Abstract
Horticulture is mainly based on transplanting seedlings produced by specialized nurseries. The recent European authorization of frass in organic farming presents new opportunities for the development of organic seedling production. Frass, a by-product of insect farming, offers innovative solutions for this sector. It mainly consists of insect excrement, exuviae, and uningested feed. Their fertilizing and biostimulating effects have been demonstrated in various pot and field crops experiments. However, the current knowledge regarding the application of frass in seedling production remains insufficient. This study aims to assess the optimal dose of mealworm frass in germination substrates for Allium cepa L., Beta vulgaris L., and Brassica rapa L. Germination and phytotoxicity tests were carried out, with seedlings evaluated one month after sowing in substrates containing frass at concentrations of 0.5%, 1%, 2%, and 3% of frass. The germination test revealed that the dilution of the frass at 1:100 produced a phytostimulant effect on A. cepa and a moderate phytotoxic effect on B. vulgaris and B. rapa. The application of mealworm frass at a concentration of 0.5–1% was generally the most effective dose, although all doses of frass in the substrate resulted in seedlings whose root length, leaf length, number of leaves, and biomass were significantly higher than the control. In conclusion, the application of low doses of mealworm frass in organic seedling production is promising and allows the management of potential phytotoxicity.
Keywords:
biostimulant; broccoli rabe; by-product; chard; edible insect; fertilization; nursery; onion; sowing substrate; Tenebrio molitor 1. Introduction
The global agriculture industry is trying to increase sustainability by focusing on the preservation of biodiversity, the reduction in non-renewable resource utilization, and the stimulation of the soil microbiome to restore its biological balance [1]. Organic farming plays a crucial role in achieving these objectives by minimizing reliance on external inputs and employing organic fertilizers, alongside effective soil and crop protection strategies. At the same time, it is essential to ensure good production efficiency by innovative techniques. Modern horticulture is based on the transplanting of seedlings produced in specialized nurseries. The quality of the seedlings, such as vigor and health, is an important prerequisite for the success of the crop in the field. Innovative techniques to produce organic seedlings have been tested, including seed priming and coating [2] and seed treatment for the control of seedborne fungal pathogens [3]. Advantages result from the biostimulant effect of coating seed treatments with vermicompost [4], chitosan [5], and dynamized plant extracts [6]. Germination and seedling growth are positively influenced by the biostimulatory effect of seed treatments with microorganisms [7] and plant growth-promoting rhizobacteria [8].
Furthermore, in organic seedling production, fertilization is allowed only through soil amendments and fertilizers of natural origin [9]. However, their slow release of nutrients [10] is unsuitable for the rapid growth of vegetable seedlings, so new organic fertilizers are of high interest.
Recently, the insect farming sector offered a by-product known as frass that has fertilizing properties. Frass is defined as “a mixture of excrements derived from farmed insects, feeding substrate, parts of farmed insects, dead eggs and with a content of dead farmed insects of not more than 5% in volume and not more than 3% in weight” [11]. Currently, the European production of frass is estimated to be of several thousand tons, but it is projected to exceed 1.5 million tons by the middle of this decade [12]. Furthermore, recent European legislation has opened new market opportunities by setting rules for the marketing of frass [11] and permitting its use in organic agriculture [13].
The nutrient composition of frass is influenced by the specific insect reared [14]. Furthermore, within the same species, the diet provided can significantly influence various physical parameters, as well as its concentrations of macro and microelements. This is attested by the variability of dry matter, pH, electrical conductivity (EC), macroelements, and especially microelements in frass of Hermetia illucens (L.) from different producers [15]. Mealworms fed different diets produced frass with significantly different EC, carbon, nitrogen, and phosphorus values [16,17]. Additionally, diet can impact the levels of heavy metals, such as As, Cd, and Pb [18], and even the presence of antioxidants, including lycopene and β-carotene [19].
The fertilizing effect of frass has been confirmed on different crops [20] with tests conducted in open fields (e.g., corn) [21] and on potted vegetables [22,23]. The increase in productivity observed has also been attributed to the biostimulating effect of frass on plants [24], as well as its beneficial impact on the soil microbiome [17]. In in vitro bioassays, H. illucens frass showed antagonistic activity against plant pathogens, such as Alternaria solani, Botrytis cinerea, Fusarium oxysporum, Rhizoctonia solani, and Sclerotinia sclerotiorum [25]; its application in pots also had a suppressive effect on F. oxysporum in tomato plants [26]. This potential activity and the simultaneous fertilizing and biostimulating properties of frass make it a valuable resource for the nursery sector. Previous studies in this sector have evaluated the frass of H. illucens as an organic fertilizer in the production of potted plants [27] and as an alternative soil amendment to peat [28]. To the best of our knowledge, no studies have specifically focused on producing vegetable seedlings for transplantation in horticulture. For this purpose, we have selected the frass of Tenebrio molitor (L.) due to its higher content of nitrate and lower ammonium compared to the frass of other farmed insects (black soldier fly and cricket) [29]; moreover, nitrogen is made available by its fast mineralization [30]. Therefore, this study aims to determine the optimal application rate of mealworm frass in the context of organic vegetable seedling production. Specifically, this study aims to evaluate the effects of different doses of mealworm frass on the germination and nursery growth of onion, Swiss chard, and broccoli rabe. These were chosen as autumn–winter vegetables and as being representative of mediterranean organic cultivation.
