Abstract
Because of the effects of climate change, a shortage of seeds from superior mother trees for forestry breeding programmes can occur. While biotechnological tools can help address this challenge, the benefits and drawbacks of each technique need to be evaluated. In this work, a comparative Pinus radiata plantlet production study was performed using two different approaches: seeds versus somatic embryos. For each procedure, the number of plantlets obtained was recorded, and the manual labour time was calculated. The skills and time required were higher for somatic embryogenesis, but so was the number of plantlets obtained: 3660 acclimatized somatic plantlets versus 1124 acclimatized zygotic plantlets. The number of different genotypes obtained was higher when germinating mature seeds, but somatic embryogenesis coupled with cryopreservation offers the advantage of having a backup for field-tested material. Additionally, analyses of carbohydrates, total protein and dry weight of somatic and zygotic embryos were carried out. These analyses revealed considerable differences between somatic and zygotic embryos but underlined the importance of high sucrose levels for germination in both systems. In conclusion, each technique offers advantages and disadvantages, and the choice will depend on the objective, the species and the value of the material to be propagated.
1. Introduction
Projections to 2050 indicate significant increases in wood demand [1]. To try to satisfy this demand while preserving native forests where possible, it is necessary to increase the efficiency of forest plantations in terms of production and resilience to climate change and its derived effects [2,3].
Radiata or Monterey pine (Pinus radiata D. Don) is one of the most widely planted tree species in the world for its timber [4]. Native to some locations in North America, it is nowadays cultivated in New Zealand, Australia, Chile, South Africa and Spain. In the Basque Country (northern Spain), this species represents 28% of the total wooded forest area (around 80–85% of the annual timber logging) [5]. However, the commercial viability of radiata pine plantations could be threatened by biotic and abiotic stressors arising from or magnified by climate change. These biotic stressors are mainly fungi such as Lecanosticta acicula, Dothistroma septosporum and Dothistroma pini [6]. Among the abiotic ones, drought and extreme climatic events are noteworthy. For this reason, and as part of other strategies in NEIKER’s radiata pine improvement program, micropropagation is being used to multiply elite plant material, selected based on its superior characteristics in terms of wood volume and optimal health condition. Among the existing in vitro techniques for propagating pines, somatic embryogenesis (SE) offers several advantages, and when coupled with conservation at ultra-low temperatures, it enables the deployment of multi-varietal forestry [7]. Moreover, this process, or at least some stages of it, can be automatized [8], and through early genomic selection, the breeding cycles could be shortened [9]. Recent advances in genome editing by CRISPR/Cas9 using embryogenic tissues [10] foresee a promising future for this propagation technique. However, one of its major drawbacks is that in vitro techniques, apart from requiring specific facilities, are more time- and labour-consuming. Several studies have analyzed the cost of SE when producing plants at a large scale [11,12,13]. NEIKER is a non-profit institution, and the SE process is mainly used for basic research and to fuel the improvement program. However, it is interesting to consider this subject for planning purposes when the different ways of producing plants, even for research, are compared.
Understanding the biochemical processes underlying embryo development and germination could also help to optimize the SE process and reduce costs. During this process, protein and carbohydrate accumulation and mobilization play a crucial role and are key factors in determining the success of embryo-to-plant conversion [14]. Modifying time frames, culture or storage conditions could adjust the molecular spectrum of embryos to maximize germination rates. Particularly, some post-maturation treatments, such as desiccation or cold storage, are known to be beneficial at this step of SE by modulating the levels of certain storage compounds [15,16]. Similarly, stratification, which is a common practice in plant nurseries to break seed dormancy [17], has been demonstrated to directly impact protein metabolism during germination [18,19,20,21]. As a result, the objectives of this research were on one hand to study if there were the differences in total protein and carbohydrate contents in mature zygotic embryos collected from brown mature cones and somatic embryos collected at different times during the maturation stage, with the aim of uncovering whether these factors influence germination and could be used to optimize the efficiency of the somatic embryogenesis process; on the other hand, we sought to compare small-scale plant production by SE and traditional techniques germination with mature seeds from the same open-pollinated previously untested mother trees.
This study shows how, starting with a similar number of seeds, plant production can vary considerably depending on the methodology used. We believe that this work presents a “problem–technology–mechanism–strategy”-oriented study that could provide practical guidance for forest propagation technologies.
2. Materials and Methods
2.1. Plant Material
Eight radiata trees with superior wood volume and health characteristics were selected (see Supplementary Table S1). These trees are candidate plus-parents under NEIKER’s radiata pine breeding program in the Basque Country. Open-pollinated brown cones (two years) were collected for traditional seed germination in January 2022 and 2023. The seeds collected from the same tree constitute a seed family, named after the mother tree. Green cones (one year) from the same trees were collected in late June and early July for SE induction.
2.2. Traditional Germination
Seeds collected from these eight trees were used in this study. Initially, the cones were carefully separated by maternal source and opened and dried under controlled conditions in well-ventilated kilns at 45–50 °C for 24 h [22]. Following dehiscence, seeds were extracted through tumbling, de-winged and subsequently cleaned using air flotation to remove debris and non-viable seeds. The conventional stratification protocol involves immersing the seeds in water for 24–48 h, which is sufficient for fresh seeds with a high germination potential [22,23]. Empty seeds, which remain buoyant, were discarded. After soaking, the seeds were drained and stored at room temperature in paper bags with silica gel until sowing. Prior to sowing, the seeds were treated with 30% (v/v) hydrogen peroxide for 5 min to enhance viability and protection [24,25]. The planting substrate was a mixture of black peat and vermiculite (Termita 2-Vermiculita., Abeto S.L) (3:1, v/v). The peat should maintain a slightly acidic to neutral pH (approximately 5.5–6.5) to optimize nutrient availability and minimize the risk of toxic salt accumulation. Maintaining low salinity is crucial to prevent stress on germinating seeds. Germination and growth of radiata pine seedlings were performed in plug trays (Herku HP 104, 37cc, supplied by PROJAR (Valencia, Spain)) in the greenhouse under controlled conditions at 20–25 °C. Proper and timely irrigation was ensured, particularly during and after germination, as well as during transplanting (Herku QP T16, 330 cc, supplied by PROJAR (Valencia, Spain)), to prevent moisture stress and promote uniform seedling growth. Except for one mother tree (Aixola 4, 100 seeds), 200 seeds per mother tree were germinated. The seeds were randomly distributed in 5 trays per mother tree, each tray containing 40 seeds. After 3–4 weeks, the number of germinated seedlings was recorded, and the percentage of germination for each family was calculated. The time for cone processing, seed planting and caring was calculated in hours. A summary of the experimental setup for the traditional germination process can be found in Figure 1.
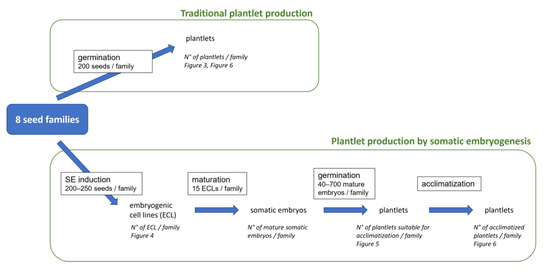
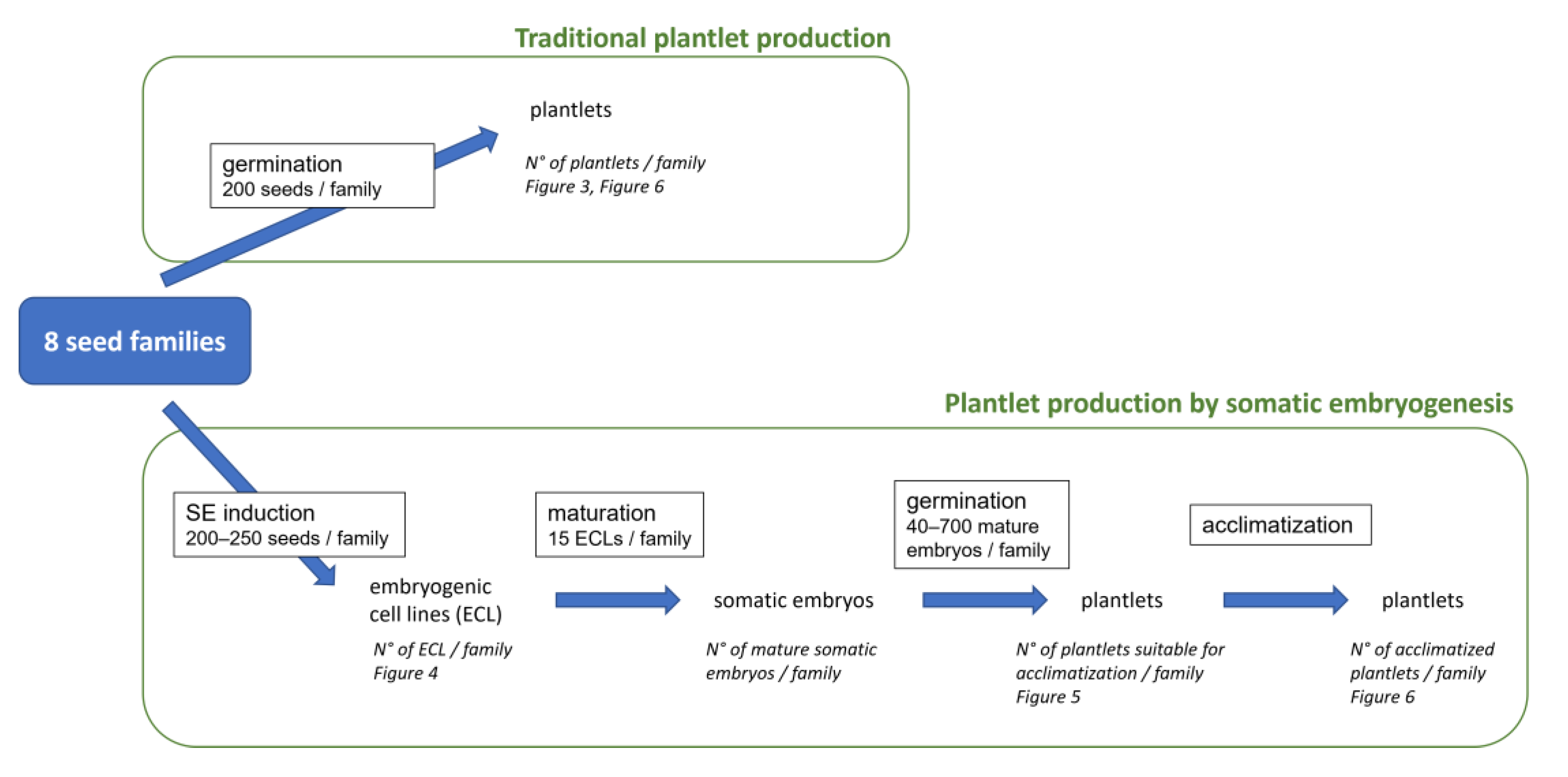
Figure 1.
