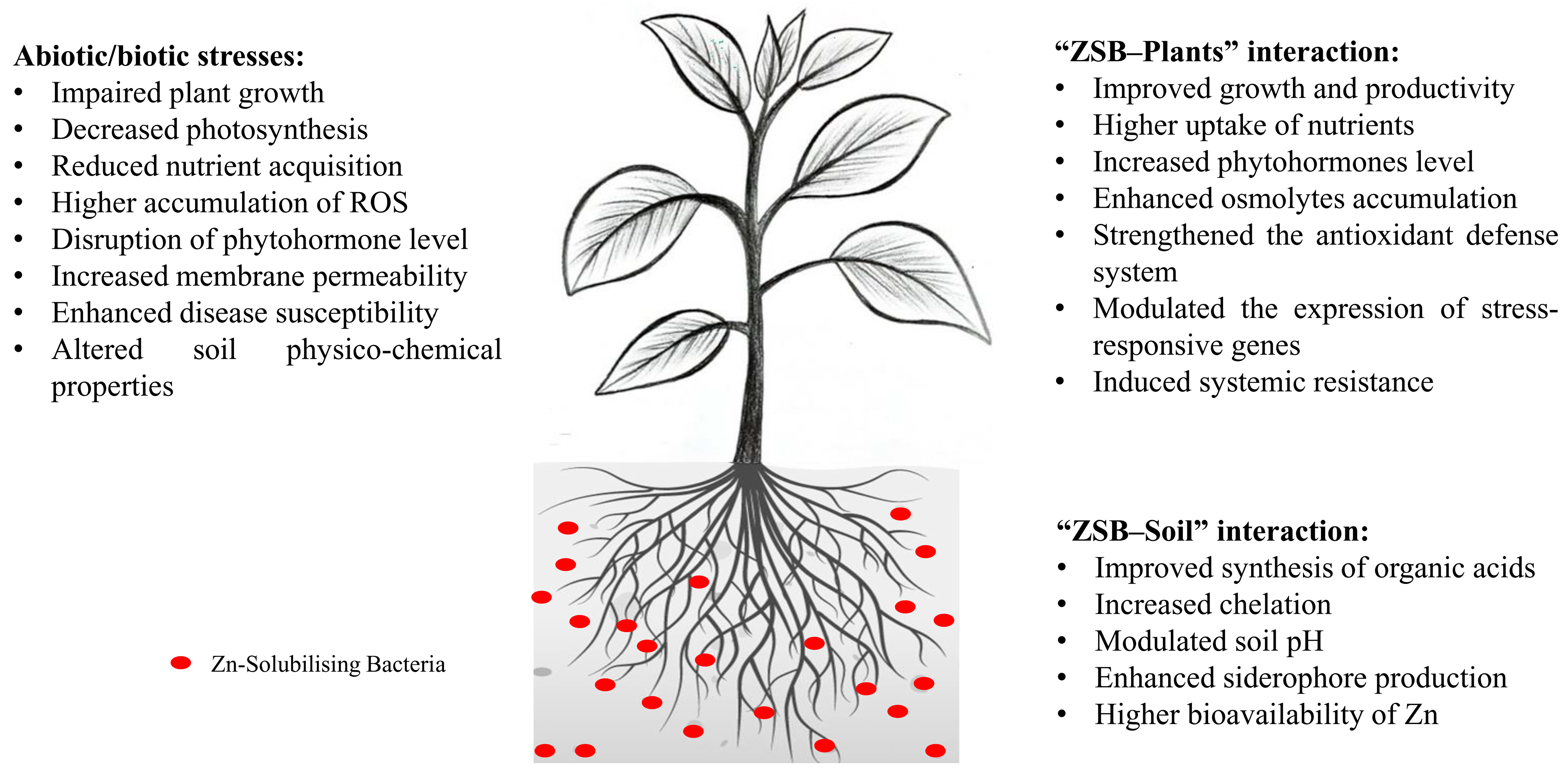
5. Zinc-Solubilizing Bacteria (ZSB) as a Biofertilizer
6. Roles of ZSB in the Biofortification of Crop Plants
6.1. Mechanism of Action
6.2. Chelation of Zn by Siderophore
6.3. Molecular Mechanism of Zn Uptake and Translocation in Plants
6.4. Zn-Assisted Biofortification
6.5. ZSB as a Stress Alleviator
7. Conclusions and Future Aspects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, A.; Zahir, Z.A.; Asghar, H.N.; Ahmad, M.; Jamil, M.; Naveed, M.; Zaman Akhtar, M.F.U. Zinc solubilizing bacteria for zinc biofortification in cereals: A step toward sustainable nutritional security. In Role of Rhizospheric Microbes in Soil: Volume 2: Nutrient Management and Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2018; pp. 203–227. [Google Scholar]
- Nitu, R.; Rajinder, K.; Sukhminderjit, K. Zinc solubilizing bacteria to augment soil fertility—A comprehensive review. Int. J. Agric. Sci. Vet. Med. 2020, 8, 38–44. [Google Scholar]
- Solanki, M.; Didwania, N.; Nandal, V. Potential of zinc solubilizing bacterial inoculants in fodder crops. Momentum 2016, 3, 1–4. [Google Scholar]
- Kumar, A.; Dewangan, S.; Lawate, P.; Bahadur, I.; Prajapati, S. Zinc-solubilizing bacteria: A boon for sustainable agriculture. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management: Volume 1: Rhizobacteria in Abiotic Stress Management; Springer: Singapore, 2019; pp. 139–155. [Google Scholar]
- Sindhu, S.S.; Sharma, R.; Sindhu, S.; Phour, M. Plant nutrient management through inoculation of zinc-solubilizing bacteria for sustainable agriculture. In Biofertilizers for Sustainable Agriculture and Environment; Springer: Cham, Switzerland, 2019; pp. 173–201. [Google Scholar]
- Upadhayay, V.K.; Singh, A.V.; Khan, A. Cross talk between zinc-solubilizing bacteria and plants: A short tale of bacterial-assisted zinc biofortification. Front. Soil Sci. 2022, 1, 788170. [Google Scholar] [CrossRef]
- Upadhayay, V.K.; Singh, A.V.; Pareek, N. An insight in decoding the multifarious and splendid role of microorganisms in crop biofortification. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2407–2418. [Google Scholar] [CrossRef]
- Prathap, S.; Thiyageshwari, S.; Krishnamoorthy, R.; Prabhaharan, J.; Vimalan, B.; Gopal, N.O.; Anandham, R. Role of zinc solubilizing bacteria in enhancing growth and nutrient accumulation in rice plants (Oryza sativa) grown on zinc (Zn) deficient submerged soil. J. Soil Sci. Plant Nutr. 2022, 22, 971–984. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z. Zinc Binding to Fulvic acids: Assessing the Impact of pH, Metal Concentrations and Chemical Properties of Fulvic Acids on the Mechanism and Stability of Formed Soluble Complexes. Molecules 2020, 25, 1297. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Hernandez-Soriano, M.C.; Jimenez-Lopez, J.C. Effects of soil water content and organic matter addition on the speciation and bioavailability of heavy metals. Sci. Total Environ. 2012, 423, 55–61. [Google Scholar] [CrossRef]
- Suganya, A.; Saravanan, A.; Manivannan, N. Role of Zinc Nutrition for Increasing Zinc Availability, Uptake, Yield, and Quality of Maize (Zea mays L.) Grains: An Overview. Commun. Soil Sci. Plant Anal. 2020, 51, 2001–2021. [Google Scholar]
- Rani, S.; Satyavan; Kumar, A.; Beniwal, S. Integrated nutrient management as a managerial tool for applying saline water in wheat crop cultivated under sub-tropic and semi-arid conditions of North-Western India. J. Plant Nutr. 2020, 43, 604–620. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Gondal, A.H.; Zafar, A.; Zainab, D.; Toor, M.D.; Sohail, S.; Ameen, S.; Younas, N. A detailed review study of zinc involvement in animal, plant and human nutrition. Indian J. Pure Appl. Biosci. 2021, 9, 262–271. [Google Scholar] [CrossRef]
- M’rah, S.; Marichali, A.; M’rabet, Y.; Chatti, S.; Casabianca, H.; Hosni, K. Morphology, physiology, and biochemistry of zinc-stressed caraway plants. Protoplasma 2023, 260, 853–868. [Google Scholar] [CrossRef]
