Antimicrobial Resistance of Neisseria gonorrhoeae in Sub-Saharan Populations
Abstract
:1. Introduction
2. Gonococcal Pathogenesis
3. Antimicrobial Resistance of N. gonorrhoeae in Africa
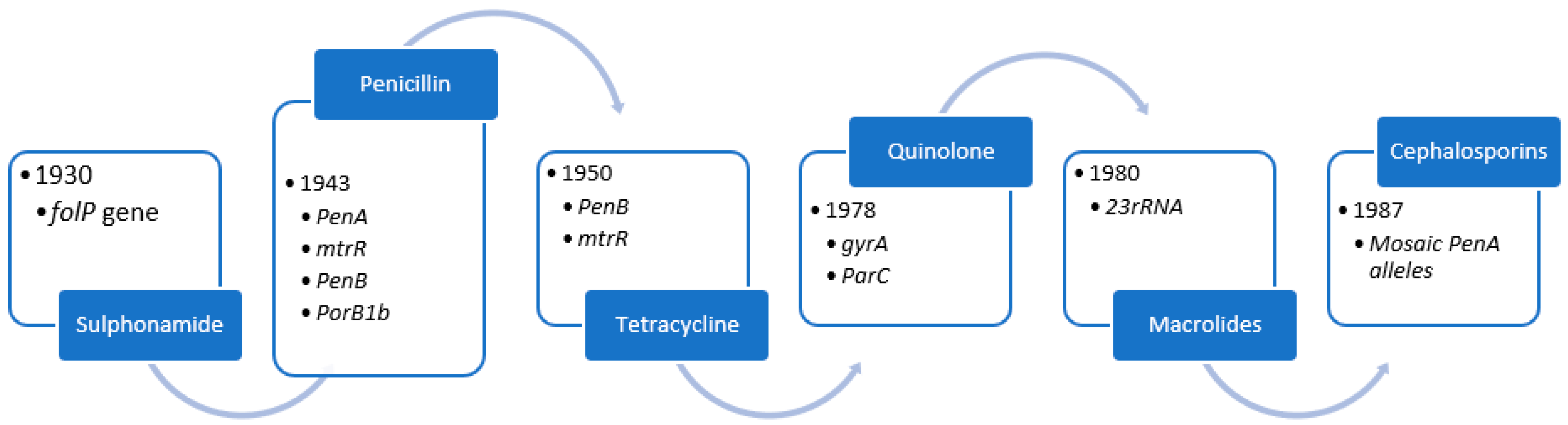
4. Evolution of Antimicrobial Resistance
4.1. Sulphonamides
4.2. Penicillin
4.3. Tetracycline
4.4. Quinolone
4.5. Azithromycin
4.6. Ceftriaxone
5. Future Treatment Objectives
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unemo, M.; Shafer, W.M. Antibiotic resistance in Neisseria gonorrhoeaee: Origin, evolution, and lessons learned for the future. Ann. N. Y. Acad. Sci. 2011, 1230, E19–E28. [Google Scholar] [CrossRef] [PubMed]
- Whittles, L.K.; White, P.J.; Paul, J.; Didelot, X. Epidemiological Trends of Antibiotic Resistant Gonorrhoeae in the United Kingdom. Antibiotics 2018, 7, 60. Available online: https://www.mdpi.com/2079-6382/7/3/60 (accessed on 14 September 2021). [CrossRef] [PubMed] [Green Version]
- Unemo, M.; Seifert, H.S.; Hook, E.W.; Hawkes, S.; Ndowa, F.; Dillon, J.-A.R. Gonorrhoeae. Nat. Rev. Dis. Prim. 2019, 5, 79. Available online: https://www.nature.com/articles/s41572-019-0128-6 (accessed on 15 September 2021). [CrossRef]
- World Health Organization. WHO Guidelines for the Treatment of Neisseria Gonorrhoeaee. WHO Library Catalog Data. 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/246114/9789241549691-eng.pdf (accessed on 15 September 2021).
- Biggel, M.; Heytens, S.; Latour, K.; Bruyndonckx, R.; Goossens, H.; Moons, P. Asymptomatic bacteriuria in older adults: The most fragile women are prone to long-term colonization. BMC Geriatr. 2019, 19, 1–11. Available online: https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-019-1181-4 (accessed on 8 November 2021). [CrossRef] [PubMed]
- Kularatne, R.; Maseko, V.; Gumede, L.; Radebe, F.; Kufa-Chakezha, T. Neisseria Gonorrhoeaee Antimicrobial Resistance Surveillance in Gauteng Province, South Africa. 2018. Available online: https://www.nicd.ac.za/wp-content/uploads/2018/08/Neisseria-gonorrhoeaee-AMR-surveillance.pdf (accessed on 15 September 2021).