Experimental plan for traditional and biotechnological production of plantlets. Each stage of the experiment is indicated with its name and the quantity of material used (in a frame), the material produced, the results recorded (in italics) and the figure (in italics) where these results are presented. N°: number.
2.3. SE Process
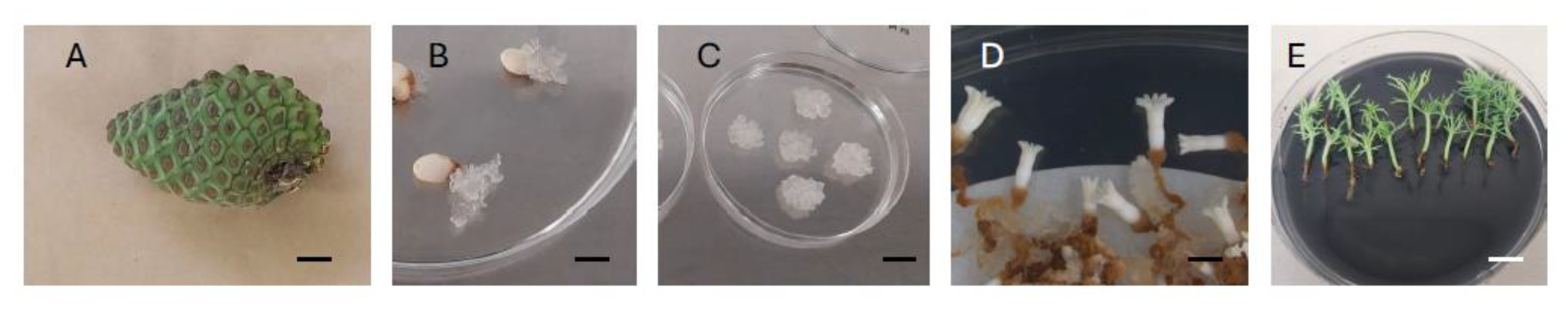
For SE induction, 1850 immature seeds from one-year old green cones (Figure 2A) of each of the above-mentioned trees (200–250 seeds per tree) were surface sterilized in 10% (v/v) hydrogen peroxide plus two drops of Tween 20® for 10 min and then rinsed three times with sterile distilled water under sterile conditions in a laminar flow unit. Seed coats were removed, and whole megagametophytes containing zygotic embryos at the precotyledonary stage were excised and placed horizontally onto Embryo Developmental Medium (EDM) for initiation as described in [16]. Each Petri plate contained 8 megagametophytes, comprising a total of 25 to 32 Petri plates per mother tree. After 4–8 weeks, embryogenic tissue (Figure 2B) larger than 3 mm in diameter was separated from the megagametophytes and subcultured in EDM proliferation medium [16] fortnightly. After 2 to 6 subculture periods, actively growing embryogenic tissue originating from each seed was recorded as an established embryogenic cell line (ECL, Figure 2C), and the percentage of ECLs from each family was calculated. To standardize downstream comparisons, no more than 15 ECLs per family were selected for maturation. Simultaneously, 4 to 10 cryotubes with embryogenic tissue per ECL were stored at −80 °C [26]. Maturation was carried out following [16]; briefly, the tissue was resuspended in liquid medium and then poured into a filter paper. After draining the liquid, the filter paper with the tissues attached (80–90 mg) was laid on Petri dishes (20 Petri dishes per ECL) with EDM maturation medium for 15 weeks. After this period, the percentage of ECLs producing somatic embryos was calculated (% of maturation), and the number of somatic embryos (Figure 2D) obtained from each family was recorded. These embryos were subjected to germination (Figure 2E) for 12 weeks following the methodology described by Montalbán et al. [16]. After in vitro germination, the number of somatic plantlets suitable for ex vitro planting was recorded (proper shoot/root balance; [16]); these plantlets were acclimatized in a greenhouse under controlled conditions at 20–25 °C. Following 8–10 weeks of ex vitro acclimatization, the number of somatic plantlets obtained from each family was recorded and the acclimatization percentage calculated. The time for media preparation, in vitro culture of megagametophytes, cell lines and their in vitro conversion to plantlets was calculated in hours. A summary of the experimental setup for the SE process can be found in Figure 1.
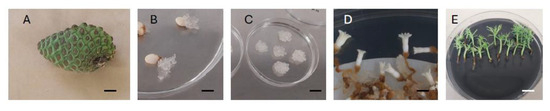
Figure 2.
Somatic embryogenesis process in Pinus radiata: one-year old green cone (bar = 20 mm) (A), extrusion of embryogenic tissue from megagametophytes (bar = 10 mm) (B), established embryogenic cell line (bar = 15 mm) (C), 15-week-old cotyledonary somatic embryos (bar = 3 mm) (D), germinated somatic plantlets (bar = 9 mm) (E).
2.4. Biochemical Analysis: Somatic vs. Zygotic Embryos
Comparative storage compound analyses were performed between somatic embryos from three different ECLs and zygotic embryos from open-pollinated cones of a genotypically related mother tree. ECLs generated following the procedure described in Section 2.3 were subjected to maturation (80 Petri dishes per ECL), and developing somatic embryos were collected at different time-points (the Petri dishes used at each time-point were discarded to avoid desiccation effects caused by repeated lid openings). For total protein analyses, somatic embryos were collected at weeks 13, 15, 17 and 19 (Supplementary Material Figure S1). At each time-point and ECL, five replicates of embryos weighing 20 mg each were used. For carbohydrate analyses, somatic embryos were collected at weeks 15, 17 and 19. In this case, three replicates of 70 mg each were used per time-point and ECL. For zygotic embryos, seeds extracted from two-year-old cones of the abovementioned mother tree were opened using forceps and scalpels, and mature zygotic embryos were excised from the surrounding megagametophytes (Supplementary Material Figure S1). The quantity of zygotic embryos required for each analysis was the same as that described for somatic embryos. Both types of embryos were immediately frozen in liquid nitrogen and stored at −80 °C up to the extraction and quantification of the storage compounds. Simultaneously, 4 replicates of 5 embryos from each embryo type, ECL and time-point were collected to determine their water content. To this aim, embryos were immediately weighed to determine their fresh weight (FW) and then introduced into an oven at 70 °C for 48 h to determine the dry weight (DW). DW percentage was calculated as DW/FW × 100. Another 15 embryos from each condition were germinated as described in Section 2.2 and Section 2.3 to determine whether the collection time had any effect on the germination success, and if this result could be linked with the biochemical profile of the somatic embryos.
2.4.1. Total Protein Analysis
Total proteins were extracted following the methodology described by Morel et al. [27]; frozen material was extracted with 0.5 mL of lysis buffer containing 2% (w/v) polyvinylpolypyrrolidone (PVPP), 5% (v/v) β-mercapto-ethanol, 2% (v/v) sodium dodecyl sulphate (SDS), 50 mM Tris HCl (pH 6.8) and 10% (v/v) glycerol. Protein content was determined using the Quick Start™ Bradford protein assay (Bio-Rad, Hercules, CA, USA) following the Bradford method. Absorbance was read at 595 nm in triplicate, and the amount of protein was calculated using a Bovine Serum Albumin (BSA) standard curve. The results were expressed as soluble protein content in mg g−1 DW.
2.4.2. Soluble Carbohydrates and Starch Estimation
For soluble carbohydrates and starch identification and quantification, the procedure described by Gautier et al. [28] was followed. Briefly, samples from Section 2.4 were freeze-dried and ground to a fine powder, and then immersed in 1 mL 80% aqueous ethanol. Extracts were then heated for 30 min at 80 °C, centrifuged and purified using activated charcoal and PVPP to remove proteins and polyphenols. After vacuum-drying, pellets were resuspended in 100 µL water and injected (10 µL) into a Chromaster high-performance liquid chromatography system (VWR Hitachi, Radnor, PA, USA) equipped with a RezexTM RPM-Monosaccharide Pb+2 (8%) column (Phenomenex, Torrance, CA, USA) and an evaporative light scattering detector (ELSD) 85 (VWR Hitachi) at a flow rate of 0.6 mL min−1. Carbohydrates were identified by co-elution with commercial standards (Sigma, Saint Louis, MO, USA) and quantified by electronic integration of the peak areas using OpenLAB CDS EZChrom (Agilent, Santa Clara, CA, USA) and comparison with the areas from the calibration curves (Supplementary Material, Figure S2). The quantification results were expressed as mg g−1 DW. Starch content was determined using the “D-glucose assay procedure” kit (Megazyme) and expressed as glucose equivalent. Briefly, residual pellets obtained after the extraction of soluble carbohydrates were incubated at 95 °C in 0.02 M NaOH for 2 h and then hydrolyzed for 1 h at 50 °C by the addition of amyloglucosidase from Aspergillus niger (Sigma). Supernatants were then used for glucose quantification.
2.5. Statistical Analyses
Statistical analyses were carried out with R software (version 4.1.3, © 2009–2022 RStudio, PBC, Boston, MA, USA). To assess the effect of the different seed families on the traditional germination process (5 replicates per mother tree) and also on the percentage of ECLs produced during the SE process (25–32 replicates per mother tree), a logistic regression followed by the corresponding analysis of deviance was performed. Multiple comparisons were based on Tukey’s post hoc test (α = 0.05). To compare the profile of proteins and carbohydrates between somatic embryos from different collection dates and mature zygotic embryos, an analysis of variance (ANOVA) was carried out, followed by Tukey’s test of Multiple Comparisons (α = 0.05). In the case of DW, the values obtained were log (x) transformed to meet homoscedasticity and then statistically analyzed following the same procedure as that described for proteins and carbohydrates.
