Abstract
(1) Background: The goal of the present study was to evaluate whether the supplementation with a multi-species probiotic in the diet of laying hens can change the microbiota and health status of the oviduct. (2) Methods: A total of 60 cages housing lightweight laying hens (36 weeks old) were randomly assigned to the following two different treatments: a control group fed a diet without probiotic, and a treatment group receiving diets supplemented with 50 g/ton of probiotics. The trial lasted for 26 weeks, after which five layers were slaughtered per treatment for oviduct (magnum) assessment, focusing on microbiome composition, oxidant and antioxidant status, and morphological analyses. Additionally, intestinal (jejunum) samples were collected to determine oxidant and antioxidant status. (3) Results: Probiotic supplementation resulted in lower counts of organisms from the RB41 order (p = 0.039) and Burkholderia genus (p = 0.017), and a total reduction in Bacillus and Corynebacterium (p = 0.050) compared to the control treatment. Genera Burkholderia (p = 0.017), Corynebacterium (p = 0.050), and Bacillus (p = 0.050) were also lower with the probiotic supplementation in relation to the control. Genera Epulopiscium (p = 0.089), Flavobacterium (p = 0.100), Ruminococcus (p = 0.089), and Staphylococcus (p = 0.100) tended to be lower in the probiotic group compared to the control. No significant differences were found between treatments for oviduct lesions. Probiotic treatment resulted in a higher protein thiol level in the intestine compared to the control (p < 0.001). However, the use of probiotics tended to reduce glutathione S-transferase levels in the oviduct compared to the control (p = 0.068). (4) Conclusions: These results suggest that dietary supplementation with probiotics can modulate the oviduct microbiota and improve the antioxidant status of laying hens, without causing tissue damage. Further research is warranted to explore the long-term implications of these changes on reproductive performance and egg quality.
1. Introduction
The oviduct is responsible for egg production and pathogen protection in laying hens [1]. Given that eggs may be exposed to bacteria before and after the shell is formed, this organ is critical for bird health and egg production with reduced bacterial contamination. As a result, limiting this exposure can extend the shelf life of the eggs. Despite it still being understudied, the microbiota of the oviduct directly influences the internal and external quality of the eggs [2]. In the case of breeder hens, the formation of the albumen microbiota plays an important role in the initial colonization of the offspring’s microbiota [2].
The microbial diversity of the reproductive tract gradually increases along the layers’ reproductive tract, starting from the infundibulum (with the lowest diversity) and progressing towards the cloaca. Thus, during egg formation in the oviduct, microbial infections within the reproductive tract pose a risk of contaminating the yolk, albumen, and shell membranes [3]. This contamination can result in foodborne infections in consumers. The main concern in this scenario is vertical transmission by Salmonella [4], Escherichia coli, and Campylobacter [5]. The overgrowth of some pathogens not only contributes to contamination but can also cause a reduction in egg production [6], which affects the system’s production costs.
Concerns about antibiotic residues in eggs and increasing regulatory restrictions have limited the use of conventional antimicrobials, especially for managing enteric, respiratory, and reproductive tract health. This scenario highlights the need for alternative strategies, such as probiotics, to support oviduct integrity and maintain egg production and quality [1]. Therefore, probiotics have become an alternative in the diet of these layers. The use of live microorganisms has improved the growth performance of birds [7], promoted animal welfare [8], maintained intestinal health [9], and improved egg quality [10]. Thus, these microorganisms have the potential to influence the intestinal microbiota and the oviduct. However, most investigations have focused on intestinal microbiota, and the literature on the reproductive tract microbiota is limited [2]. Therefore, this study aimed to investigate whether supplementation with a multi-species probiotic in laying hens can modulate the microbiota and enhance reproductive tract health in laying hens.
2. Materials and Methods
2.1. Probiotic
The multi-species probiotic additive (Protexin™ Concentrate, Elanco Animal Health, São Paulo, Brazil) included Lactobacillus acidophilus (2.06 × 108 CFU/g), Lactobacillus bulgaricus (2.06 × 108 CFU/g), Lactobacillus plantarum (1.26 × 108 CFU/g), Lactobacillus rhamnosus (2.06 × 108 CFU/g), Bifidobacterium bifidum (2.00 × 108 CFU/g), Enterococcus faecium (6.46 × 108 CFU/g), and Streptococcus thermophilus (4.10 × 108 CFU/g). The administration protocol followed the manufacturer’s guidelines and technical specifications.
2.2. Animals, Diets, and Experimental Design
The experimental protocol was approved by the Institutional Ethics Committee on the Use of Animals (CEUA/UFRGS) under protocol number 39783. Experimental units (cages with four hens each) were randomly selected from hens housed on a commercial farm (Petry Farm, Salvador do Sul, Rio Grande do Sul, Brazil), which contained approximately 14,000 lightweight laying hens (Hy-Line W-36 lineage, 36 weeks old). From this population, 30 cages per treatment were used to assess growth performance. From these cages, five hens per treatment were randomly selected and slaughtered for oviduct (magnum) assessment, focusing on microbiome composition, oxidative and antioxidative status, and morphological analyses.
These same cages had previously been used to assess performance and egg quality parameters. For the present study, five cages per treatment were selected for reproductive tract evaluation. Replicates were distributed in a completely randomized design across two experimental treatments as follows: (1) a control group receiving a basal diet without additive supplementation, and (2) a probiotic group receiving the same basal diet supplemented with a multi-species probiotic. The experiment lasted 26 weeks, with probiotic supplementation administered during the first 84 days. The basal diet (Table 1) was a corn–soybean meal-based feed formulated according to the genetic line’s nutritional requirements [11]. Inert material (kaolin) was included in the control diet to replace the volume of probiotic additives. Feed and water were provided ad libitum throughout the experimental period, using nipple drinkers and gutter feeders, respectively.

Table 1.
Composition of control diet. Reproduced from [12].
Laying hens were housed in conventional poultry sheds oriented in an east–west direction, with concrete floors and masonry walls partially enclosed with wire mesh extending to the ceiling. The facilities were equipped with adjustable side curtains, which were managed according to prevailing weather conditions to maintain thermal comfort. During the experimental period, the average minimum and maximum temperatures recorded were 18 °C and 36 °C, respectively, with relative humidity ranging from 35.8% to 94.7%. A lighting program of 16 h of light (from 04:00 a.m. to 08:00 p.m.) and 8 h of darkness was maintained daily. Hens were housed in galvanized wire cages measuring 100 cm in length × 40 cm in width × 45 cm in height, providing a floor space of 1.000 cm2 per bird. The hens remained in these cages throughout the experimental period.
2.3. Data Collection
Laying hens were randomly selected from the cages and euthanized at the end of the experiment in accordance with animal welfare and euthanasia standards, as outlined in the CONCEA euthanasia practice guidelines [13]. Samples from the oviduct (magnum) of five birds per treatment were analyzed for microbiome composition, oxidant and antioxidant status, and morphology. Hence, intestinal (jejunum) samples from the same layers were collected to determine oxidant and antioxidant status. Samples for microbiome and redox status analyses were immediately snap-frozen in liquid nitrogen and stored at −80 °C until further processing.
2.3.1. Microbiota Analyses
The DNA was extracted from the samples using the commercial kit “ZR Fecal DNA MiniPrep®” from Zymo Research (Irvine, CA, USA) following the manufacturer’s instructions. The extracted DNA was quantified by spectrophotometry at 260 nm. To assess the integrity of the extracted DNA, all the samples were subjected to 1% agarose gel electrophoresis. A segment of approximately 460 bases from the V3–V4 hypervariable region of the 16S rRNA gene was amplified using the universal primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 806R (5′-GACTACHVGGGTATCTAATCC-3′), as described by Klindworth et al. [14]. PCR conditions were as follows: 95 °C for 3 min; 25 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; followed by a final extension at 72 °C for 5 min. A metagenomic library was constructed from the amplified samples using the commercial Nextera DNA Library Preparation Kit (Illumina, San Diego, CA, USA). Amplified samples were pooled and sequenced on an Illumina MiSeq sequencer using the MiSeq Reagent Kit v3 (600-cycle), generating 2 × 300 bp paired-end reads [15].
Raw sequencing reads were processed using the QIIME (Quantitative Insights Into Microbial Ecology) platform [16,17]. The workflow included quality filtering, chimera removal, and taxonomic classification. Sequences were clustered into operational taxonomic units (OTUs) based on sequence similarity and classified taxonomically by comparison against the SILVA ribosomal RNA gene database (version 128, released in 2017) [18].
2.3.2. Oviduct Morphology
Oviduct samples from the magnum region were collected and fixed in 10% neutral-buffered formalin. Following fixation, tissue samples were processed for routine histology, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Histological images were captured using a digital microcamera (Electronic Eyepiece Camera Video, OPTON, São Carlos, SP, Brazil) mounted on a biological trinocular microscope (model TNB-41T-PL, OPTON, São Carlos, SP, Brazil). Morphometric analyses were conducted using ImageJ software (version 1.53; National Institutes of Health, Bethesda, MD, USA). This analysis was performed to confirm oviduct injury through histopathological evaluation, including the assessment of inflammatory infiltrates, epithelial degeneration, and tissue disorganization.
