UVB Stress Induced Changes in Germination and Carbohydrate Mobilization in Chenopodium Quinoa Willd. Seeds
Abstract
1. Introduction
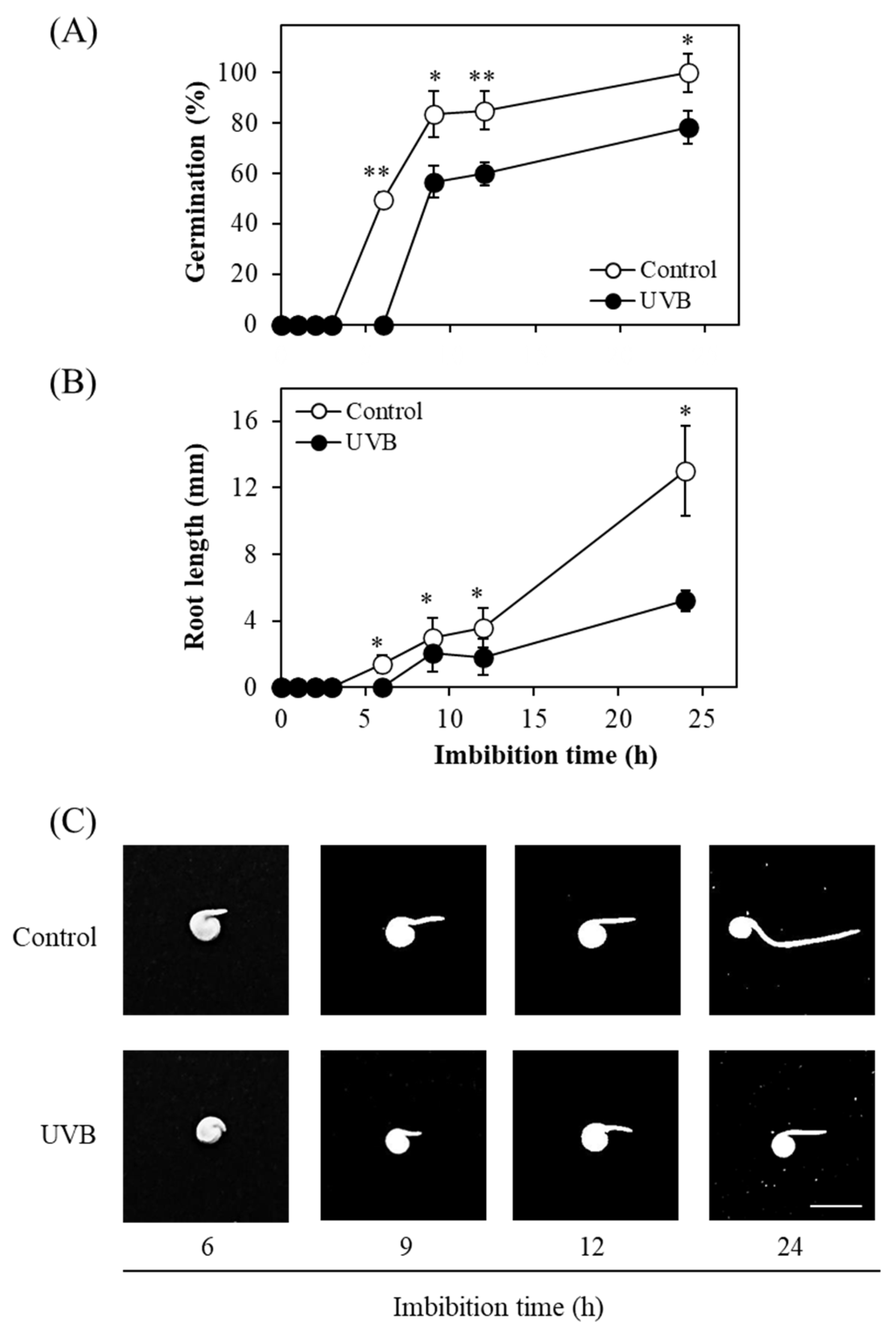
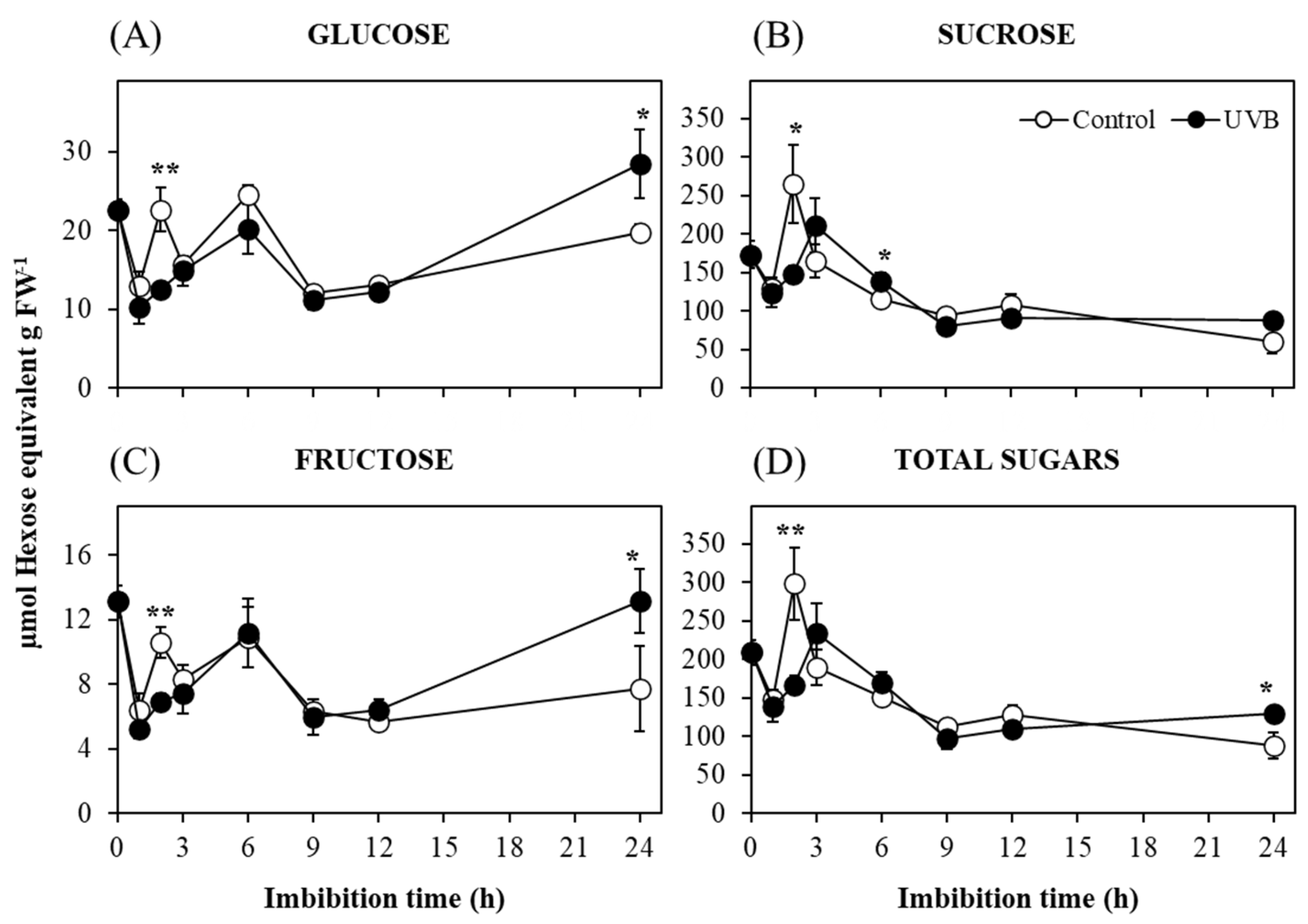
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. UVB Radiation Treatment
3.3. Biometric Analysis
3.4. Extraction and Determination of Soluble Sugars
3.5. Extraction and Determination of Starch
3.6. Protein Extraction and Zymogram of Amylolytic Activity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Barnes, P.W.; Williamson, C.E.; Lucas, R.M.; Robinson, S.A.; Madronich, S.; Paul, N.D.; Bornman, J.F.; Bais, A.F.; Sulzberger, B.; Wilson, S.R.; et al. Ozone Depletion, Ultraviolet Radiation, Climate Change and Prospects for a Sustainable Future. Nat. Sustain. 2019, 2, 569–579. [Google Scholar] [CrossRef]
- Bornman, J.F.; Barnes, P.W.; Robson, T.M.; Robinson, S.A.; Jansen, M.A.K.; Ballare, C.L.; Flint, S.D. Linkages between Stratospheric Ozone, UV Radiation and Climate Change and Their Implications for Terrestrial Ecosystems. Photochem. Photobiol. Sci. 2019, 18, 681–716. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Scartazza, A.; Castagna, A.; Cosio, E.G.; Ranieri, A.; Guglielminetti, L. Physiological Effects of Short Acute UVB Treatments in Chenopodium Quinoa Willd. Sci. Rep. 2018, 8, 371. [Google Scholar] [CrossRef]
- González Besteiro, M.A.; Bartels, S.; Albert, A.; Ulm, R. Arabidopsis MAP Kinase Phosphatase 1 and Its Target MAP Kinases 3 and 6 Antagonistically Determine UV-B Stress Tolerance, Independent of the UVR8 Photoreceptor Pathway. Plant J. 2011, 68, 727–737. [Google Scholar] [CrossRef]
- Hideg, É.; Jansen, M.A.K.; Strid, Å. UV-B Exposure, ROS, and Stress: Inseparable Companions or Loosely Linked Associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Jenkins, G.I. The UV-B Photoreceptor UVR8: From Structure to Physiology. Plant Cell 2014, 26, 21–37. [Google Scholar] [CrossRef]