- Iqbal, M.N.; Rasheed, R.; Ashraf, M.Y.; Ashraf, M.A.; Hussain, I. Exogenously applied zinc and copper mitigate salinity effect in maize (Zea mays L.) by improving key physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2018, 25, 23883–23896. [Google Scholar] [CrossRef] [PubMed]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Kumaravel, R.; Gopalsamy, J.; Sikder, M.N.A.; Sampathkumar, P. Microalgae as bio-fertilizers for rice growth and seed yield productivity. Waste Biomass Valorization 2018, 9, 793–800. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Bai, Z.; Bao, L.; Xue, L.; Zhang, S.; Wei, Y.; Zhang, Z.; Zhuang, G.; Zhuang, X. Bacillus subtilis biofertilizer mitigating agricultural ammonia emission and shifting soil nitrogen cycling microbiomes. Environ. Int. 2020, 144, 105989. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving crop yield and nutrient use efficiency via biofertilization—A global meta-analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef]
- Khan, A.; Sayyed, R.Z.; Seifi, S. Rhizobacteria: Legendary soil guards in abiotic stress management. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management: Volume 1: Rhizobacteria in Abiotic Stress Management; Springer: Singapore, 2019; pp. 327–343. [Google Scholar]
- Rehman, A.; Farooq, M.; Naveed, M.; Nawaz, A.; Shahzad, B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur. J. Agron. 2018, 94, 98–107. [Google Scholar] [CrossRef]
- Abaid-Ullah, M.; Hassan, M.N.; Nawaz, M.K.; Hafeez, F.Y. Biofortification of wheat (Triticum aestivum L.) through Zn mobilizing PGPR. In Proceedings of the International Science Conference “Prospects and Challenges to Sustainable Agriculture”, Muzaffarabad, Pakistan, 14–16 July 2011; p. 298. [Google Scholar]
- Singh, D.; Geat, N.; Rajawat, M.V.S.; Prasanna, R.; Kar, A.; Singh, A.M.; Saxena, A.K. Prospecting endophytes from different Fe or Zn accumulating wheat genotypes for their influence as inoculants on plant growth, yield, and micronutrient content. Ann. Microbiol. 2018, 68, 815–833. [Google Scholar] [CrossRef]
- Kumawat, N.; Kumar, R.; Khandkar, U.R.; Yadav, R.K.; Saurabh, K.; Mishra, J.S.; Hans, H. Silicon (Si)- and Zinc (Zn)-Solubilizing Microorganisms: Role in Sustainable Agriculture. In Biofertilizers for Sustainable Agriculture and Environment; Springer: Cham, Switzerland, 2019; pp. 109–135. [Google Scholar]
- Khalid, S.; Amanullah; Ahmed, I. Enhancing Zinc Biofortification of Wheat through Integration of Zinc, Compost, and Zinc-Solubilizing Bacteria. Agriculture 2022, 12, 968. [Google Scholar] [CrossRef]
- Yadav, R.C.; Sharma, S.K.; Varma, A.; Rajawat, M.V.S.; Khan, M.S.; Sharma, P.K.; Malviya, D.; Singh, U.B.; Rai, J.P.; Saxena, A.K. Modulation in biofertilization and biofortification of wheat crop by inoculation of zinc-solubilizing rhizobacteria. Front. Plant Sci. 2022, 13, 777771. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Patel, J.S.; Meena, V.S. Rhizospheric Microbes for Sustainable Agriculture: An Overview. In Role of Rhizospheric Microbes in Soil; Springer: Singapore, 2018; pp. 1–31. [Google Scholar]
- Verma, S.; Kumar, M.; Kumar, A.; Das, S.; Chakdar, H.; Varma, A.; Saxena, A.K. Diversity of bacterial endophytes of maize (Zea mays) and their functional potential for micronutrient biofortification. Curr. Microbiol. 2021, 79, 6. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Maheshwari, D.K. Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.M.I.; Othman, R.; Zuan, A.T.K.; Shamsuddin, A.S.; Zaman, N.B.K.; Abu Sari, N.; Panhwar, Q.A. Isolation, characterization, and identification of zinc-solubilizing bacteria (ZSB) from wetland rice fields in Peninsular Malaysia. Agriculture 2022, 12, 1823. [Google Scholar] [CrossRef]
- Hefferon, K. Biotechnological Approaches for Generating Zinc-Enriched Crops to Combat Malnutrition. Nutrients 2019, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Ajeesh Krishna, T.P.; Maharajan, T.; Victor Roch, G.; Ignacimuthu, S.; Antony Ceasar, S. Structure, function, regulation and phylogenetic relationship of ZIP family transporters of plants. Front. Plant Sci. 2020, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Y.; Liu, Z.; Tian, J.; Liang, L.; Qiu, Y.; Wang, G.; Du, Q.; Cheng, D.; Cai, H.; et al. A high activity zinc transporter OsZIP9 mediates zinc uptake in rice. Plant J. 2020, 103, 1695–1709. [Google Scholar] [CrossRef]
