Seed Nanopriming Improves Jalapeño Pepper Seedling Quality for Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
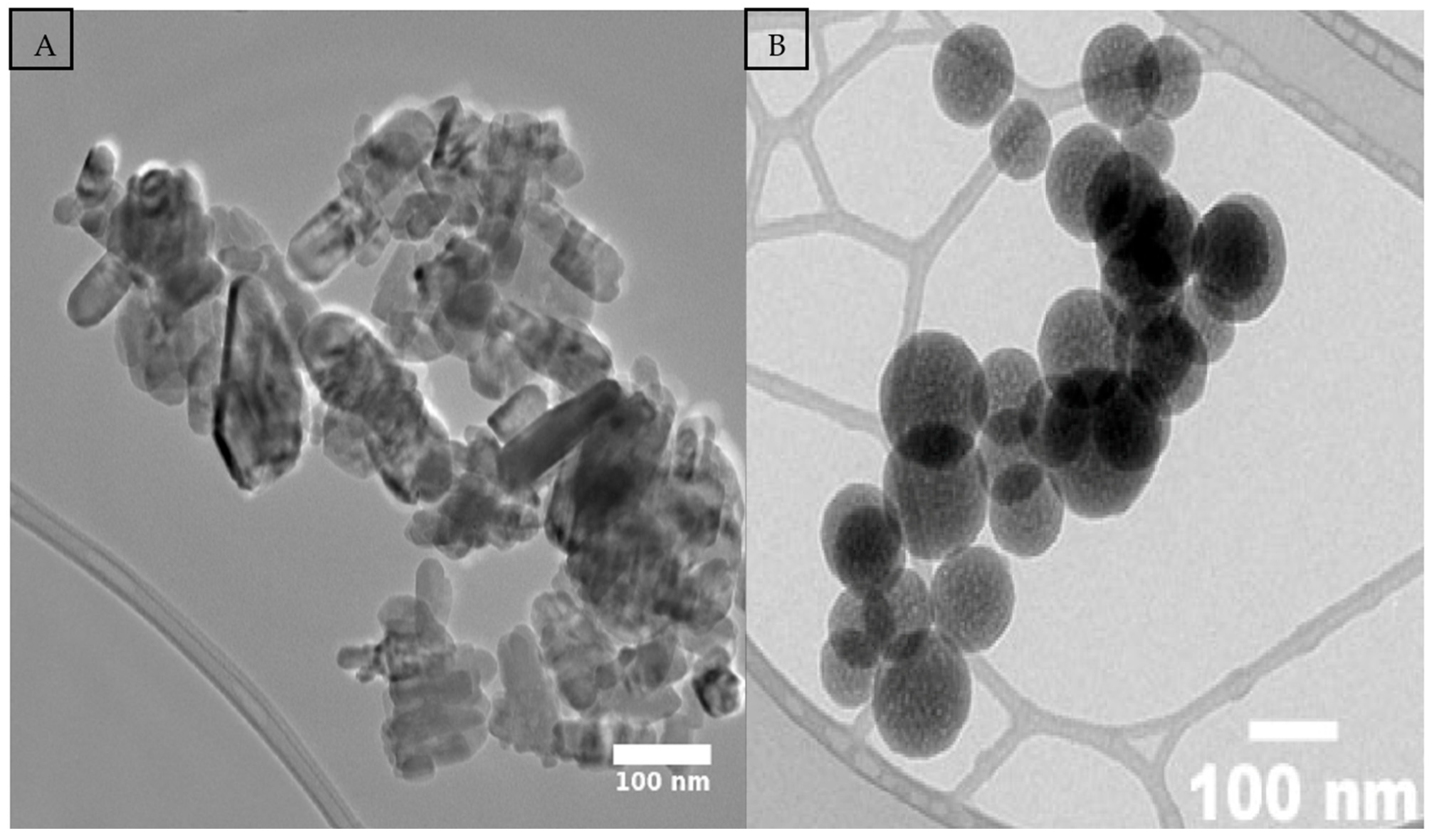
2.2. Characterization of Zinc Oxide, Silicon Dioxide NPs
2.3. Nanopriming Treatments
2.4. Experimental Design
2.5. Crop Management
2.6. Plant Sampling
2.7. Plant Analysis
2.7.1. Measurements of Morphological Parameters
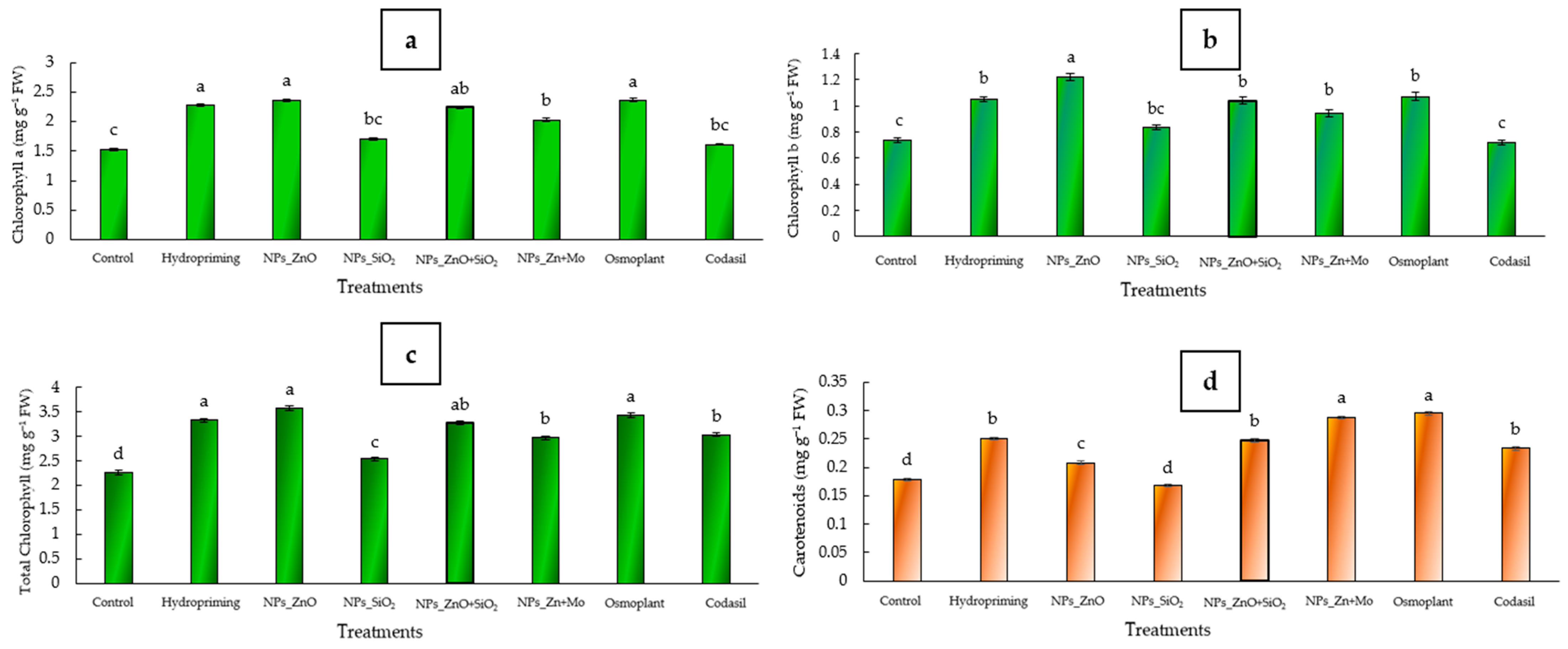
2.7.2. Concentration of Photosynthetic Pigments
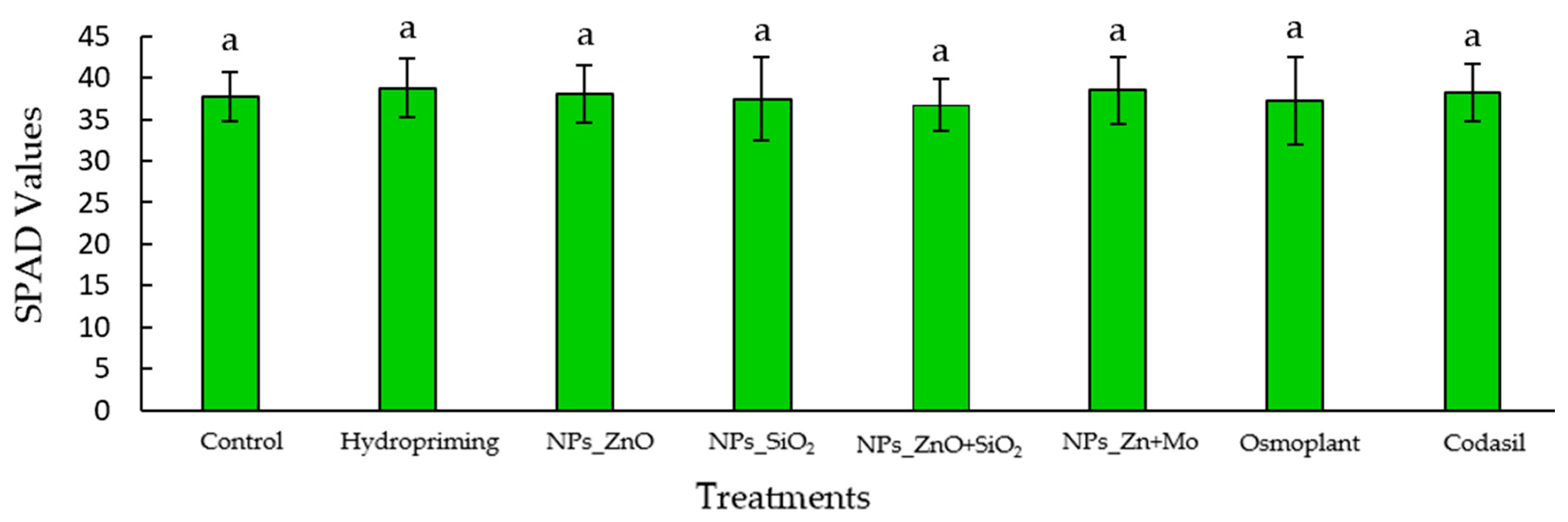
2.7.3. Measurements of Chlorophyll Index
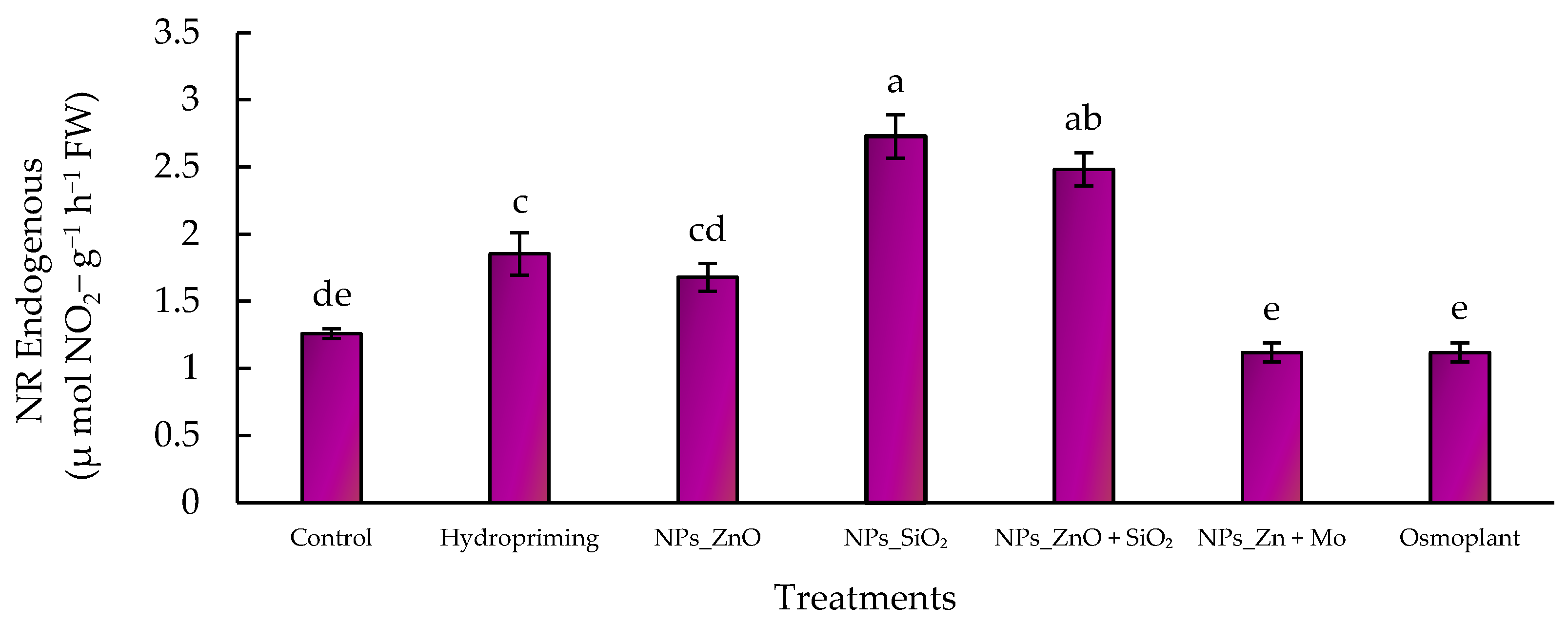
2.7.4. “In Vivo” Nitrate Reductase Activity Assay
2.8. Pearson Correlation Heatmap
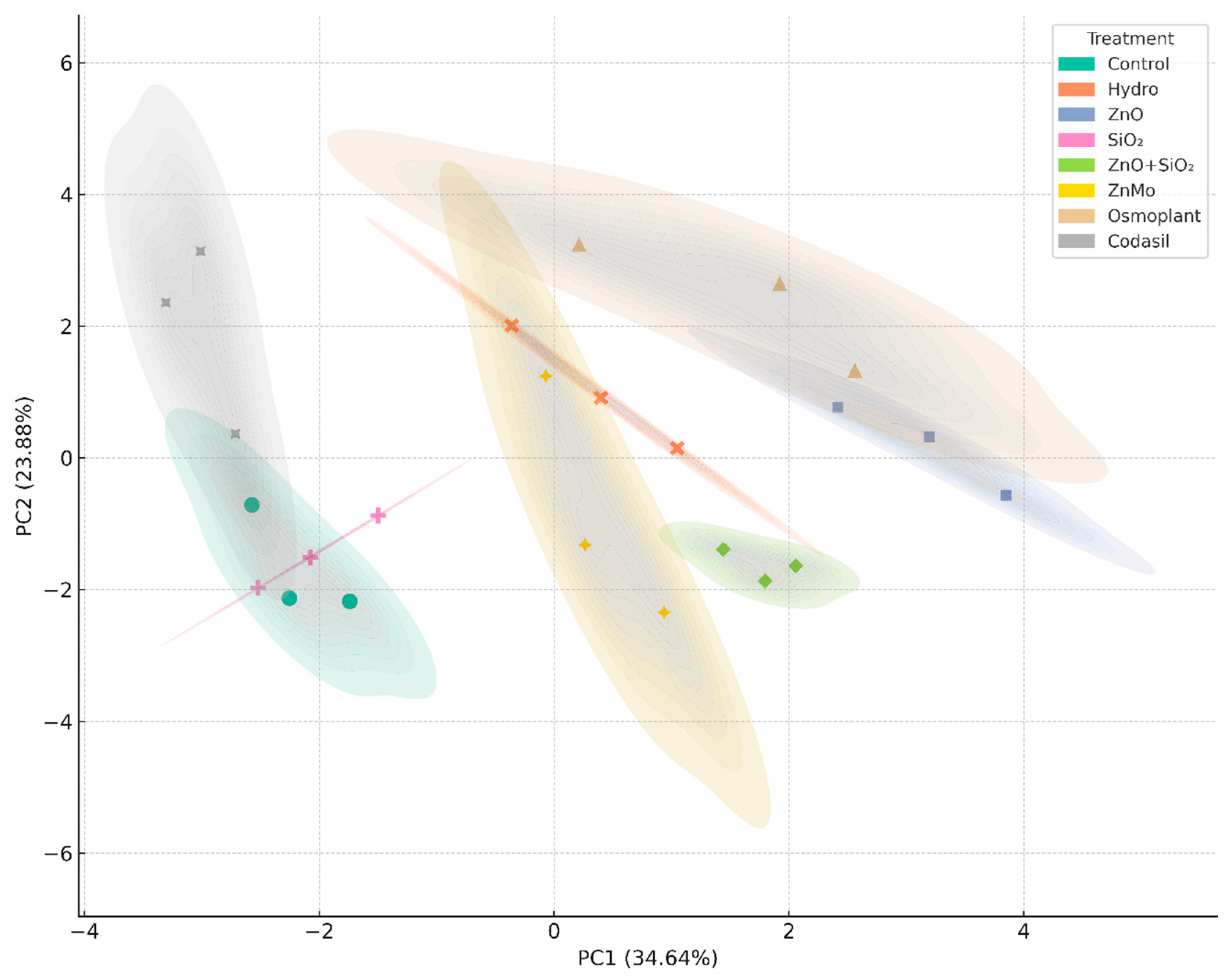
2.9. Principal Component Analysis (PCA)
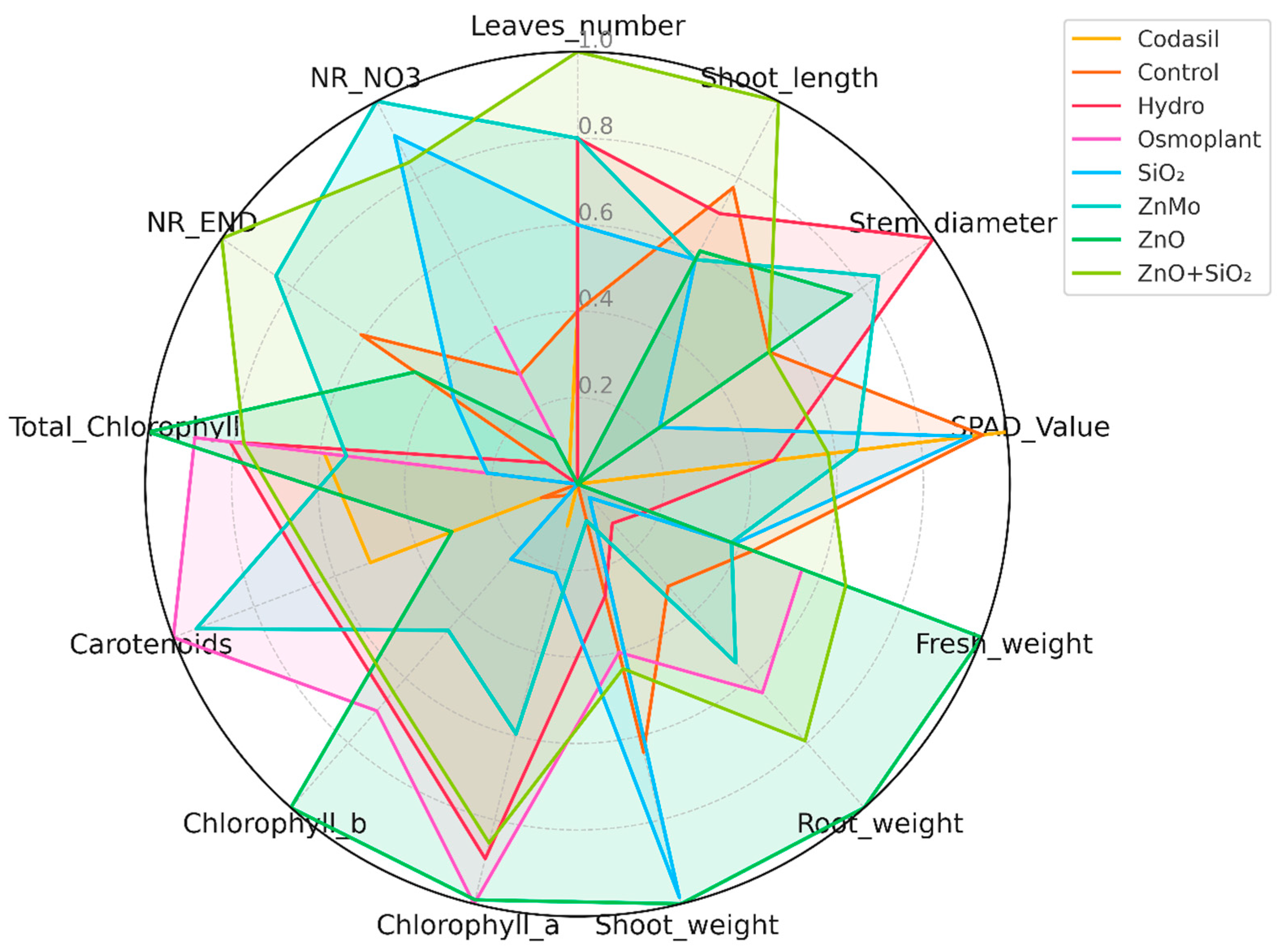
2.10. Radar Chart: Multivariate Comparison by Priming Treatment
2.11. Statistical Analysis
3. Results and Discussion
