Acinetobacter baumannii and Its Relationship to Carbapenem Resistance: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Data Analysis
3. Results
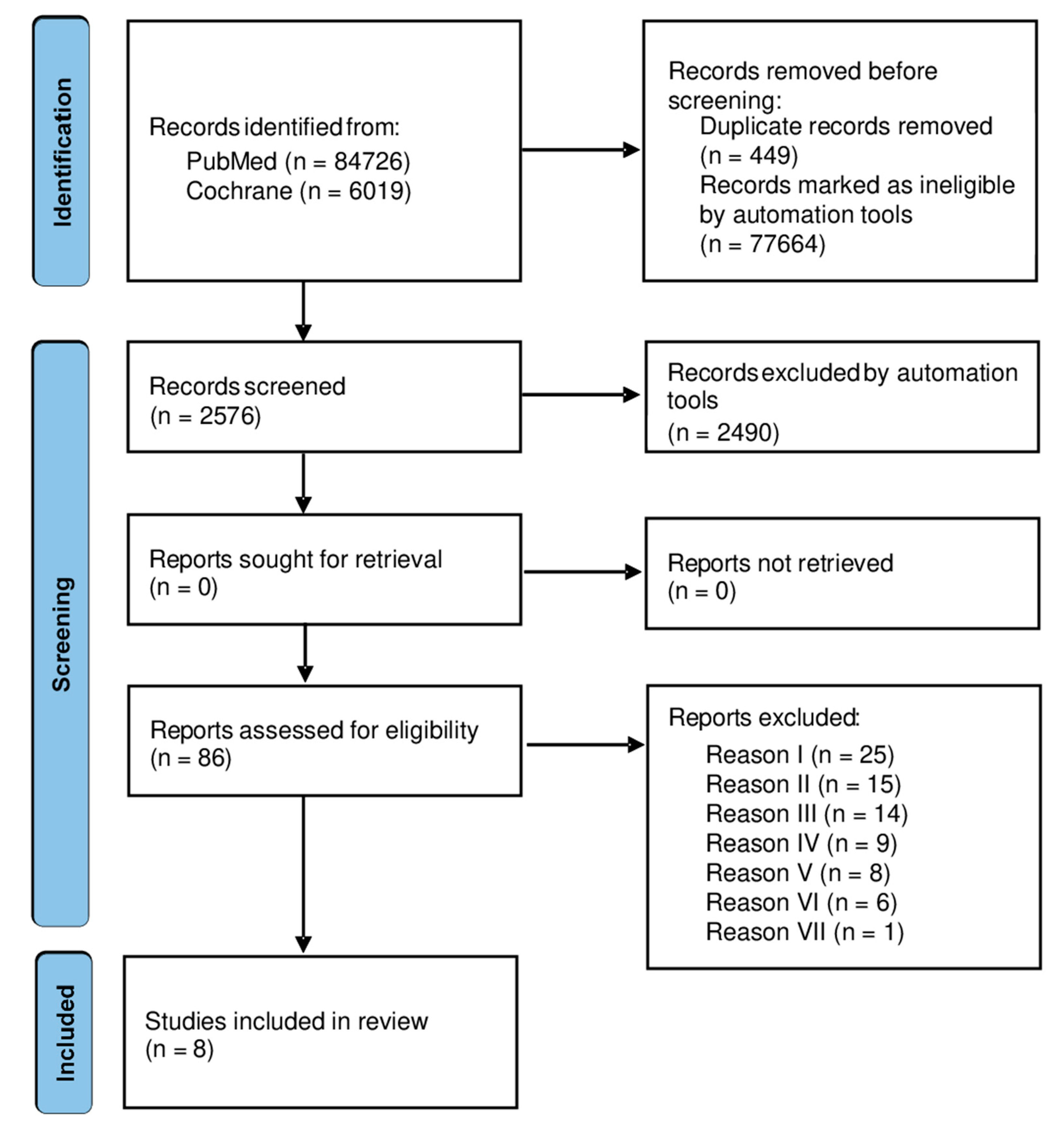
3.1. Studies Selection and Characteristics
3.2. Statistical Analysis and Synthesis
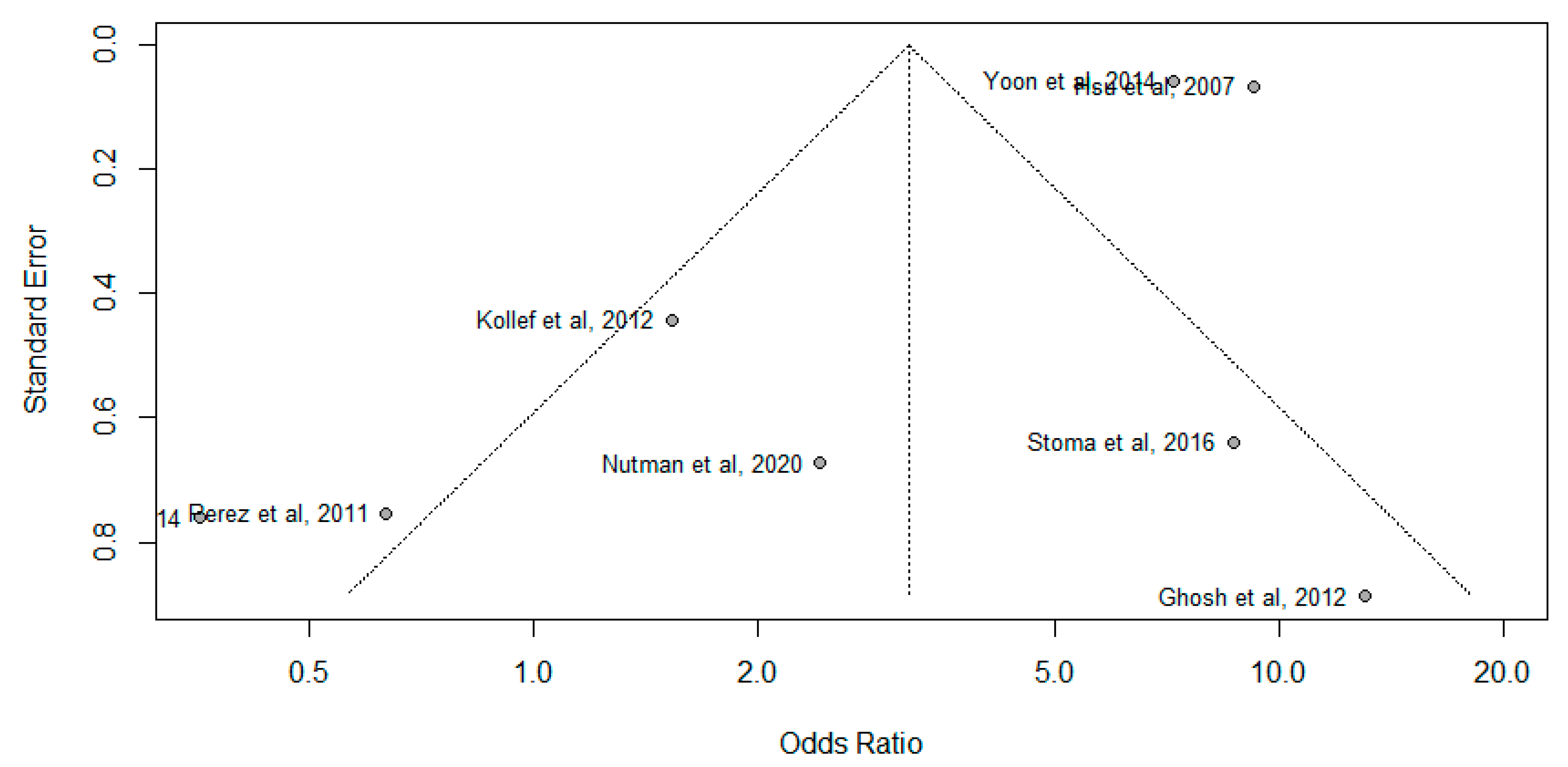
3.3. Publication Bias Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter Baumannii and Pseudomonas Aeruginosa in Health Care Facilities; World Health Organization: Geneve, Switzerland, 2017; ISBN 978-92-4-155017-8. [Google Scholar]
- Jung, S.Y.; Lee, S.H.; Lee, S.Y.; Yang, S.; Noh, H.; Chung, E.K.; Lee, J.I. Antimicrobials for the treatment of drug-resistant acinetobacter baumannii pneumonia in critically Ill Patients: A systemic review and bayesian network meta-analysis. Crit. Care 2017, 21, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliopoulos, G.M.; Maragakis, L.L.; Perl, T.M. Acinetobacter baumannii: Epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 2008, 46, 1254–1263. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Guerra, G.; Heras-Cañas, V.; Gutiérrez-Soto, M.; Del Pilar Aznarte-Padial, M.; Expósito-Ruiz, M.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Urinary tract infection by Acinetobacter baumannii and pseudomonas aeruginosa: Evolution of antimicrobial resistance and therapeutic alternatives. J. Med. Microbiol. 2018, 67, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Siegman-Igra, Y.; Bar-Yosef, S.; Gorea, A.; Avram, J. Nosocomial acinetobacter meningitis secondary to invasive procedures: Report of 25 cases and review. Clin. Infect. Dis. 1993, 17, 843–849. [Google Scholar] [CrossRef]