2. Materials and Methods
2.1. Mealworm Frass
The mature mealworm frass used in this study was sourced from a colony of Tenebrio molitor L. reared at the Insectarium CIHEAM-Bari (Apulia region, Italy). The mealworms were provided with a diet of bran and yeast in a weight ratio of 95:5, with added pumpkin as a supplementary water source. The collected frass was sieved through a mesh (hole < 0.5 mm) and subsequently stabilized in an oven (CASORI, mod. CP267-FD-RXS, Anaheim, CA, USA) at 70 °C for one hour. The nutritional composition of the frass was assessed by a private laboratory in accordance with Regulation (EU) n. 2019/1009 [31], and the following values were detected: pH 6.2, C:N ratio of 12.4, 29.3 g kg−1 of nitrogen (N), 5 g kg−1 of phosphorus (P), 15.8 g kg−1 of potassium (K), 1 g kg−1 of calcium (Ca), and 6.4 g kg−1 of magnesium (Mg).
2.2. Germination Index and Phytotoxicity Test of Mealworm Frass
Seeds of onion (Allium cepa L.), Swiss chard (Beta vulgaris L. var. cicla), and broccoli rabe or “Cima di rapa” (Brassica rapa L. subsp. sylvestris (Lam.) Janch.) were used in the bioassays. A mealworm frass extract was obtained by diluting 10 g of frass in 100 mL of distilled water, achieving a concentration of 1:10 (w/v). The mixture was shaken for 60 min at 25 °C using a magnetic stirrer (mod. BE32R, BICASA, Bernareggio (MB), Italy). Subsequently, the solution was filtered through a fine cotton cloth to remove any frass particles. This extract was found to be phytotoxic based on our preliminary observations on germination in Petri dishes. Similarly to previous studies [32,33], this extract was subsequently diluted with distilled water to obtain more suitable final dilutions, with frass–water ratios of 1:100, 1:75, and 1:50. The extracts at these dilutions were used only in the germination process in Petri dishes. They simulated frass doses of 1%, 1.3%, and 2%, and were partially similar to the doses of frass mixed with the substrate in subsequent tests, in accordance with the procedure used by other authors [32]. The electrical conductivity (EC) of each dilution was measured using a conductivity meter (mod. HI2003-02, Hanna Instruments, Ronchi di Villafranca Padovana (PD), Italy). A total of six milliliters of each dilution was placed into Petri dishes lined with filter paper, and distilled water was used as a control. For each selected species, thirty seeds were allocated to each Petri dish, with four replicates per treatment, following a completely randomized experimental design. The Petri dishes were incubated at a controlled temperature of 25 ± 2 °C in an incubator (mod. Alpha, BICASA, Bernareggio (MB), Italy). After three days, seed germination was assessed, and the root lengths were measured for A. cepa and B. rapa. In contrast, the measurements for B. vulgaris were conducted after eight days. The germination index (GI) was calculated in accordance with the equation presented by Luo et al. [34]:
where RSG (%) represents the relative seed germination, calculated as
and RRG (%) represents the relative root growth, calculated as
The assessment of frass phytotoxicity was conducted using the GI values. According to Barral et al. [35], GI values falling below 50% indicate a high level of phytotoxicity. Values ranging between 50% and 80% indicate moderate phytotoxicity, while values above 80% indicate no phytotoxicity. Notably, values exceeding 100% imply a phytonutrient or phytostimulant effect.
