Abstract
Increasing demand for high-quality food is driving the development of biologized farming methods, which involve the use of microorganisms, including endophytes, to stimulate plant growth. However, research on the composition of endosphere microbiomes is limited. The study presents an analysis of the bacterial endophytic microbiome in lettuce seeds (Lactuca sativa L., cv. Ozornik) using high-throughput sequencing of 16S rRNA amplicons. It evaluates the taxonomic composition and putative functional properties of seed endophytic bacteria. The microbial community exhibited low diversity (Shannon index ranged from 1.1 to 1.84, Simpson index from 0.57 to 0.83). The bacterial endophytic community of lettuce seeds was dominated by Pseudomonadota (83%), Actinomycetota (14%), and Bacillota (3%). The genera identified within the microbiome included Pantoea (32%), Rhodococcus (13%), Candidatus Profftella (13%), Janthinobacterium (7%), Pseudomonas (9%), Enterococcus (3%), and Alcaligenes (2%), which exhibit a broad spectrum of beneficial properties: plant growth promotion (PGPB), suppression of phytopathogens, enhanced stress tolerance, participation in contaminant biodegradation, and heavy metal detoxification. The structure and functional potential of the microbiome vary between samples, potentially due to differences in source material and cultivation conditions. The obtained results expand our understanding of the composition and functions of endophytic bacteria in lettuce seeds, which is important for the development of novel biocontrol agents for plants consumed by humans in an unprocessed form.
1. Introduction
The seed endophytic microbiome refers to communities of microorganisms, predominantly bacteria and fungi, residing within the internal tissues of seeds without causing disease [1]. These microbiomes differ from those colonizing the external surface of seeds (epiphytes) and are characterized by a close association with the embryo and endosperm, which facilitates vertical transmission (from parent to offspring) of the microbiota across plant generations [2,3]. Seed endophytes are more likely to be inherited by subsequent generations, potentially forming the initial microbial community of the seedlings.
Seed endophytic microbiomes often include representatives from various taxa such as Proteobacteria, Actinobacteria, Firmicutes, and Bacteroidetes. Among the most frequently identified genera are Bacillus, Pseudomonas, Paenibacillus, Micrococcus, Acinetobacter, and Staphylococcus [2,4]. The seed microbiome plays a crucial role in shaping plant health, influencing growth, resistance to pathogens, and adaptation to environmental conditions [5,6]. Endophytic microbes are often the primary colonizers of germinating seedlings, contributing to the early establishment of the microbiome and protecting seedlings from pathogenic microbes through competitive exclusion or colonization [1,2,7,8]. Many endophytes produce phytohormones, such as indole-3-acetic acid, secrete enzymes like ACC deaminase (which reduces plant stress hormone levels), and assist in breaking seed dormancy, thereby directly enhancing seed viability, germination rate, and seedling vigor [2,7,9]. Seed endophytes facilitate nutrient uptake by plants through atmospheric nitrogen fixation, phosphorus mobilization, siderophore production (iron chelators), and secretion of organic acids that solubilize minerals from the surrounding environment, providing competitive advantages under nutrient-limited conditions [10]. Seed endophytes also help plants cope with abiotic stresses—such as drought, salinity, and extreme temperatures—by modulating host physiology and activating systemic resistance mechanisms. These enhancements in stress tolerance and adaptive responses are particularly critical during the early stages of plant development, when seedlings are most vulnerable [2,9]. The application of beneficial seed endophytes via seed treatment or coating is being investigated as an alternative to chemical seed treatments, offering promising avenues for sustainable plant protection and yield improvement.
In recent years, increasing attention has been paid to the composition and functions of microbial communities associated with the seeds of agricultural crops [11]. The use of biocontrol agents containing seed endophytes for seed pretreatment is considered an alternative to chemical treatments, offering opportunities for sustainable plant protection and yield enhancement while simultaneously reducing the environmental pesticide burden. Lettuce (Lactuca sativa L.) is a widely consumed vegetable, often eaten raw, which increases the risk of foodborne diseases in humans [12,13]. Accordingly, the microbiome of Lactuca sativa L. seeds is attracting growing interest due to its potential impact on plant health and the safety of products consumed unprocessed by humans. Seeds serve not only as a reservoir for the transmission of microorganisms to the next plant generation, but also as a potential source of beneficial microbes capable of enhancing crop productivity and stress tolerance [14,15]. Despite the growing interest in phytomicrobiomes, the composition and functional significance of the lettuce seed microbiome remain poorly understood [16]. Low microbial biomass and diversity in seeds complicate cultivation-independent studies and often lead to pooled analyses that obscure intraspecific variability within seeds [1]. The functional roles of individual seed-transmitted endophytes remain poorly understood, particularly regarding their contributions to the host’s adaptability to various environmental conditions [17,18]. Modern molecular genetic techniques, such as high-throughput sequencing of 16S rRNA and ITS regions, enable detailed analysis of microbial diversity, identification of dominant bacterial and fungal taxa, and assessment of their potential roles in early plant development [19]. However, the principles governing the formation of endophytic microbial communities, the transmission pathways, and the ecological interactions between seed endophytes and phytopathogens in crops such as lettuce remain insufficiently understood. Studying the lettuce seed microbiome is not only of theoretical interest, but also of practical importance for the development of biological products based on beneficial microorganisms that enhance seed germination and plant resistance to biotic and abiotic stresses [20,21].
The aim of this study is a comprehensive analysis of the microbial community of L. sativa seeds using modern metagenomic methods to identify key taxa that may influence plant growth and development and, subsequently, they can be used as environmentally friendly biological control agents. Such biocontrol agents reduce reliance on chemical pesticides and enhance food safety. Additionally, the integration of seed microbiome knowledge into breeding and production processes can facilitate the development of crop varieties with improved pathogen resistance, constituting a part of a holistic approach to plant health management.
2. Materials and Methods
2.1. Seed Preparation
For the experiment, lettuce seeds (Lactuca sativa var. crispa L.) of the Ozornik variety, produced by Agrofirma AELITA LLC, Moscow, Russia, were selected. The seeds were produced in 2023 and packaged in 2024. For the study, seeds from a single lot were used to exclude heterogeneity caused by different seed production conditions. To assess the endophytic bacterial microbiome of the seeds, surface sterilization was performed. Specifically, lettuce seeds were treated with 2.5% sodium hypochlorite for 2 min, rinsed with sterile tap water, and then immersed in 70% ethanol for 3 min. The sterilized seeds were thoroughly washed five times with sterile tap water. The final rinse water was plated onto Petri dishes containing LB agar. In the absence of bacterial or fungal colony growth, the seed sterilization process was considered successful, and such seeds were used for subsequent DNA extraction from the endosphere microbiome [22].