- Wareth, G.; Abdel-Glil, M.Y.; Schmoock, G.; Steinacker, U.; Kaspar, H.; Neubauer, H.; Sprague, L.D. Draft genome sequence of an acinetobacter baumannii isolate recovered from a horse with conjunctivitis in Germany. Microbiol. Resour. Announc. 2019, 8, e01128-19. [Google Scholar] [CrossRef] [Green Version]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter Baumannii—An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Bergogne-Bérézin, E. Acinetobacter Spp., saprophytic organisms of increasing pathogenic importance. Zentralblatt für Bakteriologie 1994, 281, 389–405. [Google Scholar] [CrossRef]
- Bergogne-Bérézin, E.; Towner, K.J. Acinetobacter Spp., as nosocomial pathogens: Microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 1996, 9, 148–165. [Google Scholar] [CrossRef]
- Kämpfer, P. Acinetobacter. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 11–17. ISBN 978-0-12-384733-1. [Google Scholar]
- Huttner, B.; Jones, M.; Rubin, M.A.; Neuhauser, M.M.; Gundlapalli, A.; Samore, M. Drugs of last resort? The use of polymyxins and tigecycline at US veterans affairs medical centers, 2005–2010. PLoS ONE 2012, 7, e36649. [Google Scholar] [CrossRef]
- Viehman, J.A.; Nguyen, M.H.; Doi, Y. Treatment options for carbapenem-resistant and extensively drug-resistant acinetobacter baumannii infections. Drugs 2014, 74, 1315–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seok, H.; Choi, W.S.; Lee, S.; Moon, C.; Park, D.W.; Song, J.Y.; Cheong, H.J.; Kim, J.; Kim, J.Y.; Park, M.N.; et al. What is the optimal antibiotic treatment strategy for carbapenem-resistant acinetobacter baumannii (CRAB)? A multicentre study in Korea. J. Glob. Antimicrob. Resist. 2021, 24, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, J.H.; Harper, M.; Harrison, P.; Hale, J.D.F.; Vinogradov, E.; Seemann, T.; Henry, R.; Crane, B.; Michael, F.S.; Cox, A.D.; et al. Colistin resistance in acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 2010, 54, 4971–4977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navon-Venezia, S.; Leavitt, A.; Carmeli, Y. High tigecycline resistance in multidrug-resistant acinetobacter baumannii. J. Antimicrob. Chemother. 