- Vanhaelewyn, L.; Prinsen, E.; Van Der Straeten, D.; Vandenbussche, F. Hormone-Controlled UV-B Responses in Plants. J. Exp. Bot. 2016, 67, 4469–4482. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Yu, N.; Li, W.; Vanhaelewyn, L.; Hamshou, M.; Van Der Straeten, D.; Smagghe, G. An Ultraviolet B Condition That Affects Growth and Defense in Arabidopsis. Plant Sci. 2018, 268, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of Increasing Ultraviolet-B (UV-B) Radiation on Photosynthetic Processes. J. Photochem. Photobiol. B Biol. 2014, 137, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Cabrol, N.A.; Feister, U.; Häder, D.P.; Piazena, H.; Grin, E.A.; Klein, A. Record Solar UV Irradiance in the Tropical Andes. Front. Environ. Sci. 2014, 2, 15–20. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Esparza, E.; Crestani, G.; Limonchi, F.; Cruz, R.; Salinas, N.; Scartazza, A.; Guglielminetti, L.; Cosio, E. Physiological Responses of Maca (Lepidium meyenii Walp.) Plants to UV Radiation in Its High-Altitude Mountain Ecosystem. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Estrella, D.; Bresciani, A.; Iametti, S.; Marengo, M.; Pagani, M.A.; Marti, A. Effect of Sprouting on Proteins and Starch in Quinoa (Chenopodium quinoa Willd.). Plant Foods Hum. Nutr. 2020, 75, 635–641. [Google Scholar] [CrossRef]
- Tan, M.; Zhao, Q.; Zhao, B. Physicochemical Properties, Structural Characterization and Biological Activities of Polysaccharides from Quinoa (Chenopodium quinoa Willd.) Seeds. Int. J. Biol. Macromol. 2021, 193, 1635–1644. [Google Scholar] [CrossRef]
- Hariadi, Y.; Marandon, K.; Tian, Y.; Jacobsen, S.-E.; Shabala, S. Ionic and Osmotic Relations in Quinoa (Chenopodium quinoa Willd.) Plants Grown at Various Salinity Levels. J. Exp. Bot. 2011, 62, 185–193. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Liu, F.; Jensen, C.R. Does Root-Sourced ABA Play a Role for Regulation of Stomata under Drought in Quinoa (Chenopodium quinoa Willd.). Sci. Hortic. 2009, 122, 281–287. [Google Scholar] [CrossRef]