2.3.3. Oxidative and Antioxidant Status
Samples from the magnum region of the oviduct and the jejunum were collected to assess oxidative and antioxidant status. Tissues were homogenized on ice using a mechanical homogenizer in a saline solution supplemented with a protease inhibitor cocktail to prevent protein degradation. The homogenates were then centrifuged at 2800× g for 10 min, and the resulting supernatant was carefully collected and transferred into microtubes, which were stored at −80 °C for subsequent biochemical analyses.
Lipid peroxidation in non-homogenized oviduct and intestinal samples was evaluated by measuring thiobarbituric acid reactive substances (TBARS), as described by Ohkawa et al. [19]. The red chromogen formed was quantified spectrophotometrically at 532 nm, and results were expressed as nmol of malondialdehyde (MDA) per mg of protein.
Glutathione S-transferase (GST) activity was determined spectrophotometrically at 340 nm, according to the method of Habig et al. [20]. The reaction mixture contained the homogenate supernatant as the test sample, 0.1 M potassium phosphate buffer (pH 7.4), 100 mM reduced glutathione (GSH), and 100 mM 1-chloro-2,4-dinitrobenzene (CDNB) as the substrate. Enzymatic activity was expressed as µmol CDNB conjugated per mg of protein.
Protein thiol (PSH) content was quantified using the method described by Sedlak and Lindsay [21], employing 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB; Sigma-Aldrich, St. Louis, MO, USA). Protein pellets were resuspended in homogenization buffer, and absorbance was measured at 405 nm using a spectrofluorometer (model Synergy HT, BioTek Instruments, Winooski, VT, USA).
2.4. Statistical Analysis
Complementary approaches were applied to analyze microbiome data. Differences in alpha diversity between the control and probiotic groups were assessed using unpaired t-tests. The effect of treatments on beta diversity was evaluated using the PERMANOVA test (Permutational Multivariate Analysis of Variance), and group dispersion was assessed using PERMDISP (Permutational Analysis of Multivariate Dispersions) to verify the homogeneity of variances. Differences in the relative abundances of taxonomic groups were estimated using Welch’s t-test, which is appropriate for comparing two groups with potentially unequal variances. Differential abundance analyses of taxonomic groups were performed using Welch’s t-test in STAMP software (version 2.1.3; [22]) to compare groups. Statistical significance was considered at p ≤ 0.05, and trends were interpreted when 0.05 < p ≤ 0.10. Data on the status of antioxidants and oxidants were subjected to the Ryan-Joiner test to assess their normal distribution. Analysis of variance was performed using the General Linear Model using Minitab 21 software (State College, PA, USA). Differences between treatments were assessed with the Welch’s t-test comparison and then interpreted at p ≤ 0.05 (significant differences) and 0.05 < p ≤ 0.10 (a trend for difference).
3. Results
3.1. General Performance, Welfare, and Egg Quality
Additional experiments previously published by our group have demonstrated the consistent positive effects of probiotic supplementation on laying hen performance, egg quality, and welfare traits. In terms of performance, probiotic supplementation significantly increased the laying rate by 7% [12]. Regarding egg quality, probiotic supplementation improved albumen weight, yolk height, yolk length, yolk index, and yolk color parameters (color score, luminosity, red and yellow intensities), while reducing yolk pH and lipid oxidation during storage [8]. In addition, welfare outcomes were also improved, as probiotic supplementation increased the frequency and duration of feeding and exploratory behaviors, while decreasing the time birds spent sitting, reflecting better welfare conditions [10].
3.2. Oviduct Microbiome
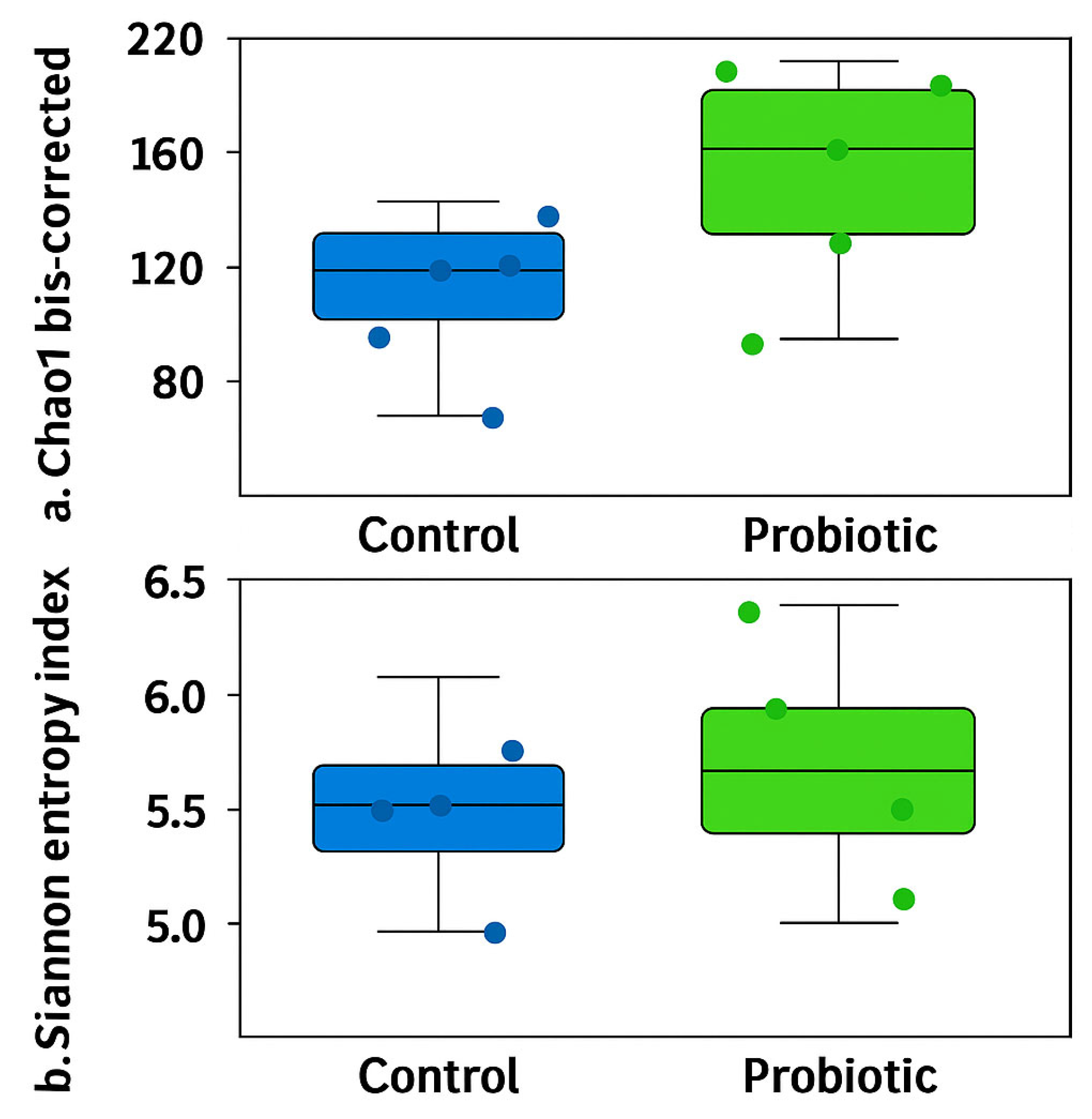
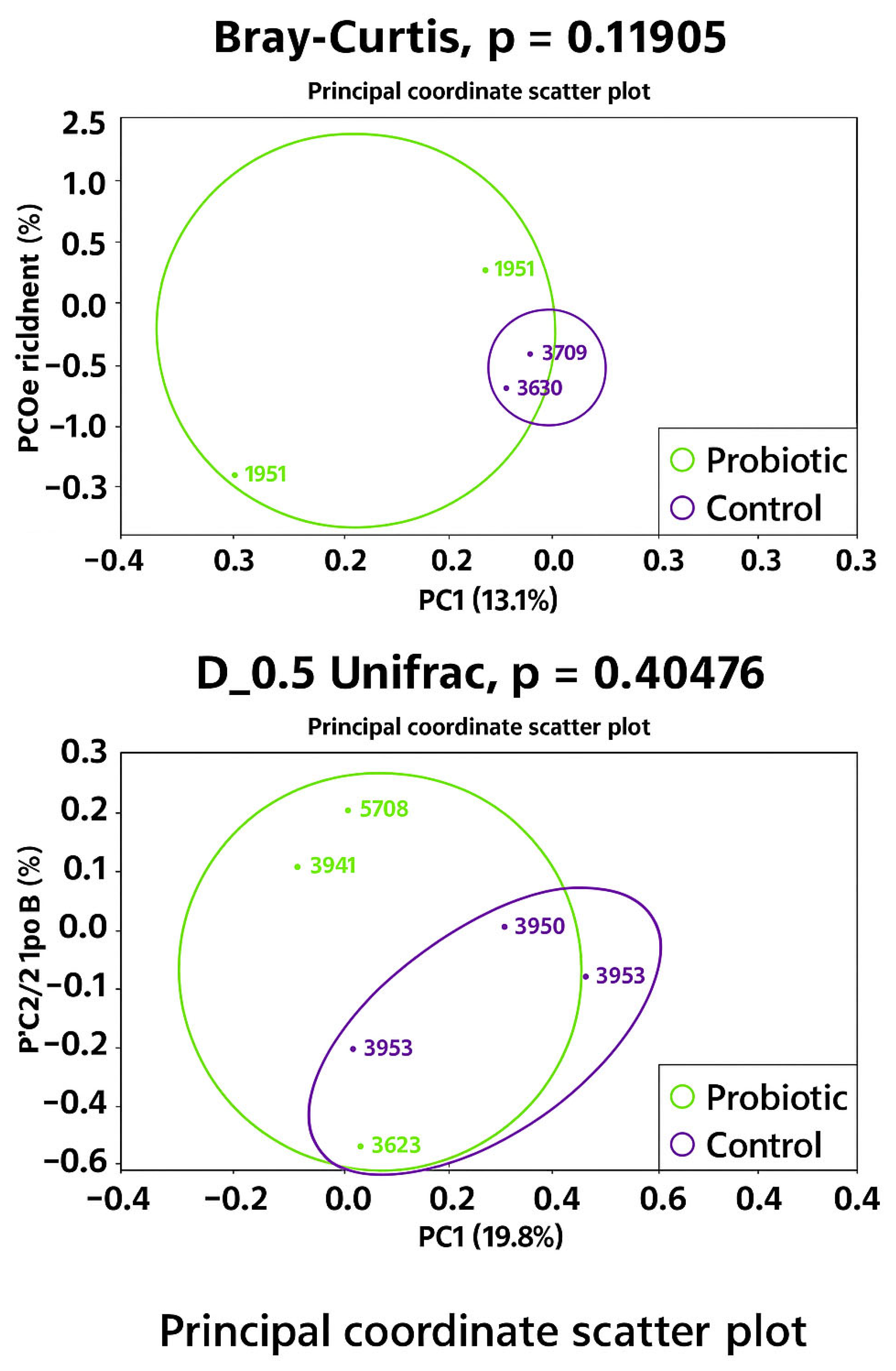
No differences were observed in the modulation of the oviduct microbiota in terms of richness (Chao1 bias-corrected) and evenness (Shannon Entropy) (Figure 1). Similarly, no significant differences were found in the abundance distributions of identified taxa between groups based on Bray–Curtis and D_0.5 Unifrac distances (Figure 2). Considering the exploratory nature of this research and the limited standardization of analytical methods for oviduct microbiota, future studies should expand the evaluation of alpha diversity by incorporating additional indices that reflect phylogenetic structure and community evenness, in order to enhance the robustness of microbial diversity interpretation.