- Deshpande, P.; Dapkekar, A.; Oak, M.; Paknikar, K.; Rajwade, J. Nanocarrier-mediated foliar zinc fertilization influences expression of metal homeostasis related genes in flag leaves and enhances gluten content in durum wheat. PLoS ONE 2018, 13, e0191035. [Google Scholar] [CrossRef] [PubMed]
- Evens, N.P.; Buchner, P.; Williams, L.E.; Hawkesford, M.J. The role of ZIP transporters and group F bZIP transcription factors in the Zn-deficiency response of wheat (Triticum aestivum). Plant J. 2017, 92, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.; Shahid, I.; Baig, D.N.; Rizwan, M.; Malik, K.A.; Mehnaz, S. Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front. Microbiol. 2017, 8, 2593. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Arshad, M.; Zahir, Z.A.; Asghar, M. Prospects of zinc solubilizing bacteria for enhancing growth of maize. Pak. J. Agric. Sci. 2015, 52, 915–922. [Google Scholar]
- Hussain, A.; Zahir, Z.A.; Asghar, H.N.; Imran, M.; Ahmad, M.; Hussain, S. Integrating the potential of Bacillus sp. Az6 and organic waste for zinc oxide bio-activation to improve growth, yield and zinc content of maize grains. Pak. J. Agric. Sci. 2020, 57, 123–130. [Google Scholar]
- Zeb, H.; Hussain, A.; Naveed, M.; Ditta, A.; Ahmad, S.; Jamshaid, M.U.; Ahmad, H.T.; Hussain, M.B.; Aziz, R.; Haider, M.S. Compost enriched with ZnO and Zn-solubilising bacteria improves yield and Zn-fortification in flooded rice. Ital. J. Agron. 2018, 13, 310–316. [Google Scholar] [CrossRef]
- Singh, K.; Batra, R.; Sharma, S.; Saripalli, G.; Gautam, T.; Singh, R.; Pal, S.; Malik, P.; Kumar, M.; Jan, I.; et al. WheatQTLdb: A QTL database for wheat. Mol. Genet. Genom. 2021, 296, 1051–1056. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, N.; Yadav, A.N.; Kumar, A.; Meena, V.S.; Singh, B.; Chauhan, V.S.; Dhaliwal, H.S.; Saxena, A.K. Rhizospheric microbiomes: Biodiversity, mechanisms of plant growth promotion, and biotechnological applications for sustainable agriculture. In Plant Growth Promoting Rhizobacteria for Agricultural Sustainability: From Theory to Practices; Springer: Singapore, 2019; pp. 19–65. [Google Scholar]
- Gontia-Mishra, I.; Sapre, S.; Tiwari, S. Zinc solubilizing bacteria from the rhizosphere of rice as prospective modulator of zinc biofortification in rice. Rhizosphere 2017, 3, 185–190. [Google Scholar] [CrossRef]
- Vaid, S.K.; Kumar, B.; Sharma, A.K.; Shukla, A.K.; Srivastava, P. Effect of Zn solubilizing bacteria on growth promotion and Zn nutrition of rice. J. Soil Sci. Plant Nutr. 2014, 14, 889–910. [Google Scholar] [CrossRef]
- Batool, S.; Asghar, H.N.; Shehzad, M.A.; Yasin, S.; Sohaib, M.; Nawaz, F.; Akhtar, G.; Mubeen, K.; Zahir, Z.A.; Uzair, M. Zinc-solubilizing bacteria-mediated enzymatic and physiological regulations confer zinc biofortification in chickpea (Cicer arietinum L.). J. Soil Sci. Plant Nutr. 2021, 21, 2456–2471. [Google Scholar] [CrossRef]
- Upadhyay, H.; Gangola, S.; Sharma, A.; Singh, A.; Maithani, D.; Joshi, S. Contribution of zinc solubilizing bacterial isolates on enhanced zinc uptake and growth promotion of maize (Zea mays L.). Folia Microbiol. 2021, 66, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Goteti, P.K.; Emmanuel, L.D.A.; Desai, S.; Shaik, M.H.A. Prospective zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int. J. Microbiol. 2013, 2013, 869697. [Google Scholar] [CrossRef] [PubMed]
- Qaisrani, M.M.; Zaheer, A.; Mirza, M.S.; Naqqash, T.; Qaisrani, T.B.; Hanif, M.K.; Rasool, G.; Malik, K.A.; Ullah, S.; Jamal, M.S.; et al. A comparative study of bacterial diversity based on culturable and culture-independent techniques in the rhizosphere of maize (Zea mays L.). Saudi J. Biol. Sci. 2019, 26, 1344–1351. [Google Scholar] [CrossRef]
- Yasmin, R.; Hussain, S.; Rasool, M.H.; Siddique, M.H.; Muzammil, S. Isolation, characterization of Zn solubilizing bacterium (Pseudomonas protegens RY2) and its contribution in growth of chickpea (Cicer arietinum L.) as deciphered by improved growth parameters and Zn content. Dose-Response 2021, 19, 5593258211036791. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ahmed, I.; Tariq, H.; Abbas, S.; Zia, M.H.; Mumtaz, A.; Sharif, M. Growth improvement of wheat (Triticum aestivum) and zinc biofortification using potent zinc-solubilizing bacteria. Front. Plant Sci. 2023, 14, 1140454. [Google Scholar] [CrossRef]