3. Results
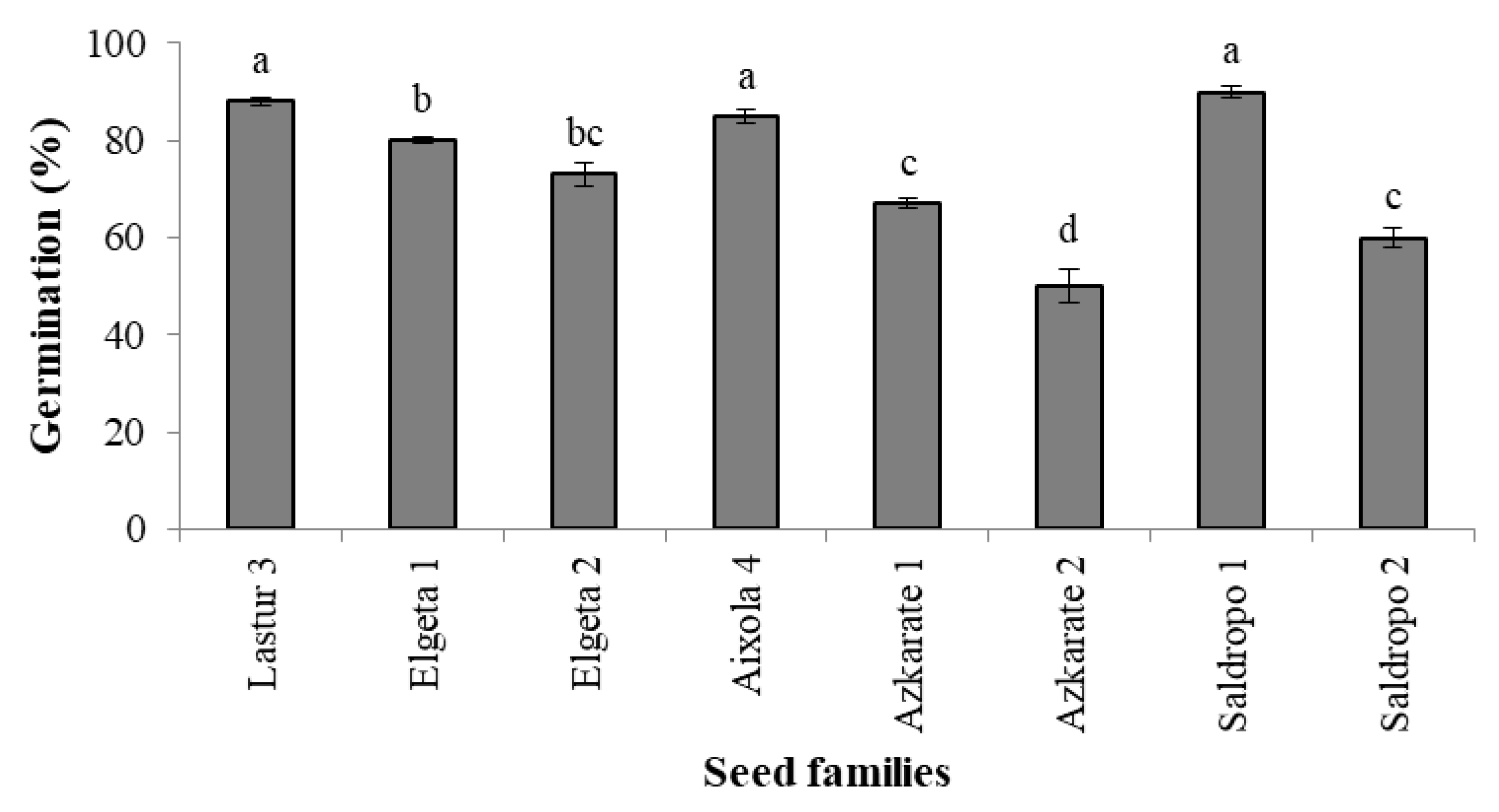
The traditional germination process led to 1124 individuals in total. The percentage of germination ranged from 50 to 90% depending on the seed family (Figure 3).
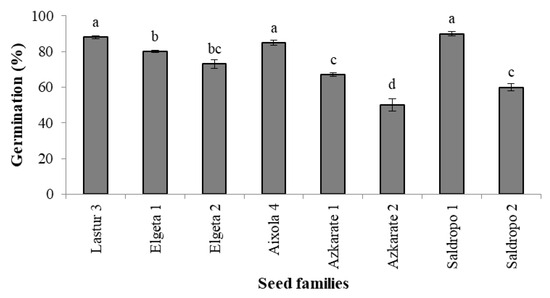
Figure 3.
Germination percentage in eight Pinus radiata mother trees (Mean ± S.E.). Significant differences at p < 0.05 are indicated by different letters.
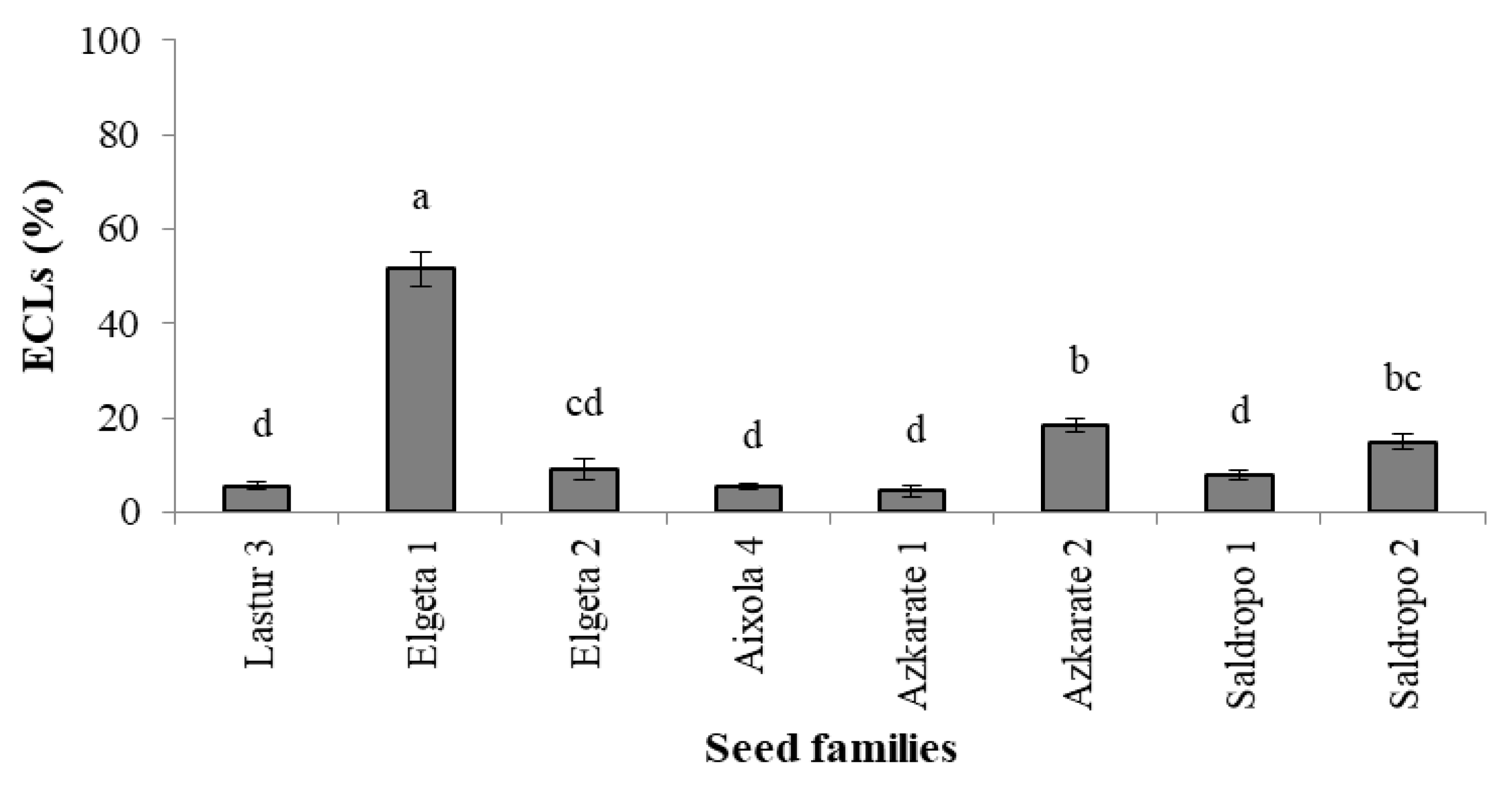
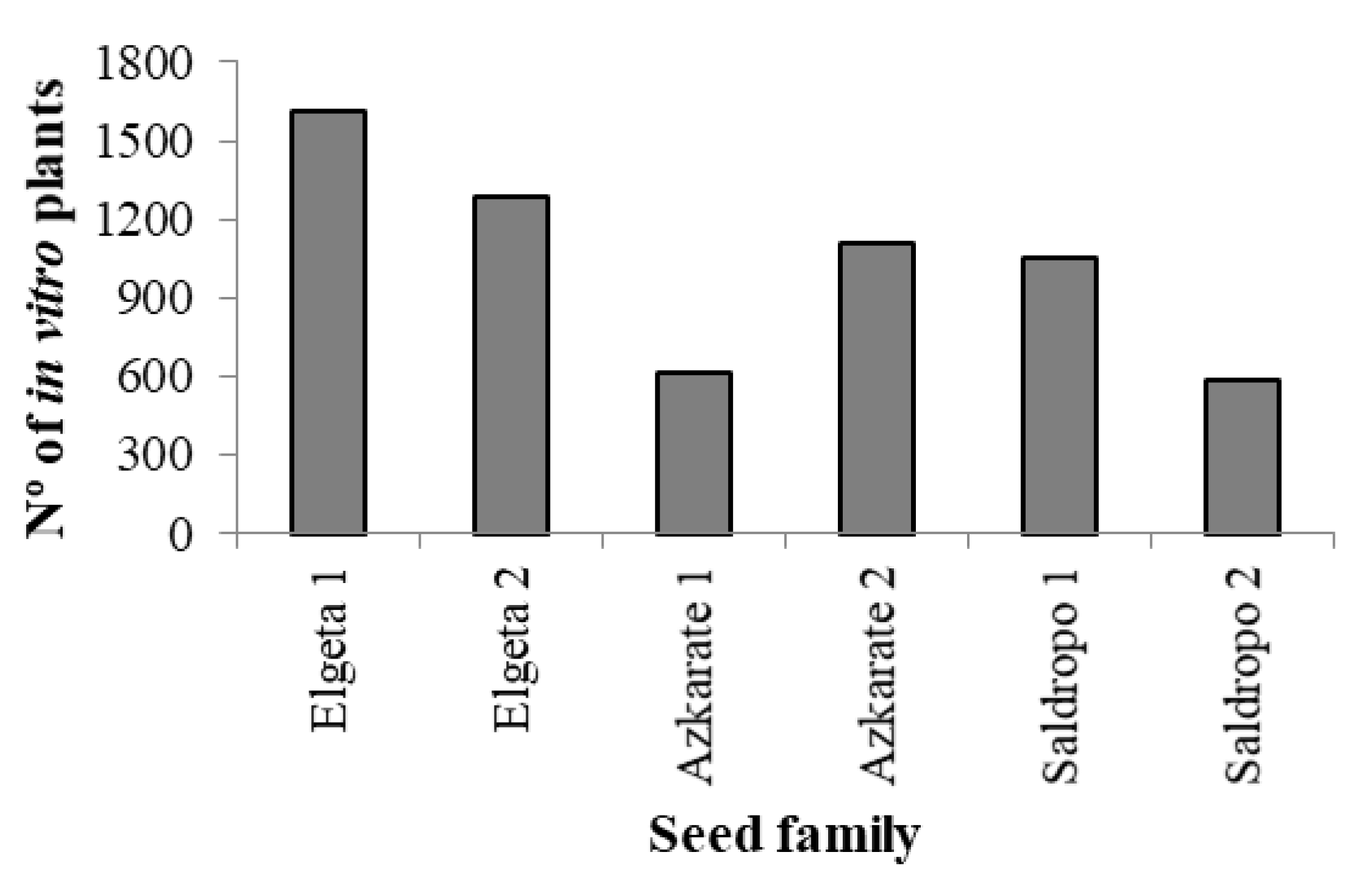
When SE was performed using 1850 immature seeds from eight mother trees, initiation rates varied greatly (Figure 4) and were below 20% for seven out of eight families (52% for Elgeta 1). Although ECLs from all seed families were subjected to maturation, it was not possible to produce somatic plantlets from two mother trees (Lastur 3 and Aixola 4). From the other seed families, it was possible to obtain somatic embryos and, after germination, 6240 in vitro plantlets from 41 ECLs. The somatic plant numbers ranged from 584 to 1608, depending on the seed family (Figure 4).