- Kirkcaldy, R.D.; Weston, E.; Segurado, A.C.; Hughes, G. Epidemiology of Gonorrhea: A Global Perspective. Sex. Health 2019, 16, 401. Available online: https://www.publish.csiro.au/SH/SH19061 (accessed on 14 September 2021). [CrossRef] [PubMed]
- Tsevat, D.G.; Wiesenfeld, H.C.; Parks, C.; Peipert, J.F. Sexually Transmitted Diseases and Infertility. Am. J. Obstet. Gynecol. 2017, 216, 1. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0002937816305737 (accessed on 8 November 2021). [CrossRef] [Green Version]
- Chesson, H.W.; Mayaud, P.; Aral, S.O. Sexually Transmitted Infections: Impact and Cost-Effectiveness of Prevention. Disease Control Priorities, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK525195/ (accessed on 14 September 2021).
- Mabonga, E.; Parkes-Ratanshi, R.; Riedel, S.; Nabweyambo, S.; Mbabazi, O.; Taylor, C.; Manabe, Y.C. Complete ciprofloxacin resistance in gonococcal isolates in an urban Ugandan clinic: Findings from a cross-sectional study. Int. J. STD AIDS 2019, 30, 256. Available online: https://journals.sagepub.com/doi/abs/10.1177/0956462418799017 (accessed on 14 September 2021). [CrossRef]
- Maduna, L.D.; Kock, M.M.; Van der Veer, B.M.; Radebe, O.; McIntyre, J.; Van Alphen, L.B.; Peters, R.P. Antimicrobial Resistance of Neisseria gonorrhoeaee Isolates from High-Risk Men in Johannesburg, South Africa. Antimicrob. Agents Chemother. 2020, 64, e00906-20. Available online: https://pubmed.ncbi.nlm.nih.gov/32868325/ (accessed on 15 September 2021). [CrossRef]
- Workneh, M.; Hamill, M.M.; Kakooza, F.; Mande, E.; Wagner, J.; Mbabazi, O.; Mugasha, R.; Kajumbula, H.; Walwema, R.; Zenilman, J.; et al. Antimicrobial Resistance of Neisseria Gonorrhoeaee in a Newly Implemented Surveillance Program in Uganda: Surveillance Report. JMIR Public Health Surveill. 2020, 6, e17009. Available online: https://publichealth.jmir.org/2020/2/e17009 (accessed on 8 November 2021). [CrossRef]
- Kharsany, A.B.M.; Karim, Q.A. HIV Infection and, A.I.DS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J. 2016, 10, 34. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4893541/ (accessed on 8 November 2021). [CrossRef] [PubMed] [Green Version]
- Torrone, E.A.; Morrison, C.S.; Chen, P.L.; Kwok, C.; Francis, S.C.; Hayes, R.J.; Looker, K.J.; McCormack, S.; McGrath, N.; van de Wijgert, J.H.; et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med. 2018, 15, e1002511. Available online: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002511 (accessed on 8 November 2021). [CrossRef] [PubMed] [Green Version]
- van Eyk, A. The treatment of sexually transmitted infections. S. Afr. Fam. Pract. 2016, 58, 12–22. [Google Scholar] [CrossRef]
- Kularatne, R.S.; Niit, R.; Rowley, J.; Kufa-Chakezha, T.; Peters, R.P.H.; Taylor, M.M.; Johnson, L.F.; Korenromp, E.L. Adult gonorrhea, chlamydia and syphilis prevalence, incidence, treatment and syndromic case reporting in South Africa: Estimates using the Spectrum-STI model, 1990–2017. PLoS ONE 2018, 13, e0205863. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0205863 (accessed on 8 November 2021). [CrossRef] [PubMed]
- da Costa-Lourenço, A.P.R.; dos Santos, K.T.B.; Moreira, B.M.; Fracalanzza, S.E.L.; Bonelli, R.R. Antimicrobial resistance in Neisseria gonorrhoeaee: History, molecular mechanisms and epidemiological aspects of an emerging global threat. Braz. J. Microbiol. 2017, 48, 617. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5628311/ (accessed on 14 September 2021). [CrossRef]
- Kularatne, R.; Maseko, V.; Gumede, L.; Kufa, T. Trends in Neisseria gonorrhoeaee Antimicrobial Resistance over a Ten-Year Surveillance Period, Johannesburg, South Africa, 2008–2017. Antibiotics 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guvenc, F.; Kaul, R.; Gray-Owen, S.D. Intimate Relations: Molecular and Immunologic Interactions Between Neisseria gonorrhoeaee and H.I.V-1. Front. Microbiol. 2020, 11, 1299. [Google Scholar] [CrossRef] [PubMed]
- Lovett, A.; Duncan, J.A. Human Immune Responses and the Natural History of Neisseria gonorrhoeaee Infection. Front. Immunol. 2018, 9, 3187. [Google Scholar] [CrossRef] [PubMed]
