Abstract
To determine if exposing embryos to light during incubation affects antibody titer and corticosterone immediately following hatch, we incubated layer eggs and exposed them to light or darkness and vaccinated a subset of each treatment against Newcastle Disease Virus (NDV) using in ovo administration on ED 18, spray application at hatch (d 0), or not at all. There were six treatments: light incubated and non-vaccinated (LNV), light incubated and in ovo vaccinated (LIV), light incubated and post-hatch vaccinated (LPHV), dark incubated and non-vaccinated (DNV), dark incubated and in ovo-vaccinated (DIV), and dark incubated and post-hatch vaccinated (DPHV). Plasma corticosterone (CORT) and NDV antibody titers were measured on d 0, 7, and 14. Light-incubated chicks had lower (p < 0.05) plasma CORT on d 0. NDV titers did not differ (p > 0.05) between light- and dark-incubated chicks on d 0, 7, or 14. However, LIV chicks had higher antibody titers than LPHV on d 14. Exposing embryos to continuous green light during incubation may reduce stress during the early post-hatch period. Vaccination method, rather than exposure to continuous green light during incubation, may have a greater impact on humoral immune response post-hatch.
1. Introduction
Lighting is an important aspect of commercial poultry management and can affect production measures as well as overall bird welfare. Commercial poultry hatcheries do not typically provide light to hatching eggs during incubation. However, a better understanding of the impact of light exposure during incubation on chick physiology and development has prompted further research in recent years. For example, some studies showed that light exposure during embryonic development can stimulate changes in post-hatch fear response [1], which could be due to the development of light-sensitive opsins in the eye [2] as well as through circadian rhythm development by the pineal gland [3]. Exposure to light for the duration of incubation can also alter the response of the hypothalamus–pituitary–adrenal (HPA) axis to environmental stressors, thereby reducing stress hormone production after hatching [4]. Melatonin has been shown to attenuate the HPA axis response to exogenous corticotropin-releasing hormone (CRH) in layer chicks [5], as well as improve post-hatch humoral immune response, when administered during incubation [6]. Indeed, Huth and Archer [7] found that providing broiler embryos with 12 hours of light per day reduced measures of fear and stress 14 days after hatch. Furthermore, layer embryos exposed to green light for 16 hours per day during incubation had higher mRNA expression of the serotonin transporter 5-HTT, and lower expression of CRH on the day of hatch compared to those incubated under white light, suggesting that light color also influences HPA axis sensitivity [8]. Broiler chicks incubated under either red, green, or white light had lower plasma CORT and higher serotonin levels after hatch compared to those incubated in darkness [9]. However, there was variation among light color treatments in terms of post-hatch fear response and humoral immune response, which were improved in the chicks exposed to white or red light but not in those exposed to green light [9]. On the other hand, green light exposure during incubation may be useful for stimulating the production of growth-related hormones after hatch, as evidenced in broilers [10].
Previous research with lighting in incubation has focused on its effects on hatchability and post-hatch growth, particularly in broilers. However, little is known about the impact of lighting during incubation on layer chick stress and humoral immune response during the early post-hatch period. In addition, previous studies have primarily used a day-night cycle, including 4, 8, 12, or 16 hours of light in a 24 h photoperiod, rather than continuous (24L:0D) light. Therefore, the objective of this research was to determine the effects of exposing layer chicken embryos to continuous green light during incubation on plasma corticosterone and antibody titer in response to in ovo and post-hatch vaccination against Newcastle Disease Virus during the first two weeks of the post-hatch period. We hypothesized that layer chicks exposed to light during incubation would have lower stress hormone levels and higher post-hatch antibody titers in response to vaccination during the first 14 d post-hatch. To test this, layer embryos were incubated under continuous green LED light or continuous darkness for the first 18 days of incubation, and either vaccinated for Newcastle Disease Virus on embryonic day (ED) 18, day of hatch (d 0), or not at all. The dark incubation served as the control within each vaccination group. The light incubated and non-vaccinated group served as the positive control, and the dark incubated and non-vaccinated group served as the negative control.