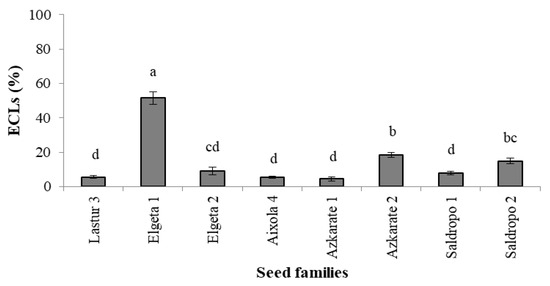
Figure 4.
Percentage of established embryogenic cell lines (ECLs) across Pinus radiata seed families (Mean ± S.E.). Significant differences at p < 0.05 are indicated by different letters.
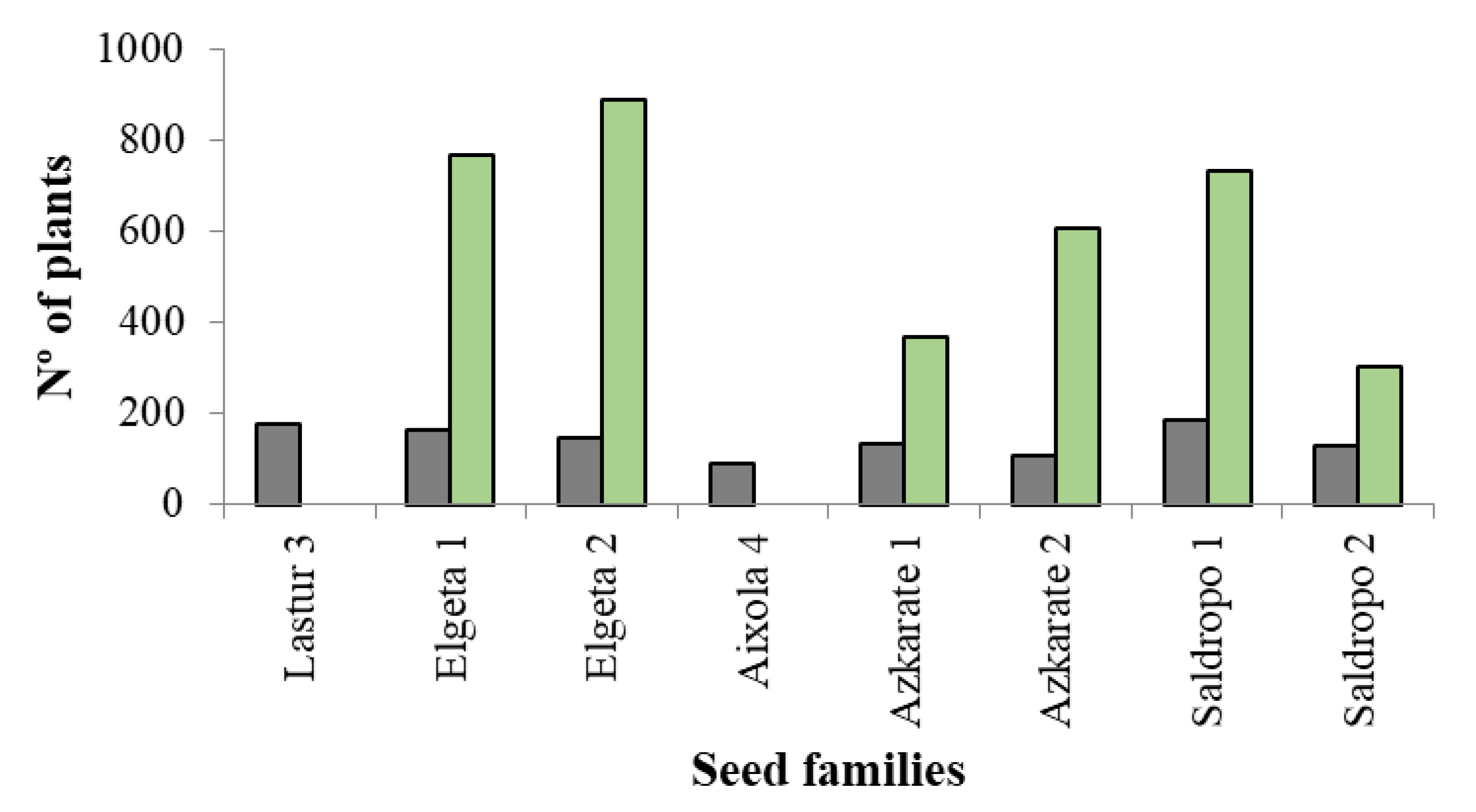
Finally, for ex vitro acclimatization, two ECLs were discarded because the number of somatic plantlets suitable for planting was below 40. In the same way, when the number of somatic plantlets per ECL was very high, no more than 700 plantlets per ECL in 2022 and no more than 500 plants per ECL in 2023 were evaluated for ex vitro plantation. Taking this into account, the total sum of in vitro plants assessed was 5780 (Figure 5), among which 3660 (63%) were well developed and suitable for ex vitro planting (Figure 6). The percentage of plantlets suitable for ex vitro planting therefore ranged from 53% (Elgeta 1) to 72% (Azkarate 1). The number of ECLs from six mother trees was six for Elgeta 1 and Azkarate 2, five for Saldropo 1 and Saldropo 2, and three for Elgeta 2 and Azkarate 1, making a total of twenty-eight ECLs. The number of plants planted per mother tree varied from 303 to 886 (Figure 6); the acclimatization of these plantlets was above 95%. The number of plantlets obtained from the traditional seed germination system was 1124 from eight mother trees (Figure 5), and the number of plants per mother tree varied between 87 plants for Aixola 4 and 184 plants for Saldropo 1 (Figure 7).
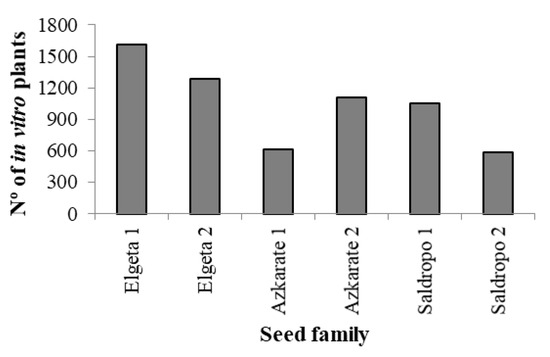
Figure 5.
Number (Nº) of in vitro somatic plantlets from different Pinus radiata seed families.

Figure 6.
(A) Somatic plant suitable for ex vitro planting (proper shoot/root balance) after germination in vitro; square = 1 cm2. (B) Acclimatized somatic plants growing in the greenhouse under controlled conditions (20–25 °C). (C) Plants produced through traditional germination (zygotic plants) growing in the greenhouse under controlled conditions (20–25 °C).
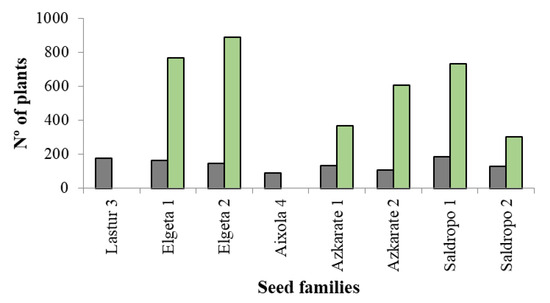
Figure 7.
Final number (Nº) of plants from different Pinus radiata seed families. In grey, those obtained from traditional seed germination, and in green, those obtained from the somatic embryogenesis process.
The traditional germination process of seeds collected from the eight mother trees started with around 5 h of cone drying, continued for 30 h to process extracted seeds (extraction, de-winging, cleaning and H2O2 treatment), and finally, sowing/transplanting took around 50 h, resulting in a total of 85 h, estimated at 100 h per year. The time consumed in the SE process per year was as follows: 40 h for opening the cones, extracting the seeds, asepsis and in vitro manipulation to isolate and place the megagametophytes onto the culture medium; 210 h for subculturing the embryonal masses during initiation and proliferation, including registering all the data to preserve the traceability of the process; 140 h for weighing, resuspending and filtering the embryonal masses at maturation stage; 90 h for weighing, tissue preconditioning and dispensing for ultra-cold preservation of ECLs; 90 h to collect the somatic embryos from maturation plates including counting them; and 80 h to collect the germinated embryos and place them in culture boxes registering the number of plantlets obtained. For the different in vitro stages described, the time spent on preparing culture media and cleaning/sterilizing the materials was also included; in total, this came to 650 h, so it was estimated at around 700 h per year. The plantlet acclimatization process took the following time: 60 h for transplanting seedlings from the ecoboxes to the plug trays, and 40 h for passing plantlets from 330 cc trays. Other tasks that were necessary to ensure successful plant acclimatization, such as peat mix preparation, inventory or data collection and processing, were estimated at 30 h, for both processes.
Biochemical Analysis: Somatic vs. Zygotic Embryos
The germination of somatic embryos collected during maturation at weeks 13, 15, 17 and 19, and mature zygotic embryos revealed only minor differences, regardless of the embryo type, the genotype or the collection date; in all cases, germination rates over 85% were obtained. Slightly lower germination rates were observed when embryos were collected during maturation at weeks 13 (93% ± 0.0) or 15 (95% ± 2.2) when compared to those maturated during 17 or 19 weeks (100% ± 0.0).
When analyzing the results obtained for DW measurements, clear and significant differences were obtained between somatic and zygotic embryos (Table 1). The DW percentage of zygotic embryos was more than three times higher than that of somatic embryos. Even if smaller, significant differences were also observed within somatic embryos from different collection times (Table 1). As maturation time increases, so does the DW percentage of somatic embryos, especially during the first weeks (13, 15 and 17). Even if higher at week 19, no significant differences were observed between embryos from weeks 17 and 19.

Table 1.
Dry weight (DW) percentage and total protein content (mg g−1 DW) for mature zygotic embryos and somatic embryos maturated for different time periods, determined with three different genotypes.