2.2. DNA Extraction, Amplicon Library Preparation and Sequencing
The MoBio PowerSoil DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA) was used for DNA extraction according to the manufacturer’s instructions. DNA concentration and quality were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). The bacterial community was sequenced using Illumina technology (Illumina, San Diego, CA, USA). Genomic library preparation was performed following the 16S metagenomic sequencing library preparation protocol (Illumina MiSeq, San Diego, CA, USA). The V3–V4 region of the 16S rRNA gene was amplified using a DNA Engine Tetrad® 2 thermal cycler (Bio-Rad, Hercules, CA, USA) with the following primers: Illumina_16S_341F (TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG) and Illumina_16S_805R (GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC). Amplification conditions were as follows: initial denaturation at 95 °C for 3 min; 27 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; followed by a final elongation at 72 °C for 3 min. Amplicons were purified using the Agencourt AMPure XP kit (Beckman Coulter, Brea, CA, USA). A second round of amplification was performed under the same conditions. Amplicon concentration was measured with a Qubit 3.0 fluorometer (Invitrogen, Carlsbad, CA, USA) using the Quant-iT™ high-sensitivity DNA assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Quality control of the amplicons was conducted on a LabChip GX Touch 24 system (PerkinElmer, Waltham, MA, USA). Sequencing was performed on a MiSeq instrument (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. The 16S rRNA sequencing data were analyzed using the DADA2 algorithm [23]. Raw paired-end reads were processed with the filterAndTrim function from the DADA2 pipeline to remove low-quality sequences and adapter contamination. Forward (R1) and reverse (R2) reads were trimmed to fixed lengths of 240 and 225 base pairs, respectively, to eliminate low-quality regions at the 3′ ends. Sequences containing ambiguous bases (N) were discarded (maxN = 0), and reads with an expected error rate higher than 2 (maxEE = c(2, 2)) were filtered out. To correct for sequencing errors, error profiles for forward and reverse reads were independently estimated using the learnErrors function. The accuracy of the error models was visually assessed with plotErrors, by comparing observed error rates to the expected quality scores (nominalQ = TRUE). Subsequently, the dada function was applied for sample-specific error correction and denoising, generating exact amplicon sequence variants (ASVs). Forward and reverse reads were merged using mergePairs. Merged sequences were compiled into an ASV abundance table using makeSequenceTable (DADA2 version 1.34.0), and the length distribution of sequences was examined. Potential chimeric sequences were identified and removed via the removeBimeraDenovo function with the consensus method, ensuring retention of only biologically valid ASVs. Finally, taxonomic classification was performed using the SILVA v138.2 reference database through the assignTaxonomy function. Prior to downstream analyses, all ASVs identified as “chloroplast” or “mitochondria” were removed.
2.3. Statistical Analysis
Further statistical analysis of the relative abundance data of amplicon sequence variants was performed using the free R software environment (version 4.4.3). Alpha diversity of bacterial communities was assessed using the Shannon and Simpson indices via the diversity function of the vegan package.
3. Results
Taxonomic Composition of the Endophytic Bacterial Community of Lettuce Seeds
Metagenomic sequencing of amplicons yielded 65,377, 73,471, and 60,108 raw reads for the 16S rRNA gene in the respective samples. After quality filtering using the filterAndTrim function, 40,515, 46,073, and 36,096 reads were retained, respectively. The DADA2 algorithm identified 6006, 6509, and 5604 unique sequences in the samples. It is noteworthy that all samples contained a high proportion of reads identified as lettuce chloroplast (39%) and mitochondrial (43%) sequences, which were removed prior to analysis. A total of 18 amplicon sequence variants (ASVs) were obtained across all samples after processing. Taxonomic composition of the bacterial endophytic bacterial microbiome of lettuce seeds is presented in Table 1. The bacterial community of the lettuce seed endosphere was dominated by representatives of the phylum Pseudomonadota (83% relative abundance), Actinomycetota (14%), and Bacillota (3%). The phylum Pseudomonadota was represented by the class Gammaproteobacteria, which included the orders Enterobacterales, Burkholderiales, and Pseudomonadales. The phylum Actinomycetota was represented by the class Actinobacteria, comprising the order Mycobacteriales. The phylum Bacillota was represented by the class Bacilli, including the order Lactobacillales. The endospheric microbial community of lettuce seeds included representatives of the genera Pantoea (32%), Rhodococcus (13%), Candidatus Profftella (13%), Janthinobacterium (7%), Pseudomonas (9%), Enterococcus (3%), and Alcaligenes (2%). The Shannon diversity indices for the three samples were 1.84, 1.51, and 1.1, respectively. The Simpson indices were 0.83, 0.76, and 0.57 for the three samples.

Table 1.
Taxonomic composition of the bacterial endophytic bacterial microbiome of lettuce seeds (Lactuca sativa L.) revealed by 16S rRNA gene amplicon sequencing. Relative abundance is indicated as a percentage (%). Values are presented for three independent samples (C1–C3). Taxa identified as “chloroplast” or “mitochondria” are not included. NA—data not identified.
4. Discussion
At the next stage, the beneficial properties of lettuce seed endophytes were analyzed (Table 2). Bacteria of the genus Pantoea are common components of the seed microbiome and perform several key functions that promote seed germination and plant growth. In particular, numerous studies have demonstrated that Pantoea spp. are plant growth-promoting bacteria (PGPB) due to their ability to produce phytohormones such as auxins, gibberellins, and cytokinins, which stimulate root system development and seed germination. They also synthesize siderophores that enhance iron availability for the plant and fix atmospheric nitrogen, thereby improving soil nutritional value [21,24,25].

Table 2.
Beneficial properties of endophytic bacterial microbiome. Reported functions from the literature include the ability to stimulate plant growth (PGPB), suppress phytopathogens, enhance tolerance to abiotic stresses, accelerate seed germination, and detoxify pollutants. Symbols: ‘+’—confirmed activity; empty cells—no data available. Percentages in parentheses represent the average relative abundance of each genus in the microbiome.
Furthermore, some strains of Pantoea exhibit antagonistic activity against phytopathogenic fungi and bacteria by producing antimicrobial compounds (e.g., pantocin and herbicides) and through strong competition for nutrients and colonization niches [16,26]. The synthesis of osmoprotectants, such as proline, enables increased plant tolerance to abiotic stresses including drought and salinity [19]. Additionally, Pantoea has been reported to enhance plant biotic resistance via the induction of systemic resistance (ISR) [20]. Studies have shown that Pantoea agglomerans and related species can accelerate lettuce seed germination by degrading growth inhibitors in the seed coat and activating metabolic processes within the embryo [14,15].
Bacteria of the genus Rhodococcus are important components of seed microbiomes and can perform several key functions that promote seed germination and enhance plant resistance. Their role in lettuce seeds has been studied less extensively than that of other endophytes; however, research on other crops suggests the following possible functions. First, Rhodococcus spp. are known for their ability to degrade a wide range of organic pollutants, including pesticides, polycyclic aromatic hydrocarbons (PAHs), and alkanes, owing to their powerful catabolic enzymes (such as cytochrome P450 and dioxygenases) [27,28]. Some strains of Rhodococcus are considered plant growth-promoting bacteria (PGPB) due to their synthesis of phytohormones (e.g., indole-3-acetic acid, IAA) and their capacity to solubilize phosphates [29]. Additionally, Rhodococcus strains can suppress pathogens through quorum sensing interference, VOC synthesis, competitive exclusion, direct antagonism (via the production of antimicrobial compounds), and the induction of systemic resistance (ISR) in plants [30,31]. Rhodococcus is frequently detected in seeds, but its mechanisms of vertical transmission and ecological roles require further investigation [14,32].