2007, 59, 772–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornsey, M.; Wareham, D.W. Effects of in vivo emergent tigecycline resistance on the pathogenic potential of acinetobacter baumannii. Sci. Rep. 2018, 8, 4234. [Google Scholar] [CrossRef]
- Sánchez-Meca, J.; Marín-Martínez, F. Meta Analysis. In International Encyclopedia of Education, 3rd ed.; Peterson, P., Baker, E., McGaw, B., Eds.; Elsevier: Oxford, UK, 2010; ISBN 978-0-08-044894-7. [Google Scholar]
- Hoffman, J.I.E. Chapter 36—Meta-Analysis. In Biostatistics for Medical and Biomedical Practitioners; Hoffman, J.I.E., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 645–653. ISBN 978-0-12-802387-7. [Google Scholar]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Kollef, M.H.; Chastre, J.; Clavel, M.; Restrepo, M.I.; Michiels, B.; Kaniga, K.; Cirillo, I.; Kimko, H.; Redman, R. A Randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit. Care 2012, 16, R218. [Google Scholar] [CrossRef] [Green Version]
- Nutman, A.; Lellouche, J.; Temkin, E.; Daikos, G.; Skiada, A.; Durante-Mangoni, E.; Dishon-Benattar, Y.; Bitterman, R.; Yahav, D.; Daitch, V.; et al. Colistin plus meropenem for carbapenem-resistant gram-negative infections: In vitro synergism is not associated with better clinical outcomes. Clin. Microbiol. Infect. 2020, 26, 1185–1191. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Yang, K.S.; Lee, S.E.; Kim, H.J.; Sohn, J.W.; Kim, M.J. Effects of Group 1 versus Group 2 carbapenems on the susceptibility of Acinetobacter baumannii to carbapenems: A before and after intervention study of carbapenem-use stewardship. PLoS ONE 2014, 9, e99101. [Google Scholar] [CrossRef]