2.3. Germination Test in Substrate
The vegetables A. cepa, B. vulgaris, and B. rapa subsp. sylvestris were subjected to a germination test using different substrates. A basic substrate was assembled by combining peat (Estonian Peat Moss©, Geosism & Nature sas, Bibbiano (RE), Italy) and sand (95% quartz sand of size 0.4–0.8 mm, Ferritalia, Padova (PD), Italy) in a ratio of 2:1 (v/v). This basic substrate is used by local nurserymen and was considered as a control. Four other substrates were added with frass at percentages of 0.5% (Fr0.5), 1% (Fr1), 2% (Fr2), and 3% (Fr3) according to the doses most tested in the field and in pots [36]. Seeds were sown individually (1 seed/hole) in polystyrene seedling trays specific to each species. The experimental design applied was structured as a randomized block design comprising 12 holes of 20 mL (14 g of substrate/hole) replicated three times for A. cepa and B. rapa, and 6 holes of 72 mL (50 g of substrate/hole) replicated four times for B. vulgaris. The trays were kept in screen houses at a controlled temperature of 26 ± 5 °C and were irrigated daily. The germination (%) was recorded after 18 days of seedling emergence.
2.4. Growth Performance of Seedlings
All the species were evaluated following a one-month growth period in the greenhouse. Seedlings were randomly collected, and the substrate was gently removed from the roots by water in the laboratory. The root length, the epicotyl length, the cotyledon length, and the length of the most developed leaf were measured manually; the number of leaves was counted, and the fresh biomass was weighed using an analytical scale (Metter-Toledo, model B2002-S, Milano (MI), Italy).
2.5. Statistical Analysis
The data were preventively assessed for normality, homogeneity, and variance homogeneity. When these criteria were satisfied, ANOVA was applied to the values, followed by a Tukey–Kramer HDS post hoc test to identify the differences between the treatments. When the assumptions were not met, the non-parametric Kruskal–Wallis test was utilized, along with pairwise multiple comparisons employing Bonferroni correction. A significance level of p < 0.05 was established. All statistical analyses were conducted using SPSS software, version 26.0 (IBM Corporation, Armonk, NY, USA).
3. Results
3.1. Effects of Frass Dilutions on Germination and Phytotoxicity in Petri Dish
The electrical conductivity (EC) of the 10% frass extract was measured to be 5330 μS/cm. The values obtained from the subsequent dilutions indicated a decrease, with EC values of 458 μS/cm, 756 μS/cm, and 924 μS/cm for the 1:100, 1:75, and 1:50 frass-to-water ratios, respectively (Table 1).

Table 1.
Electrical conductivity (EC) of different frass dilutions and value means of germination (%) of A. cepa, B. vulgaris, and B. rapa in Petri dishes. Germination evaluated after three days for A. cepa, and B. rapa, and after eight days for B. vulgaris.
The effect of the different dilutions on the germination of A. cepa showed significant differences among the treatments (F = 4.94; df = 3, 8; p = 0.032). The germination rate at the 1:50 dilution was notably reduced to 65.6 ± 7.8%, representing a decrease of 42% compared to the control. Conversely, the other dilutions, which contained lower concentrations of frass, had no significant effect on germination, with rates ranging from 88.9% to 86.7% (Table 1).
In contrast, none of the treatments had a significant effect on the germination of B. vulgaris (F = 2.02; df = 3, 8; p = 0.190), with the control already showing low germination rates at just 22.2 ± 2.9%. Additionally, the germination rates in all dilutions containing frass were cut in half. Similar findings were observed on B. rapa, where there was no difference in germination among the treatments (F = 2.96; df = 3, 12; p = 0.075). In this case, the germination rates were generally high, ranging from 90.0% to 76.7%, with the lowest rate found in the 1:50 dilution (Table 1).
The root length of A. cepa exhibited significant differences between the treatments (F = 6.05; df = 3, 8; p = 0.019). The two lower dilutions, specifically 1:100 and 1:75, resulted in the longest root lengths of 28.4 mm and 30.5 mm, respectively. This represents an increase of 23% and 32% compared to the control. However, these observed increases were not significantly different from the control, but were significantly greater than those recorded in the most concentrated dilution (1:50) (Figure 1).
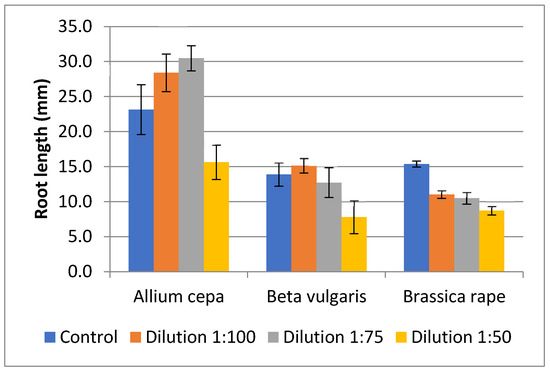
Figure 1.
Root lengths (mm) of A. cepa, B. vulgaris, and B. rapa in Petri dishes using different frass dilutions. Germination evaluated after three days for A. cepa, and B. rapa, and after eight days for B. vulgaris. Control (water). Mean ± standard error (n = 3 for A. cepa and B. vulgaris; n = 4 for B. rapa) values with same letter within columns of same vegetable are not significantly different at α = 0.05.