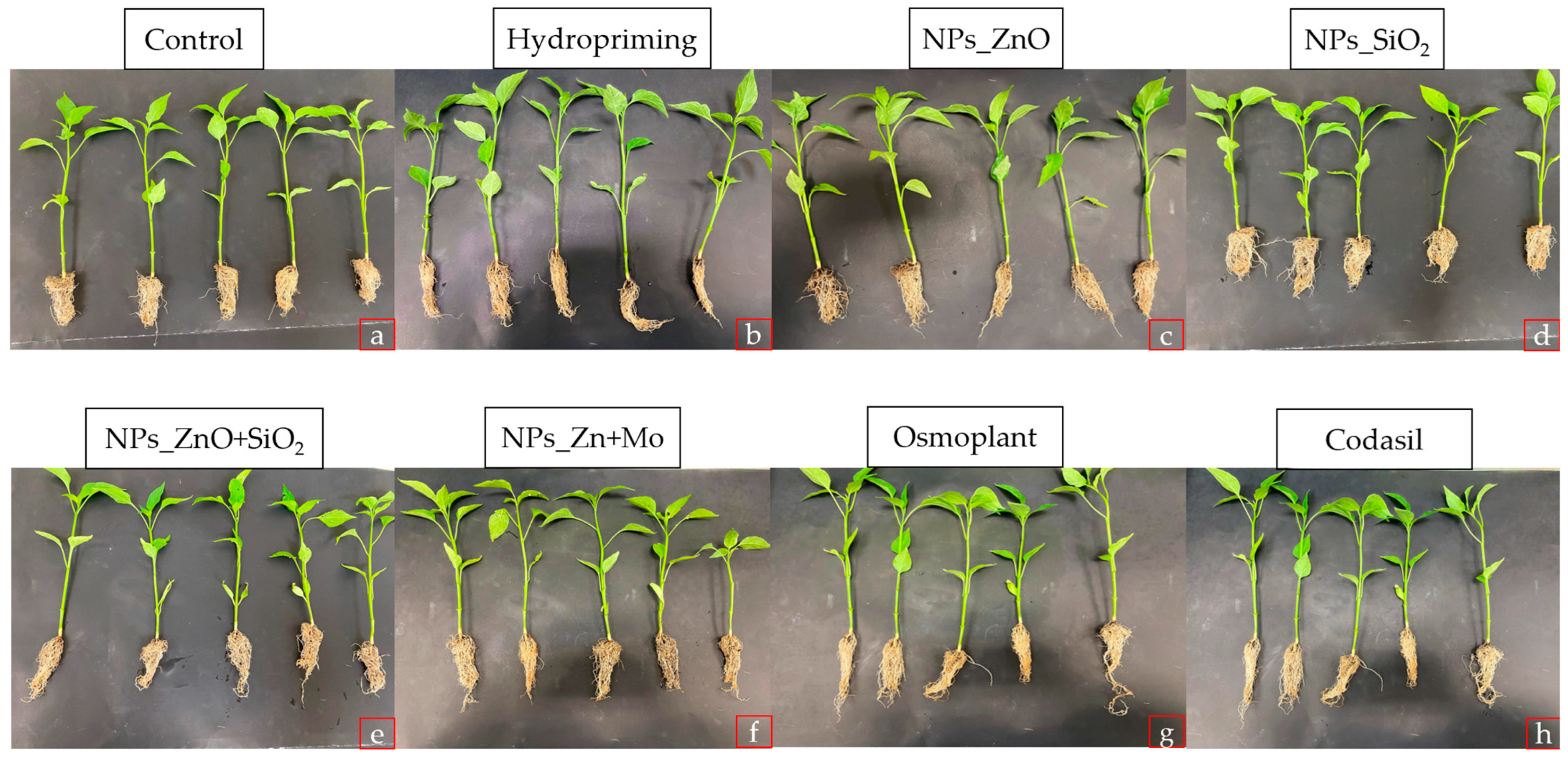
3.1. Morphological Parameters
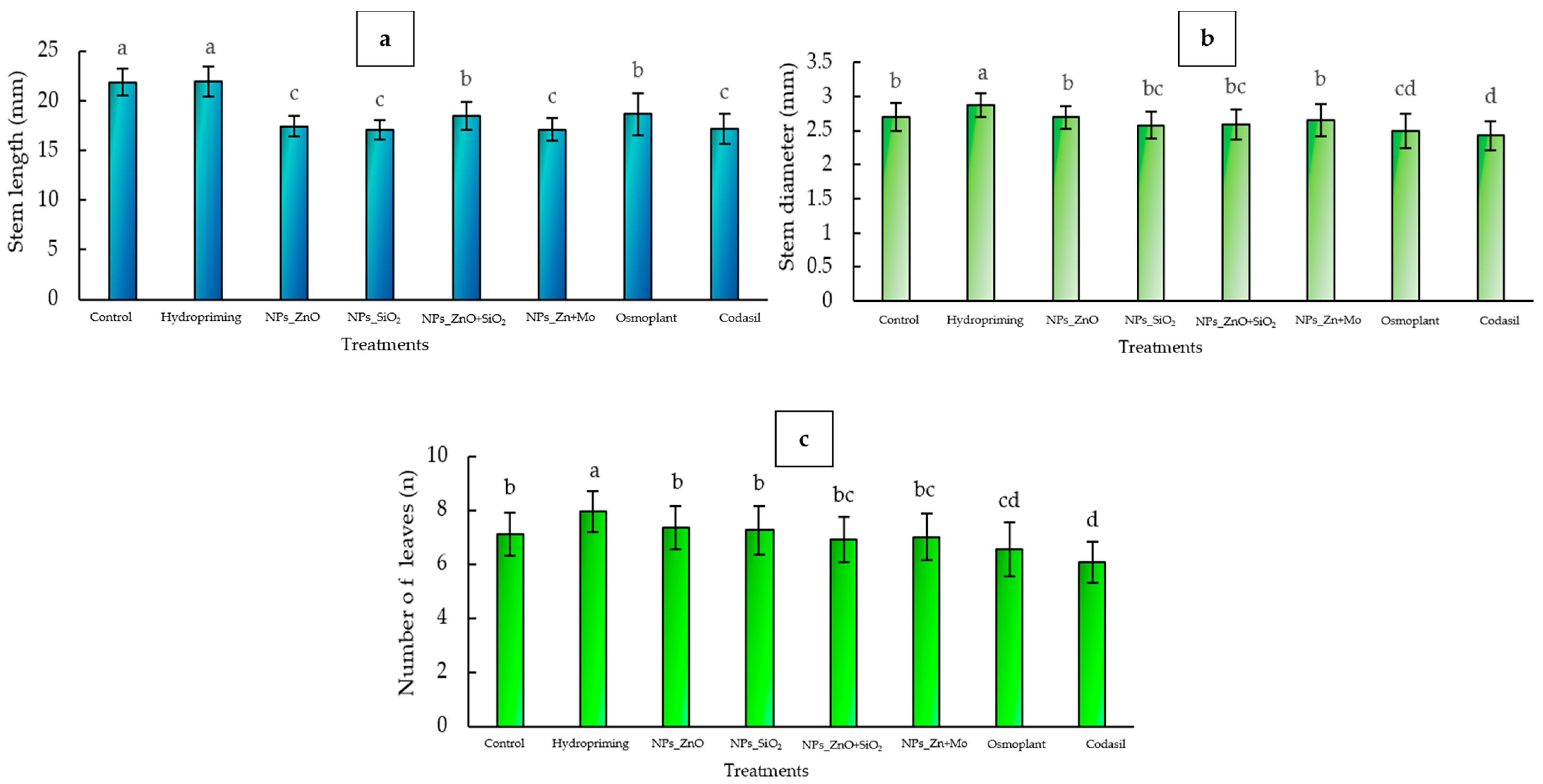
3.1.1. Stem Length
3.1.2. Stem Diameter
3.1.3. Number of Leaves
3.2. Biomass
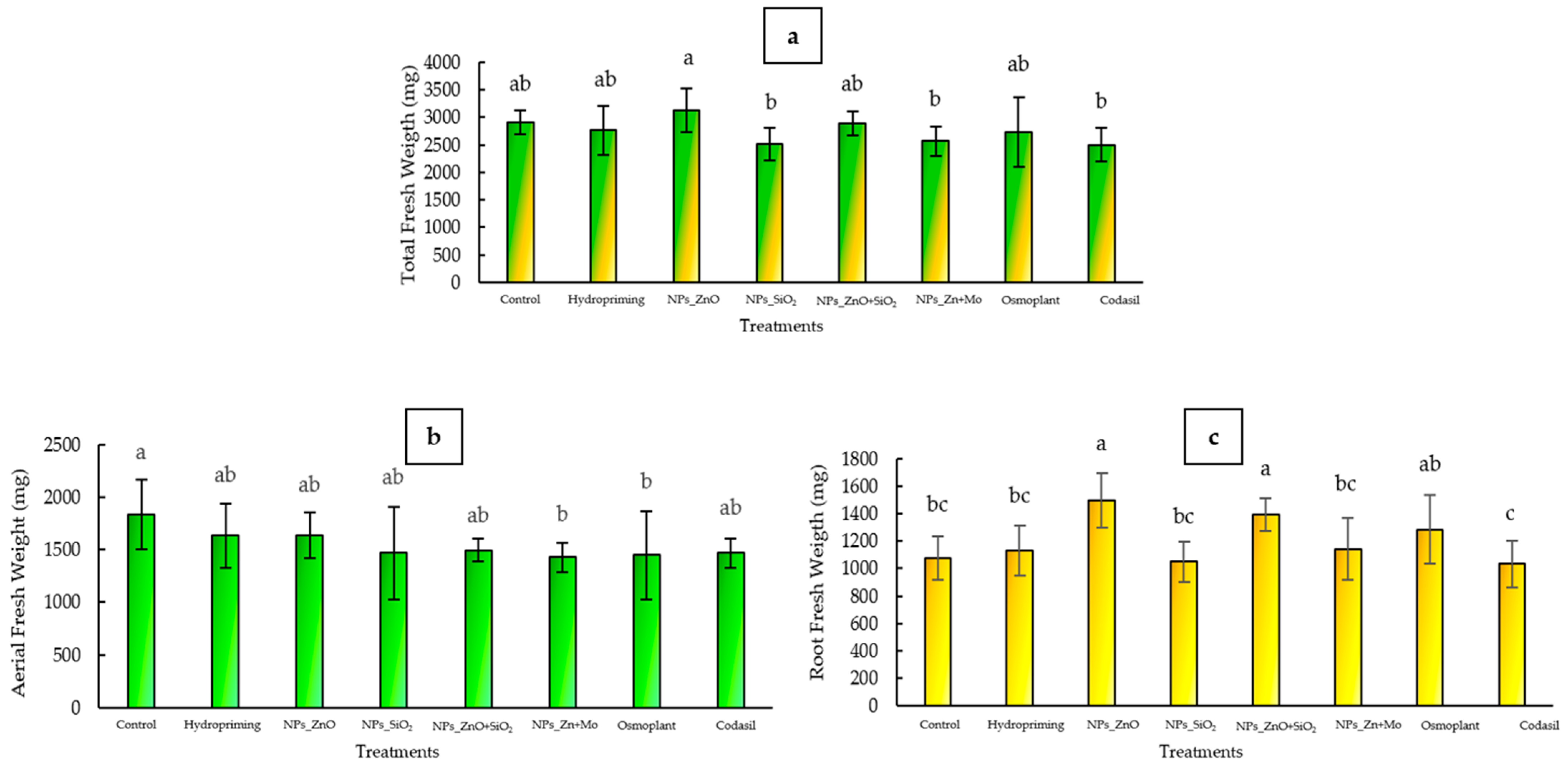
3.2.1. Total Fresh Weight
3.2.2. Aerial Fresh Weight
3.2.3. Fresh Root Weight
3.3. Photosynthetic Pigments
3.4. Chlorophyll Index
3.5. Nitrate Reductase Activity “In Vivo”
3.5.1. Endogenous Nitrate Reductase
3.5.2. NO3− Induced Nitrate Reductase
3.6. Correlation Heatmap
3.7. Multivariate Analysis
3.8. Radar Chart Analysis
3.9. General Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ch | Chitosan |
| Chl total | Chlorophyll total |
| DAS | Days after sowing |
| FW | Fresh weight |
| NPs | Nanoparticles |
| NR | Nitrate reductase |
| PCA | Principal component analysis |
References