- Perez, F.; Endimiani, A.; Ray, A.J.; Decker, B.K.; Wallace, C.J.; Hujer, K.M.; Ecker, D.J.; Adams, M.D.; Toltzis, P.; Dul, M.J.; et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: Impact of post-acute care facilities on dissemination. J. Antimicrob. Chemother. 2010, 65, 1807–1818. [Google Scholar] [CrossRef]
- Lauf, L.; Ozsvár, Z.; Mitha, I.; Regöly-Mérei, J.; Embil, J.M.; Cooper, A.; Sabol, M.B.; Castaing, N.; Dartois, N.; Yan, J.; et al. phase 3 study comparing tigecycline and ertapenem in patients with diabetic foot infections with and without osteomyelitis. Diagn. Microbiol. Infect. Dis. 2014, 78, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, I.; Raina, V.; Kumar, L.; Sharma, A.; Bakhshi, S.; Thulkar, S.; Kapil, A. Profile of infections and outcome in high-risk febrile neutropenia: Experience from a tertiary care cancer center in India. Med. Oncol. 2012, 29, 1354–1360. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Tan, T.-Y.; Jureen, R.; Koh, T.-H.; Krishnan, P.; Lin, R.T.-P.; Tee, N.W.-S.; Tambyah, P.A. Antimicrobial drug resistance in Singapore hospitals. Emerg. Infect. Dis. 2007, 13, 1944. [Google Scholar] [CrossRef] [PubMed]
- Stoma, I.; Karpov, I.; Milanovich, N.; Uss, A.; Iskrov, I. Risk factors for mortality in patients with bloodstream infections during the pre-engraftment period after hematopoietic stem cell transplantation. Blood Res. 2016, 51, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Kaitany, K.-C.J.; Klinger, N.V.; June, C.M.; Ramey, M.E.; Bonomo, R.A.; Powers, R.A.; Leonard, D.A. Structures of the class D carbapenemases OXA-23 and OXA-146: Mechanistic basis of activity against carbapenems, extended-spectrum cephalosporins, and aztreonam. Antimicrob. Agents Chemother. 2013, 57, 4848–4855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tafreshi, N.; Babaeekhou, L.; Ghane, M. Antibiotic resistance pattern of Acinetobacter baumannii from burns patients: Increase in prevalence of BlaOXA-24-like and BlaOXA-58-like genes. Iran. J. Microbiol. 2019, 11, 502–509. [Google Scholar] [CrossRef]