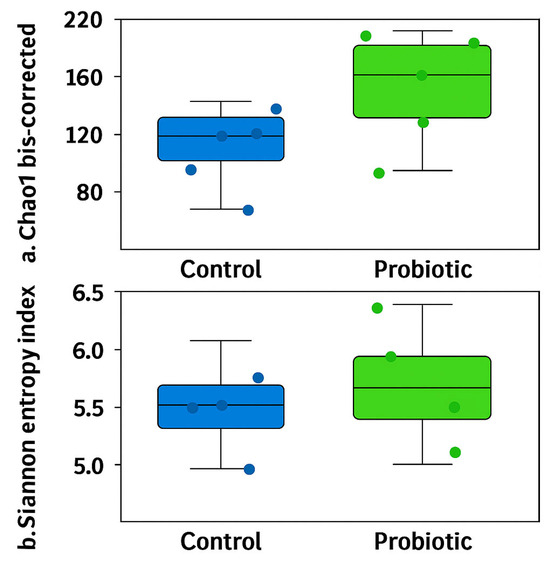
Figure 1.
Alpha diversity of the oviduct microbiota in laying hens fed a control diet or supplemented with a multi-species probiotic. Alpha diversity was assessed using the bias-corrected Chao1 (a) and Shannon entropy indices (b). There are no significant differences between groups when estimated by unpaired t-tests.
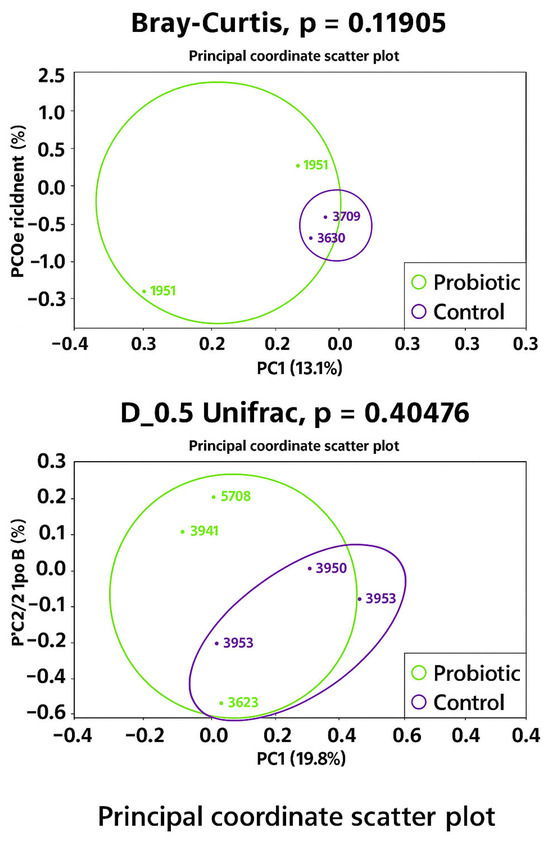
Figure 2.
Microbiota beta diversity 1 in oviduct tracts of laying hens fed a control diet (purple) or supplemented with a multi-species probiotic (green). 1 Estimated by the dissimilarity of Bray–Curtis and D_05_Unifrac.
PERMDISP analysis revealed a trend towards greater dispersion in the probiotic group compared to the control group (p = 0.055), suggesting increased variability in microbiota composition among probiotic-treated layers. This trend was particularly apparent when dispersion was estimated using Euclidean distances (p = 0.05556), with the probiotic group showing greater intra-group dissimilarity than the control. As beta dispersion evaluates the variability of samples within groups while PERMANOVA tests for differences in group centroids, both analyses were considered to ensure an accurate interpretation of beta diversity patterns.
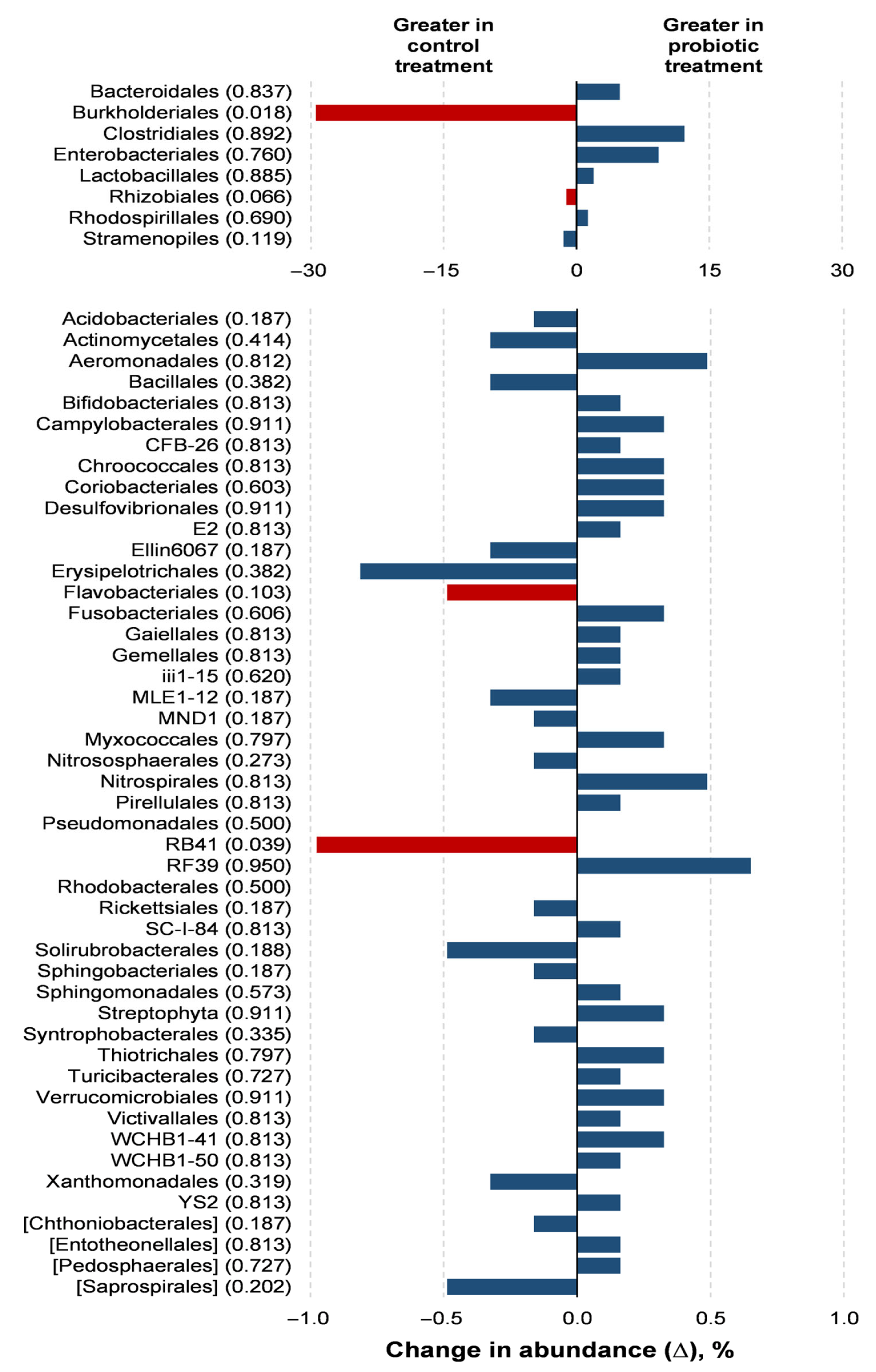
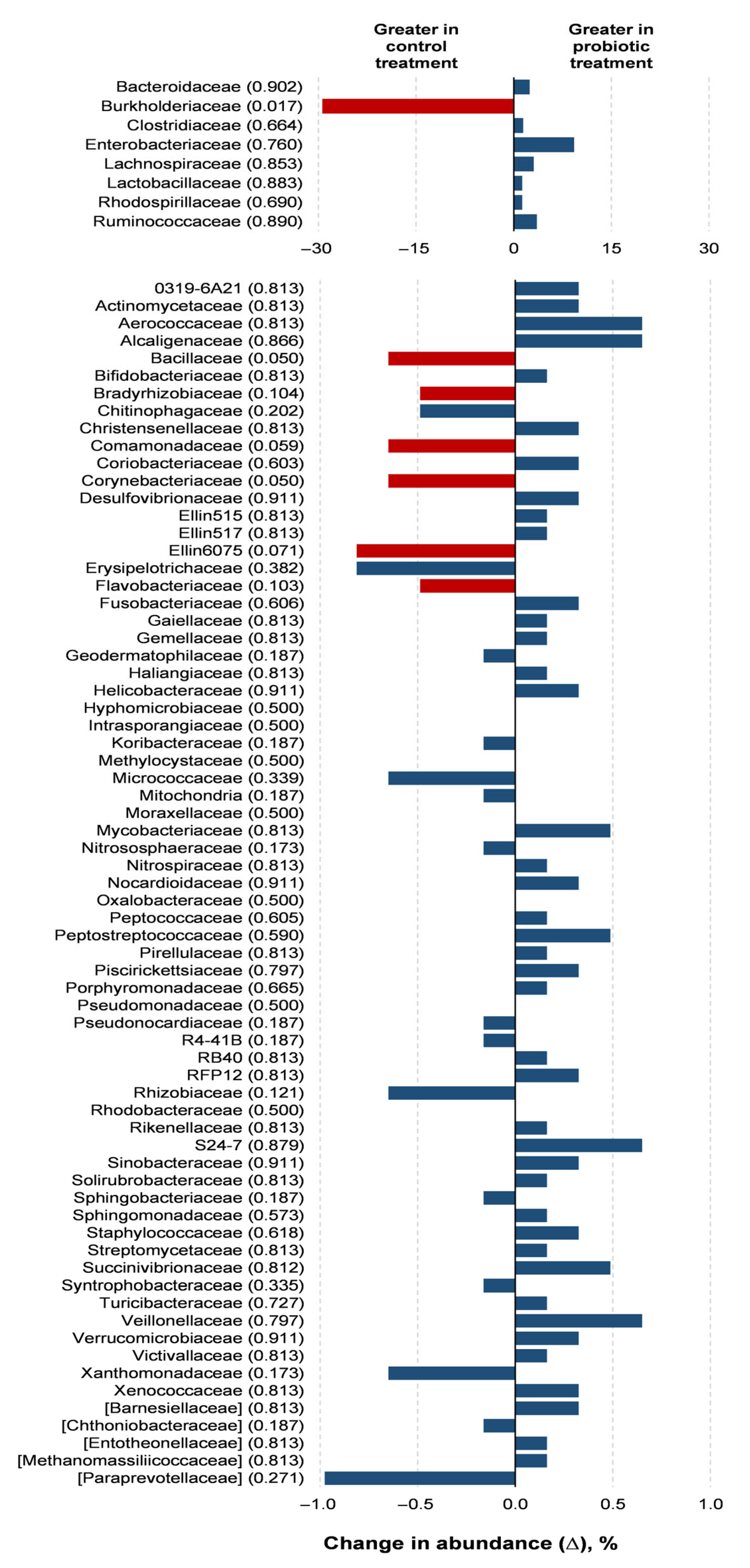
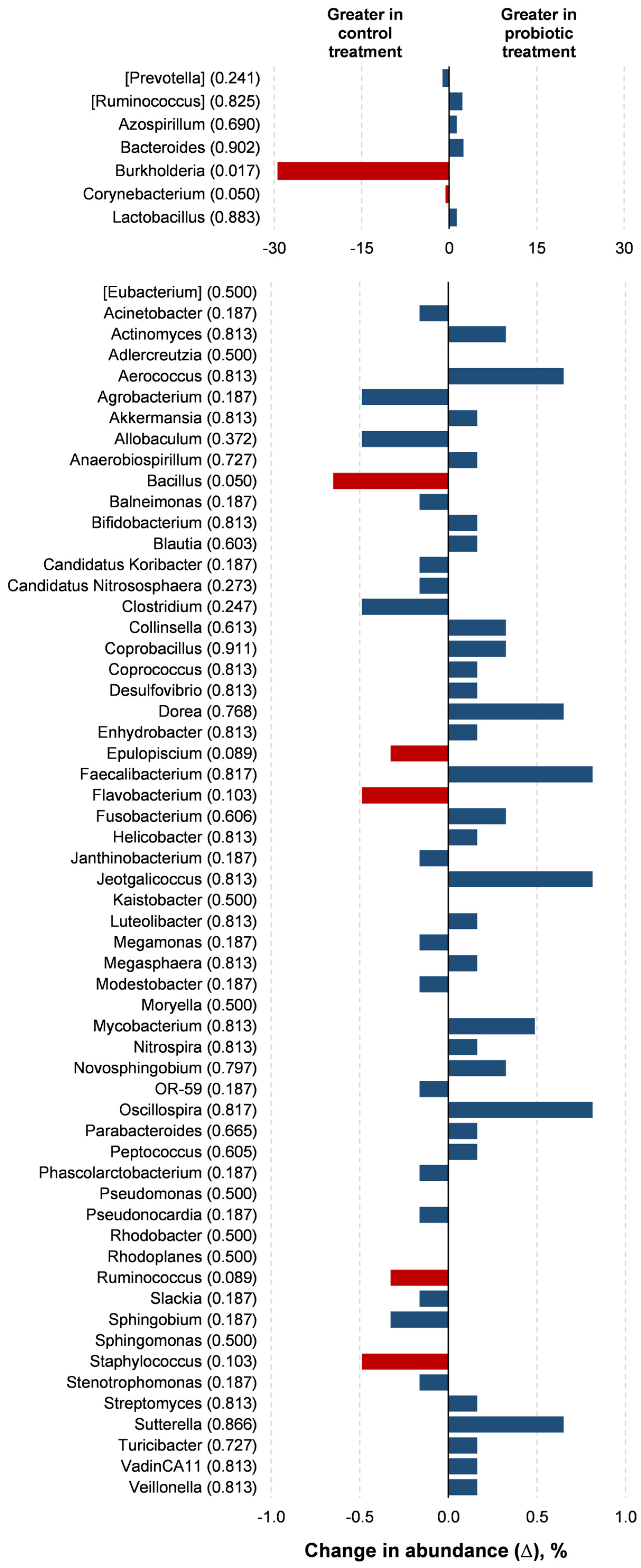
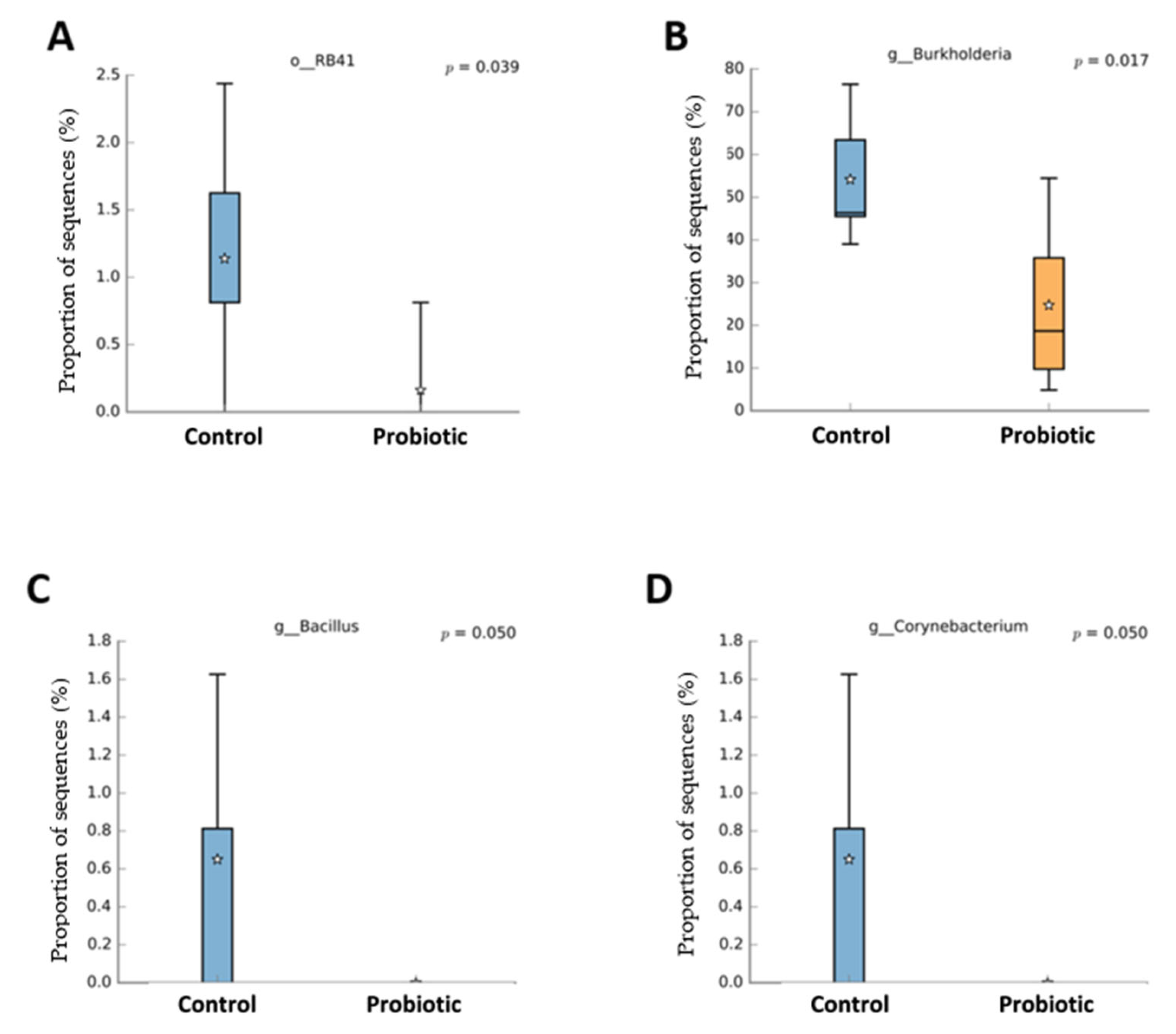