2. Materials and Methods
2.1. Animals and Experimental Design
All procedures were carried out in accordance with the guidelines established by the Texas A&M Institutional Animal Care and Use Committee (AUP#2016-0051) and birds were managed according to the guidelines described in the Guide for the Care and Use of Agricultural Animals in Research and Teaching [11]. Fertilized white layer eggs (n = 3456) were obtained from a commercial hatchery (HyLine North America, LLC) and randomly distributed among 6 treatments: light incubated and in ovo vaccinated (LIV), dark incubated and in ovo vaccinated (DIV), light incubated and post-hatch vaccinated (LPHV), dark incubated and post-hatch vaccinated (DPHV), light incubated and not vaccinated (LNV), and dark incubated and not vaccinated (DNV).
2.2. Incubation Management
Two hundred and eighty-eight eggs were set in each incubator (1500, GQF Manufacturing Company, Inc., Savannah, GA, USA), and there were two incubators in each treatment. Incubators assigned to the light treatments were equipped with four vertical LED light bars (AgriShift® TLP, Junglite Green™ technology, Once Inc., Plymouth, MN, USA). Two light bars were placed on the back wall of the incubator facing the back of the egg trays and two light bars were secured to the inside of the incubator door. The average light intensity across all levels of the incubator was 250 lux. Light intensity was the same for eggs in the middle, front, and back of the incubator because lights were placed on front and back panels so that when eggs were tilted, they were not tilted toward or away from the light. In other words, the incubator trays did not block the eggs from the light bars even when they were tilted at the most extreme angle. Figure 1 shows the absorbance spectrum for the lights used in this study. Glass windows on all the incubator doors were covered with opaque material to prevent outside light from entering the incubator. All eggs were incubated at 37.5 °C and 65% relative humidity on embryonic day (ED) 0–18. Eggs in the lit incubators were exposed to constant light (24L:0D), and eggs in the dark incubators were subject to constant darkness (0L:24D) on ED 0–18. Eggs were transferred to hatchers (1550, GQF Manufacturing Company, Inc., Savannah, GA, USA) on ED 18 and embryos in all treatments were subject to complete darkness (0L:24D) on ED 19–21.
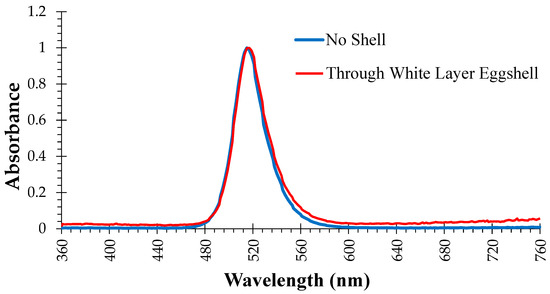
Figure 1.
Absorbance spectrum of green light emitting diode bulbs used in the incubators for treatments exposed to continuous green light (LIV—light-exposed and vaccinated against Newcastle Disease Virus in ovo on ED 18, LPHV—light-exposed and spray-vaccinated against Newcastle Disease Virus on day of hatch, and LNV—light-exposed and not vaccinated) from d 0–18 of incubation.
2.3. Vaccination Methods
On ED 18, eggs in the LIV and DIV treatments were vaccinated against Newcastle Disease Virus (NDV) (INNOVAX®-ND, Merck Animal Health, Rahway, NJ, USA) using the following method. First, an 18G needle was used to puncture the eggshell on the air cell end of the egg. Next, 100 µL of vaccine was injected into the amniotic fluid using a 1-inch 22G needle inserted through the pre-made hole. Finally, food-safe cyanoacrylate sealant was applied to the injection hole and allowed to dry. All eggs were then transferred to their respective hatchers. On the day of hatch (d 0), chicks in the LPHV and DPHV treatments were spray-vaccinated for a B1 type, C2 strain of Newcastle Disease Virus (NEWHATCH-C2®, Merck Animal Health, Rahway, NJ, USA) prior to placement in rearing pens.