- Verma, K.K.; Song, X.P.; Joshi, A.; Tian, D.D.; Rajput, V.D.; Singh, M.; Li, Y.R. Recent trends in nano-fertilizers for sustainable agriculture under climate change for global food security. Nanomaterials 2022, 12, 173. [Google Scholar] [CrossRef]
- Kaur, B.; Kaur, G.; Asthir, B. Biochemical aspects of nitrogen use efficiency: An overview. J. Plant Nutr. 2017, 40, 506–523. [Google Scholar] [CrossRef]
- Shah, A.N.; Javed, T.; Singhal, R.K.; Shabbir, R.; Wang, D.; Hussain, S.; Jaremko, M. Nitrogen use efficiency in cotton: Challenges and opportunities against environmental constraints. Front. Plant Sci. 2022, 13, 970339. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Esmaeili, S.; Eskandarzade, P.; Sharifani, M.; Lastochkina, O. Nanotechnology in regulation of growth and stress tolerance in vegetable crops. In Growth Regulation and Quality Improvement of Vegetable Crops: Physiological and Molecular Features; Springer: Singapore, 2025; pp. 653–690. [Google Scholar]
- Tang, R.; Supit, I.; Hutjes, R.; Zhang, F.; Wang, X.; Chen, X.; Chen, X. Modelling growth of chili pepper (Capsicum annuum L.) with the WOFOST model. Agric. Syst. 2023, 209, 103688. [Google Scholar] [CrossRef]
- Dobón-Suárez, A.; Zapata, P.J.; García-Pastor, M.E. A comprehensive review on characterization of pepper seeds: Unveiling potential value and sustainable agrifood applications. Foods 2025, 14, 1969. [Google Scholar] [CrossRef]
- Cañizares, E.; Giovannini, L.; Gumus, B.O.; Fotopoulos, V.; Balestrini, R.; González-Guzmán, M.; Arbona, V. Seeds of change: Exploring the transformative effects of seed priming in sustainable agriculture. Physiol. Plant. 2025, 177, e70226. [Google Scholar] [CrossRef]
- Costa, C.J.; Meneghello, G.E.; Jorge, M.H.A.; Costa, E. The importance of physiological quality of seeds for agriculture. Colloq. Agrar. 2021, 17, 102–119. [Google Scholar] [CrossRef]
