Abstract
Aquatic plants may rely on seeds to promote population persistence after severe disturbances, such as droughts. We characterized the seed germination dynamics for three Potamogeton species following seed storage under dry versus submerged conditions. Overall germination levels were highest for P. lucens and, more specifically, were higher after submerged storage (70.4%) than dry storage (56.0%). Overall germination levels were lower for the two other species and displayed a different response to storage conditions; they were higher after dry storage (P. natans: 24.6%; P. pectinatus: 28.1%) than submerged storage (10.8 and 7.1%, respectively). Only P. natans would have likely made a large seed bank contribution as follows: 42.2% of its seeds remained ungerminated and viable after submerged storage, while this figure was 17.4% for seeds that had experienced dry storage. Our results suggest the species differ in their reproductive strategies. Sexual reproduction plays an important role in Potamogeton lucens, adding new individuals to plant populations every year; however, the low viability of the species’ ungerminated seeds suggest its seed bank contribution may be small, rendering it vulnerable to long periods of unfavourable environmental conditions. In contrast, P. natans would likely make a larger seed bank contribution, underscoring the role its seeds may play in population persistence across years. Potamogeton pectinatus may minimally rely on its seeds, which fits with its predominant use of vegetative reproduction in the field. While its seeds might contribute little to population persistence, they may nonetheless promote genetic variability among populations.
1. Introduction
Aquatic plants play an important role in aquatic ecosystems because their complex architecture supplies food and shelter for many other aquatic species. Most aquatic plants can reproduce sexually and asexually [1,2]. In permanent aquatic environments, submerged macrophytes may produce dense, largely perennial vegetation. These species commonly reproduce vegetatively using turions, tubers, or even shoots, which emerge from live or senescent individuals [1,3]. In addition, aquatic plants can produce flowers and fruits, and the resulting seeds may contribute to population persistence in a species-specific manner.
Vegetative propagules tend to require a certain minimum amount of water; they can persist in submerged or wet environments but, most of the time, cannot tolerate dry conditions (but see [4]). Thus, after long dry periods or periods of very dry conditions, a population can only re-emerge if it has contributed viable seeds to the seed bank. Therefore, even though many species mainly reproduce vegetatively, sexual reproduction has an important part to play; it generates seeds that help populations persist in the face of severe disturbances and also promotes genetic diversity and plant dispersal via zoochory and other mechanisms [1]. Overall, seeds can help ensure that populations do not disappear entirely from year to year. Seed banks persist over time within soil sediment layers, and the germination of seeds from seed banks allows population re-establishment following environmental disturbances. These dynamics play a key role when vegetative propagules cannot tolerate unfavourable or very dry conditions.
Seed characteristics differ widely among aquatic plant species. Most seeds display physiological dormancy, which is usually broken by various environmental triggers (e.g., certain temperature, oxygen availability, or flooding conditions) [5]. Many species produce orthodox seeds that can tolerate desiccation periods of varying lengths, while other species produce recalcitrant or intermediate seeds that do not survive drying [6,7,8]. Past research has shown that seeds of certain Potamogeton species display physiological dormancy and are orthodox [6,9]. These seed traits may be related to the species’ need to deal with environmental disturbances, most commonly the desiccation of their aquatic habitat. Given global changes, it is important to know whether populations of aquatic plant species can be reliably re-established from seeds, information that will inform conservation management strategies.
Wetlands are among the most threatened ecosystems in the world [10,11], which means their inhabitants are under threat as well. These habitats are struggling because of the lower rainfall levels and increases in temperature caused by climate change as well as because of human activities, such as the exploitation of water resources. Aquifer overexploitation has some of the worst impacts on aquatic habitats. It modifies hydrological regimes by reducing groundwater discharge, leading to shorter inundation periods; groundwater-dependent wetlands may even dry up and become terrestrial habitats [12]. The transformation of permanent aquatic habitats into temporary aquatic habitats is a severe disturbance for resident organisms, especially those that are strictly aquatic and unable to withstand dry periods. These shifts are a challenge for aquatic plants, which generally reproduce using vegetative propagules in permanent aquatic habitats. The ability of these species to engage in sexual reproduction may be crucial in the face of severe sporadic dry periods. However, aquatic plant species vary in their use of sexual reproduction, their seed germination levels, and their seed viability levels, traits that may correlate with species resilience when experiencing major environmental disturbances [2], such as habitat desiccation.