- Springer, C.; Salen, P. Gonorrhea. Med. Monatsschr. Pharm. 2021, 44, 342–345. [Google Scholar]
- Quillin, S.J.; Seifert, H.S. Neisseria gonorrhoeaee host-adaptation and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 226. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, K.E. Lack of Sexually Transmitted Infection Treatment Accuracy When Relying on Syndromic Management in an Urgent Care Setting. Sex. Transm. Dis. 2020, 47, 625–627. Available online: https://pubmed.ncbi.nlm.nih.gov/32815903/ (accessed on 8 November 2021). [CrossRef]
- Cyr, S.S.; Barbee, L.; Workowski, K.A.; Bachmann, L.H.; Pham, C.; Schlanger, K.; Torrone, E.; Weinstock, H.; Kersh, E.N.; Thorpe, P. Update to CDC’s Treatment Guidelines for Gonococcal Infection. 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1911–1916. Available online: https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm (accessed on 8 November 2021). [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Unemo, M. Current and Future Antimicrobial Treatment of Gonorrhoeae—The Rapidly Evolving Neisseria Gonorrhoeaee Continues to Challenge. BMC Infect. Dis. 2015, 15, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hook, E.W.; Kirkcaldy, R.D., III. A Brief History of Evolving Diagnostics and Therapy for Gonorrhea: Lessons Learned. Clin. Infect. Dis. 2018, 67, 1294. [Google Scholar] [CrossRef]
- Elkashif, A.; Seleem, M.N. Investigation of auranofin and gold-containing analogues antibacterial activity against multidrug-resistant Neisseria gonorrhoeaee. Sci. Rep. 2020, 10, 5602. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.D.; Sharpe, S.; Schlanger, K.; St. Cyr, S.; Holderman, J.; Steece, R.; Soge, O.O.; Masinde, G.; Arno, J.; Schmerer, M.; et al. Emergence of Neisseria gonorrhoeaee Strains Harboring a Novel Combination of Azithromycin-Attenuating Mutations. Antimicrob. Agents Chemother. 2019, 63, e02313-18. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Connolly, K.L.; Rouquette-Loughlin, C.; Andrea, A.D.; Jerse, A.E.; Shafer, W.M. Could Dampening Expression of the Neisseria gonorrhoeaeemtrCDE-Encoded Efflux Pump Be a Strategy to Preserve Currently or Resurrect Formerly Used Antibiotics to Treat Gonorrhea? mBio 2019, 10, e01576-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangamani, S.; Mohammad, H.; Abushahba, M.F.N.; Sobreira, T.J.P.; Hedrick, V.E.; Paul, L.N.; Seleem, M.N. Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci. Rep. 2016, 6, 1–13. Available online: https://www.nature.com/articles/srep22571 (accessed on 14 September 2021). [CrossRef] [PubMed] [Green Version]
- Fourie, J.L.; Ciaassen, F.M.; Myburgh, J.J. Causative pathogens and antibiotic resistance in community-acquired urinary tract infections in central South Africa. SAMJ S. Afr. Med. J. 2021, 111, 124–128. Available online: http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S0256-95742021000200011&lng=en&nrm=iso&tlng=en (accessed on 15 September 2021). [CrossRef] [PubMed]
- Tadesse, B.T.; Ashley, E.A.; Ongarello, S.; Havumaki, J.; Wijegoonewardena, M.; González, I.J.; Dittrich, S. Antimicrobial resistance in Africa: A systematic review. BMC Infect. Dis. 2017, 17, 1–17. Available online: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-017-2713-1 (accessed on 15 September 2021). [CrossRef] [PubMed]
- Buder, S.; Dudareva, S.; Jansen, K.; Loenenbach, A.; Nikisins, S.; Sailer, A.A.; Guhl, E.; Kohl, P.K.; Bremer, V. Antimicrobial resistance of Neisseria gonorrhoeaee in Germany: Low levels of cephalosporin resistance, but high azithromycin resistance. BMC Infect. Dis. 2018, 18, 1–11. Available online: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-018-2944-9 (accessed on 14 September 2021). [CrossRef] [PubMed] [Green Version]
- Xu, S.X.; Leontyev, D.; Kaul, R.; Gray-Owen, S.D. Neisseria gonorrhoeaee co-infection exacerbates vaginal, H.I.V shedding without affecting systemic viral loads in human, C.D.34+ engrafted mice. PLoS ONE 2018, 13, e0191672. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0191672 (accessed on 14 September 2021).
