Abstract
The in vitro propagation and ex situ conservation strategy provides new means for the conservation and mass propagation of economically and medicinally important plants. The present work aimed to observe the main characteristics of the in vitro propagation of Garcinia livingstonei from matured seed segments. Successful multiple shoots were induced on a woody plant (WP) medium supplemented with cytokinins. An average of 13.0 shoots per explant were grown from matured seed segments on a WP medium containing 15.0 µM BAP after 12 weeks of culture. The shoot elongation and multiplication were achieved using a repeated and periodic subculturing of shoot clumps in the same medium. The optimum in vitro rooting of shoots was obtained on the half-strength WP medium supplemented with IBA (5.0 µM). The regenerated plantlets were successfully transplanted to pots containing soil, sand, and farmyard manure (1:1:1) and were maintained in a greenhouse with a survival frequency was 80%.
1. Introduction
Garcinia livingstonei T. Anderson (African mangosteen) belongs to the family Clusiaceae. It is a tree species that grows up to 10–16 m in height and has waxy leaves. It is native to the warmer part of Africa and exotic to India, Indonesia, Singapore, and Australia. The fruits are smaller in size, with a yellowish- to reddish-orange color and a soft, juicy edible orange pulp with a slightly acidic and sweet flavor. The fruits are rich in carbohydrates and have moderate mineral content [1]. The plants contain different phytochemicals which are accountable for anticancer [2], antimicrobial [3], antiviral [4,5], central nervous system (CNS) [6], and anti-parasitic [6,7] activities. The plant is used in traditional medicine as an aphrodisiac and to cure abdominal pain during pregnancy and after childbirth and also used as an antibiotic and for the treatment of mumps [8]. The fruits are fermented and used to prepare some alcoholic beverages [9]. The fruits possess high nutritive value and the seeds are rich in fatty acid composition [9]. Due to its horticultural and medicinal importance, the global demand for Garcinia products is increasing. The propagation of this species through seeds is difficult, its seeds have a short shelf life and propagation through softwood grafting is not effective. There are many reports on the regeneration of different Garcinia species through in vitro micropropagation such as Garcinia xanthochymus [10], G. mangostana [11,12], and G. indica [13,14,15]. However, there are no regeneration protocols for the mass multiplication and conservation of Garcinia livingstonei, therefore the present investigation was taken up to develop an efficient in vitro propagation through mature seed explants.
The in vitro culture of plants is having a tremendous role in the conservation of genetic resources. One of the areas is the regeneration of rare and threatened plant species, their reintroduction, and restoration in natural habitats or introducing clones from one population into areas where populations are dwindling. This can promote an increase in the genetic variability of the populations [16]. Another major area of application of in vitro culture is the preservation of genetic material using cryopreservation [17]. The in vitro regeneration protocol developed for Garcinia livingstonei is useful for the ex situ conservation of this useful plant.
2. Materials and Methods
2.1. Plant Material, Media Preparation, and Culture Conditions
The ripened fruits of Garcinia livingstonei (Figure 1A), which are a yellowish red or orange in color, were collected from the plant growing in the Botanical Garden, Karnatak University, Dharwad, Karnataka, India. From the collected fruits, pulp was removed with seeds washed under running tap water for about 10 min. The seeds after removing the seed coat were treated with 1% Bavistin fungicide (w/v) for 5 min followed by 10% liquid detergent Triton X 100 (v/v) for 3–4 min and Tween 20 (Himedia, India) for 5 min. Then, the seeds were treated with 0.1% Mercuric chloride (w/v) for 5 min and with 70% ethanol for 1 min under the laminar airflow chamber. After every treatment with chemical sterilant, seeds were washed with sterile distilled water three times for 3 min each. The seeds were cut into four pieces by cutting once vertically and then horizontally (Scheme 1). The seed portions were cultured on a woody plant medium (WP) [18] supplemented with 3% sucrose (w/v), 0.8% agar (w/v), and growth regulators including 6-benzyl amino purine (BAP) or kinetin (KN) or thidiazuron (TDZ) at 10, 15, and 20 µM concentrations. The pH of the medium was set at 5.8 before autoclaving the medium for 15 min at 15 psi and 121 °C. Explants were subcultured onto the fresh medium containing the same growth regulator once in 4 weeks.

Figure 1.