In this study, we characterized seed germination dynamics for three Potamogeton species found in Doñana National Park (southwestern Spain). Doñana harbours a groundwater-dependent pond network, which is presently threatened by groundwater overexploitation [13,14]. Historically, the park has been home to six species of Potamogeton, but only four may persist at present [15]. Our three study species were Potamogeton lucens, P. natans, and P. pectinatus. They are all submerqed aquatic plants, with numerous flowers, and then fruits, grouped in spikes. Each fruit (drupe) produces a single seed, and the spike may be considered a polydrupe [16]. Potamogeton natans has floating leaves which are oblong to broadly elliptical or ovate, while the submerged leaves are reduced to phyllodes. There are only submerged leaves in the other species: In P. lucens, they are oblong-lanceolate, and in P. pectinatus, submerged leaves are filiform to linear [17] (See Supplementary Materials Figure S1). Potamogeton lucens and P. natans occur exclusively in permanent ponds, which in our study area are well differentiated, P. natans appeared in areas with lower pH and conductivity than P. lucens. Potamogeton pectinatus occurs in both permanent ponds and temporary ponds with long hydroperiods, usually coexisting with one of the other two species [18]. In Doñana, most ponds are temporary. Natural permanent ponds are scarce: only 2–3 natural ponds have been classified as permanent in the last few decades; however, there are around 150 small ponds that have been artificially deepened to hold water year-round [15,19]. However, in recent years (2021–2024), all of the natural permanent ponds and many of the artificial permanent ponds have dried out during the summer.
In this study, we were interested in understanding how pond desiccation might affect seed germination in these three Potamogeton species. We conducted an experiment where we stored seeds under dry versus submerged conditions and then assessed seed germination and viability. The objective was to simulate situations in which the seeds had spent the summer in desiccated versus flooded ponds, and to evaluate whether the subsequent winter temperature had an additional effect on germination. First, we determined whether the seeds displayed species-specific germination patterns in response to the different storage conditions. Second, we characterized the viability of the seeds that remained ungerminated at the end of the germination trials, given that viable ungerminated seeds may play an important role in population persistence via their presence in the seed bank.
2. Materials and Methods
2.1. Seed Collection and Experimental Procedure
Potamogeton natans, P. lucens, and P. pectinatus used to be common aquatic plants in Doñana National Park. In May and June 2014, we collected spikes with seeds of these species from permanent ponds in the park. We only selected mature ripe seeds, discarding any that were small in size or whose seed coats were not fully developed. We gathered 550 seeds from 27 spikes for P. natans, 1465 seeds from 35 spikes for P. lucens, and 495 seeds from 30 spikes for P. pectinatus. We assigned approximately 50% of the seeds from each spike to one of the two storage treatments: seeds were either stored under dry conditions (dry treatment) or under submerged conditions (submerged treatment). For the dry treatment, we placed all the seeds from a single spike in a dry filter-paper envelope. For the submerged treatment, we placed the seeds from a single spike in 125 mL glasses filled with dechlorinated tap water. During the storage and experimental periods, all seeds were identified in relation to the spikes from which they were collected. The seeds in both treatments were kept in the same location at room temperature (26–32 °C) for around 4 months, a similar period to the start of the new hydrological cycle in our study area. On 2 October 2014, we put the seeds in 24-well dishes (4–10 seeds/well), filled the wells with dechlorinated tap water, and placed the dishes in a germination chamber. They remained in the germination chamber (temperature = 20 °C, light cycle = 12:12) until 5 March 2015, which is when the first germination trial ended (G1). Between 2 October and 5 December 2014, we checked on the seeds twice a week; after this point, we checked on the seeds once a week until no further germination was observed for more than three weeks. Each time we checked on the seeds, we counted and removed any that had germinated. We considered a seed to be germinated, when we could see that the radicle had emerged from the seed coat. On 5 March 2015, we began storing the seeds at 10 °C, simulating the winter temperatures in our study area, to assess whether the seeds required a cold stratification period to break dormancy; we removed the water from the wells in the dry treatment dishes, but we left the water in the wells in the submerged treatment dishes. On 4 May 2015, we began the second germination trial (G2). We filled all the plate wells with water and gradually raised the temperature in the germination chamber to 20 °C. We checked on the seeds twice a week until no further germination was observed, concluding on 14 January 2016. The ungerminated seeds were stored at room temperature under dry conditions.
After the germination experiment, we tested the viability of the ungerminated seeds using a tetrazolium chloride solution (TZ) (2,3,5 triphenyl tetrazolium chloride), which stains viable seeds red or pink [20]. First, we soaked the seeds in water for 24 h. We then longitudinally scarified the seeds using a razor blade, being careful to leave the embryo untouched. The seeds were then soaked in TZ for 48 h at 25 °C. The red and pink seeds were classified as viable, and the unstained seeds were classified as non-viable (Supplementary Materials Figure S2).
2.2. Statistical Analyses
We estimated the total number of seeds per spike that germinated (germination levels) for G1 and G2. We also estimated the total number of viable seeds, which was the sum of the seeds that germinated during G1 and G2 and the seeds determined to be viable via TZ testing.
We carried out generalized linear models (GLMs) in R (v. 4.3.2 [21]), to determine whether germination levels and seed viability differed among species and between the storage treatments. In these models, the independent variables were species (P. natans, P. lucens, and P. pectinatus) and storage treatment (submerged and dry); the dependent variables were the number of germinated seeds versus the number of ungerminated seeds from each spike or the number of viable versus non-viable seeds from each individual spilke. Because most of our data displayed overdispersion, we used a quasibinomial error distribution (except for the G2 models, non-overdispersed, in which we used binomial error distributions). We employed Tukey post hoc tests to identify differences among groups when a model’s interaction term was significant.