Probiotic supplementation resulted in reductions in the orders Burkholderiales (p = 0.018) and RB41 (p = 0.039) in relation to the control treatment (Figure 3). Probiotic supplementation tended to lower the abundance of the orders Rhizobiales (p = 0.066) and Flavobacteriales (p = 0.100) compared to the control treatment. Families of the Burkholderiaceae (p = 0.017), Bacillaceae (p = 0.050), and Corynebacteriaceae (p = 0.050) experienced decreases with the probiotic supplementation compared to the control (Figure 4). The probiotic supplementation resulted in a tendency to lower the abundance of the families of Bradyhizobiaceae (p = 0.100), Comamonadaceae (p = 0.059), Ellin6075 (p = 0.071), and Flavobacteriaceae (p = 0.100) compared to the control (Figure 4). Genera Burkholderia (p = 0.017), Corynebacterium (p = 0.05), and Bacillus (p = 0.050) were lower with probiotic supplementation than in the control (Figure 5). The genera Epulopiscium (p = 0.089), Flavobacterium (p = 0.10), Ruminococcus (p = 0.089), and Staphylococcus (p = 0.100) tended to be lower with the probiotic supplementation in relation to the control (Figure 5). The use of probiotic resulted in lower counts of organisms in the RB41 order (p = 0.039) and Burkholderia genus (p = 0.017), and a total reduction in Bacillus and Corynebacterium (p = 0.050) compared to the control treatment was observed (Figure 6).
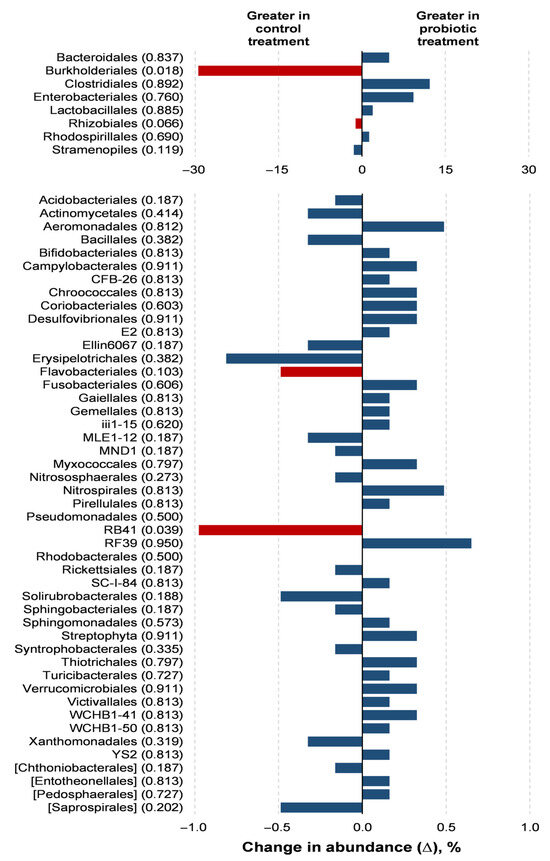
Figure 3.
