Physical Conditions That Limit Chickpea Root Growth and Emergence in Heavy-Textured Soil
Abstract
:1. Introduction
- The limitations to chickpea emergence and root growth with increasing bulk density and soil strength; and
- The extent to which chickpea emergence is limited by different above-seed or below-seed soil physical conditions.
2. Materials and Methods
2.1. Experimental Design
2.2. Details of Experiment 1
2.3. Details of Experiment 2
2.4. Plant Measurements
2.5. Statistical Analysis
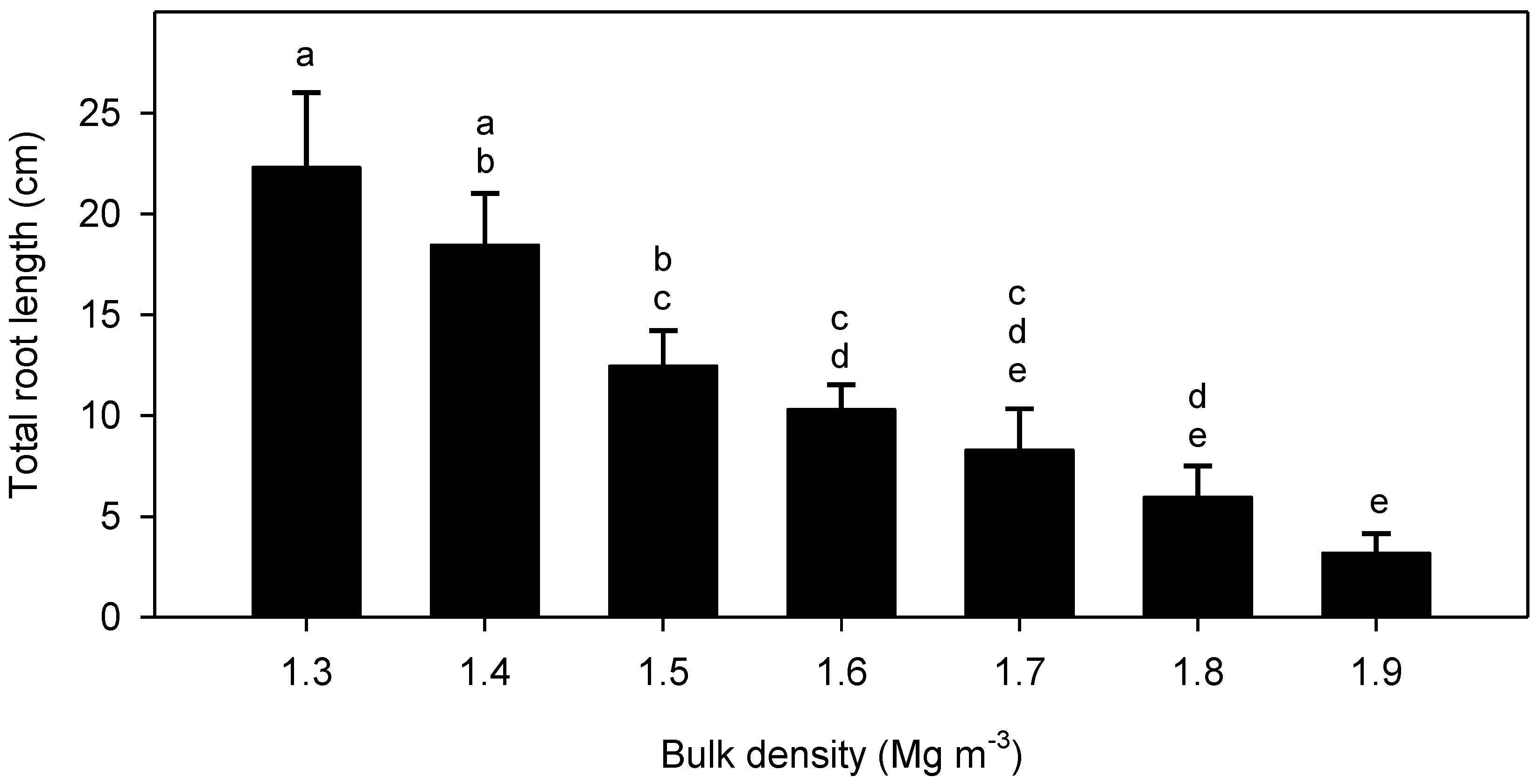
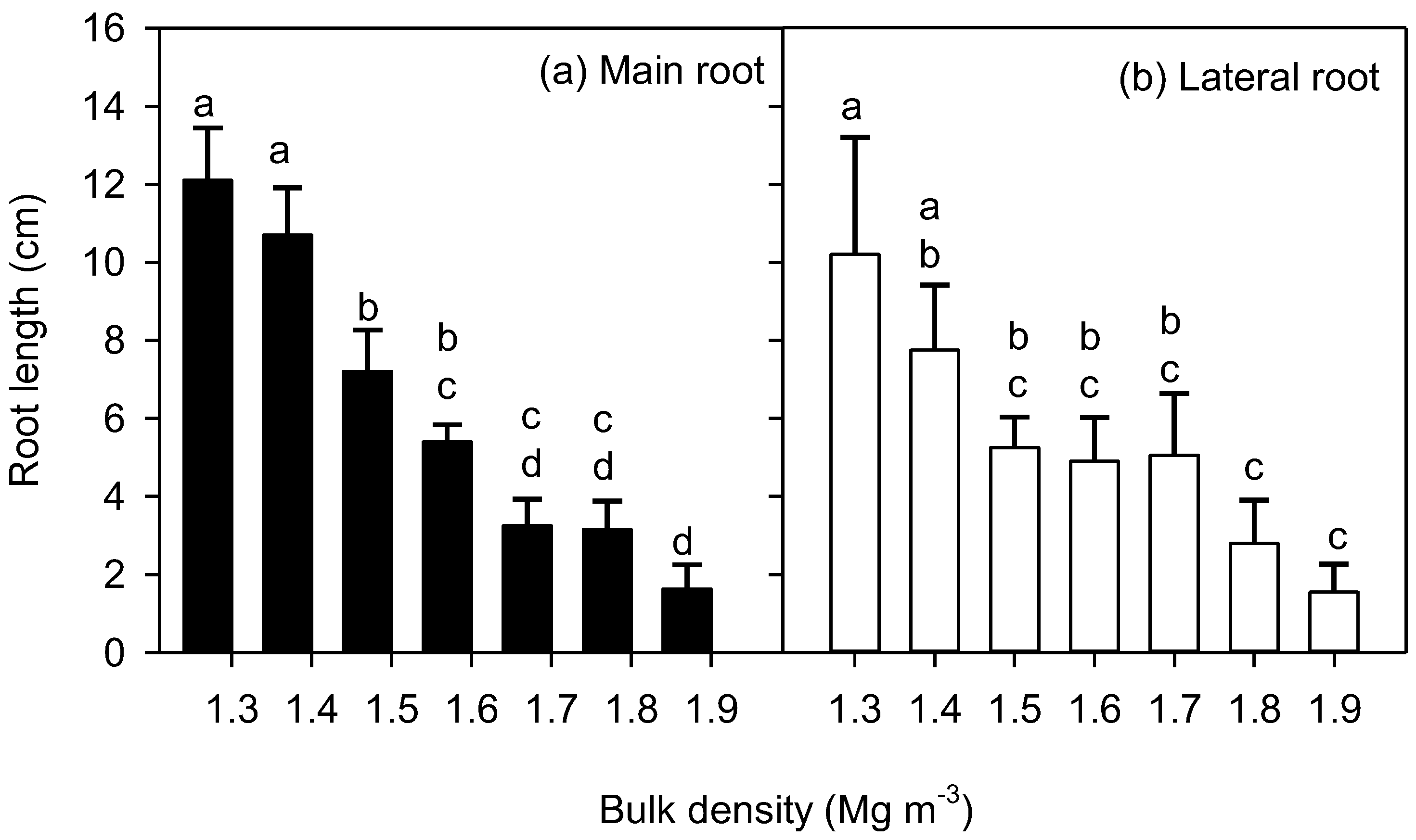
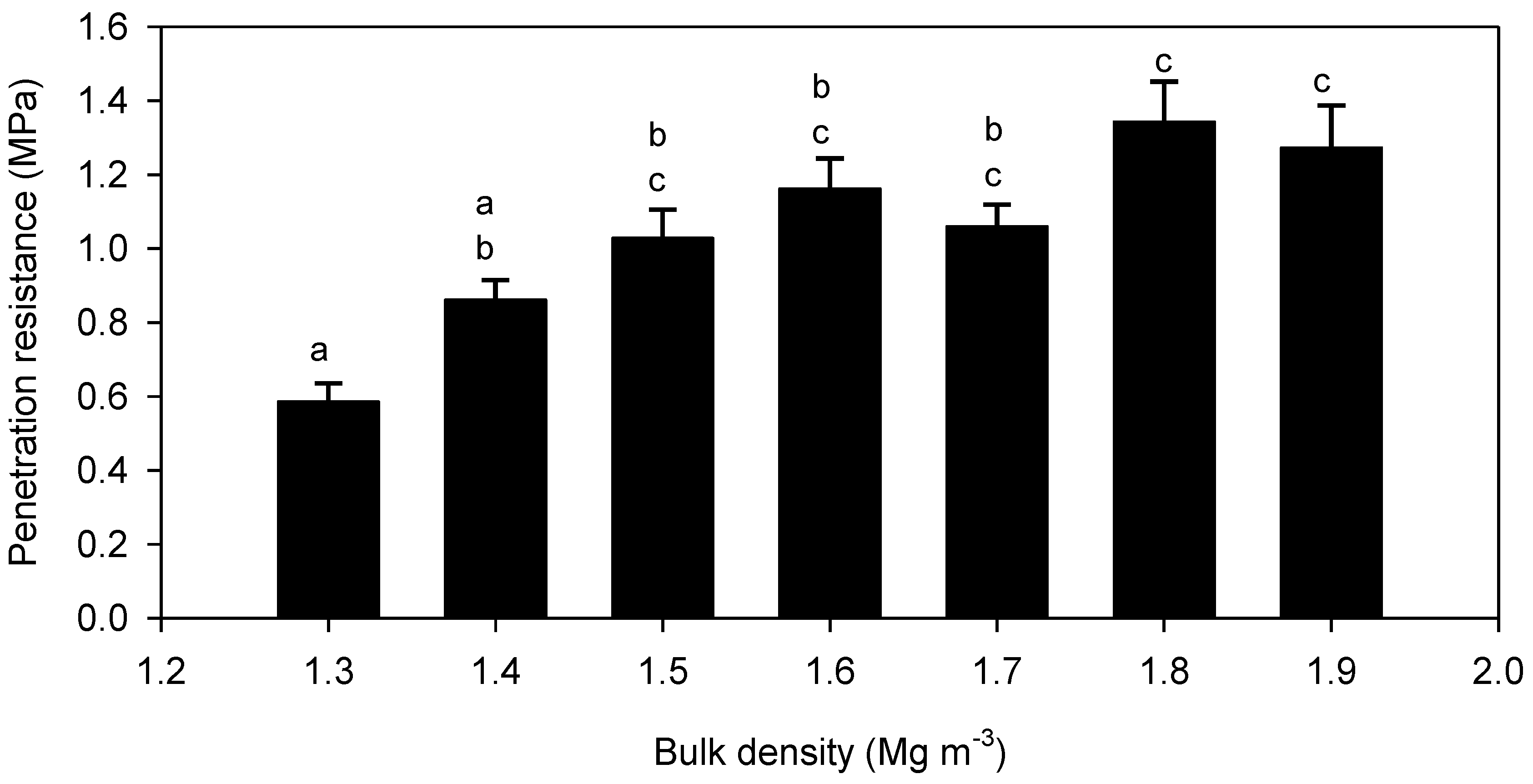
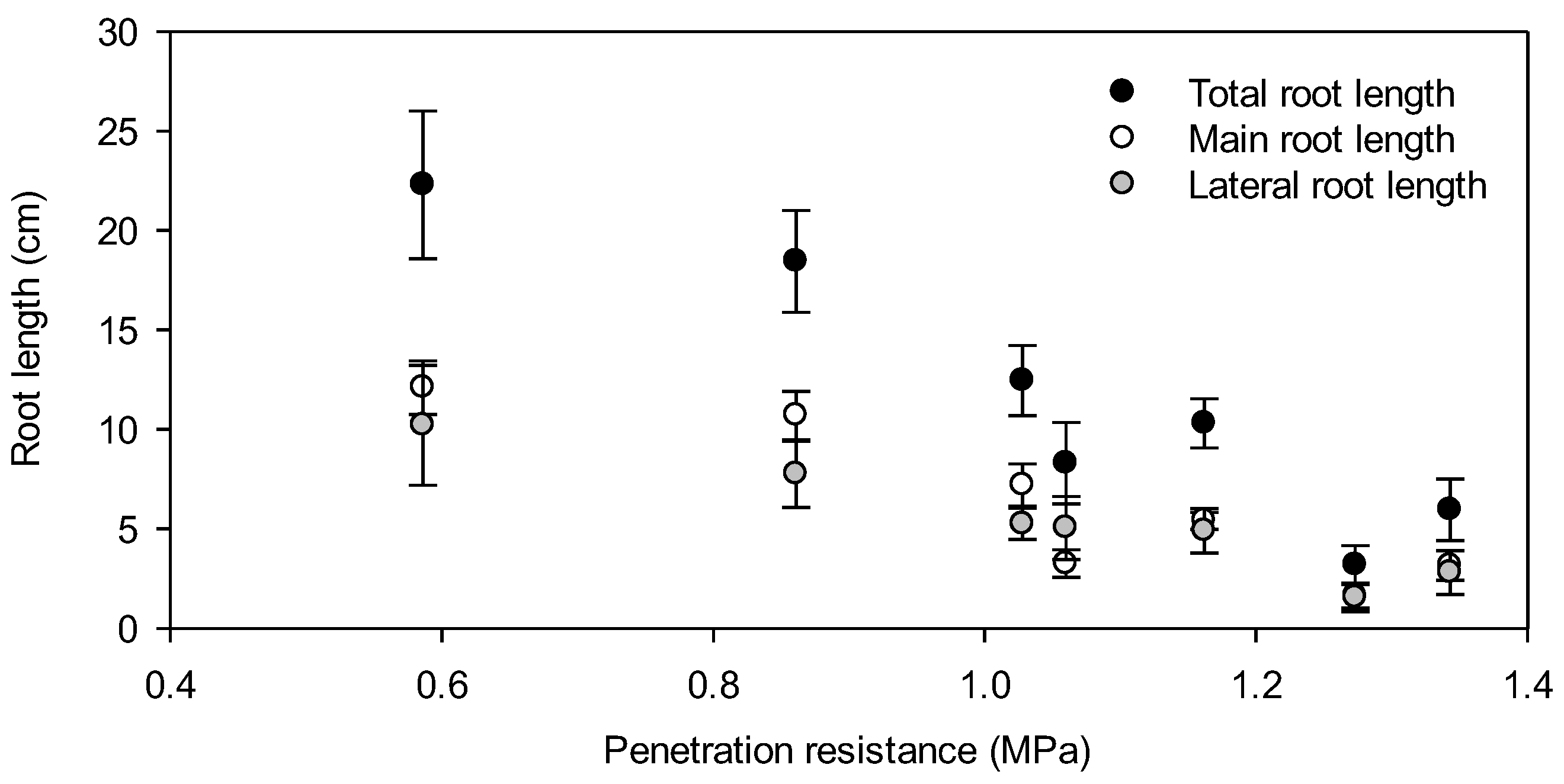
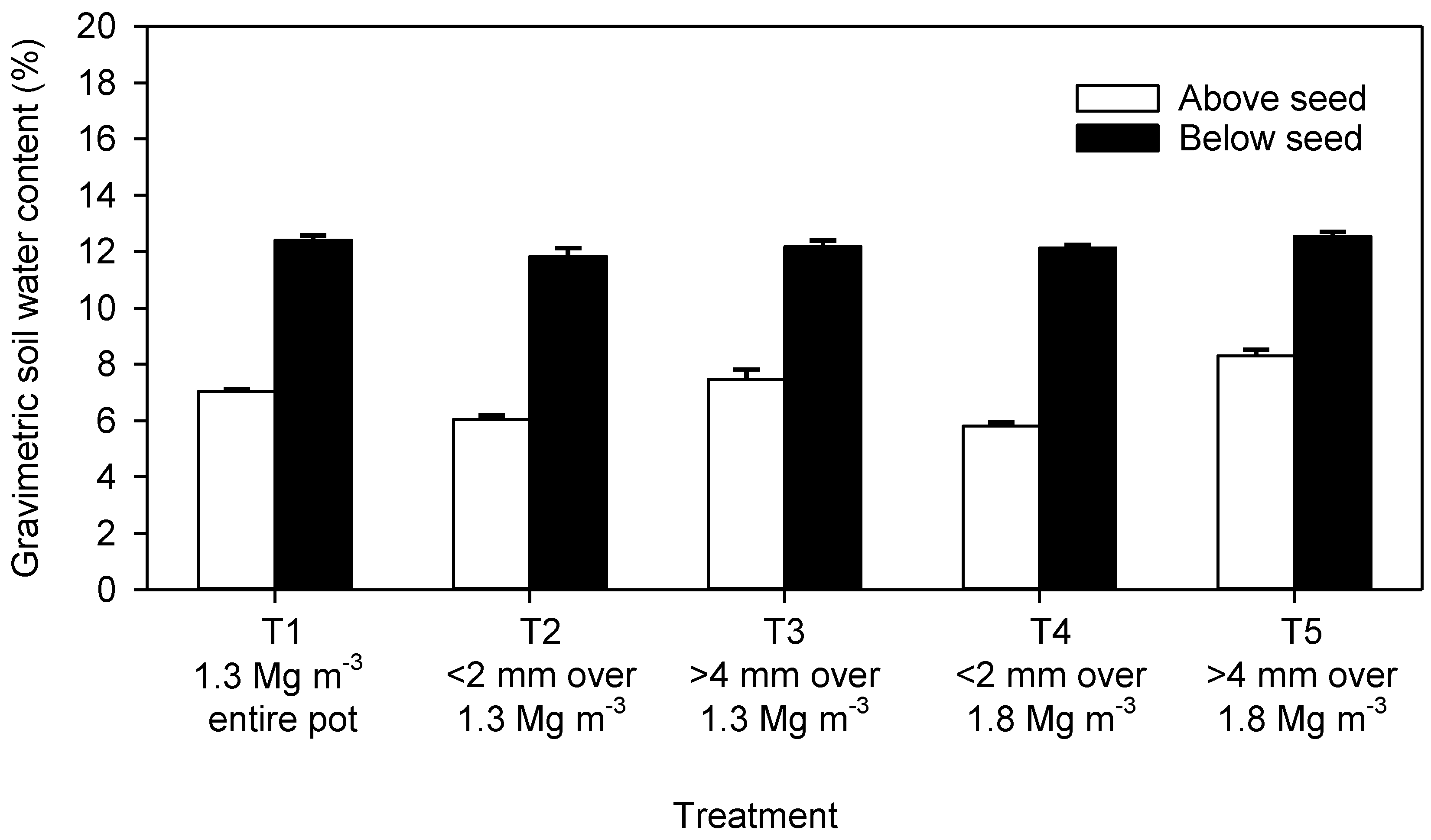
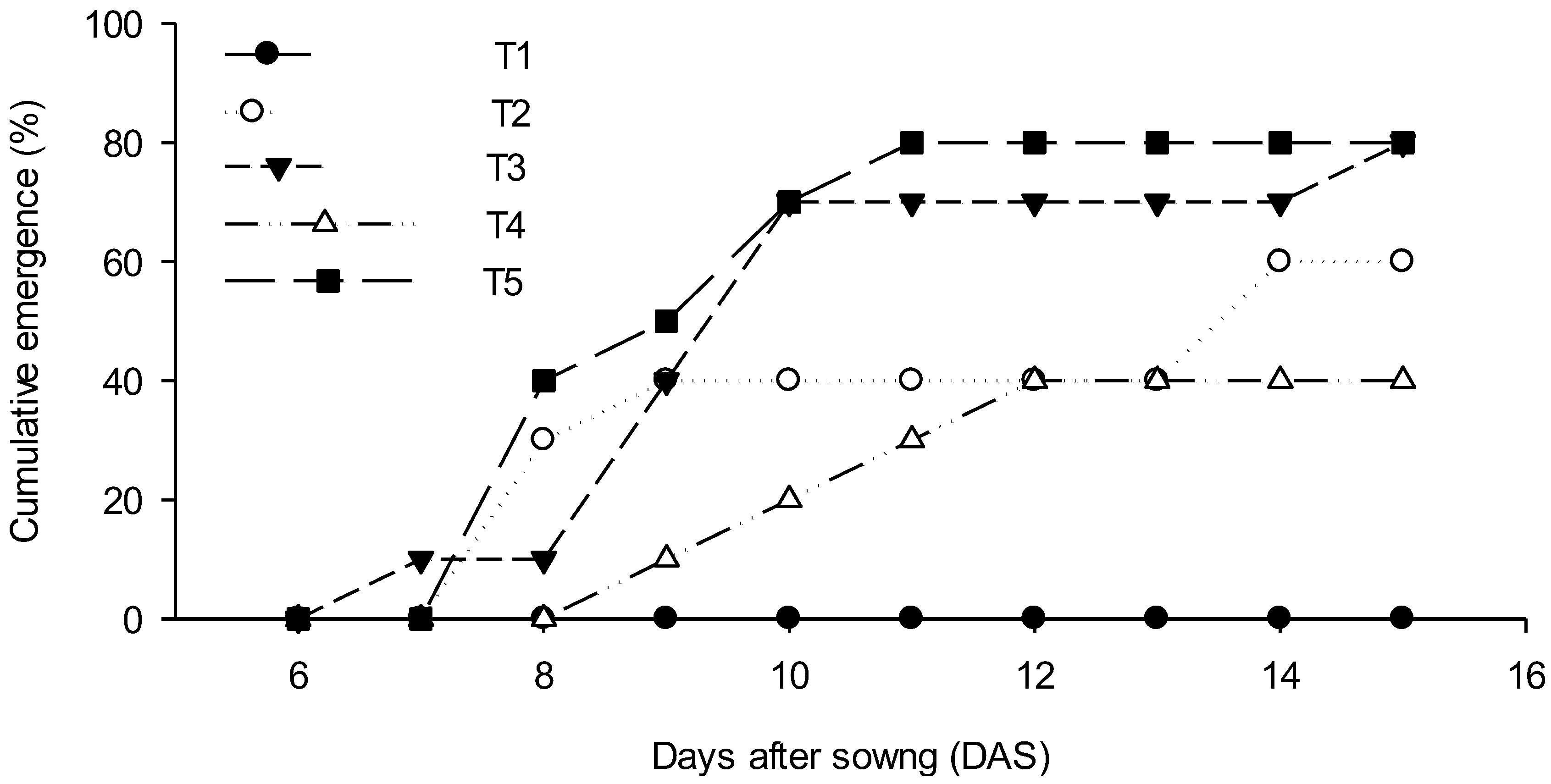
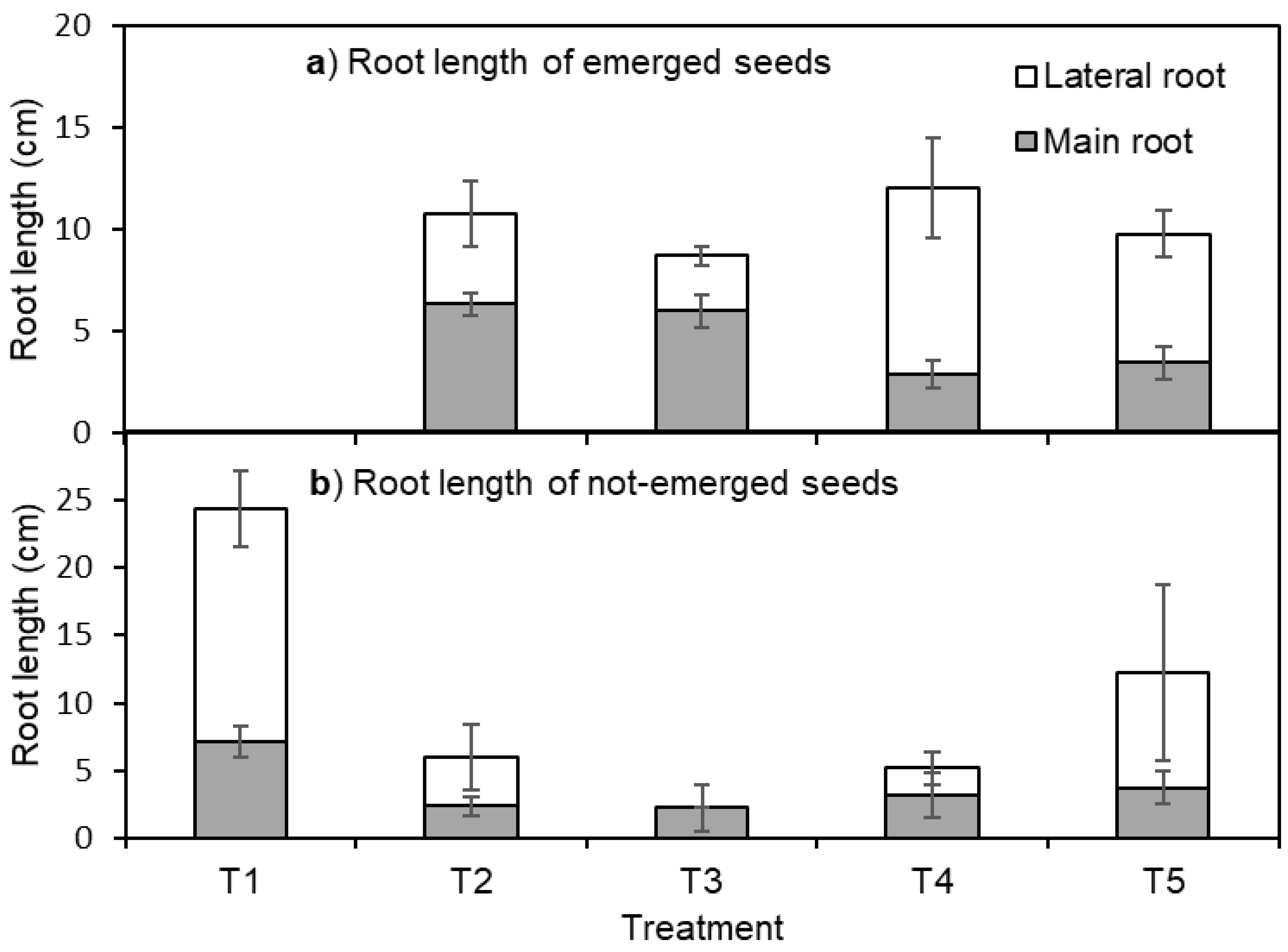
3. Results
4. Discussion
4.1. Seedling Emergence
4.2. Root Elongation
4.3. Implications for Field Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Available online: http://faostat.fao.org (accessed on 13 December 2023).
- Merga, B.; Haji, J. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Johansen, C.; Bakr, M.A.; Islam, M.S.; Mondal, N.A.; Afzal, A.; Macleod, W.J.; Pande, S.; Siddique, K.H.M. Integrated crop management of chickpea in environments of Bangladesh prone to Botrytis grey mould. Field Crop. Res. 2008, 108, 238–249. [Google Scholar] [CrossRef]