To analyze differences in germination time, we used survival analysis. The independent variables were species and storage treatment, and the dependent variable was time to seed germination. Seeds that had not germinated by the experiment’s end were treated as censored data. We specifically employed the Kaplan–Meier method in the RcmdrPlugin.survival package in R commander [22]; the command survdiff was utilized to detect differences among species and between treatments for each species (long-rank p-values).
3. Results
3.1. Germination
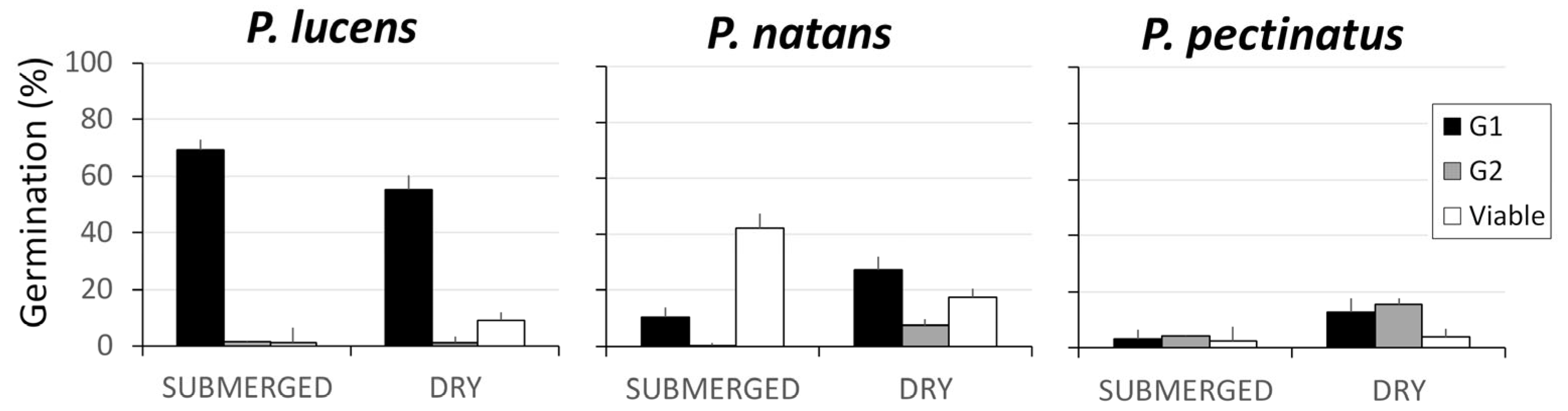
At the end of G1, we observed significant differences in seed germination among species (F2, 178 = 69.066; p < 0.0005) and between storage treatments (F1, 178 = 4.52; p = 0.035). Additionally, there was a significant interaction between species and storage treatment (F1, 178 = 14.8306; p < 0.0005), which indicates that seed germination displayed a species-specific response to storage conditions. Potamogeton lucens had the highest overall seed germination levels, where germination was greater in the submerged storage treatment (69.1 ± 5.4%) than in the dry storage treatment (55.1 ± 4.9%). The other two species had lower seed germination levels, where germination was lower in the submerged storage treatment than in the dry storage treatment (Figure 1).
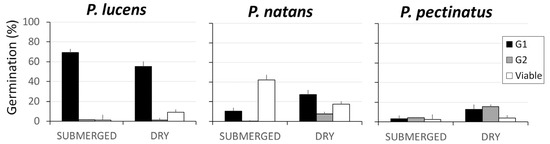
Figure 1.
Mean percentage (±SE) of the seeds per spike that germinated during the first germination trial (G1) and the second germination trial (G2), as well as the mean percentage (±SE) of viable ungerminated seeds remaining at the end of the experiment. Results are shown for the three aquatic plant species and for the submerged and dry storage treatments.
At the end of G2, only a small percentage of the remaining seeds had germinated (P. lucens: 3.0 ± 1.0%, P. natans: 5.6 ± 12.9%, and P. pectinatus: 10.3 ± 2.2%). There was a significant effect of storage treatment (χ2 1, 171 = 19.6; p < 0.0001), species (χ2 1, 171 = 18.48; p < 0.0005), and their interaction (χ2 1, 171 = 22.17; p < 0.0005). The seed germination levels of P. natans and P. pectinatus were significantly greater in the dry storage treatment than in the submerged storage treatment (Tukey post hoc test: p = 0.03 and p = 0.003, respectively). In contrast, the seed germination levels of P. lucens were lower (although not significantly so) in the dry storage treatment than in the submerged storage treatment.