2.4. Post-Hatch Management
On d 0, 100 healthy-looking chicks from each treatment were placed in floor pens measuring 1.83 m × 1.83 m and lined with 4–6 cm fresh pine shavings and equipped with nipple drinkers and tube feeders. All chicks were fed starter mash diet ad libitum. All treatments were provided with a photoperiod of 20L:4D on d 0–7, followed by 19L:5D on d 8–14. Environmental temperature was maintained at 30–34 °C on d 0–7, then reduced to 28–30 °C on d 8–14. On d 14, all chicks were humanely euthanized via exposure to carbon dioxide gas.
2.5. Blood Sampling
Blood samples were collected from 15 birds per treatment on d 0, 7, and 14 via exsanguination following decapitation. Blood samples were stored in spray-coated lithium heparin separation gel vacutainers (367884, BD Medical, Franklin Lakes, NJ, USA) and kept in an ice bath until all samples were collected. Vacutainers were then centrifuged (Centrifuge 5804, Eppendorf, Hamburg, Germany) at 4000 RPM for 15 min. Plasma was transferred to a microcentrifuge tube and stored at −20 °C, then thawed at 4 °C overnight for analysis. Commercially available ELISA kits were used to determine plasma CORT (ADI-901-097, Enzo Life Sciences, Inc., Farmingdale, NY, USA) and anti-NDV IgG antibody titers (PROFLOK®, Zoetis, Parsippany, NJ, USA).
2.6. Statistical Analysis
All data was analyzed as a 2 × 3 factorial design using the General Linear Model in Minitab 17.1.0 (Minitab, Inc., State College, PA, USA) to test the main effects of light exposure (light, no light) and vaccination method (in ovo, post-hatch spray application, or no vaccination) as well as their interaction. Pairwise comparisons were then made using Fisher’s LSD post hoc test. A significant difference was defined as p < 0.05.
3. Results
3.1. Plasma Corticosterone
Results for plasma CORT for all treatments incubated under light versus all treatments incubated under darkness are shown in Figure 2. There was a main effect of light exposure observed on d 0 (p = 0.017) and d 7 (p = 0.023), with light-incubated chicks having lower plasma CORT than dark-incubated chicks on both days, but not on d 14 (p > 0.05).
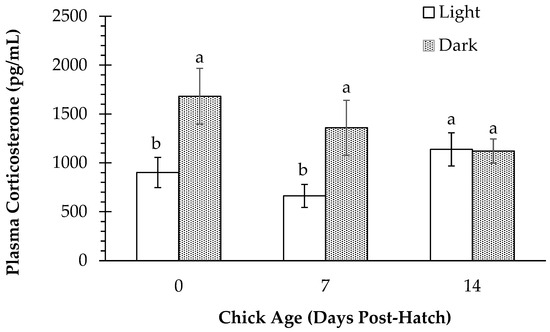
Figure 2.
Plasma corticosterone (pg/mL) in layer chicks incubated with or without light exposure at day of hatch (d 0) and d 7 and d 14 post-hatch, regardless of vaccination status. Different letters indicate a significant difference at p < 0.05.
A treatment comparison of plasma CORT is shown in Figure 3. An interaction of vaccination method and green light exposure (p = 0.03) and a main effect of vaccination method (p = 0.001) were observed on d 14 only, in which post hatch-vaccinated chicks had higher plasma CORT than in ovo- and non-vaccinated chicks. In addition, on d 14, LPHV had higher (p < 0.05) plasma CORT than all other treatments except DIV, and CORT in DIV was higher (p < 0.05) than LNV. However, d 14 CORT did not differ (p > 0.05) between DIV and LIV, nor between DNV and LNV. In addition, pairwise comparisons showed that on d 0, DIV had higher (p < 0.05) CORT than LIV and LNV. On d 7, plasma CORT was higher (p < 0.05) in DNV than LIV, LPHV, and LNV.