The root length of B. vulgaris did not show significant differences between the treatments (F = 2.97; df = 3, 8; p = 0.097). However, roots in the 1:50 dilution were shorter, measuring 56% shorter than the control, which averaged 7.8 ± 2.4 mm compared to 13.9 ± 1.7 mm in the control. In contrast, B. rapa showed significant differences between the treatments (F = 20.13; df = 3, 12; p < 0.001), with all treatments resulting in a notable reduction in root development compared to the control. Similarly, the 1:50 dilution produced roots that were 56% shorter than the control (Figure 1).
Phytotoxicity, evaluated through germination indices (GIs), revealed distinct variations among the vegetable species examined (Table 2). The GI values for A. cepa demonstrated significant differences among the treatments (F = 8.92; df = 3, 8; p = 0.006). Specifically, the GI values of the control, as well as those of the 1:100 and 1: 75 dilutions, were not significantly different to each other. However, the 1:50 dilution showed a significant difference compared to the other treatments. These results suggest a potential phytostimulating effect of the 1:100 and 1:75 dilutions, while the 1:50 dilution showed a moderately phytotoxic effect. For B. vulgaris, the GI values did not reveal significant differences among the treatments (F = 3.24; df = 3, 8; p = 0.081). Nevertheless, the 1:100 and 1:75 dilutions may induce moderate phytotoxicity, whereas the 1:50 dilution was classified as highly phytotoxic. Similar trends in phytotoxicity were noted for B. rapa; however, in this case, the GI values were significantly different between the treatments (F = 9.89; df = 3, 12; p < 0.001), with all dilutions containing frass showing notably lower values compared to the control.

Table 2.
Mean values of germination index (GI) and phytotoxicity of different frass dilutions on A. cepa, B. vulgaris, and B. rapa in Petri dishes. Germination evaluated after three days on A. cepa and B. rapa, and after eight days on B. vulgaris.
3.2. Influence of Different Frass Doses in Substrate on Seedling Production
The results of the application of frass into the seedling substrate are shown below for each species.
For A. cepa, germination on the 18th differed significantly between treatments (F = 5.78; df = 4, 10; p = 0.011) with values ranging from 91.7% to 50.0%, and the Fr3 treatment showed a significantly lower number of emerged seedlings. Furthermore, the number of emerged seedlings for the Fr3 treatment decreased progressively (due to mortality) until it had to be excluded from the subsequent final comparison of the other growth parameters (one month after sowing). The values of the other parameters were all statistically different between the treatments: root length (H = 22.87; df = 3; p < 0.001), leaf length (H = 24.42; df = 3; p < 0.001), leaf number (H = 18.86; df = 3; p < 0.001), and fresh seedling biomass (H = 32.86; df = 3; p < 0.001) (Table 3). When comparing the treatments, all of the growth parameters showed significantly lower values in the control and in Fr1. However, leaf number was significantly higher in all frass treatments compared to the control (Table 3). This resulted in an increase in seedling biomass between 50 and 150% compared to the control.

Table 3.
Mean values of growth parameters of one-month-old A. cepa in seedling tray.
For B. vulgaris, germination was not significantly different between the treatments (F = 0.27; df = 4, 15; p = 0.892), with values ranging from 57.7% to 40.5%. The results of all of the growth parameters were statistically different between the treatments: root length (H = 14.0; df = 4; p = 0.007); cotyledon length (H = 15.1; df = 4; p = 0.004); leaf length (H = 22.1; df = 4; p < 0.001); number of leaves (H = 16.9; df = 4; p = 0.002); and fresh seedling biomass (F = 10.1; df = 4, 55; p < 0.001). Generally, the frass treatments performed significantly better than the control in all of the growth parameters except for the epicotyl length, which showed no significant difference (H = 1.8; df = 4; p = 0.766) (Table 4). In the case of B. vulgaris, the values of all of the growth parameters were not significantly different between the applied frass doses.

Table 4.
Mean values of growth parameters of one-month-old B. vulgaris in seedling tray.
For B. rapa, germination was not significantly different between the treatments (F = 0.92; df = 4, 5; p = 0.520), with values between 96.7% and 83.3%. The results of all of the growth parameters were statistically different between the treatments: the root length (H = 15.7; df = 4; p = 0.003); the epicotyl length (F = 6.96; df = 4, 85; p < 0.001); the cotyledon length (F = 10.1; df = 4, 85; p < 0.001); the leaf length (H = 29.0; df = 4; p < 0.001); the number of leaves (H = 15.5; df = 4; p = 0.004); and the fresh seedling biomass (F = 18.0; df = 4, 85; p < 0.001). Generally, the treatments with frass performed significantly better than the control in all growth parameters (Table 5). Almost all of the growth parameters showed higher values in the Fr1 treatment, but they were not significantly different from the Fr0.5 and Fr2 treatments. The greatest dose of tested frass (Fr3) showed values not significantly different compared to the control for root length, epicotyl length, and leaf length.