Regarding the total protein content, significant differences were only detected between zygotic embryos and somatic embryos from early collection dates (13 and 15 weeks) (Tables S1 and S2 in Supplementary Materials). As a general trend, somatic embryos showed higher levels of total protein than zygotic embryos, but with a decreasing tendency as the maturation period extended. Nonetheless, no significant differences were observed among collection dates.
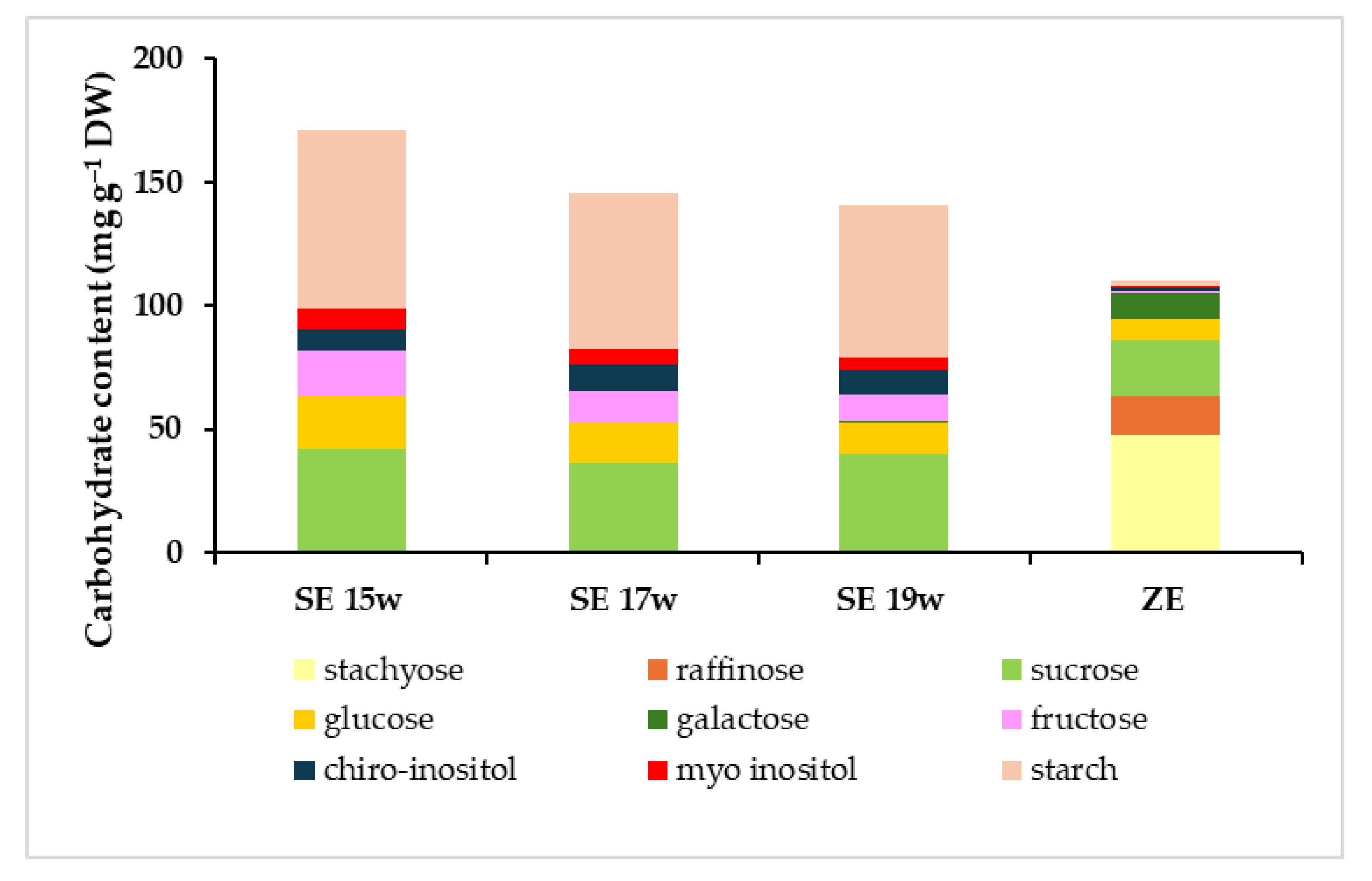
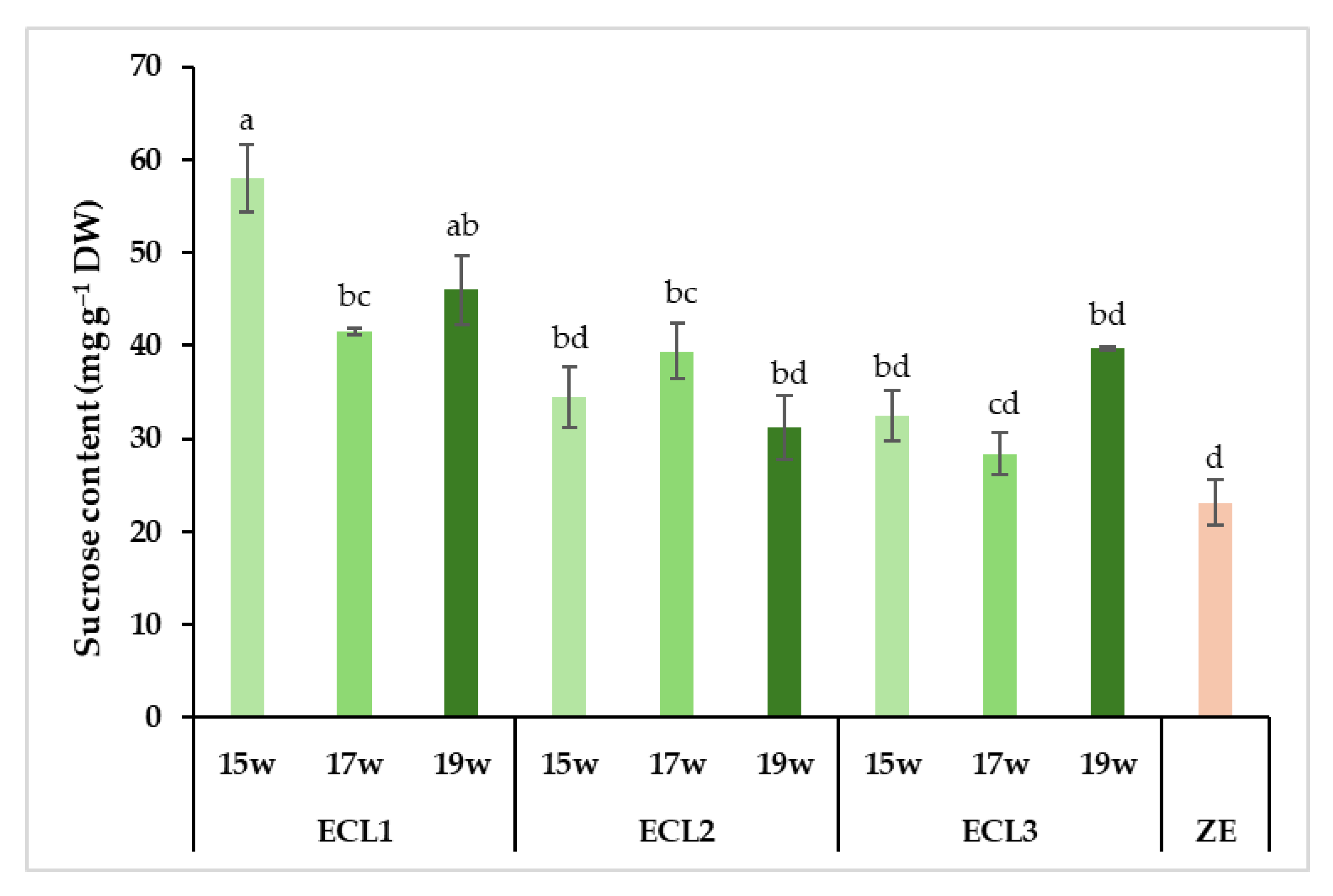
Carbohydrate analysis revealed significant differences in the total carbohydrate content between somatic and zygotic embryos (Figure 8). Zygotic embryos presented a total carbohydrate content (110 mg g−1 DW) that was lower than every somatic embryo stage analyzed (141 to 171 mg g−1 DW). In the latter, the total content appeared to decrease during the last weeks of maturation. The profile of the carbohydrates present was quite different between zygotic and somatic embryos, with more polysaccharides in the former and more monosaccharides in the latter (Figure 8). Regarding the total soluble carbohydrate profile (without starch), however, significantly lower levels were detected in somatic embryos from weeks 17 and 19 (82 and 79 mg g−1 DW, respectively), whereas no differences were observed between somatic embryos from week 15 and zygotic embryos (99 and 108 mg g−1 DW, respectively). The opposite behaviours observed between total carbohydrates and total soluble carbohydrates were caused by the marked differences in starch content between the two embryo types. In fact, somatic embryos from all collection dates showed more than thirty times higher starch content (62 to 73 mg g−1 DW) than zygotic embryos (2 mg g−1 DW, Figure 8). The highest values corresponded to somatic embryos from week 15, showing significantly higher starch levels than those from week 19 (Tables S2 and S3 in Supplementary Materials). This non-soluble carbohydrate was the most abundant carbohydrate in somatic embryos. A similar pattern between fructose, glucose and myo-inositol was observed in somatic embryos, with decreasing content according to maturation time (Figure 8; Table 2). Fructose and myo-inositol were almost absent in zygotic embryos (less than 1 mg g−1 DW). The quantity of chiro-inositol was stable in somatic embryos (9 to 10 mg g−1 DW) between the collection dates but was almost absent in zygotic embryos (1 mg g−1, Figure 8). As for sucrose, the second most abundant carbohydrate type in both embryo types, a significant interaction between genotype and condition was found (Table S4, see Supplementary Materials). In this sense, the differences observed were genotype-specific. The values observed were more similar between zygotic and somatic embryos, and also between collection dates (Figure 8). The only significant differences observed were genotype-specific, as they were detected for a single ECL (ECL 1, Figure 9). Interestingly, stachyose, which was not detected in somatic embryos, was the most abundant carbohydrate in zygotic embryos (Figure 8), representing more than 43% of the total carbohydrate content. Other sugars, such as raffinose or galactose, were only present in zygotic embryos (Figure 8) or at very low levels in somatic embryos from the last collection date (Table 2). None of these carbohydrate forms were detected in somatic embryos from weeks 15 and 17. As a result, zygotic embryos presented a very high raffinose family oligosaccharides (RFOs)/sucrose ratio (2.7) in comparison with the ratio observed in somatic embryos from the last collection date (0.02).