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [Green Version]
- Walther-Rasmussen, J.; Høiby, N. OXA-Type carbapenemases. J. Antimicrob. Chemother. 2006, 57, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- Oliveira, E.A.D.; Paula, G.R.D.; Mondino, P.J.J.; Chagas, T.P.G.; Mondino, S.S.B.D.; Mendonça-Souza, C.R.V.D. High rate of detection of OXA-23-Producing acinetobacter from two general hospitals in Brazil. Rev. Soc. Bras. Med. Trop. 2019, 52. [Google Scholar] [CrossRef]
- Rouhi, S.; Ramazanzadeh, R. Prevalence of blaOxacillinase-23and blaOxacillinase-24/40-type carbapenemases in Pseudomonas aeruginosa species isolated from patients with nosocomial and non-nosocomial infections in the West of Iran Iran. J. Pathol. 2018, 13, 348–356. [Google Scholar]
- Chetri, S.; Bhowmik, D.; Paul, D.; Pandey, P.; Chanda, D.D.; Chakravarty, A.; Bora, D.; Bhattacharjee, A. AcrAB-TolC efflux pump system plays a role in carbapenem non-susceptibility in Escherichia coli. BMC Microbiol. 2019, 19, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuhan, Y.; Ziyun, Y.; Yongbo, Z.; Fuqiang, L.; Qinghua, Z.; Yuhan, Y.; Ziyun, Y.; Yongbo, Z.; Fuqiang, L.; Qinghua, Z. Over expression of AdeABC and AcrAB-TolC efflux systems confers tigecycline resistance in clinical isolates of Acinetobacter baumannii and Klebsiella pneumoniae. Rev. Soc. Bras. Med. Trop. 2016, 49, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Baumgart, A.M.K.; Molinari, M.A.; Silveira, A.C.D.O. Prevalence of carbapenem resistant Pseudomonas aeruginosa and acinetobacter baumannii in high complexity hospital. Braz. J. Infect. Dis. 2010, 14, 433–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramette, A.; Kronenberg, A. Prevalence of carbapenem-resistant Acinetobacter baumannii from 2005 to 2016 in Switzerland. BMC Infect. Dis. 2018, 18, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