- Li, X.; Waddington, S.; Dixon, J.; Joshi, A.; de Vicente, M.C. The relative importance of drought and other water-related constraints for major food crops in South Asian farming systems. Food Sec. 2011, 3, 19–33. [Google Scholar] [CrossRef]
- Ali, M.Y. Influence of Phosphorus Fertilizer and Soil Moisture Regimes on Root System Development, Growth Dynamics and Yield of Chickpea. Ph.D. Thesis, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh, 2000. [Google Scholar]
- Johansen, C.; Musa, A.M.; Harris, D.; Islam, M.S.; Ali, M.O. Integration of chickpea and other rabi crops into rainfed rice-based systems of the High Barind Tract. In Improving Agricultural Productivity in Rice-Based Systems of the High Barind Tract of Bangladesh; Riches, C.R., Harris, D., Johnson, D.E., Hardy, B., Eds.; International Rice Research Institute: Los Banos, Phillipines, 2008; pp. 135–146. [Google Scholar]
- Johansen, C.; Haque, M.E.; Bell, R.W.; Thierfelder, C.; Esdaile, R.J. Conservation agriculture for small holder rainfed farming: Opportunities and constraints of new mechanized seeding systems. Field Crop. Res. 2012, 132, 18–32. [Google Scholar] [CrossRef]
- Gathala, M.K.; Timsina, J.; Islam, M.S.; Rahman, M.M.; Hossain, M.I.; Harun-Ar-Rashid, M.; Ghosh, A.K.; Krupnik, T.J.; Tiwari, T.P.; McDonald, A. Conservation agriculture based tillage and crop establishment options can maintain farmers’ yields and increase profits in South Asia’s rice–maize systems: Evidence from Bangladesh. Field Crop. Res. 2015, 172, 85–98. [Google Scholar] [CrossRef]