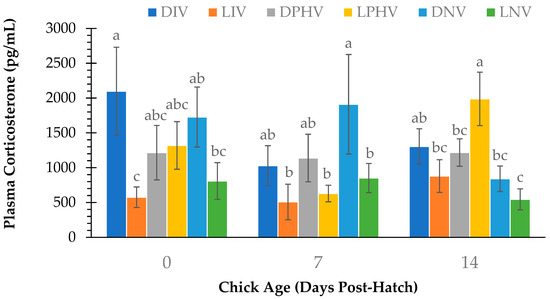
Figure 3.
Plasma corticosterone (pg/mL) at day of hatch (d 0) and d 7 and d 14 post-hatch in layer chicks incubated with or without continuous green light exposure and vaccinated against Newcastle Disease Virus in ovo on embryonic day 18, via spray application on day of hatch, or not at all. Different letters indicate a significant difference at p < 0.05. DIV = dark-incubated and in ovo vaccinated; LIV = light-exposed and in ovo vaccinated; DPHV = dark-incubated and post-hatch vaccinated; LPHV = light-exposed and post-hatch vaccinated; DNV = dark-incubated and not vaccinated; LNV = light-exposed and not vaccinated.
3.2. Anti-NDV Antibody Titer
Plasma anti-NDV antibody titers for all treatments incubated under light or dark are shown in Figure 4. A main effect of green light exposure was not observed (p > 0.05) at any time point, and antibody titers did not differ (p > 0.05) between light- and dark-incubated chicks on d 0, d 7, or d 14.
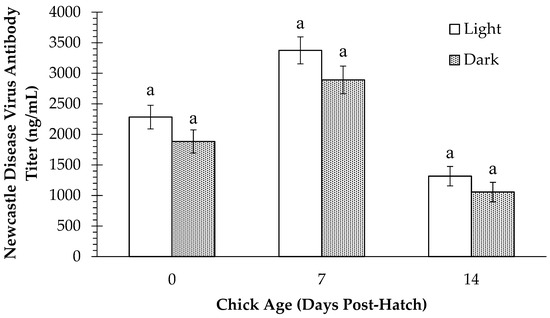
Figure 4.
Plasma Newcastle Disease Virus antibody titers (ng/mL) in layer chicks incubated with or without light exposure at day of hatch (d 0) and d 7 and d 14 post-hatch. Different letters indicate a significant difference at p < 0.05.
Figure 5 shows a treatment comparison of antibody titers on d 0, 7, and 14. There was a main effect of vaccination method on d 0 (p = 0.002), d 7 (p < 0.001), and d 14 (p < 0.001). Antibody titers were higher in the in ovo-vaccinated chicks than those in the post hatch-vaccinated or non-vaccinated groups on d 0 and d14, whereas titers were higher in both in ovo- and post hatch-vaccinated than non-vaccinated chicks on d 7.
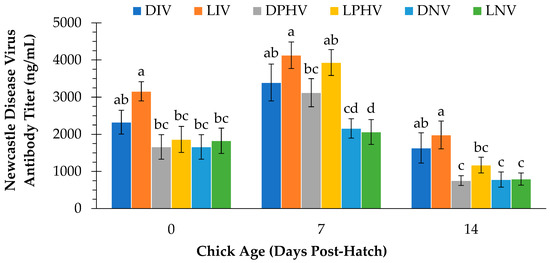
Figure 5.
Plasma Newcastle Disease Virus antibody titers (ng/mL) at day of hatch (d 0) and d 7 and d 14 post-hatch in layer chicks incubated with or without continuous green light exposure and vaccinated against Newcastle Disease Virus in ovo on embryonic day 18, via spray application on day of hatch, or not at all. Different letters indicate a significant difference at p < 0.05. DIV = dark-incubated and in ovo vaccinated; LIV = light-exposed and in ovo vaccinated; DPHV = dark-incubated and post-hatch vaccinated; LPHV = light-exposed and post-hatch vaccinated; DNV = dark-incubated and not vaccinated; LNV = light-exposed and not vaccinated.