- González, J.A.; Rosa, M.; Parrado, M.F.; Hilal, M.; Prado, F.E. Morphological and Physiological Responses of Two Varieties of a Highland Species (Chenopodium quinoa Willd.) Growing under near-Ambient and Strongly Reduced Solar UV-B in a Lowland Location. J. Photochem. Photobiol. B Biol. 2009, 96, 144–151. [Google Scholar] [CrossRef]
- Rojas, W.; Alandia, G.; Irigoyen, J.; Blajos, J.; Santivañez, T. Quinoa: An Ancient Crop to Contribute to World Food Security; FAO: Rome, Italy, 2011; pp. 1–55. Available online: https://www.fao.org/4/aq287e/aq287e.pdf (accessed on 10 September 2025).
- Tuan, P.A.; Sun, M.; Nguyen, T.-N.; Park, S.; Ayele, B.T. Molecular Mechanisms of Seed Germination. In Sprouted Grains; Feng, H., Nemzer, B., DeVries, J.W., Eds.; AACC International Press: St. Paul, MN, USA, 2019; pp. 1–24. ISBN 9780128115251. [Google Scholar]
- Hager, A.S.; Mäkinen, O.E.; Arendt, E.K. Amylolytic Activities and Starch Reserve Mobilization during the Germination of Quinoa. Eur. Food Res. Technol. 2014, 239, 621–627. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Hager, A.S.; Arendt, E.K. Localisation and Development of Proteolytic Activities in Quinoa (Chenopodium quinoa) Seeds during Germination and Early Seedling Growth. J. Cereal Sci. 2014, 60, 484–489. [Google Scholar] [CrossRef]
- Fraikin, G.Y. Signaling Mechanisms Regulating Diverse Plant Cell Responses to UVB Radiation. Biochemistry 2018, 83, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Kaiserli, E. Ultraviolet Rays Light up Transcriptional Networks Regulating Plant Growth. Dev. Cell 2018, 44, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.S.; Karami, A.; Movahhed Haghighi, T.; Maggi, F. Two Iranian Scrophularia Striata Boiss. Ecotypes under UV-B Radiation: Germination and Initial Growth Perspective. S. Afr. J. Bot. 2022, 148, 460–468. [Google Scholar] [CrossRef]