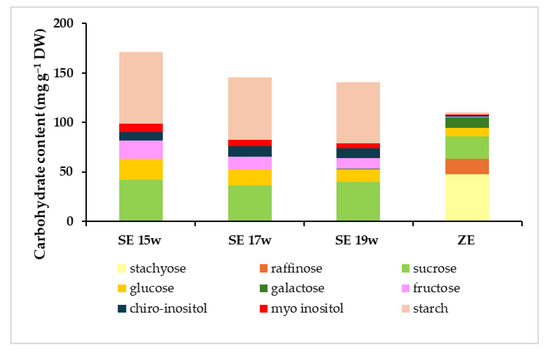
Figure 8.
Quantitative and qualitative profile of carbohydrate content of radiata pine zygotic embryos (ZE) or somatic embryos collected after 15 weeks (SE 15w), 17 weeks (SE 17w) and 19 weeks (SE 19w) on the maturation medium.

Table 2.
Different carbohydrate contents (mg g−1 DW) for mature zygotic embryos and somatic embryos maturated for different time periods, determined with three different genotypes. Data are presented as mean values ± se. Different lowercase letters within a row indicate significant differences among embryo types and collection dates at p < 0.05.
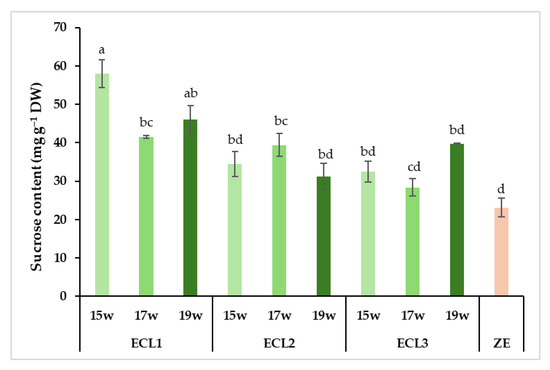
Figure 9.
Quantitative profile of sucrose content of radiata pine zygotic embryos (ZE) or somatic embryos from three different embryogenic cell lines (ECL 1, ECL 2 and ECL 3) collected after 15 weeks (15 w), 17 weeks (17 w) and 19 weeks (19 w) on the maturation medium. Different lowercase letters indicate significant differences among the interactions between the somatic embryos from different embryogenic cell lines, zygotic embryos and collection dates at p < 0.05. DW: dry weight.
4. Discussion
In this study, radiata pine plants were produced on a small scale via SE and traditional means. Starting with a similar number of seeds per mother tree, the number of acclimatized plants from each pathway was 1120 following traditional methodology and 3500 when SE was performed. The time consumed to carry out the SE process was much longer when compared to traditional germination [16]. Apart from the differences in the number of plantlets and time consumed, there are other variations depending on the production pathway chosen, as reported by [29]. First, it was not possible to obtain plant material from two mother trees. As mentioned in the Introduction of this work, it was decided to select mother trees that had not been previously tested for SE to mimic a more realistic scenario; this led to very different initiation rates depending on the mother tree, these variations have widely described in the literature [30] (and references therein); moreover, the SE initiation potential appears to be retained even in open-pollinated mother trees [9,31]. An alternative to including these non-represented female parents in an SE-based strategy could be to utilize them as male parent trees, as suggested by [32]; this alternative would imply controlled pollination, one of the next steps in our genetic improvement plan. However, it has been reported that controlled-pollinated cones tend to produce fewer seeds than open-pollinated cones [33]; so, to overcome this problem, SE could play an important role as well. Second, the somatic plantlets produced obviously came from a smaller number of genotypes when compared to traditional germination, in which each seed represents a different one. The ability of SE-derived plantations to cope with pests, given that they represent a narrower genetic background, has also been covered in different studies. Recent [34] and earlier [35] studies on this topic agree that an adequate number would be between 10 and 20 clones. Moreover, some authors suggested that increasing the number of genotypes beyond 20 unrelated clones does not contribute to reducing the risk of loss any more than it would in a wild population [36]. In our case, the plants obtained from the SE process came from 28 clones from six seed families, so it would be clearly feasible to introduce this number of clones in less than five years; the only concerns here would be to select unrelated parents for the next introductions of explants for the SE process.
The ECLs from which somatic plantlets were obtained were stored at −80 °C following [26]. Thus far, we have been able to recover them from the ultra-low freezer and regenerated plants after 6 years of storage [26], so it is likely that ECLs will maintain their regeneration potential until genomic and/or phenotypic analyses are carried out. This will lead, if desired, to the implementation of multi-varietal forestry and the production of seeds from selected plant material. As was stated by [37], a faster way to reduce the deployment timeline would be to use SE plantlets to establish new seed parents; this could reduce the deployment timeline with the aid of genomic selection from 17 years to 7 years.
Even if a higher number of plants was obtained through somatic embryogenesis, germination, and particularly the number of plantlets suitable to be planted ex vitro, could be considerably improved, as the percentage of plants suitable for ex vitro planting was 63%. This could maximize the productivity of the SE process. In this sense, successful germination and plant conversion of both somatic and zygotic embryos in conifers requires the accumulation of storage macromolecules, including carbohydrates and proteins [14]; although these contents were higher in somatic embryos, it is worth noting that during germination, zygotic embryos benefit from being surrounded by the megagamethophyte, which houses the majority of the storage reserves of the seeds, while somatic embryos lack it, this could be one of the reasons for lower levels of this metabolites found in zygotic embryos. The transition from dormancy to active growth is a demanding shift, which involves a deep reorganization of metabolism, in which reserve carbohydrates, lipids and proteins are key players in providing the energy and the structural components necessary for early development until the plant becomes autotrophic [38]. An adequate level and balance of these reserves, together with the synthesis of highly specific molecules, improve seed vigour, enhance stress tolerance and, thus, increase the probability of survival under natural conditions. Accordingly, the accumulation of high levels of certain types of carbohydrates and proteins leads to an increase in the dry mass of embryos, preventing the lethal effects of water stress (monosaccharides as glucose, participating in the regulation of osmotic pressure) and preparing them for desiccation (RFOs), a crucial step for many conifer species [39]. This desiccation tolerance is often acquired by a process induced by a drop in water content and then an increase in the DW percentage. Therefore, a comprehensive understanding of these processes is vital to optimize traditional seedling production and establishment practices, as well as new biotechnological and micropropagation approaches such as somatic embryogenesis.
In line with this idea, in this work, we analyzed the DW percentage of radiata pine somatic embryos after different weeks of maturation and compared these results to those of zygotic embryos extracted from cones stored for two years. This study demonstrated the enormous difference between somatic embryos and completely mature zygotic embryos in terms of dry matter percentage. Zygotic embryos presented DW percentage values more than three times higher (67–76%) than those of somatic embryos, regardless of the maturation period, which is in accordance with the results obtained in other conifer species [27,40]. On the other hand, differences in DW between collection dates, even if significant, were considerably smaller, reaching a maximum of 9% between embryos from the earliest and latest maturation dates. Interestingly, in previous studies carried out in our laboratory, we did not observe DW percentage differences between somatic embryos collected at week 16 of maturation and somatic embryos stored at 4 °C during 8 extra weeks [16]. These findings, however, do not correlate with germination rates. Even if slightly higher germination rates were obtained when embryos were left on the maturation medium for longer periods of time or stored under cold conditions, germination rates as high as the ones obtained in zygotic embryos under ex vitro conditions can be achieved with somatic embryos from every collection date. This suggests that the DW percentage or the water content is not a determining factor in radiata pine for in vitro germination of somatic embryos, as in Larix [41], contrasting what has been observed for other conifers [15].
No significant differences in the total amount of proteins were detected between somatic embryos collected at different time-points, although a slight decreasing tendency was observed (Table 1). The protein accumulation dynamics in conifer embryos seem to be species- and genotype-dependent. In Picea abies, results similar to those presented here were obtained when somatic embryos were kept on the maturation medium for prolonged periods of time [42]. In the Pinus genera, however, contrasting profiles have been obtained: in Pinus sylvestris, maturation periods exceeding 12 weeks provoked a drastic reduction in the protein content of somatic embryos [43], whereas in Pinus pinaster, embryos cultured on the maturation medium for long periods of time tended to accumulate more protein, even if the effect of the genotype could not be dismissed [27]. Similarly, opposing tendencies have also been observed when subjecting somatic embryos to different post-maturation treatments: desiccation typically reduces the protein content [42], whereas cold storage induces an increase [44].
The protein levels of zygotic embryos were lower than those of somatic embryos, but statistically significant only when compared to somatic embryos maturated during 13 and 15 weeks. Most of the available studies on this topic describe considerably higher amounts of proteins in zygotic embryos than in somatic embryos [45,46]. Similar protein contents have only been observed when comparing immature zygotic embryos and mature somatic embryos [27] or when subjecting somatic embryos to long cold storage periods [44]. Nonetheless, similarly to what happened with the DW, there is no clear connection between germination rates and the total protein content of embryos, which has already been observed in other conifer species. Some authors have pointed out the importance of the differential accumulation of specific protein groups, such as dehydrins, LEA proteins, HSPs and storage proteins (albumin or globulins), for a successful plant conversion, rather than the effect of the total protein pool [42,46,47].
Regarding the carbohydrate profile, somatic embryos presented higher total sugar contents than zygotic embryos, mainly attributed to the fact that somatic embryos contained very high levels of starch, whereas this polymeric carbohydrate was negligible in zygotic embryos. This is a common feature in most conifer species [44,48,49] and can be explained by the strong correlation between starch levels in somatic embryos and the sucrose and ABA utilized in the maturation culture medium [50]. In addition, the levels of starch tended to decrease along the maturation period. This has not been observed in other species after long maturation periods, but is observed when embryos start a desiccation process, suggesting that sucrose is a transient energy source that leads to the liberation of glucose acting as osmo-protectant molecules at the end of the maturation process that help to cope with water stress [42].
The second most abundant carbohydrate in both embryo types was sucrose, whose levels remained almost stable throughout the maturation period, contrasting with the profile of glucose, fructose and myo-inositol, which presented decreasing values. This carbohydrate distribution and dynamics have widely been observed in many species [40,51,52,53,54], suggesting that free hexoses are found at high concentrations during pro-embryogenic and pre-cotyledonary stages [55,56], where they interact with auxins and participate in hormone signalling to regulate cell proliferation [57]. Then, during cotyledonary embryo development, a shift towards the synthesis of disaccharides such as sucrose is prompted, which has a protective role by stabilizing proteins and membranes [58]. The capacity of sucrose to regulate osmotic pressure has been described as one of the most important factors determining germination capacity in conifer embryos [46]. The high and stable sucrose levels detected in zygotic and somatic embryos from all collection dates may be one of the factors explaining the successful germination rates obtained in all cases.