- Shaikh, S.; Saraf, M. Biofortification of Triticum aestivum through the inoculation of zinc solubilizing plant growth promoting rhizobacteria in field experiment. Biocatal. Agric. Biotechnol. 2017, 9, 120–126. [Google Scholar] [CrossRef]
- Krithika, S.; Balachandar, D. Expression of zinc transporter genes in rice as influenced by zinc-solubilizing Enterobacter cloacae strain ZSB14. Front. Plant Sci. 2016, 7, 446. [Google Scholar] [CrossRef]
- Iqbal, U.; Jamil, N.; Ali, I.; Hasnain, S. Effect of zinc-phosphate-solubilizing bacterial isolates on growth of Vigna radiata. Ann. Microbiol. 2010, 60, 243–248. [Google Scholar] [CrossRef]
- Kushwaha, P.; Srivastava, R.; Pandiyan, K.; Singh, A.; Chakdar, H.; Kashyap, P.L.; Bhardwaj, A.K.; Murugan, K.; Karthikeyan, N.; Bagul, S.Y.; et al. Enhancement in plant growth and zinc biofortification of chickpea (Cicer arietinum L.) by Bacillus altitudinis. J. Soil Sci. Plant Nutr. 2021, 21, 922–935. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M.; Nadeem, F.; Rehman, A.; Hussain, M.; Nawaz, A.; Naveed, M. Zinc application in combination with zinc solubilizing Enterobacter sp. MN17 improved productivity, profitability, zinc efficiency, and quality of desi chickpea. J. Soil Sci. Plant Nutr. 2020, 20, 2133–2144. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Sultan, A.A.Y.A.; Gebreel, H.M.; Youssef, H.I.A. Biofertilizer effect of some zinc dissolving bacteria free and encapsulated on Zea mays growth. Arch. Microbiol. 2023, 205, 202. [Google Scholar] [CrossRef]
- Jain, D.; Kour, R.; Bhojiya, A.A.; Meena, R.H.; Singh, A.; Mohanty, S.R.; Rajpurohit, D.; Ameta, K.D. Zinc tolerant plant growth promoting bacteria alleviates phytotoxic effects of zinc on maize through zinc immobilization. Sci. Rep. 2020, 10, 13865. [Google Scholar] [CrossRef] [PubMed]
- Suriyachadkun, C.; Chunhachart, O.; Srithaworn, M.; Tangchitcharoenkhul, R.; Tangjitjareonkun, J. Zinc-Solubilizing Streptomyces spp. as bioinoculants for promoting the growth of soybean (Glycine max (L.) Merrill). J. Microbiol. Biotechnol. 2022, 32, 1435. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, R.C.; Choudhary, P.; Sharma, S.K.; Bhagat, N. Mitigation of drought stress in wheat (Triticum aestivum L.) by inoculation of drought tolerant Bacillus paramycoides DT-85 and Bacillus paranthracis DT-97. J. Appl. Biol. Biotechnol. 2022, 10, 59–69. [Google Scholar] [CrossRef]
- Shakeel, M.; Rais, A.; Hassan, M.N.; Hafeez, F.Y. Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati rice (Oryza sativa) varieties. Front. Microbiol. 2015, 6, 1286. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Upadhayay, V.K.; Singh, A.V.; Khan, A.; Sharma, A. Contemplating the role of zinc-solubilizing bacteria in crop biofortification: An approach for sustainable bioeconomy. Front. Agron. 2022, 4, 903321. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Vadlamudi, S.; Samineni, S.; Kumar, C.V.S. Plant growth-promotion and biofortification of chickpea and pigeonpea through inoculation of biocontrol potential bacteria, isolated from organic soils. SpringerPlus 2016, 5, 1882. [Google Scholar] [CrossRef]
- Moreno-Lora, A.; Recena, R.; Delgado, A. Bacillus subtilis QST713 and cellulose amendment enhance phosphorus uptake while improving zinc biofortification in wheat. Appl. Soil Ecol. 2019, 142, 81–89. [Google Scholar] [CrossRef]
- Karnwal, A. Pseudomonas spp., a zinc-solubilizing vermicompost bacteria with plant growth-promoting activity moderates zinc biofortification in tomato. Int. J. Veg. Sci. 2021, 27, 398–412. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Malik, A.; Nazli, F.; Latif, M.; Zaheer, A.; Ali, Q.; Jamil, M.; Ahmad, M. Potential of zinc solubilizing Bacillus strains to improve growth, yield, and quality of maize (Zea mays). Int. J. Agric. Biol. 2020, 24, 691–698. [Google Scholar]