- Prado, F.E.; Boero, C.; Gallardo, M.; González, J.A. Effect of NaCl on Germination, Growth, and Soluble Sugar Content in Chenopodium Quinoa Willd. Seeds. Bot. Bull. Acad. Sin. 2000, 41, 27–34. [Google Scholar]
- Pompeiano, A.; Guglielminetti, L. Carbohydrate Metabolism in Germinating Caryopses of Oryza sativa L. Exposed to Prolonged Anoxia. J. Plant Res. 2016, 129, 833–840. [Google Scholar] [CrossRef]
- Liu, L.; Xia, W.; Li, H.; Zeng, H.; Wei, B.; Han, S.; Yin, C. Salinity Inhibits Rice Seed Germination by Reducing α-Amylase Activity via Decreased Bioactive Gibberellin Content. Front. Plant Sci. 2018, 9, 275. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Skalicky, M.; Brestic, M.; Pavla, V. Cross-Talk between Nitric Oxide, Hydrogen Peroxide and Calcium in Salt-Stressed Chenopodium Quinoa Willd. At Seed Germination Stage. Plant Physiol. Biochem. 2020, 154, 657–664. [Google Scholar] [CrossRef]
- Tiessen, A.; Padilla-Chacon, D. Subcellular Compartmentation of Sugar Signaling: Links among Carbon Cellular Status, Route of Sucrolysis, Sink-Source Allocation, and Metabolic Partitioning. Front. Plant Sci. 2013, 3, 306. [Google Scholar] [CrossRef]
- Guglielminetti, L.; Yamaguchi, J.; Perata, P.; Alpi, A. Amylolytic Activities in Cereal Seeds under Aerobic and Anaerobic Conditions. Plant Physiol. 1995, 109, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Guglielminetti, L.; Yamaguchi, J.; Gonzali, S.; Alpi, A.; Perata, P. Effect of Anoxia on Gibberellic Acid-Induced Protease and β-Amylase Processing in Barley Seeds. J. Plant Physiol. 1998, 152, 44–50. [Google Scholar] [CrossRef]
- Waseem, N.; Shaukat, S.S. Effect of Enhanced UV-B Radiation, Allelopathy and Their Combined Stress on Germination, Seedling Growth, Pigments, and Amylase Activity in Vigna Radiata. Int. J. Biol. Biotechnol. 2016, 13, 485–494. [Google Scholar]