- Lenz, J.D.; Dillard, J.P. Pathogenesis of neisseria gonorrhoeaeeand the host defense in ascending infections of human fallopian tube. Front. Immunol. 2018, 9, 2710. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Maland, M.; Mohammad, H.; Pascuzzi, P.E.; Avramova, L.; Koehler, C.M.; Hazbun, T.R.; Seleem, M.N. Repurposing Approach Identifies Auranofin with Broad Spectrum Antifungal Activity That Targets Mia40-Erv1 Pathway. Front. Cell. Infect. Microbiol. 2017, 18, 4. [Google Scholar] [CrossRef] [Green Version]
- Russell, M.W. Immune Responses to Neisseria gonorrhoeaee: Challenges and Opportunities with Respect to Pelvic Inflammatory Disease. J. Infect. Dis. 2021, 224 (Suppl. S2), S96–S102. Available online: https://academic.oup.com/jid/article/224/Supplement_2/S96/6352151 (accessed on 8 November 2021). [CrossRef] [PubMed]
- Russell, M.W.; Jerse, A.E.; Gray-Owen, S.D. Progress Toward a Gonococcal Vaccine: The Way Forward. Front. Immunol. 2019, 10, 2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heydarian, M.; Yang, T.; Schweinlin, M.; Steinke, M.; Walles, H.; Rudel, T.; Kozjak-Pavlovic, V. Biomimetic human tissue model for long-term study of Neisseria gonorrhoeaee infection. Front. Microbiol. 2019, 10, 1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ma, W.; Liu, B.; Wang, Y.; Chu, J.; Xiong, G.; Shen, L.; Long, C.; Lin, T.; He, D.; et al. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem. Cell Res. Ther. 2017, 8, 1–14. Available online: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-017-0500-y (accessed on 8 November 2021). [CrossRef] [Green Version]
- Budkaew, J.; Chumworathayi, B.; Pientong, C.; Ekalaksananan, T. Prevalence and factors associated with gonorrhea infection with respect to anatomic distributions among men who have sex with men. PLoS ONE 2019, 14, e0211682. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0211682 (accessed on 8 November 2021). [CrossRef] [Green Version]
- Raterman, E.L.; Jerse, A.E. Female Mouse Model of Neisseria gonorrhoeaee Infection. Methods Mol. Biol. 2019, 1997, 413–429. Available online: https://pubmed.ncbi.nlm.nih.gov/31119637/ (accessed on 8 November 2021).
- Jerse, A.E.; Wu, H.; Packiam, M.; Vonck, R.A.; Begum, A.A.; Garvin, L.E. Estradiol-treated female mice as surrogate hosts for neisseria gonorrhoeaee genital tract infections. Front. Microbiol. 2011, 2, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyal, A. Identification of Cellular Factors Involved in Neisseria Gonorrhoeae Induced Enhanced Hiv-1 Transmission in a Cervical Tissue Based Organ Culture Model. 2016. Available online: https://www.proquest.com/openview/4cd8936c51b629715033e5e569ed133b/1?pq-origsite=gscholar&cbl=18750 (accessed on 8 November 2021).
- Rouquette-Loughlin, C.E.; Zalucki, Y.M.; Dhulipala, V.L.; Balthazar, J.T.; Doyle, R.G.; Nicholas, R.A.; Begum, A.A.; Raterman, E.L.; Jerse, A.E.; Shafer, W.M. Control of gdhR Expression in Neisseria gonorrhoeaee via Autoregulation and a Master Repressor (MtrR) of a Drug Efflux Pump Operon. mBio 2017, 8, e00449-17. Available online: https://pubmed.ncbi.nlm.nih.gov/28400529/ (accessed on 8 November 2021). [CrossRef] [Green Version]
- Dubbink, J.H.; Verweij, S.P.; Struthers, H.E.; Ouburg, S.; McIntyre, J.A.; Morré, S.A.; Peters, P.R. Genital Chlamydia trachomatis and Neisseria gonorrhoeaee infections among women in sub-Saharan Africa: A structured review. Int. J. STD AIDS 2018, 29, 806–824. Available online: https://pubmed.ncbi.nlm.nih.gov/29486628/ (accessed on 8 November 2021). [CrossRef] [PubMed] [Green Version]
- Kirkcaldy, R.D. Neisseria gonorrhoeaee Antimicrobial Susceptibility Surveillance—The Gonococcal Isolate Surveillance Project, 27 Sites, United States, 2014. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2019, 65, 1–24. [Google Scholar]
- Kassa, Z.Y.; Hussen, S.; Hadra, N.; Moges, Y.; Bonja, F. Prevalence of Neisseria gonorrhoeaee infection among women of reproductive age in sub-Saharan Africa: A systematic review and meta-analysis. Eur. J. Contracept. Reprod. Health Care 2020, 25, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Wi, T.; Lahra, M.M.; Ndowa, F.; Bala, M.; Dillon, J.A.R.; Ramon-Pardo, P.; Eremin, S.R.; Bolan, G.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeaee: Global surveillance and a call for international collaborative action. PLoS Med. 2017, 14, e1002344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crucitti, T.; Belinga, S.; Fonkoua, M.C.; Abanda, M.; Mbanzouen, W.; Sokeng, E.; Nzouankeu, A. Sharp increase in ciprofloxacin resistance of Neisseria gonorrhoeaee in Yaounde, Cameroon: Analyses of a laboratory database period 2012–2018. Int. J. STD AIDS 2020, 31, 579–586. Available online: https://journals.sagepub.com/doi/abs/10.1177/0956462419897227 (accessed on 8 November 2021). [CrossRef] [PubMed]
- Meheus, A.; Widy-Wirski, R.; D’Costa, J.; Van Dyck, E.; Delgadillo, R.; Piot, P. Treatment of gonorrhoeae in males in the Central African Republic with spectinomycin and procaine penicillin. Bull. World Health Organ. 1984, 62, 89–94. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/6232015/?tool=EBI (accessed on 8 November 2021).