- Jalal, A.; Galindo, F.S.; Boleta, E.H.M.; Oliveira, C.E.d.S.; dos Reis, A.R.; Nogueira, T.A.R.; Neto, M.J.M.; Mortinho, E.S.; Fernandes, G.C.; Filho, M.C.M.T. Common bean yield and zinc use efficiency in association with diazotrophic bacteria co-inoculations. Agronomy 2021, 11, 959. [Google Scholar] [CrossRef]
- Javed, H.M.J.; Akhtar, H.N.; Jamil, A.A. Screening of zinc solubilizing bacteria and their potential to increase grain concentration in wheat (Triticum aestivum). Int. J. Agric. Biol. 2018, 20, 547–553. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Rajput, V.D.; Kumari, A.; Espinosa-Saiz, D.; Menendez, E.; Minkina, T.; Dwivedi, P.; Mandzhieva, S. Plant growth-promoting rhizobacteria: A potential bio-asset for restoration of degraded soil and crop productivity with sustainable emerging techniques. Environ. Geochem. Health 2023, 45, 9321–9344. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Wu, Q.S.; Giri, B. Salinity: An overview. In Microorganisms in Saline Environments: Strategies and Functions; Springer: Cham, Switzerland, 2019; pp. 3–18. [Google Scholar]
- Chauhan, A.; Saini, R.; Sharma, J.C. Plant growth promoting rhizobacteria and their biological properties for soil enrichment and growth promotion. J. Plant Nutr. 2021, 45, 273–299. [Google Scholar] [CrossRef]
- Janeeshma, E.; Puthur, J.T. Direct and indirect influence of arbuscular mycorrhizae on enhancing metal tolerance of plants. Arch. Microbiol. 2020, 202, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Janeeshma, E.; Rajan, V.K.; Puthur, J.T. Spectral variations associated with anthocyanin accumulation; an apt tool to evaluate zinc stress in Zea mays L. Chem. Ecol. 2021, 37, 32–49. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar]
- Bisht, A.; Garg, N. AMF modulated rhizospheric microbial enzyme activities and their impact on sulphur assimilation along with thiol metabolism in pigeonpea under Cd stress. Rhizosphere 2022, 21, 100478. [Google Scholar] [CrossRef]
- Bisht, A.; Garg, N. Harnessing the role of arbuscular mycorrhizae in arresting nodular senescence by modulating osmolyte synthesis and ascorbate-glutathione pool in cadmium stressed pigeon pea. Plant Growth Regul. 2024, 102, 409–427. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, V.K.; Tripathi, V.; Singh, P.P.; Singh, A.K. Plant growth-promoting rhizobacteria (PGPR): Perspective in agriculture under biotic and abiotic stress. In Crop Improvement through Microbial Biotechnology; Elsevier: Hoboken, NJ, USA, 2018; pp. 333–342. [Google Scholar]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B. From interaction to gene induction: An eco-friendly mechanism of PGPR-mediated stress management in the plant. In Plant Microbiome: Stress Response; Springer: Singapore, 2018; pp. 217–232. [Google Scholar]
- Grossi, C.E.M.; Fantino, E.; Serral, F.; Zawoznik, M.S.; Porto, D.A.F.D.; Ulloa, R.M. Methylobacterium sp. 2A is a plant growth-promoting rhizobacteria that has the potential to improve potato crop yield under adverse conditions. Front. Plant Sci. 2020, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Nordstedt, N.P.; Chapin, L.J.; Taylor, C.G.; Jones, M.L. Identification of Pseudomonas spp. that increase ornamental crop quality during abiotic stress. Front. Plant Sci. 2019, 10, 1754. [Google Scholar] [CrossRef] [PubMed]
- Saberi-Riseh, R.; Fathi, F.; Moradzadeh-Eskandari, M. Effect of some Pseudomonas fluorescens and Bacillus subtilis strains on osmolytes and antioxidants of cucumber under salinity stress. J. Crop Prot. 2020, 9, 1–16. [Google Scholar]
- Orozco-Mosqueda, M.d.C.; Duan, J.; DiBernardo, M.; Zetter, E.; Campos-García, J.; Glick, B.R.; Santoyo, G. The production of ACC deaminase and trehalose by the plant growth promoting bacterium Pseudomonas sp. UW4 synergistically protect tomato plants against salt stress. Front. Microbiol. 2019, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hameed, S.; Shahid, M.; Iqbal, M.; Lazarovits, G.; Imran, A. Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiol. Res. 2020, 232, 126389. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Godino, A.; Príncipe, A.; Ramírez, V.L.; Quesada, J.M.; Rigo, V.; Espinosa-Urgel, M.; Morales, G.M.; Fischer, S. Characterization of the bacteriocins and the PrtR regulator in a plant-associated Pseudomonas strain. J. Biotechnol. 2020, 307, 182–192. [Google Scholar] [CrossRef]