- Zeng, F.; Zheng, C.; Ge, W.; Gao, Y.; Pan, X.; Ye, X.; Wu, X.; Sun, Y. Regulatory Function of the Endogenous Hormone in the Germination Process of Quinoa Seeds. Front. Plant Sci. 2024, 14, 1322986. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, L.; Huarancca Reyes, T.; Ramos-Diaz, J.M.; Jouppila, K.; Guglielminetti, L. Hormonal Regulation in Different Varieties of Chenopodium Quinoa Willd. Exposed to Short Acute UV-B Irradiation. Plants 2021, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Huarancca Reyes, T.; Scartazza, A.; Pompeiano, A.; Guglielminetti, L. Physiological Responses of Lepidium Meyenii Plants to Ultraviolet-B Radiation Challenge. BMC Plant Biol. 2019, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pompeiano, A.; Damiani, C.R.; Stefanini, S.; Vernieri, P.; Huarancca Reyes, T.; Volterrani, M.; Guglielminetti, L. Seedling Establishment of Tall Fescue Exposed to Long-Term Starvation Stress. PLoS ONE 2016, 11, e0166131. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carli, M.; Guglielminetti, L.; Huarancca Reyes, T. UVB Stress Induced Changes in Germination and Carbohydrate Mobilization in Chenopodium Quinoa Willd. Seeds. Seeds 2025, 4, 46. https://doi.org/10.3390/seeds4030046
Carli M, Guglielminetti L, Huarancca Reyes T. UVB Stress Induced Changes in Germination and Carbohydrate Mobilization in Chenopodium Quinoa Willd. Seeds. Seeds. 2025; 4(3):46. https://doi.org/10.3390/seeds4030046
Chicago/Turabian StyleCarli, Marco, Lorenzo Guglielminetti, and Thais Huarancca Reyes. 2025. "UVB Stress Induced Changes in Germination and Carbohydrate Mobilization in Chenopodium Quinoa Willd. Seeds" Seeds 4, no. 3: 46. https://doi.org/10.3390/seeds4030046
APA StyleCarli, M., Guglielminetti, L., & Huarancca Reyes, T. (2025). UVB Stress Induced Changes in Germination and Carbohydrate Mobilization in Chenopodium Quinoa Willd. Seeds. Seeds, 4(3), 46. https://doi.org/10.3390/seeds4030046