Storage treatment did not have a significant effect on the sum of the number of seeds that germinated in G1 plus G2 (F1, 178 = 1.5118; p = 0.221), but there was an effect of species (F1, 178 = 53.350; p < 0.0005) and the treatment-by-species interaction (F1, 178 = 21.6863; p < 0.0005) (Figure 1).
3.2. Viability
The total number of viable seeds (i.e., those that germinated in G1 and G2 and those that remained viable but ungerminated seeds) differed significantly among species (F2, 178 = 50.991; p < 0.0005). There was no significant effect of storage treatment (F1, 178 = 1.2259; p = 0.270), but the treatment-by-species interaction was significant (F2, 178 = 7.6791; p = 0.0006). Potamogeton lucens had the greatest number of total viable seeds; there were significantly more viable seeds in the submerged storage treatment (71.7 ± 5.0%) than in the dry storage treatment (65.1 ± 3.9%). For P. natans, 52.9 ± 5.1% of seeds were viable, and there was no effect of treatment. For P. pectinatus, a low percentage of seeds were viable, and there was a significant difference between treatments: 9.5 ± 2.0% of seeds were viable in the submerged storage treatment, and 32 ± 3.6% of seeds were viable in the dry storage treatment (Figure 1).
The number of ungerminated seeds that remained viable (and could thus contribute to the seed bank) differed significantly among species (F2, 178 = 176.01; p < 0.0005) but not between treatments (F1, 178 = 0.48; p = 0.6186); the treatment-by-species interaction was significant (F2, 178 = 146.63; p < 0.0005). Potamogeton natans had the largest percentage of viable ungerminated seeds remaining after G1 and G2; there was a significant difference between the submerged storage treatment (42.2%) and the dry storage treatment (17.4%) (Tukey post hoc test: p = 0.0009). In contrast, for the other two species, these figures were much lower and were unaffected by storage treatment. For P. lucens, the percentage of viable seeds was somewhat higher in the dry storage treatment (9.0%) than in the submerged storage treatment (1.3%). For P. pectinatus, the percentage of viable seeds was similar across the two treatments (dry: 3.92%, submerged: 2.4%).
3.3. Time to Germination
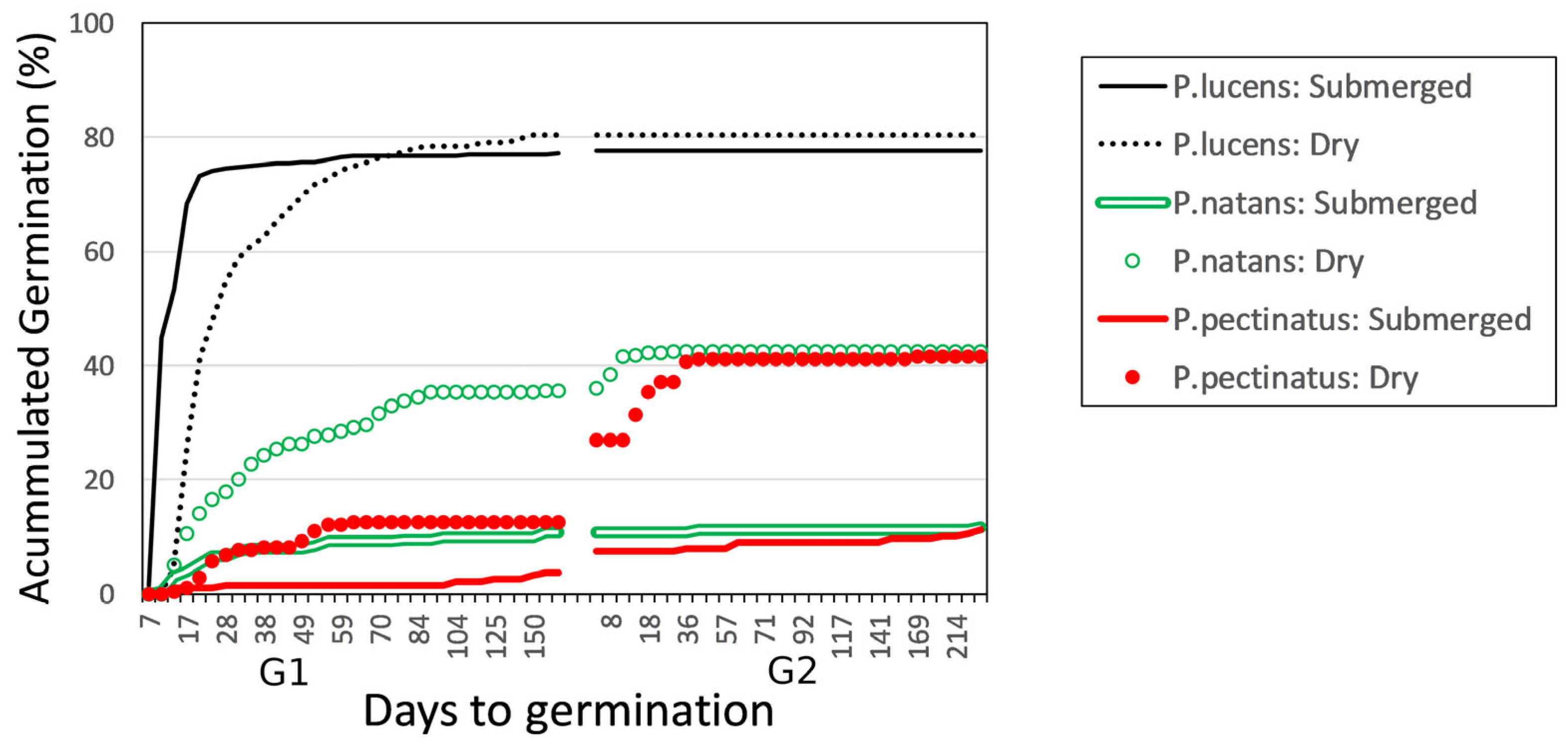
Time to germination differed among species and between treatments (χ2 = 748; df = 5; p < 0.0005). In general, seeds in the dry storage treatment germinated later than seeds in the submerged storage treatment for all species. The seeds of P. lucens germinated first. Among those seeds that experienced the submerged storage treatment, 50% germinated within the first week of the germination trial. However, the P. lucens seeds that experienced the dry storage treatment displayed significantly different germination dynamics (χ2 = 20.7; df = 1; p < 0.0005): they did not begin germinating until the second week, and it took until the third week for 50% of them to germinate (Figure 2).