- Azimi, R. Influence of nanoparticles on photosynthesis. In Nanotechnology Applications in Plant Physiology; Taylor & Francis: London, UK, 2024. [Google Scholar]
- Nitnavare, R.; Bhattacharya, J.; Ghosh, S. Nanoparticles for effective management of salinity stress in plants. In Agricultural Nanobiotechnology; Woodhead Publishing: Duxford, UK, 2022; pp. 189–216. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultivar and hybrid differing in arsenate tolerance. Front. Environ. Sci. 2016, 4, 46. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Chaparro, E.H.; Patiño-Cruz, J.J.; Anchondo-Páez, J.C.; Pérez-Álvarez, S.; Chávez-Mendoza, C.; Castruita-Esparza, L.U.; Márquez, E.M.; Sánchez, E. Seed nanopriming with ZnO and SiO2 enhances germination, seedling vigor, and antioxidant defense under drought stress. Plants 2025, 14, 1726. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.; Kumar, S.; Sakure, A.A.; Rafaliya, R.; Patil, G.B. Zinc oxide nanopriming elevates wheat drought tolerance by inducing stress-responsive genes and physio-biochemical changes. Curr. Plant Biol. 2023, 35, 100292. [Google Scholar] [CrossRef]
- Waqas Mazhar, M.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Hayat Bhatti, K.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T. Seed Nano-Priming with Zinc Oxide Nanoparticles in Rice Mitigates Drought and Enhances Agronomic Profile. PLoS ONE 2022, 17, e0264967. [Google Scholar] [CrossRef] [PubMed]
- Tamindžić, G.; Azizbekian, S.; Miljaković, D.; Ignjatov, M.; Nikolić, Z.; Budakov, D.; Vasiljević, S.; Grahovac, M. Assessment of various nanoprimings for boosting pea germination and early growth in both optimal and drought-stressed environments. Plants 2024, 13, 1547. [Google Scholar] [CrossRef] [PubMed]
- Haifa Group. NutriNet®: Precision Fertigation Recommendation Software. Available online: https://nutrinet.haifa-group.com/ (accessed on 12 September 2025).