Comparison of the relative abundance of microbiota orders in the oviduct tracts of laying hens fed a control diet or supplemented with a multi-species probiotic 1. 1 Bars indicate the absolute difference between treatments, with p-values following the name of each order. Red bars showed differences at p ≤ 0.10. Given the exploratory nature of this study and the limited knowledge on oviduct microbiota in poultry, we opted to present a broader set of taxonomic data, including non-significant differences, to provide a more comprehensive view of potential microbial shifts associated with probiotic supplementation. This approach aims to support future hypothesis-driven investigations in this emerging research area.
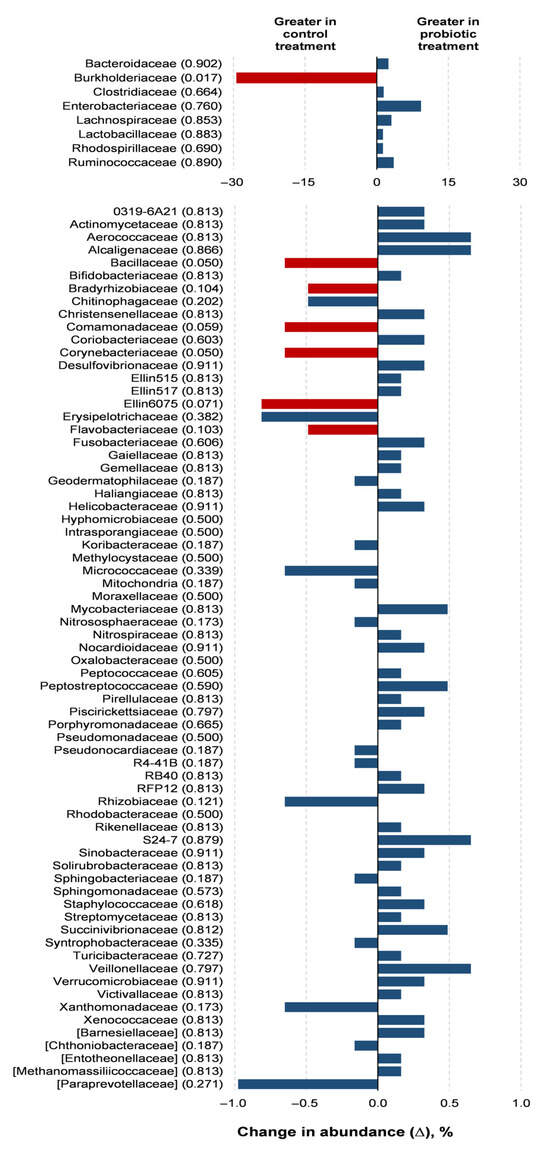
Figure 4.
Comparison of the relative abundance of microbiota families in the oviduct tracts of laying hens fed a control diet or supplemented with a multi-species probiotic 1. 1 Bars indicate the absolute difference between treatments, with p-values following the name of each family. Red bars showed differences at p ≤ 0.10. Given the exploratory nature of this study and the limited knowledge on oviduct microbiota in poultry, we opted to present a broader set of taxonomic data, including non-significant differences, to provide a more comprehensive view of potential microbial shifts associated with probiotic supplementation. This approach aims to support future hypothesis-driven investigations in this emerging research area.