Table 5.
Mean values of growth parameters of one-month-old B. rapa in seedling tray.
The increase in biomass of the B. rapa seedlings grown on the Fr0.5 and Fr1 substrates was of 112% and 187%, respectively, compared to the control.
4. Discussion
Frass utilized in substrates for the organic cultivation of vegetable seedlings must supply essential nutrients in the short term to facilitate optimal growth. Consequently, the selection of frass and its application rates are a pivotal consideration. This study utilized mealworm frass exhibiting a macroelement composition similar to those reported by other authors [14,16]. Furthermore, mealworm frass mineralizes a portion of the nitrogen in the substrate rapidly, amounting to 37% within the initial 17 days, as indicated by Houben et al. [30]. This makes it more suitable for the cultivation of seedlings in the nursery for one month, in contrast to the frass of H. illucens, which is considered a slow-release nitrogen amendment [37]. A critical factor in the application of frass is its potential phytotoxicity effect that can compromise germination and consequently affect seedling output [14,32,38].
Generally, the laboratory test results indicated that germination was not significantly influenced by the examined frass concentration. The most concentrated dilution (1:50) negatively affected the germination of A. cepa; also, the germination of B. vulgaris was diminished across all frass treatments, but it did not significantly differ from the control. In this case, the overall low germination may have influenced the results. The root length findings validated the negative effect of the 1:50 dilution in all of the tested vegetables; however, all of the tested dilutions significantly reduced root length in B. rapa. These results provided different GI values and related phytotoxicity levels [35] among the tested vegetables: low frass concentrations exhibited a stimulatory effect on A. cepa and a moderate phytotoxicity on B. vulgaris and B. rapa. The more concentrated solutions showed a moderate to high generalized phytotoxic effect. This “qualitative” approach of the level of phytotoxicity has been applied in kale seed, and a moderate level of phytotoxicity was found at the highest doses (60–80%) of thermocomposted frass from H. illucens [32]. The results of phytotoxicity are best represented by the GI values, which for B. vulgaris and B. rapa are in agreement with studies on Brassica oleracea [32], Lactuca sativa, and Raphanus sativus [38]; however, our tested mealworm frass extract dilutions were less concentrated than the H. illucens frass extracts used in the previous studies. Phytotoxicity can be caused by phenols, volatile organic acids, heavy metals, ammonium, and EC [35]. The latter two are thought to be the prevalent causes of frass phytotoxicity [38]. However, mealworm frass contains fewer ammonium ions than H. illucens frass [14,29]; therefore, the EC remain primarily responsible for its phytotoxicity. Vegetables have different salinity tolerance thresholds, beyond which the negative osmotic effect increases with increasing EC values. Furthermore, phenological stage and cultivar choice influence salinity sensitivity, making comparisons more difficult [39]. Among the vegetables tested in our study, A. cepa is highly tolerant in germination (but very susceptible during seedling growth), Brassica spp. are considered moderately sensitive, and B. vulgaris is more tolerant [39]. This is consistent with the hypothesis that our decreasing GI values are attributable to the increasing salinity of the dilutions tested [39].
The findings indicate that incorporating mealworm frass into the substrate is effective at low concentrations. In fact, one month after sowing, doses between 0.5 and 2% had a positive effect on root length, leaf length, leaf number, and seedling biomass. In a recent study, mealworm frass at low doses (0.25%, 0.5%, and 1%) demonstrated similar effects on Spinacia oleracea in pots 60 days after transplanting [22]. According to the authors of that study, all doses resulted in a substantial increase in above-ground and root biomass in comparison to the control. Nevertheless, a frass concentration of 1% resulted in the most favorable performance, which also led to a substantial increase in soil electrical conductivity. Our findings are also consistent with those of Karkanis et al. [40]. On Sonchus oleraceus and Helminthotheca echioides, the authors obtained more leaves, a higher root weight, and a higher above-ground biomass than the control, but no differences were observed between the 0.5%, 1%, and 2% doses during the 105–152-day growth period.
Our findings indicated that the incorporation of 3% mealworm frass did not provide any additional advantages. B. rapa had the same root, epicotyl, and leaf lengths as the control. Such a dose was even harmful to A. cepa, causing a reduction in developing seedlings and an increase in ultimate mortality in accordance with their poor tolerance at salinity [39]. Consequently, dosages of mealworm frass over 2% are considered an unjustified danger on the tested vegetables and under our experimental conditions, unlike high doses of H. illucens frass (up to 20%), which have shown efficacy on baby lettuce, basil, and tomato [28], as well as thermocomposted frass on Brassica oleracea [32].