- Baker, C.J.; Saxton, K.E. The ‘what’ and ‘why’ of no-tillage farming. In No-Tillage Seeding in Conservation Agriculture, 2nd ed.; Baker, C.J., Saxton, K.E., Ritchie, W.R., Chamen, W.C.T., Reicosky, D.C., Ribeiro, M.F.S., Justice, S.E., Hobbs, P.R., Eds.; FAO and CAB International: Rome, Italy, 2007; pp. 1–10. [Google Scholar]
- Vance, W.H.; Bell, R.W.; Johansen, C. Optimum soil water content for chickpea emergence in heavy-textured soils of north-west Bangladesh. J. Agron. Crop. Sci. 2015, 201, 195–205. [Google Scholar] [CrossRef]
- Hossain, I.; Esdaile, R.J.; Bell, R.B.; Holland, C.; Haque, E.; Sayre, K.; Alam, M.K. Actual challenges: Developing low cost no-till seeding technologies for heavy residues; small scale no-till seeders for two wheel tractors. In Proceedings of the 4th World Congress of Conservation Agriculture, New Delhi, India, 4–7 February 2009; pp. 171–177. [Google Scholar]
- Vance, W.H. Overcoming Soil Water Constraints to Chickpea Yield in Rainfed Environments of Western Australia and Bangladesh. Ph.D. Thesis, Murdoch University, Perth, Australia., 2013. [Google Scholar]
- Fyfield, T.P.; Gregory, P.J. Effects of temperature and water potential on germination, radicle elongation and emergence of mungbean. J. Exp. Bot. 1989, 40, 667–674. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; So, H.B.; Troedson, R.J. The effect of soil strength on the growth of pigeonpea radicles and seedlings. Plant Soil 1992, 140, 65–74. [Google Scholar] [CrossRef]