The genus Candidatus Profftella represents a poorly understood group of bacteria classified as insect endosymbionts, and their potential role in plant seeds, including those of lettuce, warrants special consideration. To date, no direct studies have confirmed the presence or functional activity of Ca. Profftella in lettuce seeds. This genus is a highly specialized insect endosymbiont with a well-established ecological niche and defined functions within the Diaphorina citri system, which is associated with citrus pathogens [33].
Bacteria of the genus Janthinobacterium are recognized as multifunctional endophytes capable of influencing plant growth and enhancing stress resistance. Janthinobacterium spp. produce several antimicrobial compounds, including violacein—a pigment with demonstrated activity against fungi (e.g., Fusarium, Pythium) and pathogenic bacteria—as well as quinazoline alkaloids that inhibit the growth of competing microorganisms [34].
Furthermore, Janthinobacterium species are classified as plant growth-promoting bacteria (PGPB) due to their synthesis of indole-3-acetic acid (IAA), phosphate solubilization, and siderophore production [35]. The induction of osmoprotectants (such as proline and glycine betaine) and the synthesis of cryoprotective proteins by Janthinobacterium strains contribute to increased bacterial resistance to drought and cold stress [36].
Bacteria of the genus Pseudomonas (particularly the species P. fluorescens, P. putida, and P. chlororaphis) are key components of the seed microbiome of lettuce and perform numerous functions critically important for plant germination, growth, and resistance. First, Pseudomonas spp. are known to suppress the growth and development of phytopathogens through the synthesis of antimicrobial metabolites such as 2,4-diacetylphloroglucinol (DAPG), phenazines, and pyoluteorin, competition for iron via siderophore production (e.g., pyoverdine), and induction of systemic resistance (ISR) in plants [37]. It has been demonstrated that P. fluorescens suppresses Fusarium by synthesizing siderophores [38], while phenazines produced by P. chlororaphis protect lettuce seeds from Pythium [39]. Pseudomonas species are classified as plant growth-promoting bacteria (PGPB) due to their ability to synthesize phytohormones (indole-3-acetic acid (IAA), gibberellins) [40], solubilize phosphates [41], and some strains can fix atmospheric nitrogen [42]. Moreover, Pseudomonas spp. have been shown to alleviate salt stress in lettuce by regulating ionic homeostasis [43], and Pseudomonas putida enhances lettuce resistance to drought by modulating phytohormones and antioxidants [44]. Pseudomonas actively colonizes seeds owing to its ability to form biofilms (facilitating adhesion to the seed coat) and to undergo horizontal transmission through soil and water [14,45].