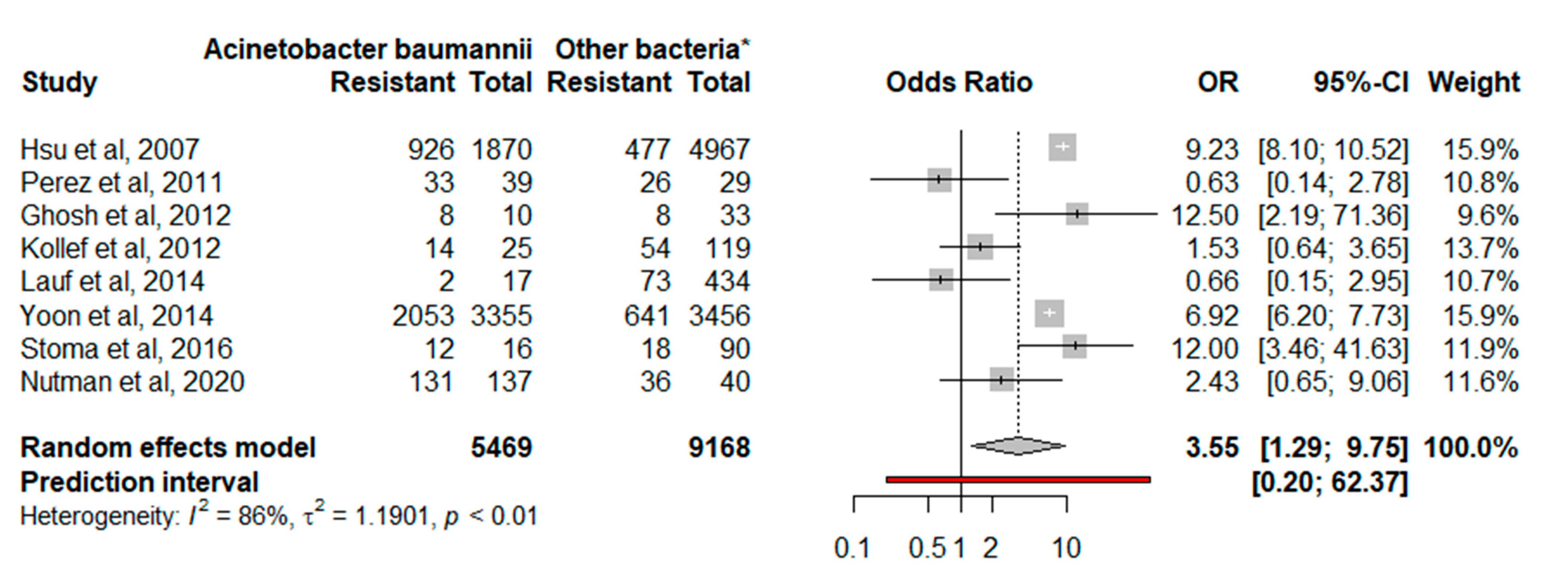
| Author | Number of Susceptible Isolates/N (%) | Year | Country | |
|---|---|---|---|---|
| Acinetobacter baumannii | Other Bacteria * | |||
| [27] | 926/1870 (49.5) | 477/4967 (9.6) | 2007 | Singapore |
| [24] | 33/39 (84.6) | 26/29 (89.6) | 2010 | USA |
| [21] | 14/25 (56) | 54/119 (45.4) | 2012 | Whole world |
| [26] | 8/10 (80) | 8/33 (24.2) | 2012 | India |
| [23] | 2053/3350 (61.2) | 641/3456 (18.5) | 2014 | South Korea |
| [25] | 2/17 (11.8) | 73/434 (16.8) | 2014 | Europe, USA, Canada, Latin America, Asia, India, Australia, and South Africa |
| [28] | 12/16 (75) | 18/90 (20) | 2016 | Belarus |
| [22] | 131/137 (95.6) | 36/40 (90) | 2020 | Italy, Greece, and Israel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, D.L.N.; Costa, F.M.R.d.; Silva, W.M.d.; Aburjaile, F.; Azevedo, V. Acinetobacter baumannii and Its Relationship to Carbapenem Resistance: A Meta-Analysis. Bacteria 2022, 1, 112-120. https://doi.org/10.3390/bacteria1020010
Rodrigues DLN, Costa FMRd, Silva WMd, Aburjaile F, Azevedo V. Acinetobacter baumannii and Its Relationship to Carbapenem Resistance: A Meta-Analysis. Bacteria. 2022; 1(2):112-120. https://doi.org/10.3390/bacteria1020010
Chicago/Turabian StyleRodrigues, Diego Lucas Neres, Francielly Morais Rodrigues da Costa, Wanderson Marques da Silva, Flavia Aburjaile, and Vasco Azevedo. 2022. "Acinetobacter baumannii and Its Relationship to Carbapenem Resistance: A Meta-Analysis" Bacteria 1, no. 2: 112-120. https://doi.org/10.3390/bacteria1020010
APA StyleRodrigues, D. L. N., Costa, F. M. R. d., Silva, W. M. d., Aburjaile, F., & Azevedo, V. (2022). Acinetobacter baumannii and Its Relationship to Carbapenem Resistance: A Meta-Analysis. Bacteria, 1(2), 112-120. https://doi.org/10.3390/bacteria1020010