- Blankhart, D.; Müller, O.; Gresenguet, G.; Weis, P. Sexually transmitted infections in young pregnant women in Bangui, Central African Republic. Int. J. STD AIDS 1999, 10, 609–614. Available online: https://pubmed.ncbi.nlm.nih.gov/10492429/ (accessed on 8 November 2021). [CrossRef]
- Connolly, S.; Wall, K.M.; Parker, R.; Kilembe, W.; Inambao, M.; Visoiu, A.M.; Sharkey, T.; Hunter, E.; Allen, S. Sociodemographic Factors and STIs associated with Chlamydia trachomatis and Neisseria gonorrhoeaee infections in Zambian female sex workers and single mothers. Int. J. STD AIDS 2020, 31, 364–374. Available online: https://journals.sagepub.com/doi/full/10.1177/0956462419894453 (accessed on 8 November 2021). [CrossRef]
- Ortashi, O.M.; El Khidir, I.; Herieka, E. Prevalence of, HIV, syphilis, Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis and candidiasis among pregnant women attending an antenatal clinic in Khartoum, Sudan. J. Obstet. Gynaecol. 2009, 24, 513–515. [Google Scholar] [CrossRef]
- Abdelrahim, N.A.; Ahmed, H.I.; Fadl-Elmula, I.M.; Bayoumi, M.A.; Homeida, M.M. Sexually transmitted infections other than, H.I.V/AIDS among women of low socio-economic class attending antenatal clinics in Khartoum, Sudan. Int. J. STD AIDS 2017, 28, 781–787. Available online: https://pubmed.ncbi.nlm.nih.gov/27582306/ (accessed on 9 November 2021). [CrossRef] [PubMed]
- Romoren, M.; Sundby, J.; Velauthapillai, M.; Rahman, M.; Klouman, E.; Hjortdahl, P. Chlamydia and gonorrhoeae in pregnant Batswana women: Time to discard the syndromic approach? BMC Infect. Dis. 2007, 7, 1–11. Available online: https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-7-27 (accessed on 11 November 2021). [CrossRef] [Green Version]
- Wynn, A.; Ramogola-Masire, D.; Gaolebale, P.; Moshashane, N.; Sickboy, O.; Duque, S.; Williams, E.; Doherty, K.; Klausner, J.D.; Morroni, C. Prevalence and treatment outcomes of routine Chlamydia trachomatis, Neisseria gonorrhoeaee and Trichomonas vaginalis testing during antenatal care, Gaborone, Botswana. Sex Transm. Infect. 2018, 94, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Maduna, L.D. Drug-Resistant Gonorrhoeae is a Growing Threat: A South African Case Study the Conversation. 2020. Available online: https://theconversation.com/drug-resistant-gonorrhoeae-is-a-growing-threat-a-south-african-case-study-148012 (accessed on 14 September 2021).