Bacteria of the genus Enterococcus are rarely considered typical components of the phytomicrobiome, but their presence in lettuce seeds may be associated with several ecological and functional roles. Some Enterococcus strains exhibit plant growth-promoting properties (PGPB) through the synthesis of phytohormones (auxins, cytokinins), phosphate solubilization, and siderophore production [46,47]. Literature evidence indicates that Enterococcus can suppress pathogens by producing bacteriocins (e.g., enterocins), demonstrate antagonistic activity against fungi of the genera Fusarium and Botrytis, and act as strong competitors for essential nutrients such as iron and carbon [48]. Enterococcus species can enhance plant resistance to oxidative stress via antioxidant production and improve tolerance to salinity through the synthesis of osmoprotectants [49]. These bacteria can colonize seeds by entering through the root system from soil or via seed treatments, such as application of organic fertilizers [50]. However, some Enterococcus strains are opportunistic human pathogens (e.g., E. faecalis) and may carry antibiotic resistance genes [51].
The genus Alcaligenes includes bacteria capable of colonizing plants and performing important ecological functions, such as plant growth promotion, atmospheric nitrogen fixation, phosphate solubilization, and synthesis of phytohormones (indole-3-acetic acid (IAA), gibberellins) [52,53]. Alcaligenes exhibits antagonistic activity against fungal pathogens (e.g., Fusarium, Rhizoctonia) and bacterial pathogens (e.g., Pseudomonas syringae) through the production of siderophores and antimicrobial compounds [54]. Additionally, Alcaligenes is capable of decomposing phenols and organochlorine compounds, as well as detoxifying heavy metals [55,56]. Consequently, Alcaligenes enhances plant resistance to salinity, drought, and heavy metal stress [49]. Alcaligenes is found in seeds, attributed to its ability to form biofilms and undergo vertical transmission from the parent plant [14].
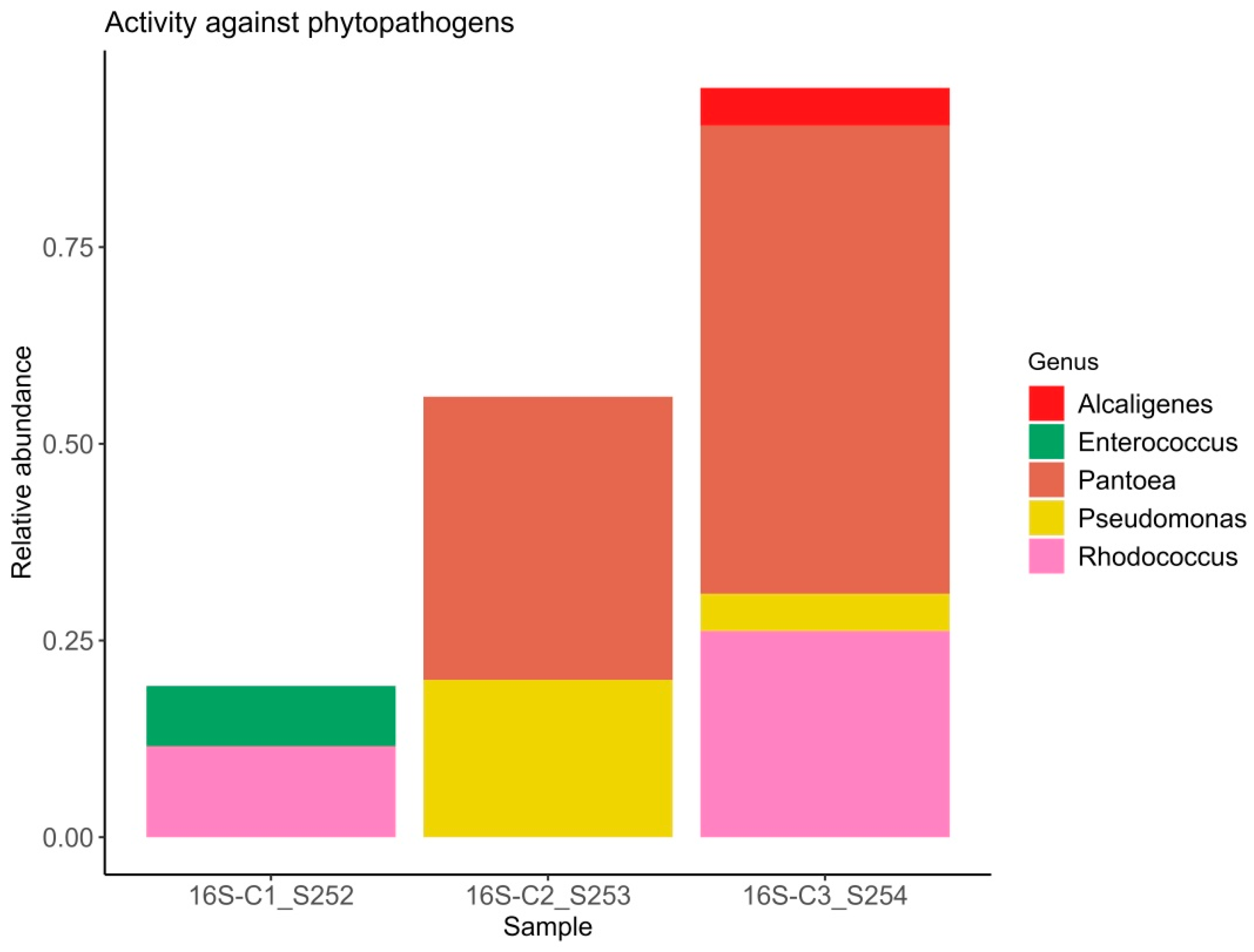
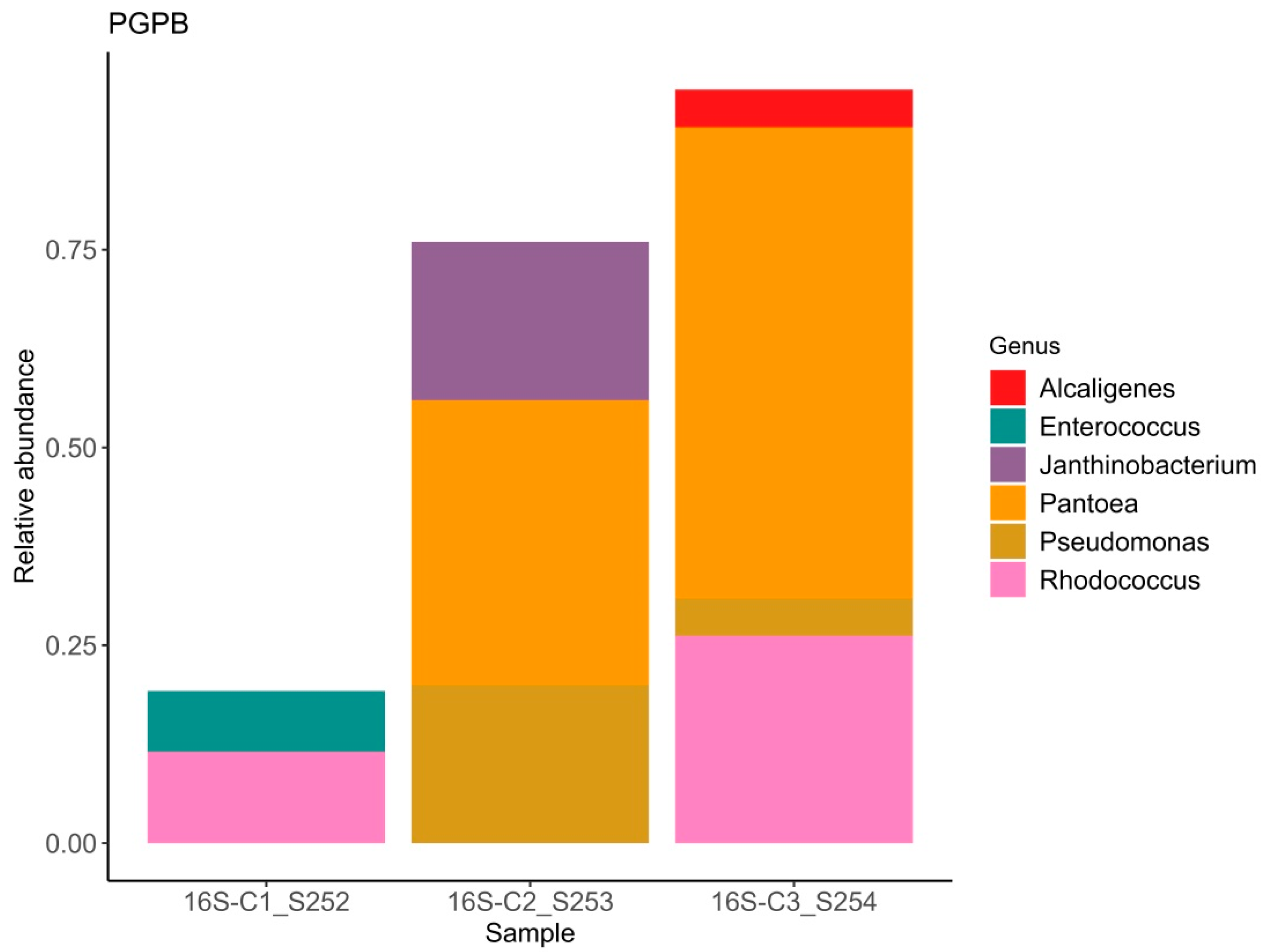
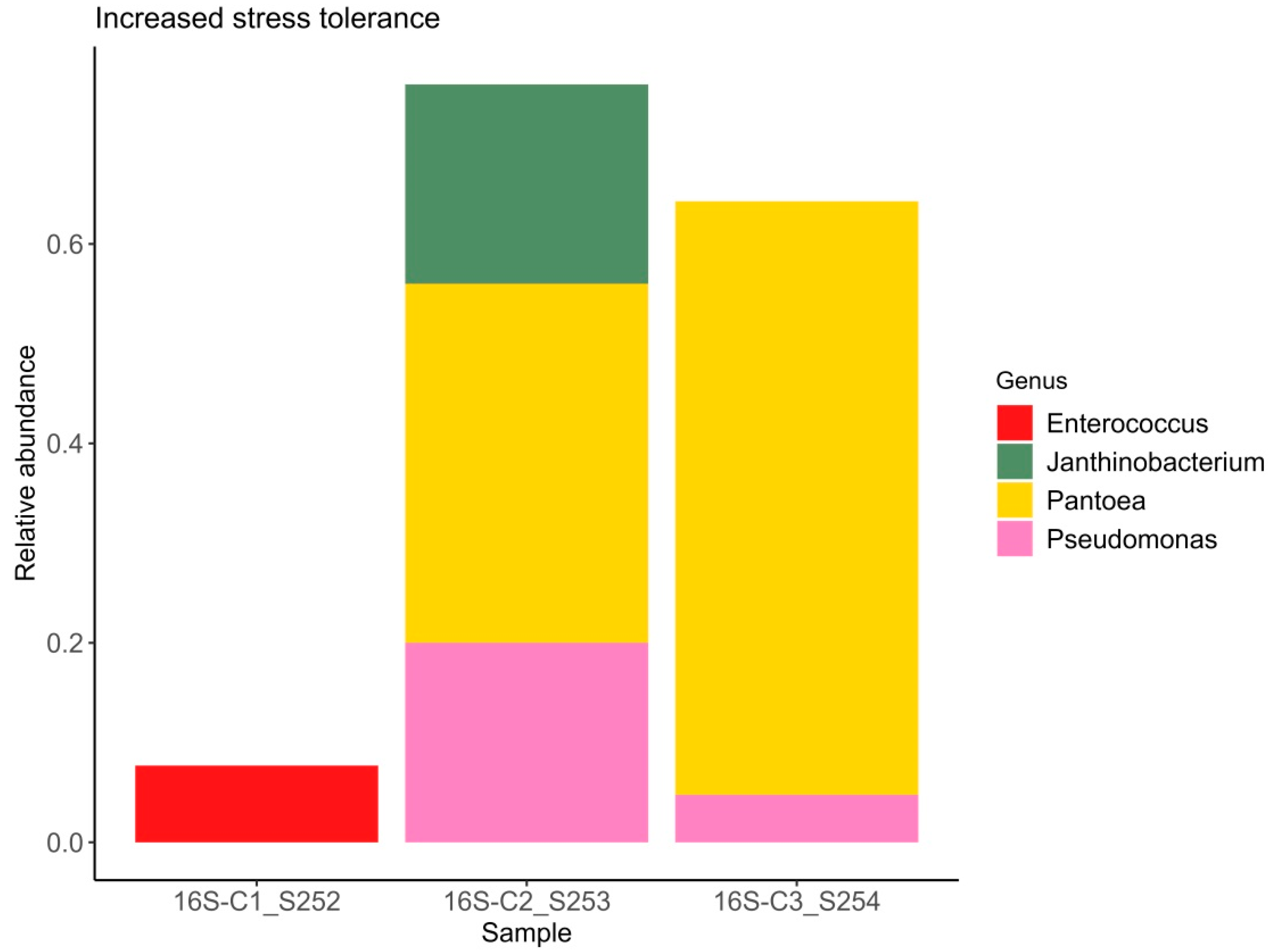
It is well established that plants recruit beneficial microorganisms into the endosphere. Moreover, vertical transmission of the endospheric microbiome from the mother plant to the offspring via the seed microbiome has been documented in the literature [57,58]. This is why the study of seed endophytic microbial communities of plants, especially those consumed by humans in raw form, represents a promising direction in the development of biological plant protection agents. The number of studies reporting on the