Although there was no interaction effect of light exposure x vaccination method on antibody titer at any time point (p > 0.05), pairwise comparisons showed some treatment differences. On d 0, antibody titer in LIV was higher (p < 0.05) than that of DPHV, LPHV, DNV, and LNV, but did not differ (p > 0.05) from DIV. Antibody titer for vaccinated treatments generally appeared higher on d 7. Among vaccinated treatments, antibody titers were higher (p < 0.05) on d 7 in LIV compared to DPHV. However, titers were not different (p > 0.05) between DIV and DPHV, nor between LIV and LPHV on d 7. In addition, antibody titers in the DPHV treatment were not different (p > 0.05) from DNV or LNV on d 7. Chicks in the LIV treatment had higher (p > 0.05) antibody titers than LPHV and DPHV on d 14 despite receiving the vaccine three days earlier than LPHV chicks. Antibody titers were also higher (p > 0.05) in DIV than DPHV on d 14. Antibody titers were not different (p > 0.05) between DIV and LIV, between DPHV and LPHV, nor between DNV and LNV at any time point.
4. Discussion
The early post-hatch period can be stressful for chicks and lead to short- and long-term consequences in terms of bird behavior and susceptibility to subsequent stressors [12]. Thus, it is important to minimize stress from handling, vaccination, and other common chick processing methods at the hatchery. This study compared both the humoral immune and stress response of embryos incubated under darkness or constant exposure to green LED light. It was noted that plasma CORT was higher in dark-incubated chicks on day of hatch and 7 days post-hatch than those exposed to light during the first 18 days of incubation. However, treatment comparisons show that DIV had significantly higher CORT than LIV and LNV chicks on d 0, which likely accounts for the difference between overall light and dark treatments. This suggests that exposure to light preceding vaccination may have attenuated the stress response in in ovo-vaccinated embryos. On the other hand, CORT did not differ between in ovo- and post hatch-vaccinated chicks except for d 14, on which CORT was higher in LPHV compared to DIV, LIV, and DPHV. Therefore, it appears that in ovo vaccination was no more stressful than post hatch spray vaccination.
Plasma CORT was also higher overall in dark-incubated chicks than light-incubated chicks on d 7 post hatch. Treatment comparisons showed that CORT was higher in DNV compared to LIV, LPHV, and LNV on d 7, again suggesting the primary reason for the difference in light- and dark-incubated treatments on d 7. One possible explanation for the differences in CORT in light- and dark-incubated chicks after hatch is that chicks exposed to light during incubation exhibit lateralization of the brain at the time of hatching, whereas the same has not been observed in those incubated in darkness [13]. This lateralization allows each hemisphere to detect, process, and respond to different aspects of the chick’s environment [14]. Chicks incubated in darkness have demonstrated greater physical asymmetry during the rearing period [15], suggesting that the absence of light itself is also a stressor [16]. Sabuncuoğlu et al. [17] concluded that exposure to green and blue light during incubation improved fearfulness in Japanese quail subjected to an open field test after hatch. In this study, chicks in the LNV and DNV treatments did not differ in plasma CORT on d 0 or d 14, although CORT was higher in DNV on d 7. This indicates that the presence or absence of light did not significantly affect the stress response upon hatching. It was, however, noted that dark-incubated chicks showed a reduction in CORT from d 0 to d 14, suggesting that these chicks experienced lateralization after hatching.