- Amna; Xia, Y.; Farooq, M.A.; Javed, M.T.; Kamran, M.A.; Mukhtar, T.; Ali, J.; Tabassum, T.; Rehman, S.U.; Munis, M.F.H.; et al. Multi-stress tolerant PGPR Bacillus xiamenensis PM14 activating sugarcane (Saccharum officinarum L.) red rot disease resistance. Plant Physiol. Biochem. 2020, 151, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ji, X.; Ge, Y.; Li, J.; Qi, W.; Qiao, K. Characterization of antagonistic Bacillus methylotrophicus isolated from rhizosphere and its biocontrol effects on maize stalk rot. Phytopathology 2019, 109, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of plants with microbial world: Exploring the regulatory networks for PGPR mediated defense signaling. Microbiol. Res. 2020, 238, 126486. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.K.; Vishnoi, V.K.; Dubey, R.; Maheshwari, D.K. A twin rhizospheric bacterial consortium induces systemic resistance to a phytopathogen Macrophomina phaseolina in mung bean. Rhizosphere 2018, 5, 71–75. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Lou, Y.; Shi, M.; Jiang, Y.; Zhou, J.; Sun, Y.; Xue, Q.; Lai, H. Bacillus amyloliquefaciens Ba13 induces plant systemic resistance and improves rhizosphere microecology against tomato yellow leaf curl virus disease. Appl. Soil Ecol. 2019, 137, 154–166. [Google Scholar] [CrossRef]
- Singh, U.B.; Malviya, D.; Singh, S.; Singh, P.; Ghatak, A.; Imran, M.; Rai, J.P.; Singh, R.K.; Manna, M.C.; Sharma, A.K.; et al. Salt-tolerant compatible microbial inoculants modulate physio-biochemical responses enhance plant growth, Zn biofortification and yield of wheat grown in saline-sodic soil. Int. J. Environ. Res. Public Health 2021, 18, 9936. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Inoculation with Lysinibacillus fusiformis strain YJ4 and Lysinibacillus sphaericus strain YJ5 alleviates the effects of cold stress in maize plants. Gesunde Pflanz. 2023, 75, 77–95. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S.; Kotra, V.; Kumar, A. Assessing the role of ACC deaminase-producing bacteria in alleviating salinity stress and enhancing zinc uptake in plants by altering the root architecture of French bean (Phaseolus vulgaris) plants. Planta 2023, 258, 3. [Google Scholar] [CrossRef]
- Ain, N.U.; Naveed, M.; Hussain, A.; Mumtaz, M.Z.; Rafique, M.; Bashir, M.A.; Alamri, S.; Siddiqui, M.H. Impact of coating of urea with bacillus-augmented zinc oxide on wheat grown under salinity stress. Plants 2020, 9, 1375. [Google Scholar] [CrossRef]
- Azeem, M.; Haider, M.Z.; Javed, S.; Saleem, M.H.; Alatawi, A. Drought stress amelioration in maize (Zea mays L.) by inoculation of Bacillus spp. strains under sterile soil conditions. Agriculture 2022, 12, 50. [Google Scholar] [CrossRef]
- Shirinbayan, S.; Khosravi, H.; Malakouti, M.J. Alleviation of drought stress in maize (Zea mays) by inoculation with Azotobacter strains isolated from semi-arid regions. Appl. Soil Ecol. 2019, 133, 138–145. [Google Scholar] [CrossRef]
- Anzuay, M.S.; Ciancio, M.G.R.; Ludueña, L.M.; Angelini, J.G.; Barros, G.; Pastor, N.; Taurian, T. Growth promotion of peanut (Arachis hypogaea L.) and maize (Zea mays L.) plants by single and mixed cultures of efficient phosphate solubilizing bacteria that are tolerant to abiotic stress and pesticides. Microbiol. Res. 2017, 199, 98–109. [Google Scholar] [CrossRef]
- Jinal, H.N.; Gopi, K.; Kumar, K.; Amaresan, N. Effect of zinc-resistant Lysinibacillus species inoculation on growth, physiological properties, and zinc uptake in maize (Zea mays L.). Environ. Sci. Pollut. Res. 2021, 28, 6540–6548. [Google Scholar] [CrossRef] [PubMed]
- Bhakat, K.; Chakraborty, A.; Islam, E. Characterization of zinc solubilization potential of arsenic tolerant Burkholderia spp. isolated from rice rhizospheric soil. World J. Microbiol. Biotechnol. 2021, 37, 39. [Google Scholar] [CrossRef] [PubMed]