composition of seed endophytic microbial communities, including those of lettuce (Lactuca sativa), is increasing annually [59]. It has been shown that the endospheric bacterial community of lettuce seeds primarily consists of representatives from the phyla Pseudomonadota (formerly classified as Proteobacteria) and Bacillota (Firmicutes). The relative abundance of these major groups can vary considerably: Pseudomonadota accounts for approximately 40–89% and Bacillota for 10–60% of the community composition in non-germinated lettuce seeds [60]. At the genera level frequently detected Pseudomonas, Pantoea, Bacillus, Serratia, Rahnella, Rhodococcus, Xanthomonas, and Streptomyces [61]. Our data also demonstrate a predominance of bacteria from the genera Pantoea, Rhodococcus, and Pseudomonas, with substantial representation of bacteria from the genera Candidatus Profftella and Janthinobacterium. In this study the potential beneficial properties of bacterial genera comprising the endospheric microbial community were assessed. According to published data, nearly all representatives of the endospheric microbial community in lettuce seeds exhibit a range of beneficial traits, including plant growth promotion (PGPB), suppression of phytopathogens, enhanced stress tolerance, positive effects on seed germination, and detoxification of environmental pollutants. Interestingly, sample C1 exhibited the highest values of the Shannon and Simpson diversity indices (1.84 and 0.83, respectively) despite having the lowest relative abundance of beneficial microorganisms. In this sample, the relative abundance of bacteria with activity against phytopathogens was 19%, PGPB accounted for 19%, and bacteria enhancing stress resistance comprised 7% (Figure 1, Figure 2 and Figure 3). Samples C2 and C3, which showed lower bacterial diversity (with Shannon and Simpson indices 1.2–1.1 and 1.7–1.5 times lower than those of sample C1, respectively), exhibited a substantially higher relative abundance of bacteria active against phytopathogens (56–95%), PGPB (76–95%), and stress-resistance-enhancing bacteria (64–76%). According to the literature, alpha diversity indices estimated for the endophytic bacterial community of lettuce seeds vary within the ranges of 0.3–1.0 for the Simpson index and 0.2–8 for the Shannon index [3,60,61]. It is important to note the limited scope of studies on the endophytic microbial community of lettuce seeds compared to those of crops such as rice and wheat [62,63,64,65].
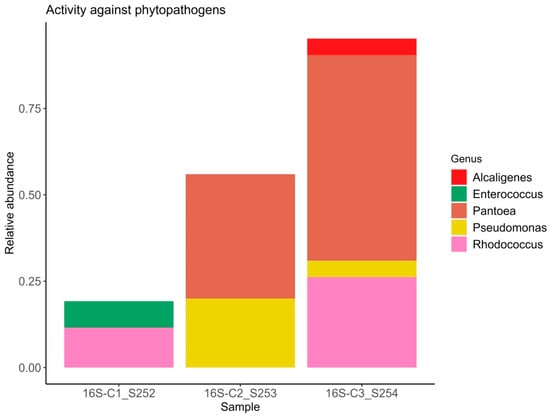
Figure 1.
Relative abundance of bacterial ASVs with phytopathogen-suppressing capabilities.
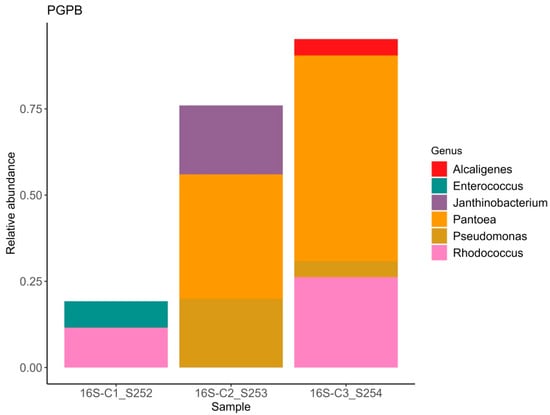
Figure 2.
Relative abundance of bacterial ASVs exhibiting plant growth-promoting traits.