Consequently, we deduce that the results obtained with low doses of mealworm frass are mostly related to its biostimulative effect on root development and the increased biomass in seedlings, which makes them more fit for transplanting. This hypothesis is consistent with previous studies on the biostimulant characteristics of mealworm frass [17,24], frass from H. illucens [41], and cricket frass [42]. This effect is attributed to the presence in the frass of plant growth-promoting microorganisms (PGPs), which induce greater growth and tolerance to abiotic stresses [17]. Furthermore, the supply of frass improves the soil microbiome by promoting the development of chitinolytic microorganisms [43].
Therefore, the early addition of low doses of mealworm frass (0.5–1%) to substrates can be an effective strategy for the growth of organic seedlings due to the immediate availability of nutrients, the biostimulating effect, and the activation of beneficial soil microbiota. In addition, such minimal doses offer the advantage of minimal cost and reduced environmental impact.
Obviously, the optimal dose of mealworm frass is relative to the vegetables tested, and further research would be necessary on other vegetables of interest that are differently tolerant to salinity. Future research should also assess the field development of seedlings cultivated in this way to validate the potential competitive advantage in the field and against soil diseases.
5. Conclusions
This study showed that mealworm frass can be usefully used in substrates for the organic production of A. cepa, B. vulgaris, and B. rapa seedlings. The application of 0.5–1% mealworm frass was sufficient to improve the growth performance and biomass of seedlings for transplanting. Higher doses (3%) did not produce improvements but increased the risk of phytotoxicity. Therefore, it is hypothesized that the results obtained derive more from a biostimulant effect than from a fertilizing effect. Further studies would be necessary on other vegetables of interest and on the productive performance of these seedlings in the field.
Author Contributions
Conceptualization, F.B. and F.L.; methodology, F.B. and F.L.; validation, F.B. and F.L.; formal analysis, F.B.; investigation, F.B. and F.L.; resources, F.B. and F.L.; data curation, F.B. and F.L.; writing—original draft preparation, F.B.; writing—review and editing, F.B. and F.L.; supervision, F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Rosa Farella for their support in data collection. We thank the anonymous referees for their constructive comments on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: Conventional vs. organic agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Szabó, O.; Pisarčik, M.; Hrevušová, Z.; Hakl, J. Seed treatment potential for the improvement of lucerne seed performance and early field growth. Agronomy 2023, 13, 2207. [Google Scholar] [CrossRef]
- Mancini, V.; Romanazzi, G. Seed treatments to control seedborne fungal pathogens of vegetable crops. Pest Manag. Sci. 2013, 70, 860–868. [Google Scholar] [CrossRef]
- Qiu, Y.; Amirkhani, M.; Mayton, H.; Chen, Z.; Taylor, A.G. Biostimulant seed coating treatments to improve cover crop germination and seedling growth. Agronomy 2020, 10, 154. [Google Scholar] [CrossRef]
- Godínez-Garrido, N.A.; Torres-Castillo, J.A.; Ramírez-Pimentel, J.G.; Covarrubias-Prieto, J.; Cervantes-Ortiz, F.; Aguirre-Mancilla, C.L. Effects on germination and plantlet development of sesame (Sesamum indicum L.) and bean (Phaseolus vulgaris L.) seeds with chitosan coatings. Agronomy 2022, 12, 666. [Google Scholar] [CrossRef]
- Do Prado Mattos, A.; Dinelli, G.; Marotti, I.; Faedo, L.F.; Boff, M.I.C.; Boff, P. Effects of dynamised high dilutions and vegetal extract based on silicon on the growth and induction of resistance in tomato plants against Rhizoctonia solani. Biol. Agric. Hortic. 2024, 1–22. [Google Scholar] [CrossRef]
- Cardarelli, M.; Woo, S.L.; Rouphael, Y.; Colla, G. Seed Treatments with Microorganisms Can Have a Biostimulant Effect by Influencing Germination and Seedling Growth of Crops. Plants 2022, 11, 259. [Google Scholar] [CrossRef]
- Kruker, G.; Guidi, E.S.; Santos, J.M.D.S.D.; Mafra, Á.L.; Almeida, J.A.D. Quality of Bokashi-type Biofertilizer Formulations and its Application in the Production of Vegetables in an Ecological System. Horticulturae 2023, 9, 1314. [Google Scholar] [CrossRef]
- Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. Available online: http://data.europa.eu/eli/reg/2018/848/oj (accessed on 15 November 2024).