On the other hand, galactose and trisaccharides and oligosaccharides of RFOs, such as raffinose and stachyose, were found at relatively high levels only in zygotic embryos or at very low levels in somatic embryos from the latest collection date. The accumulation of these types of carbohydrates is common during the development of zygotic embryos of conifers, accounting for the main part of the carbohydrate spectrum, together with sucrose [42,54,59]. During SE, however, even if low levels of RFOs have been detected at the end of the maturation process of certain conifer species such as Pinus pinaster or Larix eurolepsis [27,40], these families of carbohydrates have been specifically linked to post-maturation treatments such as desiccation or cold storage [44,52,53,60] when they reach high values and act as strong osmotic stress protectants, enhancing the role of sucrose [61]. Because of this property, several authors have highlighted the importance of a proper RFO/sucrose ratio during embryo germination [46], but this idea is not supported by the results of this study, as zygotic and somatic embryos presented very different RFO/sucrose ratios without differences in germination.
5. Conclusions
In this study, we have demonstrated that starting with a similar number of seeds, plant production can vary considerably depending on the methodology used. Each method has advantages and disadvantages that have been described throughout the manuscript; traditional germination is more economical, but can present problems such as seed scarcity or unavailability of selected material. Somatic embryogenesis is more expensive, but allows for mass propagation of selected material; furthermore, propagated material can be tested in the field, recovered from cryostorage and propagated again. However, in both techniques, several stages such as germination and plant establishment could still be optimized, and the biochemical analysis revealed that, unlike protein, the carbohydrate profile of embryos is a determining factor, suggesting that sucrose is a candidate factor involved in the successful germination of both zygotic and somatic embryos in radiata pine. Studying the storage molecule profile in ECLs with low germination rates or in aberrant embryos could provide new insights for the improvement of the process. Monitoring the protein and carbohydrate spectrum during germination could also help explain the relatively low percentage of somatic plants suitable for ex vitro planting and thus help find alternatives to maximize the productivity of the SE process. Finally, additional experiments, such as genomic or transcriptomic analyses, could be carried out to try to unravel the genotypic dependence of not only the success of the different stages of SE, but also the biochemical profile of the generated plant material.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/seeds4030041/s1, Table S1: selected radiata pine locations; Figure S1: somatic embryos collected at weeks 13 (A), 15 (B), 17 (C) and 19 (D) of maturation and mature zygotic embryos (E) used for the biochemical analyses (bar = 1 mm); Figure S2: chromatogram showing a superposition of a mixture of standards (equimolar), in blue, and one the samples (ECL 1), in green; Table S2: ANOVA for protein content. Condition: 15, 17 19 weeks somatic embryos or zygotic embryos); Table S3: ANOVA for glucose, fructose and chiro-inositol contents. Condition: 15, 17 19 weeks somatic embryos or zygotic embryos; Table S4: ANOVA for myo-inositol, starch and sucrose contents. Condition: 15, 17 19 weeks somatic embryos or zygotic embryos).
Author Contributions
Conceptualization: I.A.M. and P.M.; Funding acquisition: P.M., J.H. and I.A.M.; Investigation: A.C.-O., C.T., J.H. and I.A.M.; Data curation: A.C.-O., J.H., C.T. and I.A.M.; Formal analysis: A.C.-O., C.T. and J.H.; Visualization: A.C.-O., J.H. and I.A.M.; Writing—original draft: A.C.-O., J.H., I.A.M. and P.M.; Writing—review and editing: A.C.-O., C.T., I.A.M. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was performed through EUSKOBASOA 2050 R&D&I Programme (BASOAES II 22-00024 and FORESTEK B 21-00005 Projects) funded by the Basque Government. Project PID2020-112627RB-C32 funded by MICIU/AEI/10.13039/501100011033. MULTIFOREVER project, supported under the umbrella of ERA-NET Cofund ForestValue by ANR(FR), FNR (DE), MINCyT (AR), MINECO-AEI (ES), MMM (FI) and VINNOVA (SE). ForestValue has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement no. 773324. The authors would like to acknowledge COST Action CA21157 “European Network for Innovative Woody Plant Cloning”, www.copytree.eu (accessed on 12 March 2025), supported by COST (European Cooperation in Science and Technology) www.cost.eu (accessed on 12 March 2025).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors would like to thank the Phenobois facility for its contribution to the carbohydrate assay (Phenobois, INRAE, 2018. Wood and Tree Physicochemical Phenotyping Facility for Genetic Resources, https://doi.org/10.15454/1.5572410490640864E12).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- FAO. The State of World’s Forests. 2024. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/768ba59e-c692-47c3-9a13-3c3c10993396/content/cd1211en.html#gsc.tab=0 (accessed on 24 February 2025).
- Fenning, T.M.; Gershenzon, J. Where will the Wood come from? Plantation forests and role of biotechnology. Trends Biotechnol. 2002, 20, 291–296. [Google Scholar] [CrossRef]
- Fenning, T. The use of tissue culture and in-vitro approaches for the study of tree diseases. Plant Cell Tissue Organ Cult. 2019, 136, 415–430. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Gresham, B.A.; Meurisse, N.; Nahrung, H.F.; Perret-Gentil, A.; Pugh, A.R.; Sopow, S.L.; Turner, R.M. Pining away and at home: Global utilisation of Pinus radiata by native and non-native insects. NeoBiota 2023, 84, 137–167. [Google Scholar] [CrossRef]
- El Bosque Vasco en Cifras. Informe de HAZI Fundazioa Sobre el Inventario Forestal del País Vasco. 2022. Available online: http://www.nasdap.net/inventarioforestal (accessed on 24 February 2025).
- Mesanza, N.; García-García, D.; Raposo, E.R.; Raposo, R.; Iturbide, M.; Pascual, M.T.; Barrena, I.; Urkola, A.; Berano, N.; Sáez de Zerain, A.; et al. Weather variables associated with spore dispersal of Lecanosticta acicola causing pine needle blight in northern Spain. Plants 2021, 10, 2788. [Google Scholar] [CrossRef]
- Park, Y.S.; Barrett, J.D.; Bonga, J.M. Application of somatic embryogenesis in high-value clonal forestry: Deployment, genetic control, and stability of cryopreserved clones. Vitr. Cell Dev. Biol. Plant 1998, 34, 231–239. [Google Scholar] [CrossRef]
- Vidal, N.; Sánchez, C. Use of bioreactor systems in the propagation of forest trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef]
- Nielsen, U.B.; Hansen, C.B.; Hansen, U.; Johansen, V.K.; Egertsdotter, U. Accumulated effects of factors determining plant development from somatic embryos of Abies nordmanniana and Abies bornmuelleriana. Front. Plant Sci. 2022, 13, 989484. [Google Scholar] [CrossRef]
- Pavese, V.; Moglia, A.; Abbà, S.; Milani, A.; Torello Marinoni, D.; Corredoira, E.; Martínez, M.; Botta, R. First report on genome editing via ribonucleoprotein (RNP) in Castanea sativa Mill. Int. J. Mol. Sci. 2022, 23, 5762. [Google Scholar] [CrossRef]
- Bonga, J.M. A comparative evaluation of the application of somatic embryogenesis, rooting of cuttings, and organogenesis of conifers. Can. J. For. Res. 2015, 45, 379–383. [Google Scholar] [CrossRef]
- Park, Y.-S.; Beaulieu, J.; Bousquet, J. Multi-varietal forestry integrating genomic selection and somatic embryogenesis. In Vegetative Propagation of Forest Trees; Park, Y.-S., Bonga, J.M., Moon, H.-K., Eds.; National Institute of Forest Science: Seoul, Republic of Korea, 2016; pp. 302–322. [Google Scholar]
- Chamberland, V.; Robichaud, F.; Perron, M.; Gélinas, N.; Bousquet, J.; Beaulieu, J. Conventional versus genomic selection for white spruce improvement: A comparison of costs and benefits of plantations on Quebec public lands. Tree Genet. Genomes 2020, 16, 17. [Google Scholar] [CrossRef]
- Trontin, J.-F.; Klimaszewska, K.; Morel, A.; Hargreaves, C.; Lelu-Walter, M.-A. Molecular aspects of conifer zygotic and somatic embryo development: A review of genome-wide approaches and recent insights. In In Vitro Embryogenesis in Higher Plants; Germana, M.A., Lambardi, M., Eds.; Springer: New York, NY, USA, 2016; pp. 167–207. [Google Scholar]
- Stasolla, C.; Yeung, E.C. Recent advances in conifer somatic embryogenesis: Improving somatic embryo quality. Plant Cell Tissue Organ Cult. 2003, 74, 15–35. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Montalbán, I.A.; Moncaleán, P. Multi-strategy approach towards optimization of maturation and germination in radiata pine somatic embryogenesis. Plant Cell Tissue Organ Cult. 2023, 153, 173–190. [Google Scholar] [CrossRef]
- Cooke, J.; Cooke, B.; Gifford, D. Loblolly pine seed dormancy: Constraints to germination. New For. 2002, 23, 239–256. [Google Scholar] [CrossRef]
- Barnett, L.B.; Adams, R.E.; Ramsey, J.A. The effect of stratification on in vitro protein synthesis in seeds of Pinus lambertiana. Life Sci. 1974, 14, 653–658. [Google Scholar] [CrossRef]
- Noland, T.L.; Murphy, J.B. Protein synthesis and aminopeptidase activity in dormant sugar pine seeds during stratification and warm incubation. J. Plant Physiol. 1986, 124, 1–10. [Google Scholar] [CrossRef]
- Mullen, R.T.; King, J.E.; Gifford, D.J. Changes in mRNA populations during loblolly pine (Pinus taeda L.) seed stratification, germination, and post-germinative growth. Physiol. Plant. 1996, 9, 545–553. [Google Scholar] [CrossRef]
- Einali, A.; Valizadeh, J. Storage reserve mobilization, gluconeogenesis, and oxidative pattern in dormant pistachio (Pistacia vera L.) seeds during cold stratification. Trees 2016, 31, 659–671. [Google Scholar] [CrossRef]