- Nasr, H.M.; Selles, F. Seedling emergence as influenced by aggregate size, bulk density, and penetration resistance of the seedbed. Soil Tillage Res. 1995, 34, 61–76. [Google Scholar] [CrossRef]
- Harris, D.; Joshi, A.; Khan, P.A.; Gothkar, P.; Sodhi, P.S. On-farm seed priming in semi-arid agriculture: Development and evaluation in maize, rice and chickpea in India using participatory methods. Exp. Agric. 1999, 35, 15–29. [Google Scholar] [CrossRef]
- Soltani, A.; Robertson, M.J.; Torabi, B.; Yousefi-Daz, M.; Sarparast, R. Modelling seedling emergence in chickpea as influenced by temperature and sowing depth. Agric. For. Meteorol. 2006, 138, 156–167. [Google Scholar] [CrossRef]
- Materechera, S.A.; Dexter, A.R.; Alston, A.M. Penetration of very strong soils by seedling roots of different plant species. Plant Soil 1991, 135, 31–41. [Google Scholar] [CrossRef]
- Bengough, A.G.; Young, I.M. Root elongation of seedling peas through layered soil of different penetration resistances. Plant Soil 1993, 149, 129–139. [Google Scholar] [CrossRef]
- Unkovich, M.; McKenzie, D.; Parker, W. New insights into high soil strength and crop plants; implications for grain crop production in the Australian environment. Plant Soil 2023, 486, 183–208. [Google Scholar] [CrossRef]
- Dexter, A. Model experiments on the behaviour of roots at the interface between a tilled seed-bed and a compacted sub-soil. Plant Soil 1986, 95, 123–133. [Google Scholar] [CrossRef]