Morphogenetic response of explants obtained from mature seeds and in vitro developed plantlets of Garcinia livingstonei T. Anderson. (A) Ripened fruits of G. livingstonei (Bar = 1.5 cm), (B) Shoot buds initiated from seed segments cultured on WP medium with 15 µM BAP after 4 weeks of culture (Bar = 0.375 cm), (C) Multiple shoots developed from seed explants cultured on WP medium supplemented with 15 µM BAP after 8 weeks of culture (Bar = 0.35 cm), (D) Well-developed multiple shoots regenerated on WP medium containing 15 µM BAP after 12 weeks of culture (Bar = 1.95 cm), (E) Rooted plantlet with primary and secondary roots with 10 µM IBA (Bar = 1.25 cm), (F) Plant transferred to the pot containing a mixture of garden soil, sand, and farmyard manure (1:1:1) (Bar = 7.75 cm).
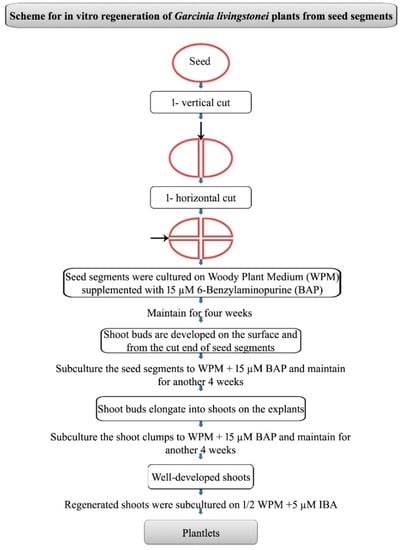
Scheme 1.
In vitro regeneration of Garcinia livingstonei from seed segments.
2.2. Root Induction
The elongated shoots were separated from the regeneration media and transferred to a rooting medium consisting of a half-strength WP medium supplemented with 3% (w/v) sucrose and 0.8% agar (w/v) and 2, 5, 10, and 20 µM IBA (Indole-3-butyric acid) or NAA (α-Naphthalene acetic acid). The pH was adjusted to 5.8 before autoclaving at 121 °C temperature for 20 min. All chemicals, growth regulators, sucrose, and agar were procured from Hi-media laboratories, Mumbai, India. The cultures were maintained in a culture room at 25 ± 2 °C under a 16/8 h (light/dark) photoperiod of 50 µmol m−2 s−1 irradiance with cool fluorescent tubes (Philips, India) and with 60% relative humidity.
2.3. Acclimatization of Regenerated Plants
The well-grown in vitro rooted plants of length 4–5 cm were removed from the culture media and the roots were thoroughly washed with distilled water to remove the adhering media from the plants. Then, the rooted plants were transferred to pots containing an autoclaved potting mixture of cocopeat: perlite: vermiculite (1:1:1). The transferred plants were allowed to grow in growth chambers, where the temperature was adjusted to 24 ± 2 °C, and the relative humidity 80%, and the irradiance of 50 µmol m−2 s−1 for 16 h light and 8 h dark photoperiods. The plants were irrigated once a week using a Hoagland solution. After 4 weeks, the plants were transferred to large pots containing soil, sand, and farmyard manure (1:1:1) and maintained in the greenhouse.
2.4. Statistical Analysis
The analysis of variance (ANOVA) was carried out to detect the difference between the treatment means and was compared using a Duncan’s Multiple Range Test (DMRT) at a 5% probability level using SPSS software version 20.0 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Multiple Shoot Regeneration
The seed segments that were cultured on the WP medium initially demonstrated swelling and developed shoot buds from the surfaces of the seed segments as well as from the cut surfaces within two weeks of culture on a medium supplemented with BAP and KN. Such shoot buds developed further in another two weeks in culture. Amongst the three cytokinins tested, BAP was most effective for adventitious shoot bud differentiation in G. livingstonei. An average of 4 to 13 shoot buds developed in 100% explants at 10 and 15 µM BAP after four weeks of culture (Figure 1B). However, the shoot buds induced on 20 µM BAP-supplemented media showed poor shoot bud differentiation (Table 1). On the other hand, 100% of the seed segments responded on the WP medium supplemented with 10 and 15 µM KN and developed 3–4 shoot buds per explant. Furthermore, an increase in KN concentration led to the poor differentiation of shoot buds (Table 1). The medium supplemented with 10–20 µM TDZ was not beneficial in triggering the shoot bud differentiation of seed explants of G. livingstonei. The subculture of explants on the medium where the shoot buds were induced was very much essential and shoot elongation was observed after another four weeks in culture (Figure 1C). One more subculture of the shoot clumps on the BAP-containing medium was responsible for the growth of the shoots (Figure 1D). After, two subcultures explants that were growing on the WP medium containing BAP (15 µM) developed an optimum of 13 shoots per explant (Table 1; Scheme 1).