- Pasternak, T.; Tietz, O.; Rapp, K.; Begheldo, M.; Nitschke, R.; Ruperti, B.; Palme, K. Protocol: An improved and universal procedure for whole mount immunolocalization in plants. Plant Methods 2015, 11, 50. [Google Scholar] [CrossRef]
- Ochoa-Chaparro, E.H.; Ramírez-Estrada, C.A.; Anchondo-Páez, J.C.; Sánchez, E.; Pérez-Álvarez, S.; Castruita-Esparza, L.U.; Muñoz-Márquez, E.; Chávez-Mendoza, C.; Patiño-Cruz, J.J.; Franco-Lagos, C.L. Nanopriming with zinc–molybdenum in jalapeño pepper on imbibition, germination, and early growth. Agronomy 2024, 14, 1609. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Shrestha, S.; Brueck, H.; Asch, F. Chlorophyll index, photochemical reflectance index and chlorophyll fluorescence measurements of rice leaves supplied with different N levels. J. Photochem. Photobiol. B 2012, 113, 7–13. [Google Scholar] [CrossRef]
- Sánchez, E.; Ruiz, J.M.; Romero, L. Compuestos nitrogenados indicadores de estrés en respuesta a las dosis tóxicas y deficientes de nitrógeno en frijol ejotero. Nova Sci. 2016, 8, 228–244. [Google Scholar] [CrossRef]
- Dudáš, A. Graphical representation of data prediction potential: Correlation graphs and correlation chains. Vis. Comput. 2024, 40, 6969–6982. [Google Scholar] [CrossRef]
- Nokeri, T.C. Principal component analysis with Scikit-Learn, PySpark, and H2O. In Data Science Solutions with Python; Apress: Berkeley, CA, USA, 2022; pp. 101–110. [Google Scholar]
- Sujata; Goyal, V.; Baliyan, V.; Avtar, R.; Mehrotra, S. Alleviating drought stress in Brassica juncea (L.) Czern & Coss. by foliar application of biostimulants—Orthosilicic acid and seaweed extract. Appl. Biochem. Biotechnol. 2023, 195, 693–721. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 15.3 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2023. [Google Scholar]
- Gallegos-Cedillo, V.M.; Diánez, F.; Nájer, C.; Santos, M. Plant agronomic features can predict quality and field performance: A bibliometric analysis. Agronomy 2021, 11, 2305. [Google Scholar] [CrossRef]
- Tatari, M.; Jafari, A.; Shirmardi, M.; Mohamadi, M. Using morphological and physiological traits to evaluate drought tolerance of pear populations (Pyrus spp.). Int. J. Fruit Sci. 2020, 20, 837–854. [Google Scholar] [CrossRef]
- Gazal, R.M.; Blanche, C.A.; Carandang, W.M. Root growth potential and seedling morphological attributes of narra (Pterocarpus indicus Willd.) transplants. For. Ecol. Manag. 2004, 195, 259–266. [Google Scholar] [CrossRef]
- Chen, J.; Ji, F.; Gao, R.; He, D. Enhancing transplant quality by optimizing LED light spectrum to advance post-transplant runner plant propagation in strawberry. Int. J. Agric. Biol. Eng. 2025, 18, 55–62. [Google Scholar]
- Leskovar, D.I.; Othman, Y.A. Direct seeding and transplanting influence root dynamics, morpho-physiology, yield, and head quality of globe artichoke. Plants 2021, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Karwat, H.; Sparke, M.-A.; Rasche, F.; Arango, J.; Nuñez, J.; Rao, I.; Moreta, D.; Cadisch, G. Nitrate reductase activity in leaves as a plant physiological indicator of in vivo biological nitrification inhibition by Brachiaria humidicola. Plant Physiol. Biochem. 2019, 135, 113–120. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Ferreira, E.V.; Ferreira Novais, R.; Aparecida dos Santos, F.; Ribeiro, C.; Barros, N.F. Nitrate reductase (NR) and glutamine synthetase (GS) can be used as indicators of nitrogen status in eucalyptus clones. Aust. J. Crop Sci. 2015, 9, 561–569. [Google Scholar]
- Cao, X.; Shen, Q.; Shang, C.; Yang, H.; Liu, L.; Cheng, J. Determinants of shoot biomass production in mulberry: Combined selection with leaf morphological and physiological traits. Plants 2019, 8, 118. [Google Scholar] [CrossRef]
- Carreño Siqueira, J.A.; Marques, D.J.; Silva, M.C.G. The use of photosynthetic pigments and SPAD can help in the selection of bean genotypes under fertilization organic and mineral. Sci. Rep. 2023, 13, 49582. [Google Scholar] [CrossRef]