- Burdon, R.D. Introduced forest trees in New Zealand: Recognition, role, and seed source. Radiata pine (Pinus radiata D. Don). In Forest Research Institute Bulletin; Miller, J.T., Ed.; New Zealand Forest Research Institute Ltd.: Rotorua, New Zealand, 1992; Volume 124, pp. 1–59. [Google Scholar]
- Escobar, R.; Sanchez, M.; Pereira, G. Forest nursery management in Chile. In National Proceedings: Forest and Conservation Nursery Associations-1999, 2000, and 2001; Dumroese, R.K., Riley, L.E., Landis, T.D., Eds.; Proceedings Rocky Mountain Research Station; US Department of Agriculture Forest Service: Ogden, UT, USA, 2002; Volume 24, pp. 219–225. [Google Scholar]
- Modi, N.R.; Radadiya, B.; Ghanchi, M.H. Effect of pre-sowing chemical treatment on selected seeds: For enhancing germination. Int. J. Sci. Res. Tech. 2025, 2, 124–129. [Google Scholar]
- Barnett, J.P.; Varela, S. A review of chemical treatments to improve germination of longleaf pine seeds. Native Plants J. 2004, 5, 18–24. [Google Scholar] [CrossRef]
- Sandoval, K.P.; Castander-Olarieta, A.; Moncaleán, P.; Montalbán, I.A. Assessment of alternative freezing methods for preservation at −80 °C of radiata pine embryogenic cultures: A six-year study. Cryobiology 2025, 119, 105217. [Google Scholar] [CrossRef]
- Morel, A.; Trontin, J.F.; Corbineau, F.; Lomenech, A.M.; Beaufour, M.; Reymond, I.; Le Metté, C.; Ader, K.; Harvengt, L.; Cadene, M.; et al. Cotyledonary somatic embryos of Pinus pinaster Ait. most closely resemble fresh, maturing cotyledonary zygotic embryos: Biological, carbohydrate and proteomic analyses. Planta 2014, 240, 1075–1095. [Google Scholar] [CrossRef]
- Gautier, F.; Label, P.; Eliášová, K.; Leplé, J.C.; Motyka, V.; Boizot, N.; Vondráková, Z.; Malbeck, J.; Trávnícková, A.; Le Metté, C.; et al. Cytological, biochemical and molecular events of embryogenic state in Douglas-fir (Pseudotsuga menziesii [Mirb.]). Front. Plant Sci. 2019, 10, 118. [Google Scholar] [CrossRef]
- Lelu-Walter, M.A.; Thompson, D.; Harvengt, L.; Sanchez, L.; Toribio, M.; Pâques, L.E. Somatic embryogenesis in forestry with a focus on Europe: State-of-art, benefits, challenges and future direction. Tree Genet. Genomes 2013, 9, 883–899. [Google Scholar] [CrossRef]
- Egerstdotter, U. Plant physiological and genetical aspects of the somatic embryogenesis process in conifers. Scand. J. Forest Res. 2019, 34, 360–369. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Zhu, L.; Liu, X.; Wang, Q.; Ye, J. Evaluation of somatic embryo production during embryogenic tissue proliferation stage using morphology, maternal genotype, proliferation rate and tissue age of Pinus thunbergii Parl. J. For. Res. 2022, 33, 445–454. [Google Scholar] [CrossRef]
- MacKay, J.J.; Becwar, M.R.; Park, Y.S.; Corderro, J.P.; Pullman, G.S. Genetic control of somatic embryogenesis initiation in loblolly pine and implications for breeding. Tree Genet. Genomes 2006, 2, 1–9. [Google Scholar] [CrossRef]
- Heine, A.J.; Walker, T.D.; Jett, J.B.; Isik, F.; McKeand, S.E. Pollination bag type affects ovule development and seed yields in Pinus taeda L. For. Sci. 2022, 69, 187–189. [Google Scholar] [CrossRef]
- Wu, H.X. Benefits and risks of using clones in forestry-A review. Scand. J. Forest Res. 2019, 34, 352–359. [Google Scholar] [CrossRef]
- Bishir, J.; Roberds, J. Limit theorems and a general framework for risk analysis in clonal forestry. Math. Biosci. 1997, 142, 1–11. [Google Scholar] [CrossRef]
- Burdon, R.D.; Aimers-Halliday, J. Managing risk in clonal forestry. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2006, 1, 1–9. [Google Scholar] [CrossRef]
- McLean, D.; Apiolaza, L.; Paget, M.; Klápště, J. Simulating deployment of genetic gain in a radiata pine breeding program with genomic selection. Tree Genet. Genomes 2023, 19, 33. [Google Scholar] [CrossRef]
- Carlsson, J.; Egertsdotter, U.; Ganeteg, U.; Svennerstam, H. Nitrogen utilization during germination of somatic embryos of Norway spruce: Revealing the importance of supplied glutamine for nitrogen metabolism. Trees Struct. Funct. 2019, 33, 383–394. [Google Scholar] [CrossRef]
- Maruyama, T.E.; Hosoi, Y. Post-maturation treatment improves and synchronizes somatic embryo germination of three species of Japanese pines. Plant Cell Tissue Organ Cult. 2012, 110, 45–52. [Google Scholar] [CrossRef]
- Savane, P.; Belmokhtar, N.; Delile, A.; Boizot, N.; Ridel, C.; Lelu-Walter, M.A.; Teyssier, C. Characterization of hybrid larch somatic embryo maturation by biochemical analyses and by a novel, fast mid-infrared approach. Physiol. Plant. 2023, 175, e13966. [Google Scholar] [CrossRef]
- Lelu-Walter, M.A.; Pâques, L.E. Simplified and improved somatic embryogenesis of hybrid larches (Larix × eurolepis and Larix × marschlinsii). Perspectives for breeding. Ann. For. Sci. 2009, 66, 104. [Google Scholar] [CrossRef]
- Eliášová, K.; Konrádová, H.; Dobrev, P.I.; Motyka, V.; Lomenech, A.M.; Fischerová, L.; Lelu-Walter, M.A.; Vondráková, Z.; Teyssier, C. Desiccation as a post-maturation treatment helps complete maturation of Norway spruce somatic embryos: Carbohydrates, phytohormones and proteomic status. Front. Plant Sci. 2022, 13, 823617. [Google Scholar] [CrossRef]
- Lelu-Walter, M.A.; Bernier-Cardou, M.; Klimaszewska, K. Clonal plant production from self- and cross-pollinated seed families of Pinus sylvestris (L.) through somatic embryogenesis. Plant Cell Tissue Organ Cult. 2008, 92, 31–45. [Google Scholar] [CrossRef]
- Välimäki, S.; Teyssier, C.; Tikkinen, M.; Delile, A.; Boizot, N.; Varis, S.; Lelu-Walter, M.A.; Aronen, T. Norway spruce somatic embryogenesis benefits from proliferation of embryogenic tissues on filter discs and cold storage of cotyledonary embryos. Front. Plant Sci. 2022, 13, 1031686. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Morency, F.; Jones-Overton, C.; Cooke, J. Accumulation pattern and identification of seed storage proteins in zygotic embryos of Pinus strobus and in somatic embryos from different maturation treatments. Physiol. Plant. 2004, 121, 682–690. [Google Scholar] [CrossRef]
- Businge, E.; Bygdell, J.; Wingsle, G.; Moritz, T.; Egertsdotter, U. The effect of carbohydrates and osmoticum on storage reserve accumulation and germination of Norway spruce somatic embryos. Physiol. Plant. 2013, 149, 273–285. [Google Scholar] [CrossRef]
- Brownfield, D.L.; Todd, C.D.; Stone, S.L.; Deyholos, M.K.; Gifford, D.J. Patterns of storage protein and triacylglycerol accumulation during loblolly pine somatic embryo maturation. Plant Cell Tissue Organ Cult. 2007, 88, 217–223. [Google Scholar] [CrossRef]
- Joy, R.W.; Yeung, E.C.; Kong, L.; Thorpe, T.A. Development of white spruce somatic embryos: I. storage product deposition. Vitr. Cell. Dev. Biol. Plant 1991, 27, 32–41. [Google Scholar] [CrossRef]
- Tereso, S.; Zoglauer, K.; Milhinhos, A.; Miguel, C.; Oliveira, M.M. Zygotic and somatic embryo morphogenesis in Pinus pinaster: Comparative histological and histochemical study. Tree Physiol. 2007, 27, 661–669. [Google Scholar] [CrossRef]
- Hazubska-Przybył, T.; Kalemba, E.M.; Ratajczak, E.; Bojarczuk, K. Effects of abscisic acid and an osmoticum on the maturation, starch accumulation and germination of Picea spp. somatic embryos. Acta Physiol. Plant. 2016, 38, 59. [Google Scholar] [CrossRef]
- Lipavská, H.; Svobodová, H.; Albrechtová, J.; Kumstýřová, L.; Vágner, M.; Vondráková, Z. Carbohydrate status during somatic embryo maturation in Norway spruce. Vitr. Cell. Dev. Biol. Plant 2000, 36, 260–267. [Google Scholar] [CrossRef]
- Kubes, M.; Drazna, N.; Konradova, H.; Lipavska, H. Robust carbohydrate dynamics based on sucrose resynthesis in developing Norway spruce somatic embryos at variable sugar supply. Vitr. Cell. Dev. Biol. Plant 2014, 50, 45–57. [Google Scholar] [CrossRef]
- Hudec, L.; Konrádová, H.; Hašková, A.; Lipavská, H. Norway spruce embryogenesis: Changes in carbohydrate profile, structural development and response to polyethylene glycol. Tree Physiol. 2016, 36, 548–561. [Google Scholar] [CrossRef]
- Goeten, D.; Farias-Soares, F.L.; Rogge-Renner, G.D.; Pereira, M.L.T.; Walters, C.; Silveira, V.; Santa Catarina, C.; Guerra, M.P.; Steiner, N. Carbohydrate and dehydrin-like protein profiles during Araucaria angustifolia seed development provides insights toward ex situ conservation. Trees 2023, 37, 1201–1215. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Pereira, C.; Mendes, V.M.; Correia, S.; Manadas, B.; Canhoto, J.; Montalbán, I.A.; Moncaleán, P. Thermopriming associated proteome and sugar content responses in Pinus radiata embryogenic tissue. Plant Sci. 2022, 321, 11132. [Google Scholar] [CrossRef]
- Navarro, B.V.; Elbl, P.; De Souza, A.P.; Jardim, V.; de Oliveira, L.F.; Macedo, A.F.; dos Santos, A.L.W.; Buckeridge, M.S.; Floh, E.I.S. Carbohydrate-mediated responses during zygotic and early somatic embryogenesis in the endangered conifer, Araucaria angustifolia. PLoS ONE 2017, 12, e0180051. [Google Scholar] [CrossRef]
- Wang, L.; Ruan, Y. Regulation of cell division and expansion by sugar and auxin signaling. Front. Plant Sci. 2013, 4, 163. [Google Scholar] [CrossRef]
- Ballesteros, D.; Pritchard, H.W.; Walters, C. Dry architecture: Towards the understanding of the variation of longevity in desiccation-tolerant germplasm. Seed Sci. Res. 2020, 30, 142–155. [Google Scholar] [CrossRef]
- Pullman, G.S.; Buchanan, M. Identification and quantitative analysis of stage-specific carbohydrates in loblolly pine (Pinus taeda) zygotic embryo and female gametophyte tissues. Tree Physiol. 2008, 28, 985–996. [Google Scholar] [CrossRef]
- Konradova, H.; Grigova, M.; Lipavska, H. Cold-induced accumulation of raffinose family oligosaccharides in somatic embryos of Norway spruce (Picea abies). Vitr. Cell. Dev. Biol. Plant 2003, 39, 425–427. [Google Scholar] [CrossRef]
- Obendorf, R.L. Oligosaccharides and galactosyl cyclitols in seed desiccation tolerance. Seed Sci. Res. 1997, 7, 63–74. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).