Light intensity also determines whether light exposure during incubation is beneficial or detrimental on chick physiology. Yu et al. [18] noted that exposure to high intensity green light (150 or 300 lux) during incubation had a negative effect on plasma thyroid and testosterone levels in broiler embryos. This study used a light intensity of 250 lux, which was chosen to ensure that all eggs received adequate light throughout the incubation period. However, 250 lux is considered high intensity [18] and therefore may have prevented some of the changes in plasma CORT we expected to see after hatch. In addition, the photoperiod provided during incubation may also be an important factor in eliciting desired responses in humoral immunity and stress response. Chicks in this study may not have developed a circadian rhythm due to the provision of constant light. Providing at least 12 h of light per day can initiate melatonin rhythmicity in broiler chicks [19]. Specifically, exposing layer embryos to green LED light for 12L:12D increased melatonin secretion compared to dark-incubated embryos [20]. Exposure to a 12L:12D photoperiod during d 13–18 of incubation was also shown to reduce measures of fear post-hatch [1]. In addition, broiler embryos exposed to 12L:12D had higher antibody titers and reduced measures of short- and long-term stress compared to those incubated under continuous darkness, 1L:23D, or 6L:18D [16]. Had we looked at measures of fearfulness and long-term stress in chicks after hatch, we may have had a better understanding of the impact of photoperiod or of light exposure on embryo behavior as well as the stress response. However, such measures were outside the scope of this study, and limitations on space and equipment prevented the investigation of other variables such as photoperiod.
In addition to plasma CORT, we also measured the effect of lighted incubation and vaccination method on humoral immune response to NDV. There were no differences in antibody titers between light and dark treatments on day of hatch, or 7- or 14-days post hatch. There are few studies to compare the results of light exposure during embryonic development and immune response after hatch. However, one study comparing exposure to different colors of monochromatic light after hatch showed that anti-NDV antibody titers of broilers reared under green light did not differ from those reared under blue, red, or yellow light on d 8, d 30, or d 42 [21]. On d 18, however, broiler chicks reared under green light had higher antibody titers than those reared under red light [21]. Archer [9] showed that white light is filtered through broiler eggshells such that embryos receive more red light. Subsequently, green light-incubated chicks showed worse levels of fearfulness and reduced antibody titers after hatch than those incubated under red or white light [9]. In this study, exposure to green light during incubation did not affect humoral immune response to in ovo or spray vaccination after hatch. Therefore, green light exposure during incubation is likely less beneficial for chicks’ early life immune response and stress susceptibility than red or white light.
A more thorough comparison can be made for vaccination method and immune response in layer chicks. Antibody titers were not different for DPHV and DNV and for LPHV and LNV on d 0 because the same chicks were obtained from either the dark or lit incubators, respectively, prior to spray-vaccination. Antibody titers in LIV were higher than all other treatments except DIV on d 0. Antibody production was expected to be higher for in ovo-vaccinated treatments on day of hatch because these were the only treatments to have received a vaccine at that point. Antibody titers were not different between LIV and LPHV on d 7 but were higher in LIV compared to LPHV on d 14. It is possible that the immune response in LPHV chicks had not peaked by d 7, and differences between LIV and LPHV could be clearer if data were analyzed on d 10 post-hatch as well. However, given the data, it appears that although LIV chicks were vaccinated three days prior to those in the LPHV treatment, they sustained a more intense immune response over a longer period than the LPHV chicks. Administering vaccines in ovo on ED 18 can stimulate an earlier immune response, which can be more beneficial in preventing disease compared to post-hatch vaccination [22]. Indeed, in ovo vaccination of some NDV vaccines can induce an effective humoral immune response despite the presence of (or without interference from) maternal antibodies [23]. In addition, Negash et al. [24] observed that chicks receiving in ovo vaccines typically demonstrate sustained high levels of antibody production throughout rearing. The reason for this could be that chicks launch an immune response not only to vaccine that is injected directly into their tissue but also a gut-associated humoral response to vaccine that is ingested from the amniotic fluid or taken up by the cloaca [24]. As such, in ovo-vaccinated chicks have demonstrated greater vaccine distribution throughout the body tissues, at higher levels, and for a prolonged period compared to those vaccinated at hatch [25]. Even though the specific NDV vaccine administered in ovo differed from that administered by spray application on day of hatch due to availability, the timing of the vaccine application may still be a more critical factor in the birds’ immune response. However, further research may clarify this detail.