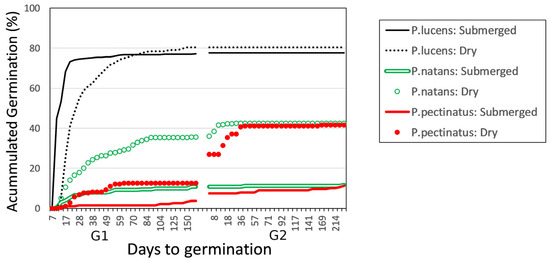
Figure 2.
Accumulated percentage of germinated Potamogeton lucens, P. natans, and P. pectinatus seeds over the two germination trials after exposure to submerged or dry storage conditions.
Time to germination also differed significantly between the dry and submerged storage treatments for P. natans (χ2 = 31.1; df = 1; p < 0.0005) and P. pectinatus (χ2 = 11; df = 1; p = 0.0009). For both species, seeds in the submerged treatment germinated earlier and faster than did seeds in the dry storage treatment.
Between the time the seeds were collected and the start of the experiment (i.e., when the seeds were placed in the germination chamber), we observed that 0.4% of P. pectinatus, 2.51% of P. lucens, and 0.27% of P. natans seeds had germinated in the submerged storage treatment.
4. Discussion
4.1. Germination and Seed Viability
We found seed germination dynamics differed significantly among the three Potamogeton species we studied. Potamogeton lucens was the only species with high levels of germination; germination levels were much lower for P. natans and P. pectinatus. In general, in species that mainly engage in asexual reproduction, the relative investment in sexual reproduction (i.e., seeds) may reflect a history of experiencing disturbance events or habitat instability, factors that may influence extinction risk [23,24]. Indeed, higher levels of seed production help populations become re-established, promote genetic variability, and facilitate species dispersal [2].
Percentages of viable ungerminated seeds differed among the three study species, which suggests that the species may have different reproductive strategies that are influenced by their specific habitat conditions and, notably, habitat stability. The species with the highest level of seed germination—P. lucens—had a very low percentage of viable seeds that remained after the germination trials, indicating that it makes a limited contribution to the seed bank. Taken together, these results suggest that sexual reproduction likely plays an important role in the annual renewal, but not the longer-term persistence, of P. lucens populations. Indeed, most of the P. lucens seeds germinated during the first germination trial; a very low number germinated during the second germination trial; and most of the seeds remaining thereafter were not viable. In nature, if catastrophic events such as severe pond desiccation occur, plant rhizomes may be eliminated and population re-establishment during the following inundation cycle would rely exclusively on seeds produced the year prior. However, the low percentage of viable ungerminated seeds we observed for P. lucens suggests that this species makes a limited contribution to the seed bank and might be less resilient if unfavourable conditions or catastrophic events last for extended periods. Thus, in such cases, species populations would be at greater risk of extinction. Although P. lucens has orthodox seeds [6], we found that dry storage conditions reduced the germination and viability levels of its seeds. Therefore, despite its high level of seed germination, P. lucens seems like it would be very sensitive to recurrent droughts.
The other two species, P. natans and P. pectinatus, had low levels of seed germination, suggesting that they may mostly depend on clonal asexual reproduction. They differed in their percentages of viable ungerminated seeds, which could mean that they have different strategies for promoting population persistence.