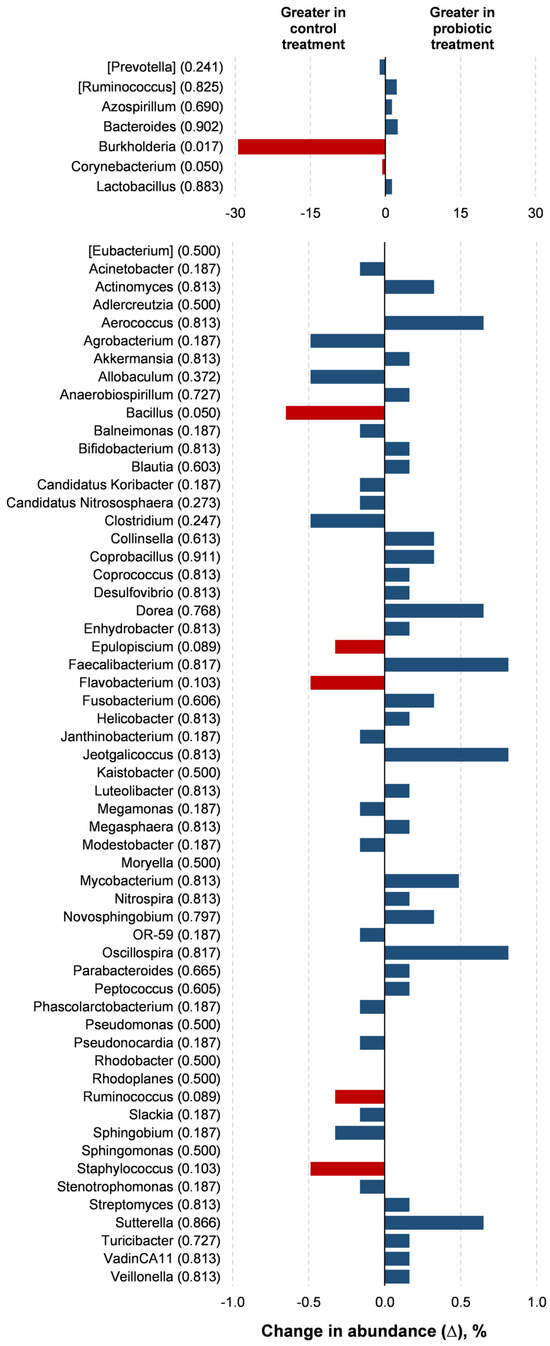
Figure 5.
Comparison of the relative abundance of microbiota genera in the oviduct tracts of laying hens fed a control diet or supplemented with a multi-species probiotic 1. 1 Bars indicate the absolute difference between treatments, with p-values following the name of each genus. Red bars showed differences at p ≤ 0.10. Given the exploratory nature of this study and the limited knowledge on oviduct microbiota in poultry, we opted to present a broader set of taxonomic data, including non-significant differences, to provide a more comprehensive view of potential microbial shifts associated with probiotic supplementation. This approach aims to support future hypothesis-driven investigations in this emerging research area.
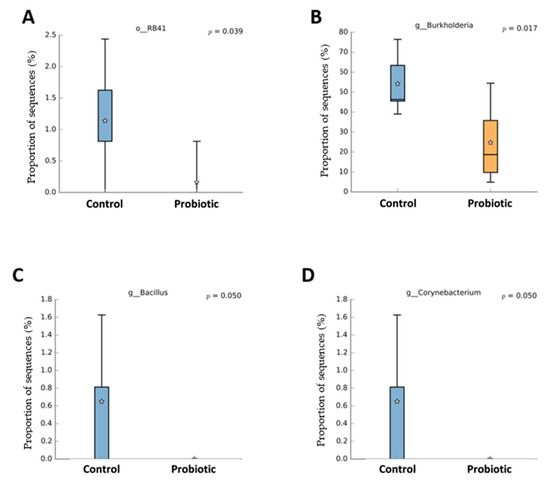
Figure 6.
Taxa with significant difference (p < 0.05) between laying hens fed a control diet or supplemented with a multi-species probiotic 1. 1 Box diagrams represent the median of the samples as a line, the mean as a star, and the 25th and 75th percentiles as the upper and lower lines of the box. The bars indicate the most extreme data points within 1.5 * (75th–25th percentile) of the median. Data points outside the bars are shown as ‘*’. (A) Relative abundance of the bacterial order RB41 was significantly reduced in the probiotic group compared to the control (p = 0.039). (B) The genus Burkholderia showed a significantly lower abundance in the probiotic group (p = 0.017). (C) The genus Bacillus was only detected in the control group, with a borderline significant difference (p = 0.050). (D) Similar to Bacillus, the genus Corynebacterium was present in the control group but nearly absent in the probiotic group (p = 0.050).
3.3. Morphology and Oxidant and Antioxidant Status
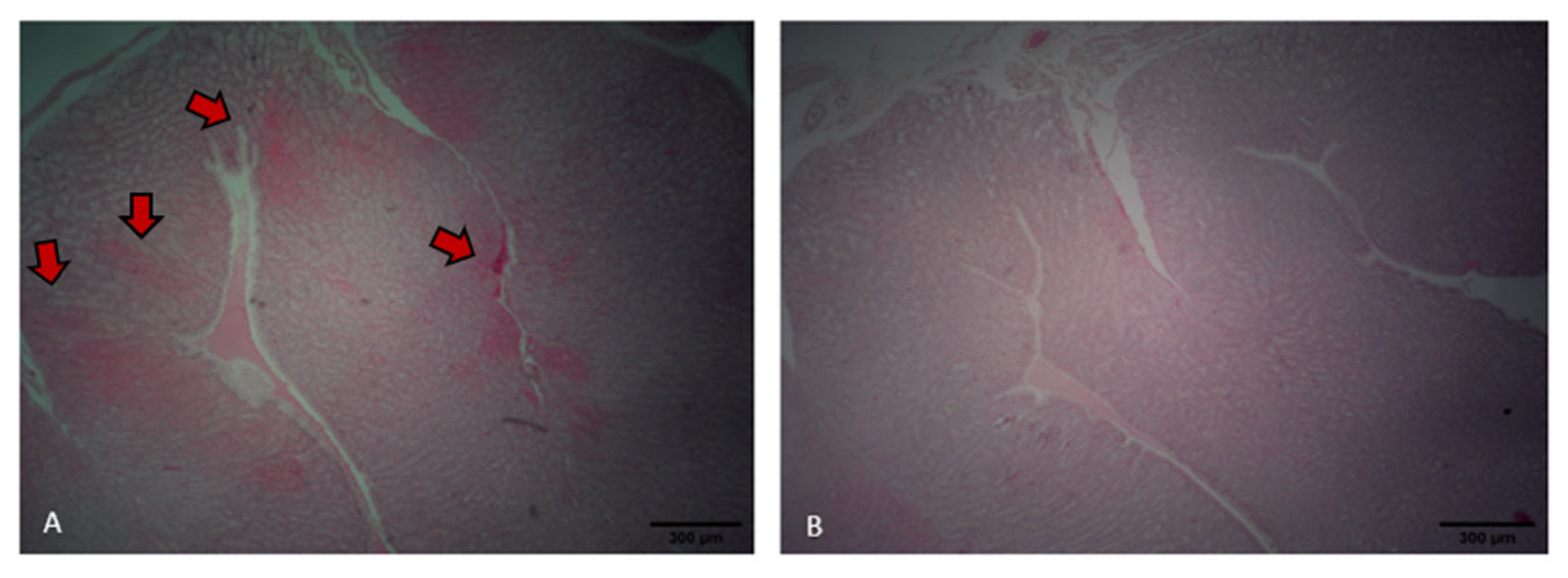
Laying hens fed the control diet exhibited areas of inflammatory infiltration, particularly around the septa and perivascular regions, as indicated by the reddish staining (Figure 7A). In contrast, hens fed the probiotic diet showed preserved oviduct tract morphology and no evident signs of inflammatory infiltration (Figure 7B). Probiotic treatment resulted in a higher PSH level in the intestine relative to that of the control (p < 0.001). However, the use of probiotic tended to reduce GST levels in the oviduct compared to the control (p = 0.068). No differences were found for TBARS and GST in the intestine and TBARS and PSH levels in the oviduct (p > 0.05; Table 2).
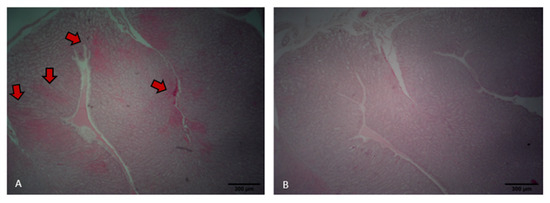
Figure 7.
Oviduct tract morphology of laying hens fed a control diet (A) or supplemented with a multi-species probiotic (B). (A) Histological section of the oviduct from a control bird showing areas of epithelial damage and inflammatory infiltration (indicated by red arrows). (B) Oviduct tissue from a probiotic-fed bird exhibiting intact epithelial structure and absence of visible inflammation.

Table 2.
Oxidant and antioxidant status in the intestinal and oviduct of laying hens fed a control diet or supplemented with a multi-species probiotic.
4. Discussion
Probiotics are known to exert beneficial effects on poultry [8,10,12] through multiple mechanisms, particularly in the gastrointestinal tract. These mechanisms include competitive exclusion of pathogenic bacteria, production of antimicrobial compounds such as bacteriocins and organic acids, and modulation of the intestinal immune response. In addition, probiotics can enhance nutrient absorption by improving gut morphology and enzyme activity, contributing to better feed efficiency and growth performance [8]. They also promote the maintenance of epithelial barrier integrity and reduce oxidative stress by stimulating the host’s antioxidant defense systems. These effects collectively support intestinal homeostasis, which is directly linked to overall bird health, productive performance, and potentially, the health of distal organs such as the reproductive tract [8,10,12].
This study was conducted to evaluate whether supplementation with a multi-species probiotic in laying hens can modulate the microbiota and health of the oviduct. The hypothesis tested in this study was that probiotic supplementation could have a beneficial effect on the oviduct microbiota and on the status of antioxidants and oxidants. Little is known about the microbiome composition in the reproductive tract of laying hens. Reproductive diseases can reduce egg production [23], resulting in economic loss. Moreover, pathogen transmission can occur through the yolk and eggshell surface [24].