Table 1.
Effect of cytokinins supplemented to WP medium on shoot induction from seed explants of Garcinia livingstonei T. Anderson.
3.2. In Vitro Root Induction
For rooting, the shoots were cultured on a half-strength WP medium with auxins such as IBA and NAA (2.0, 5.0, 10.0, and 20.0 µM). The hormone-free basal medium failed to regenerate roots. The half-strength WP medium supplemented with NAA and IBA demonstrated the rooting of shoots. The IBA supplemented to a half-strength WP medium was found superior in optimum root induction than NAA. The medium supplemented with IBA (10 µM) 100% shoot explants developed 2.0 roots per explant and the root length was also optimum with this treatment (Figure 1E; Table 2).

Table 2.
Effect of auxins supplemented to 1/2 WP medium on rooting of in vitro developed shoots of Garcinia livingstonei T. Anderson.
The well-developed plantlets were removed from the culture media and transferred to pots containing a potting mixture of cocopeat: perlite: vermiculite (1:1:1). After 4 weeks of raising under controlled conditions, the plants were transferred to large pots containing soil, sand, and farmyard manure (1:1:1) and were maintained in the greenhouse. The plantlets showed an 80% of survival rate and transferred plants grew slowly after 6 months of transplantation (Figure 1F).
4. Discussion
The Garcinia species are slow-growing plants, so conventional vegetative propagation such as softwood grafting and the rooting of cuttings are not successful [19]. Additionally, these methods are dependent on the availability of rootstock for grafting and seasonality. Therefore, the propagation of the Garcinia species is through seeds, however, seeds are recalcitrant and they do not survive desiccation or cold temperatures [20]. Additionally, Garcinia seeds exhibit a dormancy that can persist for several months [21]. Therefore, micropropagation methods have been adopted for the regeneration of many Garcinia species such as G. mangostana, G. indica, G. cambogia, G. xanthochymus, and in most of the studies, matured seeds and leaves have been used as the stock material [10,12,22,23,24,25]. Young leaves, apical, and axillary buds have been also used for the in vitro culture establishment [19,26,27]. However, no reports are available for the in vitro propagation of G. livingstonei, consequently, the present investigation was taken up. In the current study, we have used matured seeds as primary experimental material and seed segments were cultured WP medium containing BAP, KN, and TDZ (10, 15, and 20 µM). Among the different concentrations of BAP and KN tested, BAP (15 µM) was found to be an optimum medium for the regeneration of a maximum number of shoots (13 shoots per seed segment) with a high regeneration frequency (100%). Similarly, the promotive effect of BAP in inducing multiple shoots has been previously reported in G. mangostana [11,12] and G. indica [19]. A subculture of explants containing differentiated shoot buds to the fresh medium was found essential for shoot elongation. In contrast to the current results, the subculturing of explants with shoots to a hormone-free medium was reported in G. indica [19].
The rooting of shoots was achieved on the medium supplemented with auxins. Both IBA- and NAA-induced roots from the shoot, however, IBA proved better in inducing a maximum number of roots with the highest average root length. Similar results were reported in G. mangostana [11] and G. indica [19], wherein an IBA-supplemented medium was effective for root induction from the regenerated shoot. The roots were induced directly from the shoot base without an intervening callus phase on all the media tested. In contrast, is the result of Kulkarni and Deodhar [27] on G. inidca where the rooting of shoots occurred through an intervening callus phase on NAA-supplemented media. The hardening of plantlets was essential for their survival. For the hardening, the regenerated plants were initially transferred to pots containing cocopeat: perlite: vermiculite (1:1:1) and reared in controlled environmental conditions for four weeks. Plants that were transferred to pots were irrigated with a Hoagland solution. Such treatments were essential for the transplanted plants to involve in photosynthesis and for the better establishment of plants [28]. In the current study, the G. livingstonei plants were regenerated by using matured seed explants. The Garcinia species possess apomictic seeds and, thus, the regeneration of plants using apomictic seeds leads to a uniform genetic makeup; due to this reason, many researchers have used seeds and seed segments as the primary material for micropropagation [12,17,27].