5. Conclusions
Exposure to continuous green light during incubation did not consistently reduce plasma CORT in chicks after hatching. Neither did it affect anti-NDV antibody production compared to chicks incubated in darkness. As such, incubating layer eggs under continuous green light may not be effective in reducing early life stress susceptibility or humoral immune response. However, chicks that received in ovo vaccination—regardless of light exposure during incubation—launched a humoral immune response that lasted longer than chicks that were vaccinated on day of hatch. Therefore, vaccination method may have a greater influence than exposure to light during incubation on post-hatch immune response. Furthermore, in ovo vaccination increased the intensity and duration of the humoral immune response compared to post hatch vaccination, thereby improving chicks’ ability to respond to immune challenges faced during early life. These findings support the use of in ovo vaccination but not of green light exposure during incubation in commercial hatcheries.
Author Contributions
Conceptualization, methodology, and formal analysis, G.S.A.; investigation, J.R.D. and G.S.A.; writing—original draft preparation, J.R.D.; writing—review and editing, J.R.D. and G.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All experimental procedures on live animals used in this experiment were approved by the Texas A&M University Institutional Animal Care and Use Committee (AICUC Animal Use Protocol # 2016-0051, approved on 7 April 2016).
Informed Consent Statement
Not Applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful for the support of the undergraduate students who assisted with vaccination, monitoring of eggs and chicks, and data collection.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CORT | Corticosterone |
| ED | Embryonic day |
| NDV | Newcastle Disease Virus |
References
- Hill, W.L.; Bassi, K.L.; Bonaventura, L.; Sacus, J.E. Prehatch entrainment of circadian rhythms in the domestic chick using different light regimes. Dev. Psychobiol. 2004, 45, 174–186. [Google Scholar] [CrossRef]
- Bruhn, S.L.; Cepko, C.L. Development of the pattern of photoreceptors in the chick retina. J. Neurosci. 1996, 16, 1430–1439. [Google Scholar] [CrossRef]
- Okabayashi, N.; Yasuo, S.; Watanabe, M.; Namikawa, T.; Ebihara, S.; Yoshimura, T. Ontogeny of circadian clock gene expression in the pineal and the suprachiasmatic nucleus of chick embryo. Brain Res. 2003, 990, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Özkan, S.; Yalçin, S.; Babacanoğlu, E.; Koxanoğlu, H.; Karadaş, F.; Uysal, S. Photoperiodic lighting (16 hours of light:8 hours of dark) programs during incubation: 1. Effects on growth and circadian physiological traits of embryos and early stress response in broiler chickens. Poult. Sci. 2012, 91, 2912–2921. [Google Scholar] [CrossRef]
- Saito, S.; Tachibana, T.; Choi, Y.H.; Denbow, D.M.; Furuse, M. ICV melatonin reduces acute stress responses in neonatal chicks. Behav. Brain Res. 2005, 165, 197–203. [Google Scholar] [CrossRef]
- Moore, C.B.; Siopes, T.D. Enhancement of cellular and humoral immunity following embryonic exposure to melatonin in turkeys (Meleagris gallopavo). Gen. Comp. Endocrinol. 2005, 143, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Huth, J.C.; Archer, G.S. Effects of LED lighting during incubation on layer and broiler hatchability, chick quality, stress susceptibility and post-hatch growth. Poult. Sci. 2015, 94, 3052–3058. [Google Scholar] [CrossRef]
- Özkan, S.; Yalçin, S.; Bayraktar, Ö.H.; Bilgen, G.; Dayioğlu, M.; Bolhuis, J.E.; Rodenburg, T.B. Effects of incubation lighting with green or white light on brown layers: Hatching performance, feather pecking and hypothalamic expressions of genes related with photoreception, serotonin, and stress systems. Poult. Sci. 2022, 101, 102114. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.S. Exposing broiler eggs to green, red and white light during incubation. Animal 2017, 11, 1203–1209. [Google Scholar] [CrossRef]