- Kour, R.; Jain, D.; Bhojiya, A.A.; Sukhwal, A.; Sanadhya, S.; Saheewala, H.; Jat, G.; Singh, A.; Mohanty, S.R. Zinc biosorption, biochemical and molecular characterization of plant growth-promoting zinc-tolerant bacteria. 3 Biotech 2019, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Rezaee Niko, B.; Enayatizamir, N.; Norozi Masir, M. Effect of zinc solubilizing growth promoter bacterium on plant growth under laboratory conditions. J. Agric. Eng. Soil Sci. Agric. Mech. (Sci. J. Agric.) 2018, 41, 113–132. [Google Scholar]
- Pantoja-Guerra, M.; Burkett-Cadena, M.; Cadena, J.; Dunlap, C.A.; Ramírez, C.A. Lysinibacillus spp.: An IAA-producing endospore forming-bacteria that promotes plant growth. Antonie van Leeuwenhoek 2023, 116, 615–630. [Google Scholar] [CrossRef]
- Rais, A.; Jabeen, Z.; Shair, F.; Hafeez, F.Y.; Hassan, M.N. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS ONE 2017, 12, e0187412. [Google Scholar] [CrossRef]
- Sachdev, S.; Bauddh, K.; Singh, R.P. Native Rhizospheric Microbes Mediated Management of Biotic Stress and Growth Promotion of Tomato. Sustainability 2022, 15, 593. [Google Scholar] [CrossRef]
- Weinand, T.; El-Hasan, A.; Asch, F. Role of Bacillus spp. Plant Growth Promoting Properties in Mitigating Biotic and Abiotic Stresses in Lowland Rice (Oryza sativa L.). Microorganisms 2023, 11, 2327. [Google Scholar] [CrossRef]
| Mineral Zn | Complexed Zn | Adsorbed Zn |
|---|---|---|
| Smithsonite (ZnCO3) | Manure | Zn-CaCO3 |
| Sphalerite (ZnS) | Organic | Zn-MgCO3 |
| Zincite (ZnO) | Residues | Zn-FeO |
| Franklinit (ZnFe2O4) | Zn-MnO | |
| Wellemite (Zn2SiO4) Hopeite [Zn3(PO4)2·4H2O] |
| S. No. | Name of ZSB | Host Plants | Mode of Action | References |
|---|---|---|---|---|
| 1. | Pantoea dispersa, P. agglomerans, Pseudomonas fragi, Rhizobium sp., and E. cloacae | Triticum aestivum | Increased shoot dry weight and Zn uptake and accelerated the bioavailability of Zn. | [40] |
| 2. | Bacillus sp. | Zea mays | Promoted root and shoot length, dry and fresh weight, transpiration rate, and chlorophyll content. | [41,42] |
| 3. | Bacillus sp. | Oryza sativa | Higher photosynthetic rate, transpiration rate, stomatal conductance, and carbonic hydrase activity, as well as reduced electrolytic leakage. | [43] |
| 4. | Trichoderma harzianum and Bacillus amyloliquefaciens | Triticum aestivum | Upregulated the expression of ZIP transporters, caused more plant growth, and improved Zn fortification. | [44] |
| 5. | Bacillus aryabahttai | Oryza sativa | Improved plant biometrics, especially grain yield. | [45] |
| 6. | Ralstonia picketti, Pseudomonas aeruginosa, Klebsiella pneumoniae and Burkholderia cepacia | Oryza sativa | Increased Zn biofortification, growth, and Zn bioaccessibility to the plants. | [46] |
| 7. | Burkholderia and Acinetobacter | Oryza sativa | Improved dry matter production, the number of panicles, grain and straw yield, and Zn uptake. | [47] |
| 8. | Ochrobactrum intermedium, Paenibacillus polymyxa, Bacillus cereus, Stenotrophomonas maltophili, Streptomyces, and Arthrobacter globiformi | Cicer arietinum | Increased availability of Zn, increased nitrogen (N) and P content in grain, and increased Zn content in shoot, roots, and grains. | [48] |
| 9. | Burkholderia cepacia and Acinetobacter baumannii | Zea mays | Improved plant height, root length, and Zn uptake. | [49] |
| 10. | Pseudomonas and Bacillus spp. | Zea mays | Higher plant growth and increased N, K, Mn, and Zn uptake. | [50,51] |
| 11. | Pseudomonas protegens | Cicer arietinum | Enhanced shoot and root growth as well as Zn uptake. | [52] |
| 12. | Pantoea sp., Klebsiella sp., Brevibacterium sp., Klebsiella sp., Acinetobacter sp., Alcaligenes sp. NCCP-650, Citrobacter sp., Exiguobacterium sp., Raoultella sp., and Acinetobacter sp. | Triticum aestivum | Improved dry weights, fresh weights, and Zn acquisition. | [53] |
| 13. | Exiguobacterium aurantiacum | Triticum aestivum | Increased nutritional quality of seeds by enhancing the accumulation of Zn, Fe, N, P, and K. | [54] |
| 14. | Enterobacter cloacae | Oryza sativa | Upregulated the expression of ZIP genes and increased the accumulation of Zn in root and shoot. | [55] |
| 15. | Neisseria, Staphylococcus cocci, Escherichia coli, and Bacillus sp. | Vigna radiata | Improved plant growth attributes including root and shoot length and fresh and dry weight. | [56] |