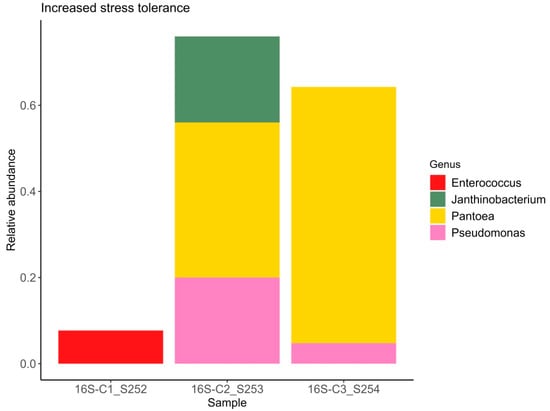
Figure 3.
Relative abundance of bacterial ASVs capable of enhancing plant stress resistance.
Furthermore, it was demonstrated that seeds of the same variety, obtained from the same producer, may differ significantly in the composition of their endospheric bacterial communities. Overall, these results indicate that the lettuce seed endosphere harbors a microbiome of relatively low diversity, yet the constituent bacteria are capable of performing a broad spectrum of functions that support effective plant development. Specifically, the microbial community includes different types of PGPB: bacteria that synthesize phytohormones, siderophores; bacteria that fix nitrogen and solubilize phosphate; bacteria that suppress phytopathogens via three main mechanisms—production of antimicrobial compounds, competitive exclusion, and induction of systemic resistance; bacteria that enhance tolerance to abiotic stresses such as cold, drought, salinity, and oxidative stress; as well as bacteria that promote seed germination and detoxify pollutants including pesticides, polycyclic aromatic hydrocarbons (PAHs), alkanes, heavy metals, phenols, and organochlorine compounds.
5. Conclusions
The conducted research revealed the taxonomic groups and functional properties of the bacterial microbiome associated with lettuce seeds. It was established that endophytic bacteria constituting the seed microbiome possess significant potential to stimulate plant growth, enhance resistance to both biotic and abiotic stresses, and suppress phytopathogens. The endophytic bacterial community of lettuce seeds includes representatives of the genera Pantoea, Rhodococcus, Candidatus Profftella, Janthinobacterium, Pseudomonas, Enterococcus, and Alcaligenes. Variation in the microbial community composition among samples indicates the influence of environmental factors on seed microbiome assembly. The results underscore the importance of further research into the functional characteristics of the seed microbiome and its targeted application in agricultural technologies aimed at increasing crop yield and product safety. The data obtained may serve as a foundation for the development of novel bioproducts based on beneficial endophytes that contribute to the sustainable advancement of crop production.
Author Contributions
Conceptualization, P.K. and S.S.; methodology, P.K.; software, D.T.; validation, G.G., N.P. and I.G.; formal analysis, N.P. and L.B.; investigation, G.G. and L.B.; resources, I.G.; data curation, P.K.; writing—original draft preparation, D.T.; writing—review and editing, P.K.; visualization, D.T.; supervision, P.K.; project administration, S.S.; funding acquisition, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
The work is carried out in accordance with the Strategic Academic Leadership Program “Priority 2030” of the Kazan Federal University of the Government of the Russian Federation.
Data Availability Statement
The original data presented in the study are openly available in sequence read archive (SRA) database under accession number PRJNA1279813.
Acknowledgments
The work is carried out in accordance with the Strategic Academic Leadership Program “Priority 2030” of the Kazan Federal University of the Government of the Russian Federation.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bintarti, A.F.; Sulesky-Grieb, A.; Stopnisek, N.; Shade, A. Endophytic Microbiome Variation Among Single Plant Seeds. Phytobiomes J. 2022, 6, 45–55. [Google Scholar] [CrossRef]
- Raheem, S.; Khan, A.L.; Saqib, B.; Sajjad, A.; Lee, I.-J. What Is There in Seeds? Vertically Transmitted Endophytic Resources for Sustainable Improvement in Plant Growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef]
- Acuña, J.J.; Hu, J.; Inostroza, N.G.; Valenzuela, T.; Perez, P.; Epstein, S.; Sessitsch, A.; Zhang, Q.; Jorquera, M.A. Endophytic Bacterial Communities in Ungerminated and Germinated Seeds of Commercial Vegetables. Sci. Rep. 2023, 13, 19829. [Google Scholar] [CrossRef]
- Krishnan, A.; Archna, S.; Sharma, P.; Singh, P.K.; Gond, S.; Pathak, D. Seed Endophytic Bacterial Profiling from Wheat Varieties of Contrasting Heat Sensitivity. Front. Plant Sci. 2023, 14, 1101818. [Google Scholar] [CrossRef]
- Shade, A.; Jacques, M.-A.; Barret, M. Ecological Patterns of Seed Microbiome Diversity, Transmission, and Assembly. Curr. Opin. Microbiol. 2017, 37, 15–22. [Google Scholar] [CrossRef]
- Nelson, E.B. The Seed Microbiome: Origins, Interactions, and Impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- L’Hoir, M.; Duponnois, R. Combining the Seed Endophytic Bacteria and the Back to the Future Approaches for Plant Holobiont Breeding. Front. Agron. 2021, 3, 724450. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, S.-H.; Yang, J.; Choi, S.; Jung, H.W.; Jeon, J. Plant Protective and Growth Promoting Effects of Seed Endophytes in Soybean Plants. Plant Pathol. J. 2023, 39, 513–521. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Assembly and Function of Seed Endophytes in Response to Environmental Stress. J. Microbiol. Biotechnol. 2023, 33, 1119–1129. [Google Scholar] [CrossRef]
- Hadian, S.; Smith, D.L.; Supronienė, S. Modulating the Plant Microbiome: Effects of Seed Inoculation with Endophytic Bacteria on Microbial Diversity and Growth Enhancement in Pea Plants. Microorganisms 2025, 13, 570. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Gollop, R.; Kroupitski, Y.; Matz, I.; Chahar, M.; Shemesh, M.; Sela Saldinger, S. Bacillus Strain BX77: A Potential Biocontrol Agent for Use against Foodborne Pathogens in Alfalfa Sprouts. Front. Plant Sci. 2024, 15, 1287184. [Google Scholar] [CrossRef]
- Reynolds, J.L. New UGA Study will Look to Lettuce Microbes for Food Safety Solutions. Available online: https://newswire.caes.uga.edu/story/8943/lettuce-microbiome.html#:~:text=By%20studying%20the%20interactions%20between,pathogen’s%20fate%20during%20produce%20processing (accessed on 25 June 2025).