In breeder hens, maintaining a healthy reproductive tract microbiota is equally or even more critical, as microbial imbalances can compromise fertility, hatchability, and progeny health. Moreover, the potential for vertical transmission of pathogens underscores the importance of strategies that promote reproductive tract integrity and microbial balance in these birds. Consequently, investigating how probiotics can alter the composition of the microbiota in the oviduct is imperative to address these concerns.
The class Betaproteobacteria, along with the genera Rhodobacter, Flavobacterium, Megamonas, Corynebacterium, and Staphylococcus, is considered important for predicting the mature hen reproductive tract [25]. Wen et al. [2] observed that the genera Staphylococcus is significantly associated with the darker brownness of the eggshell. Meanwhile, Kursa et al. [24] identified dominant orders in the turkey oviduct, with Flavobacteriales at 6.4% ± 23.52% and Burkholderiales at 4.15% ± 5.88%, alongside other orders. Both of these orders were also found in the oviducts of laying hens in our study. The high abundance of the Ruminococcus genus in the control treatment may be interpreted as a positive trait, as this genus can digest cellulose in feed and produce short-chain fatty acids through glucose metabolism [26].
Nii et al. [27] found the genus level with an abundance ratio of more than 1% across the magnum, uterus, and vagina were Corynebacterium (2.44%), Pseudomonas (7.22%, Burkholderia genera), and Staphylococcus (1.34%). Burkholderia, which was identified as a part of the Pseudomonas genus until 1992, dominated the oviduct microbiota of the layers in this study. This genus has been previously identified in the microbiota of human fallopian tubes [28] and is associated with human infections caused by the consumption of contaminated food [29] as well as septicemia in Psittaciformes [30].
Corynebacterium can be found in fecal microbiota, whereas Flavobacterium is present in the cecum [31], colon, and magnum [32]. The Corynebacterium genus is considered part of the central microbiota within the reproductive tract of adult layers, and it is predominantly abundant in segments proximate to the cloaca [2,25]. However, it is noteworthy that some species are associated with salpingitis, an oviduct inflammation in poultry [33,34]. On the other hand, the genus Flavobacterium has been identified as an opportunistic pathogen in birds [35], with reports of bacterial infections in chickens [36]. Flavobacterium causes fatal diseases in poultry and humans [35]. Therefore, the modulation caused by multi-species probiotic can be interpreted as a protective measure against pathogenic infections or contribute to controlling the growth of opportunistic pathogens. This protective effect was evident in the observed reduction in these organisms in the layer hens receiving the probiotic treatment.
The Bacillus genus has great metabolic diversity, comprising species that are not only beneficial to the host, as seen in strains used in the formulation of probiotics [37], but also including pathogens such as B. anthracis, which is associated with septicemia in ostriches [30]. Shini et al. [1] observed that the two multi-species probiotics reduced the total Bacillus genus after four weeks of supplementation for laying hens. However, these reductions were not detrimental, because the authors verified that probiotic supplementation improved reproductive tract health, reduced mortality, and increased performance (egg production and egg weight). Thus, a total reduction in Bacillus might be beneficial because it can be associated with septicemia, which reduces the egg performance and health of layer hens [30].
Epulopiscium can be found in the ileal microbiota of chickens [37] and laying hens [38]. Abaidulallah et al. [39] verified an increased abundance of pathogenic bacteria, such as Epulopiscium, in chickens infected with Newcastle disease virus. However, there is not much information about Epulopiscium and its role in layers in the literature. The order RFB41 of the diverse phylum Acidobacteria has predominantly been identified in soils [40]. However, its function in the reproductive tract microbiota still needs to be clarified.
The body has enzymatic and non-enzymatic antioxidant defense systems to combat excess free radicals that can cause oxidative stress. Glutathione S-transferase is an antioxidant enzyme found in the cytoplasm of cells, and its main role is to assist in the detoxification of organisms [41]. A reduction in GST levels in the oviduct was observed with probiotic treatment, suggesting that the body may produce fewer toxic substances, such as hydrogen peroxide and hydroperoxides. High GST levels are usually found in the presence of many toxic substances in the body [42]. Sulfhydryl proteins are markers of oxidative stress [43]. High levels of oxidant compounds can deplete intracellular thiols, resulting in their oxidation, which can change the structure and function of proteins [44]. Ficagna et al. [45] observed high levels of ROS and TBARS and lower PSH in the broiler’s intestines. Thus, in this study, a high level of intestinal protein thiols in the probiotic treatment group might indicate less oxidative stress.
Modern poultry production is moving towards more sustainable practices, including the reduction in antibiotic use and the promotion of one-health strategies. The current study was performed to further understand the composition of the oviduct microbiota and how probiotics could influence it. The results indicated this practice as an important opportunity to improve reproductive health status and thus reduce pathogen transmission through the yolk and eggshell surfaces. This not only safeguards consumers but also optimizes chick production. More studies testing the probiotic effect in the oviduct are crucial to understand the mechanism of action and the oviduct microbiota.
It is important to note that the bioinformatics pipeline used in this study, based on OTU clustering with QIIME and SILVA v128, reflects the tools available and widely adopted at the time of analysis. However, more recent platforms such as QIIME2 and updated taxonomic databases allow for higher-resolution analyses through ASV-based approaches and reflect current nomenclature standards. Future studies should adopt these updated tools to ensure greater taxonomic accuracy and comparability. In addition, future projects would benefit from a larger number of replicates, particularly for microbiota assessment, to increase statistical power and support more robust conclusions. Nevertheless, the present findings provide novel and valuable insights into the oviduct microbiota of laying hens, representing one of the first efforts to characterize this understudied microbial niche and its modulation by probiotic supplementation.
As poultry production continues to evolve toward more sustainable and health-conscious practices, understanding the role of the oviduct microbiota becomes increasingly relevant. This study provides novel insights into how probiotic supplementation can favorably modulate the reproductive tract microbiota, supporting reproductive health and potentially reducing the risk of pathogen transmission through the yolk and eggshell. Such improvements not only contribute to safer food production but also enhance hatchability and overall flock performance. Further research is essential to unravel the underlying mechanisms and fully harness the potential of probiotics in modulating the oviduct microbiome.
5. Conclusions
Supplementation with the multi-species probiotic did not affect the overall richness or diversity of the oviduct microbiota but led to a reduction in specific microbial taxa, including potentially pathogenic genera such as Burkholderia, Corynebacterium, and Staphylococcus. These findings suggest a modulatory effect on the microbial community structure, with a trend toward greater variability among supplemented birds. Although no differences were observed in oviduct morphology, the probiotic increased intestinal PSH levels and tended to reduce GST levels in the oviduct, indicating potential systemic antioxidant effects. Altogether, the results highlight the potential of targeted probiotic strategies to support reproductive tract health in laying hens, emphasizing the need for further investigation into the functional consequences of these microbial and biochemical shifts. Future studies should also incorporate multi-omics approaches and gut microbiome analysis to elucidate the mechanistic pathways through which probiotics modulate the reproductive tract microbiota and contribute to systemic health improvements.
Author Contributions
Conceptualization, G.M.G. and I.A.; methodology, G.M.G., I.A., C.L.C., A.S.d.S. and M.K.; software, I.A.; validation, G.M.G., I.A., C.L.C. and M.K.; formal analysis, G.M.G., I.A., C.L.C., A.S.d.S. and M.K.; investigation, G.M.G., I.A., C.L.C., A.S.d.S. and M.K.; resources, M.K.; data curation, G.M.G., I.A., C.L.C., A.S.d.S. and M.K.; writing—original draft preparation, G.M.G.; writing—review and editing, G.M.G., I.A., C.L.C., A.S.d.S. and M.K.; visualization, G.M.G., I.A., C.L.C., A.S.d.S. and M.K.; supervision, I.A.; project administration, I.A.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) provided partial funding for this research.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee on the Use of Animals (CEUA) of Universidade Federal do Rio Grande do Sul (protocol code 39783; approved on 21 December 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Granja Petry for the donation of the eggs and Elanco Animal Health for the donation of the products.