- Dorsainvil, F.; Durr, C.; Justes, E.; Carrera, A. Characterisation and modelling of white mustard (Sinapis alba L.) emergence under several sowing conditions. Eur. J. Agron. 2005, 23, 146–158. [Google Scholar] [CrossRef]
- Sivaprasad, B.; Sundara Sarma, K. Seedling emergence of chickpea (Cicer arietinum L.), pigeonpea (Cajanus cajan L.) and pearl millet (Pennisetum typhoides L.) Effect of differential soil crusting, as induced by raindrop size, and depth of sowing. Plant Soil 1987, 104, 263–268. [Google Scholar] [CrossRef]
- Iqbal, M.; Marley, S.J.; Erbach, D.C.; Kaspar, T.C. An evaluation of seed furrow smearing. Trans. ASAE 1998, 41, 1243–1248. [Google Scholar] [CrossRef]
- Muñoz-Romero, V.; López-Bellido, L.; López-Bellido, R.J. The effects of the tillage system on chickpea root growth. Field Crop. Res. 2012, 128, 76–81. [Google Scholar] [CrossRef]
- Isbell, R.F. The Australian Soil Classification; CSIRO: Canberra, Australia, 1996; p. 143. [Google Scholar]
- Harries, M.; Regan, K.; White, P.F. Growing chickpea. In Producing Pulses in the Northern Agricultural Region Bulletin 4656; White, P., Harries, M., Seymour, M., Burgess, P., Eds.; Department of Agriculture: Perth, Western Australia, 2005; pp. 19–28. [Google Scholar]
- van Genuchten, M.T.; Leij, F.J.; Yates, S.R. The RETC Code for Quantifying the Hydraulic Functions of Unsaturated Soils; EPA/600/2-91/065; U.S. Salinity Laboratory, U.S. Department of Agriculture: Riverside, CA, USA, 1991; p. 85. [Google Scholar]