- Dey, A.; Srivastava, P.C.; Pachauri, S.P.; Shukla, A.K. Time-dependent release of some plant nutrients from different organic amendments in a laboratory study. Int. J. Recycl. Org. Waste Agric. 2019, 8, 173–188. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2021/1925 of 5 November 2021 Amending Certain Annexes to Regulation (EU) No 142/2011 as Regards the Requirements for Placing on the Market of Certain Insect Products and the Adaptation of a Containment Method. Available online: http://data.europa.eu/eli/reg/2021/1925/oj (accessed on 15 November 2024).
- IPIFF. Contribution Paper on the Application of Insect Frass as Fertilizing Product in Agriculture. Available online: https://ipiff.org/wp-content/uploads/2019/09/19-09-2019-IPIFF-contribution-on-insect-frass-application-as-fertilising-product-final-version.pdf (accessed on 15 November 2024).
- Commission Implementing Regulation (EU) 2021/1165 of 15 July 2021 Authorising Certain Products and Substances for Use in organic Production and Establishing Their Lists. Available online: http://data.europa.eu/eli/reg_impl/2021/1165/oj (accessed on 15 November 2024).
- Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient quality and maturity status of frass fertilizer from nine edible insects. Sci. Rep. 2022, 12, 7182. [Google Scholar] [CrossRef] [PubMed]
- Gärttling, D.; Schulz, H. Compilation of black soldier fly frass analyses. J. Soil Sci. Plant Nutr. 2022, 22, 937–943. [Google Scholar] [CrossRef]
- Amorim, H.C.; Ashworth, A.J.; Arsi, K.; Rojas, M.G.; Morales-Ramos, J.A.; Donoghue, A.; Robinson, K. Insect frass composition and potential use as an organic fertilizer in circular economies. J. Econ. Entomol. 2024, 117, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Addeo, N.F.; Scivicco, M.; Vozzo, S.; Bovera, F.; Asiry, K.A.; Alqurashi, S.; Cacciola, N.A.; Severino, L. Mineral profile and heavy metals bioaccumulation in black soldier fly (Hermetia illucens, L.) larvae and frass across diverse organic substrates. Ital. J. Anim. Sci. 2024, 23, 179–188. [Google Scholar] [CrossRef]
- Baldacchino, F.; Spagnoletta, A.; Lamaj, F.; Vitale, M.L.; Verrastro, V. First optimization of tomato pomace in diets for Tenebrio molitor (L.) (Coleoptera: Tenebrionidae). Insects 2023, 14, 854. [Google Scholar] [CrossRef]
- Chavez, M.; Uchanski, M. Insect left-over substrate as plant fertiliser. J. Insects Food Feed 2021, 7, 683–694. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Musyoka, M.W.; Ekesi, S.; et al. Exploring black soldier fly frass as novel fertilizer for improved growth, yield, and nitrogen use efficiency of maize under field conditions. Front. Plant Sci. 2020, 11, 574592. [Google Scholar] [CrossRef]
- Antoniadis, V.; Molla, A.; Grammenou, A.; Apostolidis, V.; Athanassiou, C.G.; Rumbos, C.I.; Levizou, E. Insect Frass as a Novel Organic Soil Fertilizer for the Cultivation of Spinach (Spinacia oleracea): Effects on Soil Properties, Plant Physiological Parameters, and Nutrient Status. J. Soil Sci. Plant Nutr. 2023, 23, 5935–5944. [Google Scholar] [CrossRef]
- Chia, S.Y.; van Loon, J.J.; Dicke, M. Effects of frass from larvae of black soldier fly (Hermetia illucens) and yellow mealworm (Tenebrio molitor) on growth and insect resistance in field mustard (Brassica rapa): Differences between insect species and frass treatments. Entomol. Exp. Appl. 2024, 172, 394–408. [Google Scholar] [CrossRef]
- Foughar, M.; Arrobas, M.; Rodrigues, M.Â. Mealworm Larvae Frass Exhibits a Plant Biostimulant Effect on Lettuce, Boosting Productivity beyond Just Nutrient Release or Improved Soil Properties. Horticulturae 2024, 10, 711. [Google Scholar] [CrossRef]
- Arabzadeh, G.; Delisle-Houde, M.; Dorais, M.; Deschamps, M.H.; Derome, N.; Vandenberg, G.W.; Tweddell, R.J. Evaluation of the antagonistic activity of black soldier fly frass extracts against plant pathogens using single-and double-layer agar bioassays. J. Insects Food Feed 2024, 1, 1–10. [Google Scholar] [CrossRef]
- Arabzadeh, G.; Delisle-Houde, M.; Vandenberg, G.W.; Deschamps, M.H.; Dorais, M.; Derome, N.; Tweddell, R.J. Suppressive Effect of Black Soldier Fly Larvae Frass on Fusarium Wilt Disease in Tomato Plants. Insects 2024, 15, 613. [Google Scholar] [CrossRef] [PubMed]
- Borkent, S.; Hodge, S. Glasshouse Evaluation of the Black Soldier Fly Waste Product HexaFrass™ as an Organic Fertilizer. Insects 2021, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Francia, E.; Pulvirenti, A.; Gigliano, S.; Zaccardelli, M.; Pane, C.; Caradonia, F.; Bortolini, S.; Maistrello, L.; Ronga, D. Use of black soldier fly (Hermetia illucens (L.), Diptera: Stratiomyidae) larvae processing residue in peat-based growing media. Waste Manag. 2019, 95, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Praeg, N.; Klammsteiner, T. Primary study on frass fertilizers from mass-reared insects: Species variation, heat treatment effects, and implications for soil application at laboratory scale. J. Environ. Manag. 2024, 356, 120622. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.P.; Dulaurent, A.M. Potential use of mealworm frass as a fertilizer: Impact on crop growth and soil properties. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 15 November 2024).