- Rambaran, S.; Naidoo, K.; Dookie, N.; Moodley, P.; Sturm, A.W. Resistance Profile of Neisseria gonorrhoeaee in KwaZulu-Natal, South Africa Questioning the Effect of the Currently Advocated Dual Therapy. Sex. Transm. Dis. 2019, 46, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, S. The Effect of HIV and Neisseria Gonorrhoeaee on the Tight Junctions of Cervical Epithelial Cells. 2020. Available online: https://ukzn-dspace.ukzn.ac.za/handle/10413/18986 (accessed on 9 November 2021).
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Unemo, M.; Shafer, W.M. Antimicrobial Resistance in Neisseria gonorrhoeaee in the 21st Century: Past, Evolution, and Future. Clin. Microbiol. Rev. 2014, 27, 587. [Google Scholar] [CrossRef] [Green Version]
- Młynarczyk-Bonikowska, B.; Majewska, A.; Malejczyk, M.; Młynarczyk, G.; Majewski, S. Multiresistant Neisseria gonorrhoeaee: A new threat in second decade of the XXI century. Med. Microbiol. Immunol. 2019, 209, 95–108. Available online: https://link.springer.com/article/10.1007/s00430-019-00651-4 (accessed on 14 September 2021). [CrossRef] [PubMed]
- Kivata, M.W.; Mbuchi, M.; Eyase, F.; Bulimo, W.D.; Kyanya, C.K.; Oundo, V.; Mbinda, W.M.; Sang, W.; Andagalu, B.; Soge, O.O.; et al. Plasmid mediated penicillin and tetracycline resistance among Neisseria gonorrhoeaee isolates from Kenya. BMC Infect. Dis. 2020, 20, 1–11. Available online: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-020-05398-5 (accessed on 9 November 2021). [CrossRef]
- D’Atanasio, N.; de Joannon, A.C.; Sante, L.D.; Mangano, G.; Ombrato, R.; Vitiello, M.; Vitiello, M.; Bartella, C.; Magarò, G.; Prati, F.; et al. Antibacterial activity of novel dual bacterial, D.N.A type, I.I. topoisomerase inhibitors. PLoS ONE 2020, 15, e0228509. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0228509 (accessed on 15 September 2021). [CrossRef] [Green Version]
- Huband, M.D.; Bradford, P.A.; Otterson, L.G.; Basarab, G.S.; Kutschke, A.C.; Giacobbe, R.A.; Patey, S.A.; Alm, R.A.; Johnstone, M.R.; Potter, M.E.; et al. In Vitro Antibacterial Activity of, A.Z.D0914, a New Spiropyrimidinetrione, D.N.A Gyrase/Topoisomerase Inhibitor with Potent Activity against Gram-Positive, Fastidious Gram-Negative, and Atypical Bacteria. Antimicrob. Agents Chemother. 2015, 59, 467. [Google Scholar] [CrossRef] [Green Version]
- Massongo, M.; Ngando, L.; Pefura Yone, E.W.; Nzouankeu, A.; Mbanzouen, W.; Fonkoua, M.C.; Ngandjio, A.; Tchatchueng, J.; Barger, D.; Tejiokem, M.C. Trends of Antibacterial Resistance at the National Reference Laboratory in Cameroon: Comparison of the Situation between 2010 and 2017. Biomed. Res. Int. 2021, 2021. Available online: https://www.hindawi.com/journals/bmri/2021/9957112/ (accessed on 15 September 2021). [CrossRef]