- Masle, J.; Passioura, J.B. The effects of soil strength on the growth of young wheat plants. Aust. J. Plant Physiol. 1987, 14, 643–656. [Google Scholar] [CrossRef]
- Choudhary, K.; Mohanty, M.; Sinha, N.; Rawat, A.; Hati, K.; Saha, R.; Jayaraman, S.; Chaudhary, R. Rooting behaviour of chickpea (Cicer arietinum) as affected by soil compaction levels in Vertisol of central India. Indian J. Agric. Sci. 2015, 85, 1085–1091. [Google Scholar] [CrossRef]
- Murungu, F.S.; Nyamugafata, P.; Chiduza, C.; Clark, L.J.; Whalley, W.R. Effects of seed priming, aggregate size and soil matric potential on emergence of cotton (Gossypium hirsutum L.) and maize (Zea mays L.). Soil Tillage Res. 2003, 74, 161–168. [Google Scholar] [CrossRef]
- Cook, S.M.F.; Gupta, S.C.; Woodhead, T.; Larson, W.E. Soil physical constraints to establishment of mungbeans (Vigna radiata L. Wilczek) in paddy rice (Oryza sativa L.) soils. Soil Tillage Res. 1995, 33, 47–64. [Google Scholar] [CrossRef]
- Dasberg, S. Soil water movement to germinating seeds. J. Exp. Bot. 1971, 22, 999–1008. [Google Scholar] [CrossRef]
- Iijima, M.; Kato, J. Combined soil physical stress of soil drying, anaerobiosis and mechanical impedance to seedling root growth of four crop species. Plant Prod Sci. 2007, 10, 451–459. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Clark, L.J.; Whalley, W.R.; Dexter, A.R.; Barraclough, P.B.; Leigh, R.A. Complete mechanical impedance increases the turgor of cells in the apex of pea roots. Plant Cell Environ. 1996, 19, 1099–1102. [Google Scholar] [CrossRef]