- González-Lara, H.; Parra-Pacheco, B.; Aguirre-Becerra, H.; Feregrino-Perez, A.A.; Garcia-Trejo, J.F. Effects of Using Thermocomposted Frass from Black Soldier Fly Larvae as a Germination Substrate on the Phytotoxicity, Germination Index, Growth and Antioxidant Contents in Kale (Brassica oleracea). Agronomy 2024, 14, 1392. [Google Scholar] [CrossRef]
- Coviello, L.; Nuzzaci, M.; Falabella, P.; Scieuzo, C.; Salvia, R.; Ronga, D.; Vitti, A. Innovative Use of Hermetia illucens Frass Extract as Priming to Promote Tomato and Wheat Growth and Protection. J. Sustain. Agric. Environ. 2024, 3, e70030. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.T.; Paradelo, R. A review on the use of phytotoxicity as a compost quality indicator. Dyn. Soil Dyn. Plant 2011, 5, 36–44. [Google Scholar]
- Zunzunegui, I.; Martín-García, J.; Santamaría, Ó.; Poveda, J. Analysis of yellow mealworm (Tenebrio molitor) frass as a resource for a sustainable agriculture in the current context of insect farming industry growth. J. Clean. Prod. 2024, 460, 142608. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.; Musyoka, M.W.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Ekesi, S.; et al. Nitrogen fertilizer equivalence of black soldier fly frass fertilizer and synchrony of nitrogen mineralization for maize production. Agronomy 2020, 10, 1395. [Google Scholar] [CrossRef]
- Bohm, K.; Hatley, G.A.; Robinson, B.H.; Gutiérrez-Ginés, M.J. Analysis of Chemical and Phytotoxic Properties of Frass Derived from Black Soldier Fly-Based Bioconversion of Biosolids. Sustainability 2023, 15, 11526. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Karkanis, A.; Asprogeraka, A.C.; Paouris, E.; Ntanasi, T.; Karavidas, I.; Rumbos, C.I.; Ntatsi, G. Yellow mealworm frass: A promising organic fertilizer for common sowthistle (Sonchus oleraceus L.) and bristly oxtongue (Helminthotheca echioides (L.) Holub) cultivation. Heliyon 2024, 10, e35508. [Google Scholar] [CrossRef]
- Radzikowska-Kujawska, D.; Sawinska, Z.; Grzanka, M.; Kowalczewski, P.Ł.; Sobiech, Ł.; Świtek, S.; Skrzypczak, G.; Drożdżyńska, A.; Ślachciński, M.; Nowicki, M. Hermetia illucens frass improves the physiological state of basil (Ocimum basilicum L.) and its nutritional value under drought. PLoS ONE 2023, 18, e0280037. [Google Scholar] [CrossRef]
- Ferruzca-Campos, E.A.; Rico-Chavez, A.K.; Guevara-González, R.G.; Urrestarazu, M.; Cunha-Chiamolera, T.P.L.; Reynoso-Camacho, R.; Guzmán-Cruz, R. Biostimulant and elicitor responses to cricket frass (Acheta domesticus) in tomato (Solanum lycopersicum L.) under protected conditions. Plants 2023, 12, 1327. [Google Scholar] [CrossRef] [PubMed]
- Nurfikari, A.; Leite, M.F.A.; Kuramae, E.E.; de Boer, W. Microbial community dynamics during decomposition of insect exuviae and frass in soil. Soil Biol. Biochem. 2024, 194, 109426. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).