- Suay-García, B.; Pérez-Gracia, M.T. Future Prospects for Neisseria gonorrhoeaee Treatment. Antibiotics 2018, 7, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Yan, J. Antibiotic Resistance and Treatment Options for Multidrug-Resistant Gonorrhea. Infect. Microbes Dis. 2020, 2, 67–76. Available online: https://journals.lww.com/imd/Fulltext/2020/06000/Antibiotic_Resistance_and_Treatment_Options_for.6.aspx (accessed on 9 November 2021). [CrossRef]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A.; Miller, A.A.; O’Donnell, J.; Mueller, J.P. Zoliflodacin: An Oral Spiropyrimidinetrione Antibiotic for the Treatment of Neisseria gonorrheae, Including Multi-Drug-Resistant Isolates. ACS Infect. Dis. 2020, 6, 1332–1345. Available online: https://pubs.acs.org/doi/full/10.1021/acsinfecdis.0c00021 (accessed on 15 September 2021). [CrossRef] [PubMed]
- Taylor, S.N.; Marrazzo, J.; Batteiger, B.E.; Hook, E.W.; Seña, A.C.; Long, J.; Wierzbicki, M.R.; Kwak, H.; Johnson, S.M.; Lawrence, K.; et al. Single-Dose Zoliflodacin (ETX0914) for Treatment of Urogenital Gonorrhea. N. Engl. J. Med. 2018, 379, 1835–1845. [Google Scholar] [CrossRef]
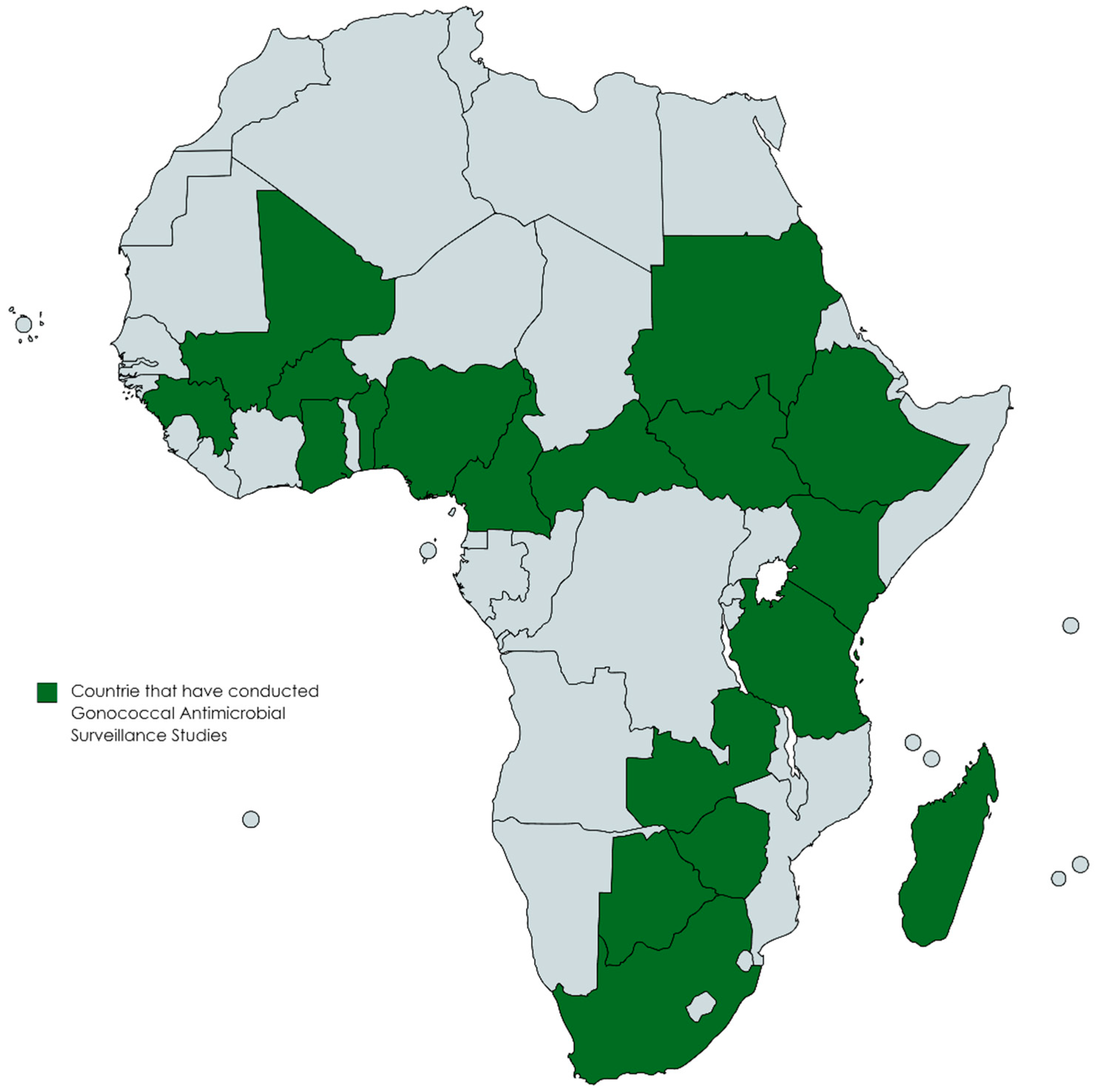
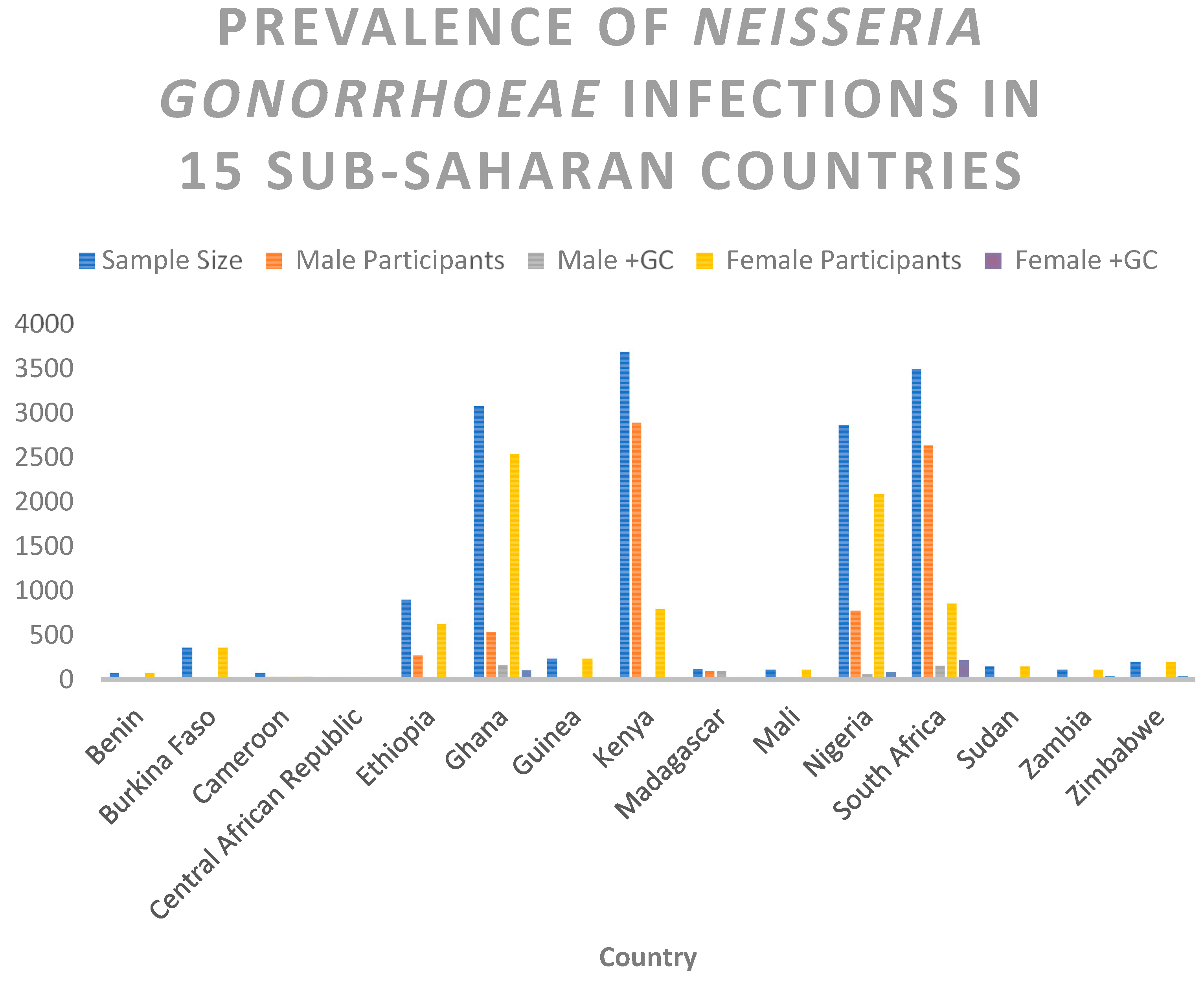
| Country | Sample Size | Male Participants | Male +GC | Female Participants | Female +GC |
|---|---|---|---|---|---|
| Benin | 81 | 0 | 0 | 81 | 1 |
| Burkina Faso | 367 | 0 | 0 | 367 | 13 |
| Cameroon | 79 | 40 | 40 | 39 | 39 |
| Central African Republic | 30 | 28 | 28 | 2 | 2 |
| Ethiopia | 907 | 274 | 17 | 633 | 26 |
| Ghana | 3079 | 539 | 173 | 2540 | 108 |
| Guinea | 237 | 0 | 0 | 237 | 9 |
| Kenya | 3696 | 2895 | 9 | 801 | 15 |
| Madagascar | 126 | 95 | 95 | 31 | 31 |
| Mali | 114 | 0 | 0 | 114 | 5 |
| Nigeria | 2868 | 777 | 61 | 2091 | 87 |
| South Africa | 3495 | 2639 | 156 | 856 | 220 |
| Sudan | 151 | 0 | 0 | 151 | 3 |
| Zambia | 116 | 0 | 0 | 116 | 43 |
| Zimbabwe | 200 | 0 | 0 | 200 | 48 |
| Total | 15546 | 7287 | 579 | 8259 | 650 |
| Antimicrobial Class | Resistance Determinants/Mechanisms |
|---|---|
| Sulphonamide | Overproduction of p-aminobenzoic acid, diluting the sulphonamide. Target affinity is reduced when folP (encoding the sulphonamide target DHPS) is mutated. SNPs or a mosaic folP gene containing sequences from commensal Neisseria spp. comprise the folP mutations [26]. |