- Clark, L.J.; Whalley, W.R.; Barraclough, P.B. How do roots penetrate strong soil? Plant Soil 2003, 255, 93–104. [Google Scholar] [CrossRef]
- Dexter, A.R. Advances in characterization of soil structure. Soil Tillage Res. 1988, 11, 199–238. [Google Scholar] [CrossRef]
- Matin, M.A.; Hossain, M.I.; Gathala, M.K.; Timsina, J.; Krupnik, T.J. Optimal design and setting of rotary strip-tiller blades to intensify dry season cropping in Asian wet clay soil conditions. Soil Tillage Res. 2021, 207, 104854. [Google Scholar] [CrossRef]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Mahmud, M.N.H. Minimum Soil Disturbance Planting for Rice-Based Rotations in Northwest Bangladesh: Effects on Plough Pan and Water Balance. Ph.D. Thesis, Murdoch University, Perth, Australia, 2021. [Google Scholar]
- Gürsoy, S.; Türk, Z. Effects of land rolling on soil properties and plant growth in chickpea production. Soil Tillage Res. 2019, 195, 104425. [Google Scholar] [CrossRef]
| Core Section | Soil Treatment | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | Treatment 5 |
|---|---|---|---|---|---|---|
| Soil aggregates | <4 mm | <2 mm | >4 mm | <2 mm | >4 mm | |
| Top core | Gravimetric soil water content (%) | 14 | 10 | 14 | 10 | 14 |
| Bulk density (Mg m−3) | 1.3 | 0.98 | 0.98 | 0.98 | 0.98 | |
| Soil aggregates | <4 mm | <4 mm | <4 mm | <4 mm | <4 mm | |