It is known that P. natans commonly employs asexual reproduction (i.e., using shoots and rhizomes [17,25]), which fits with its low levels of seed germination during the two trials. At the same time, an appreciable percentage (~30%) of its seeds remained viable at the end of the experiment and could hypothetically end up in the seed bank, where they would then experience either dry or submerged conditions. At present, P. natans is found exclusively in permanent aquatic habitats in Doñana. However, due to groundwater declines, most of these habitats have been drying out in the summer in recent years (see [13]). Given this shift, seeds may play an important role in the persistence of P. natans populations, as seeds likely function as a safeguard when populations face unfavourable environmental conditions.
Potamogeton pectinatus had a low level of germination, and only a small percentage of its seeds remained viable after the two germination events. Past research has reported the poor germination success of this species [26,27,28,29], which mainly reproduces vegetatively via propagules (e.g., tubers, turions, rhizomes), or perennial plants, which may produce new shoots to restore plants after senescence [27]. Seeds are considered to play a minimal role in population persistence, although sporadic sexual reproduction may help preserve genetic variability in populations [27]. Furthermore, seeds contribute to species dispersal and gene flow among wetlands [30,31] because they are commonly dispersed by waterfowl [32,33,34]. For P. pectinatus, seed germination and viability levels were greater following dry storage, which may be a response to the temporary desiccation they experienced; following submerged storage, seed germination and viability were poor. The positive influence of dry storage on P. pectinatus seed germination and viability is likely linked to the species’ common occurrence in temporary aquatic habitats. Indeed, this species is generally found in Doñana’s permanent ponds and temporary ponds with long hydroperiods.
4.2. Time to Germination
The ability of seeds to survive periods of desiccation is a functional trait that must be considered in population restoration efforts [35]. In all three species, seeds germinated faster following submerged storage than dry storage. This pattern is related to the fact that seeds must be rehydrated after they have dried out; such is unnecessary when seeds experience submerged storage conditions.
The seeds of P. lucens germinated the earliest, responding rapidly to the conditions that promoted seedling survival. The seeds of aquatic species commonly display non-deep physiological dormancy [5], which has differing effects on embryos and seed coats. Previous work has shown that the three Potamogeton species studied here have seeds that demonstrate this type of dormancy [5]. Given that P. lucens germinated earliest and that a large percentage of its seeds germinated even before the experiment started, it may be that this species has lower levels of physiological dormancy than the other two species [36], as well as a shorter rehydration period.
4.3. Conservation Implications
Over the last decades, the Doñana pond network has severely deteriorated: more than 60% of ponds have dried up and pond hydroperiods have become shorter overall [13]. Even natural permanent ponds and artificially deepened ponds are drying up in the summer, which has affected the survival of the strictly aquatic plants. A recent study looked at losses of vascular aquatic plants within the network: it found that 60.5% of species had experienced declines and 23.7% of species had disappeared—the hardest hit plants were associated with permanent ponds [15]. Two Potamogeton species—P. crispus and P. polygonifolius—are no longer found in the area; P. natans only occurs in the area most affected by groundwater exploitation (see [13], where it has low levels of abundance; and P. lucens has dramatically declined in abundance over the last decade [15]. The main threat to these species is that most of the permanent ponds have become temporary ponds. Our results on the germination strategies of the three Potamogeton species may explain the causes of the recent decline observed. In the case of P. natans, it does not appear that the accumulation of viable seeds in the seed bank has been enough to maintain populations in the small number of permanent ponds where the species is still found; indeed, only vegetative reproduction is observed at present [15]. Similarly, P. lucens has experienced significant population declines in recent years, which are associated with habitat desiccation. For local populations to persist, they would need to produce seeds every year, and prolonged periods of desiccation over multiple years may ultimately lead the species to disappear from the park. In contrast, although we observed low levels of seed germination for P. pectinatus, this species has increased in abundance within the park in recent years. It is currently among Doñana’s most common aquatic plant species. According to our results, P. pectinatus seeds have higher levels of germination after dry storage conditions, which means they may promote population re-establishment in ponds that experience recurrent droughts. Even with its generally low levels of germination, P. pectinatus may be able to rapidly increase in abundance after periods of desiccation thanks to its fast vegetative growth and efficient clonal reproduction, using sporadic sexual reproduction to maintain its genetic variability [27] or to establish new populations via waterfowl-mediated dispersal. Overall, sexual reproduction does not appear to be sufficient for the long-term conservation of P. lucens and P. natans in our study area. Their contribution to the seed bank is not enough to withstand the recent trends of hydroperiod shortening in this area. Only effective conservation—even through artificial maintenance—of permanent ponds in the area may prevent them from disappearing completely.