- Dishon, L.; Avital-Cohen, N.; Malamud, D.; Heiblun, R.; Druyan, S.; Porter, T.E.; Gumułka, M.; Rozenboim, I. In-ovo monochromatic green light photostimulation enhances embryonic somatotropic axis activity. Poult. Sci. 2017, 96, 1884–1890. [Google Scholar] [CrossRef]
- Federation of Animal Science Societies (FASS). Guide for The Care and Use of Agricultural Animals in Research and Teaching, 3rd ed.; Federation of Animal Science Societies: Champaign, IL, USA, 2010. [Google Scholar]
- Hedlund, L.; Whittle, R.; Jensen, P. Effects of commercial hatchery processing on short- and long-term stress responses in laying hens. Sci. Rep. 2019, 9, 2367. [Google Scholar] [CrossRef]
- Rogers, L.J. Light experience and asymmetry of brain function in chickens. Nature 1982, 297, 223–225. [Google Scholar] [CrossRef]
- Rogers, L.J. Development and function of lateralization in the avian brain. Brain Res. Bull. 2018, 76, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.S.; Shivaprasad, H.L.; Mench, J.A. Effect of providing light during incubation on the health, productivity, and behavior or broiler chickens. Poult. Sci. 2009, 88, 29–37. [Google Scholar] [CrossRef]
- Archer, G.S.; Mench, J.A. The effects of light stimulation during incubation on indicators of stress susceptibility in broilers. Poult. Sci. 2013, 92, 3103–3108. [Google Scholar] [CrossRef]
- Sabuncuoğlu, K.M.; Korkmaz, F.; Gürcan, E.K.; Narinç, D.; Samli, H.E. Effects of monochromatic light stimuli during embryogenesis on some performance traits, behavior, and fear responses in Japanese quails. Poult. Sci. 2018, 97, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, Z.; Zhong, Z.; Jin, S.; Pan, J.; Rao, X.; Yu, Y. Effect of monochromatic green LED light stimuli during incubation on embryo growth, hatching performance, and hormone levels. Trans. ASABE 2018, 61, 661–669. [Google Scholar] [CrossRef]
- Archer, G.S.; Mench, J.A. The effects of the duration and onset of light stimulation during incubation on the behavior, plasma melatonin levels, and productivity of broiler chickens. J. Anim. Sci. 2014, 92, 1753–1758. [Google Scholar] [CrossRef]
- Tang, W.Y.; Tong, Q.; Li, B.M.; Zheng, W.C.; Pan, J.M.; Wang, X.C.; Liu, X.; Jin, K. Effects of different light-emitting diode light on hatch performance, embryo development, eye structure, and plasma melatonin in layer incubation. Poult. Sci. 2023, 102, 102977. [Google Scholar] [CrossRef]
- Firouzi, S.; Nazarpak, H.H.; Habibi, H.; Jalali, S.S.; Nabizadeh, Y.; Rezaee, F.; Ardali, R.; Marzban, M. Effects of color lights on performance, immune response and hematological indices of broilers. J. World’s Poult. Res. 2014, 4, 52–55. [Google Scholar]
- Elliott, K.E.C.; Branton, S.L.; Evans, J.D.; Peebles, E.D. Early post-hatch survival and humoral immune response of layer chickens when in ovo vaccinated with strain F Mycoplasma gallisepticum. Poult. Sci. 2018, 97, 3860–3869. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, K.M.; Taylor, T.L.; Marcano, V.C.; Wiliams-Coplin, D.; Olivier, T.L.; Yu, Q.; Gogal, R.M., Jr.; Suarez, D.L.; Alfonso, C.L. Novel recombinant Newcastle Disease Virus-based in ovo vaccines bypass maternal immunity to provide full protection from early virulent challenge. Vaccines 2021, 9, 1189. [Google Scholar] [CrossRef]
- Negash, T.; Al-Garib, S.O.; Gruys, E. Comparison of in ovo and post-hatch vaccination with particular reference to infectious bursal disease. A review. Vet. Q. 2004, 26, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.M. Embryo vaccination of specific-pathogen-free chickens with Infectious Bursal Disease Virus: Tissue distribution of the vaccine virus and protection of hatched chickens against disease. Avian Dis. 1986, 30, 776–780. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).