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial Seed Endophytes: Genera, Vertical Transmission and Interaction with Plants. Environ. Microbiol. Rep. 2014, 7, 40–50. [Google Scholar] [CrossRef]
- Wassermann, B.; Cernava, T.; Müller, H.; Berg, C.; Berg, G. Seeds of Native Alpine Plants Host Unique Microbial Communities Embedded in Cross-Kingdom Networks. Microbiome 2019, 7, 108. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Wisniewski, M.; Schena, L.; Tack, A. Experimental Evidence of Microbial Inheritance in Plants and Transmission Routes from Seed to Phyllosphere and Root. Environ. Microbiol. 2021, 23, 2199–2214. [Google Scholar] [CrossRef]
- Khanal, S.; Imran, M.; Zhou, X.-G.; Antony-Babu, S. Characterization of Differences in Seed Endophytic Microbiome in Conventional and Organic Rice by Amplicon-Based Sequencing and Culturing Methods. Microbiol. Spectr. 2024, 12, e0366223. [Google Scholar] [CrossRef]
- Vimal, S.R.; Singh, J.S.; Kumar, A.; Prasad, S.M. The Plant Endomicrobiome: Structure and Strategies to Produce Stress Resilient Future Crop. Curr. Res. Microb. Sci. 2024, 6, 100236. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef]
- Rybakova, D.; Mancinelli, R.; Wikström, M.; Birch-Jensen, A.-S.; Postma, J.; Ehlers, R.-U.; Goertz, S.; Berg, G. The Structure of the Brassica Napus Seed Microbiome Is Cultivar-Dependent and Affects the Interactions of Symbionts and Pathogens. Microbiome 2017, 5, 104. [Google Scholar] [CrossRef]
- Bergna, A.; Cernava, T.; Rändler-Kleine, M.; Grosch, R.; Zachow, C.; Berg, G. Tomato Seeds Preferably Transmit Plant Beneficial Endophytes. Phytobiomes 2018, 2, 183–193. [Google Scholar] [CrossRef]
- Danilova, N.; Galieva, G.; Kuryntseva, P.; Selivanovskaya, S.; Galitskaya, P. Influence of the Antibiotic Oxytetracycline on the Morphometric Characteristics and Endophytic Bacterial Community of Lettuce (Lactuca sativa L.). Microorganisms 2023, 11, 2828. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Doni, F.; Suhaimi, N.S.M.; Irawan, B.; Mohamed, Z.; Mispan, M.S. Associations of Pantoea with Rice Plants: As Friends or Foes? Agriculture 2021, 11, 1278. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Mackiewicz, B.; Lemieszek, M.; Golec, M.; Milanowski, J. Pantoea agglomerans: A Mysterious Bacterium of Evil and Good. Part III. Deleterious Effects: Infections of Humans, Animals and Plants. Ann. Agric. Environ. Med. 2016, 23, 197–205. [Google Scholar] [CrossRef]
- Walterson, A.; Stavrinides, J. Pantoea: Insights into a Highly Versatile and Diverse Genus within the Enterobacteriaceae. FEMS Microbiol. Rev. 2015, 39, 968–984. [Google Scholar] [CrossRef]
- Chisti, Y. Bioremediation—Keeping the Earth Clean. Biotechnol. Adv. 2005, 23, 371–372. [Google Scholar] [CrossRef]
- Alvarez, H.M.; Hernández, M.A.; Lanfranconi, M.P.; Silva, R.A.; Villalba, M.S. Rhodococcus as Biofactories for Microbial Oil Production. Molecules 2021, 26, 4871. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. Bacterial Consortium for Improved Maize (Zea mays L.) Production. Microorganisms 2019, 7, 519. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, K.; Feng, J. Identification and Analysis of VOCs Released by Rhodococcus ruber GXMZU2400 to Promote Plant Growth and Inhibit Pathogen Growth. BMC Plant Biol. 2025, 25, 559. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.; Li, J.; Zhang, Y.; Huang, Y.; Zhang, W.; Shi, Y.; Wang, J.; Chen, S. A Novel Quorum Quencher, Rhodococcus pyridinivorans XN-36, Is a Powerful Agent for the Biocontrol of Soft Rot Disease in Various Host Plants. Biol. Control 2022, 169, 104889. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Ramsey, J.S.; Johnson, R.S.; Jason, S.H.; Kruse, A.; Mahoney, J.; Hilf, M.E.; Hunter, W.B.; Hall, D.G.; Schroeder, F.C.; MacCoss, M.J.; et al. Metabolic Interplay between the Asian Citrus Psyllid and Its Profftella Symbiont: An Achilles’ Heel of the Citrus Greening Insect Vector. PLoS ONE 2015, 10, e0140826. [Google Scholar] [CrossRef] [PubMed]
- Chernogor, L.; Eliseikina, M.; Petrushin, I.; Chernogor, E.; Khanaev, I.; Belikov, S.I. Janthinobacterium Sp. Strain SLB01 as Pathogenic Bacteria for Sponge Lubomirskia Baikalensis. Pathogens 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Farh, M.E.-A.; Kim, Y.-J.; Sukweenadhi, J.; Singh, P.; Yang, D.-C. Aluminium Resistant, Plant Growth Promoting Bacteria Induce Overexpression of Aluminium Stress Related Genes in Arabidopsis thaliana and Increase the Ginseng Tolerance against Aluminium Stress. Microbiol. Res. 2017, 200, 45–52. [Google Scholar] [CrossRef]
- Chowdhury, P.; Babin, D.; Sandmann, M.; Jacquiod, S.; Sommermann, L.; Sørensen, S.; Fliessbach, A.; Mäder, P.; Geistlinger, J.; Smalla, K.; et al. Effect of Long-Term Organic and Mineral Fertilization Strategies on Rhizosphere Microbiota Assemblage and Performance of Lettuce. Environ. Microbiol. 2019, 21, 2426–2439. [Google Scholar] [CrossRef]
- Hopkins, D.; Sparrow, A.D.; Gregorich, E.; Elberling, B.; Novis, P.; Fraser, F.; Scrimgeour, C.; Dennis, P.; Meier-Augenstein, W.; Greenfield, L. Isotopic Evidence for the Provenance and Turnover of Organic Carbon by Soil Microorganisms in the Antarctic Dry Valleys. Environ. Microbiol. 2009, 11, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Thorwall, S.; Trivedi, V.; Ottum, E.; Wheeldon, I. Population Genomics-Guided Engineering of Phenazine Biosynthesis in Pseudomonas chlororaphis. Metab. Eng. 2023, 78, 223–234. [Google Scholar] [CrossRef]
- Claeyssen, É.; Rivoal, J. Isozymes of Plant Hexokinase: Occurrence, Properties and Functions. Phytochemistry 2007, 68, 709–731. [Google Scholar] [CrossRef]
- Sah, S.; Krishnani, S.; Singh, R. Pseudomonas Mediated Nutritional and Growth Promotional Activities for Sustainable Food Security. Curr. Res. Microb. Sci. 2021, 2, 100084. [Google Scholar] [CrossRef]