5. Conclusions
The three Potamogeton species differed in their germination strategies, which revealed the different importance of sexual reproduction for the long-term persistence of their populations. In P. lucens, which inhabits permanent waters, the low contribution to the seed bank may be explained by the low importance of sexual reproduction for its long-term persistence, although it is important for the current incorporation of new individuals every year. In contrast, the low levels of seed germination in P. natans and P. pectinatus indicate that vegetative reproduction is more important than sexual reproduction in ensuring the long-term persistence of their populations. However, the higher contribution of P. natans to the seed bank suggests that this species may be able to resist dry periods and restore its population through seed germination, which is even increased after drying. In contrast, the low germination rates of P. pectinatus seeds, whether dry or submerged, and even the poor contribution to the seed bank, indicate that this species mainly reproduces by clonal reproduction, while seeds mainly contribute to maintaining the genetic variability and dispersal of their populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/seeds4030045/s1, Figure S1: Details of the spikes, seeds before the start of germination trials, and of germinated seeds of Potamogeton lucens, P. natans and P. pectinatus; Figure S2: Details of positive (viable: pink) or negative (non-viable) staining of seeds of the three Potamogeton species studied.
Author Contributions
Conceptualization, C.D.-P. and R.F.-Z.; methodology, C.D.-P. and R.F.-Z.; investigation, C.D.-P. and R.F.-Z.; writing—original draft preparation, C.D.-P. and R.F.-Z.; writing—review and editing, C.D.-P. and R.F.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Science, Innovation and Universities project number PID2019-104343RB-I00.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
Anna Murakozy helped with the laboratory work, especially with the seed viability testing. The Natural Processes Monitoring team at ICTS-Doñana helped with assistance in the field and facilities at Doñana National Park. The study has been funded by the Spanish Ministry of Science, Innovation and Universities (project PID2019-104343RB-I00).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cronk, J.K.; Fennessy, M.S. Wetland Plants: Biology and Ecology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Eckert, C.G.; Doken, M.E.; Barret, S.C.H. Ecological and evolutionary consequences of sexual and clonal reproduction in aquatic plants. Aquat. Bot. 2016, 135, 46–61. [Google Scholar] [CrossRef]
- Sculthorpe, C.D. The Biology of Aquatic Vascular Plants; Edward Arnold Ltd.: London, UK, 1967. [Google Scholar]
- Adamec, L. Ecophysiological characteristics of turions of aquatic plants: A review. Aquat. Bot. 2018, 148, 64–77. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Hay, F.R.; Probert, R.J.; Marro, J.; Dawson, M. Towards the Ex Situ Conservation of Aquatic Angiosperms: A Review of Seed Storage Behaviour. In Seed Biology: Advances and Applications, Proceedings of the Sixth International Workshop on Seeds, Merida, Mexico, January 1999; Black, M., Bradford, K., Vazquez-Ramos, J., Eds.; CABI Publishing: Oxford, UK, 2000; pp. 161–177. [Google Scholar]
- Pammeter, N.W.; Berjak, P. A review of recalcitrant seed physiology in relation to desiccation-tolerance mechanisms. Seed Sci. Res. 1999, 9, 13–37. [Google Scholar] [CrossRef]
- Pammeter, N.W.; Berjak, P. Evolutionary and ecological aspects of recalcitrant seed biology. Seed Sci. Res. 2000, 10, 301–306. [Google Scholar] [CrossRef]
- Hay, F.R.; Probert, R.J.; Dawson, M. Laboratory germination of seeds from 10 British species of Potamogeton. Aquat. Bot. 2008, 88, 353–357. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.; Knowler, D.J.; Leveque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Davidson, N.C. Wetland Loss and the Status of Wetland-Dependent Species. In The Wetland Book: II. Distribution, Description, and Conservation; Finlayson, M., Milton, R., Prentice, C., Davidson, N., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 369–381. [Google Scholar]
- Froend, R.H.; Horwitz, P.; Sommer, B. Groundwater Dependent Wetlands. In The Wetland Book: II. Distribution, Description, and Conservation; Finlayson, M., Milton, R., Prentice, C., Davidson, N., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 345–355. [Google Scholar]
- de Felipe, M.; Aragonés, D.; Díaz-Paniagua, C. Thirty-four years of Landsat monitoring reveal longterm effects of groundwater abstractions on a World Heritage Site wetland. Sci. Total Environ. 2023, 880, 163329. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Paniagua, C.; Ramírez-Soto, M.; Aragonés, D. Pond basin colonization by terrestrial vegetation indicates wetland deterioration. Aquat. Conserv. 2023, 33, 798–809. [Google Scholar] [CrossRef]