| 16. | Bacillus altitudinis | Cicer arietinum | Improved growth attributes and higher Zn uptake. | [57] |
| 17. | Enterobacter sp. | Cicer arietinum | Improved yield, bioavailability of Zn, and grain quality. | [58] |
| 18. | Bacillus aryabhattai | Triticum aestivum, Glycine max | Reduced soil pH, increased the production of total organic acid, and improved soil enzymatic activities. | [59] |
| 19. | Acinetobacter calcoaceticus, Bacillus proteolyticus and Stenotrophomonas pavanii | Zea mays | Higher Zn content and plant dry weight. | [60] |
| 20. | Serratia sp. | Zea mays | Increased peroxidase, superoxide dismutase, catalase, and polyphenol activity. | [61] |
| 21. | Streptomyces spp. | Glycine max | Increased root and shoot length, dry weight of plants, and number of pods. | [62] |
| 22. | Bacillus spp. | Triticum aestivum | Enhanced nutrient use efficacy, growth, yield, and Zn biofortification. | [63] |
| Stress | ZSB | Plant | Mechanism of Action | References |
|---|---|---|---|---|
| Salinity | Bacillus amyloliquefaciens B-16 | Triticum aestivum L. | Increased uptake and translocation of potassium and calcium. | [98] |
| Bacillus pumilus and Pseudomonas pseudoalcaligenes | Oryza sativa | Improved chlorophyll, carotenoids, and antioxidant enzymes activity. | [99] | |
| Pantoea agglomerans R1 and Pseudomonas fragi R4 | Phaseolus vulgaris | Higher chlorophyll, carotenoid, and osmoprotectants levels, and improved antioxidative enzymes activity. | [100] | |
| Bacillus spp. | Triticum aestivum L. | Increased plant growth parameters and Zn content in shoots as well as grains. | [101] | |
| Drought | Bacillus spp. | Zea mays | Improved physiological and biochemical traits, alongside reduced antioxidant enzyme activity. | [102] |
| Azotobacter | Zea mays | Enhanced plant growth. | [103] | |
| Heavy metals | Serratia spp. | Zea mays | Improved plant growth parameter and antioxidant enzyme activity. | [104] |
| Lysinibacillus spp. | Zea mays L. | Increased chlorophyll a and b, proline, total phenol, and ascorbic acid content. | [105] | |
| Burkholderia vietnamiensis and Burkholderia seminalis | Oryza sativa | Induced the production of indole acetic acid (IAA) and the solubilization of potassium and phosphate. | [106] | |
| Serratia sp. | Zea mays | Enhanced shoot length, root length, and total chlorophyll content. | [107] | |
| Temperature | Stenotrophomonas | Zea mays | Increased carbohydrates, auxins, and chlorophyll contents, and imparted heat stress resilience. | [108] |
| L. fusiformis and L. sphaericus | Zea mays | Improved lignin content, cell viability, osmolytes (proline, glycine betaine, and soluble sugars) accumulation, total phenols and 1-aminocyclopropane-1-carboxylic acid (ACC) contents, and upregulated the antioxidant defense system. | [109] | |
| Disease | Bacillus sp. and Bacillus cereus | Oryza sativa | Suppressed the growth of Pyricularia oryzae and Fusarium moniliforme, and increased the yield. | [110] |
| T. lixii | Solanum lycopersicum | Reduced Fusarium wilt and early blight severity. | [111] | |
| B. pumilus | Oryza sativa | Inhibited fungal growth and reduced brown spot disease. | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Chhabra, R.; Sharma, A.; Bisht, A. Harnessing the Power of Zinc-Solubilizing Bacteria: A Catalyst for a Sustainable Agrosystem. Bacteria 2024, 3, 15-29. https://doi.org/10.3390/bacteria3010002
Singh S, Chhabra R, Sharma A, Bisht A. Harnessing the Power of Zinc-Solubilizing Bacteria: A Catalyst for a Sustainable Agrosystem. Bacteria. 2024; 3(1):15-29. https://doi.org/10.3390/bacteria3010002
Chicago/Turabian StyleSingh, Swapnil, Rohit Chhabra, Ashish Sharma, and Aditi Bisht. 2024. "Harnessing the Power of Zinc-Solubilizing Bacteria: A Catalyst for a Sustainable Agrosystem" Bacteria 3, no. 1: 15-29. https://doi.org/10.3390/bacteria3010002
APA StyleSingh, S., Chhabra, R., Sharma, A., & Bisht, A. (2024). Harnessing the Power of Zinc-Solubilizing Bacteria: A Catalyst for a Sustainable Agrosystem. Bacteria, 3(1), 15-29. https://doi.org/10.3390/bacteria3010002