| Penicillin | PenA mutations (encoding the main lethal target PBP2). Traditionally, the mutations were a single amino acid insertion D345 in PBP2 and 4 to 8 concomitant mutations in the PBP2 carboxyl-terminal region, which reduced PBP2 acylation rate and susceptibility by a factor of 6 to 8. Many mosaic penA alleles with up to 70 amino acid changes, reducing PBP2 acylation, have been described in the last decade. Overexpression and increased efflux from the MtrCDE efflux pump are caused by mutations in mtrR, the promoter (most commonly a single nucleotide [A] deletion in the 13-bp inverted repeat sequence), or the coding sequence (most commonly a G45D substitution). PorB1b SNPs, such as those encoding G120K and G120D/A121D mutations in PorB1b loop 3, reduce influx (penB resistance determinants). Surprisingly, the penB phenotype is only seen in strains carrying the mtrR resistance determinant [17,26]. |
| Tetracycline | Mutations in penB and mtrR (see above). |
| Quinolone | gyrA SNPs in the QRDR, such as S91F, D95N, and D95G, reduce quinolone binding to DNA gyrase. ParC SNPs in the QRDR, such as D86N, S88P, and E91K, reduce quinolone binding to topoisomerase IV [66,67]. |
| Macrolides (Azithromycin) | C2611T and A2059G, 23S rRNA SNPs (in 1 to 4 alleles) result in a 23S rRNA target (peptidyltransferase loop of domain V) with a lower affinity for the 50S ribosomal macrolide target [29]. |
| Cephalosporins (Ceftriaxone) | Mosaic penA alleles encoding mosaic PBP2s with a lower rate of PBP2 acylation These proteins have up to 70 amino acid changes and are the result of horizontal transfer of partial penA genes from primarily commensal Neisseria spp. A311V, I312M, V316T, V316P, T483S, A501P, A501V, N512Y, and G545S are mosaic PBP2 mutations known to contribute to resistance. Other epistatic mutations in the mosaic penA allele are required for resistance mutations [17,65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakobi, S.H.; Pooe, O.J. Antimicrobial Resistance of Neisseria gonorrhoeae in Sub-Saharan Populations. Bacteria 2022, 1, 96-111. https://doi.org/10.3390/bacteria1020009
Yakobi SH, Pooe OJ. Antimicrobial Resistance of Neisseria gonorrhoeae in Sub-Saharan Populations. Bacteria. 2022; 1(2):96-111. https://doi.org/10.3390/bacteria1020009
Chicago/Turabian StyleYakobi, Sinethemba H., and Ofentse J. Pooe. 2022. "Antimicrobial Resistance of Neisseria gonorrhoeae in Sub-Saharan Populations" Bacteria 1, no. 2: 96-111. https://doi.org/10.3390/bacteria1020009
APA StyleYakobi, S. H., & Pooe, O. J. (2022). Antimicrobial Resistance of Neisseria gonorrhoeae in Sub-Saharan Populations. Bacteria, 1(2), 96-111. https://doi.org/10.3390/bacteria1020009