Conflicts of Interest
Kipper, M. was employed by Elanco Animal Health, São Paulo, Brazil. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Shini, S.; Shini, A.; Blackall, P.J. The potential for probiotics to prevent reproductive tract lesions in free-range laying hens. Anim. Prod. Sci. 2013, 53, 1298–1308. [Google Scholar] [CrossRef]
- Wen, C.; Li, Q.; Lan, F.; Li, X.; Li, G.; Yan, Y.; Wu, G.; Yang, N.; Sun, C. Microbiota continuum along the chicken oviduct and its association with host genetics and egg formation. Poult. Sci. 2021, 100, 101104. [Google Scholar] [CrossRef]
- De Reu, K.; Messens, W.; Heyndrickx, M.; Rodenburg, T.B.; Uyttendaele, M.; Herman, L. Bacterial contamination of table eggs and the influence of housing systems. J. World‘s Poult. Sci. 2008, 64, 5–19. [Google Scholar] [CrossRef]
- Gast, R.K.; Rupa, G.; Jean, G. Salmonella enteritidis deposition in eggs after experimental infection of laying hens with different oral doses. J. Food. Prot. 2013, 76, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Hope, B.K.; Baker, R.; Edel, E.D.; Hogue, A.T.; Schlosser, R.; Whiting, R.; McDowell, R.M.; Morales, R.A. An overview of the Salmonella enteritidis risk assessment for shell eggs and egg products. Risk Anal. 2002, 22, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Lu, Y.; Li, X.; Yang, X.; Wang, H.; Liu, L.; Chen, J.; Zhao, C.; Chang, Y. In vitro adherence and invasion of primary chicken oviduct epithelial cells by Gallibacterium antis. Vet. Microbiol. 2017, 203, 136–142. [Google Scholar] [CrossRef]
- Lv, J.; Guo, J.; Chen, B.; Hao, K.; Ma, H.; Liu, Y.; Min, Y. Effects of different probiotic fermented feeds on production performance and intestinal health of laying hens. Poult. Sci. 2022, 10, 101570. [Google Scholar] [CrossRef]
- Carvalho, C.L.; Andretta, I.; Galli, G.M.; Martins, G.B.; Camargo, N.; De, O.T.; Stefanello, T.B.; Melchior, R.; Da Silva, M.K. Dietary supplementation with β-mannanase and probiotics as a strategy to improve laying hen‘s welfare. Front. Vet. Sci. 2022, 9, 985947. [Google Scholar] [CrossRef]
- Xue, M.; Zhang, L.; Yang, Y.; Wang, Y.; Lv, F. Compound probiotics can improve intestinal health by affecting the gut microbiota of broilers. Poult. Sci. 2022, 101, 102208. [Google Scholar]
- Carvalho, C.L.; Andretta, I.; Galli, G.M.; Stefanello, T.B.; Camargo, N.d.O.T.; Marchiori, M.; Melchior, R.; Kipper, M. Effects of Dietary Probiotic Supplementation on Egg Quality during Storage. Poultry 2022, 1, 180–192. [Google Scholar] [CrossRef]
- Hy-Line. Management Guide for W-36 Commercial Layers. 2020. Available online: https://www.hyline.com/filesimages/Hy-Line-Products/Hy-Line-Product-PDFs/W-36/36%20COM%20ENG.pdf (accessed on 10 September 2024).
- Carvalho, C.L.; Andretta, I.; Galli, G.M.; Stefanello, T.B.; Camargo, N.D.O.T.; Mendes, R.E.; Pelisser, G.; Balamuralikrishnan, B.; Melchior, R.; Kipper, M. Dietary supplementation with β-mannanase and probiotics as a strategy to improve laying hen performance and egg quality. Front. Vet. Sci. 2023, 10, 1229485. [Google Scholar] [CrossRef]
- Brasil Ministério da Ciência. Tecnologia e Inovação. In Diretrizes Para a Prática de Eutanásia do CONCEA; MCTI: Brasília, Brazil, 2013. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Degnan, P.H.; Ochman, H. Illumina-based analysis of microbial community diversity. ISME J. 2012, 6, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “all-species living tree project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2013, 42, gkt1209. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1978, 95, 351–358. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Kursa, O.; Tomczyk, G.; Sawicka-Durkalec, A.; Adamska, K. Bacterial communities of the oviduct of turkeys. Sci. Rep. 2022, 12, 14884. [Google Scholar] [CrossRef]
- Lee, S.; La, T.M.; Lee, H.J.; Choi, I.S.; Song, C.S.; Park, S.Y.; Lee, J.B.; Lee, S.W. Characterization of microbial communities in the chicken oviduct and the origin of chicken embryo gut microbiota. Sci. Rep. 2019, 9, 6839. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Q.; Huang, Z.; Lv, L.; Liu, X.; Yin, C.; Yan, H.; Yuan, J. Effect of Bacillus subtilis CGMCC 1. 1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016, 120, 195–204. [Google Scholar]
- Nii, T.; Shinkoda, T.; Isobe, N.; Yoshimura, Y. Intravaginal injection of Lactobacillus johnsonii may modulates oviductal microbiota and mucosal barrier function of laying hens. Poult. Sci. 2023, 102, 102699. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, E.S.; Willner, D.; Buttini, M.; Hafner, L.M.; Theodoropoulos, C.; Huygens, F. The fallopian tube microbiome: Implications for reproductive health. Oncotarget 2018, 9, 21541–21551. [Google Scholar] [CrossRef] [PubMed]
- Lupo, A.; Isis, E.; Perreten, V.; Endimiani, A. Raw meat contaminated with epidemic clones of Burkholderia multivorans found in cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, 150–152. [Google Scholar] [CrossRef]
- Shivaprasad, H.L. Pathology of birds—An overview. C. L. Davis Found. Conf. Gross Morb. Anat. Anim. 2002, 1–50. [Google Scholar]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics to Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef]
- Lee, S.J.; Cho, S.; La, T.M.; Lee, H.J.; Lee, J.B.; Park, S.Y.; Song, C.S.; Choi, I.S.; Lee, S.W. Comparison of microbiota in the cloaca, colon, and magnum of layer chicken. PLoS ONE 2020, 15, e0237108. [Google Scholar] [CrossRef]
- Greenacre, C.B.; Morishita, T.Y. Backyard Poultry Medicine and Surgery: A Guide for Veterinary Practitioners, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Poultry DVM. SALPINGITIS. 2021. Available online: http://www.poultrydvm.com/condition/salpingitis (accessed on 8 January 2025).
- Oh, J.K.; Pajarillo, E.A.B.; Chae, J.P.; Kim, I.H.; Kang, D.K. Protective effects of Bacillus subtilis against Salmonella infection in the microbiome of Hy-Line Brown layers. Asian-Australas J. Anim. Sci. 2017, 30, 1332–1339. [Google Scholar] [CrossRef]
- Vancanneyt, M.; Segers, P.; Hauben, L.; Hommez, L.; Devriese, L.A.; Hoste, B.; Vandamme, P.; Kersters, K. Flavobacterium meningosepticum, a pathogen in birds. J. Clin. Microbiol. 1994, 32, 2398–2403. [Google Scholar] [CrossRef]
- Ramlucken, U.; Lalloo, R.; Roets, Y.; Moonsamy, G.; Van Rensburg, C.J.; Thantsha, M.S. Advantages of Bacillus-based probiotics in poultry production. Livest. Sci. 2020, 241, 104215. [Google Scholar] [CrossRef]
- Gao, M.; Wang, J.; Lv, Z. Supplementing Genistein for Breeder Hens Alters the Growth Performance and Intestinal Health of Offspring. Life 2023, 13, 1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Meng, J.X.; Ren, W.X.; Ma, H.; Liu, G.; Liu, R.; Geng, H.L.; Zhao, Q.; Zhang, X.X.; Ni, H.B. Amplicon-based metagenomic association analysis of gut microbiota in relation to egg-laying period and breeds of hens. BMC Microbiol. 2023, 23, 138. [Google Scholar] [CrossRef] [PubMed]
- Abaidulallah, M.; Peng, S.; Kamran, M.; Song, X.; Yin, Z. Current findings on gut microbiota mediated immune modulation against viral diseases in chicken. Viruses 2019, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Quaiser, A.; Ochsenreiter, T.; Lanz, C.; Schuster, S.C.; Treusch, A.H.; Eck, J.; Schleper, C. Acidobacteria form a coherent but highly diverse group within the bacterial domain: Evidence from environmental genomics. Mol. Microbiol. 2003, 50, 563–575. [Google Scholar] [CrossRef]
- Kherouf, A.; Aouacheri, O.; Tichati, L.; Tebboub, I.; Kherouf, M.; Saka, S. Potential antioxidant properties and anti-diabetic and hepatic/pancreatic protective effects of dietary Boswellia serrata gum resin powder against oxidative damage in streptozotocin-induced diabetic rats. Comp. Clin. Path. 2021, 30, 891–904. [Google Scholar] [CrossRef]
- Tarasconi, L.; Dazuk, V.; Molosse, V.L.; Cecere, B.G.O.; Deolindo, G.L.; Mendes, R.R.; Gloria, E.M.; Ternus, E.M.; Galli, G.M.; Paiano, D.; et al. Nursery pigs fed with feed contaminated by aflatoxin B1 (Aspergillus flavus) and anti-mycotoxin blend: Pathogenesis and negative impact on animal health and weight gain. Microb. Pathog. 2024, 186, 106464. [Google Scholar] [CrossRef]
- Galli, G.M.; Aniecevski, E.; Petrolli, T.G.; Da Rosa, G.; Boiago, M.M.; Simões, C.A.D.P.; Wagner, R.; Copetti, P.M.; Morsch, V.R.; Araujo, D.N.; et al. Growth performance and meat quality of broilers fed with microencapsulated organic acids. Anim. Feed Sci. Technol. 2021, 271, 114706. [Google Scholar] [CrossRef]
- Baba, S.P.; Bhatnagar, A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef]
- Ficagna, C.A.; Galli, G.M.; Zatti, E.; Sponchiado, B.M.; Cecere, B.G.O.; Deolindo, G.L.; Tarasconi, L.; Horn, V.W.; Mendes, R.E.; Bissacotti, B.F.; et al. Butyric acid glycerides in the diet of broilers to replace conventional growth promoters: Effects on performance, metabolism, and intestinal health. Arch. Anim. Nutr. 2022, 76, 191–204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).