5. Conclusions
The present study demonstrated a simple and efficient method for high-frequency direct shoot regeneration from the mature seed of G. livingstonei. This present protocol is an attempt to conserve the economically important horticultural tree species with high medicinal value. This protocol could be used for the large-scale multiplication of elite clones of G. livingstonei.
Author Contributions
Conceptualization, H.N.M. and S.-Y.P.; methodology, investigation, S.S., J.-D.L., E.-B.J.; writing—review and editing, S.S., H.N.M., S.-Y.P., J.-D.L. and E.-B.J.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Acknowledgments
H.N.M. is thankful to the National Foundation of Korea for the award Brain Pool Fellowship (2022H1D3A2A02056665).
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO Traditional Food Plants. A Resource Book for Promoting the Exploitation and Consumption of Food Plants in Arid, Semi-Arid, and Sub-Humid Lands of East Africa; FAO Food and Nutrition Paper 42; FAO: Rome, Italy, 1988. [Google Scholar]
- Yang, H.; Figueroa, M.; Satoshi, T.; Baggett, S.; Jiang, B.; Basile, M.J.; Weinstein, I.B.; Kennelly, E.J. Benzophenones and bioflavonoids from Garcinia livingstonei fruits. J. Agric. Food Chem. 2010, 58, 4749–4755. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, F.J.; Claudino, A.L.; Cruz, J.W., Jr.; Chavasco, J.K.; Faria e Silva, P.M.; Veloso, M.P.; Dos Santos, M.H. Antimicrobial activity of benzophenones and extracts from the fruits of Garcinia brasiliensis. J. Med. Food 2009, 12, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.R.; Blunt, J.W.; Munro, M.H.G.; Fuller, R.W.; McKee, T.C.; Cardellina, J.H.I.I.; McMahon, J.B.; Cragg, G.M.; Boyd, M.R. The guttiferones, HIV-inhibitory benzophenones from Symphonia globuliera, Garcinia livingstonei, Garcinia ovalifolia and Clusia rosea. Tetrahedron 1992, 48, 10093–10102. [Google Scholar] [CrossRef]
- Magadula, J.J.; Suleimani, H.O. Cytotoxic and anti-HIV activities of some Tanzanian Garcinia species. Tanzan. J. Health Res. 2010, 12, 1–7. [Google Scholar] [CrossRef]
- Sordat-Diserens, I.; Hamburger, M.; Rogers, C.; Hostettmann, K. Dimeric xanthones from Garcinia livingstonei. Phytochemistry 1992, 31, 3589–3593. [Google Scholar] [CrossRef]
- Lenta, B.N.; Vonthron-Senecheau, C.; Weniger, B.; Devkot, K.P.; Ngoupayo, J.; Kaiser, M.; Naz, Q.; Choudhary, M.I.; Tsamo, E.; Sewald, N. Leishmanicidal and cholinesterase inhibiting activities of phenolic compounds from Allanblackia monticola and Symphonia globulifera. Molecules 2007, 12, 1548–1557. [Google Scholar] [CrossRef]
- Palgrave, M.C. Keith Coates Palgrave trees of Southern Africa, 3rd ed.; Struik Publishers: Cape Town, South Africa, 2002. [Google Scholar]
- Joseph, K.S.; Bolla, S.; Joshi, K.; Bhat, M.A.; Naik, K.; Patil, S.; Bendre, S.; Gangappa, B.; Haibatti, V.; Payamalle, S.; et al. Determination of chemical composition and nutritive value with fatty acid compositions of African Mangosteen (Garcinia livingstonei). Erwerbs-Obstbau 2017, 59, 195–202. [Google Scholar] [CrossRef]
- Patil, L.M.; Murthy, H.N.; Dandin, V.S.; Koli, S.P.; Manohar, S.H.; Payamalle, S. Micropropagation of yellow mangosteen: A valuable endemic tree of India. J. For. Res. 2016, 27, 161–165. [Google Scholar] [CrossRef]
- Goh, H.K.L.; Rao, A.N.; Loh, C.S. Direct shoot bud formation from leaf explant of seedlings and mature mangosteen (Garcinia mangostana L.) trees. Plant Sci. 1990, 68, 113–121. [Google Scholar] [CrossRef]