- Wei, L.; Lu, L.; Shang, Y.; Ran, X.; Liu, Y.; Fang, Y. Can SPAD Values and CIE L*a*b* Scales Predict Chlorophyll and Carotenoid Concentrations in Leaves and Diagnose the Growth Potential of Trees? An Empirical Study of Four Tree Species. Horticulturae 2024, 10, 548. [Google Scholar] [CrossRef]
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Liu, X.; Rebezov, M.B.; Kai, G. Nano-priming as emerging seed priming technology for sustainable agriculture—Recent developments and future perspectives. J. Nanobiotechnol. 2022, 20, 254. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Liu, H.; Chen, S.; Zaman, Q.U. Variability in morpho-biochemical, photosynthetic pigmentation, enzymatic and quality attributes of potato for salinity stress tolerance. Sci. Hortic. 2023, 316, 111236. [Google Scholar] [CrossRef]
- Boonupara, T.; Udomkun, P.; Kajitvichyanukul, P. Quantitative analysis of atrazine impact on UAV-derived multispectral indices and correlated plant pigment alterations: A heatmap approach. Agronomy 2024, 14, 814. [Google Scholar] [CrossRef]
- Gewers, F.L.; Ferreira, G.R.; de Arruda, H.F.; Silva, F.N.; Comin, C.H.; Amancio, D.R.; Costa, L.d.F. Principal component analysis: A natural approach to data exploration. arXiv 2018, arXiv:1804.02502. [Google Scholar] [CrossRef]
- Sivakumar, J.; Sharma, L.; Thiruppathi, S.; Manikandan, R.; Subramanian, K.S. Principal component analysis approach for comprehensive assessment of salt tolerance among tomato germplasm at the seedling stage. J. Biosci. 2020, 45, 144. [Google Scholar] [CrossRef]
- Shlens, J. A Tutorial on Principal Component Analysis. arXiv 2014, arXiv:1404.1100. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.D.; Ullah, A.; Bhutta, M.A.; Bibi, A.; Farooq, U. Radar Analysis of Spring Wheat Genotypes at Seedling Stage against Limited Water Conditions. Agriculture 2022, 12, 1153. [Google Scholar] [CrossRef]
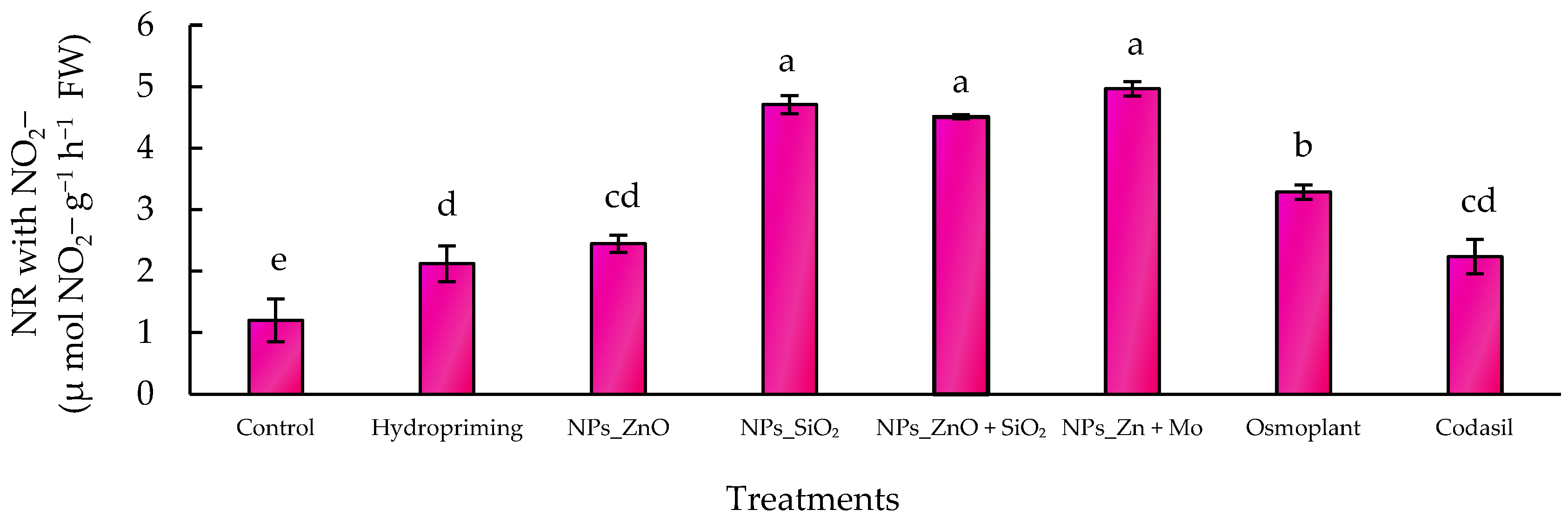

| Treatment | Chemical Composition | Doses |
|---|---|---|
| Control | Not applicable | Not applicable |
| Hydropriming | Tridistilled water | Not applicable |
| NPs ZnO + Ch | 50 nm, 99.9% and Poli-D-glucosamine | (100 and 100 mg L−1) |
| NPs SiO2 + Ch | 80 nm, 99.9% and Poli-D-glucosamine | (10 and 100 mg L−1) |
| NPs ZnO + SiO2 + Ch | 50 nm, 99.9%, <80 nm, 99.9% and Poli-D-glucosamine | (100, 10 and 100 mg L−1) |
| NPs Zn + Mo | Liquid solution composed of 62% Zn, 5% Mo, and 5% of an algae extract-based chelating agent. | (124 and 10 mg L−1) |
| Osmoplant® | Liquid solution composed of 6% free amino acids, 2.4% nitrogen and 3.3% potassium. | (2000 mL L−1) |
| Codasil® | Liquid solution with a high concentration of soluble silicon composed of 20% silicon, 4% free amino acids and 11.20% potassium. | (2000 mL L−1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa-Chaparro, E.H.; Patiño-Cruz, J.J.; Anchondo-Páez, J.C.; Alvarez-Monge, A.; Franco-Lagos, C.L.; Sánchez, E. Seed Nanopriming Improves Jalapeño Pepper Seedling Quality for Transplantation. Seeds 2025, 4, 47. https://doi.org/10.3390/seeds4030047
Ochoa-Chaparro EH, Patiño-Cruz JJ, Anchondo-Páez JC, Alvarez-Monge A, Franco-Lagos CL, Sánchez E. Seed Nanopriming Improves Jalapeño Pepper Seedling Quality for Transplantation. Seeds. 2025; 4(3):47. https://doi.org/10.3390/seeds4030047
Chicago/Turabian StyleOchoa-Chaparro, Erick H., Juan J. Patiño-Cruz, Julio C. Anchondo-Páez, Alan Alvarez-Monge, Cristina L. Franco-Lagos, and Esteban Sánchez. 2025. "Seed Nanopriming Improves Jalapeño Pepper Seedling Quality for Transplantation" Seeds 4, no. 3: 47. https://doi.org/10.3390/seeds4030047
APA StyleOchoa-Chaparro, E. H., Patiño-Cruz, J. J., Anchondo-Páez, J. C., Alvarez-Monge, A., Franco-Lagos, C. L., & Sánchez, E. (2025). Seed Nanopriming Improves Jalapeño Pepper Seedling Quality for Transplantation. Seeds, 4(3), 47. https://doi.org/10.3390/seeds4030047