- Sanow, S.; Kuang, W.; Schaaf, G.; Huesgen, P.; Schurr, U.; Roessner, U.; Watt, M.; Arsova, B. Molecular Mechanisms of Pseudomonas-Assisted Plant Nitrogen Uptake: Opportunities for Modern Agriculture. Mol. Plant Microbe Interact. 2023, 36, 536–548. [Google Scholar] [CrossRef]
- Wang, M.; Bian, Z.; Shi, J.; Wu, Y.; Yu, X.; Yang, Y.; Ni, H.; Chen, H.; Bian, X.; Li, T.; et al. Effect of the Nitrogen-Fixing Bacterium Pseudomonas protegens CHA0-ΔretS-Nif on Garlic Growth under Different Field Conditions. Ind. Crops Prod. 2020, 145, 111982. [Google Scholar] [CrossRef]
- Cassán, F.; Vanderleyden, J.; Spaepen, S. Physiological and Agronomical Aspects of Phytohormone Production by Model Plant-Growth-Promoting Rhizobacteria (PGPR) Belonging to the Genus Azospirillum. J. Plant Growth Regul. 2014, 33, 440–459. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, C.; Kang, X.; Zhang, L.; Wang, J.; Zheng, S.; Zhang, T. Hydrogen Sulfide and Nitric Oxide Are Involved in Melatonin-Induced Salt Tolerance in Cucumber. Plant Physiol. Biochem. 2021, 167, 101–112. [Google Scholar] [CrossRef]
- Matthieu, B.; Martial, B.; Sophie, B.; Anne, P.; Sophie, V.; Olivier, B.; Gilles, H.; Philippe, S.; Marie-Agnès, J. Emergence Shapes the Structure of the Seed Microbiota. Appl. Environ. Microbiol. 2015, 81, 1257–1266. [Google Scholar] [CrossRef]
- Mendoza-Hernández, J.C.; Vázquez-Delgado, O.R.; Castillo-Morales, M.; Varela-Caselis, J.L.; Santamaría-Juárez, J.D.; Olivares-Xometl, O.; Arriola Morales, J.; Pérez-Osorio, G. Phytoremediation of Mine Tailings by Brassica juncea Inoculated with Plant Growth-Promoting Bacteria. Microbiol. Res. 2019, 228, 126308. [Google Scholar] [CrossRef]
- Hale, L.; Curtis, D.; Azeem, M.; Montgomery, J.; Crowley, D.E.; McGiffen, M.E. Influence of Compost and Biochar on Soil Biological Properties under Turfgrass Supplied Deficit Irrigation. Appl. Soil Ecol. 2021, 168, 104134. [Google Scholar] [CrossRef]
- Hynes, T.M.; Mc Donnell, R.J.; Kirsch, A.; Dillon, R.J.; O’Hora, R.; Gormally, M.J. Effect of Temperature on the Larval Stage of Tetanocera elata (Diptera: Sciomyzidae)—Potential Biological Control Agent of Pestiferous Slugs. Biol. Control 2014, 74, 45–51. [Google Scholar] [CrossRef]
- Mafa, M.S.; Rufetu, E.; Alexander, O.; Kemp, G.; Mohase, L. Cell-Wall Structural Carbohydrates Reinforcements Are Part of the Defence Mechanisms of Wheat against Russian Wheat Aphid (Diuraphis noxia) Infestation. Plant Physiol. Biochem. 2022, 179, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Zhang, H.; Shim, W. Application of Game Theory to Explore the Dynamics of Host−Pathogen Association in Phytobiomes. Phytobiomes 2018, 2, 111–116. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. The Ecology, Epidemiology and Virulence of Enterococcus. Microbiology 2009, 155, 1749–1757. [Google Scholar] [CrossRef]
- Jiménez, J.; Novinscak, A.; Filion, M. Pseudomonas fluorescens LBUM677 Differentially Increases Plant Biomass, Total Oil Content and Lipid Composition in Three Oilseed Crops. J. Appl. Microbiol. 2019, 128, 1119–1127. [Google Scholar] [CrossRef]
- Jaksomsak, P.; Rerkasem, B. Responses of Grain Zinc and Nitrogen Concentration to Nitrogen Fertilizer Application in Rice Varieties with High-Yielding Low-Grain Zinc and Low-Yielding High Grain Zinc Concentration. Plant Soil 2017, 411, 101–109. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Zhang, G.-F.; Liu, W.-X.; Wan, F.-H. Continuous Heat Waves Change the Life History of a Host-Feeding Parasitoid. Biol. Control 2019, 135, 57–65. [Google Scholar] [CrossRef]
- Jiang, Y.; Wen, J.; Bai, J.; Jia, X.; Hu, Z. Biodegradation of Phenol at High Initial Concentration by Alcaligenes faecalis. J. Hazard. Mater. 2007, 147, 672–676. [Google Scholar] [CrossRef] [PubMed]
- De, J.; Nagappa, R.; Vardanyan, L. Detoxification of Toxic Heavy Metals by Marine Bacteria Highly Resistant to Mercury. Mar. Biotechnol. 2008, 10, 471–477. [Google Scholar] [CrossRef]
- Sanz-Puente, I.; Redondo-Salvo, S.; Torres-Cortés, G.; de Toro, M.; Fernandes, S.; Börner, A.; Lorenzo, Ó.; de la Cruz, F.; Robledo, M. Vertical Transmission of Core Endophytes through the Seeds. bioRxiv 2025. [CrossRef]
- Frank, A.C.; Saldierna Guzmán, J.P.; Shay, J.E. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef]
- Vasileva, E.N.; Akhtemova, G.A.; Zhukov, V.A.; Tikhonovich, I.A. Endophytic Microorganisms in Fundamental Research and Agriculture. Ecol. Genet. 2019, 17, 19–32. [Google Scholar] [CrossRef]
- Kuryntseva, P.; Pronovich, N.; Galieva, G.; Galitskaya, P.; Selivanovskaya, S. Exploring the Role of Vertical and Horizontal Pathways in the Formation of Lettuce Plant Endospheric Bacterial Communities: A Comparative Study of Hydroponic and Soil Systems. Horticulturae 2025, 11, 762. [Google Scholar] [CrossRef]
- Raimi, A.; Adeleke, R. High-Throughput Sequencing Analysis of Community Diversity and Functional Structure of Endophytic Bacteria in Edible Vegetable Crops: Potential Implication on Plant Microbiological Quality. 3 Biotech 2025, 15, 216. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A Review on the Plant Microbiome: Ecology, Functions, and Emerging Trends in Microbial Application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Eyre, A.W.; Wang, M.; Oh, Y.; Dean, R.A. Identification and Characterization of the Core Rice Seed Microbiome. Phytobiomes J. 2019, 3, 148–157. [Google Scholar] [CrossRef]
- Wang, M.; Eyre, A.W.; Thon, M.R.; Oh, Y.; Dean, R.A. Dynamic Changes in the Microbiome of Rice During Shoot and Root Growth Derived from Seeds. Front. Microbiol. 2020, 11, 559728. [Google Scholar] [CrossRef]
- Nunes, I.; Hansen, V.; Bak, F.; Bonnichsen, L.; Su, J.; Hao, X.; Raymond, N.S.; Nicolaisen, M.H.; Jensen, L.S.; Nybroe, O. Succession of the Wheat Seed-Associated Microbiome as Affected by Soil Fertility Level and Introduction of Penicillium and Bacillus Inoculants in the Field. FEMS Microbiol. Ecol. 2022, 98, fiac028. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).