| Base core | Gravimetric soil water content (%) | 14 | 14 | 14 | 14 | 14 |
| Bulk density (Mg m−3) | 1.3 | 1.3 | 1.3 | 1.8 | 1.8 |
| Treatment | Treatment Description (Above-Seed Aggregates Size, Below-Seed Bulk Density) | Emerged (%) | Not-Emerged Root and Shoot Development (%) | Not-Emerged Root Development Only (%) | Not-Germinated (%) |
|---|---|---|---|---|---|
| T1 | T1, 1.3 Mg m−3 entire pot | 0 | 70 | 30 | 0 |
| T2 | T2, <2 mm over 1.3 Mg m−3 | 60 | 30 | 0 | 10 |
| T3 | T3, >4 mm over 1.3 Mg m−3 | 80 | 10 | 0 | 10 |
| T4 | T4, <2 mm over 1.8 Mg m−3 | 40 | 30 | 30 | 0 |
| T5 | T5, >4 mm over 1.8 Mg m−3 | 80 | 20 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vance, W.H.; Bell, R.W.; Johansen, C. Physical Conditions That Limit Chickpea Root Growth and Emergence in Heavy-Textured Soil. Seeds 2024, 3, 26-39. https://doi.org/10.3390/seeds3010003
Vance WH, Bell RW, Johansen C. Physical Conditions That Limit Chickpea Root Growth and Emergence in Heavy-Textured Soil. Seeds. 2024; 3(1):26-39. https://doi.org/10.3390/seeds3010003
Chicago/Turabian StyleVance, Wendy H., Richard W. Bell, and Chris Johansen. 2024. "Physical Conditions That Limit Chickpea Root Growth and Emergence in Heavy-Textured Soil" Seeds 3, no. 1: 26-39. https://doi.org/10.3390/seeds3010003
APA StyleVance, W. H., Bell, R. W., & Johansen, C. (2024). Physical Conditions That Limit Chickpea Root Growth and Emergence in Heavy-Textured Soil. Seeds, 3(1), 26-39. https://doi.org/10.3390/seeds3010003