- Huang, L.C.; Huang, B.L.; Wang, C.H.; Kuo, C.I.; Murashige, T. Developing improved in vitro propagation system for slow growing species using Garcinia mangostana L. (mangosteen). In Vitro Cell. Dev. Biol.—Plant 2000, 36, 501–504. [Google Scholar] [CrossRef]
- Baskaran, M.; Krishnan, S. High-frequency plant regeneration from the mature seeds of Garcinia indica. Biol. Plant 2011, 55, 554–558. [Google Scholar] [CrossRef]
- Deodhar, S.R.; Thengane, R.J.; Thengane, S.R. De nova shoot regeneration from root culture of Garcinia indica Choisy. Indian J. Exp. Biol. 2008, 46, 482–486. [Google Scholar] [PubMed]
- Thengane, S.R.; Deodhar, S.R.; Bhosle, S.V.; Rawal, S.K. Direct somatic embryogenesis and plant regeneration in Garcinia indica Choisy. Curr. Sci. 2006, 91, 1074–1078. [Google Scholar]
- Sheikholeslami, B.; Shukla, M.R.; Turi, C.E.; Harpur, C.; Saxena, P.K. Saving threatened plant species: Reintroduction of Hills Thistle (Cirsium hilli (Canby) Fernald) to its natural habitat. PLoS ONE 2020, 15, e0231741. [Google Scholar] [CrossRef]
- Kulak, V.; Longboat, S.; Brunet, N.D.; Shukla, M.; Saxena, P. In vitro technology in plant conservation: Relevance to biocultural diversity. Plants 2022, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.B.; McCown, B.H. Commercially feasible micropropagation of mountain laurel Kalmia latifolia by use of shoot tip culture. Comb. Proc.—Int. Plant Propag. Soc. 1980, 30, 412–427. [Google Scholar]
- Malik, S.K.; Chaudhury, R.; Kalia, R.K. Rapid in vitro multiplication and conservation of Garcinia indica: A tropical medicinal tree species. Sci. Hortic. 2005, 106, 519–523. [Google Scholar] [CrossRef]
- Noor, N.M.; Aizat, W.M.; Hussin, K.; Rohani, E.R. Seed characteristics and germination properties of four Garcinia (Clusiaceae) fruit species. Fruits 2016, 71, 199–207. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. The great diversity in kinds of seed dormancy: A revision of the Nikolaeva-Baskin classification system for primary seed dormancy. Seed Sci. Res. 2021, 31, 249–277. [Google Scholar] [CrossRef]
- Teo, C.K.H. In vitro culture of mangosteen seed. Acta Hort. 1992, 292, 81–85. [Google Scholar] [CrossRef]
- Goh, C.J.; Prakash, L.; Loh, C.S. High frequency direct shoot bud regeneration from excised leaves of mangosteen (Garcinia mangostana L) trees. Plant Sci. 1994, 68, 113–121. [Google Scholar] [CrossRef]
- Goh, C.J.; Ng, S.K.; Prakash, L.; Loh, C.S. The role of ethylene on direct shoot bud regeneration from mangosteen (Garcinia mangostana L) leave cultured in vitro. Plant Sci. 1997, 124, 193–202. [Google Scholar]
- Normah, M.N.; Noor-Azza, A.B.; Aliudin, R. Factors affecting in vitro shoot proliferation and ex vitro establishment of mangosteen. Plant Cell Tissue Organ Cult. 1995, 43, 291–294. [Google Scholar] [CrossRef]
- Mathew, K.M.; Rao, Y.S.; Kuruvilla, K.M.; Lakshmanan, R.; George, G.L.; Madhusoodanan, K.J.; Potty, S.N. Multiple shoot regeneration in kokum and gamboge. J. Spices Aromatic Crops 2001, 10, 151–152. [Google Scholar]
- Kulkarni, M.D.; Deodhar, M.A. In vitro regeneration and hydroxycitric acid production in tissue cultures of Garcinia indica Choisy. Indian J. Biotech. 2002, 1, 301–304. [Google Scholar]
- Kozai, T.; Koyama, Y.; Watanabe, I. Multiplication of potato plantlets in vitro with sugar free medium under high photosynthetic photon flux. Acta Hort. 1988, 230, 121–127. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).