- García-Murillo, P.; Díaz-Paniagua, C.; Fernández-Zamudio, R. Decline of aquatic plants in an iconic European protected natural area. J. Nat. Conserv. 2025, 84, 126814. [Google Scholar] [CrossRef]
- García-Murillo, P.; Potamogeton, L. Flora Iberica; Castroviejo, S., Morales, R., Quintanar, A., Cabezas, F., Pujadas, A.J., Cirujano, S., Eds.; Real Jardín Botánico CSIC: Madrid, Spain, 2010; Volume 17, pp. 64–85. [Google Scholar]
- Wiegleb, G.; Kaplan, Z. An account of the species of Potamogeton L. (Potamogetonaceae). Folia Geobot. 1998, 33, 241–316. [Google Scholar] [CrossRef]
- Fernández-Zamudio, R.; García-Murillo, P.; Díaz-Paniagua, C. Physical and chemical features and water permanence determine aquatic plant distribution in a temporary pond network (Doñana National Park). Hydrobiologia 2016, 774, 123–135. [Google Scholar] [CrossRef]
- Díaz-Paniagua, C.; Fernandez-Zamudio, R.; Serrano, L.; Florencio, M.; Gómez-Rodríguez, C.; Sousa, A.; Sánchez Castillo, P.; García-Murillo, P.; Siljeström, P. El Sistema de Lagunas Temporales de Doñana, una Red de Hábitats Acuáticos Singulares. Organismo Autónomo de Parques Nacionales; Ministerio de Agricultura; Alimentación y Medio Ambiente: Madrid, Spain, 2015. [Google Scholar]
- Porter, R.; Durrell, M.; Romm, H. The use of 2,3,5-triphenyl-tetrazolium chlorid as a measure of seed germinability. Plant Physiol. 1947, 22, 149–159. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Fox, J.; Carvalho, M.S. The RcmdrPlugin.survival Package: Extending the R Commander Interface to Survival Analysis. J. Stat. Softw. 2012, 49, 1–32. [Google Scholar] [CrossRef]
- Pollux, B.J.A.; Jong, M.D.E.; Steegh, A.; Verbruggen, E.; Van Groenendael, J.M.; Ouborg, N.J. Reproductive strategy, clonal structure and genetic diversity in populations of the aquatic macrophyte Sparganium emersum in river systems. Mol. Ecol. 2007, 16, 313–325. [Google Scholar] [CrossRef]
- Okada, M.; Grewell, B.J.; Jasieniuk, M. Clonal spread of invasive Ludwigia hexapetala and L. grandiflora in freshwater wetlands of California. Aquat. Bot. 2009, 91, 123–129. [Google Scholar] [CrossRef]
- Wiegleb, G.; Brux, H.; Herr, W. Human impact on the ecological performance of Potamogeton species in northwestern Germany. Vegetatio 1991, 97, 161–172. Available online: http://www.jstor.org/stable/20046095 (accessed on 5 October 2024). [CrossRef]
- Van Wijk, R.J. Ecological studies on Potamogeton pectinatus L.I. General characteristics, biomass production and life cycles under field conditions. Aquat. Bot. 1988, 31, 211–258. [Google Scholar] [CrossRef]
- Van Wijk, R.J. Ecological studies on Potamogeton pectinatus L. III. Reproductive strategies and germination ecology. Aquat. Bot. 1989, 33, 271–299. [Google Scholar] [CrossRef]
- Rodrigo, M.A.; Calero, S. Phenology of macrophytes in coastal environments: Utricularia australis (R. Br.) and Stuckenia pectinata (L.) Börner in an interdunal pond within the Albufera de València Natural Park. Limnetica 2019, 38, 317–334. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, R.; Liu, Y.; Yin, L.; Wang, C.; Li, W. The effect of storage condition on seed germination of six Hydrocharitaceae and Potamogetonaceae species. Aquat. Bot. 2017, 143, 49–53. [Google Scholar] [CrossRef]
- Clausen, P.; Nolet, B.A.; Fox, A.D.; Klaassen, M. Long-distance endozoochorous dispersal of submerged macrophyte seeds by migratory waterbirds in northern Europe—A critical review of possibilities and limitations. Acta Oecologica 2002, 23, 191–203. [Google Scholar] [CrossRef]
- Santamaría, L.; Charalambidou, I.; Figuerola, J.; Green, A.J. Effect of passage through duck gut on germination of fennel pondweed seeds. Arch. Hydrobiol. 2002, 156, 11–22. [Google Scholar] [CrossRef]
- Figuerola, J.; Charalambidou, I.; Santamaria, L.; Green, A.J. Internal dispersal of seeds by waterfowl: Effect of seed size on gut passage time and germination patterns. Naturwissenschaften 2010, 97, 555–565. [Google Scholar] [CrossRef]
- Mader, E.; van Vierssen, W.; Schwenk, K. Clonal diversity in the submerged macrophyte Potamogeton pectinatus L. inferred from nuclear and cytoplasmic variation. Aquat. Bot. 1998, 62, 147–160. [Google Scholar] [CrossRef]
- Tóth, P.; Green, A.J.; Wilkinson, D.M.; Brides, K.; Lovas-Kiss, Á. Plant traits associated with seed dispersal by ducks and geese in urban and natural habitats. Ecol. Evol. 2023, 13, e10677. [Google Scholar] [CrossRef]
- Tweddle, J.C.; Dickie, J.B.; Baskin, C.C.; Baskin, J.M. Ecological aspects of seed desiccation sensitivity. J. Ecol. 2003, 91, 294–304. Available online: http://www.jstor.org/stable/3599764 (accessed on 3 December 2024). [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).